Abstract
Research on the concept of craving may lead to a better understanding of the biobehavioral circuitries that contribute to the complexity of Alcohol Use Disorders (AUD). The experiences described as craving or desire to drink, are often associated with physical responses such as increased salivation, heart rate and alteration of stress hormones as well as psychological responses such as anxiety and depression. Greater craving has been associated with the increased probability of alcohol relapse. Reversal of craving, understood as a symptom of protracted abstinence, offers the possibility of preventing relapses and treating alcoholism. Various medications have been studied to establish whether they are able to reduce craving; however, the results obtained from clinical studies have been inconsistent. Here we review the interdisciplinary models developed to evaluate craving, then the different approaches used to assess and measure craving, and finally, the medications utilized and tested to lessen craving in patients suffering from AUD.
1. Background
As a subjective experience, craving is a difficult phenomenon to define, but is used by patients, clinicians and researchers to describe a strong desire to drink alcohol. It was first recognized as a component of alcoholism in 1955 (1). The term, however, was acknowledged later in the scientific and clinical communities (2) and investigated for its association with alcohol withdrawal and relapse (3-5). The experience of craving has been identified as on a continuum, rather than an on-and-off symptom. Craving is also positively correlated with negative emotions (6, 7), stress and anxiety (8-10), and negatively correlated with length of alcohol abstinence (11).
Researchers have used different definitions for craving. For example, craving has been described by some as “desire and urge,” while others have suggested the definition of craving to be only the “desire” for experiencing the effects of a drug, while specifying the term “urge” to the behavioral intention to use it (4). Additionally, in clinical practice, it is a difficult symptom to assess because patients may deny experiencing it (2). Moreover they may be unable to recognize it because of the overwhelming emotional experience during abstinence and withdrawal (12), or simply because they do not remember having experienced craving before the relapse occurred (13). Proposing a uniform definition for craving has been challenging due to the lack of a valid method to measure psychological, behavioral and brain function of subjective experiences (14, 15). Furthermore, because of the lack of a standardized definition, operationalizing valid and reliable preclinical models that can be used as predictors of human craving is especially challenging. (16).
The understanding of craving has evolved considerably due to progress in many cross-interdisciplinary studies. For example, elucidating neurobiological circuitries (17, 18), developing preclinical models (19-21), and evolving cue-elicitation investigation in human studies (22) are several approaches that researchers have investigated to understand the role of craving in Alcohol Use Disorder (AUD). In addition, cognitive psychology (23), have advanced neuroimaging techniques (24-26), and pharmacological studies (27-29) have inferred that overlapping neurochemical systems contribute to alcohol craving.
While actively discussed as a symptom in addictive behavior disorders (30-33) craving is called “a strong desire or sense of compulsion to take the substance” in the International Statistical Classification of Diseases and Related Health Problems 10th Revision (ICD-10). The inclusion of a “craving” criterion to the just released fifth revision of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) was predicted to bring an improvement in the application of the diagnostic criteria used to assess alcohol problems (35). In addition to the merger of the two diagnoses of Alcohol Abuse and Alcohol Dependence into a monothetic diagnosis of AUD, recent research evaluated the addition of the “craving” criterion proposed to the DSM-5 diagnosis of AUD (36, 37). Empirically, while the AUD coalesced criterion of the DSM-5 includes individuals less severely affected than those meeting only DSM-IV criteria , the consequences of adding the craving criterion could not be fully evaluated due to the lack of relevant comparative data regarding craving (36).
The hypothesis that an ideal pharmacotherapy to treat alcoholism should target a decrease in alcohol craving was first suggested in the 1990s (38). While many behavioral and pharmacotherapy approaches targeting craving are shown to reduce alcohol consumption in randomized controlled trials (RCTs) (12), craving has not consistently been associated with reduced alcohol relapse (4, 39-43). Moreover, pharmacological approches alone or combined with behavioral interventions that reduced drinking by attenuating craving did not always improve drinking outcomes (4, 44).
2. Alcohol Craving: Models and Neurobiology
Several clinical and preclinical models and approaches have been proposed to assess craving and relapse. One of the first approaches to establish a comprehensive definition for alcohol craving was derived from the hypothesis that numerous mechanisms were responsible for the urge to drink, and therefore a multidisciplinary approach was necessary to elucidate the basis of alcohol craving (45).
Psychological approaches to assess craving include: the study of the conditioning (alcohol-related cues; i.e. association of experience with alcohol ingestion, which become conditioned stimuli), and cognitive models (cognitive processes, which dictate response to alcohol and alcohol-related cues during abstinence). Further, alcohol cue-exposure techniques have been utilized to evaluate cognitive changes in alcoholics (46). According to the conditioning model, relapse situations originate from neutral stimuli that produce conditioned craving after repeated pairing of environmental stimuli and withdrawal. Many human studies targeting experimental approaches have used the cue-exposure paradigm to test pharmacotherapies for potential alcohol anti-craving properties (47). Cue-reactivity models developed in laboratory studies allow assessing the potential risk factors for alcohol relapse. In addition, cue-reactivity sessions provide a platform to evaluate potential medications for protracted abstinence, as an alternative to laboratory methods involving alcohol administration (48, 49). Because of the simplicity of the model adopted in original studies, craving has not always predicted alcohol relapses. The dynamics of craving (tonic, phasic, pulsatile, continuous, discontinuous) (50) and integration of neurochemical circuitry in the three-pathway psychobiological model (the reward, relief and obsessive craving) offered the opportunity to simultaneously evaluate multiple psychoneuro-networks in AUD (51). From the dual process model (23), craving was conceptualized as the result of an imbalance between reflective and reflexive systems. Alcohol may increase hyper-excitability in reflexive brain areas (prefrontal cortex) and reduce reflective circuitries (amygdala). Later experimental psychology approaches corroborated that drugs (as well as alcohol) have the ability to trigger specific inner mechanisms (from the locus of the emotions, such as the amygdala) that modulate, or even take over, individual cognitive resources that are needed for exercising willpower to resist drugs (52).
Reaching for alcohol, rather than trying to avoid it, is a more common behavior in heavy-drinking alcohol dependent individuals. This harmful behavior is hypothesized to be related to cue-induced craving (53). Attention bias has also been associated with subjective craving (54), however the “substance-seeking” is not always a conscious experience (55). Finally, lack of self-control, impulsivity and suppression of unwanted affects are also attributed to “resource depletion,” that may trigger urges and craving (56).
Preclinical models have generated many hypotheses to explain the neurobiological mechanisms associated with anticipatory states that have significant relevance for studies on the neurobiology of craving (57) and relapse (58). A dysregulation of the brain reward processing produced by alteration on mesolimbic dopamine (DA), glutamate, γ-aminobutyric acid (GABA), and stress circuitries, may be responsible for the compulsive drug use and a loss of control over drug taking (59). This neuroadaptive model for craving was based on the theory that hyper-sensitization to drugs (including alcohol) and drug-associated stimuli converts ordinary 'wanting' into craving, and prolonged abstinence may prompt reward memory, thereby inducing craving (55).
The effects of alcohol in the development of craving has been hypothesized to directly affect the mesolimbic DA pathway (60). The implication of the limbic system in the mediation of craving and loss of control is described as the incentive sensitization model. The acquisition and sensitization of craving for alcohol (and other addictive drugs) may develop by repeated exposure and release of DA in response to cues (55, 61, 62). Additionally, the dopamine receptor subtypes described as D1-like (D1 and D5) and D2-like (D2-4) are expressed in brain structures such as the amygdala and hippocampus, both of which have been implicated in human and preclinical models of addiction (55, 63-65). The mesolimbic DA pathway projecting from the ventral tegmental area (VTA) to the nucleus accumbens (NAcc) has been recognized as a site for the reinforcing actions of ethanol and other addictive drugs in preclinical models (Figure 1, blue arrow) (66-68). In addition, glutamatergic neurotransmission projecting from the prefrontal cortex (PFC), amygdala and hippocampus to the NAcc and VTA are associated with relapse (Figure 1, red arrows) (69). Alcohol is shown to modulate mesolimbic pathways and induce the urge to drink and loss of control (55).
Figure 1. Involvement of the Limbic System in the Mediation of Craving.
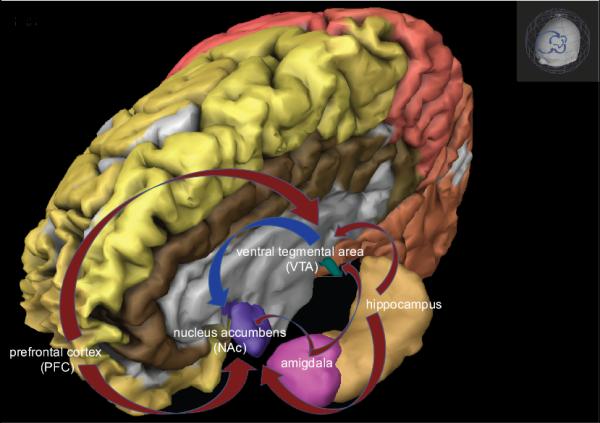
Mesolimbic dopamine pathways that project from the ventral tegmental area (VTA) to the nucleus accumbens (NAcc) are recognized as sites for the reinforcing actions of ethanol and other addictive drugs, blue arrow; glutamatergic projections from the prefrontal cortex (PFC), amygdala and hippocampus to the NAcc and VTA are associated with relapse, red arrows (Image created using Brain Explore2, Allen Human Brain Atlas, available to download from http://human.brain-map.org/static/brainexplorer 9/12/2013).
The loss of control, with the manifestation of withdrawal symptoms (51), may be due to the alteration of the increased dopaminergic and glutamatergic action and a decrease of GABAergic neurotransmission (25, 70, 71). The modulation of serotonin (5-HT), or 5-hydroxytryptamine-mediated signal transmission has been linked to increased alcohol consumption (72, 73) and to the obsessive-compulsive behavior in alcohol craving (74, 75). Serotonergic neurons, located in the raphe nucleus, project to the amygdala, VTA, hippocampus and to the NAcc. Serotonin traverses the synaptic clefts, tightly regulated by transporters and bind to a discrete group of 5-HT-subtype receptors, which selectively can affect behaviors and mood states. Furthermore, the serotonergic system interacts with other neurotransmitters such as GABA in the hippocampal formation (influencing cognition and judgment) and with DA in the VTA (mediating rewarding effects).
Alcohol alters the serotonergic system in both acute and chronic exposure. Single alcohol ingestion may increase central 5-HT levels (72) and activate 5-HT3 receptors (76), which are widely distributed in the brain. Chronic alcohol exposure may be responsible for the 5-HT2 receptor adaptive changes resulting from the effort to compensate for the continuous alcohol-induced alterations (such as increasing receptor number) (77). Augmented activation of the 5-HT3 receptors in the GABAergic neurons results in increased neuronal inhibition, and in the increase in the mesolimbic DA activity contributes to enhancement of alcohol rewarding effects. This dual mechanism has been described as the two-stage process of craving (55, 78, 79). Moreover, the deleterious neuroadaptations of the 5-HT2 receptor in the effort to re-establish normal homeostasis functions, may be responsible for the individual’s anxiety (80) and withdrawal symptoms (81), mood dysregulation and obsessive alcohol-seeking behaviors (51) that may result in the development of craving. A deficiency of serotonergic function at any level of this highly structured system may contribute to craving and increase the vulnerability to develop AUD (78). The role of serotonergic neurotransmission in alcohol craving has been evaluated using several methodologies, which include interventions favoring serotonergic neurotransmission, stabilization of mood, and reduction of obsessional thinking (51).
Extensive preclinical data demonstrate an essential role for the endogenous opioid system in stimulation of DA release by ethanol in the NAcc (82). More recently, positron emission tomography (PET) studies show a correlation between alcohol craving and μ-opioid receptors in alcoholic individuals during abstinence (24, 83). Under physiological conditions, the action of the endogenous opioid system is very subtle. Acute alcohol exposure stimulates the release of brain β-endorphins, which may interact with brain regions that affect many neurobiological and behavioral effects of alcohol, either by opioid-receptor binding in the mesolimbic region, or by distant action such as activating the hypothalamic-pituitary axis (HPA). As we noted previously for other neurobiological systems affected by alcohol ingestion, chronic alcohol exposure induces adaptive changes to maintain normal homeostatic levels to overcome the effects of alcohol deprivation. Therefore, the decrease in β-endorphin activity during alcohol abstinence may favor alcohol consumption through the mechanisms of negative reinforcement, inducing craving and promoting relapses. Interventions on both direct and endocrine-like functions of the endogenous opioid system may alter mood and motivation, and may modify drinking behaviors, control alcohol craving, and prevent relapses in individuals suffering from AUD (84).
In summary, human and animal models have been developed to elucidate the underlying mechanism of craving both at psychological, neurobiological, behavioral, cellular and molecular levels. However the subjective, self-reported cognition of a state obtained in human laboratory studies is not feasible to measure in preclinical setting (16). Paradigms such as stress-induced techniques, conditioning, extinction and self-operant chambers are utilized to evaluate alcohol consumption and deprivation prior to drinking or during protracted abstinence (85). More recently, others have developed novel preclinical paradigms to test tolerance during intoxication following withdrawal periods (86).
Future craving research should incorporate a large array of interdisciplinary theories since little is known of craving in the naturalistic environment. Our current research is based mostly on assessing craving under detoxification (withdrawal craving) or undergoing laboratory sessions (cue-reactivity craving) (50). From a neurobiological perspective, addictive drugs produce incremental neuroadaptations, which may encompass many systems and consequent behavioral and psychological effects. To facilitate the translation of these findings into the clinical setting, neuroadaptive mechanisms entail an integration of basic neuroscience with psychology, including genetics. Neuroadaptations may be increased in subjects who have inherited a genetic predisposition (87-89) or because they have acquired susceptibility through repeated experience of intense stress (90, 91).
3. Measuring Craving
Because there is no uniformly accepted definition for craving (15), a measure that captures both the psychological and neurobiological aspects of urge and desire has been elusive (14). The different types of craving such as physical craving (with manifestation of withdrawal symptoms) or impulsive craving (excitability triggered by cues) (50) are not experienced by all alcohol-dependent individuals or may be experienced at different times during alcohol abstinence. Craving variability may be due to the individual salience of drinking, tolerance, intensity of withdrawal symptoms, and subjective awareness of a compulsion to drink alcohol (16). Measurement error in assessing craving has limited the selection and monitoring of pharmacotherapies that target the desire and urge to drink during abstinence.
The major problem with assessing and measuring craving is that it is a self-reported event and the validation of subjective craving is a major issue in measuring craving in alcoholic individuals. In clinical research studies, craving assessments have included both subjective approaches (questionnaires and drinking behaviors) and objective parameters (measures of autonomic responses such as heart rate changes and salivary output). Physical responses, however, do not assure that craving measurements are precise. Autonomic responses can be triggered by numerous homeostatic imbalances, (thermoregulation, circadian rhythm, baroreceptors), which are very common in patients during withdrawal or in those suffering from other comorbidities. The retrospective nature of the self-reported measurement of subjective experiences by patients is widely accepted both in research and clinical settings to assess alcohol urge and desire despite the lack of an absolute scale for craving (92). The measurement of craving should include a series of items that have common meaning for the participants and the researchers. Cravingis a continuous experience that cannot be exclusively restricted to a specific point during an episode of craving (93).
A commonly used measure of alcohol craving is the single-item measure which assesses craving in terms of frequency or intensity on a continuous scale (i.e. Likert or Visual Analogue Scales; VAS) (94). This approach is easy to implement, but focuses only on the current state and does not provide robust prediction over time (95, 96). A more robust measurement of alcohol craving is the multiple-item Penn Alcohol Craving Scale. It is comprised of five items that assess frequency, intensity, and duration of craving (97), and is widely used and accepted in RCTs (14). The Yale–Brown Obsessive Compulsive Scale—heavy drinkers was developed based on the theory that craving is similar to obsessive-compulsive disorder behaviors (74, 98). Its modified version, the Obsessive Compulsive Drinking Scale (OCDS) (2, 3, 99, 100) is regarded among the better performing multi-item measures for alcohol craving (14). The Questionnaire of Alcohol Urges, Alcohol Urge Questionnaire (101) and Alcohol Craving Questionnaire (ACQ), were derived from the 32-item Questionnaire for Smoking Urges (13). Desire to drink is also a fluctuating experience assessed during abstinence (102) and the Desires for Alcohol Questionnaire (103) attempts to capture this subjective experience as well. These questions seek to assess the desire, pleasure, reinforcement, and control over drinking. Currently, it is impossible to assess if one questionnaire is more suitable than another in assessing alcohol craving. Factors affecting self-reported measure of craving are extensive, and due to the individual differences in the severity of alcohol dependence, are conceptually complex and have challenged researchers to develop additional questionnaires such as the Jellinek Craving Questionnaire (96) and Alcohol Craving Experience Questionnaire (94, 103, 104).
Craving may also result from an affective state; therefore, analysis of facial expressions has been utilized to measure craving in clinical studies. Facial muscle activity, measured by facial electromyography (EMG), has been used to effectively assess global positive and negative facial expressions (105) and previously utilized in measuring craving in smoking studies (106). Measuring craving though imaging studies showing specific craving manipulation, has been widely used to determine the participation of isolated brain structures. For example, using PET (107) and functional Magnetic Resonance Imaging (fMRI) (108), it was possible to assess craving in real time (as measured on a visual analog scale) during alcohol cue-induced activation. Using these approaches, it was possible to visualize the involvement of specific neuroanatomical regions such as the amygdala (emotional locus), hippocampus (cognitive nucleus), and ventral striatum (with the NAcc being one of the primary neural substrates mediating addiction) specifically at the moment when patients were experiencing craving.
In summary, several tools and questionnaires are available to measure craving, but no single approach available today represents a state-of-the-art instrument to measure and capture craving from psychological and neurobiological perspectives (14).
4. Current Pharmacotherapy Treatments to Reduce Craving for Alcohol
Specifically targeting the psychological aspects associated with the three pathway psychological model of alcohol craving (51),experimental approaches to evaluate pharmacotherapies to reduce craving have been based on a theory of dysregulation of neurobiological mechanisms of craving associated with clinical symptoms in alcoholic individuals. First, targeting of the reward pathways, which involve the stimulating effects of alcohol, may result from DA and/or opioid dysregulation. Therefore, opioid receptor antagonists (e.g., naltrexone) and DA receptor antagonists (e.g. atypical antipsychotics) have been evaluated as anti-craving medications. Another possible approach targets obsessive mechanisms. The second pathway affects the dysregulation of the 5-HT system, resulting in intrusive thoughts about drinking with impaired social functioning. As such, 5-HT3 antagonists (e.g. ondansetron) and serotonin specific re-uptake inhibitors (SSRIs; e.g. sertraline) have been tested extensively. Finally, the dysregulation of relief circuitry, resulting from GABAergic and glutamatergic activity, hypothesized to be responsible for several withdrawal symptoms (anxiety, tension, and arousal), was evaluated with pharmacological treatments (e.g. baclofen, gabapentin, topiramate). Please see Table 1. for the medications discussed in this section and their mechanisms of action.
Table 1.
Medications and their mechanisms of action
| Medications | Receptor Selectivity and other Mechanisms of Action | System |
|---|---|---|
| naltrexone | μ, κ and δ competitive antagonist | opioid |
| nalmefene | μ, κ and δ competitive antagonist | |
| haloperidol | D2-antagonist | dopamine |
| aripiprazole | D2 and 5-HT1A/2C partial agonist 5-HT2A antagonist |
|
| quetiapine | D1-2 antagonist 5-HT1A partial agonist 5-HT2A antagonist adrenergic α1-2 antagonist |
|
| olanzapine | D2,4 and 5-HT2 antagonist | |
| ondansetron | 5-HT3 antagonist | serotonin |
| fluoxetine | serotonin-specific reuptake inhibitor (SSRI) | |
| topiramate | α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPA)/kainate glutamate antagonist gamma-amino butyric acid (GABA)a agonist carbonic anhydrase enzyme antagonist |
inhibition |
| lamotrigine | sodium channel blocker | |
| levetiracetam | not fully elucidated, but it has been found to target high-voltage, N-type calcium channels, the synaptic vesicle protein 2A (SV2A) |
|
| zonisamide | modulation of voltage-gated ion channels, enhancement of synaptic inhibition, and inhibition of synaptic excitation |
|
| baclofen | GABAB agonist | |
| gamma- hydroxybutyrlc acid (GHB) |
GABAA agonist GHB antagonist |
|
| memantlne | n-methyl d-aspartate (NMDA) antagonist | glutamate |
| acamprosate | NMDA antagonist metabotropic glutamate receptor-5 (mGluR5) antagonist |
|
| disulflram | acetaldehyde dehydrogenase inhibition | alcohol metabolism |
| varenlcllne | α4β2 partial agonist | adrenergic |
| prazosin | α1 antagonist |
4.1 Pharmacological Interventions that Target the Reward Pathway
4.1.1 Opioid Receptors Antagonists
Naltrexone is an opioid-receptor antagonist that has Food and Drug Administration (FDA)-approved indication to treat alcoholism. In the last 20 years, many RCTs evaluating naltrexone for alcoholism, included craving as an outcome. Randomized clinical trials data show that naltrexone-treated patients drink less both in terms of quantity and frequency when compared with placebo-treated subjects (109) and craved less alcohol compared to acamprosate-treated subjects (15). Naltrexone, at a dose of 50 mg/day in combination with a behavioral therapy, decreases alcohol relapse and was also attributed to a reduction of craving (110). Later clinical studies showed that naltrexone was more effective in heavy drinking alcoholics (111) and in patients with a strong family history of alcoholism (112). Heavy drinking early-onset alcoholics, defined as Type B alcoholics, tend to crave alcohol more intensely than moderate drinking late-onset (or Type A) alcoholics (113). These data support the usefulness of using a craving measurement as a clinical guide in assessing relapse risk in heavy drinking alcoholics; however, the role of craving in treatment outcomes and as a predictor of drinking in moderate drinking alcoholics remains unclear.
The fact that naltrexone compared to placebo or acamprosate was effective in reducing overall alcohol consumption in heavy drinking alcoholics brought about the possibility of a feedback loop between craving and amount of drinking in the COMBINE trial (44). Interestingly, it was demonstrated that naltrexone was more successful in reducing relapse when patients experienced a high level of alcohol craving at study randomization (38, 114-116). This observation suggests that naltrexone may represent a pharmacotherapy to help patients at the initial state of recovery, when craving is at its most intense.
To establish whether opioid antagonists may be an effective anti-craving pharmacotherapy, clinical researchers have used different approaches. For example, a study was designed to administer naltrexone “as-needed” in anticipation of high-risk drinking, when triggers were expected, or when drinking was felt to be imminent (117). Compared to placebo, patients in the naltrexone intervention reported less craving and showed reduced drinking after 12 weeks. This approach targeted two important aspects: (1) craving intensity, which addresses the primary impact on relapse issues when the craving experience is acute (14); and (2) craving frequency and duration, which addresses the concept of time of maximum desire (50, 94). A subsequent trial corroborated the targeted naltrexone data showing that naltrexone (compared to placebo) taken only when craving occurs was effective in maintaining alcohol reduction by heavy drinking alcoholics (118). The efficacy of “as-needed” modification of the opioid system to reduce alcohol consumption in patients suffering from AUD has also been used with nalmefene, an opioid antagonist with a longer half-life, greater oral bioavailability, and no observed dose-dependent liver toxicity compared to naltrexone (119). Nalmefene was recently approved, “as needed” by European Medicine Agency as a treatment for alcoholism.
Researchers adopted the use of a combination of pharmaceutical and behavioral therapies and evaluated the results compared with monotherapies (44, 120-124). Investigators of the Combined Pharmacotherapies and Behavioral Interventions for Alcohol Dependence (COMBINE) study demonstrated that naltrexone alone, not in combination with Combined Behavioral Intervention (CBI), improved alcohol drinking outcomes (125). However, both naltrexone and CBI were shown to reduce craving; yet naltrexone’s anti-craving effects were only visible after four weeks of treatment, while CBI required 12 weeks of intervention before reducing craving episodes (44).
The anti-craving effect of naltrexone was also evaluated in longer studies. A 12-month-long open-label study in India compared the efficacy of naltrexone and disulfiram in preventing alcohol relapses in conditions similar to those in routine clinical practice (126). The study demonstrated that naltrexone was superior to disulfiram in reducing craving (126). This study supported the beneficial anti-craving effect of naltrexone in another long-term (12-month) randomized single-blind study comparing the effect of naltrexone to acamprosate (15). While the mean time to “first drink” was similar between the naltrexone (44 days) and acamprosate (39 days) groups, the time to “first relapse” (five or more drinks) was longer in the naltrexone (63 days) than acamprosate (42 days) group. At the end of treatment, participants in the naltrexone group experienced less craving than in the acamprosate group. Alternately, despite the positive results in reducing craving when compared to other medications, naltrexone failed to be effective in reducing relapse in chronic heavy-drinking alcoholics, in another study (127).
As a large result of genetic heritability, alcoholic individuals with a family history of alcoholism appear to have greater benefits with naltrexone therapy compared with those who do not (128).. The effect of naltrexone as an anti-craving medication may be moderated by variations in the μ-opioid receptor gene OPRM1 (89, 129, 130). The A118G single nucleotide polymorphism (SNP) of the OPRM1 (μ) gene was tested in a double-blind, placebo-controlled laboratory trial of naltrexone in heavy drinking alcoholics (88). Individuals with at least one copy of the G allele reported lower alcohol craving and higher alcohol-induced “high” than patients with the A homozygous allele. In other words naltrexone attenuated alcohol craving and an alcohol-induced “high” with a stronger effect among patients with the heterozygous G allele (88). The efficacy of naltrexone in reducing relapses however, was not confirmed by a study of polymorphic variants at each of the three opioid receptor genes, OPRM1, OPRK1 (κ) and OPRD1 (δ) (131). The study specifically focused on the OPRM1 Asn40Asp variant, that was previously identified as a predictor of response to naltrexone (51, 89, 129, 130). The moderating effects of Asn40Asp variant on the relation between craving, alcohol consumption and the attenuating effects of naltrexone was however, confirmed by a recent 12-week, RCT (132). In summary, naltrexone is one of the most evaluated medications in clinical research for reducing craving. It was superior to placebo in lessening craving, preventing relapse to heavy drinking, and in increasing the percentage of abstinent days. The results obtained from numerous studies are consistent with providing evidence that naltrexone may reduce craving when compared to other medications (acamprosate and disulfiram); however, it was not effective in reducing alcohol drinking as a long-term clinical outcome.
4.1.2 Dopamine Receptor Antagonists
The involvement of the dopaminergic limbic system (133) and the ‘incentive sensitization’ model of craving (55, 62) suggests that dopamine antagonists may counteract the DA moderated effects of alcohol intoxication (134). A RCT investigating the effect of the D2-antagonist haloperidol in alcohol-dependent individuals showed that pre-treatment with haloperidol significantly reduced alcohol craving, amount of alcohol ingested, and reduced impulsivity (134). The detrimental consequences associated with the first generation of antipsychotics, such as the extrapyramidal effects involving motor control (akathisia) and involuntary movement disorder (tardive dyskinesia), have limited their use both in research and clinical applications in treating AUD (135-137). Subsequently, it was hypothesized that if the first generation of antipsychotics proved to block alcohol cue-elicitation and blunt craving experiences, newer atypical antipsychotics might reduce craving symptoms without the substantial adverse effect profile associated with first-generation antipsychotics (138). Ultimately, the use of atypical antipsychotics was shown to provide some benefit to patients suffering from AUD and those with concomitant psychiatric illness (139-143).
Aripiprazole is an atypical antipsychotic that acts as a partial agonist both at D2 and at the 5-HT1A receptors, and like the other atypical antipsychotics, also displays an antagonist profile at the 5-HT2A receptor. Aripiprazole, first proposed in 2003 for use in alcoholism (144), also antagonizes the 5-HT7 receptor and acts as a partial agonist at the 5-HT2C receptor. Aripiprazole was subsequently tested in a multi-site RCT, but failed to meet its primary outcomes (145). Aripiprazole, in another double-blind, comparison trial was shown to reduce craving (146), but to a lesser extent than with naltrexone (147).
Quetiapine, which binds to D1-2, 5-HT1A-2A, and adrenergic α1-2 receptors, has shown to improve abstinence possibly by addressing sleep disturbances in alcohol-dependent patients (148, 149). Furthermore, quetiapine was also shown to provide benefits in reducing craving in patients suffering from the more complicated type B alcoholism (139). In a case study of alcoholics with mood disorder comorbidity and persistent craving after withdrawal, eight of nine individuals who received quetiapine , remained abstinent during a two-to-seven-month period (150). In a later open-label study, quetiapine was used as the sole therapy for patients suffering from alcohol dependence with a comorbid diagnosis of bipolar disorder, schizophrenia, or other borderline personality disorder (151). In this study of alcoholic patients with a concurrent Axis I disorder, quetiapine not only reduced overall alcohol consumption and psychiatric symptom intensity , but also reduced craving (151). The extended release formulation of quetiapine was also evaluated in a larger multi-site trial, however was no better than placebo in reducing heavy drinking in alcoholic patients and also failed to improve craving (152).
Olanzapine, an atypical D2,4 and 5-HT2 antagonist, was investigated in a placebo-controlled RCT where participants were subsequently exposed to both the control and alcohol cues in each session (138). The results demonstrated that a single dose of olanzapine was capable of reducing alcohol urge to drink and loss of control without affecting the alcohol reward mechanism (138). Because olanzapine possesses a high affinity for the D4 receptor, clinical researchers continue to investigate whether the DRD4 gene may influence the effect of a priming dose of alcohol on craving in alcohol-dependent patients. This pharmacogenomic approach targets the effect of craving in heavy drinking alcoholics with different variable numbers of tandem repeat (VNTR) polymorphisms of the D4 DA receptor gene (DRD4 VNTR). Olanzapine was capable of reducing craving exclusively in patients who possess a long sequence of DRD4 VNTR repeat alleles after exposure to alcohol cues (87). A follow-up 12-week study using olanzapine showed that individuals with the DRD4 7-repeat allele or greater reported reductions in cue-elicited alcohol craving and consumption, while olanzapine did not attenuate alcohol craving in subjects with less than the DRD4 7-repeat allele (153).
Researchers reporting the results of a recent study that evaluated the genetic influences of the dopaminergic pathways involving DA receptor genes (DRD1-4), and the gene that encodes the DA transporter, (solute carrier family 6, member 3;SLC6A3), concluded that despite the large sample size (n = 3,976), it was not possible to identify a strong enough genetic influence by DA to moderate craving with the severity of alcohol dependence (154).
In summary, pharmacological interventions that target the antagonist effect at the limbic system by counteracting the dopaminergic effects of alcohol intoxication with the intent of mediating craving has not provided consistent results even though it had provided some benefit in patients suffering from AUD and those with concomitant psychiatric illness.
4.2 Pharmacological Interventions that Target the Obsessive Mechanisms
4.2.1 Serotonin System: 5-HT Receptors Antagonists and SSRIs
The role of the serotonergic system in alcohol craving has focused mainly on the participation of the impulse-control processes as meditators of addictive behaviors (78), targeting both antagonism at the widely expressed 5-HT3 receptor and inhibitory action of SSRIs. Ondansetron is a 5-HT3 receptor antagonist used as an antiemetic for postoperative nausea and has been shown to have beneficial effects in reducing drinking behavior in early onset drinkers (155, 156). An fMRI study that targeted the ventral striatum, an area of the brain identified as being cue-stimulated with release of alcohol-induced DA output (157), demonstrated that naltrexone and ondansetron, alone or in combination, decreased alcohol cue-induced activation (108).
The serotonergic system has also been identified as a critical component of the craving experience (78). In an early placebo-controlled trial, the SSRI fluoxetine, reduced craving for alcohol (158). However, in a subsequent trial, while it was reported that craving was reduced, there were no differences in alcohol drinking outcomes (159). Despite the intense research dedicated to understanding the effects of alcohol on the serotonergic system, RCTs utilizing SSRIs have reported conflicting results (160-162). The lack of clinical consensus regarding the beneficial outcomes using SSRIs may be due to the complexity of the serotonergic system, since distinct 5-HT receptor subtypes act on several neural mechanisms associated with alcohol consumption (163, 164). In addition, different genetic variations in the 5-HT system suggest that diverse populations previously discussed such as Type A and Type B alcoholics (113) may respond differentially to SSRI treatment (165-167) or even benefit from treatment using specific 5-HT3-receptor antagonists (155, 168-170). The Type B group may benefit from a pharmacotherapy that offers more than blocking 5-HT reuptake (139).
Genetic influences may be responsible for some of the conflicting results in the study of the relationship between 5-HT and alcohol dependence. For example, the gene that encodes the 5-HT transporter, (solute carrier family 6, member 4;SLC6A4) contains a SNP (rs1042173) that may be a genetic marker for cue-induced alcohol craving among alcoholics (171). More specifically these data support the hypothesis that an underlying neurobiological mechanism associated with the rs1042173-TT genotype may trigger a disproportionate craving in response to alcohol consumption (171).
4.3 Pharmacological Interventions that Target a Relief Mechanism
4.3.1 Inhibition System
Topiramate is a FDA-approved antiepileptic drug with multiple mechanisms of action including antagonism of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPA)/kainate glutamate receptors, potentiation of GABA, enhancement of GABAA receptor function, and inhibition of the carbonic anhydrase enzyme. Topiramate, at 300mg/day (after titration), was evaluated as a possible treatment to reduce drinking days with positive outcomes compared to placebo in reducing the percentage of heavy drinking days from baseline and overall drinking days (172). It was also suggested that topiramate may attenuate drinking consumption by lessening alcohol craving (173). A laboratory study evaluated a lower dose of topiramate (200mg/day) in an alcohol-cue reactivity paradigm showing that topiramate reduced drinking during the titration period as compared to placebo, but did not alter craving (43). The results from this study suggested that topiramate might reduce drinking at lower doses than previously tested, and that the reduced drinking may not be linked to the reduction of craving. A pharmacogenetic investigation also reported that a SNP (rs2832407) of the glutamate receptor GluR5 gene (GRIK1) was associated with the serum level of topiramate, affecting the severity of topiramate-induced side effects based on genotype (174).
Lamotrigine, levetiracetam and zonisamide are other anti-epileptic medications with a broad combination of mechanisms of action (175-177) evaluated for treating alcohol craving or urge. Lamotrigine is a sodium channel blocker with many indeterminate mechanisms of action. Lamotrigine reduced craving both in preclinical models (178) and in human trials (142). In combination with clozapine, lamotrigine was shown to reduce craving among patients suffering from schizophrenia and comorbid alcohol dependence (142). Levetiracetam has been shown to reduce alcohol craving in open-label studies (179); however, in a double-blind, placebo-controlled RCT, levetiracetam did not show benefits in alcohol consumption in patients suffering from severe AUD (180). In an open-label study of alcohol-dependent patients, zonisamide reduced craving, weekly drinking and urge (181). Zonisamide also reduced heavy drinking, overall drinking and urge in a double-blind RCT compared to placebo (182).
Baclofen, a GABAB receptor agonist approved by the FDA to control muscular spasticity, has been used with beneficial effect to treat AUD (183-185). Based on its limited liver metabolism, baclofen was hypothesized as a pharmacotherapy intervention suitable for alcoholic patients with cirrhosis (186), including those with Hepatitis C virus (HCV) infection (187), and effective in suppressing alcohol withdrawal (183, 185, 188). The positive outcomes, measured as lessening craving in alcoholics (189-192), were suggested by the effects of baclofen in reducing anxiety (190, 193), which is often comorbid in alcoholics (194). It was also demonstrated that despite the previous positive drinking outcomes and the reduction of anxiety, baclofen was not superior to placebo in the treatment of alcohol dependence (195). In addition to the difference in severity of alcohol dependence (196) and high placebo effect (197), a reason for these conflicting results might be the large variability of baclofen’s pharmacokinetics profile in alcoholics (198). Finally, a recent pilot laboratory study provided preliminary evidence that baclofen may not reduce cue-induced craving. By contrast, this study suggested that baclofen’s ability to reduce alcohol drinking might be secondary to its ability to alter alcohol-related biphasic effects (199).
Gamma-hydroxybutyric acid (GHB), approved in Italy and Austria as a treatment for alcohol dependence, has been investigated as an anti-craving medication for alcohol dependence (200). GHB is a naturally occurring substance that is approved for restricted use by the FDA to treat narcolepsy. GHB has also been used as a general anesthetic and to treat conditions such as insomnia, clinical depression and alcoholism in Europe. It is also used as an illegal intoxicant or as a date rape drug (200). The transient beneficial effect of GHB in reducing craving compared to placebo (201, 202) may be due to the short half-life of the drug (203). In one study, patients receiving the same dose of GHB (50 mg/kg) from three to six times per day reported less craving than at baseline (204). Despite the encouraging results from investigations of GHB in lessening craving, the clinical application of a drug that requires administration six times per day in patients suffering from AUD, often with other comorbidities, and is itself an addicting substance, is difficult to justify. Nonetheless, GHB is widely used in some European countries but its distribution is done under very strict medical monitoring (205).
4.3.2 Glutamate System: NMDA Receptor Antagonists
Glutamate, the major excitatory neurotransmitter in the brain, utilizes both inotropic and metabotropic receptors to transduce excitatory signals. The NMDA receptors are gated by membrane potentials and provide binding sites simultaneously for both glycine and glutamate. NMDA receptors represent one of the highest affinity ethanol targets in the central nervous system (CNS;206-209). Specifically, ethanol inhibits responses to n-methyl-d-aspartate (NMDA) in distinct regional variants in the NMDA receptor subunit composition. Preclinical glutamatergic studies have therefore dedicated intense research in exploring novel pharmacotherapies for alcoholism (210, 211). In clinical studies, NMDA antagonists have been used to produce a state of intoxication that resembles ethanol’s effects (212). Therefore, pharmacotherapies that could counteract this effect by acting on NMDA receptors have been investigated in alcohol-dependent patients (213). Moreover, the potential benefits of NMDA receptor antagonists as treatments for craving in alcohol-dependent patients have also been investigated in preclinical (214) and clinical trials (215).
Human studies using memantine, an NMDA receptor (NMDAR) antagonist, show that NMDAR neurotransmission may be involved in both alcohol craving and alcohol-induced subjective dissociative effects (215, 216). While a human laboratory study reported that pretreatment with memantine but not treatment of post-alcohol consumption, lessened craving (215), memantine had no effect on drinking in a placebo-controlled trial (217)
Though approved in Europe since 1989, acamprosate (N-acetyl homotaurine) was only approved in the United States (US) for treating alcohol dependence in 2004. In vitro studies show that acamprosate may attenuate excessive glutamatergic activity by blocking toxicity produced by abrupt ethanol withdrawal (218). The action of acamprosate on the metabotropic glutamate receptor 5 (mGluR5) has been shown both in preclinical models (219) and in clinical findings (220). The inclusion of acamprosate as an adjunct pharmacotherapy to psychotherapy was shown to be successful in reducing the incidence of relapse (221) and maintaining abstinence in alcohol-dependent patients (222, 223). A double-blind RCT that administered acamprosate for 21 days demonstrated that acamprosate-treated patients showed significantly reduced craving compared to placebo (224).
The effective acamprosate response in alcohol-dependent subjects may be influenced by genetically-controlled variation of NMDA receptors and mGLUR5 (219). Pharmacogenetically-relevant variants for alcohol-dependent patients were explored in a genome-wide association study that included a follow-up study with relapse behavior and pharmacological treatment response in patients suffering from AUD (225). This double-blind, placebo-controlled RCT with acamprosate or naltrexone, reported that the intronic SNP rs13273672, an SP encoded by the gene for the euthroid transcription factor GATA-binding protein 4 (GATA4), was associated with relapse in the acamprosate-treated arm only, and a potential predictor of response to acamprosate among alcoholic individuals. However, the genetically moderated response to acamprosate was not associated with craving.
4.4 Pharmacological Interventions that Target other Mechanisms
4.4.1 Alcohol Metabolism
Disulfiram was the first medication that received an FDA indication to treat alcoholism. It produces aversive effects by interfering with alcohol metabolism and increasing acetaldehyde concentration (226). In RCTs, disulfiram has demonstrated inconsistent results as a pharmacotherapy to increase abstinence in alcoholics by reducing craving. As an adjunct therapy, disulfiram has facilitated clinical benefits in reducing alcohol craving by providing additional psychological support (227). An eight-month study comparing disulfiram with acamprosate demonstrated that despite disulfiram showing more beneficial effects in preventing relapses in alcoholics, the acamprosate group reported lower craving (228).
Another study testing disulfiram in patients with a concurrent Axis I psychiatric disorder and alcohol dependence suggested that disulfiram might have some benefits in reducing alcohol craving (229). This effect may be attributed to disulfiram interfering with the metabolism of DA (230), and therefore potentially influencing the development of craving. Additionally, subjects with post-traumatic stress disorder (PTSD) had better alcohol outcomes with disulfiram when compared to naltrexone and placebo (231). Patients diagnosed with PTSD receiving disulfiram responded better than non-PTSD patients, possibly because of disulfiram inhibition of DA beta-hydroxylase in the CNS, potentially resulted in an excess of DA and decreased synthesis of norepinephrine (232). The beneficial effect of disulfiram is limited to trials where the drug is administered under supervision (233) and does not provide enough evidence to support its use to treat AUD or to reduce craving in the clinical setting (234).
4.4.2 Adrenergic System: Nicotinic Receptor Antagonists
Because both nicotine and ethanol increase DA release in the reward pathway, it has been hypothesized that they may play a synergistic role when administered simultaneously (235). In addition, some of the effects of ethanol in the brain seem mediated by the activation of neuronal nicotinic acetylcholine receptors (nAChRs) (236). Varenicline is an FDA-approved treatment for smoking cessation that targets the nAChRs and has been investigated both in preclinical (237-239) and clinical (240, 241) settings as a medication to reduce alcohol consumption. Varenicline has been shown to have beneficial effects in preclinical craving models in reducing cue-induced ethanol relapses, but not nicotine seeking in self-administration-reinstatement models (242). A double-blind, placebo-controlled study examined the effect of varenicline following a priming alcohol drink, and showed that varenicline attenuated alcohol craving and reduced subjective reinforcing alcohol effects (243). Furthermore, a randomized, double-blind, 16-week study in heavy-drinking smokers designed to determine the effects of varenicline on alcohol craving revealed that patients allocated to the varenicline-arm reported less craving than the placebo group (240). In a recent pilot study, the varenicline effects in term of drinking outcomes were not significantly more effective than placebo (244), however, a multi-site 13-week RCT that assessed varenicline in alcohol-dependent patients (both smokers and nonsmokers) showed that the varenicline group reported significantly lower alcohol craving than the placebo contol (245).
Prazosin, a FDA-approved α1 receptor antagonist medication approved to treat blood pressure and benign prostatic hyperplasia, was demonstrated to be efficacious in stress-induced alcohol reinstatement in preclinical models (246-248). The rationale to utilize prazosin to treat AUD was based on the potential to antagonize postsynaptic α1 activity involved in stress-induced reinstatement of alcohol seeking and modulate craving experiences. A preliminary clinical study peformed during early recovery from alcoholism reported a favorable outcome in reducing alcohol craving, anxiety, and negative emotion following stress exposure in patients taking prazosin(249). In a second study using 16mg/day of prazosin compared to placebo, among the 20 completers, the prazosin group reported fewer drinking days per week than the placebo group during the final 3 weeks of the study (250).
5. Conclusion
The challenges associated with the understanding of alcohol craving and its role in AUD has been a difficult task to operationalize in both in the research and clinical settings. Researchers have developed interdisciplinary craving models to reduce the knowledge gap attempt to explain betweenthe brain circuitry pathways and the central behavioral feature of addiction. Clinicians have assessed and measured craving to monitor the efficacy of pharmacotherapies in patients suffering from AUD. However, based on a relatively rudimentary understanding of the subjective nature of craving, accurate assessment of what experiencing craving is to both the patient and clinician at this time is impossible. Scientists though continue to strive to find medications that reduce drinking and craving, with the assumption that treating both will result in better outcomes. As a result, many drugs have been studied with mixed results.
Naltrexone, a medication evaluated extensively in clinical research was shown to be superior to placebo and other interventions (CBI, disulfiram and acamprosate) in lessening craving; however, it was not effective in reducing alcohol drinking as a long-term clinical outcome. Pharmacological interventions that target the antagonist effect at the limbic system (antipsychotics) have not provided consistent results even though they demonstrated benefit in patients with concomitant psychiatric illness. RCTs Randomized controlled trials utilizing SSRIs have reported inconclusive results on their therapeutic effect in preventing relapses and lessening craving for alcohol. Varenicline, which utilizes the similar craving aspects and the synergistic effects of smoking and alcohol, was shown to reduce craving and smoking both in smokers and in nonsmokers; however, craving is was not always associated with reducing alcohol drinkingconsumption. A better understanding of the neurobiology associated with AUD and more comprehensive methods to measure and assess craving may favor more effective pharmacological interventions that lead to beneficial clinical outcomes
The development of alternative approaches that aim to reduce craving has yielded interesting interventions that need further evaluation. For example, by easing stress-induced alcohol seeking, it may be possible to reduce craving experiences, diminish risk of relapses and facilitate the formation of memories with less deleterious behavioral consequences (251). There are no current medications that target stress-induced craving at the extrahypothalamic brain level in order to reduce compulsive alcohol seeking, although non-peptidic, blood-brain barrier penetrating corticotropin releasing factor (CRF)-receptor 1 antagonists have shown efficacy in animal models to treat alcoholism (252, 253). A pharmacological intervention that targets stress-induced alcohol craving may be more suitable for patients that suffer anxiety or are prone to stress-triggered situations such as cue-induced relapses (254). CRF-antagonists that target the effect of stress in alcohol consumption, despite evidence that stress increases risk of relapses and induces alcohol craving, demonstrated in clinical investigation that not all alcoholics during abstinence experience anxiety, therefore limiting CRF-antagonists for patients who are prone to stress-triggered situations such as cue-induced relapses. Craving is dictated by intertwined mechanisms that affect adjacent neuronal or distant physiologic systems. Current research on evaluating medications that target multi-systems may lead to new pharmacological approaches to treat AUDs.
Key points.
Now recognized by the DSM-5 as an important diagnostic assessment for alcoholism, craving is a superfluous subjective concept difficult to express and accurately assess across the heterogeneous population of alcoholics.
Though related to alcohol relapse, a reduction in craving seen with pharmacotherapy has not consistently been associated with a reduction in drinking.
A better understanding of craving is needed to help advance the successful treatment of alcoholism.
ACKNOWLEDGEMENTS
Drs. Haass-Koffler, Leggio and Kennahave no conflicts of interest that are directly relevant to the content of this review. We would like to thank the 5T32AA007459-28 training grant from the National Institute on Alcohol Abuse and Alcoholism (NIAAA) that supports the work of Dr. Haass-Koffler. Dr. Leggio’s current work is supported by the Division of Intramural Clinical and Biological Research of the National Institute on Alcohol Abuse and Alcoholism (NIAAA) and the Intramural Research Program of the National Institute on Drug Abuse (NIDA). Dr. Kenna has received funding from the National Institute on Alcohol Abuse and Alcoholism for the following drugs: R01-AA016079 (ondansetron and sertraline), R03AA020169 (baclofen), R21AA019709 (ghrelin), R01 AA015753 (aripiprazole and topiramate), R21AA019994 (doxazosin), R21AA021128 (metadoxine) and has received a consultant fee from CT San Remo (GET73).
REFERENCES
- 1.Jellinek EM. The craving for alcohol. Q J Stud Alcohol. 1955 Mar;16(1):35–8. [PubMed] [Google Scholar]
- 2.Anton RF. What is craving? Models and implications for treatment. Alcohol Res Health. 1999;23(3):165–73. [Research Support, U.S. Gov't, P.H.S.Review] [PMC free article] [PubMed] [Google Scholar]
- 3.Anton RF, Moak DH, Latham PK. The obsessive compulsive drinking scale: A new method of assessing outcome in alcoholism treatment studies. Arch Gen Psychiatry. 1996 Mar;53(3):225–31. doi: 10.1001/archpsyc.1996.01830030047008. [Clinical TrialRandomized Controlled TrialResearch Support, U.S. Gov't, P.H.S.] [DOI] [PubMed] [Google Scholar]
- 4.Swift RM. Medications and alcohol craving. Alcohol Res Health. 1999;23(3):207–13. [Review] [PMC free article] [PubMed] [Google Scholar]
- 5.Kenna GA, Swift RM, Hillemacher T, Leggio L. The relationship of appetitive, reproductive and posterior pituitary hormones to alcoholism and craving in humans. Neuropsychol Rev. 2012 Sep;22(3):211–28. doi: 10.1007/s11065-012-9209-y. [Review] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Litt MD, Cooney NL, Kadden RM, Gaupp L. Reactivity to alcohol cues and induced moods in alcoholics. Addict Behav. 1990;15(2):137–46. doi: 10.1016/0306-4603(90)90017-r. [Clinical TrialRandomized Controlled TrialResearch Support, U.S. Gov't, P.H.S.] [DOI] [PubMed] [Google Scholar]
- 7.Cooney NL, Litt MD, Morse PA, Bauer LO, Gaupp L. Alcohol cue reactivity, negative-mood reactivity, and relapse in treated alcoholic men. J Abnorm Psychol. 1997 May;106(2):243–50. doi: 10.1037//0021-843x.106.2.243. [Research Support, U.S. Gov't, Non-P.H.S.Research Support, U.S. Gov't, P.H.S.] [DOI] [PubMed] [Google Scholar]
- 8.Fox HC, Bergquist KL, Hong KI, Sinha R. Stress-induced and alcohol cue-induced craving in recently abstinent alcohol-dependent individuals. Alcohol Clin Exp Res. 2007 Mar;31(3):395–403. doi: 10.1111/j.1530-0277.2006.00320.x. [Research Support, N.I.H., Extramural] [DOI] [PubMed] [Google Scholar]
- 9.Breese GR, Chu K, Dayas CV, Funk D, Knapp DJ, Koob GF, et al. Stress enhancement of craving during sobriety: a risk for relapse. Alcohol Clin Exp Res. 2005 Feb;29(2):185–95. doi: 10.1097/01.alc.0000153544.83656.3c. [Congresses Research Support, U.S. Gov't, P.H.S.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sinha R, Fox HC, Hong KA, Bergquist K, Bhagwagar Z, Siedlarz KM. Enhanced negative emotion and alcohol craving, and altered physiological responses following stress and cue exposure in alcohol dependent individuals. Neuropsychopharmacology. 2009 Apr;34(5):1198–208. doi: 10.1038/npp.2008.78. [Research Support, N.I.H., Extramural] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mathew RJ, Claghorn JL, Largen J. Craving for alcohol in sober alcoholics. Am J Psychiatry. 1979 Apr;136(4B):603–6. [PubMed] [Google Scholar]
- 12.Monti PM, Rohsenow DJ, Rubonis AV, Niaura RS, Sirota AD, Colby SM, et al. Cue exposure with coping skills treatment for male alcoholics: a preliminary investigation. J Consult Clin Psychol. 1993 Dec;61(6):1011–9. doi: 10.1037//0022-006x.61.6.1011. [Clinical TrialRandomized Controlled TrialResearch Support, U.S. Gov't, Non-P.H.S.Research Support, U.S. Gov't, P.H.S.] [DOI] [PubMed] [Google Scholar]
- 13.Tiffany ST. A cognitive model of drug urges and drug-use behavior: role of automatic and nonautomatic processes. Psychol Rev. 1990 Apr;97(2):147–68. doi: 10.1037/0033-295x.97.2.147. [Review] [DOI] [PubMed] [Google Scholar]
- 14.Kavanagh DJ, Statham DJ, Feeney GF, Young RM, May J, Andrade J, et al. Measurement of alcohol craving. Addict Behav. 2013 Feb;38(2):1572–84. doi: 10.1016/j.addbeh.2012.08.004. [Research Support, Non-U.S. Gov'tReview] [DOI] [PubMed] [Google Scholar]
- 15.Rubio G, Jimenez-Arriero MA, Ponce G, Palomo T. Naltrexone versus acamprosate: one year follow-up of alcohol dependence treatment. Alcohol Alcohol. 2001 Sep-Oct;36(5):419–25. doi: 10.1093/alcalc/36.5.419. [Clinical TrialComparative StudyRandomized Controlled TrialResearch Support, Non-U.S. Gov't] [DOI] [PubMed] [Google Scholar]
- 16.Li TK. Clinical perspectives for the study of craving and relapse in animal models. Addiction. 2000 Aug;95(Suppl 2):S55–60. doi: 10.1080/09652140050111645. [Review] [DOI] [PubMed] [Google Scholar]
- 17.Childress AR, Mozley PD, McElgin W, Fitzgerald J, Reivich M, O'Brien CP. Limbic activation during cue-induced cocaine craving. Am J Psychiatry. 1999 Jan;156(1):11–8. doi: 10.1176/ajp.156.1.11. [Research Support, Non-U.S. Gov'tResearch Support, U.S. Gov't, Non-P.H.S.Research Support, U.S. Gov't, P.H.S.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kalivas PW, O'Brien C. Drug addiction as a pathology of staged neuroplasticity. Neuropsychopharmacology. 2008 Jan;33(1):166–80. doi: 10.1038/sj.npp.1301564. [Research Support, N.I.H., ExtramuralReview] [DOI] [PubMed] [Google Scholar]
- 19.Shaham Y, Shalev U, Lu L, De Wit H, Stewart J. The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology (Berl) 2003 Jul;168(1-2):3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- 20.Katz JL, Higgins ST. The validity of the reinstatement model of craving and relapse to drug use. Psychopharmacology (Berl) 2003 Jul;168(1-2):21–30. doi: 10.1007/s00213-003-1441-y. [DOI] [PubMed] [Google Scholar]
- 21.Fuchs RA, Tran-Nguyen LT, Specio SE, Groff RS, Neisewander JL. Predictive validity of the extinction/reinstatement model of drug craving. Psychopharmacology (Berl) 1998 Jan;135(2):151–60. doi: 10.1007/s002130050496. [DOI] [PubMed] [Google Scholar]
- 22.Monti PM, Rohsenow DJ, Hutchison KE, Swift RM, Mueller TI, Colby SM, et al. Naltrexone's effect on cue-elicited craving among alcoholics in treatment. Alcohol Clin Exp Res. 1999 Aug;23(8):1386–94. [Clinical TrialRandomized Controlled TrialResearch Support, Non-U.S. Gov'tResearch Support, U.S. Gov't, Non-P.H.S.Research Support, U.S. Gov't, P.H.S.] [PubMed] [Google Scholar]
- 23.Tiffany ST. Cognitive concepts of craving. Alcohol Res Health. 1999;23(3):215–24. [Review] [PMC free article] [PubMed] [Google Scholar]
- 24.Bencherif B, Wand GS, McCaul ME, Kim YK, Ilgin N, Dannals RF, et al. Mu-opioid receptor binding measured by [11C]carfentanil positron emission tomography is related to craving and mood in alcohol dependence. Biol Psychiatry. 2004 Feb 1;55(3):255–62. doi: 10.1016/j.biopsych.2003.07.007. [Research Support, U.S. Gov't, P.H.S.] [DOI] [PubMed] [Google Scholar]
- 25.Heinz A, Beck A, Grusser SM, Grace AA, Wrase J. Identifying the neural circuitry of alcohol craving and relapse vulnerability. Addict Biol. 2009 Jan;14(1):108–18. doi: 10.1111/j.1369-1600.2008.00136.x. [Review] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sinha R, Li CS. Imaging stress- and cue-induced drug and alcohol craving: association with relapse and clinical implications. Drug Alcohol Rev. 2007 Jan;26(1):25–31. doi: 10.1080/09595230601036960. [Research Support, N.I.H., Extramural Review] [DOI] [PubMed] [Google Scholar]
- 27.Anton RF, Moak DH, Waid LR, Latham PK, Malcolm RJ, Dias JK. Naltrexone and cognitive behavioral therapy for the treatment of outpatient alcoholics: results of a placebo-controlled trial. Am J Psychiatry. 1999 Nov;156(11):1758–64. doi: 10.1176/ajp.156.11.1758. [DOI] [PubMed] [Google Scholar]
- 28.Volpicelli JR, Alterman AI, Hayashida M, O'Brien CP. Naltrexone in the treatment of alcohol dependence. Arch Gen Psychiatry. 1992 Nov;49(11):876–80. doi: 10.1001/archpsyc.1992.01820110040006. [DOI] [PubMed] [Google Scholar]
- 29.O'Malley SS, Jaffe AJ, Chang G, Schottenfeld RS, Meyer RE, Rounsaville B. Naltrexone and coping skills therapy for alcohol dependence. A controlled study. Arch Gen Psychiatry. 1992 Nov;49(11):881–7. doi: 10.1001/archpsyc.1992.01820110045007. [DOI] [PubMed] [Google Scholar]
- 30.Casey M, Adamson G, Shevlin M, McKinney A. The role of craving in AUDs: dimensionality and Differential Functioning in the DSM-5. Drug Alcohol Depend. 2012 Sep 1;125(1-2):75–80. doi: 10.1016/j.drugalcdep.2012.03.019. [Research Support, Non-U.S. Gov't] [DOI] [PubMed] [Google Scholar]
- 31.Keyes KM, Krueger RF, Grant BF, Hasin DS. Alcohol craving and the dimensionality of alcohol disorders. Psychol Med. 2011 Mar;41(3):629–40. doi: 10.1017/S003329171000053X. [Research Support, N.I.H., ExtramuralResearch Support, Non-U.S. Gov't] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cherpitel CJ, Borges G, Ye Y, Bond J, Cremonte M, Moskalewicz J, et al. Performance of a craving criterion in DSM alcohol use disorders. J Stud Alcohol Drugs. 2010 Sep;71(5):674–84. doi: 10.15288/jsad.2010.71.674. [Comparative Study Multicenter StudyResearch Support, N.I.H., Extramural] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mewton L, Slade T, McBride O, Grove R, Teesson M. An evaluation of the proposed DSM-5 alcohol use disorder criteria using Australian national data. Addiction. 2011 May;106(5):941–50. doi: 10.1111/j.1360-0443.2010.03340.x. [Evaluation Studies] [DOI] [PubMed] [Google Scholar]
- 34.Janca A, Ustun TB, Early TS, Sartorius N. The ICD-10 symptom checklist: a companion to the ICD-10 classification of mental and behavioural disorders. Soc Psychiatry Psychiatr Epidemiol. 1993 Oct;28(5):239–42. doi: 10.1007/BF00788743. [DOI] [PubMed] [Google Scholar]
- 35.Kupfer DJ, Regier DA. Neuroscience, clinical evidence, and the future of psychiatric classification in DSM-5. Am J Psychiatry. 2011 Jul;168(7):672–4. doi: 10.1176/appi.ajp.2011.11020219. [DOI] [PubMed] [Google Scholar]
- 36.Edwards AC, Gillespie NA, Aggen SH, Kendler KS. Assessment of a modified DSM-5 diagnosis of alcohol use disorder in a genetically informative population. Alcohol Clin Exp Res. 2013 Mar;37(3):443–51. doi: 10.1111/j.1530-0277.2012.01954.x. [Research Support, N.I.H., Extramural] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Haass-Koffler CL, Kenna GA. Bacchus by Caravaggio as the Visual Diagnosis of Alcohol Use Disorder from the Fifth Edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) Frontiers in psychiatry. 2013;4:86. doi: 10.3389/fpsyt.2013.00086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Volpicelli JR, Alterman AI, Hayashida M, O'Brien CP. Naltrexone in the treatment of alcohol dependence. Arch Gen Psychiatry. 1992 Nov;49(11):876–80. doi: 10.1001/archpsyc.1992.01820110040006. [Clinical TrialRandomized Controlled TrialResearch Support, Non-U.S. Gov'tResearch Support, U.S. Gov't, P.H.S.] [DOI] [PubMed] [Google Scholar]
- 39.Drummond DC, Glautier S. A controlled trial of cue exposure treatment in alcohol dependence. J Consult Clin Psychol. 1994 Aug;62(4):809–17. doi: 10.1037//0022-006x.62.4.809. [Clinical TrialControlled Clinical TrialResearch Support, Non-U.S. Gov't] [DOI] [PubMed] [Google Scholar]
- 40.Grusser SM, Wrase J, Klein S, Hermann D, Smolka MN, Ruf M, et al. Cue-induced activation of the striatum and medial prefrontal cortex is associated with subsequent relapse in abstinent alcoholics. Psychopharmacology (Berl) 2004 Sep;175(3):296–302. doi: 10.1007/s00213-004-1828-4. [Research Support, Non-U.S. Gov't] [DOI] [PubMed] [Google Scholar]
- 41.Litt MD, Cooney NL, Morse P. Reactivity to alcohol-related stimuli in the laboratory and in the field: predictors of craving in treated alcoholics. Addiction. 2000 Jun;95(6):889–900. doi: 10.1046/j.1360-0443.2000.9568896.x. [Research Support, U.S. Gov't, P.H.S.] [DOI] [PubMed] [Google Scholar]
- 42.Verheul R, van den Brink W, Geerlings P. A three-pathway psychobiological model of craving for alcohol. Alcohol Alcohol. 1999 Mar-Apr;34(2):197–222. doi: 10.1093/alcalc/34.2.197. [DOI] [PubMed] [Google Scholar]
- 43.Miranda R, Jr., MacKillop J, Monti PM, Rohsenow DJ, Tidey J, Gwaltney C, et al. Effects of topiramate on urge to drink and the subjective effects of alcohol: a preliminary laboratory study. Alcohol Clin Exp Res. 2008 Mar;32(3):489–97. doi: 10.1111/j.1530-0277.2007.00592.x. [Comparative StudyRandomized Controlled TrialResearch Support, N.I.H., ExtramuralResearch Support, U.S. Gov't, Non-P.H.S.] [DOI] [PubMed] [Google Scholar]
- 44.Subbaraman MS, Lendle S, van der Laan M, Kaskutas LA, Ahern J. Cravings as a mediator and moderator of drinking outcomes in the COMBINE Study. Addiction. 2013 May 14; doi: 10.1111/add.12238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Singleton EG, Gorelick DA. Mechanisms of alcohol craving and their clinical implications. Recent Dev Alcohol. 1998;14:177–95. doi: 10.1007/0-306-47148-5_8. [Review] [DOI] [PubMed] [Google Scholar]
- 46.Cooney NL, Gillespie RA, Baker LH, Kaplan RF. Cognitive changes after alcohol cue exposure. J Consult Clin Psychol. 1987 Apr;55(2):150–5. doi: 10.1037//0022-006x.55.2.150. [Research Support, U.S. Gov't, P.H.S.] [DOI] [PubMed] [Google Scholar]
- 47.Monti PM, Rohsenow DJ, Hutchison KE. Toward bridging the gap between biological, psychobiological and psychosocial models of alcohol craving. Addiction. 2000 Aug;95(Suppl 2):S229–36. doi: 10.1080/09652140050111799. [Research Support, U.S. Gov't, Non-P.H.S.Research Support, U.S. Gov't, P.H.S.Review] [DOI] [PubMed] [Google Scholar]
- 48.Mason BJ, Light JM, Williams LD, Drobes DJ. Proof-of-concept human laboratory study for protracted abstinence in alcohol dependence: effects of gabapentin. Addict Biol. 2009 Jan;14(1):73–83. doi: 10.1111/j.1369-1600.2008.00133.x. [Clinical Trial, Phase IIIRandomized Controlled TrialResearch Support, N.I.H., Extramural] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Miranda R, Ray L, Blanchard A, Reynolds EK, Monti PM, Chun T, et al. Effects of naltrexone on adolescent alcohol cue reactivity and sensitivity: an initial randomized trial. Addict Biol. 2013 Mar 13; doi: 10.1111/adb.12050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Drummond DC, Litten RZ, Lowman C, Hunt WA. Craving research: future directions. Addiction. 2000 Aug;95(Suppl 2):S247–55. doi: 10.1080/09652140050111816. [Review] [DOI] [PubMed] [Google Scholar]
- 51.Verheul R, van den Brink W, Geerlings P. A three-pathway psychobiological model of craving for alcohol. Alcohol Alcohol. 1999 Mar-Apr;34(2):197–222. doi: 10.1093/alcalc/34.2.197. [Review] [DOI] [PubMed] [Google Scholar]
- 52.2006 May;3(5):30–41. [Google Scholar]
- 53.Stacy AW, Wiers RW. Implicit cognition and addiction: a tool for explaining paradoxical behavior. Annu Rev Clin Psychol. 2010;6:551–75. doi: 10.1146/annurev.clinpsy.121208.131444. [Research Support, N.I.H., ExtramuralResearch Support, Non-U.S. Gov'tReview] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Field M, Munafo MR, Franken IH. A meta-analytic investigation of the relationship between attentional bias and subjective craving in substance abuse. Psychol Bull. 2009 Jul;135(4):589–607. doi: 10.1037/a0015843. [Meta-Analysis] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993 Sep-Dec;18(3):247–91. doi: 10.1016/0165-0173(93)90013-p. [Research Support, U.S. Gov't, P.H.S.Review] [DOI] [PubMed] [Google Scholar]
- 56.Hofmann W, Schmeichel BJ, Baddeley AD. Executive functions and self-regulation. Trends Cogn Sci. 2012 Mar;16(3):174–80. doi: 10.1016/j.tics.2012.01.006. [Review] [DOI] [PubMed] [Google Scholar]
- 57.Meyer RE. Craving: what can be done to bring the insights of neuroscience, behavioral science and clinical science into synchrony. Addiction. 2000 Aug;95(Suppl 2):S219–27. doi: 10.1080/09652140050111780. [Review] [DOI] [PubMed] [Google Scholar]
- 58.imms JA, Haass-Koffler CL, Bito-Onon J, Li R, Bartlett SE. Mifepristone in the central nucleus of the amygdala reduces yohimbine stress-induced reinstatement of ethanol-seeking. Neuropsychopharmacology. 2012 Mar;37(4):906–18. doi: 10.1038/npp.2011.268. [Research Support, N.I.H., ExtramuralResearch Support, Non-U.S. Gov'tResearch Support, U.S. Gov't, Non-P.H.S.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Koob GF, Le Moal M. Drug abuse: hedonic homeostatic dysregulation. Science. 1997 Oct 3;278(5335):52–8. doi: 10.1126/science.278.5335.52. [Research Support, Non-U.S. Gov'tResearch Support, U.S. Gov't, P.H.S.] [DOI] [PubMed] [Google Scholar]
- 60.Modell JG, Mountz JM. Focal cerebral blood flow change during craving for alcohol measured by SPECT. J Neuropsychiatry Clin Neurosci. 1995 Winter;7(1):15–22. doi: 10.1176/jnp.7.1.15. [Research Support, U.S. Gov't, P.H.S.] [DOI] [PubMed] [Google Scholar]
- 61.Berridge KC, Robinson TE. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res Brain Res Rev. 1998 Dec;28(3):309–69. doi: 10.1016/s0165-0173(98)00019-8. Review. [DOI] [PubMed] [Google Scholar]
- 62.Wise RA. The neurobiology of craving: implications for the understanding and treatment of addiction. J Abnorm Psychol. 1988 May;97(2):118–32. doi: 10.1037//0021-843x.97.2.118. [Review] [DOI] [PubMed] [Google Scholar]
- 63.Oak JN, Oldenhof J, Van Tol HH. The dopamine D(4) receptor: one decade of research. Eur J Pharmacol. 2000 Sep 29;405(1-3):303–27. doi: 10.1016/s0014-2999(00)00562-8. [Research Support, Non-U.S. Gov'tReview] [DOI] [PubMed] [Google Scholar]
- 64.Asghari V, Sanyal S, Buchwaldt S, Paterson A, Jovanovic V, Van Tol HH. Modulation of intracellular cyclic AMP levels by different human dopamine D4 receptor variants. J Neurochem. 1995 Sep;65(3):1157–65. doi: 10.1046/j.1471-4159.1995.65031157.x. [Research Support, Non-U.S. Gov't] [DOI] [PubMed] [Google Scholar]
- 65.Noain D, Avale ME, Wedemeyer C, Calvo D, Peper M. Rubinstein M. Identification of brain neurons expressing the dopamine D4 receptor gene using BAC transgenic mice. Eur J Neurosci. 2006 Nov;24(9):2429–38. doi: 10.1111/j.1460-9568.2006.05148.x. [Research Support, Non-U.S. Gov't] [DOI] [PubMed] [Google Scholar]
- 66.Katner SN, Weiss F. Neurochemical characteristics associated with ethanol preference in selected alcohol-preferring and -nonpreferring rats: a quantitative microdialysis study. Alcohol Clin Exp Res. 2001 Feb;25(2):198–205. [Comparative StudyResearch Support, U.S. Gov't, P.H.S.] [PubMed] [Google Scholar]
- 67.Koob GF. Neural mechanisms of drug reinforcement. Ann N Y Acad Sci. 1992 Jun 28;654:171–91. doi: 10.1111/j.1749-6632.1992.tb25966.x. [Research Support, U.S. Gov't, P.H.S.Review] [DOI] [PubMed] [Google Scholar]
- 68.Melendez RI, Rodd-Henricks ZA, Engleman EA, Li TK, McBride WJ, Murphy JM. Microdialysis of dopamine in the nucleus accumbens of alcohol-preferring (P) rats during anticipation and operant self-administration of ethanol. Alcohol Clin Exp Res. 2002 Mar;26(3):318–25. [Comparative StudyResearch Support, Non-U.S. Gov'tResearch Support, U.S. Gov't, P.H.S.] [PubMed] [Google Scholar]
- 69.Kalivas PW, Volkow N, Seamans J. Unmanageable motivation in addiction: a pathology in prefrontal-accumbens glutamate transmission. Neuron. 2005 Mar 3;45(5):647–50. doi: 10.1016/j.neuron.2005.02.005. [Review] [DOI] [PubMed] [Google Scholar]
- 70.Heinz A, Siessmeier T, Wrase J, Hermann D, Klein S, Grusser SM, et al. Correlation between dopamine D(2) receptors in the ventral striatum and central processing of alcohol cues and craving. Am J Psychiatry. 2004 Oct;161(10):1783–9. doi: 10.1176/appi.ajp.161.10.1783. [Comparative StudyResearch Support, Non-U.S. Gov't] [DOI] [PubMed] [Google Scholar]
- 71.Heinz A, Lober S, Georgi A, Wrase J, Hermann D, Rey ER, et al. Reward craving and withdrawal relief craving: assessment of different motivational pathways to alcohol intake. Alcohol Alcohol. 2003 Jan-Feb;38(1):35–9. doi: 10.1093/alcalc/agg005. [Research Support, Non-U.S. Gov't] [DOI] [PubMed] [Google Scholar]
- 72.LeMarquand D, Pihl RO, Benkelfat C. Serotonin and alcohol intake, abuse, and dependence: clinical evidence. Biol Psychiatry. 1994 Sep 1;36(5):326–37. doi: 10.1016/0006-3223(94)90630-0. [Research Support, Non-U.S. Gov'tReview] [DOI] [PubMed] [Google Scholar]
- 73.Johnson BA. Serotonergic agents and alcoholism treatment: rebirth of the subtype concept--an hypothesis. Alcohol Clin Exp Res. 2000 Oct;24(10):1597–601. [CommentResearch Support, U.S. Gov't, P.H.S.Review] [PubMed] [Google Scholar]
- 74.Modell JG, Glaser FB, Cyr L, Mountz JM. Obsessive and compulsive characteristics of craving for alcohol in alcohol abuse and dependence. Alcohol Clin Exp Res. 1992 Apr;16(2):272–4. doi: 10.1111/j.1530-0277.1992.tb01375.x. [Research Support, U.S. Gov't, P.H.S.] [DOI] [PubMed] [Google Scholar]
- 75.Roberts JS, Anton RF, Latham PK, Moak DH. Factor structure and predictive validity of the Obsessive Compulsive Drinking Scale. Alcohol Clin Exp Res. 1999 Sep;23(9):1484–91. [Clinical TrialRandomized Controlled TrialResearch Support, U.S. Gov't, P.H.S.] [PubMed] [Google Scholar]
- 76.Lovinger DM, Zhou Q. Alcohols potentiate ion current mediated by recombinant 5-HT3RA receptors expressed in a mammalian cell line. Neuropharmacology. 1994 Dec;33(12):1567–72. doi: 10.1016/0028-3908(94)90131-7. [Research Support, Non-U.S. Gov'tResearch Support, U.S. Gov't, P.H.S.] [DOI] [PubMed] [Google Scholar]
- 77.Pandey SC, Davis JM, Pandey GN. Phosphoinositide system-linked serotonin receptor subtypes and their pharmacological properties and clinical correlates. J Psychiatry Neurosci. 1995 May;20(3):215–25. [Review] [PMC free article] [PubMed] [Google Scholar]
- 78.Ciccocioppo R. The role of serotonin in craving: from basic research to human studies. Alcohol Alcohol. 1999 Mar-Apr;34(2):244–53. doi: 10.1093/alcalc/34.2.244. [Research Support, Non-U.S. Gov'tReview] [DOI] [PubMed] [Google Scholar]
- 79.Di Chiara G. A motivational learning hypothesis of the role of mesolimbic dopamine in compulsive drug use. J Psychopharmacol. 1998;12(1):54–67. doi: 10.1177/026988119801200108. [DOI] [PubMed] [Google Scholar]
- 80.Lal H, Prather PL, Rezazadeh SM. Potential role of 5HT1C and/or 5HT2 receptors in the mianserin-induced prevention of anxiogenic behaviors occurring during ethanol withdrawal. Alcohol Clin Exp Res. 1993 Apr;17(2):411–7. doi: 10.1111/j.1530-0277.1993.tb00785.x. [Research Support, U.S. Gov't, P.H.S.] [DOI] [PubMed] [Google Scholar]
- 81.Lovinger DM. 5-HT3 receptors and the neural actions of alcohols: an increasingly exciting topic. Neurochem Int. 1999 Aug;35(2):125–30. doi: 10.1016/s0197-0186(99)00054-6. [Review] [DOI] [PubMed] [Google Scholar]
- 82.Di Chiara G, Imperato A. Ethanol preferentially stimulates dopamine release in the nucleus accumbens of freely moving rats. Eur J Pharmacol. 1985 Sep 10;115(1):131–2. doi: 10.1016/0014-2999(85)90598-9. [DOI] [PubMed] [Google Scholar]
- 83.Heinz A, Reimold M, Wrase J, Hermann D, Croissant B, Mundle G, et al. Correlation of stable elevations in striatal mu-opioid receptor availability in detoxified alcoholic patients with alcohol craving: a positron emission tomography study using carbon 11-labeled carfentanil. Arch Gen Psychiatry. 2005 Jan;62(1):57–64. doi: 10.1001/archpsyc.62.1.57. [Research Support, Non-U.S. Gov't] [DOI] [PubMed] [Google Scholar]
- 84.Gianoulakis C. Influence of the endogenous opioid system on high alcohol consumption and genetic predisposition to alcoholism. J Psychiatry Neurosci. 2001 Sep;26(4):304–18. [Research Support, Non-U.S. Gov'tReview] [PMC free article] [PubMed] [Google Scholar]
- 85.Koob GF, Weiss F, Tiffany ST, Zieglgansberger W, Spanagel R. Animal models of craving: a roundtable discussion. Alcohol Res Health. 1999;23(3):233–6. [PMC free article] [PubMed] [Google Scholar]
- 86.Rhodes JS, Best K, Belknap JK, Finn DA, Crabbe JC. Evaluation of a simple model of ethanol drinking to intoxication in C57BL/6J mice. Physiol Behav. 2005 Jan 31;84(1):53–63. doi: 10.1016/j.physbeh.2004.10.007. [Comparative Study Evaluation StudiesResearch Support, U.S. Gov't, Non-P.H.S.Research Support, U.S. Gov't, P.H.S.] [DOI] [PubMed] [Google Scholar]
- 87.Hutchison KE, Wooden A, Swift RM, Smolen A, McGeary J, Adler L, et al. Olanzapine reduces craving for alcohol: a DRD4 VNTR polymorphism by pharmacotherapy interaction. Neuropsychopharmacology. 2003 Oct;28(10):1882–8. doi: 10.1038/sj.npp.1300264. [Clinical TrialComparative StudyRandomized Controlled TrialResearch Support, U.S. Gov't, P.H.S.] [DOI] [PubMed] [Google Scholar]
- 88.Ray LA, Hutchison KE. Effects of naltrexone on alcohol sensitivity and genetic moderators of medication response: a double-blind placebo-controlled study. Arch Gen Psychiatry. 2007 Sep;64(9):1069–77. doi: 10.1001/archpsyc.64.9.1069. [Clinical TrialComparative StudyResearch Support, N.I.H., Extramural] [DOI] [PubMed] [Google Scholar]
- 89.Oslin DW, Berrettini W, Kranzler HR, Pettinati H, Gelernter J, Volpicelli JR, et al. A functional polymorphism of the mu-opioid receptor gene is associated with naltrexone response in alcohol-dependent patients. Neuropsychopharmacology. 2003 Aug;28(8):1546–52. doi: 10.1038/sj.npp.1300219. [Clinical Trial Comparative StudyRandomized Controlled TrialResearch Support, U.S. Gov't, Non-P.H.S. Research Support, U.S. Gov't, P.H.S.] [DOI] [PubMed] [Google Scholar]
- 90.Brown SA, Vik PW, McQuaid JR, Patterson TL, Irwin MR, Grant I. Severity of psychosocial stress and outcome of alcoholism treatment. J Abnorm Psychol. 1990 Nov;99(4):344–8. doi: 10.1037//0021-843x.99.4.344. [Research Support, U.S. Gov't, Non-P.H.S.Research Support, U.S. Gov't, P.H.S.] [DOI] [PubMed] [Google Scholar]
- 91.Breese GR, Knapp DJ, Overstreet DH. Stress sensitization of ethanol withdrawal-induced reduction in social interaction: inhibition by CRF-1 and benzodiazepine receptor antagonists and a 5-HT1A-receptor agonist. Neuropsychopharmacology. 2004 Mar;29(3):470–82. doi: 10.1038/sj.npp.1300282. [Comparative StudyResearch Support, U.S. Gov't, P.H.S.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sloan JA, Aaronson N, Cappelleri JC, Fairclough DL, Varricchio C. Assessing the clinical significance of single items relative to summated scores. Mayo Clin Proc. 2002 May;77(5):479–87. [Consensus Development ConferenceResearch Support, U.S. Gov't, P.H.S.Review] [PubMed] [Google Scholar]
- 93.Sitharthan T, McGrath D, Sitharthan G, Saunders JB. Meaning of craving in research on addiction. Psychol Rep. 1992 Dec;71:823–6. doi: 10.2466/pr0.1992.71.3.823. 3 Pt 1. [DOI] [PubMed] [Google Scholar]
- 94.Drobes DJ, Thomas SE. Assessing craving for alcohol. Alcohol Res Health. 1999;23(3):179–86. [Review] [PMC free article] [PubMed] [Google Scholar]
- 95.Ooteman W, Koeter MW, Vserheul R, Schippers GM, van den Brink W. Measuring craving: an attempt to connect subjective craving with cue reactivity. Alcohol Clin Exp Res. 2006 Jan;30(1):57–69. doi: 10.1111/j.1530-0277.2006.00019.x. [Comparative StudyResearch Support, Non-U.S. Gov't] [DOI] [PubMed] [Google Scholar]
- 96.Connor JP, Feeney GF, Young RM. A comparison of the Yale-Brown Obsessive Compulsive Scale for "heavy drinking" with a single item craving measure: construct validity and clinical utility. Subst Use Misuse. 2005;40(4):551–61. doi: 10.1081/ja-200030723. [Comparative StudyValidation Studies] [DOI] [PubMed] [Google Scholar]
- 97.Flannery BA, Volpicelli JR, Pettinati HM. Psychometric properties of the Penn Alcohol Craving Scale. Alcohol Clin Exp Res. 1999 Aug;23(8):1289–95. [Research Support, U.S. Gov't, P.H.S.] [PubMed] [Google Scholar]
- 98.Modell JG, Glaser FB, Mountz JM, Schmaltz S, Cyr L. Obsessive and compulsive characteristics of alcohol abuse and dependence: quantification by a newly developed questionnaire. Alcohol Clin Exp Res. 1992 Apr;16(2):266–71. doi: 10.1111/j.1530-0277.1992.tb01374.x. [DOI] [PubMed] [Google Scholar]
- 99.Anton RF, Moak DH, Latham P. The Obsessive Compulsive Drinking Scale: a self-rated instrument for the quantification of thoughts about alcohol and drinking behavior. Alcohol Clin Exp Res. 1995 Feb;19(1):92–9. doi: 10.1111/j.1530-0277.1995.tb01475.x. [Comparative StudyResearch Support, U.S. Gov't, P.H.S.] [DOI] [PubMed] [Google Scholar]
- 100.Anton RF. Obsessive-compulsive aspects of craving: development of the Obsessive Compulsive Drinking Scale. Addiction. 2000 Aug;95(Suppl 2):S211–7. doi: 10.1080/09652140050111771. [Research Support, U.S. Gov't, P.H.S.Review] [DOI] [PubMed] [Google Scholar]
- 101.Bohn MJ, Krahn DD, Staehler BA. Development and initial validation of a measure of drinking urges in abstinent alcoholics. Alcohol Clin Exp Res. 1995 Jun;19(3):600–6. doi: 10.1111/j.1530-0277.1995.tb01554.x. [Research Support, Non-U.S. Gov'tResearch Support, U.S. Gov't, P.H.S.] [DOI] [PubMed] [Google Scholar]
- 102.Kavanagh DJ, Andrade J, May J. Imaginary relish and exquisite torture: the elaborated intrusion theory of desire. Psychol Rev. 2005;112(2):446–67. doi: 10.1037/0033-295X.112.2.446. [DOI] [PubMed] [Google Scholar]
- 103.Kavanagh DJ, May J, Andrade J. Tests of the elaborated intrusion theory of craving and desire: Features of alcohol craving during treatment for an alcohol disorder. Br J Clin Psychol. 2009 Sep 48;:241–54. doi: 10.1348/014466508X387071. [Comparative StudyResearch Support, Non-U.S. Gov't] Pt 3. [DOI] [PubMed] [Google Scholar]
- 104.Statham DJ, Connor JP, Kavanagh DJ, Feeney GF, Young RM, May J, et al. Measuring alcohol craving: development of the Alcohol Craving Experience questionnaire. Addiction. 2011 Jul;106(7):1230–8. doi: 10.1111/j.1360-0443.2011.03442.x. [Research Support, Non-U.S. Gov'tValidation Studies] [DOI] [PubMed] [Google Scholar]
- 105.Cacioppo JT, Martzke JS, Petty RE, Tassinary LG. Specific forms of facial EMG response index emotions during an interview: from Darwin to the continuous flow hypothesis affect-laden information processing. J Pers Soc Psychol. 1988 Apr;54(4):592–604. doi: 10.1037//0022-3514.54.4.592. [Research Support, U.S. Gov't, Non-P.H.S.] [DOI] [PubMed] [Google Scholar]
- 106.Drobes DJ, Tiffany ST. Induction of smoking urge through imaginal and in vivo procedures: physiological and self-report manifestations. J Abnorm Psychol. 1997 Feb;106(1):15–25. doi: 10.1037//0021-843x.106.1.15. [Research Support, Non-U.S. Gov't] [DOI] [PubMed] [Google Scholar]
- 107.Everitt B. Craving cocaine cues: cognitive neuroscience meets drug addiction research. Trends Cogn Sci. 1997 Apr;1(1):1–2. doi: 10.1016/S1364-6613(97)01009-7. [DOI] [PubMed] [Google Scholar]
- 108.Myrick H, Anton RF, Li X, Henderson S, Randall PK, Voronin K. Effect of naltrexone and ondansetron on alcohol cue-induced activation of the ventral striatum in alcohol-dependent people. Arch Gen Psychiatry. 2008 Apr;65(4):466–75. doi: 10.1001/archpsyc.65.4.466. [Randomized Controlled Trial Research Support, N.I.H., ExtramuralResearch Support, U.S. Gov't, Non-P.H.S.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chick J, Anton R, Checinski K, Croop R, Drummond DC, Farmer R, et al. A multicentre, randomized, double-blind, placebo-controlled trial of naltrexone in the treatment of alcohol dependence or abuse. Alcohol Alcohol. 2000 Nov-Dec;35(6):587–93. doi: 10.1093/alcalc/35.6.587. [Clinical TrialMulticenter StudyRandomized Controlled TrialResearch Support, Non-U.S. Gov't] [DOI] [PubMed] [Google Scholar]
- 110.O'Malley SS, Krishnan-Sarin S, Farren C, Sinha R, Kreek MJ. Naltrexone decreases craving and alcohol self-administration in alcohol-dependent subjects and activates the hypothalamo-pituitary-adrenocortical axis. Psychopharmacology (Berl) 2002 Feb;160(1):19–29. doi: 10.1007/s002130100919. [Clinical TrialRandomized Controlled TrialResearch Support, U.S. Gov't, P.H.S.] [DOI] [PubMed] [Google Scholar]
- 111.Davidson D, Palfai T, Bird C, Swift R. Effects of naltrexone on alcohol self-administration in heavy drinkers. Alcohol Clin Exp Res. 1999 Feb;23(2):195–203. [Clinical TrialRandomized Controlled TrialResearch Support, Non-U.S. Gov't] [PubMed] [Google Scholar]
- 112.Monterosso JR, Flannery BA, Pettinati HM, Oslin DW, Rukstalis M, O'Brien CP, et al. Predicting treatment response to naltrexone: the influence of craving and family history. Am J Addict. 2001 Summer;10(3):258–68. doi: 10.1080/105504901750532148. [Clinical TrialRandomized Controlled TrialResearch Support, U.S. Gov't, P.H.S.] [DOI] [PubMed] [Google Scholar]
- 113.Babor TF, Hofmann M, DelBoca FK, Hesselbrock V, Meyer RE, Dolinsky ZS, et al. Types of alcoholics, I. Evidence for an empirically derived typology based on indicators of vulnerability and severity. Arch Gen Psychiatry. 1992 Aug;49(8):599–608. doi: 10.1001/archpsyc.1992.01820080007002. [Research Support, U.S. Gov't, P.H.S.] [DOI] [PubMed] [Google Scholar]
- 114.Jaffe AJ, Rounsaville B, Chang G, Schottenfeld RS, Meyer RE, O'Malley SS. Naltrexone, relapse prevention, and supportive therapy with alcoholics: an analysis of patient treatment matching. J Consult Clin Psychol. 1996 Oct;64(5):1044–53. doi: 10.1037//0022-006x.64.5.1044. [DOI] [PubMed] [Google Scholar]
- 115.O'Malley SS, Jaffe AJ, Chang G, Schottenfeld RS, Meyer RE, Rounsaville B. Naltrexone and coping skills therapy for alcohol dependence. A controlled study. Arch Gen Psychiatry. 1992 Nov;49(11):881–7. doi: 10.1001/archpsyc.1992.01820110045007. [Clinical TrialRandomized Controlled TrialResearch Support, U.S. Gov't, P.H.S.] [DOI] [PubMed] [Google Scholar]
- 116.Volpicelli JR, Clay KL, Watson NT, O'Brien CP. Naltrexone in the treatment of alcoholism: predicting response to naltrexone. J Clin Psychiatry. 1995;56(Suppl 7):39–44. [Clinical TrialRandomized Controlled TrialResearch Support, U.S. Gov't, P.H.S.] [PubMed] [Google Scholar]
- 117.Kranzler HR, Tennen H, Penta C, Bohn MJ. Targeted naltrexone treatment of early problem drinkers. Addict Behav. 1997 May-Jun;22(3):431–6. doi: 10.1016/s0306-4603(96)00064-0. [Research Support, U.S. Gov't, P.H.S.] [DOI] [PubMed] [Google Scholar]
- 118.Heinala P, Alho H, Kiianmaa K, Lonnqvist J, Kuoppasalmi K, Sinclair JD. Targeted use of naltrexone without prior detoxification in the treatment of alcohol dependence: a factorial double-blind, placebo-controlled trial. J Clin Psychopharmacol. 2001 Jun;21(3):287–92. doi: 10.1097/00004714-200106000-00006. [Clinical Trial Randomized Controlled TrialResearch Support, Non-U.S. Gov't] [DOI] [PubMed] [Google Scholar]
- 119.Mann K, Bladstrom A, Torup L, Gual A, van den Brink W. Extending the treatment options in alcohol dependence: a randomized controlled study of as-needed nalmefene. Biol Psychiatry. 2013 Apr 15;73(8):706–13. doi: 10.1016/j.biopsych.2012.10.020. [Research Support, Non-U.S. Gov't] [DOI] [PubMed] [Google Scholar]
- 120.Anton RF, Moak DH, Latham P, Waid LR, Myrick H, Voronin K, et al. Naltrexone combined with either cognitive behavioral or motivational enhancement therapy for alcohol dependence. J Clin Psychopharmacol. 2005 Aug;25(4):349–57. doi: 10.1097/01.jcp.0000172071.81258.04. [Clinical TrialComparative StudyRandomized Controlled TrialResearch Support, N.I.H., ExtramuralResearch Support, U.S. Gov't, P.H.S.] [DOI] [PubMed] [Google Scholar]
- 121.Anton RF, Moak DH, Latham PK, Waid LR, Malcolm RJ, Dias JK, et al. Posttreatment results of combining naltrexone with cognitive-behavior therapy for the treatment of alcoholism. J Clin Psychopharmacol. 2001 Feb;21(1):72–7. doi: 10.1097/00004714-200102000-00013. [Clinical TrialComparative StudyRandomized Controlled TrialResearch Support, U.S. Gov't, P.H.S.] [DOI] [PubMed] [Google Scholar]
- 122.Anton RF, Moak DH, Waid LR, Latham PK, Malcolm RJ, Dias JK. Naltrexone and cognitive behavioral therapy for the treatment of outpatient alcoholics: results of a placebo-controlled trial. Am J Psychiatry. 1999 Nov;156(11):1758–64. doi: 10.1176/ajp.156.11.1758. [Clinical TrialRandomized Controlled TrialResearch Support, U.S. Gov't, P.H.S.] [DOI] [PubMed] [Google Scholar]
- 123.Heilig M, Egli M. Pharmacological treatment of alcohol dependence: target symptoms and target mechanisms. Pharmacol Ther. 2006 Sep;111(3):855–76. doi: 10.1016/j.pharmthera.2006.02.001. [Review] [DOI] [PubMed] [Google Scholar]
- 124.Weiss RD, Kueppenbender KD. Combining psychosocial treatment with pharmacotherapy for alcohol dependence. J Clin Psychopharmacol. 2006 Dec;26(Suppl 1):S37–42. doi: 10.1097/01.jcp.0000248604.58305.b3. [Research Support, N.I.H., ExtramuralReview] [DOI] [PubMed] [Google Scholar]
- 125.Anton RF, O'Malley SS, Ciraulo DA, Cisler RA, Couper D, Donovan DM, et al. Combined pharmacotherapies and behavioral interventions for alcohol dependence: the COMBINE study: a randomized controlled trial. Jama. 2006 May 3;295(17):2003–17. doi: 10.1001/jama.295.17.2003. [Multicenter StudyRandomized Controlled TrialResearch Support, N.I.H., Extramural] [DOI] [PubMed] [Google Scholar]
- 126.De Sousa A. A one-year pragmatic trial of naltrexone vs disulfiram in the treatment of alcohol dependence. Alcohol Alcohol. 2004 Nov-Dec;39(6):528–31. doi: 10.1093/alcalc/agh104. [Clinical TrialComparative StudyRandomized Controlled Trial] [DOI] [PubMed] [Google Scholar]
- 127.Krystal JH, Cramer JA, Krol WF, Kirk GF, Rosenheck RA. Naltrexone in the treatment of alcohol dependence. N Engl J Med. 2001 Dec 13;345(24):1734–9. doi: 10.1056/NEJMoa011127. [Clinical TrialMulticenter StudyRandomized Controlled TrialResearch Support, Non-U.S. Gov'tResearch Support, U.S. Gov't, Non-P.H.S.] [DOI] [PubMed] [Google Scholar]
- 128.Krishnan-Sarin S, Krystal JH, Shi J, Pittman B, O'Malley SS. Family history of alcoholism influences naltrexone-induced reduction in alcohol drinking. Biol Psychiatry. 2007 Sep 15;62(6):694–7. doi: 10.1016/j.biopsych.2006.11.018. [Comparative StudyResearch Support, N.I.H., ExtramuralResearch Support, Non-U.S. Gov't] [DOI] [PubMed] [Google Scholar]
- 129.McGeary JE, Monti PM, Rohsenow DJ, Tidey J, Swift R, Miranda R., Jr Genetic moderators of naltrexone's effects on alcohol cue reactivity. Alcohol Clin Exp Res. 2006 Aug;30(8):1288–96. doi: 10.1111/j.1530-0277.2006.00156.x. [Comparative StudyRandomized Controlled TrialResearch Support, N.I.H., ExtramuralResearch Support, U.S. Gov't, Non-P.H.S.] [DOI] [PubMed] [Google Scholar]
- 130.Anton RF, Oroszi G, O'Malley S, Couper D, Swift R, Pettinati H, et al. An evaluation of mu-opioid receptor (OPRM1) as a predictor of naltrexone response in the treatment of alcohol dependence: results from the Combined Pharmacotherapies and Behavioral Interventions for Alcohol Dependence (COMBINE) study. Arch Gen Psychiatry. 2008 Feb;65(2):135–44. doi: 10.1001/archpsyc.65.2.135. [Multicenter StudyRandomized Controlled TrialResearch Support, N.I.H., ExtramuralResearch Support, N.I.H., Intramural] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Gelernter J, Gueorguieva R, Kranzler HR, Zhang H, Cramer J, Rosenheck R, et al. Opioid receptor gene (OPRM1, OPRK1, and OPRD1) variants and response to naltrexone treatment for alcohol dependence: results from the VA Cooperative Study. Alcohol Clin Exp Res. 2007 Apr;31(4):555–63. doi: 10.1111/j.1530-0277.2007.00339.x. [Randomized Controlled TrialResearch Support, N.I.H., ExtramuralResearch Support, U.S. Gov't, Non-P.H.S.] [DOI] [PubMed] [Google Scholar]
- 132.Kranzler HR, Armeli S, Covault J, Tennen H. Variation in OPRM1 moderates the effect of desire to drink on subsequent drinking and its attenuation by naltrexone treatment. Addict Biol. 2013 Jan;18(1):193–201. doi: 10.1111/j.1369-1600.2012.00471.x. [Randomized Controlled TrialResearch Support, N.I.H., ExtramuralResearch Support, Non-U.S. Gov't] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Modell JG, Mountz JM, Beresford TP. Basal ganglia/limbic striatal and thalamocortical involvement in craving and loss of control in alcoholism. J Neuropsychiatry Clin Neurosci. 1990 Spring;2(2):123–44. doi: 10.1176/jnp.2.2.123. [Review] [DOI] [PubMed] [Google Scholar]
- 134.Modell JG, Mountz JM, Glaser FB, Lee JY. Effect of haloperidol on measures of craving and impaired control in alcoholic subjects. Alcohol Clin Exp Res. 1993 Apr;17(2):234–40. doi: 10.1111/j.1530-0277.1993.tb00755.x. [Clinical TrialRandomized Controlled TrialResearch Support, U.S. Gov't, P.H.S.] [DOI] [PubMed] [Google Scholar]
- 135.Sussman N. The implications of weight changes with antipsychotic treatment. J Clin Psychopharmacol. 2003 Jun;23(3 Suppl 1):S21–6. doi: 10.1097/01.jcp.0000084037.22282.a3. [Review] [DOI] [PubMed] [Google Scholar]
- 136.Osser DN, Najarian DM, Dufresne RL. Olanzapine increases weight and serum triglyceride levels. J Clin Psychiatry. 1999 Nov;60(11):767–70. doi: 10.4088/jcp.v60n1109. [Clinical Trial] [DOI] [PubMed] [Google Scholar]
- 137.Heiskanen T, Niskanen L, Lyytikainen R, Saarinen PI, Hintikka J. Metabolic syndrome in patients with schizophrenia. J Clin Psychiatry. 2003 May;64(5):575–9. doi: 10.4088/jcp.v64n0513. [Comparative Study] [DOI] [PubMed] [Google Scholar]
- 138.Hutchison KE, Swift R, Rohsenow DJ, Monti PM, Davidson D, Almeida A. Olanzapine reduces urge to drink after drinking cues and a priming dose of alcohol. Psychopharmacology (Berl) 2001 Apr;155(1):27–34. doi: 10.1007/s002130000629. [Clinical TrialRandomized Controlled TrialResearch Support, Non-U.S. Gov'tResearch Support, U.S. Gov't, Non-P.H.S.Research Support, U.S. Gov't, P.H.S.] [DOI] [PubMed] [Google Scholar]
- 139.Kampman KM, Pettinati HM, Lynch KG, Whittingham T, Macfadden W, Dackis C, et al. A double-blind, placebo-controlled pilot trial of quetiapine for the treatment of Type A and Type B alcoholism. J Clin Psychopharmacol. 2007 Aug;27(4):344–51. doi: 10.1097/JCP.0b013e3180ca86e5. [Randomized Controlled TrialResearch Support, Non-U.S. Gov't] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Scheller-Gilkey G, Woolwine BJ, Cooper I, Gay O, Moynes KA, Miller AH. Relationship of clinical symptoms and substance use in schizophrenia patients on conventional versus atypical antipsychotics. Am J Drug Alcohol Abuse. 2003 Aug;29(3):553–66. doi: 10.1081/ada-120023458. [Comparative StudyResearch Support, Non-U.S. Gov'tResearch Support, U.S. Gov't, P.H.S.] [DOI] [PubMed] [Google Scholar]
- 141.Green AI, Burgess ES, Dawson R, Zimmet SV, Strous RD. Alcohol and cannabis use in schizophrenia: effects of clozapine vs. risperidone. Schizophr Res. 2003 Mar 1;60(1):81–5. doi: 10.1016/s0920-9964(02)00231-1. [Comparative StudyResearch Support, Non-U.S. Gov'tResearch Support, U.S. Gov't, P.H.S.] [DOI] [PubMed] [Google Scholar]
- 142.Kalyoncu A, Mirsal H, Pektas O, Unsalan N, Tan D, Beyazyurek M. Use of lamotrigine to augment clozapine in patients with resistant schizophrenia and comorbid alcohol dependence: a potent anti-craving effect? J Psychopharmacol. 2005 May;19(3):301–5. doi: 10.1177/0269881105051542. [Case Reports] [DOI] [PubMed] [Google Scholar]
- 143.Drake RE, Xie H, McHugo GJ, Green AI. The effects of clozapine on alcohol and drug use disorders among patients with schizophrenia. Schizophr Bull. 2000;26(2):441–9. doi: 10.1093/oxfordjournals.schbul.a033464. [Clinical TrialResearch Support, U.S. Gov't, P.H.S.] [DOI] [PubMed] [Google Scholar]
- 144.Kenna GA. Rationale for use of aripiprazole for alcohol dependence treatment. Drugs of the Future. 2003;28(12):1227–35. [Google Scholar]
- 145.Anton RF, Kranzler H, Breder C, Marcus RN, Carson WH, Han J. A randomized, multicenter, double-blind, placebo-controlled study of the efficacy and safety of aripiprazole for the treatment of alcohol dependence. J Clin Psychopharmacol. 2008 Feb;28(1):5–12. doi: 10.1097/jcp.0b013e3181602fd4. [Multicenter StudyRandomized Controlled TrialResearch Support, Non-U.S. Gov't] [DOI] [PubMed] [Google Scholar]
- 146.Martinotti G, Di Nicola M, Janiri L. Efficacy and safety of aripiprazole in alcohol dependence. Am J Drug Alcohol Abuse. 2007;33(3):393–401. doi: 10.1080/00952990701313660. [Clinical TrialResearch Support, Non-U.S. Gov't] [DOI] [PubMed] [Google Scholar]
- 147.Martinotti G, Di Nicola M, Di Giannantonio M, Janiri L. Aripiprazole in the treatment of patients with alcohol dependence: a double-blind, comparison trial vs. naltrexone. J Psychopharmacol. 2009 Mar;23(2):123–9. doi: 10.1177/0269881108089596. [Comparative StudyRandomized Controlled Trial] [DOI] [PubMed] [Google Scholar]
- 148.Monnelly EP, Ciraulo DA, Knapp C, LoCastro J, Sepulveda I. Quetiapine for treatment of alcohol dependence. J Clin Psychopharmacol. 2004 Oct;24(5):532–5. doi: 10.1097/01.jcp.0000138763.23482.2a. [Research Support, U.S. Gov't, Non-P.H.S.] [DOI] [PubMed] [Google Scholar]
- 149.Landolt HP, Gillin JC. Sleep abnormalities during abstinence in alcohol-dependent patients. Aetiology and management. CNS Drugs. 2001;15(5):413–25. doi: 10.2165/00023210-200115050-00006. [Research Support, Non-U.S. Gov'tResearch Support, U.S. Gov't, Non-P.H.S.Research Support, U.S. Gov't, P.H.S.Review] [DOI] [PubMed] [Google Scholar]
- 150.Croissant B, Klein O, Gehrlein L, Kniest A, Hermann D, Diehl A, et al. Quetiapine in relapse prevention in alcoholics suffering from craving and affective symptoms: a case series. Eur Psychiatry. 2006 Dec;21(8):570–3. doi: 10.1016/j.eurpsy.2006.04.007. [Case Reports] [DOI] [PubMed] [Google Scholar]
- 151.Martinotti G, Andreoli S, Di Nicola M, Di Giannantonio M, Sarchiapone M, Janiri L. Quetiapine decreases alcohol consumption, craving, and psychiatric symptoms in dually diagnosed alcoholics. Hum Psychopharmacol. 2008 Jul;23(5):417–24. doi: 10.1002/hup.944. [Clinical Trial] [DOI] [PubMed] [Google Scholar]
- 152.Litten RZ, Fertig JB, Falk DE, Ryan ML, Mattson ME, Collins JF, et al. A double-blind, placebo-controlled trial to assess the efficacy of quetiapine fumarate XR in very heavy-drinking alcohol-dependent patients. Alcohol Clin Exp Res. 2012 Mar;36(3):406–16. doi: 10.1111/j.1530-0277.2011.01649.x. [Multicenter StudyRandomized Controlled TrialResearch Support, N.I.H., ExtramuralResearch Support, Non-U.S. Gov'tResearch Support, U.S. Gov't, Non-P.H.S.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Hutchison KE, Ray L, Sandman E, Rutter MC, Peters A, Davidson D, et al. The effect of olanzapine on craving and alcohol consumption. Neuropsychopharmacology. 2006 Jun;31(6):1310–7. doi: 10.1038/sj.npp.1300917. [Clinical TrialComparative StudyRandomized Controlled TrialResearch Support, N.I.H., ExtramuralResearch Support, Non-U.S. Gov't] [DOI] [PubMed] [Google Scholar]
- 154.Agrawal A, Wetherill L, Bucholz KK, Kramer J, Kuperman S, Lynskey MT, et al. Genetic influences on craving for alcohol. Addict Behav. 2013 Feb;38(2):1501–8. doi: 10.1016/j.addbeh.2012.03.021. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov'tResearch Support, U.S. Gov't, P.H.S.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Johnson BA, Roache JD, Javors MA, DiClemente CC, Cloninger CR, Prihoda TJ, et al. Ondansetron for reduction of drinking among biologically predisposed alcoholic patients: A randomized controlled trial. Jama. 2000 Aug 23-30;284(8):963–71. doi: 10.1001/jama.284.8.963. [Clinical TrialRandomized Controlled TrialResearch Support, U.S. Gov't, P.H.S.] [DOI] [PubMed] [Google Scholar]
- 156.Johnson BA, Roache JD, Ait-Daoud N, Zanca NA, Velazquez M. Ondansetron reduces the craving of biologically predisposed alcoholics. Psychopharmacology (Berl) 2002 Apr;160(4):408–13. doi: 10.1007/s00213-002-1002-9. [Clinical Trial Comparative StudyRandomized Controlled TrialResearch Support, U.S. Gov't, P.H.S.] [DOI] [PubMed] [Google Scholar]
- 157.Myrick H, Anton RF, Li X, Henderson S, Drobes D, Voronin K, et al. Differential brain activity in alcoholics and social drinkers to alcohol cues: relationship to craving. Neuropsychopharmacology. 2004 Feb;29(2):393–402. doi: 10.1038/sj.npp.1300295. [DOI] [PubMed] [Google Scholar]
- 158.Naranjo CA, Poulos CX, Bremner KE, Lanctot KL. Fluoxetine attenuates alcohol intake and desire to drink. Int Clin Psychopharmacol. 1994 Sep;9(3):163–72. doi: 10.1097/00004850-199409000-00004. [Clinical TrialRandomized Controlled TrialResearch Support, Non-U.S. Gov't] [DOI] [PubMed] [Google Scholar]
- 159.Kabel DI, Petty F. A placebo-controlled, double-blind study of fluoxetine in severe alcohol dependence: adjunctive pharmacotherapy during and after inpatient treatment. Alcohol Clin Exp Res. 1996 Jun;20(4):780–4. doi: 10.1111/j.1530-0277.1996.tb01686.x. [Clinical TrialComparative StudyRandomized Controlled TrialResearch Support, Non-U.S. Gov'tResearch Support, U.S. Gov't, Non-P.H.S.Research Support, U.S. Gov't, P.H.S.] [DOI] [PubMed] [Google Scholar]
- 160.Pettinati HM. The use of selective serotonin reuptake inhibitors in treating alcoholic subtypes. J Clin Psychiatry. 2001;62(Suppl 20):26–31. [Research Support, U.S. Gov't, P.H.S.Review] [PubMed] [Google Scholar]
- 161.Pettinati HM, Volpicelli JR, Luck G, Kranzler HR, Rukstalis MR, Cnaan A. Double-blind clinical trial of sertraline treatment for alcohol dependence. J Clin Psychopharmacol. 2001 Apr;21(2):143–53. doi: 10.1097/00004714-200104000-00005. [Clinical TrialRandomized Controlled TrialResearch Support, U.S. Gov't, Non-P.H.S.Research Support, U.S. Gov't, P.H.S.] [DOI] [PubMed] [Google Scholar]
- 162.Kranzler HR, Burleson JA, Brown J, Babor TF. Fluoxetine treatment seems to reduce the beneficial effects of cognitive-behavioral therapy in type B alcoholics. Alcohol Clin Exp Res. 1996 Dec;20(9):1534–41. doi: 10.1111/j.1530-0277.1996.tb01696.x. [Clinical TrialControlled Clinical TrialResearch Support, U.S. Gov't, P.H.S.] [DOI] [PubMed] [Google Scholar]
- 163.Lovinger DM. Serotonin's role in alcohol's effects on the brain. Alcohol Health Res World. 1997;21(2):114–20. [Review] [PMC free article] [PubMed] [Google Scholar]
- 164.Kenna GA, McGeary JE, Swift RM. Pharmacotherapy, pharmacogenomics, and the future of alcohol dependence treatment, Part 2. Am J Health Syst Pharm. 2004 Nov 15;61(22):2380–8. doi: 10.1093/ajhp/61.22.2380. [Review] [DOI] [PubMed] [Google Scholar]
- 165.Pettinati HM, Volpicelli JR, Kranzler HR, Luck G, Rukstalis MR, Cnaan A. Sertraline treatment for alcohol dependence: interactive effects of medication and alcoholic subtype. Alcohol Clin Exp Res. 2000 Jul;24(7):1041–9. [Clinical TrialRandomized Controlled TrialResearch Support, U.S. Gov't, Non-P.H.S.Research Support, U.S. Gov't, P.H.S.] [PubMed] [Google Scholar]
- 166.Johnson BA. The role of serotonergic agents as treatments for alcoholism. Drugs Today (Barc) 2003 Sep;39(9):665–72. doi: 10.1358/dot.2003.39.9.799475. [Research Support, U.S. Gov't, P.H.S.Review] [DOI] [PubMed] [Google Scholar]
- 167.Johnson BA, Seneviratne C, Wang XQ, Ait-Daoud N, Li MD. Determination of genotype combinations that can predict the outcome of the treatment of alcohol dependence using the 5-HT(3) antagonist ondansetron. Am J Psychiatry. 2013 Sep 1;170(9):1020–31. doi: 10.1176/appi.ajp.2013.12091163. [Randomized Controlled TrialResearch Support, N.I.H., Extramural] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Johnson BA, Ait-Daoud N, Ma JZ, Wang Y. Ondansetron reduces mood disturbance among biologically predisposed, alcohol-dependent individuals. Alcohol Clin Exp Res. 2003 Nov;27(11):1773–9. doi: 10.1097/01.ALC.0000095635.46911.5D. [Clinical TrialRandomized Controlled TrialResearch Support, U.S. Gov't, P.H.S.] [DOI] [PubMed] [Google Scholar]
- 169.Kenna GA, Zywiak WH, McGeary JE, Leggio L, McGeary C, Wang S, et al. A within-group design of nontreatment seeking 5-HTTLPR genotyped alcohol-dependent subjects receiving ondansetron and sertraline. Alcohol Clin Exp Res. 2009 Feb;33(2):315–23. doi: 10.1111/j.1530-0277.2008.00835.x. [Randomized Controlled Trial] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Kranzler HR, Armeli S, Tennen H, Covault J, Feinn R, Arias AJ, et al. A double-blind, randomized trial of sertraline for alcohol dependence: moderation by age of onset [corrected] and 5-hydroxytryptamine transporter-linked promoter region genotype. J Clin Psychopharmacol. 2011 Feb;31(1):22–30. doi: 10.1097/JCP.0b013e31820465fa. [Comparative StudyRandomized Controlled TrialResearch Support, N.I.H., ExtramuralResearch Support, Non-U.S. Gov't] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Ait-Daoud N, Seneviratne C, Smith JB, Roache JD, Dawes MA, Liu L, et al. Preliminary Evidence for cue-induced Alcohol Craving Modulated by Serotonin Transporter Gene Polymorphism rs1042173. Front Psychiatry. 2012;3:6. doi: 10.3389/fpsyt.2012.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Johnson BA, Rosenthal N, Capece JA, Wiegand F, Mao L, Beyers K, et al. Topiramate for treating alcohol dependence: a randomized controlled trial. Jama. 2007 Oct 10;298(14):1641–51. doi: 10.1001/jama.298.14.1641. [Multicenter StudyRandomized Controlled TrialResearch Support, Non-U.S. Gov't] [DOI] [PubMed] [Google Scholar]
- 173.Johnson BA, Ait-Daoud N, Bowden CL, DiClemente CC, Roache JD, Lawson K, et al. Oral topiramate for treatment of alcohol dependence: a randomised controlled trial. Lancet. 2003 May 17;361(9370):1677–85. doi: 10.1016/S0140-6736(03)13370-3. [Clinical TrialRandomized Controlled TrialResearch Support, Non-U.S. Gov'tResearch Support, U.S. Gov't, P.H.S.] [DOI] [PubMed] [Google Scholar]
- 174.Ray LA, Miranda R, Jr., MacKillop J, McGeary J, Tidey JW, Rohsenow DJ, et al. A preliminary pharmacogenetic investigation of adverse events from topiramate in heavy drinkers. Exp Clin Psychopharmacol. 2009 Apr;17(2):122–9. doi: 10.1037/a0015700. [Controlled Clinical TrialResearch Support, N.I.H., ExtramuralResearch Support, U.S. Gov't, Non-P.H.S.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Biton V. Clinical pharmacology and mechanism of action of zonisamide. Clin Neuropharmacol. 2007 Jul-Aug;30(4):230–40. doi: 10.1097/wnf.0b013e3180413d7d. [Research Support, Non-U.S. Gov'tReview] [DOI] [PubMed] [Google Scholar]
- 176.Ulloa CM, Towfigh A, Safdieh J. Review of levetiracetam, with a focus on the extended release formulation, as adjuvant therapy in controlling partial-onset seizures. Neuropsychiatr Dis Treat. 2009;5:467–76. doi: 10.2147/ndt.s4844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Coulter DA. Antiepileptic drug cellular mechanisms of action: where does lamotrigine fit in? J Child Neurol. 1997 Nov;12(Suppl 1):S2–9. doi: 10.1177/0883073897012001031. [Research Support, Non-U.S. Gov'tResearch Support, U.S. Gov't, P.H.S.Review] [DOI] [PubMed] [Google Scholar]
- 178.Vengeliene V, Heidbreder CA, Spanagel R. The effects of lamotrigine on alcohol seeking and relapse. Neuropharmacology. 2007 Dec;53(8):951–7. doi: 10.1016/j.neuropharm.2007.09.006. [Research Support, Non-U.S. Gov't] [DOI] [PubMed] [Google Scholar]
- 179.Sarid-Segal O, Piechniczek-Buczek J, Knapp C, Afshar M, Devine E, Sickles L, et al. The effects of levetiracetam on alcohol consumption in alcohol-dependent subjects: an open label study. Am J Drug Alcohol Abuse. 2008;34(4):441–7. doi: 10.1080/00952990802082180. [Randomized Controlled TrialResearch Support, Non-U.S. Gov't] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Fertig JB, Ryan ML, Falk DE, Litten RZ, Mattson ME, Ransom J, et al. A double-blind, placebo-controlled trial assessing the efficacy of levetiracetam extended-release in very heavy drinking alcohol-dependent patients. Alcohol Clin Exp Res. 2012 Aug;36(8):1421–30. doi: 10.1111/j.1530-0277.2011.01716.x. [Randomized Controlled TrialResearch Support, Non-U.S. Gov't] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Rubio G, Lopez-Munoz F, Ferre F, Martinez-Gras I, Ponce G, Pascual JM, et al. Effects of zonisamide in the treatment of alcohol dependence. Clin Neuropharmacol. 2010 Sep-Oct;33(5):250–3. doi: 10.1097/WNF.0b013e3181f0ed9a. [Clinical Trial Multicenter Study] [DOI] [PubMed] [Google Scholar]
- 182.Arias AJ, Feinn R, Oncken C, Covault J, Kranzler HR. Placebo-controlled trial of zonisamide for the treatment of alcohol dependence. J Clin Psychopharmacol. 2010 Jun;30(3):318–22. doi: 10.1097/JCP.0b013e3181db38bb. [Clinical TrialComparative StudyRandomized Controlled TrialResearch Support, N.I.H., Extramural] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Addolorato G, Caputo F, Capristo E, Janiri L, Bernardi M, Agabio R, et al. Rapid suppression of alcohol withdrawal syndrome by baclofen. Am J Med. 2002 Feb 15;112(3):226–9. doi: 10.1016/s0002-9343(01)01088-9. [Case Reports] [DOI] [PubMed] [Google Scholar]
- 184.Addolorato G, Leggio L, Abenavoli L, Caputo F, Gasbarrini G. Baclofen for outpatient treatment of alcohol withdrawal syndrome. J Fam Pract. 2005 Jan;54(1):24. [CommentLetter] [PubMed] [Google Scholar]
- 185.Addolorato G, Leggio L, Abenavoli L, Agabio R, Caputo F, Capristo E, et al. Baclofen in the treatment of alcohol withdrawal syndrome: a comparative study vs diazepam. Am J Med. 2006 Mar;119(3):276 e13–8. doi: 10.1016/j.amjmed.2005.08.042. [Comparative StudyRandomized Controlled TrialResearch Support, Non-U.S. Gov't] [DOI] [PubMed] [Google Scholar]
- 186.Addolorato G, Leggio L, Ferrulli A, Cardone S, Vonghia L, Mirijello A, et al. Effectiveness and safety of baclofen for maintenance of alcohol abstinence in alcohol-dependent patients with liver cirrhosis: randomised, double-blind controlled study. Lancet. 2007 Dec 8;370(9603):1915–22. doi: 10.1016/S0140-6736(07)61814-5. [Randomized Controlled TrialResearch Support, Non-U.S. Gov't] [DOI] [PubMed] [Google Scholar]
- 187.Leggio L, Ferrulli A, Zambon A, Caputo F, Kenna GA, Swift RM, et al. Baclofen promotes alcohol abstinence in alcohol dependent cirrhotic patients with hepatitis C virus (HCV) infection. Addict Behav. 2012 Apr;37(4):561–4. doi: 10.1016/j.addbeh.2011.12.010. [Randomized Controlled TrialResearch Support, Non-U.S. Gov't] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Lyon JE, Khan RA, Gessert CE, Larson PM, Renier CM. Treating alcohol withdrawal with oral baclofen: a randomized, double-blind, placebo-controlled trial. J Hosp Med. 2011 Oct;6(8):469–74. doi: 10.1002/jhm.928. [Multicenter StudyRandomized Controlled TrialResearch Support, Non-U.S. Gov't] [DOI] [PubMed] [Google Scholar]
- 189.Addolorato G, Caputo F, Capristo E, Colombo G, Gessa GL, Gasbarrini G. Ability of baclofen in reducing alcohol craving and intake: II--Preliminary clinical evidence. Alcohol Clin Exp Res. 2000 Jan;24(1):67–71. [PubMed] [Google Scholar]
- 190.Addolorato G, Caputo F, Capristo E, Domenicali M, Bernardi M, Janiri L, et al. Baclofen efficacy in reducing alcohol craving and intake: a preliminary double-blind randomized controlled study. Alcohol Alcohol. 2002 Sep-Oct;37(5):504–8. doi: 10.1093/alcalc/37.5.504. [DOI] [PubMed] [Google Scholar]
- 191.Bucknam W. Suppression of symptoms of alcohol dependence and craving using high-dose baclofen. Alcohol Alcohol. 2007 Mar-Apr;42(2):158–60. doi: 10.1093/alcalc/agl091. [DOI] [PubMed] [Google Scholar]
- 192.Flannery BA, Garbutt JC, Cody MW, Renn W, Grace K, Osborne M, et al. Baclofen for alcohol dependence: a preliminary open-label study. Alcohol Clin Exp Res. 2004 Oct;28(10):1517–23. doi: 10.1097/01.alc.0000141640.48924.14. [Clinical Trial Comparative StudyResearch Support, Non-U.S. Gov'tResearch Support, U.S. Gov't, P.H.S.] [DOI] [PubMed] [Google Scholar]
- 193.Drake RG, Davis LL, Cates ME, Jewell ME, Ambrose SM, Lowe JS. Baclofen treatment for chronic posttraumatic stress disorder. Ann Pharmacother. 2003 Sep;37(9):1177–81. doi: 10.1345/aph.1C465. [DOI] [PubMed] [Google Scholar]
- 194.Grant BF, Stinson FS, Dawson DA, Chou SP, Dufour MC, Compton W, et al. Prevalence and co-occurrence of substance use disorders and independent mood and anxiety disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch Gen Psychiatry. 2004 Aug;61(8):807–16. doi: 10.1001/archpsyc.61.8.807. [DOI] [PubMed] [Google Scholar]
- 195.Garbutt JC, Kampov-Polevoy AB, Gallop R, Kalka-Juhl L, Flannery BA. Efficacy and safety of baclofen for alcohol dependence: a randomized, double-blind, placebo-controlled trial. Alcohol Clin Exp Res. 2010 Nov;34(11):1849–57. doi: 10.1111/j.1530-0277.2010.01273.x. [Randomized Controlled TrialResearch Support, N.I.H., Extramural] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Leggio L, Garbutt JC, Addolorato G. Effectiveness and safety of baclofen in the treatment of alcohol dependent patients. CNS Neurol Disord Drug Targets. 2010 Mar;9(1):33–44. doi: 10.2174/187152710790966614. [Review] [DOI] [PubMed] [Google Scholar]
- 197.Colloca L, Miller FG. How placebo responses are formed: a learning perspective. Philos Trans R Soc Lond B Biol Sci. 2011 Jun 27;366(1572):1859–69. doi: 10.1098/rstb.2010.0398. [Research Support, N.I.H., IntramuralReview] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Marsot A, Imbert B, Alvarez JC, Grassin-Delyle S, Jaquet I, Lancon C, et al. High Variability in the Exposure of Baclofen in Alcohol-Dependent Patients. Alcohol Clin Exp Res. 2013 Aug 22; doi: 10.1111/acer.12235. [DOI] [PubMed] [Google Scholar]
- 199.Leggio L, Zywiak WH, McGeary JE, Edwards S, Fricchione SR, Shoaff JR, et al. A human laboratory pilot study with baclofen in alcoholic individuals. Pharmacol Biochem Behav. 2013 Feb;103(4):784–91. doi: 10.1016/j.pbb.2012.11.013. [Research Support, Non-U.S. Gov't] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Johnson BA, Swift RM, Addolorato G, Ciraulo DA, Myrick H. Safety and efficacy of GABAergic medications for treating alcoholism. Alcohol Clin Exp Res. 2005 Feb;29(2):248–54. doi: 10.1097/01.alc.0000153542.10188.b0. [Congresses] [DOI] [PubMed] [Google Scholar]
- 201.Gallimberti L, Ferri M, Ferrara SD, Fadda F, Gessa GL. gamma-Hydroxybutyric acid in the treatment of alcohol dependence: a double-blind study. Alcohol Clin Exp Res. 1992 Aug;16(4):673–6. doi: 10.1111/j.1530-0277.1992.tb00658.x. [Clinical TrialRandomized Controlled Trial] [DOI] [PubMed] [Google Scholar]
- 202.Addolorato G, Castelli E, Stefanini GF, Casella G, Caputo F, Marsigli L, et al. An open multicentric study evaluating 4-hydroxybutyric acid sodium salt in the medium-term treatment of 179 alcohol dependent subjects. GHB Study Group. Alcohol Alcohol. 1996 Jul;31(4):341–5. doi: 10.1093/oxfordjournals.alcalc.a008160. [Multicenter StudyResearch Support, Non-U.S. Gov't] [DOI] [PubMed] [Google Scholar]
- 203.Ferrara SD, Zotti S, Tedeschi L, Frison G, Castagna F, Gallimberti L, et al. Pharmacokinetics of gamma-hydroxybutyric acid in alcohol dependent patients after single and repeated oral doses. Br J Clin Pharmacol. 1992 Sep;34(3):231–5. doi: 10.1111/j.1365-2125.1992.tb04129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Addolorato G, Cibin M, Caputo F, Capristo E, Gessa GL, Stefanini GF, et al. Gamma-hydroxybutyric acid in the treatment of alcoholism: dosage fractioning utility in non-responder alcoholic patients. Drug Alcohol Depend. 1998 Dec 1;53(1):7–10. doi: 10.1016/s0376-8716(98)00094-5. [Comparative StudyResearch Support, Non-U.S. Gov't] [DOI] [PubMed] [Google Scholar]
- 205.Addolorato G, Leggio L, Ferrulli A, Caputo F, Gasbarrini A. The therapeutic potential of gamma-hydroxybutyric acid for alcohol dependence: balancing the risks and benefits. A focus on clinical data. Expert Opin Investig Drugs. 2009 May;18(5):675–86. doi: 10.1517/13543780902905855. [Review] [DOI] [PubMed] [Google Scholar]
- 206.Weight FF. Cellular and molecular physiology of alcohol actions in the nervous system. Int Rev Neurobiol. 1992;33:289–348. doi: 10.1016/s0074-7742(08)60694-7. [Review] [DOI] [PubMed] [Google Scholar]
- 207.Lovinger DM, White G, Weight FF. Ethanol inhibits NMDA-activated ion current in hippocampal neurons. Science. 1989 Mar 31;243(4899):1721–4. doi: 10.1126/science.2467382. [Comparative StudyResearch Support, U.S. Gov't, P.H.S.] [DOI] [PubMed] [Google Scholar]
- 208.Peoples RW, Weight FF. Ethanol inhibition of N-methyl-D-aspartate-activated ion current in rat hippocampal neurons is not competitive with glycine. Brain Res. 1992 Feb 7;571(2):342–4. doi: 10.1016/0006-8993(92)90674-x. [DOI] [PubMed] [Google Scholar]
- 209.Lovinger DM. Developmental decrease in ethanol inhibition of N-methyl-D-aspartate receptors in rat neocortical neurons: relation to the actions of ifenprodil. J Pharmacol Exp Ther. 1995 Jul;274(1):164–72. [Research Support, U.S. Gov't, P.H.S.] [PubMed] [Google Scholar]
- 210.Grant KA, Colombo G. Discriminative stimulus effects of ethanol: effect of training dose on the substitution of N-methyl-D-aspartate antagonists. J Pharmacol Exp Ther. 1993 Mar;264(3):1241–7. [Research Support, U.S. Gov't, P.H.S.] [PubMed] [Google Scholar]
- 211.Hundt W, Danysz W, Holter SM, Spanagel R. Ethanol and N-methyl-D-aspartate receptor complex interactions: a detailed drug discrimination study in the rat. Psychopharmacology (Berl) 1998 Jan;135(1):44–51. doi: 10.1007/s002130050484. [Comparative StudyResearch Support, Non-U.S. Gov't] [DOI] [PubMed] [Google Scholar]
- 212.Krystal JH, Petrakis IL, Webb E, Cooney NL, Karper LP, Namanworth S, et al. Dose-related ethanol-like effects of the NMDA antagonist, ketamine, in recently detoxified alcoholics. Arch Gen Psychiatry. 1998 Apr;55(4):354–60. doi: 10.1001/archpsyc.55.4.354. [Clinical TrialRandomized Controlled TrialResearch Support, U.S. Gov't, Non-P.H.S.Research Support, U.S. Gov't, P.H.S.] [DOI] [PubMed] [Google Scholar]
- 213.Krupitsky EM, Neznanova O, Masalov D, Burakov AM, Didenko T, Romanova T, et al. Effect of memantine on cue-induced alcohol craving in recovering alcohol-dependent patients. Am J Psychiatry. 2007 Mar;164(3):519–23. doi: 10.1176/ajp.2007.164.3.519. [Comparative StudyRandomized Controlled TrialResearch Support, N.I.H., ExtramuralResearch Support, Non-U.S. Gov'tResearch Support, U.S. Gov't, Non-P.H.S.] [DOI] [PubMed] [Google Scholar]
- 214.Bienkowski P, Koros E, Kostowski W, Danysz W. Effects of N-methyl-D-aspartate receptor antagonists on reinforced and nonreinforced responding for ethanol in rats. Alcohol. 1999 Jun-Jul;(2-3):131–7. doi: 10.1016/s0741-8329(98)00075-5. [Research Support, Non-U.S. Gov't] [DOI] [PubMed] [Google Scholar]
- 215.Bisaga A, Evans SM. Acute effects of memantine in combination with alcohol in moderate drinkers. Psychopharmacology (Berl) 2004 Feb;172(1):16–24. doi: 10.1007/s00213-003-1617-5. [Clinical TrialRandomized Controlled TrialResearch Support, U.S. Gov't, P.H.S.] [DOI] [PubMed] [Google Scholar]
- 216.Nagy J. Renaissance of NMDA receptor antagonists: do they have a role in the pharmacotherapy for alcoholism? IDrugs. 2004 Apr;7(4):339–50. [Review] [PubMed] [Google Scholar]
- 217.Evans SM, Levin FR, Brooks DJ, Garawi F. A pilot double-blind treatment trial of memantine for alcohol dependence. Alcohol Clin Exp Res. 2007 May;31(5):775–82. doi: 10.1111/j.1530-0277.2007.00360.x. [Randomized Controlled Trial Research Support, N.I.H., Extramural] [DOI] [PubMed] [Google Scholar]
- 218.De Witte P, Littleton J, Parot P, Koob G. Neuroprotective and abstinence-promoting effects of acamprosate: elucidating the mechanism of action. CNS Drugs. 2005;19(6):517–37. doi: 10.2165/00023210-200519060-00004. [Research Support, Non-U.S. Gov'tReview] [DOI] [PubMed] [Google Scholar]
- 219.Blednov YA, Harris RA. Metabotropic glutamate receptor 5 (mGluR5) regulation of ethanol sedation, dependence and consumption: relationship to acamprosate actions. Int J Neuropsychopharmacol. 2008 Sep;11(6):775–93. doi: 10.1017/S1461145708008584. [Research Support, N.I.H., Extramural] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Mann K, Lehert P, Morgan MY. The efficacy of acamprosate in the maintenance of abstinence in alcohol-dependent individuals: results of a meta-analysis. Alcohol Clin Exp Res. 2004 Jan;28(1):51–63. doi: 10.1097/01.ALC.0000108656.81563.05. [Comparative StudyMeta-Analysis] [DOI] [PubMed] [Google Scholar]
- 221.Lhuintre JP, Daoust M, Moore ND, Chretien P, Saligaut C, Tran G, et al. Ability of calcium bis acetyl homotaurine, a GABA agonist, to prevent relapse in weaned alcoholics. Lancet. 1985 May 4;1(8436):1014–6. doi: 10.1016/s0140-6736(85)91615-0. [Clinical TrialControlled Clinical Trial] [DOI] [PubMed] [Google Scholar]
- 222.Paille FM, Guelfi JD, Perkins AC, Royer RJ, Steru L, Parot P. Double-blind randomized multicentre trial of acamprosate in maintaining abstinence from alcohol. Alcohol Alcohol. 1995 Mar;30(2):239–47. [Clinical TrialMulticenter StudyRandomized Controlled Trial] [PubMed] [Google Scholar]
- 223.Pelc I, Verbanck P, Le Bon O, Gavrilovic M, Lion K, Lehert P. Efficacy and safety of acamprosate in the treatment of detoxified alcohol-dependent patients. A 90-day placebo-controlled dose-finding study. Br J Psychiatry. 1997 Jul;171:73–7. doi: 10.1192/bjp.171.1.73. [Clinical TrialMulticenter StudyRandomized Controlled TrialResearch Support, Non-U.S. Gov't] [DOI] [PubMed] [Google Scholar]
- 224.Hammarberg A, Nylander I, Zhou Q, Jayaram-Lindstrom N, Reid MS, Franck J. The effect of acamprosate on alcohol craving and correlation with hypothalamic pituitary adrenal (HPA) axis hormones and beta-endorphin. Brain Res. 2009 Dec 11;1305(Suppl):S2–6. doi: 10.1016/j.brainres.2009.09.093. [Clinical TrialRandomized Controlled TrialResearch Support, Non-U.S. Gov't] [DOI] [PubMed] [Google Scholar]
- 225.Kiefer F, Witt SH, Frank J, Richter A, Treutlein J, Lemenager T, et al. Involvement of the atrial natriuretic peptide transcription factor GATA4 in alcohol dependence, relapse risk and treatment response to acamprosate. Pharmacogenomics J. 2011 Oct;11(5):368–74. doi: 10.1038/tpj.2010.51. [Research Support, Non-U.S. Gov't] [DOI] [PubMed] [Google Scholar]
- 226.Savas MC, Gullu IH. Disulfiram-ethanol test reaction: significance of supervision. Ann Pharmacother. 1997 Mar;31(3):374–5. doi: 10.1177/106002809703100325. [Case ReportsLetter] [DOI] [PubMed] [Google Scholar]
- 227.Suh JJ, Pettinati HM, Kampman KM, O'Brien CP. The status of disulfiram: a half of a century later. J Clin Psychopharmacol. 2006 Jun;26(3):290–302. doi: 10.1097/01.jcp.0000222512.25649.08. [Historical ArticleResearch Support, N.I.H., ExtramuralResearch Support, U.S. Gov't, Non-P.H.S.Review] [DOI] [PubMed] [Google Scholar]
- 228.de Sousa A. An open randomized study comparing disulfiram and acamprosate in the treatment of alcohol dependence. Alcohol Alcohol. 2005 Nov-Dec;40(6):545–8. doi: 10.1093/alcalc/agh187. [Comparative StudyRandomized Controlled Trial] [DOI] [PubMed] [Google Scholar]
- 229.Petrakis IL, Poling J, Levinson C, Nich C, Carroll K, Rounsaville B. Naltrexone and disulfiram in patients with alcohol dependence and comorbid psychiatric disorders. Biol Psychiatry. 2005 May 15;57(10):1128–37. doi: 10.1016/j.biopsych.2005.02.016. [Clinical TrialRandomized Controlled TrialResearch Support, U.S. Gov't, Non-P.H.S.] [DOI] [PubMed] [Google Scholar]
- 230.Rogers WK, Benowitz NL, Wilson KM, Abbott JA. Effect of disulfiram on adrenergic function. Clin Pharmacol Ther. 1979 Apr;25(4):469–77. doi: 10.1002/cpt1979254469. [Research Support, U.S. Gov't, P.H.S.] [DOI] [PubMed] [Google Scholar]
- 231.Petrakis IL, Poling J, Levinson C, Nich C, Carroll K, Ralevski E, et al. Naltrexone and disulfiram in patients with alcohol dependence and comorbid post-traumatic stress disorder. Biol Psychiatry. 2006 Oct 1;60(7):777–83. doi: 10.1016/j.biopsych.2006.03.074. [Comparative StudyRandomized Controlled TrialResearch Support, Non-U.S. Gov'tResearch Support, U.S. Gov't, Non-P.H.S.] [DOI] [PubMed] [Google Scholar]
- 232.Karamanakos PN, Pappas P, Stephanou P, Marselos M. Differentiation of disulfiram effects on central catecholamines and hepatic ethanol metabolism. Pharmacol Toxicol. 2001 Feb;88(2):106–10. [PubMed] [Google Scholar]
- 233.Krampe H, Ehrenreich H. Supervised disulfiram as adjunct to psychotherapy in alcoholism treatment. Curr Pharm Des. 2010;16(19):2076–90. doi: 10.2174/138161210791516431. [Review] [DOI] [PubMed] [Google Scholar]
- 234.Franck J, Jayaram-Lindstrom N. Pharmacotherapy for alcohol dependence: status of current treatments. Curr Opin Neurobiol. 2013 Jun 26; doi: 10.1016/j.conb.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 235.Tizabi Y, Bai L, Copeland RL, Jr., Taylor RE. Combined effects of systemic alcohol and nicotine on dopamine release in the nucleus accumbens shell. Alcohol Alcohol. 2007 Sep-Oct;42(5):413–6. doi: 10.1093/alcalc/agm057. [Research Support, N.I.H., Extramural] [DOI] [PubMed] [Google Scholar]
- 236.Chatterjee S, Bartlett SE. Neuronal nicotinic acetylcholine receptors as pharmacotherapeutic targets for the treatment of alcohol use disorders. CNS Neurol Disord Drug Targets. 2010 Mar;9(1):60–76. doi: 10.2174/187152710790966597. [Research Support, N.I.H., ExtramuralResearch Support, Non-U.S. Gov'tResearch Support, U.S. Gov't, Non-P.H.S.Review] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Ericson M, Lof E, Stomberg R, Soderpalm B. The smoking cessation medication varenicline attenuates alcohol and nicotine interactions in the rat mesolimbic dopamine system. J Pharmacol Exp Ther. 2009 Apr;329(1):225–30. doi: 10.1124/jpet.108.147058. [Research Support, Non-U.S. Gov't] [DOI] [PubMed] [Google Scholar]
- 238.Bito-Onon JJ, Simms JA, Chatterjee S, Holgate J, Bartlett SE. Varenicline, a partial agonist at neuronal nicotinic acetylcholine receptors, reduces nicotine-induced increases in 20% ethanol operant self-administration in Sprague-Dawley rats. Addict Biol. 2011 Jul;16(3):440–9. doi: 10.1111/j.1369-1600.2010.00309.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Steensland P, Simms JA, Holgate J, Richards JK, Bartlett SE. Varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, selectively decreases ethanol consumption and seeking. Proc Natl Acad Sci U S A. 2007 Jul 24;104(30):12518–23. doi: 10.1073/pnas.0705368104. [Research Support, Non-U.S. Gov't] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Mitchell JM, Teague CH, Kayser AS, Bartlett SE, Fields HL. Varenicline decreases alcohol consumption in heavy-drinking smokers. Psychopharmacology (Berl) 2012 Oct;223(3):299–306. doi: 10.1007/s00213-012-2717-x. [Randomized Controlled TrialResearch Support, N.I.H., ExtramuralResearch Support, Non-U.S. Gov't] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Fucito LM, Toll BA, Wu R, Romano DM, Tek E, O'Malley SS. A preliminary investigation of varenicline for heavy drinking smokers. Psychopharmacology (Berl) 2011 Jun;215(4):655–63. doi: 10.1007/s00213-010-2160-9. [Randomized Controlled TrialResearch Support, N.I.H., ExtramuralResearch Support, Non-U.S. Gov't] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Wouda JA, Riga D, De Vries W, Stegeman M, van Mourik Y, Schetters D, et al. Varenicline attenuates cue-induced relapse to alcohol, but not nicotine seeking, while reducing inhibitory response control. Psychopharmacology (Berl) 2011 Jul;216(2):267–77. doi: 10.1007/s00213-011-2213-8. [Research Support, Non-U.S. Gov't] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.McKee SA, Harrison EL, O'Malley SS, Krishnan-Sarin S, Shi J, Tetrault JM, et al. Varenicline reduces alcohol self-administration in heavy-drinking smokers. Biol Psychiatry. 2009 Jul 15;66(2):185–90. doi: 10.1016/j.biopsych.2009.01.029. [Randomized Controlled TrialResearch Support, N.I.H., ExtramuralResearch Support, U.S. Gov't, Non-P.H.S.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Plebani JG, Lynch KG, Rennert L, Pettinati HM, O'Brien CP, Kampman KM. Results from a pilot clinical trial of varenicline for the treatment of alcohol dependence. Drug Alcohol Depend. 2013 Dec 1;133(2):754–8. doi: 10.1016/j.drugalcdep.2013.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Litten RZ, Ryan ML, Fertig JB, Falk DE, Johnson B, Dunn KE, et al. A Double-Blind, Placebo-Controlled Trial Assessing the Efficacy of Varenicline Tartrate for Alcohol Dependence. J Addict Med. 2013 May 30; doi: 10.1097/ADM.0b013e31829623f4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Le AD, Funk D, Juzytsch W, Coen K, Navarre BM, Cifani C, et al. Effect of prazosin and guanfacine on stress-induced reinstatement of alcohol and food seeking in rats. Psychopharmacology (Berl) 2011 Nov;218(1):89–99. doi: 10.1007/s00213-011-2178-7. [Research Support, N.I.H., Extramural Research Support, N.I.H., Intramural] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Walker BM, Rasmussen DD, Raskind MA, Koob GF. alpha1-noradrenergic receptor antagonism blocks dependence-induced increases in responding for ethanol. Alcohol. 2008 Mar;42(2):91–7. doi: 10.1016/j.alcohol.2007.12.002. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Rasmussen DD, Alexander LL, Raskind MA, Froehlich JC. The alpha1-adrenergic receptor antagonist, prazosin, reduces alcohol drinking in alcohol-preferring (P) rats. Alcohol Clin Exp Res. 2009 Feb;33(2):264–72. doi: 10.1111/j.1530-0277.2008.00829.x. [Research Support, N.I.H., Extramural] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Fox HC, Anderson GM, Tuit K, Hansen J, Kimmerling A, Siedlarz KM, et al. Prazosin effects on stress- and cue-induced craving and stress response in alcohol-dependent individuals: preliminary findings. Alcohol Clin Exp Res. 2012 Feb;36(2):351–60. doi: 10.1111/j.1530-0277.2011.01628.x. [Randomized Controlled TrialResearch Support, N.I.H., ExtramuralResearch Support, Non-U.S. Gov't] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Simpson TL, Saxon AJ, Meredith CW, Malte CA, McBride B, Ferguson LC, et al. A pilot trial of the alpha-1 adrenergic antagonist, prazosin, for alcohol dependence. Alcohol Clin Exp Res. 2009 Feb;33(2):255–63. doi: 10.1111/j.1530-0277.2008.00807.x. [DOI] [PubMed] [Google Scholar]
- 251.Heilig M, Thorsell A, Sommer WH, Hansson AC, Ramchandani VA, George DT, et al. Translating the neuroscience of alcoholism into clinical treatments: from blocking the buzz to curing the blues. Neurosci Biobehav Rev. 2010 Nov;35(2):334–44. doi: 10.1016/j.neubiorev.2009.11.018. [Research Support, U.S. Gov't, Non-P.H.S.Review] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Gehlert DR, Cramer J, Morin SM. Effects of corticotropin-releasing factor 1 receptor antagonism on the hypothalamic-pituitary-adrenal axis of rodents. J Pharmacol Exp Ther. 2012 Jun;341(3):672–80. doi: 10.1124/jpet.111.189753. [DOI] [PubMed] [Google Scholar]
- 253.Gehlert DR, Cippitelli A, Thorsell A, Le AD, Hipskind PA, Hamdouchi C, et al. 3-(4-Chloro-2-morpholin-4-yl-thiazol-5-yl)-8-(1-ethylpropyl)-2,6-dimethyl-imidazo [1,2-b]pyridazine: a novel brain-penetrant, orally available corticotropin-releasing factor receptor 1 antagonist with efficacy in animal models of alcoholism. J Neurosci. 2007 Mar 7;27(10):2718–26. doi: 10.1523/JNEUROSCI.4985-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Zorrilla EP, Heilig M, de Wit H, Shaham Y. Behavioral, biological, and chemical perspectives on targeting CRF(1) receptor antagonists to treat alcoholism. Drug Alcohol Depend. 2013 Mar 1;128(3):175–86. doi: 10.1016/j.drugalcdep.2012.12.017. [Research Support, N.I.H., ExtramuralResearch Support, N.I.H., Intramural] [DOI] [PMC free article] [PubMed] [Google Scholar]


