Abstract
Background
Retinal detachment (RD) with proliferative vitreoretinopathy (PVR) often requires surgery to restore normal anatomy and to stabilize or improve vision. PVR usually occurs in association with recurrent RD (that is, after initial retinal re-attachment surgery) but occasionally may be associated with primary RD. Either way, a tamponade agent (gas or silicone oil) is needed during surgery to reduce the rate of postoperative recurrent RD.
Objectives
The objective of this review was to assess the relative safety and effectiveness of various tamponade agents used with surgery for retinal detachment (RD) complicated by proliferative vitreoretinopathy (PVR).
Search methods
We searched CENTRAL (which contains the Cochrane Eyes and Vision Group Trials Register) (The Cochrane Library 2013, Issue 5), Ovid MEDLINE, Ovid MEDLINE In-Process and Other Non-Indexed Citations, Ovid MEDLINE Daily, Ovid OLDMEDLINE (January 1946 to June 2013), EMBASE (January 1980 to June 2013), Latin American and Caribbean Literature on Health Sciences (LILACS) (January 1982 to June 2013), the metaRegister of Controlled Trials (mRCT) (www.controlled-trials.com), ClinicalTrials.gov (www.clinicaltrials.gov) and the WHO International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp/search/en). We did not use any date or language restrictions in the electronic searches for trials. We last searched the electronic databases on 26 June 2013.
Selection criteria
We included randomized controlled trials (RCTs) of participants undergoing surgery for RD associated with PVR that compared various tamponade agents.
Data collection and analysis
Two review authors screened the search results independently. We used the standard methodological procedures expected by The Cochrane Collaboration.
Keywords: Fluorocarbons [*administration & dosage]; Randomized Controlled Trials as Topic; Recurrence [prevention & control]; Retinal Detachment [etiology; prevention & control; *therapy]; Silicone Oils [*administration & dosage]; Sulfur Hexafluoride [*administration & dosage]; Vitreoretinopathy, Proliferative [*complications]; Humans
Main results
The review included 516 participants from three RCTs. One study was conducted in the USA and consisted of two trials: the first trial randomized 151 adults to receive either silicone oil or sulfur hexafluoride (SF6) gas tamponades; and the second trial randomized 271 adults to receive either silicone oil or perfluropropane (C3F8) gas tamponades. The third trial was a multi-center international trial and randomized 94 participants (age range not specified) to receive heavy silicone oil (a mixture of perfluorohexyloctane (F6H8) and silicone oil) versus standard silicone oil (either 1000 centistokes or 5000 centistokes, per the surgeon’s preference).
In participants with RD associated with PVR, outcomes after pars plana vitrectomy and infusion of either silicone oil, perfluropropane gas, or sulfur hexafluoride gas appeared comparable for a broad variety of cases. There were no significant differences between silicone oil and perfluoropropane gas in terms of the proportion of participants achieving at least 5/200 visual acuity (risk ratio (RR) 0.97; 95% confidence interval (CI) 0.73 to 1.31) or achieving macular attachment (RR 1.00; 95% CI 0.86 to 1.15) at a minimum of one year. Although sulfur hexafluoride gas was reported to be associated with significantly worse anatomic and visual outcomes than was silicone oil at one year (quantitative data not reported), there were no significant differences between silicone oil and sulfur hexafluoride gas in terms of achieving at least 5/200 visual acuity at two years (RR 1.57; 95% CI 0.93 to 2.66). For macular attachment, participants treated with silicone oil received significantly more favourable outcomes than did participants who received sulfur hexafluoride at both one year (quantitative data not reported) and two years (RR 1.37; 95% CI 1.01 to 1.86). The first two trials did not perform any sample size calculation or power detection. In the third trial, which had a power of 80% to detect differences, heavy silicone oil was not shown to be superior to standard silicone oil. There were no significant differences between standard silicone oil and heavy silicone oil in the change in visual acuity at one year using adjusted mean logMAR visual acuity (mean difference -0.03 logMAR; 95% CI -0.35 to 0.29). Adverse events were not reported for the first two trials. For the third trial, only the total number of adverse events was reported, and adverse events for each group were not specified. Of the 94 participants, four died, 26 had recurrent retinal detachment, 22 developed glaucoma, four developed a cataract, and two had capsular fibrosis.
All three trials employed adequate methods for random sequence generation and allocation concealment. None of the trials employed masking of participants and surgeons, and only the third trial masked outcome assessors. The first trial had a large portion of participants excluded from the final analyses, while the other two trials were at low risk of attrition bias. All trials appear to be free of reporting bias. The first two trials were funded by the National Eye Institute, and the third trial was funded by the German Research Foundation.
Authors’ conclusions
The use of either perfluropropane or standard silicone oil appears reasonable for most patients with RD associated with PVR. Because there do not appear to be any major differences in outcomes between the two agents, the choice of a tamponade agent should be individualized for each patient. Heavy silicone oil, which is not available for routine clinical use in the USA, has not demonstrated evidence of superiority over standard silicone oil.
PLAIN LANGUAGE SUMMARY
Tamponade in surgery for retinal detachment associated with proliferative vitreoretinopathy
Review question
We reviewed the effect of tamponade agents used in surgery involving pars plana vitrectomy in participants with retinal detachment (RD) associated with proliferative vitreoretinopathy (PVR).
Background
The retina is the light-sensing tissue in the back of the eye (similar to the film within a camera), and its normal function depends on its attachment to an underlying layer called the retinal pigment epithelium (RPE). RD, a physical separation of the retina from the RPE, remains an important cause of visual loss. The macula is the centermost part of the retina and is responsible for central vision and perception of fine details. RD may or may not involve the macula, but patients with macular detachment typically have more severe visual loss than patients without macular detachment. RD is generally treated with surgery, but surgery is not always successful. In some patients surgery is initially successful but RD may recur months or years later. Most recurrent RDs, and some primary RDs, are associated with varying degrees of PVR, or the growth of fibrous membranes (similar to scar tissue) along the surface of the retina. The only proven therapy for RD with PVR is further surgery; where the membranes must be physically removed from the surface of the retina. In addition, injection of a material to hold the newly attached retina in position, called a tamponade agent, is performed to reduce the rate of fluid flow through open retinal tears, which would cause recurrent RD. The major tamponade agents that are available today are various gases and silicone oils.
Study characteristics
We found three randomized controlled trials (RCTs) involving 516 participants that compared tamponade agents. All participants underwent surgery to treat RD associated with PVR. The Silicone Study compared the use of silicone oil to either sulfur hexafluoride (SF6) gas or perfluropropane (C3F8) gas in two RCTs. Both gases and silicone oil are less dense than water, so that they float upwards or towards the top of the eye while a patient is sitting or standing. This is sometimes but not always beneficial, so a denser-than-water silicone oil called heavy silicone oil has been investigated, primarily outside the US. The Heavy Silicone Oil Study compared the use of heavy silicone oil to standard silicone oil in participants with RD involving the lower parts of the retina. The evidence was current to June 2013.
Key results
When silicone oil was compared to SF6 gas, eyes randomized to receive silicone oil more often achieved a visual acuity of 5/200 or better at one year, and more often achieved macular attachment at one year but with no difference at two years. When silicone oil was compared with C3F8 gas, there were no significant differences between the groups with respect to visual acuity or macular attachment at one year. When heavy silicone oil was compared to standard silicone oil, there were no significant differences between the groups with respect to retinal re-attachment or visual acuity at one year. Heavy silicone oil did not demonstrate any significant benefit over standard silicone oil. Adverse events were only reported for the Heavy Silicone Oil Study; however, only the total number of adverse events was reported, and the numbers for each group were not specified: of the 94 participants, there were four deaths, 26 recurrent RDs, 22 patients with glaucoma, four patients with cataract, and two patients with capsular fibrosis (scarring behind a lens implant).
Quality of evidence
The overall quality of these studies was moderately satisfactory. Although all trials employed proper randomization methods for participants, the masking of participants was unclear in all of the three RCTs, and masking of outcome assessors was not performed in two RCTs.
BACKGROUND
Description of the condition
Introduction
Retinal detachment (RD) remains a significant cause of vision loss. A variety of surgical techniques are available to treat RD. For primary RD, these procedures have a very high rate of successful anatomic retinal reattachment (overall above 90%) (Schwartz 2004). The Scleral Buckling versus Primary Vitrectomy in Rhegmatogenous Retinal Detachment (SPR) study, which excluded many relatively straightforward cases, reported single operation success rates between 60% to 80%, depending on the subgroup, and 73% overall (Heimann 2007). Most recurrent RDs, and some primary RDs, are associated with varying degrees of proliferative vitreoretinopathy (PVR), or the growth of fibrous membranes (similar to scar tissue) along the surface of the retina, which leads to traction on the retina (TRSTC 1983).
Epidemiology
Recurrent RD with PVR occurs in about 5% to 10% of patients (Charteris 2002). Major risk factors for recurrent RD with PVR include RD in the inferior (lower) portion of the eye (Singh 1986), severe ocular trauma (Kruger 2002), and giant retinal tears (Scott 2002). Other reported risk factors for recurrent RD with PVR include the inability to identify a retinal break, the use of pars plana vitrectomy in the initial repair, preoperative PVR, preoperative choroidal detachment, and a relatively greater use of cryopexy (Cowley 1989). Recurrent RD with PVR may require multiple additional surgeries and is associated with poorer visual outcomes. These additional surgeries are associated with significantly increased costs (Patel 2004). Some patients with primary RD may also present with PVR; risk factors include large or giant retinal tears, longstanding RD, and other factors (Garweq 2013).
Presentation and diagnosis
PVR is usually diagnosed within the first few months after RD surgery. Symptoms include decreased vision in the affected eye. The diagnosis is made by dilated fundus examination in the office or outpatient clinic.
Description of the intervention
Vitreoretinal surgery is standard treatment for RD with PVR. Pars plana vitrectomy (PPV), removal of the epiretinal membranes; treatment of the retinal breaks; and injection of a tamponade agent are performed. In some cases, removal of the lens (either the crystalline lens or a previously placed intraocular lens) is performed. Tamponade is necessary to reduce the rate of fluid flow through open retinal tears, which would cause recurrent RD. The major tamponade agents available today are various gases and silicone oils. Currently available gases include air, sulfur hexafluoride (SF6), hexafluroethane (C2F6), and perfluropropane (C3F8). The major advantage of gas tamponade is that the gas spontaneously dissipates, usually over several weeks. Currently available silicone oils come in 1000 centistoke and 5000 centistoke viscosities. Silicone oil is permanent and may eventually require surgical removal.
There are several investigational tamponade agents, including polydimethylsiloxane (PDMS) 1000 (Tognetto 2005), perfluorohexylethan (O62) (Hoerauf 2005), perfluoro-n-octane (PFO) (Rofail 2005), a mixture of perfluorohexyloctane (F6H8) in silicone oil (Stappler 2008), and a mixture of perfluorohexyloctane (F6H8) in PDMS 1000 (Heimann 2008; Tognetto 2008). Various tamponade agents with a specific gravity greater than that of water have shown evidence of toxicity in animal models, in rat retinal cell cultures in vitro, and in clinical reports (Eckardt 1990; Matteucci 2007; Singh 2001). These investigational agents are not available for routine clinical use in the USA.
Tamponade agents are useful in four broad categories of patients with RD.
Patients with primary RD, treated with PPV as a first-line procedure. These patients are usually treated with gas tamponade rather than silicone oil.
Patients with complex or recurrent RD associated with PVR. These patients are the focus of this review. These patients are typically treated with either gas or silicone oil.
Patients with RD associated with a giant retinal tear. These patients are treated with either gas or silicone oil.
Patients with inferior RD, treated with PPV as a first-line procedure. Some surgeons use heavy liquids, such as PFO or heavy silicone oil, as investigational agents in these patients.
How the intervention might work
Tamponade agents are believed to work by reducing or eliminating fluid vectors through open retinal breaks until the applied retinopexy (typically photocoagulation or cryopexy) creates a permanent seal. Gases such as SF6 and C3F8 spontaneously dissipate, while silicone oil is permanent and may eventually require removal.
Why it is important to do this review
The various tamponade agents offer different advantages and disadvantages in terms of safety and effectiveness (Krzystolik 2000; Young 2005). A systematic review may assist surgeons in the selection of a tamponade agent.
OBJECTIVES
The objective of this review was to assess the relative safety and effectiveness of various tamponade agents used with surgery for retinal detachment (RD) complicated by proliferative vitreoretinopathy (PVR).
The specific comparisons depended on the trials we identified in the search. The secondary objectives of the review were to examine quality of life measures such as patient satisfaction and subjective visual improvement, and to summarize economic data such as direct and indirect costs of surgery and rehabilitation. We intended to compare:
the various gas tamponade agents with each other;
the two silicone oil preparations to each other;
the various gas agents versus the various silicone oils;
the established agents (gases, silicone oil) versus the investigational agents.
METHODS
Criteria for considering studies for this review
Types of studies
We included randomized controlled trials only. We set no limitations on the various treatment arms compared.
Types of participants
We included trials in which participants underwent surgical repair of RD associated with PVR. We employed no restrictions with respect to age or cause of RD.
Types of interventions
We included trials which studied agents used as tamponade in the treatment of RD associated with PVR, such as air, sulfur hexafluoride (SF6), hexafluroethane (C2F6), perfluropropane (C3F8), and silicone oil, as well as investigational agents such as heavy silicone oil (polydimethylsiloxane 1000), perfluorohexylethan (O62), and perfluoro-n-octane (PFO).
Types of outcome measures
Primary outcomes
The primary outcome for this review was visual acuity at one year. We analyzed outcomes at additional times of follow-up as reported in the included trials. We intended to compare visual acuity as a dichotomous outcome (the proportion of participants who lost three or more lines of logMAR visual acuity; participants who lost one or two lines of logMAR visual acuity were considered stabilized) and also as a continuous outcome (mean logMAR scores). We considered other dichotomous and continuous visual acuity outcomes at other time points as reported in the included trials.
Secondary outcomes
The secondary outcome for this review was macular attachment at one year. This was chosen because in some patients with PVR complete retinal re-attachment is not possible, but macular attachment yields generally better visual results than does persistent macular detachment. We also presented secondary outcomes measured at other time points as reported in the included trials.
Adverse effects (severe and minor)
Severe
Retina detached at one year
Visual acuity worse than 20/200 (regardless of anatomic outcome)
Minor
Intraocular pressure (IOP) greater than 21 mmHg
Visually significant cataract
Quality of life measures
We intended to examine patient satisfaction, subjective visual improvement, and other quality of life measures evaluated using a validated scale.
Economic data
We intended to summarize direct and indirect costs of surgery and rehabilitation and any other economic data in the included studies.
Follow-up
We restricted studies to those with at least one year of follow-up. We believe that shorter follow-up periods are less clinically relevant.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) (which contains the Cochrane Eyes and Vision Group Trials Register) 2013, Issue 5, part of The Cochrane Library (www.thecochranelibrary.com) (accessed 26 June 2013), Ovid MEDLINE, Ovid MEDLINE In-Process and Other Non-Indexed Citations, Ovid MEDLINE Daily, Ovid OLDMEDLINE (January 1946 to June 2013), EMBASE (January 1980 to June 2013), Latin American and Caribbean Health Sciences Literature Database (LILACS) (January 1982 to June 2013), the metaRegister of Controlled Trials (mRCT) (www.controlled-trials.com), ClinicalTrials.gov (www.clinicaltrials.gov) and the WHO International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp/search/en). We did not use any date or language restrictions in the electronic searches for trials. We last searched the electronic databases on 27 June 2013.
See: Appendices for details of search strategies for CENTRAL (Appendix 1), MEDLINE (Appendix 2), EMBASE (Appendix 3), LILACS (Appendix 4), mRCT (Appendix 5), ClinicalTrials.gov (Appendix 6) and the ICTRP (Appendix 7).
Searching other resources
We searched the reference lists of the studies included in the review for other potential inclusions. We did not search conference proceedings for the purpose of this review. Although we initially did not intend to contact individuals or organizations specifically for this review, because we did not believe that doing so would add significantly to the data obtainable through published trials, we contacted the investigators of included studies for clarification of methods and other data reported in published manuscripts.
Data collection and analysis
Selection of studies
At least two authors, working independently, reviewed the titles and abstracts resulting from the searches. Two authors reviewed the full-text manuscripts of all possibly or definitely relevant studies to determine eligibility for inclusion. We resolved any discrepancies through discussion when screening titles and abstracts and assessing the eligibility for full-text reports. We did not mask trial details in this process. For unclear information, we contacted the study investigators for further clarification. For the studies we excluded during full-text assessment, we recorded them and described the reasons for exclusion in the ’Characteristics of excluded studies’ table.
Data extraction and management
Extraction of study characteristics
We extracted the following information for each trial.
Methods: method of allocation, masking (blinding), exclusions after randomization, losses to follow-up and compliance, unusual study design.
Participants: country where participants enrolled, number randomized, age, sex, inclusion and exclusion criteria.
Interventions: test intervention, comparison intervention (control), duration of intervention.
Outcomes: visual acuity, macular attachment, complication rates, adverse effects, quality of life, and economic outcomes.
Notes: additional details (such as funding sources).
Data extraction and entry
Two authors, working independently, extracted data using a paper data extraction form developed and piloted by the Cochrane Eyes and Vision Group. We resolved discrepancies by discussion. One review author entered the data into RevMan 5.2 (RevMan 2012) and a second author verified the data entry. The main outcome measures were visual acuity, macular attachment, and various complication rates. This included dichotomous data (such as retinal detachment, proportion of participants who lost three or more lines of logMAR visual acuity) as well as continuous data (such as mean logMAR visual acuity).
Assessment of risk of bias in included studies
We reviewed the risk of bias of included studies as outlined in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). At least two authors assessed the risk of bias for each included study according to the following criteria.
Selection bias (randomized sequence generation and allocation concealment).
Performance bias (masking of participants and researchers).
Attrition bias (incomplete outcome data adequately addressed).
Detection bias (masking of outcome assessors).
Reporting bias (free of selective outcome reporting).
We judged each area of potential bias as low risk of bias, high risk of bias, or unclear risk of bias. We considered methods such as central randomization and use of sequential opaque envelopes as evidence of adequate allocation concealment. We evaluated any exclusions after randomization, losses to follow-up and differential reasons for losses to follow-up in the treatment groups. Any discrepancies were resolved through discussion.
We recognized that masking of participants and surgeons (performance bias) and masking of persons assessing retinal detachment (detection bias) may not be possible in studies comparing gas to silicone oil. However, studies that had successfully masked outcome data (such as studies in which visual acuity was measured by an examiner masked to the tamponade agent) were emphasized.
Measures of treatment effect
We reported unpooled risk ratios (RRs) with 95% confidence intervals (CIs) for the dichotomous outcomes of visual acuity and macular attachment for Silicone Study 1992a and Silicone Study 1992b; RR with 95% CI for recurrent RDs and mean difference (MD) with 95% CI for visual acuity for HSO Study. If other continuous outcomes are included in future updates of the review, we will calculate mean differences or standardized mean differences depending on the types of measurement scales.
We initially intended to compare ’all gases’ (that is, SF6, C2F6, and C3F8) versus silicone oil, but the included studies did not compare tamponade agents in this manner. Specifically, the Silicone Study conducted two trials, one comparing silicone oil (1000 centistokes) with SF6, and one comparing silicone oil (1000 centistokes) with C3F8. Accordingly, this review used the same comparisons.
Unit of analysis issues
The unit of analysis for outcomes was eyes of individuals. All three trials included only one eye per participant. For future updates of the review, if a trial randomized one eye to one tamponade group and the other eye of the same person to the other group, we will only include such a trial design when it appropriately considered intra-person correlation in their analyses. We will refer to the guidelines in Chapter 9 of the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2011).
Dealing with missing data
We contacted the primary authors of the included studies to provide 12 month visual acuity and macula status outcome data when not reported in the published papers. We did not impute data for this review, but we will consider imputation for future updates of the review and discuss the assumptions made during imputation.
Assessment of heterogeneity
We intended to assess for statistical heterogeneity using the Chi2 test and the I2 statistic, but since no pooled estimates were included these assessments of heterogeneity were not applicable. If data synthesis is considered at the time of an update to this review, we will follow the following guidelines. We will consider an I2 value greater than 50% to indicate substantial statistical heterogeneity. In such a situation we will not report a pooled estimate. We also will not report a pooled estimate when clinical or methodological heterogeneity (from details listed in the table ’Characteristics of included studies’) is detected. Instead, we will report a narrative or tabulated summary of the included studies. We will use a random-effects model to incorporate the heterogeneity if the I2 value is less than 50%, unless there are fewer than three studies. If we detect no statistical heterogeneity (I2 value of 0) or there are fewer than three studies, we will use a fixed-effect model.
Assessment of reporting biases
We assessed selective outcome reporting by comparing outcomes listed in the protocol of the trials and the outcomes analyzed in the final published report. For future updates of the review, when the protocol is not available we will compare the outcomes prespecified in the methods section and outcomes analyzed in the results section, and will follow the guidelines in Chapter 10 of the Cochrane Handbook for Systematic Review of Interventions (Sterne 2011). We planned to examine funnel plots from each meta-analysis to assess reporting bias when at least 10 studies are included.
Data synthesis
No pooled estimates of included studies are reported. If pooled estimates are considered for future updates of the review, we will follow the guidelines in Chapter 9 of the Cochrane Handbook for Systematic Review of Interventions (Deeks 2011).
Subgroup analysis and investigation of heterogeneity
We will consider subgroup analyses, as appropriate, in future updates of this review and will consult the guidelines for investigating heterogeneity in Chapter 9 of the Cochrane Handbook for Systematic Review of Interventions (Deeks 2011). One possible strategy is to divide participants by surgical history, such as participants with chronic RD with PVR and no previous surgery, participants with recurrent RD following scleral buckling only, and participants with recurrent RD following PPV and previous intravitreal tamponade (gas or oil). Another possible strategy is to divide participants with certain high-risk clinical features, such as participants with giant retinal tear, participants with open-globe trauma, and participants under 18 years of age.
Sensitivity analysis
We planned to examine the impact of the exclusion of unpublished and industry-funded studies in sensitivity analyses, but these exclusions were not applicable to the current systematic review.
RESULTS
Description of studies
Results of the search
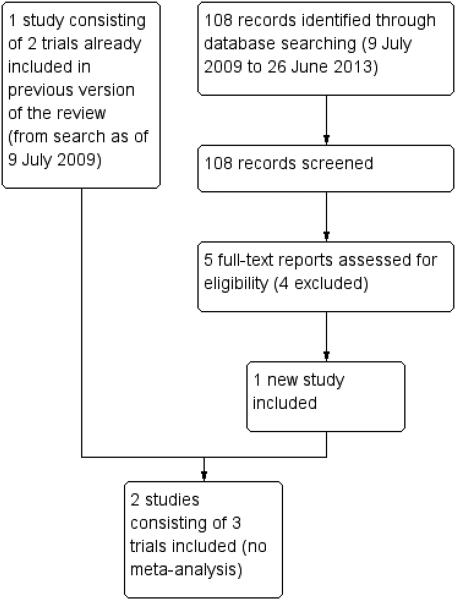
Our initial literature search yielded a total of 348 titles and abstracts. After independent review of the references by two review authors, we identified 19 studies which appeared to be relevant. After retrieving and reviewing of the full text, we found one study consisting of two trials that met our inclusion criteria and recorded one ongoing study. An updated search conducted on 26 June 2013 yielded another 102 titles and abstracts, one registered trial from the ISRCTN Register and five records from ClinicalTrials.gov. After independent review of the records, we identified five potentially relevant studies. After retrieval and review of the full-text reports, one additional study was considered eligible (HSO Study). A study flow chart is shown in Figure 1.
Figure 1.
Results from searching for studies for inclusion in the review.
Included studies
We identified two studies that met our inclusion criteria. One study consisted of two trials conducted in the USA. Enrollment for the first trial comparing silicone oil to SF6 gas occurred from September 1985 to September 1987 (Silicone Study 1992a). For the second part of the study period, SF6 gas was replaced with the longer-lasting C3F8 gas. Enrollment for the second trial comparing silicone oil to C3F8 occurred between September 1987 to October 1990 (Silicone Study 1992b). Participants aged 18 years or older and with RD associated with PVR were offered randomization. One eye per patient was randomized and grouped as eyes that had not undergone prior vitrectomy (Group 1) or eyes that had undergone vitrectomy but without silicone oil injection (Group 2). The first trial included 113 eyes in Group 1 and 38 eyes in Group 2; the second trial included 132 eyes in Group 1 and 139 eyes in Group 2. The exclusion criteria were uncontrolled concomitant eye disease, a history of blunt trauma within three months of entry into the study, a history of penetrating trauma, a giant retinal tear of 90 ° or greater, proliferative diabetic retinopathy, and any medical condition that could preclude participation in a three year study.
The other study, the Heavy Silicone Oil Study (HSO Study), compared vitrectomy with heavy silicone oil (a mixture of perfluorohexyloctane (F6H8) and silicone oil) versus standard silicone oil (either 1000 centistokes or 5000 centistokes, per the surgeon’s preference) and was performed between December 2003 and February 2008. The HSO Study was a multi-center study conducted in Germany, Austria, Sweden, Great Britain, China, Poland, Portugal, the Netherlands, Italy, Hungary, and USA. Ninety-four participants with RD associated with inferior and posterior PVR or inferior RD with inferior giant retinal tear were randomized into the two intervention groups, with 46 participants in the heavy silicone oil group and 48 in the standard silicone oil group. The exclusion criteria included: RD associated with superior anterior PVR; superior giant retinal tear; retinotomies, holes or tears between 10 and 2 o’clock; diabetic retinopathy requiring treatment; glaucoma resulting in visual field defects requiring treatment; no written informed consent; age below 18 years; participation in another clinical trial; or pregnancy. An American Academy of Ophthalmology (AAO) abstract (Oncel 2006) referenced a study that may be relevant to this review, and that is listed in the ’Characteristics of studies awaiting classification’ table until further details are reported.
The Silicone Study was funded by the National Eye Institute, National Institutes of Heath, US, and the HSO Study was funded by the German Research Foundation (Deutsche Forschungsgemeinschaft). None of the study investigators reported declaration of interests.
Excluded studies
We excluded 20 studies and listed them in the ’Characteristics of excluded studies’ table with reasons for exclusion. Twelve of the 20 studies were not randomized controlled trials.
Risk of bias in included studies
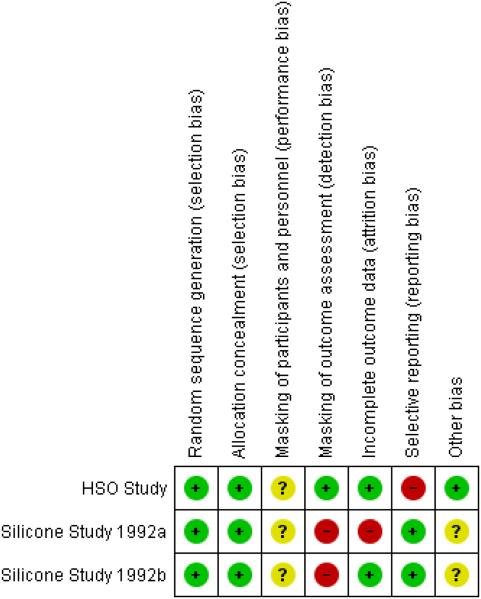
Two studies, including two trials reported by the Silicone Study investigators, met the inclusion criteria for this review (HSO Study; Silicone Study 1992a; Silicone Study 1992b). Since the two trials of the Silicone Study were part of the same study protocol, they follow the same design, methods, and analyses (Azen 1991 in Silicone Study 1992a). Both the Silicone Study and HSO Study were of good methodological quality and at low risk of bias (Figure 2) except that whether the participants were masked was not reported explicitly in any of these trials and the Silicone Study did not mask all outcome assessors.
Figure 2.
Methodological quality summary: review authors’ judgements about each methodological quality item for each included study.
Allocation
For the Silicone Study, the randomization scheme was administered centrally through the Data Coordinating Center and employed stratification and blocking to ensure equal treatment assignments within each clinical center. Treatment allocation was adequately concealed with sequential opaque envelopes delivered to each study site and opened at the time of tamponade injection. For the HSO Study, randomization was generated using permuted blocks of varying sizes, stratified by surgeon. Treatment allocation was adequately concealed with sealed envelopes opened after study enrollment.
Masking (performance bias and detection bias)
None of the trials reported masking of participants or surgeons. The study outcome assessors and surgeons were not masked for the two trials of the Silicone Study but were masked in the HSO Study.
Incomplete outcome data
For the Silicone Study, the last observation carried forward method was used for missing data. Data were imputed for participants who missed intermediate examinations, but attended prior and subsequent examinations, only when findings were deemed consistent. In the event that a retinal detachment recurred during the study period and required surgery, participants were analysed using the original random treatment allocation. Randomized participants from a study centre that ceased recruitment during the study period were excluded from the analysis (12 out of 151 participants from Silicone Study 1992a and six out of 271 participants from Silicone Study 1992b). However, the first trial (Silicone Study 1992a) also excluded 38 participants that had previous vitrectomy from the final analyses, therefore, almost a third of participants in this study did not contribute to any outcome data (51 participants out of 151), so we assessed the risk of bias as high.
For the HSO Study (HSO Study), participants who did not satisfy the major inclusion criteria but were already randomized (performed preoperatively) were all included in the full analysis set; three participants in the heavy silicone oil group and five participants in the standard silicone group fulfilled intraoperative exclusion criteria. One participant was excluded from analysis due to a lack of pre- and post-surgical assessment and data (only randomization sheet present).
Selective reporting
Both the trials from the Silicone Study (Silicone Study 1992a; Silicone Study 1992b) appeared to be free of selective reporting since the primary and secondary outcomes were published a priori in their respective methods paper (Azen 1991 in Silicone Study 1992a). However, in the methods paper for the HSO Study (Joussen 2007 in HSO Study), it pre-specified to measure quality of life outcomes, but no data were reported. Therefore, we assessed the reporting bias as low for the Silicone Study and high for the HSO Study.
Other potential sources of bias
Fourteen baseline characteristics were compared between treatment arms in the Silicone Study (age, sex, study eye, prior scleral buckle, other ocular surgery, mean duration of RD, Retina Society classification, visual acuity, refractive status, IOP, corneal status, aqueous flare, aqueous cell, and neovascularization). The Silicone Study investigators reported one statistically significant difference in baseline characteristics between the eyes of participants assigned to receive SF6 gas and those assigned to receive silicone oil (Silicone Study 1992a). The estimated duration of RD was greater in Group 2 eyes (eyes of participants with prior vitrectomy but without silicone oil injection) randomized to SF6 compared to Group 2 eyes randomized to silicone oil. We identified no other potential bias for the HSO Study.
Effects of interventions
Silicone oil versus gas tamponades
The Silicone Study conducted two trials, one comparing silicone oil (1000 centistokes) with SF6 and one comparing silicone oil (1000 centistokes) with C3F8. Below we have described results for each outcome we pre-specified in the methods section of this review. For the first trial comparing silicone oil (1000 centistokes) with SF6, the study investigators performed statistical analyses only on non-vitrectomized eyes (group 1) because the sample size of eyes that had already undergone vitrectomy (group 2) was small (38 participants).
Visual acuity
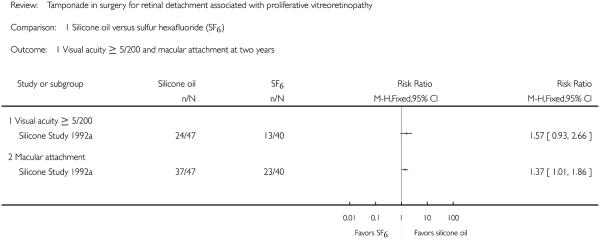
We intended to compare visual acuity as a dichotomous outcome (the proportion of participants who lost three or more lines of logMAR visual acuity; participants who lost one or two lines of logMAR visual acuity were considered stabilized) and also as a continuous outcome (mean logMAR scores); however, no studies reported the proportion of participants who lost three or more lines of visual acuity, instead, participants achieving 5/200 or better visual acuity were reported for both groups. The cut-off point of 5/200 was chosen because 5/200 is considered ’ambulatory vision’ (enough vision not to bump into large objects while walking) and is used in some clinical trials with severe diseases and generally bad outcomes. The Silicone Study recorded visual acuity using the Diabetic Retinopathy Vitrectomy Study protocol and charts. Two trials including 352 eyes of 352 participants contributed to this outcome at 24 months (87 eyes in Silicone Study 1992a) or at the last follow-up evaluation (265 eyes in Silicone Study 1992b). When silicone oil was compared with SF6, the study investigators reported that eyes that had not undergone prior vitrectomy (Group 1) and were randomized to receive silicone oil more often achieved a visual acuity of 5/200 or better at one year (P < 0.05; visual acuity data not reported), but there was no statistically significant difference between the groups at two years (RR 1.57; 95% CI 0.93 to 2.66) (Figure 3).
Figure 3.
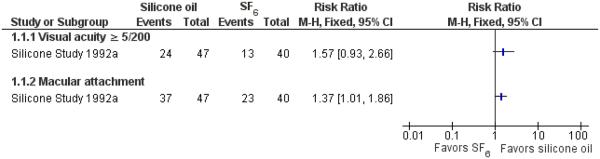
Forest plot of comparison: 1 Silicone oil versus SF6, outcome: 1.1 Visual acuity ≥ 5/200 and macular attachment at 24 months.
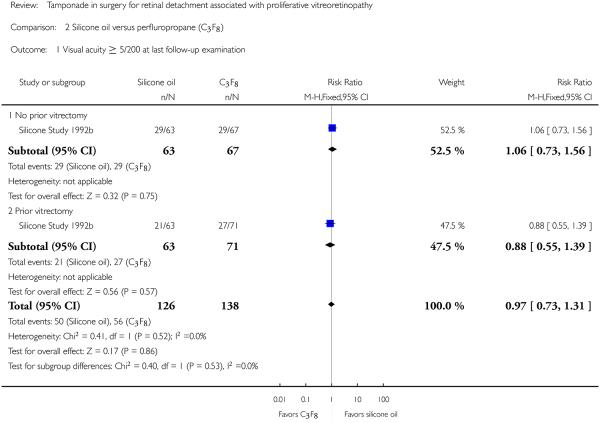
When silicone oil (1000 centistokes) was compared with C3F8, there were no statistically significant differences between the groups with respect to visual acuity of 5/200 or better at a minimum of one year (RR 0.97; 95% CI 0.73 to 1.31) (Figure 4).
Figure 4.
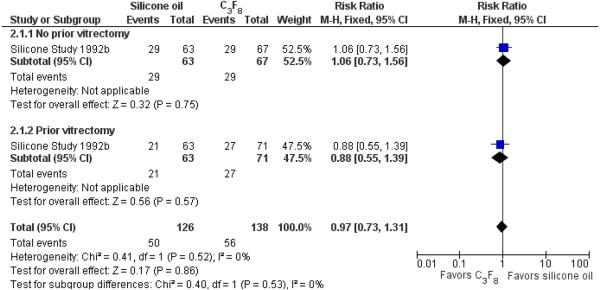
Forest plot of comparison: 2 Silicone oil versus perfluropropane (C3F8), outcome: 2.1 Visual acuity ≥ 5/200 at last follow-up examination.
Macular attachment
Two trials including 352 eyes of 352 participants contributed to this outcome at 24 months (87 eyes in Silicone Study 1992a) or at last follow-up evaluation (265 eyes in Silicone Study 1992b). When silicone oil was compared with SF6, the study investigators reported that eyes that had not undergone prior vitrectomy (Group 1) and were randomized to receive silicone oil were 37% more likely to achieve macular attachment at both one year (P < 0.05; data not reported) and two years (RR 1.37; 95% CI 1.01 to 1.86) (Figure 3).
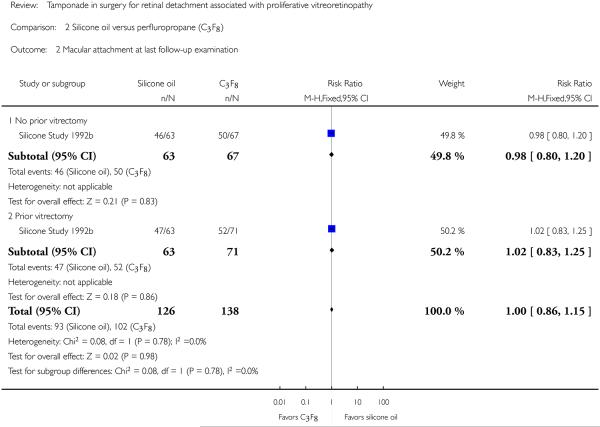
When silicone oil (1000 centistokes) was compared with C3F8, there were no statistically significant differences between the groups with respect to macular attachment at a minimum of one year follow-up (RR 1.00; 95% CI 0.86 to 1.15) (Figure 5). However, the proportions of eyes with postoperative macular attachment were higher in eyes randomized to C3F8 versus silicone oil at each time point, and this difference was of borderline statistical significance at 36 months (83% versus 60%; P = 0.045; standard deviation or 95% CI not provided).
Figure 5.
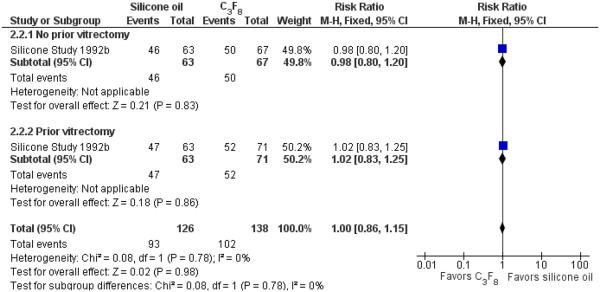
Forest plot of comparison: 2 Silicone oil versus perfluropropane (C3F8), outcome: 2.2 Macular attachment at last follow-up examination.
Adverse effects (severe, minor)
Two trials comprising 366 eyes of 366 participants contributed to the adverse event outcomes.
Severe (retina detached at one year and visual acuity worse than 20/200)
Retina detachment and visual acuity worse than 20/200 were not reported in the two trials.
Minor (intraocular pressure (IOP) greater than 21 mmHg and visually significant cataract)
IOP
IOP greater than 21 mmHg was not reported in the trials, however, IOP greater than or equal to 30 mmHg was reported in one eye treated with SF6 gas and no eyes treated with silicone oil in the first trial, and two eyes treated with C3F8 gas and one eye with the silicone oil for the second trial (RR 1.04; 95% CI 0.10 to 11.40) during the follow-up for three years.
Visually significant cataract
SF6, C3F8, and silicone oil can worsen cataracts. However, it was unlikely that cataract progression played a major role in the visual outcomes because most eyes were pseudophakic or aphakic at one year. In the silicone oil versus SF6 study, about 40% of the eyes were phakic at baseline, and the lens was subsequently removed in 69% of the eyes in the silicone oil group and 90% in the SF6 group for the non-vitrectomized eyes (RR 0.76; 95% CI 0.53 to 1.09) (Silicone Study 1992a). In the silicone oil versus C3F8 study, 48% of eyes were phakic at baseline, and the lens was subsequently removed in 91% of these eyes in the silicone oil group and 86% in the C3F8 gas group for the non-vitrectomized eyes (RR 1.06; 95% CI 0.88 to 1.26), and in 93% of the eyes in the silicone oil group and 100% in the C3F8 group (RR 0.71; 95% CI 0.53 to 0.95) (Silicone Study 1992b).
Quality of life measures
The Silicone Study did not specifically address quality of life measurements.
Economic data
The Silicone Study did not specifically address economic analysis, but a subsequent economic model including data from the Silicone Study reported that surgery for RD associated with PVR was cost-effective. In eyes that had not undergone previous PPV, silicone oil (USD per quality-adjusted life year (QALY) gained of USD 40,252) was slightly more cost-effective than C3F8 (USD per QALY gained of USD 46,926). In eyes that had undergone previous PPV, C3F8 (USD per QALY gained of USD 46,162) was more cost-effective than silicone oil (USD per QALY gained of USD 62,383) (Brown 2002).
Standard silicone oil versus heavy silicone oil
The HSO Study conducted one trial, comparing standard silicone oil (either 1000 centistokes or 5000 centistokes, per the surgeon’s preference) and heavy silicone oil (a mixture of perfluorohexyloctane (F6H8) and silicone oil) in participants with inferior RD associated with PVR.
Visual acuity
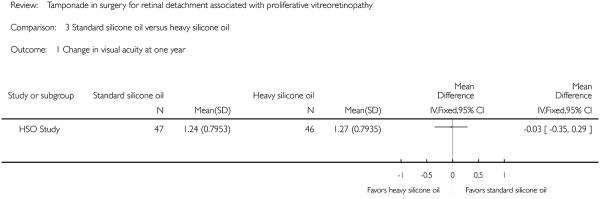
We intended to compare visual acuity as a dichotomous outcome (the proportion of participants who lost three or more lines of logMAR visual acuity; participants who lost one or two lines of logMAR visual acuity were considered stabilized) and also as a continuous outcome (mean logMAR scores); however, change in visual acuity as a dichotomous outcome was not reported, instead the investigators reported mean change in visual acuity and rates of recurrent RD. A total of 93 eyes of 93 participants contributed to this outcome at one year. The adjusted mean logMAR visual acuity was 1.24 (standard error (SE) 0.116) in the standard silicone oil group and 1.27 (SE 0.117) in the heavy silicone oil group. Non-inferiority of heavy silicone oil compared to standard silicone oil could not be demonstrated with respect to change in visual acuity at 12 months (mean difference -0.03; 95% CI -0.35 to 0.29) (Figure 6).
Figure 6.
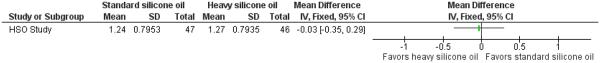
Forest plot of comparison: 3 Standard silicone oil versus heavy silicone oil, outcome: 3.1 Change in visual acuity at one year.
Macular attachment
Macular attachment was not reported in the HSO Study.
Adverse effects (severe, minor)
The HSO Study had 94 eyes of 94 participants that contributed to the adverse events at one year.
Severe (retina detached at one year and visual acuity worse than 20/200)
Retina detachment
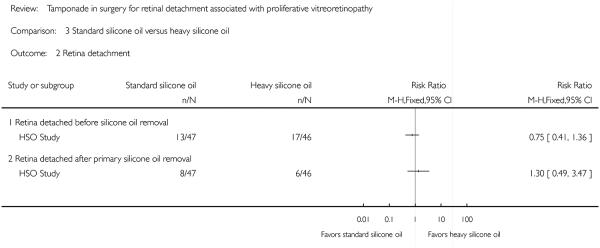
Retinal detachments were reported both before and after the removal of silicone oil, and no statistically significant differences were found between the two tamponade agents with respect to rates of recurrent RD at one year (before removal: RR 0.75; 95% CI 0.41 to 1.36; after removal: RR 1.30; 95% CI 0.49 to 3.47).
Visual acuity worse than 20/200
As described above, visual acuity was not reported as a dichotomous outcome.
Minor (intraocular pressure (IOP) greater than 21 mmHg and visually significant cataract)
Minor adverse events including IOP greater than 21 mmHg and cataract were not reported in the HSO Study. Instead, the HSO Study reported that of the 94 participants, four died, 26 had recurrent retinal detachment, 22 developed glaucoma, four developed cataract, and two had capsular fibrosis. However, numbers for each silicone oil group were not specified.
Quality of life measures and economic data
The HSO Study did not specifically address quality of life measurements or economic data.
DISCUSSION
Summary of main results
The Silicone Study comprised two well-designed prospective, multicenter, randomized controlled trials of participants with RD associated with PVR. The first trial, comparing silicone oil to SF6, was conducted between 1985 and 1987. The second trial, comparing silicone oil to C3F8, was conducted between 1987 and 1990. Pars plana vitrectomy and infusion of either silicone oil or C3F8 gas appeared to show comparable results for final visual acuities of 5/200 or better at one year and macular attachments at one year. SF6 gas was associated with worse anatomic and visual outcomes than silicone oil, although some of these differences diminished after two years.
The HSO Study was a well-designed prospective, multicenter, randomized controlled trial of participants with RD associated with PVR. The trial compared standard silicone oil (either 1000 centistokes or 5000 centistokes, per the surgeon’s preference) with heavy silicone oil (a mixture of perfluorohexyloctane (F6H8) and silicone oil), which is not approved by the US Food and Drug Administration (FDA) and is not available outside a clinical trial. Despite the many theoretical benefits of a heavier-than-water tamponade agent in treating participants with inferior vitreoretinal pathology, no important advantages were reported in this study.
Overall completeness and applicability of evidence
In the intervening two decades since the Silicone Study began, there have been many advances in vitrectomy instrumentation, intraoperative viewing systems, and surgical techniques. The silicone oil used in the Silicone Study was not approved by the US FDA and differed in many respects from the higher quality, more purified oils used today.
In addition, although SF6 and C3F8 are still used today, many surgeons now prefer 5000 centistoke silicone oil to the 1000 centistoke oil used in the Silicone Study, although anatomic and visual outcomes are similar with either oil viscosity (Scott 2005).
Perfluoro-n-octane (PFO) became available in 1988 as an intraoperative tool to achieve retinal re-attachment. PFO was not available for any of the participants enrolled in the first trial of the Silicone Study (oil versus SF6), which completed enrollment in 1987. PFO was available for some, but not all, participants enrolled in the second trial (oil versus C3F8). In addition, the investigational use of PFO and other heavy liquids as intermediate-term tamponade agents was not described until more recently.
The Silicone Study also excluded participants with a history of penetrating trauma, giant retinal tears greater than 90 °, and proliferative diabetic retinopathy. Similarly, the HSO Study excluded participants with active diabetic retinopathy, visually significant glaucoma, pregnancy, and participants under 18 years of age. For these reasons, the results reported in these trials may not be applicable to many participants undergoing contemporary surgical procedures.
Quality of the evidence
The overall quality of the evidence is moderately satisfactory. All three trials employed proper methodology for random sequence generation and allocation concealment. None of the trials clearly stated whether the study participants were masked, so we assessed performance bias as unclear for all three trials. The HSO Study (HSO Study) performed masking of outcome assessors while the Silicone Study (Silicone Study 1992a; Silicone Study 1992b) did not, so we assessed two trials from the Silicone Study as at high risk of detection bias, and the trial from HSO Study as at low risk. Two out of the three trials had less than 10% of participants lost to follow-up (low risk of attrition bias for Silicone Study 1992b from the Silicone Study, and the HSO Study). The Silicone Study 1992a had almost a third of participants lost to follow-up (50/151 were excluded from analyses), so we assessed it as at high risk of attrition bias. We assessed the Silicone Study as at low risk of reporting bias and the HSO Study at high risk because it prespecified measurement of a quality of life outcome but no data were reported.
Potential biases in the review process
Although conducting a highly sensitive search for studies, we identified only three trials relevant to this review topic. These trials compared different tamponade agents, used different statistical methods, and reported outcomes at different time points. Due to the heterogeneity among the trials, comparing the treatment effects in meta-analysis was not possible. Rather, we presented the results of the individual studies, which carry their own potential biases as discussed in other sections of this review. If adequately designed randomized controlled trials are published in the future with standardized outcomes, then additional data could improve the overall evidence in this review.
Agreements and disagreements with other studies or reviews
This is an update of a review initially published in 2009 (Schwartz 2009). This update is broadly consistent with the prior version; the HSO Study (HSO Study), which was published since the last version, has been added. To our knowledge, no other reviews on this specific topic have been published during this timeframe.
AUTHORS’ CONCLUSIONS
Implications for practice
Based on results from the Silicone Study, participants with RD associated with PVR had good results with PPV with either C3F8 gas or silicone oil tamponades. There is a suggestion that C3F8 may have certain advantages with respect to long-term anatomic outcomes in some participants, although the visual results appear similar between the tamponade agents. The choice of tamponade agent is usually made on an individual, patient-by-patient basis. Factors to be considered include the configuration of the detachment, the location of the retinal breaks, the lens status, the visual status of the fellow eye, the patient’s ability to comply with postoperative positioning requirements, the patient’s need to travel by air in the early postoperative period, and individual physician and patient preferences.
As tamponade agents, C3F8 and silicone oil appear to have visual and anatomic advantages over SF6, especially within the first year after surgery, but SF6 may be a reasonable choice in certain clinical situations.
Based on the results of the HSO Study, the heavy silicone oil mixture used in this trial (a mixture of perfluorohexyloctane (F6H8) and silicone oil) does not offer any additional benefits relative to standard silicone oil (either 1000 centistokes or 5000 centistokes, per the surgeon’s preference) in participants with complex or recurrent RD associated with PVR.
Implications for research
The Silicone Study delineated various relative advantages and disadvantages of 1000 centistoke silicone oil, SF6, and C3F8 as tamponade agents. Formal evaluation of 5000 centistoke silicone oil in a prospective clinical trial appears warranted, but to our knowledge there are no plans for such a trial at this time. Future research may develop alternative tamponade agents, particularly with a density greater than water, which would reduce the postoperative positioning requirements for many patients. Properties of an ideal tamponade agent include optical clarity, lack of toxicity, no effect on the eye’s refractive state, no effect on IOP or cataract formation, inhibition of cellular migration, and inhibition of gliosis or glial proliferation.
CHARACTERISTICS OF STUDIES
Characteristics of included studies [ordered by study ID]
HSO Study
| Methods | Study design: “prospective, multicentre, randomized, controlled, clinical trial” Number randomized (total and per group): 94 participants total, 46 in the heavy silicone oil group and 48 in the standard silicone oil group Number analyzed (total and per group): 93 participants total, 46 in the heavy silicone oil group and 47 in the standard silicone oil group Exclusions and loss to follow-up: “One control patient having no data except the randomization sheet was excluded from the analysis” Study follow-up: 12 months Sample size calculation: power of 80% |
|
| Participants | Country: Germany, Austria, Sweden, Great Britain, China, Poland, Portugal, the Netherlands, Italy, Hungary, US Age (mean ± SD): Heavy silicone oil group: 65.54 years + 12.20 Standard silicone oil group: 61.87 years + 15.69 Gender: M:F = 19:28 for the standard silicone oil group and 35:11 for the heavy silicone oil group Inclusion criteria: “Inferior and posterior PVR grade C-A6, P12 according to Machemer at 10 over 6 to 2 hrs (PVR only as rhegmatogenous retinal detachment or a complication of trauma) or inferior retinal detachment with giant retinal tear in the inferior hemisphere (10 over 6 to 2 hrs)” Exclusion criteria: ”-Superior anterior PVR grade C A6 between 2, 12 and 10 o’clock -Superior giant retinal tear retinal detachment between 2, 12 and 10 o’clock -Retinotomy holes tears above 2 and 10 o’clock -Diabetic retinopathy requiring treatment -Glaucoma resulting in visual field defects requiring treatment -No written informed consent -Age below 18 years -Participation at another trial -Pregnancy“ |
|
| Interventions | Intervention 1: heavy silicone oil (a mixture of perfluorohexyloctane (F6H8) in silicone oil) as a tamponade agent: endotamponade with heavy silicone oil Intervention 2: standard silicone oil as a tamponade agent: endotamponade with silicone oil of 1000 or 5000 cSt viscosity according to the surgeon’s preference The surgical procedure for both groups included: ”encircling band according to the surgeon’s preference, PPV via conventional three-port approach, removal of the flap of the retinal tear, if present, usage of perfuordecalin fluid (PFCL) to unfold the retina, retinopexy by cryopexy or laser coagulation, and relaxing retinotomies, if necessary. PFCL standard silicone exchange or PFCL-air-silicone exchange according to the individual surgeon’s preferences.“ |
|
| Outcomes | Primary outcome(s): Anatomical success - complete retinal reattachment at 12 months Visual acuity - mean change from baseline to 12 months (logMAR) Secondary outcome(s): “combined the evaluation of the complete retinal attachment before endotamponade removal, a quality of life analysis, and the evaluation of the number of retina-affecting reoperations within 1 year of follow-up.” Time points measurements were taken: ”participants were examined postoperatively within the one week of surgery, preremoval, 2 months after removal surgery, and one year after initial surgery“ ”Attachment of the retina is assessed blindly by the endpoint committee, who compare the preoperative fundus documentation with that taken 12 months after initial surgery. Fundus photos are taken according to the nine field regimen introduced by Irvine et al. (1988) and Azen et al. (1998); fundus drawings are in accordance with Meyer-Schwickerath & Wessing (1975). Visual acuity is the main subjective criterion for assessing the function of an eye, reflecting the patient’s point of view that the improvement of VA is the most important parameter of a successful operation. The endpoint is defined as a change of VA 12 months after initial surgery compared with the preoperative measurement using letter- by-letter scoring on ETDRS charts.“ Unit of analysis: individual - only one eye per participant was included in the study. When both eyes of a participant were eligible, the surgeon determined the study eye Other issues with outcome assessment: none |
|
| Notes | Study dates: December 2003 to February 2008 Funding source(s): Deutsche Forschungsgemeinschaft (DFG Ki 743 2-1 and DFG Hi 541 1-1) (Germany Research Foundation) Declaration of interests: not reported Publication language: English |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) |
Low risk | “The randomization list was generated using permuted blocks of varying sizes, stratified by surgeon. No further stratification will take place.” |
| Allocation concealment (selection bias) | Low risk | “After verification of the eligibility criteria (including informed consent), randomization (opening of the prepared sealed envelopes) took place.” |
| Masking of participants and personnel (performance bias) |
Unclear risk | No information provided with respect to masking of participants or study investigators |
| Masking of outcome assessment (detection bias) |
Low risk | “The primary end-point ’12 month attachment’ as well as the secondary end-points ’attachment (before removal)’ and number of re-operations will be assessed by masked examiners within the end-point committee meetings based on photo documentation or surrogates. Anonymous documentation, which does not allow identification of the applied treatment, will be presented at the end-point committee meetings by the documentation centre. In this sense, the evaluation of these endpoint criteria can be considered as single-blind.” |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | “None of the randomized patients was excluded by the end-point committee. As patients who did not satisfy the major inclusion criteria were in all cases already randomized (performed preoperatively), all of them are therefore part of the full analysis set. One patient (‘8701001’) was excluded due to a lack of pre- and postsurgical assessment and data (only randomization sheet present). Three patients in the HSO group and five patients in the standard silicone group fulfilled intraoperative exclusion criteria.” “Concerning the evaluation of the anatomical success, in 20 cases the 12-month visit was substituted by the delayed 12-month visit. In cases where a 12-month visit was not available, the anatomical success was assessed as treatment failures. This is applied to 18 cases in the HSO group and 16 in the standard group.” “For the analysis of VA, in cases of no valid 12-month visit, the last available observation was used (last observation carried forward) for 18 patients in the HSO group and 15 patients in the standard group.” |
| Selective reporting (reporting bias) | High risk | The sample size of the study changed from what was specified in the protocol because of the low recruitment rate, although the study investigators recalculated the sample size and it still had 80% power for detection the difference. Also, the quality of life data was pre-specified in the methods section, but it was not reported in the results |
| Other bias | Low risk | No issues identified |
Silicone Study 1992a
| Methods | Study design: unmasked, multicenter randomized controlled trial Number randomized (total and per group): 151 participants in total, number per group was not reported Eyes of participants were stratified as follows (only one eye per participant randomized): Eyes that had not undergone prior vitrectomy (Group 1): 113 in total, 47 in the standard silicone oil group and 46 in the heavy silicone oil group Eyes that had undergone vitrectomy but without silicone oil injection (Group 2): 38 in total, number per group was not reported Number analyzed (total and per group): Statistical analyses were performed only on group 1 data because the sample size of group 2 was small: total: 101 participants; 49 in the SF6 gas group and 52 in the silicone oil group Exclusions and loss to follow-up: 12 participants in group 1 were excluded, all participants in group 2 were excluded Study follow-up: 36 months Sample size calculation: no |
|
| Participants | Country: USA Age (mean ± SD): mean ± SD not reported, median age was 62.1 years (range 25 to 84) for the SF6 gas group and 66.2 years (range 24 to 84) for the silicone oil group Gender: M:F=16:33 for the SF6 gas group and 17:35 for the silicone oil group Inclusion criteria: Participants with proliferative vitreoretinopathy with a classification of at least C-3 grade, at least age 18, visual acuity better than light perception, and sufficient contracture so intraocular dissection was required Exclusion criteria: Participants with uncontrolled concomitant eye disease, occurrence of blunt trauma to the eye within 3 months of randomization, history of penetrating trauma to the eye, presence of a giant tear ≥90°, presence of proliferative diabetic retinopathy, and the existence of any condition that would prevent 3 year follow-up |
|
| Interventions | Intervention 1: sulfur hexafluoride gas (SF6): 49 eyes (Group 1), 15 eyes (Group 2) Intervention 2: silicone oil: 52 eyes (Group 1), 23 eyes (Group 2) |
|
| Outcomes | The study did not separate primary or secondary outcomes. All outcomes included: visual acuity, retinal reattachment, refraction; development or change in ocular complications affecting the cornea, iris, or lens; and measurements of intraocular pressure at 10 days, and 1, 3, 6, 12, 18, 24, and 36 months following randomization Secondary outcome(s): N/A Time points measurements were taken: 1,3,6,12,18,24, and 36 months |
|
| Notes | Study dates: 1 September 1985 to 30 June 30 1991 Funding source(s): trial sponsored by the National Eye Institute. Silicone oil provided by the Dow Corning Corporation Declaration of interests: not reported Publication language: English |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) |
Low risk | Randomization scheme generated by the Data Coordinating Center; stratification and blocking methods employed to ensure equal treatment assignments within each clinical center |
| Allocation concealment (selection bias) | Low risk | Investigators used sealed opaque envelopes supplied in limited numbers by the Data Coordinating Center |
| Masking of participants and personnel (performance bias) |
Unclear risk | No information provided with respect to masking of participants or study investigators |
| Masking of outcome assessment (detection bias) |
High risk | “A surgeon cannot be masked to the treatment during the operative procedure. During follow-up examinations, silicone fluid produces a characteristic appearance in the eye unlike that of a long-acting gas, making it impossible to mask study technicians.” |
| Incomplete outcome data (attrition bias) All outcomes |
High risk | Last observation carried forward method used for missing data, but data inferred only if “consistent” findings for prior and subsequent examinations. Randomized participants from a center that ceased recruitment (n = 12) and randomized participants with a history of prior vitrectomy (n = 38) were excluded from the analysis |
| Selective reporting (reporting bias) | Low risk | This study appeared to be free of selective reporting. Outcomes were reported in a prior manuscript describing trial design and participant baseline characteristics |
| Other bias | Unclear risk | The baseline estimated duration of retinal detachment was greater in Group 2 eyes (eyes of participants with prior vitrectomy but without silicone oil injection) randomized to SF6 compared to Group 2 eyes randomized to silicone oil |
Silicone Study 1992b
| Methods | Study design: unmasked, multicenter randomized controlled trial Number randomized (total and per group): 271 participants in total, number per group was not reported Eyes of participants were stratified as follows (only one eye per participant randomized): Eyes that had not undergone prior vitrectomy (Group 1): 132 in total, number per group not reported Eyes that had undergone vitrectomy but without silicone oil injection (Group 2): 139 in total, number per group not reported Number analyzed (total and per group): 265 participants in total, 138 in the C3F8 gas group and 127 in the silicone oil group Eyes that had not undergone prior vitrectomy (Group 1): 131 in total, 67 in the C3F8 gas group and 64 in the silicone oil group Eyes that had undergone vitrectomy but without silicone oil injection (Group 2): 134 in total, 71 in the C3F8 gas group and 63 in the silicone oil group Exclusions and loss to follow-up: 6 participants were excluded Study follow-up: 36 months Sample size calculation: no |
|
| Participants | Country: USA Age (mean ± SD): mean ± SD was not reported, median age for the groups were listed below: Group 1: 66.2 years (range 20-86) for the C3F8 gas group and 66.0 years (range 21-89) for the silicone oil group Group 2: 63.3 years (range 22-88) for the C3F8 gas group and 61.6 years (range 27-84) for the silicone oil group Gender: Group 1: M:F=47:20 for the C3F8 gas group and 43:21 for the silicone oil group Group 2: M:F=48:23 for the C3F8 gas group and 49:14 for the silicone oil group Inclusion criteria: Participants with proliferative vitreoretinopathy with a classification of at least C-3 grade, at least age 18, visual acuity better than light perception, and sufficient contracture so intraocular dissection was required Exclusion criteria: Participants with uncontrolled concomitant eye disease, occurrence of blunt trauma to the eye within 3 months of randomization, history of penetrating trauma to the eye, presence of a giant tear ≥90°, presence of proliferative diabetic retinopathy, and the existence of any condition that would prevent 3-year follow-up |
|
| Interventions | Intervention 1: Perfluropropane gas (C3F8): 67 eyes (Group 1), 71 eyes (Group 2) Intervention 2: Silicone oil: 64 eyes (Group 1), 63 eyes (Group 2) |
|
| Outcomes | Primary outcome(s): Visual acuity, retinal reattachment, refraction; development or change in ocular complications affecting the cornea, iris, or lens; and measurements of intraocular pressure at 10 days, and 1, 3, 6, 12, 18, 24, and 36 months following randomization Secondary outcome(s): N/A Time points measurements were taken: 1,3,6,12,18,24, and 36 months. Number of eyes included in the last follow-up analysis: 67 of 67 eyes (Group 1) and 71 or 71 eyes (Group 2) randomized to C3F8; 63 of 64 eyes (Group 1) and 63 of 63 eyes (Group 2) randomized to silicone oil. One participant randomized to silicone oil in Group 1 died after the baseline visit |
|
| Notes | Study dates: September 1, 1987 to June 30, 1991; “one center terminated follow-up in 1988 and patient data were excluded (n = 1 from Group 1; n = 5 from Group 2)” Twelve month visual acuity and macula status outcomes were displayed in graphs; investigators contacted for 12 month outcomes Funding source(s): trial sponsored by the National Eye Institute. Silicone oil provided by the Dow Corning Corporation Declaration of interests: not reported Publication language: English |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) |
Low risk | Randomization scheme generated by the Data Coordinating Center; stratification and blocking methods employed to ensure equal treatment assignments within each clinical center |
| Allocation concealment (selection bias) | Low risk | Investigators used sealed envelopes supplied in limited numbers by the Data Coordinating Center |
| Masking of participants and personnel (performance bias) |
Unclear risk | No information provided with respect to masking of participants or study investigators |
| Masking of outcome assessment (detection bias) |
High risk | “A surgeon cannot be masked to the treatment during the operative procedure. During follow-up examinations, silicone fluid produces a characteristic appearance in the eye unlike that of a long-acting gas, making it impossible to mask study technicians.” |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | Last observation carried forward method used for missing data, but data imputed only if “consistent” findings for prior and subsequent examinations. Randomized participants (n = 6) from center that ceased recruitment were excluded from the analysis |
| Selective reporting (reporting bias) | Low risk | This study appeared to be free of selective reporting. Outcomes were reported in a prior manuscript describing trial design and participant baseline characteristics |
| Other bias | Unclear risk | No differences in baseline characteristics |
cSt: centistokes
ETDRS: Early Treatment Diabetic Retinopathy Study
HSO: heavy silicone oil
PPV: pars plana vitrectomy
PVR: proliferative vitreoretinopathy
SD: standard deviation
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Avci 2001 | Not a randomized trial |
| Brazitikos 2005 | RCT that excluded patients with PVR stage C or greater, and not all patients received a tamponade agent |
| Chang 1988 | Not a randomized trial |
| Gao 1993 | Case series |
| Hammer 1997 | RCT that had compared sulfur hexafluoride gas and silicone oil fro PVR patients, however, study reported only 180 days of follow-up |
| Hutchins 2003 | Case series |
| Kralinger 2010 | RCT that had compared silicone oil and acetyl-salicylic acid suspension for PVR patients, however, study reported a follow-up period of six months |
| Krasnik 1998 | Not a randomized trial |
| Latecka-Krajewska 1998 | Not a randomized trial |
| Lean 1989 | Not a randomized trial |
| Li 1995 | Case series |
| Lu 2002 | Retrospective study |
| Oncel 2005 | Conference abstract, the study was never published |
| Pastor 1998 | Retrospective study |
| Pertile 1999 | Not a randomized trial, did not include patients with RD and PVR (macular hole study) |
| Peyman 1987 | RCT, but average follow-up 8.4 months |
| Soheilian 2006 | Retrospective study |
| Stefer 1991 | Case series |
| Tufail 1997 | Not a randomized trial, did not compare tamponade agents |
| van Effenterre 1987 | Case series |
Characteristics of studies awaiting assessment [ordered by study ID]
Oncel 2006
| Methods | Randomized controlled trial |
| Participants | 45 participants with complicated retinal detachments |
| Interventions | Heavy silicone oil (viscosity of 1400 cSt, density = 1.06 g/cm3) Standard silicone oil |
| Outcomes | Retinal re-attachment (time of follow-up unknown) |
| Notes | Conference abstract from the American Academy of Ophthalmology Meeting (2006). This trial does not appear to have ever been published |
cm: centimeter
cSt: centistokes
g: grams
DATA AND ANALYSES
Comparison 1.
Silicone oil versus sulfur hexafluoride (SF6)
| Outcome or subgroup title | No. of studies |
No. of participants |
Statistical method | Effect size |
|---|---|---|---|---|
| 1 Visual acuity ≥ 5/200 and macular attachment at two years |
1 | Risk Ratio (M-H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Visual acuity ≥ 5/200 | 1 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Macular attachment | 1 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 2.
Silicone oil versus perfluropropane (C3F8)
| Outcome or subgroup title | No. of studies |
No. of participants |
Statistical method | Effect size |
|---|---|---|---|---|
| 1 Visual acuity ≥ 5/200 at last follow-up examination |
1 | 264 | Risk Ratio (M-H, Fixed, 95% CI) | 0.97 [0.73, 1.31] |
| 1.1 No prior vitrectomy | 1 | 130 | Risk Ratio (M-H, Fixed, 95% CI) | 1.06 [0.73, 1.56] |
| 1.2 Prior vitrectomy | 1 | 134 | Risk Ratio (M-H, Fixed, 95% CI) | 0.88 [0.55, 1.39] |
| 2 Macular attachment at last follow-up examination |
1 | 264 | Risk Ratio (M-H, Fixed, 95% CI) | 1.00 [0.86, 1.15] |
| 2.1 No prior vitrectomy | 1 | 130 | Risk Ratio (M-H, Fixed, 95% CI) | 0.98 [0.80, 1.20] |
| 2.2 Prior vitrectomy | 1 | 134 | Risk Ratio (M-H, Fixed, 95% CI) | 1.02 [0.83, 1.25] |
Comparison 3.
Standard silicone oil versus heavy silicone oil
| Outcome or subgroup title | No. of studies |
No. of participants |
Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in visual acuity at one year |
1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Retina detachment | 1 | Risk Ratio (M-H, Fixed, 95% CI) | Totals not selected | |
| 2.1 Retina detached before silicone oil removal |
1 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Retina detached after primary silicone oil removal |
1 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Analysis 1.1. Comparison 1 Silicone oil versus sulfur hexafluoride (SF6), Outcome 1 Visual acuity ≥ 5/200 and macular attachment at two years.
Analysis 2.1. Comparison 2 Silicone oil versus perfluropropane (C3F8), Outcome 1 Visual acuity ≥ 5/200 at last follow-up examination.
Analysis 2.2. Comparison 2 Silicone oil versus perfluropropane (C3F8), Outcome 2 Macular attachment at last follow-up examination.
Analysis 3.1. Comparison 3 Standard silicone oil versus heavy silicone oil, Outcome 1 Change in visual acuity at one year.
Analysis 3.2. Comparison 3 Standard silicone oil versus heavy silicone oil, Outcome 2 Retina detachment.
ACKNOWLEDGEMENTS
We acknowledge Iris Gordon, the Trials Search Co-ordinators for the Cochrane Eyes and Vision Group (CEVG), for designing and conducting the electronic searches. We acknowledge support provided by the CEVG US Project, which is funded by Grant 1 U01 EY020522-01, National Eye Institute, National Institutes of Health. We also acknowledge Peter Gehlbach, Barbara Hawkins, Kate Henshaw and Tianjing Li for their comments on the protocol version of this review (Schwartz 2006). We acknowledge Ann-Margret Ervin (AE) and Elizabeth Ssemanda (ES) for their contribution to the previous published version of the review (Schwartz 2009), and Michael Marrone for his assistance in data extraction during the update of this review.
Richard Wormald (Co-ordinating Editor for CEVG) acknowledges financial support for his CEVG research sessions from the Department of Health through the award made by the National Institute for Health Research to Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology for a Specialist Biomedical Research Centre for Ophthalmology. The views expressed in this publication are those of the authors and not necessarily those of the Department of Health.
SOURCES OF SUPPORT
Internal sources
Bascom Palmer Eye Institute, USA.
External sources
Partially supported by NIH center grant P30-EY014801, USA.
Unrestricted grant to the University of Miami from Research to Prevent Blindness, USA.
National Eye Institute, National Institutes of Health, USA.
Xue Wang is supported by the Cochrane Eyes and Vision Group US Project through the National Eye Institute, Grant 1 U01 EY020522
Appendix 1. CENTRAL search strategy
#1 MeSH descriptor Retinal Detachment
#2 MeSH descriptor Retinal Perforations
#3 MeSH descriptor Vitreous Detachment
#4 retina* near/2 break*
#5 retina* near/2 tear*
#6 retina* near/2 detach*
#7 retina* near/2 perforat*
#8 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7)
#9 MeSH descriptor Silicone Oils
#10 silicone oil*
#11 tamponade*
#12 MeSH descriptor Sulfur Hexafluoride
#13 sulfur hexafluoride*
#14 hexafluoroethane*
#15 MeSH descriptor Fluorocarbons
#16 MeSH descriptor Dimethylpolysiloxanes
#17 perfluoropropane*
#18 polydimethylsiloxane*
#19 perfluorohexylethan*
#20 perfluoro-n-octane
#21 (#9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20)
#22 (#8 AND #21)
Appendix 2. MEDLINE (OvidSP) search strategy
1. randomized controlled trial.pt.
2. (randomized or randomised).ab,ti.
3. placebo.ab,ti.
4. dt.fs.
5. randomly.ab,ti.
6. trial.ab,ti.
7. groups.ab,ti.
8. or/1-7
9. exp animals/
10. exp humans/
11. 9 not (9 and 10)
12. 8 not 11
13. exp retinal detachment/
14. exp retinal perforation/
15. exp vitreous detachment/
16. (retina$ adj2 break$).tw.
17. (retina$ adj2 tear$).tw.
18. (retina$ adj2 detach$).tw.
19. (retina$ adj2 perforat$).tw.
20. or/13-19
21. exp silicone oils/
22. silicone oil$.tw.
23. tamponade$.tw.
24. exp sulfur hexafluoride/
25. sulfur hexafluoride$.tw.
26. hexafluoroethane$.tw.
27. exp fluorocarbons/
28. exp dimethylpolysiloxanes/
29. perfluoropropane$.tw.
30. polydimethylsiloxane$.tw.
31. perfluorohexylethan$.tw.
32. perfluoro-n-octane.tw.
33. or/21-32
34. 20 and 33
35. 12 and 34
The search filter for trials at the beginning of the MEDLINE strategy is from the published paper by Glanville et al (Glanville 2006).
Appendix 3. EMBASE (OvidSP) search strategy
1. exp randomized controlled trial/
2. exp randomization/
3. exp double blind procedure/
4. exp single blind procedure/
5. random$.tw.
6. or/1-5
7. (animal or animal experiment).sh.
8. human.sh.
9. 7 and 8
10. 7 not 9
11. 6 not 10
12. exp clinical trial/
13. (clin$ adj3 trial$).tw.
14. ((singl$ or doubl$ or trebl$ or tripl$) adj3 (blind$ or mask$)).tw.
15. exp placebo/
16. placebo$.tw.
17. random$.tw.
18. exp experimental design/
19. exp crossover procedure/
20. exp control group/
21. exp latin square design/
22. or/12-21
23. 22 not 10
24. 23 not 11
25. exp comparative study/
26. exp evaluation/
27. exp prospective study/
28. (control$ or prospectiv$ or volunteer$).tw.
29. or/25-28
30. 29 not 10
31. 30 not (11 or 23)
32. 11 or 24 or 31
33. retina detachment/
34. retina tear/
35. vitreous body detachment/
36. (retina$ adj2 break$).tw.
37. (retina$ adj2 tear$).tw.
38. (retina$ adj2 detach$).tw.
39. (retina$ adj2 perforat$).tw.
40. or/33-39
41. silicone oil/
42. silicone oil$.tw.
43. tamponade$.tw.
44. sulfur hexafluoride/
45. sulfur hexafluoride$.tw.
46. hexafluoroethane$.tw.
47. fluorocarbon/
48. dimeticone/
49. perfluoropropane$.tw.
50. polydimethylsiloxane$.tw.
51. perfluorohexylethan$.tw.
52. perfluoro-n-octane.tw.
53. or/41-52
54. 40 and 53
55. 32 and 54
Appendix 4. LILACS search strategy
retina$ and detach$ or perforat$ or break$ or tear and silicone or sulfur hexafluoride$ or hexafluoroethane$ or fluorocarbon$ or dimethylpolysiloxane$ or perfluoropropane$ or polydimethylsiloxane$ or perfluorohexylethan$
Appendix 5. metaRegister of Controlled Trials search strategy
(tamponade or oil) and retina
Appendix 6. ClinicalTrials.gov search strategy
(Tamponade OR Silicone Oil) AND Retina
Appendix 7. ICTRP search strategy
Retinal detachment = condition AND (Tamponade OR Silicone Oil) = intervention
CONTRIBUTIONS OF AUTHORS
For the original review
Conceiving the review: SGS
Designing the review: SGS
Coordinating the review: SGS
Undertaking manual searches: SGS, ES
Screening search results: SGS, ES
Organizing retrieval of papers: SGS, ES
Screening retrieved papers against inclusion criteria: SGS, ES
Appraising quality of papers: SGS, ES
Abstracting data from papers: SGS, ES, AE
Writing to authors of papers for additional information: SGS
Obtaining and screening data on unpublished studies: SGS
Data management for the review: SGS, ES, AE
Entering data into RevMan: SGS, ES, AE
Analysis of data: SGS, HWF, ES, AE
Interpretation of data: SGS, HWF
Writing the review: SGS, WHL, HWF, ES, AE
Securing funding for the review: SGS
Guarantor for the review: SGS
For the update of the review
Screening search results: SGS, XW
Organizing retrieval of papers: SGS, XW
Screening retrieved papers against inclusion criteria: SGS, XW
Appraising quality of papers: SGS, XW
Abstracting data from papers: SGS, XW
Writing to authors of papers for additional information: SGS
Obtaining and screening data on unpublished studies: SGS
Data management for the review: SGS, XW
Entering data into RevMan: SGS, XW
Analysis of data: SGS, HWF, XW
Interpretation of data: SGS, HWF
Writing the review: SGS, WHL, HWF, XW
Securing funding for the review: SGS
Guarantor for the review: SGS
Footnotes
DECLARATIONS OF INTEREST
Harry W Flynn, Jr, MD is a co-author on several of the studies that were eligible for inclusion in this review. Dr Flynn also has served on advisory boards for Santen and has served on lecture panels for Vindico.
Stephen G Schwartz, MD, MBA has served on advisory boards for the following entities: Alimera, Bausch and Lomb, and Santen, and has received lecture fees from Regneron and ThromboGenics.
REFERENCES
- References to studies included in this review
- HSO Study {published data only}
- Joussen AM, Kirchhof B, Schrage N, Ocklenburg C, Hilgers RD. Heavy silicone oil versus standard silicone oil as vitreous tamponade in inferior PVR (HSO Study): design issues and implications. Acta Ophthalmologica Scandinavica. 2007;85(6):623–30. doi: 10.1111/j.1600-0420.2007.00898.x. [DOI] [PubMed] [Google Scholar]
- *.Joussen AM, Rizzo S, Kirchhof B, Schrage N, Li X, Lente C, HSO-Study Group Heavy silicone oil versus standard silicone oil in as vitreoustamponade in inferior PVR (HSO Study): interim analysis. Acta Ophthalmologica. 2011;89(6):e483–9. doi: 10.1111/j.1755-3768.2011.02139.x. [DOI] [PubMed] [Google Scholar]
- Silicone Study 1992a {published data only}
- Abrams GW, Azen SP, Barr CC, Lai MY, Hutton WL, Trese MT, et al. The incidence of corneal abnormalities in the Silicone Study. Silicone Study Report 7. Archives of Ophthalmology. 1995;113(6):764–9. doi: 10.1001/archopht.1995.01100060090039. [DOI] [PubMed] [Google Scholar]
- Abrams GW, Azen SP, McCuen BW, 2nd, Flynn HW, Jr, Lai MY, Ryan SJ. Vitrectomy with silicone oil or long-acting gas in eyes with severe proliferative vitreoretinopathy: results of additional and long-term follow-up. Silicone Study Report 11. Archives of Ophthalmology. 1997;115(3):335–43. doi: 10.1001/archopht.1997.01100150337005. [DOI] [PubMed] [Google Scholar]
- *.Anonymous Vitrectomy with silicone oil or sulfur hexafluoride gas in eyes with severe proliferative vitreoretinopathy: results of a randomized clinical trial. Silicone Study Report 1. Archives of Ophthalmology. 1992;110(6):770–9. doi: 10.1001/archopht.1992.01080180042027. [DOI] [PubMed] [Google Scholar]
- Azen SP, Boone DC, Barlow W, McCuen BW, Walonker AF, Anderson MM, et al. Methods, statistical features, and baseline results of a standardized, multicentered ophthalmologic surgical trial: the Silicone Study. Controlled Clinical Trials. 1991;12(3):438–55. doi: 10.1016/0197-2456(91)90022-e. [DOI] [PubMed] [Google Scholar]
- Barr CC, Lai MY, Lean JS, Linton KL, Trese M, Abrams G, et al. Postoperative intraocular pressure abnormalities in the Silicone Study. Silicone Study Report 4. Ophthalmology. 1993;100(11):1629–35. doi: 10.1016/s0161-6420(93)31425-9. [DOI] [PubMed] [Google Scholar]
- Blumenkranz MS, Azen SP, Aaberg T, Boone DC, Lewis H, Radtke N, et al. Relaxing retinotomy with silicone oil or long-acting gas in eyes with severe proliferative vitreoretinopathy. Silicone Study Report 5. The Silicone Study Group. American Journal of Ophthalmology. 1993;116(5):557–64. doi: 10.1016/s0002-9394(14)73196-4. [DOI] [PubMed] [Google Scholar]
- Brown GC, Brown MM, Sharma S, Busbee B, Landy J. A cost-utility analysis of interventions for severe proliferative vitreoretinopathy. American Journal of Ophthalmology. 2002;133(3):365–71. doi: 10.1016/s0002-9394(01)01423-4. [DOI] [PubMed] [Google Scholar]
- Cox MS, Azen SP, Barr CC, Linton KL, Diddie KR, Lai MY, et al. Macular pucker after successful surgery for proliferative vitreoretinopathy. Silicone Study Report 8. Ophthalmology. 1995;102(12):1884–91. doi: 10.1016/s0161-6420(95)30779-8. [DOI] [PubMed] [Google Scholar]
- Diddie KR, Azen SP, Freeman HM, Boone DC, Aaberg TM, Lewis H. Anterior proliferative vitreoretinopathy in the silicone study. Silicone Study Report 10. Ophthalmology. 1996;103(7):1092–9. doi: 10.1016/s0161-6420(96)30562-9. [DOI] [PubMed] [Google Scholar]
- Hutton WL, Azen SP, Blumenkranz MS, Lai MY, McCuen BW, Han DP, et al. The effects of silicone oil removal. Silicone Study Report 6. Archives of Ophthalmology. 1994;112(6):778–85. doi: 10.1001/archopht.1994.01090180076038. [DOI] [PubMed] [Google Scholar]
- Lean J, Azen SP, Lopez PF, Qian D, Lai MY, McCuen B. The prognostic utility of the Silicone Study Classification System. Silicone Study Report 9. Silicone Study Group. Archives of Ophthalmology. 1996;114(3):286–92. doi: 10.1001/archopht.1996.01100130282009. [DOI] [PubMed] [Google Scholar]
- McCuen BW, 2nd, Azen SP, Stern W, Lai MY, Lean JS, Linton KL, et al. Vitrectomy with silicone oil or perfluoropropane gas in eyes with severe proliferative vitreoretinopathy. Silicone Study Report 3. Retina. 1993;13(4):279–84. doi: 10.1097/00006982-199313040-00002. [DOI] [PubMed] [Google Scholar]
- Silicone Study 1992b {published data only}
- Anonymous Vitrectomy with silicone oil or perfluoropropane gas in eyes with severe proliferative vitreoretinopathy: results of a randomized clinical trial. Silicone Study Report 2. Archives of Ophthalmology. 1992;110(6):780–92. doi: 10.1001/archopht.1992.01080180052028. [DOI] [PubMed] [Google Scholar]
- References to studies excluded from this review
- Avci 2001 {published data only}
- Avci R. Cataract surgery and transpupillary silicone oil removal through a single scleral tunnel incision under topical anesthesia; sutureless surgery. International Ophthalmology. 2001;24(6):337–41. doi: 10.1023/b:inte.0000006833.64874.55. [DOI] [PubMed] [Google Scholar]
- Brazitikos 2005 {published data only}
- Brazitikos PD, Androudi S, Christen WG, Stangos NT. Primary pars plana vitrectomy versus scleral buckle surgery for the treatment of pseudophakic retinal detachment: a randomized clinical trial. Retina. 2005;25(8):957–64. doi: 10.1097/00006982-200512000-00001. [DOI] [PubMed] [Google Scholar]
- Chang 1988 {published data only}
- Chang S, Ozmert E, Zimmerman NJ. Intraoperative perfluorocarbon liquids in the management of proliferative vitreoretinopathy. American Journal of Ophthalmology. 1988;106(6):668–74. doi: 10.1016/0002-9394(88)90698-8. [DOI] [PubMed] [Google Scholar]
- Gao 1993 {published data only}
- Gao R, Lu L, Zhang S, Tang S, Liu Q, Hu Z. Study on application of the silicone oil in the reattachment of complicated retinal detachment. Eye Science. 1993;9(3):146–8. [PubMed] [Google Scholar]
- Hammer 1997 {published data only}
- Hammer M, Margo CE, Grizzard WS. Complex retinal detachment treated with silicone oil or sulfur hexafluoride gas: a randomized clinical trial. Ophthalmic Surgery and Lasers. 1997;28(11):926–31. [PubMed] [Google Scholar]
- Hutchins 2003 {published data only}
- Hutchins RK, Kaufman AH, Augsburger JJ. Perfluorooctane internal tamponade when using a temporary keratoprosthesis during retinal detachment repair. Retina. 2003;23(1):106–10. doi: 10.1097/00006982-200302000-00020. [DOI] [PubMed] [Google Scholar]
- Kralinger 2010 {published data only}
- Kralinger MT, Stolba U, Velikay M, Egger S, Binder S, Wedrich A, et al. Safety and feasibility of a novel intravitreal tamponade using a silicone oil/acetyl-salicylic acid suspension for proliferative vitreoretinopathy: first results of the Austrian Clinical Multicenter Study. Graefe’s Archive for Clinical and Experimental Ophthalmology. 2010;248(8):1193–8. doi: 10.1007/s00417-010-1389-7. [DOI] [PubMed] [Google Scholar]
- Krasnik 1998 {published data only}
- Krásnik V, Strmen P, Furdová A, Hasa J. Ultrasonic diagnosis of retinal detachment after internal tamponade with silicone oil [Ultrazvukova diagnostika amocie sietnice po vnutornej tamponade silikonovym olejom] Ceska a Slovenska Oftalmologie. 1998;54(5):305–9. [PubMed] [Google Scholar]
- Latecka-Krajewska 1998 {published data only}
- Latecka-Krajewska B, Nawrocki J, Bogorodzki B. Usefulness of different silicone oils for intraocular tamponade [Ocena przydatnosci roznych rodzajow oleju sylikonowego do tamponady wewnatrzgalkowej] Klinka Oczna. 1998;100(5):295–300. [PubMed] [Google Scholar]
- Lean 1989 {published data only}
- Lean JS, Stern WH, Irvine AR, Azen SP, Boone D, Quillen-Thomas B, et al. Classification of proliferative vitrereotinopathy used in the Silicone Study. Ophthalmology. 1989;96(6):765–71. doi: 10.1016/s0161-6420(89)32821-1. [DOI] [PubMed] [Google Scholar]
- Li 1995 {published data only}
- Li X, Jiang Y, Zkang X. A comparison of attachment rates between SF6 and silicone oil tamponades following vitrectomy for treatment of complicated retinal detachment. Chinese Journal of Ophthalmology. 1995;31(4):250–4. [PubMed] [Google Scholar]
- Lu 2002 {published data only}
- Lu L, Li Y, Cai S, Yang J. Vitreous surgery in highly myopic retinal detachment resulting from a macular hole. Clinical and Experimental Ophthalmology. 2002;30(4):261–5. doi: 10.1046/j.1442-9071.2002.00530.x. [DOI] [PubMed] [Google Scholar]
- Oncel 2005 {published data only}
- Oncel M. American Academy of Ophthalmology. 2005. Heavy silicone oil as long-term internal tamponade for complicated retinal detachment. abstract 255. [Google Scholar]
- Pastor 1998 {published data only}
- Pastor JC, Zarco JM, Del Nozal MJ, Pampliega A, Marinero P. Clinical consequences of the use of highly purified silicone oil: comparative study of highly and less purified silicone oil. European Journal of Ophthalmology. 1998;8(3):179–83. doi: 10.1177/112067219800800311. [DOI] [PubMed] [Google Scholar]
- Pertile 1999 {published data only}
- Pertile G, Claes C. Silicone oil vs. gas for the treatment of full-thickness macular hole. Bulletin de la Société Belge d’Ophtalmologie. 1999;274:31–6. [PubMed] [Google Scholar]
- Peyman 1987 {published data only}
- Peyman GA, Kao GW, de Corral LR. Randomized clinical trial of intraocular silicone vs. gas in the management of complicated retinal detachment and vitreous hemorrhage. International Ophthalmology. 1987;10(4):221–34. doi: 10.1007/BF00155629. [DOI] [PubMed] [Google Scholar]
- Soheilian 2006 {published data only}
- Soheilian M, Mazareei M, Mohammadpour M, Rahmani B. Comparison of silicon oil removal with various viscosities after complex retinal detachment surgery. BMC Ophthalmology. 2006;6:21. doi: 10.1186/1471-2415-6-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefer 1991 {published data only}
- Stefer U, Wiedemann P, Weber J, Heimann K. Functional results after vitrectomy with silicone oil injection. European Journal of Ophthalmology. 1991;1(2):89–95. doi: 10.1177/112067219100100207. [DOI] [PubMed] [Google Scholar]
- Tufail 1997 {published data only}
- Tufail A, Schwartz SD, Gregor ZJ. Prophylactic argon laser retinopexy prior to removal of silicone oil: a pilot study. Eye. 1997;11(Pt 3):328–30. doi: 10.1038/eye.1997.69. [DOI] [PubMed] [Google Scholar]
- van Effenterre 1987 {published data only}
- van Effenterre G, Haut J, Larricart P, Abi-Rached J, Vachet JM. Gas tamponade as a single technique in the treatment of retinal detachment: is vitrectomy needed? A comparative study of 120 cases. Graefe’s Archive for Clinical and Experimental Ophthalmology. 1987;225(4):254–8. doi: 10.1007/BF02150143. [DOI] [PubMed] [Google Scholar]
- References to studies awaiting assessment
- Oncel 2006 {published data only}
- Oncel M, Acikalin B. Heavy silicone oil vs. standard silicone oil for the management of complicated retinal detachment: a prospective randomized study. American Academy of Ophthalmology. 2006 abstract 301. [Google Scholar]
- Additional references
- Brown 2002
- Brown GC, Brown MM, Sharma S, Busbee B, Landy J. A cost-utility analysis of interventions for severe proliferative vitreoretinopathy. American Journal of Ophthalmology. 2002;133(3):365–72. doi: 10.1016/s0002-9394(01)01423-4. [DOI] [PubMed] [Google Scholar]
- Charteris 2002
- Charteris DG, Sethi CS, Lewis GP, Fisher SK. Proliferative vitreoretinopathy - developments in adjunctive treatment and retinal pathology. Eye. 2002;16(4):369–74. doi: 10.1038/sj.eye.6700194. [DOI] [PubMed] [Google Scholar]
- Cowley 1989
- Cowley M, Conway BP, Campochiaro PA, Kaiser D, Gaskin H. Clinical risk factors for proliferative vitreoretinopathy. Archives of Ophthalmology. 1989;107(8):1147–51. doi: 10.1001/archopht.1989.01070020213027. [DOI] [PubMed] [Google Scholar]
- Deeks 2011
- Deeks JJ, Higgins JPT, Altman DG, Higgins JPT, Green S, editors. Chapter 9: Analysing data and undertaking meta-analyses. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. 2011 (updated March 2011). The Cochrane Collaboration. Available from www.cochrane-handbook.org.
- Eckardt 1990
- Eckardt C, Schmidt D, Czank M. Intraocular tolerance to silicone oils of different specific gravities: an experimental study. Ophthalmologica. 1990;201(3):133–9. doi: 10.1159/000310141. [DOI] [PubMed] [Google Scholar]
- Garweq 2013
- Garweq JG, Tappeiner C, Halberstadt M. Pathophysiology of proliferative vitreoretinopathy in retinal detachment. Survey of Ophthalmology. 2013;58(4):321–9. doi: 10.1016/j.survophthal.2012.12.004. [DOI] [PubMed] [Google Scholar]
- Glanville 2006
- Glanville JM, Lefebvre C, Miles JN, Camosso-Stefinovic J. How to identify randomized controlled trials in MEDLINE: ten years on. Journal of the Medical Library Association. 2006;94(2):130–6. [PMC free article] [PubMed] [Google Scholar]
- Heimann 2007
- Heimann H, Bartz-Schmidt KU, Bornfeld N, Weiss C, Hilgers RD, Foerster MH. Scleral Buckling versus Primary Vitrectomy in Rhegmatogenous Retinal Detachment Study Group. Scleral buckling versus primary vitrectomy in rhegmatogenous retinal detachment: a prospective randomized multicenter clinical study. Ophthalmology. 2007;114(12):2142–54. doi: 10.1016/j.ophtha.2007.09.013. [DOI] [PubMed] [Google Scholar]
- Heimann 2008
- Heimann H, Stappler T, Wong D. Heavy tamponade 1: a review of indications, use, and complications. Eye. 2008;22(10):1342–59. doi: 10.1038/eye.2008.61. [DOI] [PubMed] [Google Scholar]
- Higgins 2011
- Higgins JPT, Altman DG, Higgins JPT, Green S, editors. Chapter 8: Assessing risk of bias in included studies. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. 2011 (updated March 2011). The Cochrane Collaboration. Available from www.cochrane-handbook.org.
- Hoerauf 2005
- Hoerauf H, Roider J, Kobuch K, Laqua H. Perfluorohexylethan (O62) as ocular endotamponade in complex vitreoretinal surgery. Retina. 2005;25(4):479–88. doi: 10.1097/00006982-200506000-00014. [DOI] [PubMed] [Google Scholar]
- Kruger 2002
- Kruger EF, Nguyen QD, Ramos-Lopez M, Lashkari K. Proliferative vitreoretinopathy after trauma. International Ophthalmology Clinics. 2002;42(3):129–43. doi: 10.1097/00004397-200207000-00015. [DOI] [PubMed] [Google Scholar]
- Krzystolik 2000
- Krzystolik MG, D’Amico DJ. Complications of intraocular tamponade: silicone oil versus intraocular gas. International Ophthalmology Clinics. 2000;40(1):187–200. doi: 10.1097/00004397-200040010-00018. [DOI] [PubMed] [Google Scholar]
- Matteucci 2007
- Matteucci A, Formisano G, Paradisi S, Carnovale-Scalzo G, Scorcia G, Caiazza S, et al. Biocompatibility assessment of liquid artificial vitreous replacements: relevance of in vitro studies. Survey of Ophthalmology. 2007;52(3):289–99. doi: 10.1016/j.survophthal.2007.02.004. [DOI] [PubMed] [Google Scholar]
- Patel 2004
- Patel NN, Bunce C, Asaria RH, Charteris DG. Resources involved in managing retinal detachment complicated by proliferative vitreoretinopathy. Retina. 2004;24(6):883–7. doi: 10.1097/00006982-200412000-00007. [DOI] [PubMed] [Google Scholar]
- RevMan 2012
- Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
- Rofail 2005
- Rofail M, Lee LR. Perfluoro-n-octane as a postoperative vitreoretinal tamponade in the management of giant retinal tears. Retina. 2005;25(7):897–901. doi: 10.1097/00006982-200510000-00013. [DOI] [PubMed] [Google Scholar]
- Schwartz 2004
- Schwartz SG, Mieler WF. Management of primary rhegmatogenous retinal detachment. Comprehensive Ophthalmology Update. 2004;5:285–94. [Google Scholar]
- Scott 2002
- Scott IU, Murray TG, Flynn HW, Jr, Feuer WJ, Schiffman JC, Perfluoron Study Group Outcomes and complications associated with giant retinal tear management using perfluoro-n-octane. Ophthalmology. 2002;109(10):1828–33. doi: 10.1016/s0161-6420(02)01184-3. [DOI] [PubMed] [Google Scholar]
- Scott 2005
- Scott IU, Flynn HW, Jr, Murray TG, Smiddy WE, Davis JL, Feuer WJ. Outcomes of complex retinal detachment repair using 1000- vs 5000-centistoke silicone oil. Archives of Ophthalmology. 2005;123(4):473–8. doi: 10.1001/archopht.123.4.473. [DOI] [PubMed] [Google Scholar]
- Singh 1986
- Singh AK, Glaser BM, Lemor M, Michels RG. Gravity-dependent distribution of retinal pigment epithelial cells dispersed into the vitreous cavity. Retina. 1986;6(2):77–80. doi: 10.1097/00006982-198600620-00002. [DOI] [PubMed] [Google Scholar]
- Singh 2001
- Singh J, Ramaesh K, Wharton SB, Cormack G, Chawla HB. Perfluorodecalin-induced intravitreal inflammation. Retina. 2001;21(3):247–51. doi: 10.1097/00006982-200106000-00009. [DOI] [PubMed] [Google Scholar]
- Stappler 2008
- Stappler T, Heimann H, Wong D, Gibran SK, Groenewald C, Pearce IA. Heavy tamponade 2 Densiron 68 in routine clinical practice: anatomical and functional outcomes of a consecutive case series. Eye. 2008;22(10):1360–5. doi: 10.1038/eye.2008.62. [DOI] [PubMed] [Google Scholar]
- Sterne 2011
- Sterne JAC, Egger M, Moher D, Higgins JPT, Green S, editors. Chapter 10: Addressing reporting biases. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. 2011 (updated March 2011). The Cochrane Collaboration. Available from www.cochrane-handbook.org. [Google Scholar]
- Tognetto 2005
- Tognetto D, Minutola D, Sanguinetti G, Ravalico G. Anatomical and functional outcomes after heavy silicone oil tamponade in vitreoretinal surgery for complicated retinal detachment. A pilot study. Ophthalmology. 2005;112(9):1574–8. doi: 10.1016/j.ophtha.2005.04.013. [DOI] [PubMed] [Google Scholar]
- Tognetto 2008
- Tognetto D, Lepori L, Lapasin R, Minutola D, Sanguinetti G, Michelone L, et al. A new heavy internal tamponade in vitreoretinal surgery: an in vitro study. Eye. 2008;22(8):1082–8. doi: 10.1038/eye.2008.144. [DOI] [PubMed] [Google Scholar]
- TRSTC 1983
- The Retina Society Terminology Committee The classification of retinal detachment with proliferative vitreoretinopathy. Ophthalmology. 1983;90(2):121–5. doi: 10.1016/s0161-6420(83)34588-7. [DOI] [PubMed] [Google Scholar]
- Young 2005
- Young TA, D’Amico DJ. Controversies in proliferative vitreoretinopathy tamponade and pharmacologic agents. International Ophthalmology Clinics. 2005;45(5):163–71. doi: 10.1097/01.iio.0000176368.93887.2c. [DOI] [PubMed] [Google Scholar]
- References to other published versions of this review
- Schwartz 2006
- Schwartz SG, Lee WH, Flynn HW., Jr Tamponade in surgery for retinal detachment associated with proliferative vitreoretinopathy. Cochrane Database of Systematic Reviews. 2006;(3) doi: 10.1002/14651858.CD006126.pub3. [DOI: 10.1002/14651858.CD006126] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz 2009
- Schwartz SG, Flynn HW, Jr, Lee WH, Ssemanda E, Ervin AM. Tamponade in surgery for retinal detachment associated with proliferative vitreoretinopathy. Cochrane Database of Systematic Reviews. 2009;(4) doi: 10.1002/14651858.CD006126.pub2. [DOI: 10.1002/14651858.CD006126.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Indicates the major publication for the study