Abstract
AIM: To investigate the ultrastructure of abnormal hepatocyte mitochondria, including their cellular and hepatic zonal distribution, in bioptates in pediatric non-alcoholic steatohepatitis (NASH).
METHODS: Ultrastructural investigations were conducted on biopsy liver specimens obtained from 10 children (6 boys and 4 girls) aged 2-14 years with previously clinicopathologically diagnosed NASH. The disease was diagnosed if liver biopsy revealed steatosis, inflammation, ballooned hepatocytes, Mallory hyaline, or focal necrosis, varying degrees of fibrosis in the absence of clinical, serological, or histological findings of infectious liver diseases, autoimmune hepatitis, metabolic liver diseases, or celiac disease. For ultrastructural analysis, fresh small liver blocks (1 mm3 volume) were fixed in a solution containing 2% paraformaldehyde and 2.5% glutaraldehyde in 0.1 mol/L cacodylate buffer. The specimens were postfixed in osmium tetroxide, subsequently dehydrated through a graded series of ethanols and propylene oxide, and embedded in Epon 812. The material was sectioned on a Reichert ultramicrotome to obtain semithin sections, which were stained with methylene blue in sodium borate. Ultrathin sections were contrasted with uranyl acetate and lead citrate, and examined using an Opton EM 900 transmission electron microscope.
RESULTS: Ultrastructural analysis of bioptates obtained from children with non-alcoholic steatohepatitis revealed characteristic repetitive mitochondrial abnormalities within hepatocytes; mainly mitochondrial polymorphisms such as megamitochondria, loss of mitochondrial cristae, and the presence of linear crystalline inclusions within the mitochondrial matrix of an increased electron density. The crystalline inclusions were particularly evident within megamitochondria (MMC), which seemed to be distributed randomly both within the hepatic parenchymal cell and the zones of hepatic lobule, without special variations in abundance. The inclusions appeared as bundles viewed longitudinally, or as an evenly spaced matrix in cross section, and frequently caused mitochondrial deformation. The average diameter of these linear structures was 10 nm and the average space between them 20 nm. Sometimes enlarged intramitochondrial granules were seen in their vicinity. Foamy cytoplasm of hepatocytes was found, resulting from the proliferation of smooth endoplasmic reticulum and glycogen accumulation. The perivascular space of Disse was frequently dilated, and contained transitional hepatic stellate cells, as well as mature and/or newly forming collagen fiber bundles.
CONCLUSION: Marked ultrastructural abnormalities observed in hepatocyte mitochondria, especially their polymorphism in the form of MMC and loss of mitochondrial cristae, accompanied by foamy cytoplasm, clearly indicate a major role of these organelles in the morphogenesis of pediatric NASH. Our findings seem to prove the high effectiveness of electron microscopy in the diagnosis of the disease.
Keywords: Pediatric non-alcoholic steatohepatitis, Hepatocyte ultrastructure, Megamitochondria with crystalline inclusions, Foamy cytoplasm of hepatocytes
Core tip: Our electron-microscopic analysis of liver bioptates, being the first known investigation into pediatric non-alcoholic steatohepatitis (NASH), revealed characteristic repetitive mitochondrial abnormalities within hepatocytes, mainly mitochondrial polymorphism in the form of megamitochondria, loss of cristae, and the presence of linear crystalline inclusions within the mitochondrial matrix of increased electron density, frequently accompanied by foamy cytoplasm. As the discovery of these morphological indices of hepatocyte injury may be very useful in the diagnosis of NASH in pediatric patients, the ultrastructural analysis of liver biopsy can be recommended as an effective diagnostic method.
INTRODUCTION
Non-alcoholic fatty liver disease (NAFLD), regarded as a hepatic manifestation of metabolic syndrome, is a growing health problem worldwide both among children and adults. Importantly, the more severe form of this condition, non-alcoholic steatohepatitis (NASH), may progress to fibrosis, as well as cryptogenic cirrhosis and its complications, including hepatocellular carcinoma[1-6]. Unfortunately, the pathogenesis of NASH still remains unknown.
The last decade has brought increasing evidence that mitochondrial dysfunction, more specifically respiratory chain deficiency, plays a causative role in the pathophysiology of NASH, whatever its initial cause[2,7-14]. Basaranoglu et al[2] have noted that the pathogenesis of NASH is multifactorial, and that excess intracellular fatty acids, oxidant stress, ATP depletion, and mitochondrial dysfunction are essential for hepatocyte injury in this condition.
Despite clinical symptoms, laboratory data, and imaging findings, the microscopic analysis of liver biopsies still remains the golden standard for the diagnosis of non-alcoholic steatohepatitis[4,7].
Histopathological investigations of liver biopsy specimens from NASH patients have revealed, aside from steatosis accompanied by lobular inflammatory infiltration, evidence of hepatocyte injury in the absence of clinical, serological, or histological findings of infectious liver diseases, autoimmune hepatitis, metabolic liver disease, or celiac disease[1,4,7,8,15]. Damage to liver parenchymal cells have been demonstrated in the form of hydropic change (ballooning cell injury), Mallory’s hyaline bodies, and focal lytic necrosis causing sinusoidal distortion, and reduced intrasinusoidal volume and microvascular blood flow[1,3,4,7,8,15-17].
It has been reported that in the course of non-alcoholic steatohepatitis, cell types other than hepatocytes are involved, i.e., non-parenchymal hepatic cells: hepatic stellate cells (HSCs), Kupffer cells, sinusoidal endothelial cells, and inflammatory cells and platelets. This leads to deregulation of microvascular blood flow and may result in pericellular/perisinusoidal fibrosis and cirrhosis[5,6,17-19]. However, the diagnostic criteria of NASH have not yet been established.
In spite of increasing interest in the morphogenesis of non-alcoholic steatohepatitis, there are only a few reports, mainly limited to adult patients, on the ultrastructure of hepatocytes in this pathology[20-24]. We could not find any other publications to compare to the current findings, apart from our Letter to the Editor of the American Journal of Gastroenterology[25] on the ultrastructure of hepatocyte mitochondria in five children with NASH published 10 years ago.
Thus, the study objective was to analyze the image of abnormal electron microscopic findings observed in the hepatocytes of liver biopsy material collected from a larger group of children with clinicopathologically diagnosed NASH. The search for repetitive specific ultrastructural indices of this disease in liver bioptates would facilitate its early morphological diagnosis, which may result in undertaking targeted therapy.
MATERIALS AND METHODS
Patients
This study was carried out in accordance with the Declaration of Helsinki (2000) of the World Medical Association. Ultrastructural investigations were conducted on liver specimens obtained by needle biopsy from 10 children (6 boys and 4 girls) aged 2-14 years (mean 5 years) with previously clinicopathologically diagnosed NASH. Laboratory tests revealed increased serum alanine transaminase activity, and ultrasound examination showed liver brightness in all study patients. NASH was diagnosed if the liver biopsy revealed steatosis, inflammation, ballooned hepatocytes, Mallory hyaline, focal necrosis, or varying degrees of fibrosis in the absence of clinical, serological, or histological findings of infectious liver diseases (hepatitis B, hepatitis C, cytomegalovirus, or Toxoplasma gondii), autoimmune hepatitis, metabolic liver diseases (Wilson’s disease, cystic fibrosis, galactosemia, or alpha 1-antitrypsin deficiency), and celiac disease. Alcohol consumption was excluded in all the children.
The study was carried out in the Department of Medical Pathomorphology, Medical University of Bialystok, by an electron microscopist blinded to the clinical information.
Ultrastructural analysis
For transmission electron microscopic investigations, fresh small tissue blocks (1 mm3 volume) from the liver bioptates were fixed in a solution containing 2% paraformaldehyde and 2.5% glutaraldehyde in 0.1 mol/L cacodylate buffer (pH 7.4) at room temperature. Subsequently, the specimens were postfixed in 2% osmium tetroxide (OsO4) in 0.1 mol/L cacodylate buffer (pH 7.4) for 1 h. The material was then dehydrated through a graded series of ethanols and propylene oxide, embedded in Epon 812, and sectioned on a Reichert ultramicrotome (Reichert Ultracut S) to obtain semithin sections. Next, the sections were stained with 1% methylene blue in 1% sodium borate and preliminarily examined under a light microscope to select Epon blocks. The blocks were then used to prepare ultrathin sections ca. 75 nm thick, which were placed on grids, double stained with uranyl acetate and lead citrate, examined, and photographed via an Opton EM 900 transmission electron microscope (Zeiss, Oberkochen, Germany). This processing procedure had been used in our earlier ultrastructural investigations of the liver in children[18,25,26].
RESULTS
Out of 10 clinicopathologically diagnosed cases of pediatric NASH, nine presented with repetitive mitochondrial abnormalities observed within hepatic parenchymal cells under transmission electron microscope. In 7 patients, the majority of mitochondria were affected, especially within ballooned hepatocytes; in 2 cases the abnormalities were found in a smaller number of hepatic parenchymal cells. The mitochondrial lesions showed no particular differences in quality or topography. They were similarly pronounced in the organelles located both in the vicinity of the cell nucleus, at a certain distance from the nucleus, and on the cell periphery (Figure 1).
Figure 1.
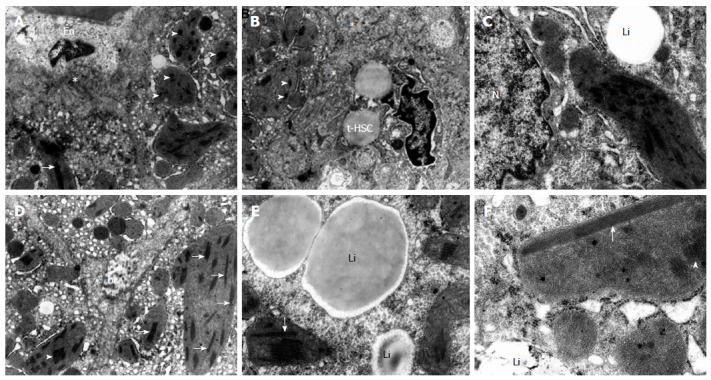
Ultrastructural appearance of polymorphic hepatic mitochondria, with megamitochondria at the foreground, containing linear crystalline inclusions and reduced cristae in bioptates from different pediatric patients with non-alcoholic steatohepatitis. Abnormal mitochondria distributed randomly in topographically varied parts of hepatocytes. A-F: Linear inclusions - cut longitudinally (arrows) and in cross section (arrowheads) in the matrix of moderately or markedly increased electron density in the majority of altered mitochondria, especially elongated and rounded megamitochondria. A, B: Megamitochondria (MMC) located in the vascular pole of hepatocytes; the mitochondrial lesions are accompanied by the proliferation of smooth endoplasmic reticulum; considerably swollen endothelial cell (En) of the sinusoidal vessel; transitional hepatic stellate cells (t-HSC), accumulation of electron-dense material, and collagen formed (asterisk) in the dilated space of Disse (original magnification × 7000); C: The MMC located in the nuclear region of hepatocyte is accompanied by glycogen accumulation; the megamitochondrium shows enlarged intramitochondrial dense granules in the vicinity of linear crystalline inclusions; hepatocyte nucleus (N); lipid material (Li) within hepatocyte cytoplasm (original magnification × 12000); D: MMC distributed randomly within the biliary pole of hepatocytes accompanied by the proliferation of smooth endoplasmic reticulum; bc - biliary canaliculus (original magnification × 7000); E, F: MMC located at a certain distance from the nucleus of hepatocyte; mitochondrial abnormalities are accompanied by glycogen accumulation; some MMC show enlarged intramitochondrial dense granules in the vicinity of linear crystalline inclusions; lipid material (Li) (original magnification × 12000, × 20000, respectively).
The major submicroscopical exponent was the polymorphism of these organelles, with the presence of the so-called megamitochondria, accompanied by variously pronounced defects of the cristae, including their complete loss and increased electron-density of the matrix to its condensation (Figure 1). Frequently, remnants of such cristae, resembling bristles, were found on the periphery of enlarged organelles.
The characteristic feature of the ultrastructure of the altered mitochondria, especially megamitochondria, was the presence of intramitochondrial linear crystalline inclusions within the changed matrix, seen as long parallel strands that often caused deformation of these organelles. They appeared as bundles when viewed longitudinally, or as an evenly-spaced matrix in cross section (Figure 1). The average diameter of these linear structures was 10 nm and the average space between them 20 nm. Sometimes in their vicinity there were enlarged intramitochondrial dense granules (Figure 1C, E, F).
The megamitochondria with characteristic crystalline inclusions (MMC) seemed to be distributed randomly, both within the hepatic parenchymal cell and the zones of the hepatic lobule, without special variations in abundance. They were found in close vicinity to lipid material (Figure 1C, E, F), and also in hepatocytes with no distinct features of steatosis.
We also observed a wide range of changes in hepatocellular ultrastructure, including the foamy cytoplasmic appearance resulting from smooth endoplasmic reticulum proliferation (Figure 1A, B, D) and glycogen accumulation (Figure 1C, E, F), mainly in the nuclear regions (Figure 1C). Heterogeneous lipofuscin pigment granules of various size and shapes were occasionally found.
The sinusoids with the adherent vascular pole of hepatocytes that showed distinct mitochondrial lesions had markedly swollen endothelial linings (Figure 1A). Kupffer cells in the sinusoidal lumen were considerably activated, sometimes showing features of erythrophagocytosis (their morphology in children with NASH was described in our earlier paper)[18].
The perivascular space of Disse was frequently dilated and contained transitional (i.e., activated) hepatic stellate cells (t-HSCs) (Figure 1B), deposits of electron-dense material, as well as mature and/or collagen forming fiber bundles (Figure 1A, B).
DISCUSSION
Although light microscopy is routinely used to evaluate liver biopsy specimens from NASH patients, the ultrastructural investigations that make the diagnosis more profound are conducted in a limited number of cases, especially with respect to children. No reports have been published, apart from those in our center, on the electron-microscopic picture of liver bioptates from pediatric patients with NASH. We focused not only on hepatocytes[25,27], but also on the changes concerning non-parenchymal liver cells, especially Kupffer and liver progenitor/oval cells[18,26,28].
The current ultrastructural observations of hepatocytes obtained from pediatric NASH biopsies were consistent with our preliminary findings presented recently at the 186th Falk Symposium[27], as well as in the previously mentioned Letter to the Editor[25]. The studies revealed numerous mitochondrial abnormalities similar to those found in adults[20,24], including polymorphisms such as megamitochondria, loss of cristae, increased electron-density of the matrix, and the presence of characteristic linear crystalline inclusions within the matrix. The megamitochondria with crystalline inclusions were seen as parallel strands, and reduced cristae were the major repetitive ultrastructural abnormalities in pediatric NASH cases analyzed in the current study. These MMC seemed to be distributed randomly, both within the hepatocytes and the zones of the hepatic lobule.
In addition to mitochondrial alterations, we quite frequently observed foamy cytoplasm of hepatocytes resulting from the proliferation of smooth endoplasmic reticulum and glycogen accumulation, which was also reported by Ahishai et al[20] in adult patients with NASH. The space of Disse was frequently dilated and contained t-HSCs, which were accompanied by electron-dense material and collagen accumulation. However, the sinusoids were lined with distinctly swollen endothelium and markedly activated Kupffer cells.
The mitochondrial abnormalities showing varying degrees of cristae loss and increased matrix density evidently indicate decreased intra-mitochondrial protein synthesis and respiratory chain dysfunction, which was also emphasized by Ahishali et al[20] with respect to adult patients with NASH. However, according to Caldwell et al[21] and Le et al[22], the formation of megamitochondria with crystalline inclusions of unknown composition may, in patients with NASH, represent an adaptive process to oxidative stress rather than a secondary injury.
The formation of crystalline inclusions in the mitochondrial matrix has been noted in a variety of other conditions. Similar crystal-like structures have been reported mainly in the early phase of alcoholic steatohepatitis or in copper metabolism disorders such as Wilson’s disease[29,30]. As already mentioned, they have been recently described in non-alcoholic fatty liver in adult patients, where their presence is correlated indirectly with oxidative stress[20,24], and their absence is noted when cirrhosis develops[21,24]. According to Le et al[22], as well as in our own observations concerning children, in NASH the MMC seem to be distributed randomly within the hepatic zones and without variation in abundance, regardless of the stage of fibrosis. In contrast, rodent models of NASH do not develop mitochondrial crystalline inclusions. The major electron-microscopic changes in rodents include severe mitochondrial swelling with coexisting proliferation of the smooth endoplasmic reticulum and abnormal granular endoplasmic reticulum[31].
Considering the role of mitochondria in the pathophysiology of NASH, it has been emphasized that hepatic oxidative stress increases the release of lipid peroxidation products, as reflected by increased fatty acid beta-oxidation and cytokines, which together trigger liver lesions[2,7,9,10,12,13,19,24].
In conclusion, this is the first full report on the hepatocyte ultrastructure in pediatric NASH. In our study, the most significant repetitive electron microscopic alterations in this pathology involved the appearance of randomly distributed megamitochondria within hepatocytes, with characteristic crystalline inclusions seen as parallel strands and reduced mitochondrial cristae, including their complete loss. Additionally, foamy cytoplasm of liver parenchymal cells was found, resulting from the proliferation of smooth endoplasmic reticulum and glycogen accumulation. The submicroscopic changes observed in hepatocytes, especially mitochondrial abnormalities, may play a major role in the morphogenesis of pediatric non-alcoholic steatohepatitis.
We hope that similar reports will appear in other centers to provide comparative material for our current study in children. The discovery of submicroscopic repetitive indices of damage to liver parenchymal cells, specific to this pathology, may have both diagnostic and therapeutic implications for the treatment of fatty liver disease in pediatric patients.
Considering the great usefulness of transmission electron microscopy in the diagnosis of NASH in children, ultrastructural analysis of liver bioptates should be recommended as an effective diagnostic tool, at least in diagnostically difficult cases, despite its high costs and effort.
COMMENTS
Background
Despite increasing interest in non-alcoholic steatohepatitis (NASH), there are only a few reports on the hepatocyte ultrastructure in this pathology. This manuscript is the first full original paper known to be conducted on biopsy material in the course of this pathology in children.
Innovations and breakthroughs
The main objective of the study was ultrastructural analysis of the image of abnormal hepatocyte mitochondria, including their cellular and hepatic zonal distribution in biopsy material in clinicopathologically diagnosed NASH in children. The electron microscopic investigations revealed characteristic mitochondrial structural defects within hepatocytes, mainly mitochondrial polymorphism (megamitochondria), loss of mitochondrial cristae, and the presence of linear crystalline inclusions within the mitochondrial matrix of increased electron density. The crystalline inclusions were particularly evident within megamitochondria. The megamitochondria with characteristic inclusions (MMC) seemed to be distributed randomly, both within the cell and in the hepatic zones, without any special variation in abundance. In addition to mitochondrial alterations, the authors could quite frequently observe foamy cytoplasm of hepatocytes resulting from the proliferation of smooth endoplasmic reticulum and glycogen accumulation.
Applications
The authors’ ultrastructural investigations indicate that pediatric NASH is associated with marked abnormalities in hepatocyte mitochondria, especially their polymorphism in the form of MMC, as well as the loss of mitochondrial cristae accompanied by foamy cytoplasm of hepatocytes. These findings seem to prove the high effectiveness of electron microscopy in the diagnosis of the disease.
Peer review
This study appears to be properly conducted form a technical view-point. The conclusions of the authors are supported by the data reported.
Footnotes
P- Reviewers: Romani A, Tanaka N, Williams GM S- Editor: Wen LL L- Editor: Rutherford A E- Editor: Zhang DN
References
- 1.Alexander J, Torbenson M, Wu TT, Yeh MM. Non-alcoholic fatty liver disease contributes to hepatocarcinogenesis in non-cirrhotic liver: a clinical and pathological study. J Gastroenterol Hepatol. 2013;28:848–854. doi: 10.1111/jgh.12116. [DOI] [PubMed] [Google Scholar]
- 2.Basaranoglu M, Basaranoglu G, Sentürk H. From fatty liver to fibrosis: a tale of “second hit”. World J Gastroenterol. 2013;19:1158–1165. doi: 10.3748/wjg.v19.i8.1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Caldwell SH, Lee VD, Kleiner DE, Al-Osaimi AM, Argo CK, Northup PG, Berg CL. NASH and cryptogenic cirrhosis: a histological analysis. Ann Hepatol. 2009;8:346–352. [PMC free article] [PubMed] [Google Scholar]
- 4.Brunt EM, Tiniakos DG. Histopathology of nonalcoholic fatty liver disease. World J Gastroenterol. 2010;16:5286–5296. doi: 10.3748/wjg.v16.i42.5286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jeen YM, Jin SY. [Pathology of nonalcoholic steatohepatitis] Korean J Hepatol. 2009;15:122–130. doi: 10.3350/kjhep.2009.15.2.122. [DOI] [PubMed] [Google Scholar]
- 6.Farrell GC, Teoh NC, McCuskey RS. Hepatic microcirculation in fatty liver disease. Anat Rec (Hoboken) 2008;291:684–692. doi: 10.1002/ar.20715. [DOI] [PubMed] [Google Scholar]
- 7.Takahashi Y, Fukusato T. Pediatric nonalcoholic fatty liver disease: overview with emphasis on histology. World J Gastroenterol. 2010;16:5280–5285. doi: 10.3748/wjg.v16.i42.5280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Basaranoglu M, Turhan N, Sonsuz A, Basaranoglu G. Mallory-Denk Bodies in chronic hepatitis. World J Gastroenterol. 2011;17:2172–2177. doi: 10.3748/wjg.v17.i17.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fromenty B, Robin MA, Igoudjil A, Mansouri A, Pessayre D. The ins and outs of mitochondrial dysfunction in NASH. Diabetes Metab. 2004;30:121–138. doi: 10.1016/s1262-3636(07)70098-8. [DOI] [PubMed] [Google Scholar]
- 10.Henkel J, Frede K, Schanze N, Vogel H, Schürmann A, Spruss A, Bergheim I, Püschel GP. Stimulation of fat accumulation in hepatocytes by PGE2-dependent repression of hepatic lipolysis, β-oxidation and VLDL-synthesis. Lab Invest. 2012;92:1597–1606. doi: 10.1038/labinvest.2012.128. [DOI] [PubMed] [Google Scholar]
- 11.Jiang Y, Zhao M, An W. Increased hepatic apoptosis in high-fat diet-induced NASH in rats may be associated with downregulation of hepatic stimulator substance. J Mol Med (Berl) 2011;89:1207–1217. doi: 10.1007/s00109-011-0790-y. [DOI] [PubMed] [Google Scholar]
- 12.Pessayre D. Role of mitochondria in non-alcoholic fatty liver disease. J Gastroenterol Hepatol. 2007;22 Suppl 1:S20–S27. doi: 10.1111/j.1440-1746.2006.04640.x. [DOI] [PubMed] [Google Scholar]
- 13.Rolo AP, Teodoro JS, Palmeira CM. Role of oxidative stress in the pathogenesis of nonalcoholic steatohepatitis. Free Radic Biol Med. 2012;52:59–69. doi: 10.1016/j.freeradbiomed.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 14.Thomas A, Klein MS, Stevens AP, Reinders Y, Hellerbrand C, Dettmer K, Gronwald W, Oefner PJ, Reinders J. Changes in the hepatic mitochondrial and membrane proteome in mice fed a non-alcoholic steatohepatitis inducing diet. J Proteomics. 2013;80C:107–122. doi: 10.1016/j.jprot.2012.12.027. [DOI] [PubMed] [Google Scholar]
- 15.Brunt EM, Janney CG, Di Bisceglie AM, Neuschwander-Tetri BA, Bacon BR. Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am J Gastroenterol. 1999;94:2467–2474. doi: 10.1111/j.1572-0241.1999.01377.x. [DOI] [PubMed] [Google Scholar]
- 16.Tandra S, Yeh MM, Brunt EM, Vuppalanchi R, Cummings OW, Unalp-Arida A, Wilson LA, Chalasani N. Presence and significance of microvesicular steatosis in nonalcoholic fatty liver disease. J Hepatol. 2011;55:654–659. doi: 10.1016/j.jhep.2010.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tiniakos DG. Liver biopsy in alcoholic and non-alcoholic steatohepatitis patients. Gastroenterol Clin Biol. 2009;33:930–939. doi: 10.1016/j.gcb.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 18.Lotowska JM, Sobaniec-Lotowska ME, Lebensztejn DM. The role of Kupffer cells in the morphogenesis of nonalcoholic steatohepatitis - ultrastructural findings. The first report in pediatric patients. Scand J Gastroenterol. 2013;48:352–357. doi: 10.3109/00365521.2012.746390. [DOI] [PubMed] [Google Scholar]
- 19.Rombouts K, Marra F. Molecular mechanisms of hepatic fibrosis in non-alcoholic steatohepatitis. Dig Dis. 2010;28:229–235. doi: 10.1159/000282094. [DOI] [PubMed] [Google Scholar]
- 20.Ahishali E, Demir K, Ahishali B, Akyuz F, Pinarbasi B, Poturoglu S, Ibrisim D, Gulluoglu M, Ozdil S, Besisik F, et al. Electron microscopic findings in non-alcoholic fatty liver disease: is there a difference between hepatosteatosis and steatohepatitis? J Gastroenterol Hepatol. 2010;25:619–626. doi: 10.1111/j.1440-1746.2009.06142.x. [DOI] [PubMed] [Google Scholar]
- 21.Caldwell SH, Swerdlow RH, Khan EM, Iezzoni JC, Hespenheide EE, Parks JK, Parker WD. Mitochondrial abnormalities in non-alcoholic steatohepatitis. J Hepatol. 1999;31:430–434. doi: 10.1016/s0168-8278(99)80033-6. [DOI] [PubMed] [Google Scholar]
- 22.Le TH, Caldwell SH, Redick JA, Sheppard BL, Davis CA, Arseneau KO, Iezzoni JC, Hespenheide EE, Al-Osaimi A, Peterson TC. The zonal distribution of megamitochondria with crystalline inclusions in nonalcoholic steatohepatitis. Hepatology. 2004;39:1423–1429. doi: 10.1002/hep.20202. [DOI] [PubMed] [Google Scholar]
- 23.Park KS, Jang BK, Chung WJ, Cho KB, Hwang JS, Ahn SH, Kang YN, Hwang JB, Keum DY. [Abnormal electron microscopic findings of nonalcoholic steatohepatitis and related factors] Korean J Gastroenterol. 2005;45:417–424. [PubMed] [Google Scholar]
- 24.Sanyal AJ, Campbell-Sargent C, Mirshahi F, Rizzo WB, Contos MJ, Sterling RK, Luketic VA, Shiffman ML, Clore JN. Nonalcoholic steatohepatitis: association of insulin resistance and mitochondrial abnormalities. Gastroenterology. 2001;120:1183–1192. doi: 10.1053/gast.2001.23256. [DOI] [PubMed] [Google Scholar]
- 25.Sobaniec-Lotowska ME, Lebensztejn DM. Ultrastructure of hepatocyte mitochondria in nonalcoholic steatohepatitis in pediatric patients: usefulness of electron microscopy in the diagnosis of the disease. Am J Gastroenterol. 2003;98:1664–1665. doi: 10.1111/j.1572-0241.2003.07561.x. [DOI] [PubMed] [Google Scholar]
- 26.Sobaniec-Łotowska ME, Lebensztejn DM, Lotowska JM, Kańczuga-Koda L, Sulkowski S. Ultrastructure of liver progenitor/oval cells in children with nonalcoholic steatohepatitis. Adv Med Sci. 2011;56:172–179. doi: 10.2478/v10039-011-0037-8. [DOI] [PubMed] [Google Scholar]
- 27.Lotowska JM, Sobaniec-Lotowska ME, Sulkowska U, Lebensztejn DM. Mitochondrial abnormalities in nonalcoholic steatohepatitis in pediatric patients. Falk Symposium 186. Challenges of Liver Cirrhosis and Tumors: Prevent it, Treat it, Manage Consequences; October 5-6, 2012, Mainz, Germany, 46. Available from: http://www.drfalkpharma.de/uploads/tx_tocfpshoperw/FS186_Program.pdf.
- 28.Sobaniec-Lotowska ME, Lotowska JM, Bockowska SB, Lebensztejn DM. The role of Kupffer cells in the pathogenesis of non-alcoholic steatohepatitis: immunohistochemical and ultrastructural findings in pediatric patients. Falk Symposium 181. Innate Immunity in Gastrointestinal Disorders: Basic and Therapeutic Concepts; February 8-9, 2012, Munich, Germany, 28. Available from: http://www.drfalkpharma.de/uploads/tx_tocfpshoperw/FS181_Program.pdf.
- 29.Chedid A, Mendenhall CL, Tosch T, Chen T, Rabin L, Garcia-Pont P, Goldberg SJ, Kiernan T, Seeff LB, Sorrell M. Significance of megamitochondria in alcoholic liver disease. Gastroenterology. 1986;90:1858–1864. doi: 10.1016/0016-5085(86)90253-2. [DOI] [PubMed] [Google Scholar]
- 30.Phillips MJ, Poucell S, Patterson J, Valencia P. The Liver: An Atlas and Text of Ultrastructural Pathology. New York: Raven Press; 1987. pp. 239–446. [Google Scholar]
- 31.Kirsch R, Clarkson V, Shephard EG, Marais DA, Jaffer MA, Woodburne VE, Kirsch RE, Hall Pde L. Rodent nutritional model of non-alcoholic steatohepatitis: species, strain and sex difference studies. J Gastroenterol Hepatol. 2003;18:1272–1282. doi: 10.1046/j.1440-1746.2003.03198.x. [DOI] [PubMed] [Google Scholar]


