Abstract
AIM: To study the relationship between the CX3CL1 chemokine, its receptor CX3CR1, and gastric carcinoma/gastric carcinoma perineural invasion (PNI).
METHODS: Thirty cases of gastric carcinoma were surgically resected (radical resection or palliative resection) between February 2012 and July 2012. Tumour and tumour-adjacent tissues were evaluated for the presence of CX3CL1 (ELISA) and CX3CR1 (immunohistochemistry and Western blotting) in an effort to analyse the relationship between CX3CL1/CX3CR1 and gastric carcinoma/gastric carcinoma PNI.
RESULTS: Of these 30 cases, 14 were PNI-positive (46.7%). No significant differences in CX3CL and CX3CR1 expression in tumour-adjacent tissues were found between the PNI positive and negative groups. Expression levels of CX3CL and CX3CR1 in tumour tissues were significantly higher than those in adjacent tissues (P < 0.01), and were significantly higher in tumour tissues from the PNI-positive group compared to the PNI-negative group (P < 0.01).
CONCLUSION: CX3CL1/CX3CR1 expression may be associated with the occurrence and development of gastric carcinoma as well as gastric carcinoma PNI.
Keywords: Gastric carcinoma, Perineural invasion, CX3CL1, CX3CR1
Core tip: This preliminary study determined the relationship between the CX3CL1/CX3CR1 system and gastric carcinoma/gastric carcinoma perineural invasion (PNI). The results revealed that the CX3CL1/CX3CR1 system may be associated with the occurrence and development of gastric carcinoma, and had an obvious relationship with gastric carcinoma PNI.
INTRODUCTION
Gastric carcinomas are one of the most common malignant tumours, and often undergo metastasis. Studies have increasingly reported tumour perineural invasion, although this phenomenon has not been explored. In 1835, Cruveilhier[1] reported perineural invasion (PNI), a particular biological behaviour in tumour cells, in which the tumour approaches nerves and covers approximately 33% (or more) of the nerve perimeter or penetrates any of the 3 nerve-sheath layers[2]. The role of chemokines in the pathogenesis of gastric carcinoma and gastric carcinoma PNI is of interest. However, relative to other subfamilies, the current research on CX3CL1 is relatively sparse.
The aim of this study was to study the expression of CX3CL1 and CX3CR1 in gastric carcinoma and explore their relationship with gastric carcinoma and gastric carcinoma PNI.
MATERIALS AND METHODS
Specimens
Thirty patients underwent gastric carcinoma surgery in the Department of General Surgery, Nanjing Affiliated Hospital of Nanjing Medical University, between February 2012 and July 2012. Tumour tissue and 5 cm of adjacent tissue samples from the tumour boundary (control) were collected.
CX3CL1 ELISA
Proteins were extracted from the samples and the protein content was quantified using the Bradford method. Samples were evaluated using the Chemokine C-X3-C- basic ligand 1 (CX3CL1) ELISA kit (USCN Co., Ltd.) according to the instructions provided with the kit, and read at 450 nm. The CX3CL1 concentration in each sample was determined by comparison to appropriately-diluted standards.
Immunohistochemical detection of CX3CR1 (LP method)
Paraffin sections were dewaxed in water, treated with 3% H2O2 to inactivate endogenous peroxidase and incubated with CX3CR1 rabbit anti-human monoclonal antibody (Abcam Co., Ltd.). Bound antibody was detected with the Universal One-Step Hypersensitive Detection Kit (Xiya Golden Bridge Biotechnology Co. Ltd.) using DAB as the peroxidase substrate (brown precipitate). Slides were counterstained with hematoxylin, dehydrated and coverslipped.
Ten randomly-selected high-power fields (400 ×) were evaluated in a double-blind manner and the following metric used: If the cytoplasm of the tumour cells contained stained granules and the tumour cell staining was > 10%, it was considered positive; if the tumour was unstained or the staining was less than 10%, the result was considered negative.
Western blot assay for CX3CR1 protein expression
Tissues were extracted using the whole protein extraction kit KGP250 (Nanjing KGI Biological Technology Development Co. Ltd.). Extracted proteins (X amount) were mixed with loading buffer, denatured, and polyacrylamide gel electrophoresis performed to separate the proteins. The gel was blotted to a nitrocellulose membrane, the membrane was stained with Ponceau red, and the band(s) of interest excised and blocked with 8% skim milk. Rabbit anti-human CX3CR1 antibody (Abcam Co. Ltd.) was added and incubated overnight at 4 °C. The membrane was washed and goat anti-rabbit IgG (Jackson Co. Ltd.) added and incubated for X time at Y temperature. The membrane was washed and electrochemiluminescence (ECL) was used to detect the labelled protein(s).
Statistical analysis
SPSS17.0 was used for statistical analysis of the data, and the t-test was used where needed to compare data sets. P < 0.05 was considered statistically significant.
RESULTS
CX3CL1 expression in PNI-positive and negative groups (ELISA)
Samples were divided into PNI-positive and PNI-negative groups based on CX3CL1 expression in the tumour and adjacent tissues detected by ELISA (Table 1). Of the 30 cases of gastric carcinoma, 14 cases were PNI-positive (46.7%). In tumour tissues, CX3CL1 levels in the PNI-positive group were significantly higher than those in the PNI-negative group (P < 0.05). When the PNI-negative and PNI-positive groups were compared, CX3CL1 expression in the tumour tissue was significantly higher (P < 0.05) than that in the adjacent tissue.
Table 1.
Comparison of CX3CL1 levels (pg/mg) in gastric carcinoma perineural invasion-positive and negative groups
| PNI positive group | PNI negative group | t | |
| (mean ± SD) | (mean ± SD) | ||
| Tumor tissue | 674 ± 16.09 | 515 ± 12.31 | 4.859 (P < 0.05) |
| Adjacent tissue | 479 ± 14.51 | 445 ± 11.13 | 1.190 (P > 0.05) |
| t | 5.888 (P < 0.01) | 2.916 (P < 0.02) |
PNI: Perineural invasion.
CX3CR1 expression in the PNI-positive and negative groups (immunohistochemistry)
CX3CR1 expression was evaluated in the tumour and adjacent tissues (Figure 1). Tumour tissues from the PNI-negative group contained CX3CR1 positive cells and nerve cells were unstained. In the PNI-positive group, CX3CR1 expression was seen in both tumour and nerve cells.
Figure 1.
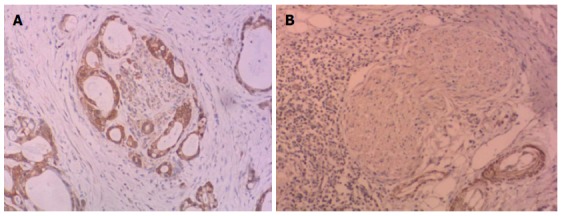
CX3CR1 expression. A: CX3CR1 expression (× 200) in perineural invasion (PNI)-positive tumour cells and nerve cells; B: CX3CR1 expression was present in tumour cells and absent in nerve cells from the PNI-negative group (× 200).
CX3CR1 expression levels in the tumour tissues and adjacent tissues of PNI positive/negative groups by Western blot assay
CX3CR1 expression in tumour tissues from the PNI positive/negative groups was higher than that in adjacent tissues (Figure 2) and the expression of CX3CR1 in the tumour tissues from the PNI-positive group was significantly higher than that in the PNI-negative group (P < 0.01) (Table 2). No statistically significant differences were seen in CX3CR1 levels in the adjacent tissues of the PNI-positive and negative groups (P > 0.05). Mean CX3CR1-positive values in the PNI positive/negative groups were significantly higher than those in the adjacent tissues, and the difference was statistically significant (P < 0.01).
Figure 2.
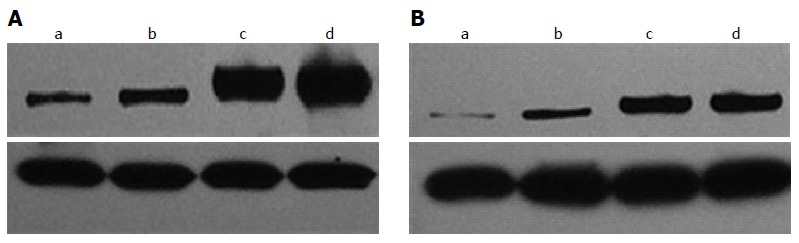
CX3CR1 expression levels in the perineural invasion-positive group (A) and perineural invasion-negative groups (B). a and b are from tissues adjacent to the tumour, while c and d are tumour tissues, and β-actin is shown below.
Table 2.
Comparison of CX3CR1 levels in gastric carcinoma perineural invasion positive/negative groups
| PNI positive group | PNI negative group | t | |
| (mean ± SD) | (mean ± SD) | ||
| Tumor tissues | 1.01 ± 0.13 | 0.59 ± 0.11 | 4.632 (P < 0.01) |
| Adjacent tissues | 0.34 ± 0.06 | 0.28 ± 0.08 | 1.175 (P > 0.05) |
| t | 7.572 (P < 0.01) | 8.538 (P < 0.01) |
PNI: Perineural invasion.
DISCUSSION
PNI is significantly correlated with a poor prognosis in prostate, pancreatic and especially head and neck cancer patients, and is an important pathological index, indicating a shorter survival period, increased local recurrence rate, and shorter recurrence time[3]. While PNI is an important factor which affects survival, according to the reports of the Association of American Pathologists[4], head and neck cancers are the only tumours which require the assessment of the perineural invasion state.
The relationship between PNI and the prognosis of gastric carcinoma patients has attracted more attention in recent years, and although no consensus has been reached, some scholars have indicated that PNI is not an independent factor affecting prognosis[5]. Clinical pathology data[6] from 178 cases of gastric carcinoma revealed that overall survival time and disease-free survival time in PNI-positive patients were statistically different when compared with PNI-negative patients. Multi-factor analysis also revealed that PNI was an independent risk factor affecting the prognosis of gastric carcinoma patients, which was consistent with data reported by Bilici et al[7] and Tianhang et al[8]. Although there was significant correlation between PNI and the prognosis of tumour patients, the pathogenesis is still unclear.
Among the 4 chemokine subfamilies, relatively little work has been carried out on CX3CL1. CX3CL1 is reported[9] to have a dual function, acting as a chemical inducer to stimulate the anti-tumour effect of leukocytes, while the combination of CX3CL1 and CX3CR1 can induce the aggregation/adherence of NK cells, CD8+T and CD4+T on/near tumour cells, where they can exert their anti-tumoural effect. However, little has been published on the role of CX3CL1 in clinical assessments. Ohta et al[10] found that colorectal cancer patients with high expression of CX3CL1 had a higher density of tumour-infiltrating immune cells, and therefore better prognosis than patients with low CX3CL expression. Others[11] detected CX3CL1 expression by immunohistochemistry, and combined with the prognosis, suggested patients with high CX3CL1 expression had a longer survival time than those with low expression. Vitale et al[12] reported that invasion and metastasis of colon cancer cells which expressed CX3CL1 was reduced, and the growth of metastases slowed, the membrane type and secreting type performed different roles in tumour development.
For gastric carcinoma, few reports exploring the relationship between CX3CL1 and gastric carcinoma have been published. Hyakudomi[13] evaluated CX3CL1 expression in 158 cases of T2/T3 stage gastric carcinoma and used two antibodies (anti-CD57 and anti-CD8) to assess the infiltration of NK cells and CD8+T cells, respectively. These data, combined with clinical pathology and follow-up, suggested that patients in the CX3CL1-high-expression group had a better prognosis and disease-free survival period compared to the CX3CL1-low-expression group. Multivariate analysis showed the expression of CX3CL1 was the independent factor which affected the disease-free survival period of gastric carcinoma patients.
In this study, ELISA data indicated that CX3CL1 content in the tumour tissues, irrespective of PNI-negative or PNI-positive, was higher than in adjacent tissues. Western blotting revealed that expression of the receptor CX3CR1 in PNI-positive and negative groups was significantly higher in the tumour tissues than in the adjacent tissues. Taken together, these data suggested that CX3CL1/CX3CR1 may be associated with the occurrence and development of gastric carcinoma.
Another function of CX3CL1 is to act as an adhesion molecule, and combined with the receptors expressed by tumour cells, may be the key factor leading to the clinical differences seen in the CX3CL1/CX3CR1 system response against tumours[9]. In certain tumours, the potential anticancer effect of CX3CL1 was overcome by its actions promoting tumour development. In prostate cancer, CX3CL1 expressed by bone marrow endothelial cells could induce the aggregation of CX3CR1 inside tumour cells, playing an important role in the bone metastasis of prostate cancer[14]. In breast cancer, although there was no significant correlation between CX3CL1/CX3CR1 and the overall survival rate and disease-free survival rate of patients, expression did indicate the occurrence of brain metastases[15]. Recently, the CX3CL1/CX3CR1 system has been reported to play an important role in the bone metastasis of neuroblastoma[16]. The adhesion effect of CX3CL1 in the nervous system also suggests a role in nerves peripheral to other tumours, which may be an underrated source of metastases. In addition to the traditional routes of tumour metastasis (blood, lymphatic and implantation metastasis), cancer cells could also move along the nerve (PNI).
Literature addressing the relationship between CX3CL1 and PNI in digestive tract tumours primarily studied pro-nerve tumours, such as pancreatic ductal adenocarcinoma (PDA) which accounted for the majority, while no relevant literature on tumours in the gastrointestinal tract have been found. In PDA, PNI occurs in the early stage of the disease[17-19], and when biopsies are evaluated, tumour cells show an elevated expression of CX3CR1 compared to normal pancreatic cells. When examined in vitro, the CX3CR1-positive PDA cells directionally migrate towards the CX3CL1 expressed on other cells and specifically adhere to the nerve cells, where pathogenesis involved the activation of a Gi protein and adhesion molecules (β1 integrin and focal adhesion kinase)[17]. Postoperative pathology confirmed that for PNI-positive pancreatic cancer, 90% were CX3CR1 positive and 56% exhibited a strong positive expression, while systematic assessment of the relationship between tumour CX3CR1 expression and PNI revealed that elevated levels of CX3CR1 were closely related to the early localized recurrence of the tumour.
In this study, the results of immunohistochemistry revealed that when PNI occurred, tumour and nerve cells expressed CX3CR1, whereas when PNI was absent, CX3CR1 expression was seen in tumour cells only. ELISA and Western blotting of CX3CL1/CX3CR1 revealed that CX3CL1 and CX3CR1 expression in tumour tissues was significantly higher in the PNI-positive group compared to the PNI-negative group. No statistical significance was seen for CX3CL1 and CX3CR1 in the adjacent tissues, suggesting that high expression of CX3CL1/CX3CR1 may be correlated with gastric carcinoma PNI. However, whether expression of the CX3CL1/CX3CR1 system was involved in the stimulation of gastric carcinoma-associated PNI could not be clearly determined in this study, and will be further explored.
In summary, this research determined the relationship between the CX3CL1/CX3CR1 system and gastric carcinoma/gastric carcinoma PNI, and indicated that PNI may be related to the occurrence and development of gastric carcinoma. Although overexpression could be significantly associated with gastric carcinoma PNI, how the combination of CX3CL1 and CX3CR1 complete signal transduction inside the intracellular pathway, whether or not it is involved in the occurrence of PNI, and the specific relationship between CX3CL1/CX3CR1 and the prognosis of gastric carcinoma should be studied further.
COMMENTS
Background
Gastric carcinoma is one of the most common malignant tumours, and easily undergoes metastasis. Perineural invasion (PNI) occurs when the tumour approaches nerves and encompasses at least 33% of the nerve perimeter or tumour cells penetrate any of the 3 nerve sheath layers.
Research frontiers
With respect to the pathogenesis of gastric carcinoma and gastric carcinoma PNI, the role of chemokines has attracted the attention of researchers. However, relative to other subfamilies, the current research on CX3CL1 and its receptor, CX3CR1, is minimal.
Innovations and breakthroughs
This research determined the relationship between the CX3CL1/CX3CR1 system and gastric carcinoma/gastric carcinoma PNI. The data suggested that CX3CL1/CX3CR1 expression may be associated with the occurrence and development of gastric carcinoma and also plays a role in gastric carcinoma PNI.
Applications
The CX3CL1/CX3CR1 system plays an important role in a series of physiological and pathological processes. The dynamic changes, regulatory mechanisms and clinical significance of the CX3CL1/CX3CR1 system and its interaction with other chemokines and cytokines have significant potential application in preventing and treating cancer and PNI. The development of a CX3CL1 gene knockout/transgenic or monoclonal antibody technology, and directing them toward clinical applications, will be the focus of future research.
Peer review
In this manuscript, the authors performed a preliminary study on the correlation between CX3CL1/CX3CR1 and gastric carcinoma/gastric carcinoma PNI. The manuscript is interesting in that it explores the potential of using an assessment of the CX3CL1/CX3CR1 system to the prevention and treatment of cancer and PNI.
Footnotes
Supported by Nanjing Science and Technology Project, No. 201106016
P- Reviewers: Forns X, Sinatra RS, Thuss-Patience PC S- Editor: Wang JL L- Editor: Webster JR E- Editor: Liu XM
References
- 1.Cruveilhier J, et al. Maladies des Nerfs: anatomie Pathologique du Corp Humain. 2nd ed. Paris: Bailliere; 1835. p. 1835.p. 1842. [Google Scholar]
- 2.Liebig C, Ayala G, Wilks JA, Berger DH, Albo D. Perineural invasion in cancer: a review of the literature. Cancer. 2009;115:3379–3391. doi: 10.1002/cncr.24396. [DOI] [PubMed] [Google Scholar]
- 3.Rodin AE, Larson DL, Roberts DK. Nature of the perineural space invaded by prostatic carcinoma. Cancer. 1967;20:1772–1779. doi: 10.1002/1097-0142(196710)20:10<1772::aid-cncr2820201028>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 4.Pilch BZ, Gillies E, Houck JR Jr, Min KW, Novis D, Shah J, Zarbo RJ, Wenig MB. Upper aerodigestic tract: Head and Neck. New York: College of American Pathologists; 2005. pp. 12–16. [Google Scholar]
- 5.Johnston M, Yu E, Kim J. Perineural invasion and spread in head and neck cancer. Expert Rev Anticancer Ther. 2012;12:359–371. doi: 10.1586/era.12.9. [DOI] [PubMed] [Google Scholar]
- 6.Xie HH, Lv CY, Huang WB. [Analysis of clinical characteristics and prognosis of perineural invasion in patients with gastric carcinoma] Zhonghua Weichang Waike Zazhi. 2010;13:413–416. [PubMed] [Google Scholar]
- 7.Bilici A, Seker M, Ustaalioglu BB, Kefeli U, Yildirim E, Yavuzer D, Aydin FM, Salepci T, Oncel M, Gumus M. Prognostic significance of perineural invasion in patients with gastric cancer who underwent curative resection. Ann Surg Oncol. 2010;17:2037–2044. doi: 10.1245/s10434-010-1027-y. [DOI] [PubMed] [Google Scholar]
- 8.Tianhang L, Guoen F, Jianwei B, Liye M. The effect of perineural invasion on overall survival in patients with gastric carcinoma. J Gastrointest Surg. 2008;12:1263–1267. doi: 10.1007/s11605-008-0529-4. [DOI] [PubMed] [Google Scholar]
- 9.Pan Y, Lloyd C, Zhou H, Dolich S, Deeds J, Gonzalo JA, Vath J, Gosselin M, Ma J, Dussault B, et al. Neurotactin, a membrane-anchored chemokine upregulated in brain inflammation. Nature. 1997;387:611–617. doi: 10.1038/42491. [DOI] [PubMed] [Google Scholar]
- 10.Ohta M, Tanaka F, Yamaguchi H, Sadanaga N, Inoue H, Mori M. The high expression of Fractalkine results in a better prognosis for colorectal cancer patients. Int J Oncol. 2005;26:41–47. [PubMed] [Google Scholar]
- 11.Zeng SQ, Cao J, Zhang XY, Zeng J, Chen JP, Zhang WJ, Liu HJ. Increased expression of CX3CL1 is correlated with a better prognosis in colon carcinoma patients. Redai Yixue Zazhi. 2010;10:784–787. [Google Scholar]
- 12.Vitale S, Cambien B, Karimdjee BF, Barthel R, Staccini P, Luci C, Breittmayer V, Anjuère F, Schmid-Alliana A, Schmid-Antomarchi H. Tissue-specific differential antitumour effect of molecular forms of fractalkine in a mouse model of metastatic colon cancer. Gut. 2007;56:365–372. doi: 10.1136/gut.2005.088989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hyakudomi M, Matsubara T, Hyakudomi R, Yamamoto T, Kinugasa S, Yamanoi A, Maruyama R, Tanaka T. Increased expression of fractalkine is correlated with a better prognosis and an increased number of both CD8+ T cells and natural killer cells in gastric adenocarcinoma. Ann Surg Oncol. 2008;15:1775–1782. doi: 10.1245/s10434-008-9876-3. [DOI] [PubMed] [Google Scholar]
- 14.Shulby SA, Dolloff NG, Stearns ME, Meucci O, Fatatis A. CX3CR1-fractalkine expression regulates cellular mechanisms involved in adhesion, migration, and survival of human prostate cancer cells. Cancer Res. 2004;64:4693–4698. doi: 10.1158/0008-5472.CAN-03-3437. [DOI] [PubMed] [Google Scholar]
- 15.Andre F, Cabioglu N, Assi H, Sabourin JC, Delaloge S, Sahin A, Broglio K, Spano JP, Combadiere C, Bucana C, et al. Expression of chemokine receptors predicts the site of metastatic relapse in patients with axillary node positive primary breast cancer. Ann Oncol. 2006;17:945–951. doi: 10.1093/annonc/mdl053. [DOI] [PubMed] [Google Scholar]
- 16.Nevo I, Sagi-Assif O, Meshel T, Ben-Baruch A, Jöhrer K, Greil R, Trejo LE, Kharenko O, Feinmesser M, Yron I, et al. The involvement of the fractalkine receptor in the transmigration of neuroblastoma cells through bone-marrow endothelial cells. Cancer Lett. 2009;273:127–139. doi: 10.1016/j.canlet.2008.07.029. [DOI] [PubMed] [Google Scholar]
- 17.Marchesi F, Piemonti L, Fedele G, Destro A, Roncalli M, Albarello L, Doglioni C, Anselmo A, Doni A, Bianchi P, et al. The chemokine receptor CX3CR1 is involved in the neural tropism and malignant behavior of pancreatic ductal adenocarcinoma. Cancer Res. 2008;68:9060–9069. doi: 10.1158/0008-5472.CAN-08-1810. [DOI] [PubMed] [Google Scholar]
- 18.D'Haese JG, Demir IE, Friess H, Ceyhan GO. Fractalkine/CX3CR1: why a single chemokine-receptor duo bears a major and unique therapeutic potential. Expert Opin Ther Targets. 2010;14:207–219. doi: 10.1517/14728220903540265. [DOI] [PubMed] [Google Scholar]
- 19.Pour PM, Bell RH, Batra SK. Neural invasion in the staging of pancreatic cancer. Pancreas. 2003;26:322–325. doi: 10.1097/00006676-200305000-00002. [DOI] [PubMed] [Google Scholar]


