Abstract
Radiofrequency ablation (RFA) is commonly applied for the treatment of hepatocellular carcinoma (HCC) because of the facile procedure, and the safety and effectiveness for the treatment of this type of tumor. On the other hand, it is believed that HCC cells should spread predominantly through the blood flow of the portal vein, which could lead to the formation of intrahepatic micrometastases. Therefore, monitoring tumor response after the treatment is quite important and accurate assessment of treatment response is critical to obtain the most favorable outcome after the RFA. Indeed, several reports suggested that even small HCCs of ≤ 3 cm in diameter might carry intrahepatic micrometastases and/or microvascular invasion. From this point of view, for preventing local recurrences, RFA should be performed ablating a main tumor as well as its surrounding non-tumorous liver tissue where micrometastases and microvascular invasion might exist. Recent advancement of imaging modalities such as contrast-enhanced ultrasonic, computed tomography, and magnetic resonance imaging are playing an important role on assessing the therapeutic effects of RFA. The local recurrence rate tends to be low in HCC patients who were proven to have adequate ablation margin after RFA; namely, not only disappearance of vascular enhancement of main tumor, but also an adequate ablation margin. Therefore, contrast enhancement gives important findings for the diagnosis of recurrent HCCs on each imaging. However, hyperemia of non-tumorous liver surrounding the ablated lesion, which could be attributed to an inflammation after RFA, may well obscure the findings of local recurrence of HCCs after RFA. Therefore, we need to carefully address to these imaging findings given the fact that diagnostic difficulties of local recurrence of HCC. Here, we give an overview of the current status of the imaging assessment of HCC response to RFA.
Keywords: Hepatocellular carcinoma, Micrometastasis, Microvascular invasion, Radiofrequency ablation, Safety margin
Core tip: Radiofrequency ablation (RFA) therapy is needed to ablate wider range of region than targeted tumor, including surrounding liver tissues that involve micrometastases and microvascular invasion. The local recurrence rate tends to be lower in hepatocellular carcinoma patients with an adequate ablation margin, and thus, it is essential to assess safety margin accurately to reduce local recurrence. From this point of view, we need to focus on the achievement of a sufficient ablation margin as well the lack of tumor vascular enhancement for the assessment of successful RFA. However, inflammatory hyperemia due to RFA which often appears as peripheral rim enhancement, and non-typical imaging features of tumor recurrence sometimes lead to the inappropriate diagnosis.
INTRODUCTION
Surgical resection is the first treatment of choice for hepatocellular carcinoma (HCC). Unfortunately, the majority of HCC patients are not suitable for curative resection at the time of diagnosis because of large tumor size, multifocal disease, vascular involvement, extrahepatic spread, poor liver function, etc.[1-6]. Therefore, there is a need to develop a simple and effective technique to treat unresectable HCC. Several local, minimally invasive hepatic therapies [percutaneous ethanol injection (PEI), acetic acid injection, microwave coagulation therapy, and radiofrequency ablation (RFA)] have been developed to prolong survival in unresectable HCC patients over the past few decades[7-13]. Especially, RFA is currently performed widely due to its ease of use, safety and effectiveness for managing HCC in patients with cirrhosis[14-17], while its high repeatability makes it particularly valuable for controlling intrahepatic recurrences[18].
Monitoring tumor response to therapy is an important part of the clinical management of cancer patients, and accurate assessment of tumor response is essential for favorable outcomes. Imaging techniques such as contrast-enhanced ultrasound (US), computed tomography (CT) and magnetic resonance imaging (MRI) are generally used to diagnose HCC or assess therapeutic effects[19-21]. However, these techniques naturally use different principles to generate images, and the type and dose of contrast agents are different. Contrast enhancement is an important finding on imaging; however, enhancement does not necessarily depict the same phenomenon between US, CT and MRI. Therefore, we need to be familiar with the findings on each modality after ablation to evaluate the success of treatment, detect residual or recurrent tumors, and diagnose new lesions.
In this review, we focus our discussion on the imaging assessment of HCC response to RFA.
CLINICOPATHOLOGICAL FEATURES OF HCC FOR ABLATION
HCC cells spread mainly via the portal system and form intrahepatic micrometastases[22,23]. Among risk factors for recurrences, tumor size, portal vein invasion, and intrahepatic metastasis are generally considered the major causes of intrahepatic HCC recurrence after treatment. A previous pathologic study showed that intrahepatic metastasis occurs in 10% of cases even in early HCC (lesions < 2 cm in diameter)[22]. Okusaka et al[24] reported that 19% of HCC nodules of 3.0 cm or less in diameter had satellite lesions that were not detected during pre-treatment evaluation. Nakashima et al[25] revealed that 59.1% of small HCC of ≤ 3.0 cm in diameter had micrometastases within 5 mm of each micrometastatic lesion and its primary HCC. Especially, among single nodular type HCC, micrometastases were shown in 77.8% within 5 mm.
Several studies address the relation between microvascular invasion of HCC cells and tumor size. Kojiro et al[22] reported that the tumor invades the portal vein in 27% of cases even in early HCC (lesions < 2 cm in dimension). Esnaola et al[26] found the frequency of microvascular invasion to be 25% and 31% for tumors < 2 cm and 2-4 cm in the greatest dimension, respectively. On the other hand, microvascular invasion was shown in 17% of patients with tumors < 2 cm and 20% of patients with tumors 2-3 cm[24]. The reported frequency of microvascular invasion in patients with an HCC tumor of 2-3 cm ranges from approximately 20%-30%. Thus, it has reported that the risk factors for early local tumor recurrence were larger tumor size, poor pathologic differentiation of tumor cells and advanced tumor staging[27,28].
PRINCIPLE OF TUMOR RESPONSE ASSESSMENT TO RFA FOR HCC
Efficacy of treatment is usually monitored radiologically. Effective treatment is indicated by not only lack of vascular enhancement of HCC, but also the safety margin. The safety margin is ablated peritumoral liver tissue that is located between a necrotic tumor and unablated liver tissue (Figure 1). For the RFA procedure to be considered technically successful, the tumor and at least a 5 mm safety margin must be included in the ablation zone[29]. The local recurrence rate differs markedly depending on whether or not a 5 mm safety margin is secured. Kudo et al[14] reported that the local recurrence rate was 2.6% in HCC patients with a ≥ 5 mm safety margin at 2 years after RFA, whereas it was 20.8% in HCC patients without safety margin (P = 0.01). Another report indicated that the significant risk factors for local recurrence of RFA were a tumor with a diameter ≥ 2.3 cm, an insufficient safety margin, and a multinodular tumor[30]. In addition, the safety margin has one more important role as security in avoiding limitations on CT assessment due to a partial volume effect.
Figure 1.
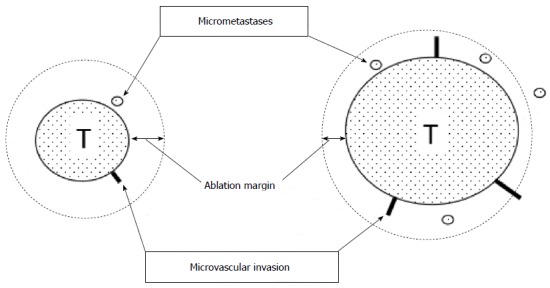
Ablation margin and micrometastases/microvascular invasion. Radiofrequency ablation (RFA) therapy is required to ablate the main tumor and its surrounding liver tissues involving micrometastases and microvascular invasion. However, as the tumor get bigger, micrometastases and microcascular invasion frequently occur. Unablated lesions lead to local recurrences after RFA. T: Tumor.
Alpha-fetoprotein (AFP), lens culinaris agglutinin-reactive fraction of AFP (AFP-L3), and des-γ-carboxy prothrombin (DCP) have been used as tumor markers for HCC[31-34]. Levels of tumor markers often fall to within the normal range after effective treatment and rise before tumor relapse is detected by imaging studies. However, sensitivity and specificity of tumor markers are insufficient to detect HCC in all patient samples, and the monitoring of tumor marker levels after therapy does not replace imaging[35].
Recurrence of tumors in the treated area or elsewhere is defined as re-appearance of vascular enhancement. The ideal imaging interval is unknown, but initially a 3-4 mo interval is commonly used to monitor HCC lesions after initial treatment. After about 2 years of recurrence-free survival, the interval of follow-up imaging examinations can be at less frequent intervals[35].
Imaging
CT: Contrast-enhanced CT has been most widely used for the evaluation of treatment response after RFA because of the advantages of CT: the rapid acquisition of images, clear and specific information, and the referring of a wide range of the abdomen including the liver. After a bolus dispense of contrast agent, tissue contrast enhancement depends on arterial blood flow, capillary permeability, rate of diffusion, and extravascular extracellular space volume. In clinical practice, evaluation of successful treatment was based on a visual comparison of the pre- and post-RFA CT images by referring specific landmarks such as hepatic vessels and the liver surface[14,36,37]. If the non-enhancing ablation zone included the original tumor and an adequate safety margin in all directions, the RFA should be regarded as technically successful[38]. Sala et al[39] revealed that the independent predictors of survival were Child-Pugh class (P = 0.0001) and initial complete response to percutaneous ablation (P = 0.006). Among patients classified as Child-Pugh grade A, a 20% difference of survival rate was achieved at 5 years (42% in responders vs 18% in non-responders), while among patients classified as Child-Pugh grade B, the difference of survival rate was observed at 3 years (42% in responders vs 16% in non-responders).
Compared with RFA alone, the combination of RFA and transcatheter arterial chemoembolization (TACE) markedly increased the extent of induced coagulation of RFA[40]. Combined TACE and RFA have several advantages over RFA treatment alone. Theoretically, embolization along with the chemotherapy is synergic to thermal ablation by lowering the convection by vascular flow, decreasing the impedance in the tumor and facilitating heat distribution within the tumor. Moreover, satellite nodules, which are found more commonly around large HCCs can be depicted by Lipiodol spots. Thus, RFA combined with TACE has been reported to be promising for local control of medium-size of tumors[41-44]. In addition, Lipiodol-TACE could offer another advantage for assessment of following RFA procedure. When the ablated area could cover the whole HCC with a sufficient margin, the ablative margin can be shown as the boundary between the high density area as Lipiodol accumulation in HCC and the enhancing area as surrounding normal liver parenchyma. As the area of Lipiodol deposition is an ideal landmark of the tumor margin, the successful safety margin can be easily evaluated only by post-RFA dynamic CT images in HCC patients treated by RFA combined with TACE, without a comparison of the pre- and post-RFA CT images (Figure 2)[45].
Figure 2.
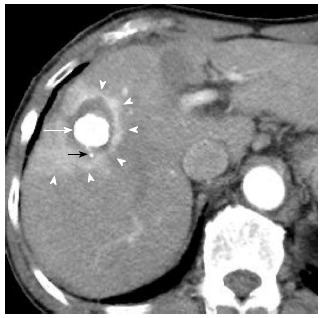
A 80-year-old woman with 2.5 cm hepatocellular carcinoma after radiofrequency ablation combined with transcatheter arterial chemoembolization. Early-phase dynamic computed tomography shows a high-density center indicating Lipiodol deposition in hepatocellular carcinoma (white arrow) and a surrounding low-density zone indicating radiofrequency ablation-induced coagulation necrosis of the liver. A microsatellite (black arrow) was depicted as a high-density spot in the low-density zone. Therefore, this ablation therapy achieved complete necrosis of chief tumor and micrometastasis. Moreover, hyperemia surrounding the ablated lesion is depicted as peripheral rim enhancement (arrowheads).
Hyperemia in tissue surrounding the ablated lesion can represent an inflammatory reaction due to thermal injury. Peripheral rim enhancement resulting from reactive hyperemia is usually uniform in thickness and envelops the ablated lesion (Figure 2), whereas residual HCC demonstrates focal and irregular peripheral enhancement[46,47]. However, differentiation of reactive hyperemia from residual HCCs is sometimes difficult. Moreover, typical imaging features (arterial enhancement followed by delayed washout on dynamic contrast-enhanced CT) are not usually depicted for the diagnosis of recurrent HCCs. Mikami et al[48] reported that 17.5% of patients were diagnosed as local recurrent HCC with typical enhancement pattern, while 40.6% had arterial hypervascularity without washout in the portal venous phase and 11.9% showed washout in portal venous phase without arterial hypervascularity. A non-typical enhancement pattern of local HCC recurrence may reflect the fact that insufficient RFA therapy could lead to further malignant transformation of HCC[49]. Therefore, careful comparison with imaging before ablation and close follow-up are necessary in in patients who showed unusual pattern of enhancement in the liver after RFA.
US: US contrast agents consist of gas-cored microbubbles that are encapsulated by a shell constructed of a lipid monolayer or cross-linked albumin. Each bubble acts as a harmonic oscillator and contrast-enhanced echo signals contain significant energy components at higher harmonics, while tissue echoes do not. With the use of a contrast agent, contrast harmonic imaging possesses not only a very high sensitivity to contrast agents but also a high spatial resolution, and can depict signals from microbubbles with a very slow flow. Several researchers have reported that contrast-enhanced US is a useful tool for assessing the vascularity of local recurrence of HCCs[50-54]. The detectability of viable HCCs was 83.5% in B-mode US and increased to 93.2% in contrast-enhanced US, using contrast-enhanced CT was used as the reference standard[55]. As reported by Kim et al[56], the diagnostic concordance between the contrast-enhanced US just after the RFA and the CT after the 1-mo follow-up was 99% in terms of the assessment of the therapeutic response to RFA. The sensitivity, specificity, and diagnostic accuracy of contrast-enhanced US were 95.3%, 100%, and 98.1%, respectively[57]. Consequently, contrast-enhanced US may provide an alternative approach that shows high diagnostic concordance with dynamic CT in assessing the therapeutic response of RFA in hypervascular HCC (Figure 3). However, it is often difficult to identify the safety margin on US in the some cases. Zhou et al[58] found that contrast-enhanced US could not evaluate safety margin in 34.8% of HCC nodules because the tumor boundary could not be identified clearly by US after RFA. Therefore, contrast-enhanced US and contrast-enhanced CT should carry a complementary role for the evaluation of the treatment response after RFA.
Figure 3.
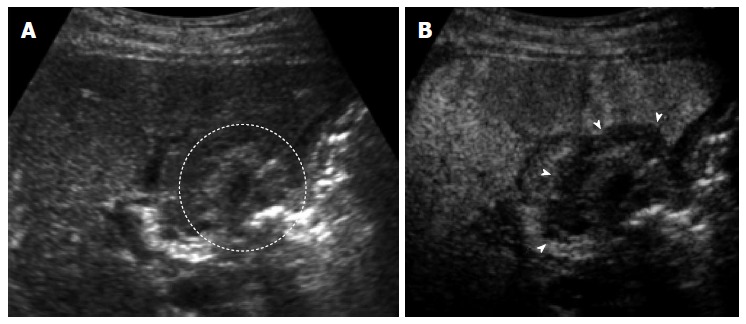
A 70-year-old man with 1.5 cm hepatocellular carcinoma after radiofrequency ablation. A: The ablated tumor is depicted as hyper echoic lesion (circle) on B-mode ultrasound (US). However, the boundary between ablated area and unablated liver tissue could not be identified clearly; B: Contrast-enhanced US using Sonazoid shows the defect (arrows) in the Kupffer phase. The ablation margin is shown as low echoic zone surrounding the ablated tumor.
Perfluorocarbon microbubbles (Sonazoid) is classified as second-generation US-contrast agents. Unlike others, perfluorocarbon microbubbles are not trapped by Kupffer cells. A double contrast US technique using Sonazoid reinjection has been developed on the basis of two specific characteristics of Sonazoid: real-time blood flow images with low acoustic power and robust Kupffer images tolerable for repeated scanning in the Kupffer phase[59-61]. According to contrast-enhanced US using Sonazoid, peripheral hyperemia areas show hyper-echogenicity during the early vascular phase and iso-echogenicity as adjacent liver parenchyma during the Kupffer phase. On the other hand, residual HCC demonstrates a focal defect during the Kupffer phase and represents hypervascular enhancement by the reinjection of Sonazoid. Therefore, differentiation of reactive hyperemia from residual HCCs is not difficult. Dynamic contrast-enhanced US guidance in ablation therapy for locally recurrent HCCs should be an efficient approach[61].
MRI: MRI provides better contrast between the different soft tissues and higher spatial resolution with sensitivity than CT. Recent advances in MRI allow imaging of the liver with a high spatial resolution during a single breath-hold. Khankan et al[62] reported that a hyperintense zone on non-enhanced T1-weighted MRI within 2 d after RFA reflected the extent of the ablated region. Evaluation of the safety margin also needs comparisons of the pre- and post-RFA images because of the blurriness of tumor boundary on non-enhanced MRI after RFA.
Hepatocyte-specific MRI contrast agents were developed for detection and characterization of focal liver lesions. Gadolinium-ethoxybenzyl-diethylenetriamine pentaacetic acid (Gd-EOB-DTPA) is a contrast agent with combined properties of a conventional non-specific extracellular and hepatocyte-specific contrast agent[63]. It is recognized that hepatocyte phase images help to distinguish vascular pseudolesions (e.g., those due to arterioportal shunting, portal venule obstruction, nonportal splanchnic veins, or rib compression) from hypervascular tumors[64]. Meanwhile, a recent study[65] reported that more than 10% of vascular pseudolesions showed hypointensity on hepatocyte-phase images and that those pseudolesions occasionally mimicked the configurations and signal intensities of HCCs. Watanabe et al[66] analyzed the image of HCC tumors using the area under the receiver operating characteristic (ROC) curve, and concluded that the incorporation of hepatocyte phase images did not improve the diagnostic accuracy of Gd-EOB-DTPA-enhanced MRI for locally recurrent HCCs after RFA. On the other hand, Koda et al[67] reported the ablative margin grading assessment using superparamagnetic iron oxide (SPIO)-enhanced MRI. They intravenously injected ferucarbotran (0.016 mL/kg body weight) 20-60 min before RFA, and performed MRI at 7 d after RFA. Because SPIO remained in ablated hepatic parenchyma, post-ablation MRI showed a high-intensity area of HCC surrounding by low-intensity area of ablative margin.
CONCLUSION
The prognosis of patients with small HCC is still unsatisfactory because of frequent recurrence even after complete ablation. The high recurrence rate may be attributed to the undefined satellite lesions or microvascular invasion before treatment, which are too small to be detected with the current imaging modality. For the procedure of local ablation therapies including RFA, we need to ablate wider range of region than targeted tumor, including surrounding non-tumorous liver tissues that could involve micrometastases and microvascular invasion. The local recurrence rate tends to be lower in HCC patients with an adequate ablation margin, and thus, it is essential to assess safety margin accurately to reduce local recurrence. From this point of view, we need to focus on the achievement of a sufficient ablation margin as well the lack of tumor vascular enhancement for the assessment of successful RFA. However, inflammatory hyperemia due to RFA which often appears as peripheral rim enhancement, and non-typical imaging features of tumor recurrence sometimes lead to the inappropriate diagnosis. Therefore, we need to be careful for the imaging findings given the fact that the diagnostic difficulties for local recurrence of HCC. Careful comparison of imaging before ablation and close follow-up are critical in HCC patients treated with RFA.
Footnotes
P- Reviewers: Beierle EA, Nagai H, Tanaka K S- Editor: Wen LL L- Editor: A E- Editor: Liu XM
References
- 1.Minagawa M, Makuuchi M, Takayama T, Kokudo N. Selection criteria for repeat hepatectomy in patients with recurrent hepatocellular carcinoma. Ann Surg. 2003;238:703–710. doi: 10.1097/01.sla.0000094549.11754.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Capussotti L, Muratore A, Massucco P, Ferrero A, Polastri R, Bouzari H. Major liver resections for hepatocellular carcinoma on cirrhosis: early and long-term outcomes. Liver Transpl. 2004;10:S64–S68. doi: 10.1002/lt.20035. [DOI] [PubMed] [Google Scholar]
- 3.Poon RT, Fan ST. Hepatectomy for hepatocellular carcinoma: patient selection and postoperative outcome. Liver Transpl. 2004;10:S39–S45. doi: 10.1002/lt.20040. [DOI] [PubMed] [Google Scholar]
- 4.Song TJ, Ip EW, Fong Y. Hepatocellular carcinoma: current surgical management. Gastroenterology. 2004;127:S248–S260. doi: 10.1053/j.gastro.2004.09.039. [DOI] [PubMed] [Google Scholar]
- 5.Llovet JM, Schwartz M, Mazzaferro V. Resection and liver transplantation for hepatocellular carcinoma. Semin Liver Dis. 2005;25:181–200. doi: 10.1055/s-2005-871198. [DOI] [PubMed] [Google Scholar]
- 6.Lin S, Hoffmann K, Schemmer P. Treatment of Hepatocellular Carcinoma: A Systematic Review. Liver Cancer. 2012;1:144–158. doi: 10.1159/000343828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shiina S, Tagawa K, Unuma T, Takanashi R, Yoshiura K, Komatsu Y, Hata Y, Niwa Y, Shiratori Y, Terano A. Percutaneous ethanol injection therapy for hepatocellular carcinoma. A histopathologic study. Cancer. 1991;68:1524–1530. doi: 10.1002/1097-0142(19911001)68:7<1524::aid-cncr2820680711>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 8.Livraghi T, Bolondi L, Lazzaroni S, Marin G, Morabito A, Rapaccini GL, Salmi A, Torzilli G. Percutaneous ethanol injection in the treatment of hepatocellular carcinoma in cirrhosis. A study on 207 patients. Cancer. 1992;69:925–929. doi: 10.1002/1097-0142(19920215)69:4<925::aid-cncr2820690415>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 9.Seki T, Wakabayashi M, Nakagawa T, Itho T, Shiro T, Kunieda K, Sato M, Uchiyama S, Inoue K. Ultrasonically guided percutaneous microwave coagulation therapy for small hepatocellular carcinoma. Cancer. 1994;74:817–825. doi: 10.1002/1097-0142(19940801)74:3<817::aid-cncr2820740306>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 10.Seki T, Wakabayashi M, Nakagawa T, Imamura M, Tamai T, Nishimura A, Yamashiki N, Okamura A, Inoue K. Percutaneous microwave coagulation therapy for patients with small hepatocellular carcinoma: comparison with percutaneous ethanol injection therapy. Cancer. 1999;85:1694–1702. [PubMed] [Google Scholar]
- 11.Ohnishi K, Yoshioka H, Ito S, Fujiwara K. Prospective randomized controlled trial comparing percutaneous acetic acid injection and percutaneous ethanol injection for small hepatocellular carcinoma. Hepatology. 1998;27:67–72. doi: 10.1002/hep.510270112. [DOI] [PubMed] [Google Scholar]
- 12.Rossi S, Di Stasi M, Buscarini E, Cavanna L, Quaretti P, Squassante E, Garbagnati F, Buscarini L. Percutaneous radiofrequency interstitial thermal ablation in the treatment of small hepatocellular carcinoma. Cancer J Sci Am. 1995;1:73–81. [PubMed] [Google Scholar]
- 13.Curley SA, Izzo F, Ellis LM, Nicolas Vauthey J, Vallone P. Radiofrequency ablation of hepatocellular cancer in 110 patients with cirrhosis. Ann Surg. 2000;232:381–391. doi: 10.1097/00000658-200009000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kudo M. Local ablation therapy for hepatocellular carcinoma: current status and future perspectives. J Gastroenterol. 2004;39:205–214. doi: 10.1007/s00535-003-1280-y. [DOI] [PubMed] [Google Scholar]
- 15.Shiina S, Teratani T, Obi S, Sato S, Tateishi R, Fujishima T, Ishikawa T, Koike Y, Yoshida H, Kawabe T, et al. A randomized controlled trial of radiofrequency ablation with ethanol injection for small hepatocellular carcinoma. Gastroenterology. 2005;129:122–130. doi: 10.1053/j.gastro.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 16.Llovet JM, Bruix J. Novel advancements in the management of hepatocellular carcinoma in 2008. J Hepatol. 2008;48 Suppl 1:S20–S37. doi: 10.1016/j.jhep.2008.01.022. [DOI] [PubMed] [Google Scholar]
- 17.Lencioni R, Crocetti L. Local-regional treatment of hepatocellular carcinoma. Radiology. 2012;262:43–58. doi: 10.1148/radiol.11110144. [DOI] [PubMed] [Google Scholar]
- 18.Rossi S, Ravetta V, Rosa L, Ghittoni G, Viera FT, Garbagnati F, Silini EM, Dionigi P, Calliada F, Quaretti P, et al. Repeated radiofrequency ablation for management of patients with cirrhosis with small hepatocellular carcinomas: a long-term cohort study. Hepatology. 2011;53:136–147. doi: 10.1002/hep.23965. [DOI] [PubMed] [Google Scholar]
- 19.Lee JM, Yoon JH, Joo I, Woo HS. Recent Advances in CT and MR Imaging for Evaluation of Hepatocellular Carcinoma. Liver Cancer. 2012;1:22–40. doi: 10.1159/000339018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Minami Y, Kudo M. Therapeutic response assessment of transcatheter arterial chemoembolization for hepatocellular carcinoma: ultrasonography, CT and MR imaging. Oncology. 2013;84 Suppl 1:58–63. doi: 10.1159/000345891. [DOI] [PubMed] [Google Scholar]
- 21.Minami Y, Kitai S, Kudo M. Treatment response assessment of radiofrequency ablation for hepatocellular carcinoma: usefulness of virtual CT sonography with magnetic navigation. Eur J Radiol. 2012;81:e277–e280. doi: 10.1016/j.ejrad.2011.02.026. [DOI] [PubMed] [Google Scholar]
- 22.Kojiro M. Pathology of early hepatocellular carcinoma: progression from early to advanced. Hepatogastroenterology. 1998;45 Suppl 3:1203–1205. [PubMed] [Google Scholar]
- 23.Sasaki A, Kai S, Iwashita Y, Hirano S, Ohta M, Kitano S. Microsatellite distribution and indication for locoregional therapy in small hepatocellular carcinoma. Cancer. 2005;103:299–306. doi: 10.1002/cncr.20798. [DOI] [PubMed] [Google Scholar]
- 24.Okusaka T, Okada S, Ueno H, Ikeda M, Shimada K, Yamamoto J, Kosuge T, Yamasaki S, Fukushima N, Sakamoto M. Satellite lesions in patients with small hepatocellular carcinoma with reference to clinicopathologic features. Cancer. 2002;95:1931–1937. doi: 10.1002/cncr.10892. [DOI] [PubMed] [Google Scholar]
- 25.Nakashima Y, Nakashima O, Tanaka M, Okuda K, Nakashima M, Kojiro M. Portal vein invasion and intrahepatic micrometastasis in small hepatocellular carcinoma by gross type. Hepatol Res. 2003;26:142–147. doi: 10.1016/s1386-6346(03)00007-x. [DOI] [PubMed] [Google Scholar]
- 26.Esnaola NF, Lauwers GY, Mirza NQ, Nagorney DM, Doherty D, Ikai I, Yamaoka Y, Regimbeau JM, Belghiti J, Curley SA, et al. Predictors of microvascular invasion in patients with hepatocellular carcinoma who are candidates for orthotopic liver transplantation. J Gastrointest Surg. 2002;6:224–232; discussion 232. doi: 10.1016/s1091-255x(01)00015-4. [DOI] [PubMed] [Google Scholar]
- 27.Lin SM, Lin CJ, Lin CC, Hsu CW, Chen YC. Randomised controlled trial comparing percutaneous radiofrequency thermal ablation, percutaneous ethanol injection, and percutaneous acetic acid injection to treat hepatocellular carcinoma of 3 cm or less. Gut. 2005;54:1151–1156. doi: 10.1136/gut.2004.045203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yu HC, Cheng JS, Lai KH, Lin CP, Lo GH, Lin CK, Hsu PI, Chan HH, Lo CC, Tsai WL, et al. Factors for early tumor recurrence of single small hepatocellular carcinoma after percutaneous radiofrequency ablation therapy. World J Gastroenterol. 2005;11:1439–1444. doi: 10.3748/wjg.v11.i10.1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goldberg SN, Grassi CJ, Cardella JF, Charboneau JW, Dodd GD, Dupuy DE, Gervais D, Gillams AR, Kane RA, Lee FT, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria. J Vasc Interv Radiol. 2005;16:765–778. doi: 10.1097/01.RVI.0000170858.46668.65. [DOI] [PubMed] [Google Scholar]
- 30.Zytoon AA, Ishii H, Murakami K, El-Kholy MR, Furuse J, El-Dorry A, El-Malah A. Recurrence-free survival after radiofrequency ablation of hepatocellular carcinoma. A registry report of the impact of risk factors on outcome. Jpn J Clin Oncol. 2007;37:658–672. doi: 10.1093/jjco/hym086. [DOI] [PubMed] [Google Scholar]
- 31.Marrero JA, Feng Z, Wang Y, Nguyen MH, Befeler AS, Roberts LR, Reddy KR, Harnois D, Llovet JM, Normolle D, et al. Alpha-fetoprotein, des-gamma carboxyprothrombin, and lectin-bound alpha-fetoprotein in early hepatocellular carcinoma. Gastroenterology. 2009;137:110–118. doi: 10.1053/j.gastro.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yamamoto K, Imamura H, Matsuyama Y, Kume Y, Ikeda H, Norman GL, Shums Z, Aoki T, Hasegawa K, Beck Y, et al. AFP, AFP-L3, DCP, and GP73 as markers for monitoring treatment response and recurrence and as surrogate markers of clinicopathological variables of HCC. J Gastroenterol. 2010;45:1272–1282. doi: 10.1007/s00535-010-0278-5. [DOI] [PubMed] [Google Scholar]
- 33.Toyoda H, Kumada T, Tada T, Niinomi T, Ito T, Kaneoka Y, Maeda A. Prognostic significance of a combination of pre- and post-treatment tumor markers for hepatocellular carcinoma curatively treated with hepatectomy. J Hepatol. 2012;57:1251–1257. doi: 10.1016/j.jhep.2012.07.018. [DOI] [PubMed] [Google Scholar]
- 34.Song P, Gao J, Inagaki Y, Kokudo N, Hasegawa K, Sugawara Y, Tang W. Biomarkers: Evaluation of Screening for and Early Diagnosis of Hepatocellular Carcinoma in Japan and China. Liver Cancer. 2013;2:31–39. doi: 10.1159/000346220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–1022. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Okuwaki Y, Nakazawa T, Shibuya A, Ono K, Hidaka H, Watanabe M, Kokubu S, Saigenji K. Intrahepatic distant recurrence after radiofrequency ablation for a single small hepatocellular carcinoma: risk factors and patterns. J Gastroenterol. 2008;43:71–78. doi: 10.1007/s00535-007-2123-z. [DOI] [PubMed] [Google Scholar]
- 37.Tateishi R, Shiina S, Ohki T, Sato T, Masuzaki R, Imamura J, Goto E, Goto T, Yoshida H, Obi S, et al. Treatment strategy for hepatocellular carcinoma: expanding the indications for radiofrequency ablation. J Gastroenterol. 2009;44 Suppl 19:142–146. doi: 10.1007/s00535-008-2247-9. [DOI] [PubMed] [Google Scholar]
- 38.Kim KW, Lee JM, Klotz E, Kim SJ, Kim SH, Kim JY, Han JK, Choi BI. Safety margin assessment after radiofrequency ablation of the liver using registration of preprocedure and postprocedure CT images. AJR Am J Roentgenol. 2011;196:W565–W572. doi: 10.2214/AJR.10.5122. [DOI] [PubMed] [Google Scholar]
- 39.Sala M, Llovet JM, Vilana R, Bianchi L, Solé M, Ayuso C, Brú C, Bruix J. Initial response to percutaneous ablation predicts survival in patients with hepatocellular carcinoma. Hepatology. 2004;40:1352–1360. doi: 10.1002/hep.20465. [DOI] [PubMed] [Google Scholar]
- 40.Kitamoto M, Imagawa M, Yamada H, Watanabe C, Sumioka M, Satoh O, Shimamoto M, Kodama M, Kimura S, Kishimoto K, et al. Radiofrequency ablation in the treatment of small hepatocellular carcinomas: comparison of the radiofrequency effect with and without chemoembolization. AJR Am J Roentgenol. 2003;181:997–1003. doi: 10.2214/ajr.181.4.1810997. [DOI] [PubMed] [Google Scholar]
- 41.Kagawa T, Koizumi J, Kojima S, Nagata N, Numata M, Watanabe N, Watanabe T, Mine T; Tokai RFA Study Group. Transcatheter arterial chemoembolization plus radiofrequency ablation therapy for early stage hepatocellular carcinoma: comparison with surgical resection. Cancer. 2010;116:3638–3644. doi: 10.1002/cncr.25142. [DOI] [PubMed] [Google Scholar]
- 42.Peng ZW, Zhang YJ, Liang HH, Lin XJ, Guo RP, Chen MS. Recurrent hepatocellular carcinoma treated with sequential transcatheter arterial chemoembolization and RF ablation versus RF ablation alone: a prospective randomized trial. Radiology. 2012;262:689–700. doi: 10.1148/radiol.11110637. [DOI] [PubMed] [Google Scholar]
- 43.Lu Z, Wen F, Guo Q, Liang H, Mao X, Sun H. Radiofrequency ablation plus chemoembolization versus radiofrequency ablation alone for hepatocellular carcinoma: a meta-analysis of randomized-controlled trials. Eur J Gastroenterol Hepatol. 2013;25:187–194. doi: 10.1097/MEG.0b013e32835a0a07. [DOI] [PubMed] [Google Scholar]
- 44.Peng ZW, Zhang YJ, Chen MS, Xu L, Liang HH, Lin XJ, Guo RP, Zhang YQ, Lau WY. Radiofrequency ablation with or without transcatheter arterial chemoembolization in the treatment of hepatocellular carcinoma: a prospective randomized trial. J Clin Oncol. 2013;31:426–432. doi: 10.1200/JCO.2012.42.9936. [DOI] [PubMed] [Google Scholar]
- 45.Nishikawa H, Inuzuka T, Takeda H, Nakajima J, Sakamoto A, Henmi S, Matsuda F, Eso Y, Ishikawa T, Saito S, et al. Percutaneous radiofrequency ablation therapy for hepatocellular carcinoma: a proposed new grading system for the ablative margin and prediction of local tumor progression and its validation. J Gastroenterol. 2011;46:1418–1426. doi: 10.1007/s00535-011-0452-4. [DOI] [PubMed] [Google Scholar]
- 46.Limanond P, Zimmerman P, Raman SS, Kadell BM, Lu DS. Interpretation of CT and MRI after radiofrequency ablation of hepatic malignancies. AJR Am J Roentgenol. 2003;181:1635–1640. doi: 10.2214/ajr.181.6.1811635. [DOI] [PubMed] [Google Scholar]
- 47.Kim SK, Lim HK, Kim YH, Lee WJ, Lee SJ, Kim SH, Lim JH, Kim SA. Hepatocellular carcinoma treated with radio-frequency ablation: spectrum of imaging findings. Radiographics. 2003;23:107–121. doi: 10.1148/rg.231025055. [DOI] [PubMed] [Google Scholar]
- 48.Mikami S, Tateishi R, Akahane M, Asaoka Y, Kondo Y, Goto T, Shiina S, Yoshida H, Koike K. Computed tomography follow-up for the detection of hepatocellular carcinoma recurrence after initial radiofrequency ablation: a single-center experience. J Vasc Interv Radiol. 2012;23:1269–1275. doi: 10.1016/j.jvir.2012.06.032. [DOI] [PubMed] [Google Scholar]
- 49.Obara K, Matsumoto N, Okamoto M, Kobayashi M, Ikeda H, Takahashi H, Katakura Y, Matsunaga K, Ishii T, Okuse C, et al. Insufficient radiofrequency ablation therapy may induce further malignant transformation of hepatocellular carcinoma. Hepatol Int. 2008;2:116–123. doi: 10.1007/s12072-007-9040-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Meloni MF, Goldberg SN, Livraghi T, Calliada F, Ricci P, Rossi M, Pallavicini D, Campani R. Hepatocellular carcinoma treated with radiofrequency ablation: comparison of pulse inversion contrast-enhanced harmonic sonography, contrast-enhanced power Doppler sonography, and helical CT. AJR Am J Roentgenol. 2001;177:375–380. doi: 10.2214/ajr.177.2.1770375. [DOI] [PubMed] [Google Scholar]
- 51.Vilana R1, Bianchi L, Varela M, Nicolau C, Sánchez M, Ayuso C, García M, Sala M, Llovet JM, Bruix J, Bru C; BCLC Group. Is microbubble-enhanced ultrasonography sufficient for assessment of response to percutaneous treatment in patients with early hepatocellular carcinoma? Eur Radiol. 2006;16:2454–2462. doi: 10.1007/s00330-006-0264-8. [DOI] [PubMed] [Google Scholar]
- 52.Minami Y, Kudo M. Review of dynamic contrast-enhanced ultrasound guidance in ablation therapy for hepatocellular carcinoma. World J Gastroenterol. 2011;17:4952–4959. doi: 10.3748/wjg.v17.i45.4952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Inoue T, Kudo M, Hatanaka K, Arizumi T, Takita M, Kitai S, Yada N, Hagiwara S, Minami Y, Sakurai T, et al. Usefulness of contrast-enhanced ultrasonography to evaluate the post-treatment responses of radiofrequency ablation for hepatocellular carcinoma: comparison with dynamic CT. Oncology. 2013;84 Suppl 1:51–57. doi: 10.1159/000345890. [DOI] [PubMed] [Google Scholar]
- 54.Salvatore V, Bolondi L. Clinical Impact of Ultrasound-Related Techniques on the Diagnosis of Focal Liver Lesions. Liver Cancer. 2012;1:238–246. doi: 10.1159/000343838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Masuzaki R, Shiina S, Tateishi R, Yoshida H, Goto E, Sugioka Y, Kondo Y, Goto T, Ikeda H, Omata M, et al. Utility of contrast-enhanced ultrasonography with Sonazoid in radiofrequency ablation for hepatocellular carcinoma. J Gastroenterol Hepatol. 2011;26:759–764. doi: 10.1111/j.1440-1746.2010.06559.x. [DOI] [PubMed] [Google Scholar]
- 56.Kim CK, Choi D, Lim HK, Kim SH, Lee WJ, Kim MJ, Lee JY, Jeon YH, Lee J, Lee SJ, et al. Therapeutic response assessment of percutaneous radiofrequency ablation for hepatocellular carcinoma: utility of contrast-enhanced agent detection imaging. Eur J Radiol. 2005;56:66–73. doi: 10.1016/j.ejrad.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 57.Wen YL, Kudo M, Zheng RQ, Minami Y, Chung H, Suetomi Y, Onda H, Kitano M, Kawasaki T, Maekawa K. Radiofrequency ablation of hepatocellular carcinoma: therapeutic response using contrast-enhanced coded phase-inversion harmonic sonography. AJR Am J Roentgenol. 2003;181:57–63. doi: 10.2214/ajr.181.1.1810057. [DOI] [PubMed] [Google Scholar]
- 58.Zhou P, Kudo M, Minami Y, Chung H, Inoue T, Fukunaga T, Maekawa K. What is the best time to evaluate treatment response after radiofrequency ablation of hepatocellular carcinoma using contrast-enhanced sonography? Oncology. 2007;72 Suppl 1:92–97. doi: 10.1159/000111713. [DOI] [PubMed] [Google Scholar]
- 59.Kudo M. New sonographic techniques for the diagnosis and treatment of hepatocellular carcinoma. Hepatol Res. 2007;37 Suppl 2:S193–S199. doi: 10.1111/j.1872-034X.2007.00184.x. [DOI] [PubMed] [Google Scholar]
- 60.Kudo M, Hatanaka K, Maekawa K. Newly developed novel ultrasound technique, defect reperfusion ultrasound imaging, using sonazoid in the management of hepatocellular carcinoma. Oncology. 2010;78 Suppl 1:40–45. doi: 10.1159/000315229. [DOI] [PubMed] [Google Scholar]
- 61.Kudo M, Hatanaka K, Kumada T, Toyoda H, Tada T. Double-contrast ultrasound: a novel surveillance tool for hepatocellular carcinoma. Am J Gastroenterol. 2011;106:368–370. doi: 10.1038/ajg.2010.432. [DOI] [PubMed] [Google Scholar]
- 62.Khankan AA, Murakami T, Onishi H, Matsushita M, Iannaccone R, Aoki Y, Tono T, Kim T, Hori M, Osuga K, et al. Hepatocellular carcinoma treated with radio frequency ablation: an early evaluation with magnetic resonance imaging. J Magn Reson Imaging. 2008;27:546–551. doi: 10.1002/jmri.21050. [DOI] [PubMed] [Google Scholar]
- 63.Campos JT, Sirlin CB, Choi JY. Focal hepatic lesions in Gd-EOB-DTPA enhanced MRI: the atlas. Insights Imaging. 2012;3:451–474. doi: 10.1007/s13244-012-0179-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yoon JH, Lee EJ, Cha SS, Han SS, Choi SJ, Juhn JR, Kim MH, Lee YJ, Park SJ. Comparison of gadoxetic acid-enhanced MR imaging versus four-phase multi-detector row computed tomography in assessing tumor regression after radiofrequency ablation in subjects with hepatocellular carcinomas. J Vasc Interv Radiol. 2010;21:348–356. doi: 10.1016/j.jvir.2009.11.014. [DOI] [PubMed] [Google Scholar]
- 65.Motosugi U, Ichikawa T, Sou H, Sano K, Tominaga L, Muhi A, Araki T. Distinguishing hypervascular pseudolesions of the liver from hypervascular hepatocellular carcinomas with gadoxetic acid-enhanced MR imaging. Radiology. 2010;256:151–158. doi: 10.1148/radiol.10091885. [DOI] [PubMed] [Google Scholar]
- 66.Watanabe H, Kanematsu M, Goshima S, Yoshida M, Kawada H, Kondo H, Moriyama N. Is gadoxetate disodium-enhanced MRI useful for detecting local recurrence of hepatocellular carcinoma after radiofrequency ablation therapy? AJR Am J Roentgenol. 2012;198:589–595. doi: 10.2214/AJR.11.6844. [DOI] [PubMed] [Google Scholar]
- 67.Koda M, Tokunaga S, Miyoshi K, Kishina M, Fujise Y, Kato J, Matono T, Murawaki Y, Kakite S, Yamashita E. Ablative margin states by magnetic resonance imaging with ferucarbotran in radiofrequency ablation for hepatocellular carcinoma can predict local tumor progression. J Gastroenterol. 2013;48:1283–1292. doi: 10.1007/s00535-012-0747-0. [DOI] [PubMed] [Google Scholar]


