Abstract
AIM: To evaluate human epidermal growth factor receptor 2 (HER2) and death decoy receptor (DcR3) as colorectal cancer prognostic indicators.
METHODS: Colorectal carcinoma specimens from 300 patients were analyzed by immunohistochemistry to detect the staining patterns of HER2 and DcR3. Classification of HER2 staining was carried out using the United States Food and Drug Administration semi-quantitative scoring system, with scores of 0 or 1+ indicating a tumor-negative (normal expression) status and scores of 2+ and 3+ indicating a tumor-positive (overexpression) status. Classification of DcR3 was carried out by quantitating the percentage of positive cells within the stained section, with < 10% indicating a tumor-negative status and ≥ 10% indicating a tumor-positive status. Correlation of the HER2 and DcR3 staining status with clinicopathological parameters [age, sex, tumor size, differentiation, and the tumor, node, metastasis (pTNM) classification] and survival was statistically assessed.
RESULTS: Tumor-positive status for HER2 and DcR3 was found in 18.33% and 58.33% of the 300 colorectal carcinoma specimens, respectively. HER2 tumor-positive status showed a significant correlation with tumor size (P = 0.003) but not with other clinicopathological parameters. DcR3 tumor-positive status showed a significant correlation with tumor differentiation (P < 0.001), pTNM stage (P < 0.001), and lymph node metastasis (P < 0.001). However, correlation coefficient analysis did not indicate that a statistically significant correlation exists between tumor-positive status for the HER2 and DcR3 overexpression (P = 0.236). Patients with specimens classified as DcR3-overexpressing had a significantly worse overall survival (OS) rate than those without DcR3 overexpression (median OS: 42.11 vs 61.21 mo; HR = 50.27, 95%CI: 44.90-55.64, P < 0.001). HER2 overexpression had no significant impact on median OS (35.10 mo vs 45.25 mo; HR = 44.40, 95%CI: 39.32-49.48, P = 0.344). However, patients with specimens classified as both HER2- and DcR3-overexpressing had a significantly poorer median OS than those with only HER2 overexpression (31.80 mo vs 52.20 mo; HR = 35.10, 95%CI: 22.04-48.16, P = 0.006).
CONCLUSION: HER2 overexpression is not an independent prognostic marker of colorectal cancer, but DcR3 overexpression is highly correlated with lymph node metastasis and poor OS.
Keywords: Colorectal carcinoma, Human epidermal growth factor receptor 2, Death decoy receptor, Immunohistochemistry, Prognosis
Core tip: Overexpression of the human epidermal growth factor receptor 2 (HER2) and death decoy receptor (DcR3) has been observed in clinical specimens of colorectal cancer, but their roles in prognosis remain unknown. In this systematic investigation of the immunohistochemistry staining patterns of HER2 and DcR3 in 300 clinical specimens, only DcR3 overexpression was identified as a potential prognostic marker of colorectal cancer. Specifically, DcR3 tumor-positive staining showed a strong statistical correlation with lymph node metastasis and poor overall survival. Moreover, HER2-positive patients with DcR3 overexpression had poorer overall survival than their DcR3-negative counterparts.
INTRODUCTION
Colorectal cancer remains one of the most common malignancies diagnosed worldwide. Extensive research effort has been put forth to elucidate the molecular mechanisms underlying progression to metastatic colorectal carcinoma, yet there remains a notable absence of accurate and convenient prognostic biomarkers[1]. Disease management of both non-metastatic and metastatic cases consists of surgical resection and chemotherapeutic drug delivery (systemic, as well as cancer cell-targeted). However, cases of resistance to the cytotoxic agents are not infrequent, and this condition represents a particular clinical challenge as no other treatment options are available.
The multistep carcinogenic process of colorectal cancer is known to involve perturbed activation and signaling of inflammation and oxidative stress pathways. Two key factors of these pathways are the pro-inflammatory human epidermal growth factor receptor 2 (HER2) and the anti-inflammatory death decoy receptor 3 (DcR3), both of which have been observed as overexpressed in clinical samples of colorectal cancer[2,3]. Moreover, response to adjuvant chemotherapy with 5-fluorouracil is associated with the DcR3 chromosomal locus[4].
HER2 is a transmembrane tyrosine kinase receptor that regulates cell growth and differentiation. As such, abnormal HER2 expression and/or activity can promote growth and progression of malignant cells[5,6], and its carcinogenic role has been defined in several human cancers[5-11]. In breast cancer, chemotherapy treatment based on herceptin, an anti-HER2 monoclonal antibody, has been shown to significantly improve the overall survival (OS) rate[12,13].
DcR3 is a tumor necrosis factor (TNF) receptor that counteracts activation of signals of the inflammation pathway by competitively binding to the TNF ligands [Fas ligand (FasL), LIGHT, and TL1A][14-17]. The DcR3-mediated inhibition of TNF signaling has been shown to protect tumor cells from apoptosis and to promote invasiveness and metastasis. Abnormal DcR3 expression has been reported in clinical specimens of lung, gastrointestinal, renal and ovarian cancers, as well as colon cancer[18-22].
Thus, the protein expression levels of HER2 and DcR3 may represent prognostic markers of colorectal cancer and may reflect malignant potential of resected tumor specimens. This study was designed to investigate the correlations between immunohistochemical staining patterns of HER2 and DcR3, individually and in combination, and clinicopathological parameters of colorectal cancer in order to evaluate their potential prognostic value.
MATERIALS AND METHODS
Patient information
This retrospective study involved 300 treatment-naïve patients who had undergone elective surgical resection for colorectal cancer at the Department of General Surgery of Su Bei People’s Hospital of Yangzhou University (China) between January 2003 and December 2010. The study population was composed of 177 males and 123 females, ranging in age from 21 to 85 years (mean age: 56.3 years). For each patient, data on the clinicopathological parameters were collected from the medical records database and included patient age and sex, and tumor size, differentiation, and pathological classification according to the tumor, node, and metastasis (pTNM) scoring system.
All patients provided informed consent for storage and future use of their resected tumor specimens for research purposes, and this study was authorized by the Ethics Committee of Su Bei People’s Hospital of Jiangsu Province.
Immunohistochemical staining of HER2 and DcR3
Formalin-fixed paraffin-embedded sections of surgical specimens on glass slides were deparaffinized in xylene and rehydrated through an ethanol gradient. Endogenous peroxidase was blocked by a 10-min incubation with 0.3% H2O2 in methanol, after which antigen retrieval was performed by 15 min of heating in 10 mmol/L sodium citrate (pH 6.0). After the sample was cooled for 20 min to room temperature and washed with phosphate-buffered saline (PBS), non-specific binding sites were blocked by a 10 min pre-incubation with 10% fetal calf serum in PBS with 0.01% sodium azide. The primary antibody against HER2 (1:100 dilution; HercepTest™ kit from Dako, Glostrup, Denmark) or DcR3 (1:200; Dako) was added and the slide incubated for 1 h in a humidified chamber. Following a triplicate wash with PBS, the appropriate secondary antibody (undiluted; Envision-HRP Complex from Dako) was added and the slide incubated for an additional 60 min in the humidified chamber. Immunoreactivity was visualized by incubation with the chromagen 3’-3’-diaminobenzidine. All sections were counterstained with hematoxylin. For each specimen, a negative control was generated by replacing the primary antibody with PBS alone.
Scoring of HER2 and DcR3 immunostaining
Each processed slide was assessed by two pathologists working independently. Instances of interobserver variability were resolved by consensus discussion during simultaneous dual re-evaluation.
Scoring of the HER2 staining was carried out according to the semi-quantitative strategy recommended by the United States Food and Drug Administration[23], as follows: no staining or membrane staining in < 10% of the tumor cells = 0; incomplete membrane staining in > 10% of tumor cells = 1+; weak-to-moderate complete membrane staining in > 10% of tumor cells = 2+; moderate-to-strong complete membrane staining in > 10% of tumor cells = 3+. Specimens with scores of 0 or 1+ were classified as tumor-negative for HER2 (normal expression), and specimens with scores of 2+ or 3+ were classified as tumor-positive for HER2 (overexpression).
Classification of DcR3 was carried out by quantitating the percentage of positive cells within the stained section, with < 10% indicating a tumor-negative status and ≥ 10% indicating a tumor-positive status.
Follow-up
Patients were followed to December 2010 or until death. The median follow-up interval was 50.3 mo (2-93 mo). Follow-up examinations included imaging analysis to detect any signs of recurrence.
Statistical analysis
All statistical analyses were carried out with the SPSS statistical software for Windows, version 17 (SPSS Inc., Chicago, IL, United States). The significance of associations between HER2 and DcR3 immunostaining and the various clinicopathological parameters was analyzed by χ2 test. OS curves were generated using the Kaplan-Meier method, and the significance of differences between the curves was analyzed by log-rank testing. The threshold for statistical significance was set at a P value < 0.05.
RESULTS
Immunohistochemical staining patterns of HER2 and DcR3 in human colorectal cancer specimens
Of the total 300 colorectal cancer specimens examined, 55 (18.33%) showed HER2 overexpression, with the immunostaining scores being mostly moderate (2+, n = 45) (Figure 1A) and only 10 having strong scores (3+) (Figure 1B). In contrast, a remarkably larger percentage of the specimens showed DcR3 overexpression (58.33%) (Figure 1C).
Figure 1.
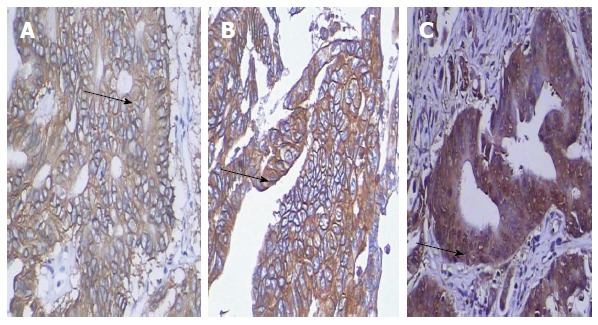
Immunohistochemical detection of human epidermal growth factor receptor 2 and death decoy receptor expression in colorectal cancer. Serial-section specimens from one representative case are shown (× 200). A: Human epidermal growth factor receptor 2 (HER2)-positive tumor section, scored as 2+ (arrow); B: HER2-positive tumor section, scored as 3+ (arrow); C: Death decoy receptor-positive tumor section showing staining in > 10% of the tumor cells (arrow).
Correlation of HER2 or DcR3 overexpression with clinicopathological features of colorectal cancer
The correlation of HER2 and DcR3 immunostaining patterns with clinicopathological features is presented in Table 1. HER2 overexpression was found to be significantly correlated only with tumor size. In contrast, DcR3 overexpression was found to be significantly correlated with tumor differentiation, lymph node metastases, and pTNM stage (P < 0.05).
Table 1.
Correlation of colorectal cancer clinicopathological variables with immunohistochemical staining patterns of human epidermal growth factor receptor 2 and death decoy receptor expression
| Variable | n |
HER2 |
P value1 |
DcR3 |
P value1 | ||
| Positive | Negative | Positive | Negative | ||||
| Age | |||||||
| < 60 | 182 | 34 | 148 | 0.847 | 109 | 73 | 0.497 |
| ≥ 60 | 118 | 21 | 97 | 66 | 52 | ||
| Sex | |||||||
| Male | 171 | 30 | 141 | 0.684 | 102 | 69 | 0.595 |
| Female | 129 | 25 | 104 | 73 | 56 | ||
| Tumor size (cm) | |||||||
| < 5 | 194 | 26 | 168 | 0.003 | 106 | 88 | 0.279 |
| ≥ 5 | 106 | 29 | 77 | 51 | 55 | ||
| Differentiation grade | |||||||
| Well | 73 | 11 | 62 | 0.385 | 29 | 44 | |
| Moderate | 174 | 31 | 143 | 101 | 73 | < 0.001 | |
| Poor | 53 | 13 | 40 | 45 | 8 | ||
| Lymph node status | |||||||
| Negative | 161 | 32 | 129 | 0.457 | 74 | 87 | < 0.001 |
| Positive | 139 | 23 | 116 | 101 | 38 | ||
| Distant metastasis | |||||||
| M0 | 273 | 49 | 224 | 0.774 | 157 | 116 | 0.357 |
| M1 | 27 | 6 | 21 | 18 | 9 | ||
| Perineural invasion | |||||||
| Negative | 285 | 51 | 231 | 0.9002 | 163 | 119 | 0.459 |
| Positive | 18 | 4 | 14 | 12 | 6 | ||
| pTNM stage | |||||||
| I | 50 | 4 | 46 | 0.0692 | 16 | 34 | < 0.001 |
| II | 110 | 18 | 92 | 52 | 58 | ||
| III | 90 | 23 | 67 | 66 | 24 | ||
| IV | 50 | 10 | 40 | 41 | 9 | ||
Pearson’s χ2 test;
Adjusted χ2 test. HER2: Human epidermal growth factor receptor 2; DcR3: Death decoy receptor; pTNM: The tumor, node, metastasis classification.
Association between HER2 and DcR3 overexpression in colorectal cancer specimens
A little more than half (65.45%) of the specimens showing HER2 overexpression also showed DcR3 overexpression, which was not significantly different from the percentage of specimens showing normal HER2 expression and DcR3 overexpression (56.73%) (P = 0.236).
Correlation of HER2 or DcR3 overexpression with survival
HER2 immunostaining status had no impact on OS; the median OS of patients with HER2-positive tumor specimens was 35.10 mo, compared to 45.25 mo for the patients with HER2-negative tumor specimens (HR = 44.40, 95%CI: 39.32-49.48, P = 0.344) (Figure 2A). In contrast, DcR3-positive status was associated with a poorer OS (median OS of 42.11 mo vs DcR3-negative status: 61.21 mo; HR = 50.27, 95%CI: 44.90-55.64, P < 0.001) (Figure 2B). Moreover, patients whose tumor specimens showed double positivity [HER-2(+)/DcR3(+)] had worse OS than their counterparts without DcR3 overexpression [HER-2(+)/DcR3(-)] (median OS: 31.80 mo vs 52.20 mo, respectively; HR = 35.10, 95%CI: 22.04-48.16, P = 0.006) (Figure 2C).
Figure 2.
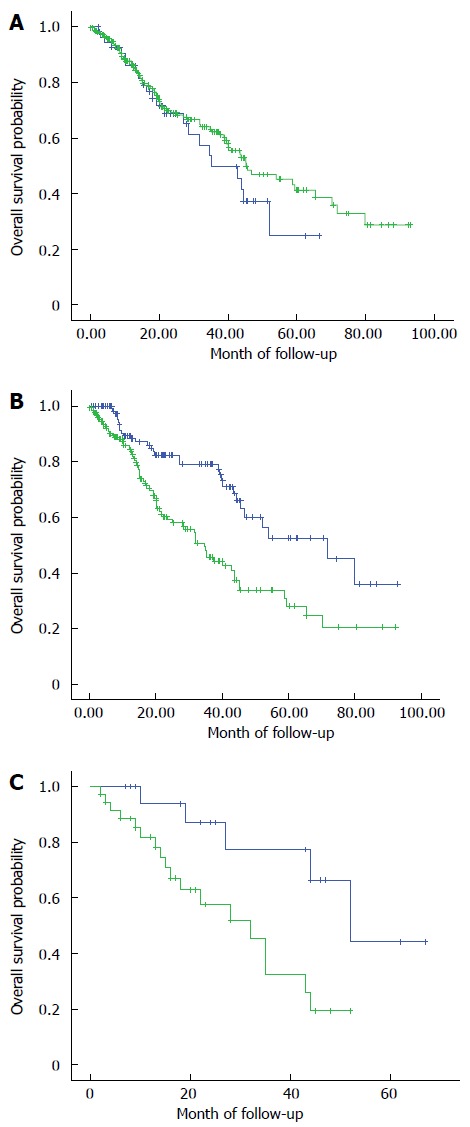
Kaplan-Meier overall survival curves. A: Kaplan-Meier overall survival curves for the 300 colorectal cancer patients, according to immunohistochemical detection of human epidermal growth factor receptor 2 normal (green) and overexpression (blue). The difference between the curves did not reach statistical significance (P = 0.344); B: Kaplan-Meier overall survival curves for the 300 colorectal cancer patients, according to immunohistochemical detection of death decoy receptor normal (green) and overexpression (blue). The difference between the curves was significant (P < 0.001); C: Kaplan-Meier overall survival curves for the 300 colorectal cancer patients, according to immunohistochemical detection of human epidermal growth factor receptor 2 overexpression in isolation (blue) or coincident with (green) death decoy receptor overexpression. The difference between the curves was significant (P = 0.006).
DISCUSSION
In this investigation of 300 human colorectal cancer specimens, immunohistochemical detection of DcR3, and not HER2, overexpression showed promise as a clinical marker of disease prognosis. Similar to the findings from a previous study of growth factor expression in Chinese patient specimens (i.e., the pro-angiogenic vascular endothelial growth factor (VEGF) in relation to the HER2 growth factor receptor)[24], immunohistochemical detection of HER2 overexpression showed a correlation with only tumor size and had no significant relation with survival. However, in the previous study, HER2 overexpression showed a statistical correlation with distant metastasis; this inconsistency could be explained by a variance due to limited sample size.
In the present study, immunohistochemically detected DcR3 overexpression reflected features of colorectal cancer progression. Specifically, the specimens showing DcR3-positive immunostaining were less differentiated and more invasive. Moreover, the patients with DcR3-positive tumors were more likely to have lymph node metastases at the time of resection and poorer OS. Thus, DcR3 may represent a useful prognostic marker of colorectal cancer malignancy and risk of death.
Coincident overexpression of DcR3 significantly worsened the OS in patients with HER2 overexpression. Malignant transformation of a tumor cell requires molecular signals that promote growth and inhibit apoptosis, as well as those that allow a cell to migrate and invade surrounding tissues. While HER2 overexpression may contribute to the unrestricted growth of a cancer cell, DcR3 may play a key role in promoting metastasis, which is related to higher risk of cancer-related death and complicates the clinical management.
In summary, colorectal cancer specimens from Chinese patients show both isolated and coincident overexpression of HER2 and DcR3. Isolated DcR3 overexpression may be a clinical prognostic marker of malignancy and survival, and coincident DcR3/HER2 overexpression may indicate more robust malignancy risk. Future studies need to elucidate the molecular mechanism of DcR3-mediated carcinogenesis to further define its relation to patient prognosis (in various ethnicities and disease conditions) and its potential as a target of molecular-based therapies.
COMMENTS
Background
Overexpression of the pro-inflammatory human epidermal growth factor receptor 2 (HER2) and anti-inflammatory death decoy receptor 3 (DcR3) has been observed in various human cancers, including colorectal carcinoma. Yet, their roles in malignant transformation of colorectal cancer cells and potential as clinical markers of disease prognosis remain unknown.
Research frontiers
Immunohistochemical detection of DcR3 overexpression in human colorectal cancer specimens resected from Chinese patients is correlated with malignancy and overall survival. DcR3 may represent a useful prognostic biomarker of colorectal cancer in this patient population. Moreover, while overexpression of HER2 may promote the unrestricted growth of colorectal tumor cells, the overexpression of DcR3 may promote cell invasiveness and migration to increase the risk of metastasis and cancer-related death.
Innovations and breakthroughs
To date, no study in the publicly available literature has reported data on the coincident overexpression of HER2 and DcR3 in human colorectal cancer specimens. Clinical detection of the immunohistochemical staining patterns of these two cancer-related proteins may represent a convenient and accurate approach for determining the prognosis of Chinese patients with colorectal cancer.
Applications
Immunohistochemical detection of DcR3 overexpression, in isolation or coincident with HER2 overexpression, may be a useful clinical approach for predicting risk of malignancy in and survival of colorectal cancer patients.
Peer review
This study investigated the potential association of two cancer-related proteins, DcR3 and HER2, with colorectal cancer, and evaluated whether isolated or coincident overexpression may be indicative of increased risk of malignancy and/or overall survival. According to statistical correlation analysis of the HER2 and DcR3 immunohistochemical staining patterns and clinicopathological parameters, DcR3 overexpression, in isolation or coincident with HER2 overexpression, was highly associated with increased malignancy and poorer survival. Specifically, DcR3 overexpression was shown to be significantly correlated with less differentiated tumors, worse pTNM stage, and presence of lymph node metastasis. Thus, the study provides novel evidence supporting the potential utility of DcR3 as a prognostic marker for clinical evaluation of colorectal cancer patients, and provides a foundation for future studies to explore the role of this receptor in carcinogenesis and its potential as a novel target of anticancer drugs.
Footnotes
P- Reviewers: Michael L, Harry HXX S- Editor: Ma YJ L- Editor: Wang TQ E- Editor: Zhang DN
References
- 1.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.Nathanson DR, Culliford AT, Shia J, Chen B, D’Alessio M, Zeng ZS, Nash GM, Gerald W, Barany F, Paty PB. HER 2/neu expression and gene amplification in colon cancer. Int J Cancer. 2003;105:796–802. doi: 10.1002/ijc.11137. [DOI] [PubMed] [Google Scholar]
- 3.Wu Y, Han B, Sheng H, Lin M, Moore PA, Zhang J, Wu J. Clinical significance of detecting elevated serum DcR3/TR6/M68 in malignant tumor patients. Int J Cancer. 2003;105:724–732. doi: 10.1002/ijc.11138. [DOI] [PubMed] [Google Scholar]
- 4.Mild G, Bachmann F, Boulay JL, Glatz K, Laffer U, Lowy A, Metzger U, Reuter J, Terracciano L, Herrmann R, et al. DCR3 locus is a predictive marker for 5-fluorouracil-based adjuvant chemotherapy in colorectal cancer. Int J Cancer. 2002;102:254–257. doi: 10.1002/ijc.10711. [DOI] [PubMed] [Google Scholar]
- 5.Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 6.Slamon DJ, Godolphin W, Jones LA, Holt JA, Wong SG, Keith DE, Levin WJ, Stuart SG, Udove J, Ullrich A. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science. 1989;244:707–712. doi: 10.1126/science.2470152. [DOI] [PubMed] [Google Scholar]
- 7.Han H, Landreneau RJ, Santucci TS, Tung MY, Macherey RS, Shackney SE, Sturgis CD, Raab SS, Silverman JF. Prognostic value of immunohistochemical expressions of p53, HER-2/neu, and bcl-2 in stage I non-small-cell lung cancer. Hum Pathol. 2002;33:105–110. doi: 10.1053/hupa.2002.30183. [DOI] [PubMed] [Google Scholar]
- 8.Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687–697. doi: 10.1016/S0140-6736(10)61121-X. [DOI] [PubMed] [Google Scholar]
- 9.Caruso ML, Valentini AM. Immunohistochemical p53 overexpression correlated to c-erbB-2 and cathepsin D proteins in colorectal cancer. Anticancer Res. 1996;16:3813–3818. [PubMed] [Google Scholar]
- 10.Osako T, Miyahara M, Uchino S, Inomata M, Kitano S, Kobayashi M. Immunohistochemical study of c-erbB-2 protein in colorectal cancer and the correlation with patient survival. Oncology. 1998;55:548–555. doi: 10.1159/000011911. [DOI] [PubMed] [Google Scholar]
- 11.Yang JL, Ow KT, Russell PJ, Ham JM, Crowe PJ. Higher expression of oncoproteins c-myc, c-erb B-2/neu, PCNA, and p53 in metastasizing colorectal cancer than in nonmetastasizing tumors. Ann Surg Oncol. 1996;3:574–579. doi: 10.1007/BF02306092. [DOI] [PubMed] [Google Scholar]
- 12.Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 13.Vogel CL, Cobleigh MA, Tripathy D, Gutheil JC, Harris LN, Fehrenbacher L, Slamon DJ, Murphy M, Novotny WF, Burchmore M, et al. Efficacy and safety of trastuzumab as a single agent in first-line treatment of HER2-overexpressing metastatic breast cancer. J Clin Oncol. 2002;20:719–726. doi: 10.1200/JCO.2002.20.3.719. [DOI] [PubMed] [Google Scholar]
- 14.Houghton JA, Harwood FG, Tillman DM. Thymineless death in colon carcinoma cells is mediated via fas signaling. Proc Natl Acad Sci USA. 1997;94:8144–8149. doi: 10.1073/pnas.94.15.8144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roth W, Isenmann S, Nakamura M, Platten M, Wick W, Kleihues P, Bähr M, Ohgaki H, Ashkenazi A, Weller M. Soluble decoy receptor 3 is expressed by malignant gliomas and suppresses CD95 ligand-induced apoptosis and chemotaxis. Cancer Res. 2001;61:2759–2765. [PubMed] [Google Scholar]
- 16.Chen PH, Yang CR. Decoy receptor 3 expression in AsPC-1 human pancreatic adenocarcinoma cells via the phosphatidylinositol 3-kinase-, Akt-, and NF-kappa B-dependent pathway. J Immunol. 2008;181:8441–8449. doi: 10.4049/jimmunol.181.12.8441. [DOI] [PubMed] [Google Scholar]
- 17.Elnemr A, Ohta T, Yachie A, Kayahara M, Kitagawa H, Fujimura T, Ninomiya I, Fushida S, Nishimura GI, Shimizu K, et al. Human pancreatic cancer cells disable function of Fas receptors at several levels in Fas signal transduction pathway. Int J Oncol. 2001;18:311–316. doi: 10.3892/ijo.18.2.311. [DOI] [PubMed] [Google Scholar]
- 18.Pitti RM, Marsters SA, Lawrence DA, Roy M, Kischkel FC, Dowd P, Huang A, Donahue CJ, Sherwood SW, Baldwin DT, et al. Genomic amplification of a decoy receptor for Fas ligand in lung and colon cancer. Nature. 1998;396:699–703. doi: 10.1038/25387. [DOI] [PubMed] [Google Scholar]
- 19.Bai C, Connolly B, Metzker ML, Hilliard CA, Liu X, Sandig V, Soderman A, Galloway SM, Liu Q, Austin CP, et al. Overexpression of M68/DcR3 in human gastrointestinal tract tumors independent of gene amplification and its location in a four-gene cluster. Proc Natl Acad Sci USA. 2000;97:1230–1235. doi: 10.1073/pnas.97.3.1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takahama Y, Yamada Y, Emoto K, Fujimoto H, Takayama T, Ueno M, Uchida H, Hirao S, Mizuno T, Nakajima Y. The prognostic significance of overexpression of the decoy receptor for Fas ligand (DcR3) in patients with gastric carcinomas. Gastric Cancer. 2002;5:61–68. doi: 10.1007/s101200200011. [DOI] [PubMed] [Google Scholar]
- 21.Macher-Goeppinger S, Aulmann S, Wagener N, Funke B, Tagscherer KE, Haferkamp A, Hohenfellner M, Kim S, Autschbach F, Schirmacher P, et al. Decoy receptor 3 is a prognostic factor in renal cell cancer. Neoplasia. 2008;10:1049–1056. doi: 10.1593/neo.08626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Connor JP, Felder M. Ascites from epithelial ovarian cancer contain high levels of functional decoy receptor 3 (DcR3) and is associated with platinum resistance. Gynecol Oncol. 2008;111:330–335. doi: 10.1016/j.ygyno.2008.07.012. [DOI] [PubMed] [Google Scholar]
- 23.Jacobs TW, Gown AM, Yaziji H, Barnes MJ, Schnitt SJ. Specificity of HercepTest in determining HER-2/neu status of breast cancers using the United States Food and Drug Administration-approved scoring system. J Clin Oncol. 1999;17:1983–1987. doi: 10.1200/JCO.1999.17.7.1983. [DOI] [PubMed] [Google Scholar]
- 24.Li Q, Wang D, Li J, Chen P. Clinicopathological and prognostic significance of HER-2/neu and VEGF expression in colon carcinomas. BMC Cancer. 2011;11:277. doi: 10.1186/1471-2407-11-277. [DOI] [PMC free article] [PubMed] [Google Scholar]


