Abstract
Normal myotubes and adult innervated skeletal myofibers express the glycoprotein pannexin1 (Panx1). Six of them form a “gap junction hemichannel-like” structure that connects the cytoplasm with the extracellular space; here they will be called Panx1 channels. These are poorly selective channels permeable to ions, small metabolic substrate, and signaling molecules. So far little is known about the role of Panx1 channels in muscles but skeletal muscles of Panx1−/− mice do not show an evident phenotype. Innervated adult fast and slow skeletal myofibers show Panx1 reactivity in close proximity to dihydropyridine receptors in the sarcolemma of T-tubules. These Panx1 channels are activated by electrical stimulation and extracellular ATP. Panx1 channels play a relevant role in potentiation of muscle contraction because they allow release of ATP and uptake of glucose, two molecules required for this response. In support of this notion, the absence of Panx1 abrogates the potentiation of muscle contraction elicited by repetitive electrical stimulation, which is reversed by exogenously applied ATP. Phosphorylation of Panx1 Thr and Ser residues might be involved in Panx1 channel activation since it is enhanced during potentiation of muscle contraction. Under denervation, Panx1 levels are upregulated and this partially explains the reduction in electrochemical gradient, however its absence does not prevent denervation-induced atrophy but prevents the higher oxidative state. Panx1 also forms functional channels at the cell surface of myotubes and their functional state has been associated with intracellular Ca2+ signals and regulation of myotube plasticity evoked by electrical stimulation. We proposed that Panx1 channels participate as ATP channels and help to keep a normal oxidative state in skeletal muscles.
Keywords: potentiation, sarcolemma leakage, muscular plasticity, phosphorylation, ATP
Pannexin 1 based channels: structure and expression
Myotubes (Buvinic et al., 2009) and innervated myofibers (Cea et al., 2013; Riquelme et al., 2013) express pannexin1 (Panx1). Panx genes (PANX) are a small family; three of them have been cloned from mammals and are called PANX1, PANX2, and PANX3. They are orthologs of invertebrate innexins (Inxs) (Ambrosi et al., 2010), which form gap junction channels allowing the direct cytoplasm communication between adjacent cells (Sáez et al., 2003). Presently, it has been proposed that gap junction channels of chordates have to be formed exclusively by connexins (Cxs) (Dahl and Keane, 2012). Gap junction channel formation requires plasma membrane proximity of two cells and the docking of two hemichannels (half of a gap junction channel), each one provided by one of two adjacent cells (Sáez et al., 2003). However, mammalian Panxs and Inxs do not share significant gene and protein sequence homology with chordate Cxs. Nevertheless, Panx channels share some pharmacological properties with Cx-based channels and present some differences in sensitivity to different compounds and conditions including Probenecid, carbenoxolone, flufenamic acid, La3+ and cytoplasmic acidification (D'Hondt et al., 2009). Panx1 channels are also inhibited by brilliant blue G, a P2X receptor blocker, and by the food dye FD&C blue N° 1 [Brilliant Blue FCF (BB FCF) or Blue 1] (Wang et al., 2013), present in Blue Gatorade (see in nutritional facts on official web site www.gatorade.com) and Blue M&M (see in ingredients on official web site www.mms.com). Also, they share some permeability properties with Cx hemichannels, since they are permeable to small positively [i.e., ethidium (Etd+), DAPI] and negatively (i.e., gluconate, glutamate, ATP, and aspartate) charged organic molecules and inorganic ions (Na+, K+, Ca2+, and Cl−). In addition, Panx1 forms gap junction channels in exogenous expression systems including Xenopus laevis oocytes and some mammalian cells lines (Vanden Abeele et al., 2006). Nevertheless, up to the present there is no in vivo evidence that Panxs form gap junction channels, as compared to the evidence for Cxs or Inxs (Dahl and Keane, 2012). Therefore, these channels seem to work exclusively as channels in the nonjunctional membrane, allowing the exchange of molecules between the cytoplasm and the extracellular space. Panx1 channels present in the reticular system and the plasma membrane are activated by positive membrane potentials, high extracellular K+, extracellular ATP via P2 receptors, stretch-induced membrane deformation and posttranslational modifications (D'Hondt et al., 2013).
Panx1 is ubiquitously expressed in several tissues. Different levels of mRNA are detected in northern blots, being strongly expressed in heart, skeletal muscle, testis, and ovary. Medium levels are present in brain (Bruzzone et al., 2003), placenta, thymus, kidney, prostate, and small intestine, and low to almost undetectable levels are found in lung, liver, pancreas, spleen, colon, and peripheral blood (Baranova et al., 2004). The pattern of expression of the other two Panxs is completely different; Panx2 is preferentially expressed in brain (Baranova et al., 2004) and Panx3, mostly expressed in bone and skin (Sandilos and Bayliss, 2012). Panx1 channels are oligomeric structures formed by 6 subunits of Panx1 protein (Boassa et al., 2007). Panx1 shows similar membrane topology than Cxs and is characterized by four transmembrane (TM) segments, two extracellular loops, and cytoplasmic localization of both amino and C-termini. Panxs 1 and 3 are glycoproteins, unlike Cxs that are not glycosylated. It has been proposed that glycosylation of Panxs prevents formation of gap junction channels (Boassa et al., 2008). Panx1 and Panx3 suffer N-linked glycosylation at Asp254 present in the second extracellular loop and Asp71 found at the first extracellular loop, respectively. However, a fraction of Panx1 mutant that is not glycosylated still traffics to the cell surface, suggesting that Panx1 glycosylated protein could interact and form channels with unglycosylated forms and/or that the unglycosylated Panx1 fraction forms gap junction channel-like structures (Penuela et al., 2009).
Structural analyses of the lining residues of the Panx1 pore using the substituted cysteine accessibility method (SCAM) have revealed that the TM1 region and domains of the first extracellular loop (E1) are exposed to the channel lumen. Also, C-terminal amino acids substitution and reagent perturbation suggest the contribution of this segment to the permeation pathway (Wang and Dahl, 2010). Panx1 channels activated by positive membrane potentials show several substates. The full open state presents a characteristically high conductance in different cell types including Xenopus oocytes, insulinoma cells and cardio myocytes (Bao et al., 2004; Iglesias et al., 2009; Kienitz et al., 2011). However, recent studies using different Panx1 transfectants revealed a characteristic unitary conductance of ~60 pS (Ma et al., 2009; Romanov et al., 2012). Moreover, the permeability of Panx1 channels has been recently shown to be negligible to anions exceeding 250 Da, which would exclude ATP (Romanov et al., 2012). This apparent controversy might be explained by Panx1 channel variations due to pore properties, such as diameter and length changes caused by different interactions with other cellular proteins or due to different post-translational modifications in different cell types. Thus further studies maybe required to clarify this issue.
Activation of Panx1 channels
Several stimuli increase the activity of Panx1 channels. Among them are: increase in extracellular K+ concentration, positive membrane voltage over +40 mv, extracellular ligands, such as ATP [which activates Panx1 channels in micromolar concentrations (Locovei et al., 2006) but inhibit them in milimolar concentrations (Qiu and Dahl, 2009)], that enhance the intracellular free Ca2+ levels including like P2Y1−2 receptors coupled to Gq proteins or P2X7 receptors that are non-selective cationic channels with a slow kinetic of inactivation (Locovei et al., 2006, 2007; Iglesias et al., 2008). Panx1 channel opening has been induced by glutamate through NMDA receptor activation in neurons (Thompson et al., 2008; Orellana et al., 2011), neuronal stress induced by oxygen glucose deprivation (Thompson et al., 2008), hypertonic stress in lymphocytes (Woehrle et al., 2010) and increase in intracellular free Ca2+ levels induced by Ca2+ ionophore (Locovei et al., 2006). Although Panx1 does not have putative Ca2+ biding sites, it possesses multiple phosphorylation consensus sites in the C-terminal tail to several serine and threonine kinases (Penuela et al., 2007; Riquelme et al., 2013). A possible mechanism of activation of Panx1 channels that involves phosphorylation has been recently suggested. During repetitive skeletal muscle contraction, the Panx1 channel activity increases and the state of phosphorylation of Panx1 Ser and Thr residues is also increased (Riquelme et al., 2013). In contrast, phosphorylation of Tyr residues in Panx1 has not been detected yet (Iglesias et al., 2008; Riquelme et al., 2013). A protein phosphorylation-dependent activation could be followed by inactivation via a phosphoprotein phosphatase or by phosphorylation of a different amino acid residue by another protein kinase with less Ca2+ affinity, followed by complete dephosphorylation via a protein phosphatase.
An alternative mechanism of Panx1 channel activation in skeletal muscle could be a direct protein-protein dependent mechanism (Panx1 channel/dihydropyridine receptor) mediated by conformational changes of voltage activated dihydropyridine receptors induced by depolarizing membrane potentials. In support of this view it is possible to say that electrical stimulation induces ATP release and uptake of Etd+ and fluorescent glucose derivatives in myofibers, indicating opening of Panx1 channels (Riquelme et al., 2013). Also, myotubes lacking the Cav1.1-α1 subunit released almost no ATP upon electrical stimulation, suggesting that Cav1.1 plays a critical role in this process (Jorquera et al., 2013).
Role of Panx1 based channels in normal skeletal muscles
Possible role of Panx1 channels in muscular ontogeny
Skeletal muscles develop through a process partially coordinated by extracellular signaling. The coordinate response of cell groups includes the myogenic commitment of mesodermal pluripotent cells, myoblast alignment and fusion. In mice, this process requires the presence of Cx43 expression and functional gap junction channels (Kalderon et al., 1977; Proulx et al., 1997; Araya et al., 2003). In rats, these channels disappear at about 1 week postnatal age when skeletal muscles become innervated (Cea et al., 2012).
The acquisition of myogenic commitment requires increase of [Ca2+]i, and activation of calcineurin, a Ca2+-dependent protein phosphatase that induces expression of the transcription factor myf-5 (Friday and Pavlath, 2001). Increases in [Ca2+]i could be induced by activation of P2 receptors with extracellular ATP/ADP. In addition, activation of P2X receptors 2, 4 or 7 increases the cell membrane permeability to small molecules, including Lucifer yellow, Etd+ and YO-PRO-1, in diverse cell types such as myoblasts and macrophages (North, 2002; Araya et al., 2005; Pelegrin and Surprenant, 2006). However, Panx1 has been proposed to mediate the plasma membrane permeabilization to dyes after activation of P2X/Y receptors (Pelegrin and Surprenant, 2006; Locovei et al., 2006, 2007).
Treatment with a concentration of β-glycyrrhetinic acid that blocks connnexin-based channels (gap junction channels and hemichannels) and Panx1 channels (Bruzzone et al., 2005) prevents the expression of myogenin and MRF4, two transcription factors that promote myogenesis and myotubes formation (Proulx et al., 1997). However, treatment with octanol, blocker of Cx-based channels but not Panx1 channels (Bruzzone et al., 2005; Pelegrin and Surprenant, 2006), does not block myogenesis as evaluated by the expression of the pro-myogenic transcription factor Myf-5 (Proulx et al., 1997). Thus, the presence of functional Panx1 channels might be enough to promote commitment and myogenesis in vitro, and probably Cx hemichannels and/or other Ca2+ channels have a redundant role that overcomes the lack of Panx1, since Panx1−/− mice do not show evident muscular phenotype changes. In agreement with the role of Cx-based channels coordinating the commitment of myoblast, Cx43 deficient muscles show a delay regeneration response after BaCl2-induced damage (Araya et al., 2005).
Potentiation of muscular contraction
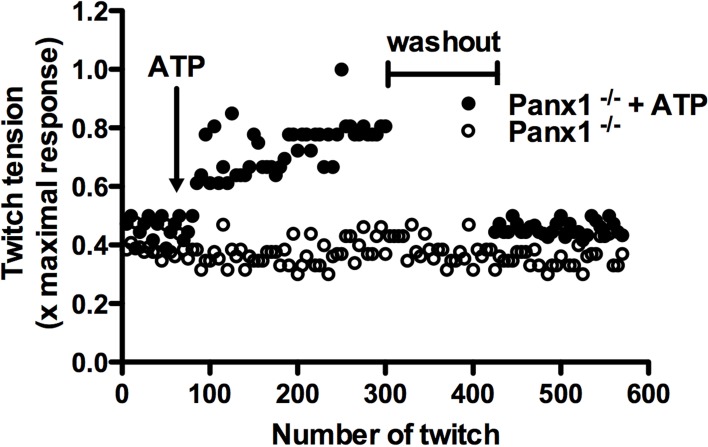
The force generated in muscular contraction increases after repetitive twitches. This response is called potentiation and depends on an increase of intracellular free Ca2+ concentration (Sandonà et al., 2005; Zhi et al., 2005) due to Ca2+ release from intracellular stores and Ca2+ inflow from the extracellular space in fast and slow twitch muscles, respectively. In the latter, Ca2+ uptake depends on the activation of purinergic ionotropic P2X4 receptors (Sandonà et al., 2005), which are highly expressed in this type of muscle. For fast twitch muscles, the potentiation response is independent of the extracellular Ca2+ concentration (Louboutin et al., 1996), but requires extracellular ATP. Accordingly, extracellular ATPases inhibit the potentiation response (Sandonà et al., 2005) and, together with the absence of P2X receptors, have led to suggest that the potentiation response in these muscles is mediated by activation of metabotropic P2 receptors as P2Y1 receptors (Riquelme et al., 2013). Recently, Panx1 channels were proposed as a possible pathway for ATP release in skeletal muscles (Riquelme et al., 2013). In normal adult skeletal muscle, Panx1 was localized in the sarcolemma of the T-tubules system (Cea et al., 2012; Riquelme et al., 2013; Jorquera et al., 2013). Moreover, Panx1 channels were activated by electrical stimulation allowing the uptake of glucose (a fluorescent glucose analog, 2-NBDG) and release of ATP, which are necessary for potentiation of muscular contraction (Sandonà et al., 2005; Riquelme et al., 2013). In addition, the absence of Panx1 in myofibers of Panx1 deficient mice (Panx1−/− mice) does not exhibit potentiation of muscle contraction induced by electrical stimulation. Therefore, it was suggested that this response is due to the absence of ATP release (Riquelme et al., 2013). In agreement with this notion, the lost potentiation response of skeletal muscles from Panx1−/− mice can be reversed by addition of exogenous ATP to the bath, shown for the first time here (Figure 1). This finding confirms that Panx1 channels are necessary for the potentiation response, and is consistent with the hypothesis that potentiation in vivo could be due to permeability of Panx1 channels to ATP, at least in activated skeletal muscles. It further suggests that the presumptive Panx1 channel-dependent mechanism for ATP release is not compensated by other pathways of ATP release, or other possible mechanisms of potentiation.
Figure 1.
Exogenous ATP reverses the absence of potentiation of muscular contraction in Panx1 deficient mice. Potentiation of muscular contraction was induced by electrical transmural stimulation (0.03 Hz, 45 V, 100 ms stimuli duration) in isolated Soleus muscle from Pannexin 1 deficient mice (Panx1−/−), and exogenous ATP was applied (200 μ M, final concentration, closed circles) after 65 twitches. Then, the muscle was rinsed during 2 min and the potentiation response was re-evaluated. In addition, the potentiation response with the same number of twitches was evaluated in absence of ATP and washout (open circles).
Role of Panx1 channels in pathological conditions of skeletal muscles
Recently, denervation was shown to induce de novo expression of Cx-based hemichannels that mediate a drastic increase in sarcolemma permeability and leads to muscular atrophy (Cea et al., 2013). In addition, it was found that Panx1 channels do not play a crucial role on this phenomenon, since muscles of Panx1−/− mice showed similar increase in membrane permeability and atrophy to those observed in denervated muscles of wild type animals (Cea et al., 2013). Until now, this is the only work published in which a possible pathological role of Panx1-based channels in skeletal muscles has been evaluated. In addition, it was analyzed the production of thiobarbituric reactive substances (TBARS), including malondialdehyde (MDA), as a measure of oxidative stress in denervated skeletal muscles of Panx1−/− mice because denervation is known to increase the skeletal muscle levels of reactive oxygen species (ROS) (Abruzzo et al., 2010). We found, in this work, that the absence of Panx1 does not affect the basal levels of TBARS but prevented the increase in TBARS levels present in 7-day denervated wild type muscles (Figure 2), suggesting that Panx1 channels might allow loss of reducing agents and thus their absence would protect against ROS generation. Alternatively, constitutive Panx1−/− muscles might have developed more antioxidant phenotype and thus could be more resistant to the denervation-induced ROS generation.
Figure 2.
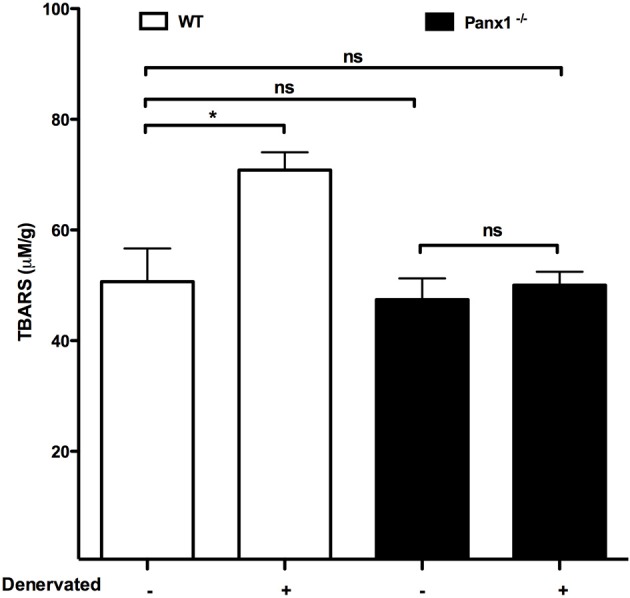
The absence of Panx1 prevents the increase in levels of thiobarbituric reactive substances in denervated skeletal muscles. Seven days after denervation gastrocnemius muscles were isolated from wild type (wt, white bar), or Panx1 deficient mice (Panx1−/−, black bar), and levels of thiobarbituric acid reactive substances (TBARS) were evaluated in denervated vs. not denervated muscles. *P < 0.05; ns, not significant.
Skeletal muscle plasticity
The skeletal muscle activity induces remodeling of structure and functional performance of myofibers, changing the muscular force output, endurance and contractile velocity with respect to a functional demand (Tavi and Westerblad, 2011). All these changes are called skeletal muscle plasticity. This adaptive process depends on the frequency of repetitive fiber contraction, activation of intracellular signal pathways and gene expression that promotes the establishment of new myofiber characteristics (Tavi and Westerblad, 2011). The physiological importance of skeletal muscle plasticity is evident; however, the molecular mechanisms involved in muscle plasticity remain elusive. Electrical stimuli at frequencies that activate Panx1 channels (20 Hz) induce the expression of a molecular marker that reflects the transition between a fast to slow myofiber (Jorquera et al., 2013). Panx1 channels present in the T-tubule membrane are regulated for the dihydropyridine receptors; with high frequencies (90 Hz) the activity of Panx1 channels is low and the expression of plasticity marker in myofibers does not change (Jorquera et al., 2013). The electrical stimulation induces ATP release through Panx1 channels, eliciting an IP3-dependent intracellular Ca2+ signal (Eltit et al., 2006) which is directly associated with gene expression changes (Semsarian et al., 1999; Jaimovich and Carrasco, 2002; Carrasco et al., 2003; Buvinic et al., 2009). These responses depend on the activation of P2 receptors by extracellular ATP because apyrase, an ATP hydrolase, or suramin, a general P2 receptors inhibitor, blocks both signals. The metabotropic Ca2+ signal induced by extracellular ATP was prevented by Panx1 channel inhibitors (10Panx1 and oleamide) that reduced the calcium transients and the ATP release (Buvinic et al., 2009). Consequently, it was proposed that a train of action potentials with a defined frequency induces Ca2+ release events that differentially activate Ca2+-dependent signaling pathways, which determine the expression of genes responsible for the slow or fast muscle phenotype (Tavi and Westerblad, 2011). These signaling pathways include calcineurin–NFAT-, Ca2+/calmodulin-dependent kinases II and IV- and protein kinase C-dependent pathways (Tavi and Westerblad, 2011). These Ca2+ signals lead to muscular plasticity by modulating the expression of several genes including IL-6 and c-fos, and the switch between troponin isoform from fast to slow fiber (Buvinic et al., 2009; Jorquera et al., 2013).
Concluding remarks
Panx1 channels are involved in several relevant physiological skeletal muscle processes, such as potentiation of skeletal muscle contraction since they do not occur in the absence of these channels. Currently, Panx1 channels have been clearly involved in potentiation of contraction of skeletal muscles. In addition, Panx1 channels appear to mediate the release of ATP involved in muscle remodeling. People who are active in sports should be cautious with food and drink additives that could reduce the Panx1 channel activity and consequently the potentiation response of muscle contraction. Moreover, to further understand the possible role of Panx1 channels in pathologic responses it may be needed to develop highly selective Panx1 channels inhibitors and/or acute down regulation of Panx1 expression using inducible KO animals or transfections with siRNA or morpholines. Finally, Panx1 channels act as ATP channels and the absence prevent the induction of reactive oxygen species; measured as levels of TBARS in this work (Figure 2), suggesting that Panx1 channels are relevant to keep healthy skeletal muscles.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was partially supported by grants Fondecyt 1111033 (to Luis A. Cea and Juan C. Sáez) and Chilean Science Millennium Institute P09-022-F (to Juan C. Sáez).
References
- Abruzzo P. M., di Tullio S., Marchionni C., Belia S., Fanó G., Zampieri S., et al. (2010). Oxidative stress in the denervated muscle. Free Radic. Res. 44, 563–576 10.3109/10715761003692487 [DOI] [PubMed] [Google Scholar]
- Ambrosi C., Gassmann O., Pranskevich J. N., Boassa D., Smock A., Wang J., et al. (2010). Pannexin1 and Pannexin2 channels show quaternary similarities to connexons and different oligomerization numbers from each other. J. Biol. Chem. 285, 24420–24431 10.1074/jbc.M110.115444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araya R., Eckardt D., Maxeiner S., Krüger O., Theis M., Willecke K., et al. (2005). Expression of connexins during differentiation and regeneration of skeletal muscle: functional relevance of connexin43. J. Cell Sci. 118, 27–37 10.1242/jcs.01553 [DOI] [PubMed] [Google Scholar]
- Araya R., Eckardt D., Riquelme M. A., Willecke K., Sáez J. C. (2003). Presence and importance of connexin43 during myogenesis. Cell Commun. Adhes. 10, 451–456 10.1080/cac.10.4-6.451.456 [DOI] [PubMed] [Google Scholar]
- Bao L., Locovei S., Dahl G. (2004). Pannexin membrane channels are mechanosensitive conduits for ATP. FEBS Lett. 572, 65–68 10.1016/j.febslet.2004.07.009 [DOI] [PubMed] [Google Scholar]
- Baranova A., Ivanov D., Petrash N., Pestova A., Skoblov M., Kelmanson I., et al. (2004). The mammalian pannexin family is homologous to the invertebrate innexin gap junction proteins. Genomics 83, 706–716 10.1016/j.ygeno.2003.09.025 [DOI] [PubMed] [Google Scholar]
- Boassa D., Ambrosi C., Qiu F., Dahl G., Gaietta G., Sosinsky G. (2007). Pannexin1 channels contain a glycosylation site that targets the hexamer to the plasma membrane. J. Biol. Chem. 282, 31733–31743 10.1074/jbc.M702422200 [DOI] [PubMed] [Google Scholar]
- Boassa D., Qiu F., Dahl G., Sosinsky G. (2008). Trafficking dynamics of glycosylated pannexin 1 proteins. Cell Commun. Adhes. 15, 119–132 10.1080/15419060802013885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruzzone R., Barbe M. T., Jakob N. J., Monyer H. (2005). Pharmacological properties of homomeric and heteromeric pannexin hemichannels expressed in xenopus oocytes. J. Neurochem. 92, 1033–1043 10.1111/j.1471-4159.2004.02947.x [DOI] [PubMed] [Google Scholar]
- Bruzzone R., Hormuzdi S. G., Barbe M. T., Herb A., Monyer H. (2003). Pannexins, a family of gap junction proteins expressed in brain. Proc. Natl. Acad. Sci. U.S.A. 100, 13644–13649 10.1073/pnas.2233464100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buvinic S., Almarza G., Bustamante M., Casas M., López J., Riquelme M., et al. (2009). ATP released by electrical stimuli elicits calcium transients and gene expression in skeletal muscle. J. Biol. Chem. 284, 34490–34505 10.1074/jbc.M109.057315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasco M. A., Riveros N., Ríos J., Müller M., Torres F., Pineda J., et al. (2003). Depolarization-induced slow calcium transients activate early genes in skeletal muscle cells. Am. J. Physiol. Cell Physiol. 284, C1438–C1447 10.1152/ajpcell.00117.2002 [DOI] [PubMed] [Google Scholar]
- Cea L. A., Cisterna B. A., Puebla C., Frank M., Figueroa X. F., Cardozo C., et al. (2013). De novo expression of connexin hemichannels in denervated fast skeletal muscles leads to atrophy. Proc. Natl. Acad. Sci. U.S.A. 110, 16229–16234 10.1073/pnas.1312331110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cea L. A., Riquelme M. A., Cisterna B. A., Puebla C., Vega J. L., Rovegno M., et al. (2012). Connexin- and pannexin-based channels in normal skeletal muscles and their possible role in muscle atrophy. J. Membr. Biol. 245, 423–436 10.1007/s00232-012-9485-8 [DOI] [PubMed] [Google Scholar]
- Dahl G., Keane R. W. (2012). Pannexin: from discovery to bedside in 11±4 years? Brain Res. 1487, 150–159 10.1016/j.brainres.2012.04.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Hondt C., Iyyathurai J., Vinken M., Rogiers V., Leybaert L., Himpens B., et al. (2013). Regulation of connexin- and pannexin-based channels by post-translational modifications. Biol. Cell. 10, 373–398 10.1111/boc.201200096 [DOI] [PubMed] [Google Scholar]
- D'Hondt C., Ponsaerts R., De Smedt H., Bultynck G., Himpens B. (2009). Pannexins, distant relatives of the connexin family with specific cellular functions? Bioessays 31, 953–974 10.1002/bies.200800236 [DOI] [PubMed] [Google Scholar]
- Eltit J. M., García A. A., Hidalgo J., Liberona J. L., Chiong M., Lavandero S., et al. (2006). Membrane electrical activity elicits inositol 1,4,5-trisphosphate-dependent slow Ca2+ signals through a gbetagamma/phosphatidylinositol 3-kinase gamma pathway in skeletal myotubes. J. Biol. Chem. 281, 12143–12154 10.1074/jbc.M511218200 [DOI] [PubMed] [Google Scholar]
- Friday B. B., Pavlath G. K. (2001). A calcineurin- and NFAT-dependent pathway regulates Myf5 gene expression in skeletal muscle reserve cells. J. Cell Sci. 114, 303–310 [DOI] [PubMed] [Google Scholar]
- Iglesias R., Locovei S., Roque A., Alberto A. P., Dahl G., Spray D. C., et al. (2008). P2X7 receptor-Pannexin1 complex: pharmacology and signaling. Am. J. Physiol. Cell Physiol. 25, C752–C760 10.1152/ajpcell.00228.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iglesias R., Spray D. C., Scemes E. (2009). Mefloquine blockade of Pannexin1 currents: resolution of a conflict. Cell Commun. Adhes. 16, 131–137 10.3109/15419061003642618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaimovich E., Carrasco M. A. (2002). IP3 dependent Ca2+ signals in muscle cells are involved in regulation of gene expression. Biol. Res. 35, 195–202 10.4067/S0716-97602002000200010 [DOI] [PubMed] [Google Scholar]
- Jorquera G., Altamirano F., Contreras-Ferrat A., Almarza G., Buvinic S., Jacquemond V., et al. (2013). Cav1.1 controls frequency-dependent events regulating adult skeletal muscle plasticity. J. Cell Sci. 126, 1189–1198 10.1242/jcs.116855 [DOI] [PubMed] [Google Scholar]
- Kalderon N., Epstein M. L., Gilula N. B. (1977). Cell-to-cell communication and myogenesis. J. Cell Biol. 75, 788–806 10.1083/jcb.75.3.788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kienitz M. C., Bender K., Dermietzel R., Pott L., Zoidl G. (2011). Pannexin 1 constitutes the large conductance cation channel of cardiac myocytes. J. Biol. Chem. 286, 290–298 10.1074/jbc.M110.163477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locovei S., Scemes E., Qiu F., Spray D. C., Dahl G. (2007). Pannexin1 is part of the pore forming unit of the P2X(7) receptor death complex. FEBS Lett. 581, 483–488 10.1016/j.febslet.2006.12.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locovei S., Wang J., Dahl G. (2006). Activation of pannexin 1 channels by ATP through P2Y receptors and by cytoplasmic calcium. FEBS Lett. 580, 239–244 10.1016/j.febslet.2005.12.004 [DOI] [PubMed] [Google Scholar]
- Louboutin J. P., Fichter-Gagnepain V., Noireaud J. (1996). External calcium dependence of extensor digitorum longus muscle contractility during bupivacaine-induced regeneration. Muscle Nerve 19, 994–1002 [DOI] [PubMed] [Google Scholar]
- Ma W., Hui H., Pelegrin P., Surprenant A. (2009).Pharmacological characterization of pannexin-1 currents expressed in mammalian cells. J. Pharmacol. Exp. Ther. 328, 409–418 10.1124/jpet.108.146365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- North R. A. (2002). Molecular physiology of P2X receptors. Physiol. Rev. 82, 1013–10167 10.1152/physrev.00015.2002 [DOI] [PubMed] [Google Scholar]
- Orellana J. A., Froger N., Ezan P., Jiang J. X., Bennett M. V., Naus C. C., et al. (2011). ATP and glutamate released via astroglial connexin 43 hemichannels mediate neuronal death through activation of pannexin 1 hemichannels. J. Neurochem. 118, 826–840 10.1111/j.1471-4159.2011.07210.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelegrin P., Surprenant A. (2006). Pannexin-1 mediates large pore formation and interleukin-1beta release by the ATP-gated P2X7 receptor. EMBO J. 25, 5071–5082 10.1038/sj.emboj.7601378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penuela S., Bhalla R., Gong X. Q., Cowan K. N., Celetti S. J., Cowan B. J., et al. (2007). Pannexin 1 and pannexin 3 are glycoproteins that exhibit many distinct characteristics from the connexin family of gap junction proteins. J. Cell Sci. 120, 3772–3783 10.1242/jcs.009514 [DOI] [PubMed] [Google Scholar]
- Penuela S., Bhalla R., Nag K., Laird D. W. (2009). Glycosylation regulates pannexin intermixing and cellular localization. Mol. Biol. Cell. 20, 4313–4323 10.1091/mbc.E09-01-0067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proulx A., Merrifield P. A., Naus C. C. (1997). Blocking gap junctional intercellular communication in myoblasts inhibits myogenin and MRF4 expression. Dev. Genet. 20, 133–144 [DOI] [PubMed] [Google Scholar]
- Qiu F., Dahl G. (2009). A permeant regulating its permeation pore: inhibition of pannexin 1 channels by ATP. Am. J. Physiol. Cell Physiol. 296, C250–C255 10.1152/ajpcell.00433.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riquelme M. A., Cea L. A., Vega J. L., Boric M. P., Monyer H., Bennett M. V., et al. (2013). The ATP required for potentiation of skeletal muscle contraction is released via pannexin hemichannels. Neuropharmacology 75, 594–603 . 10.1016/j.neuropharm.2013.03.022 [DOI] [PubMed] [Google Scholar]
- Romanov R. A., Bystrova M. F., Rogachevskaya O. A., Sadovnikov V. B., Shestopalov V. I., Kolesnikov S. S. (2012). The ATP permeability of pannexin 1 channels in a heterologous system and in mammalian taste cells is dispensable. J. Cell Sci. 125, 5514–5523 10.1242/jcs.111062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sáez J. C., Berthoud V. M., Brañes M. C., Martínez A. D., Beyer E. C. (2003). Plasma membrane channels formed by connexins: their regulation and functions. Physiol. Rev. 83, 1359–1400 10.1152/physrev.00007.2003 [DOI] [PubMed] [Google Scholar]
- Sandilos J. K., Bayliss D. A. (2012). Physiological mechanisms for the modulation of pannexin 1 channel activity. J. Physiol. 590, 6257–6266 10.1113/jphysiol.2012.240911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandonà D., Danieli-Betto D., Germinario E., Biral D., Martinello T., Lioy A., et al. (2005). The T-tubule membrane ATP-operated P2X4 receptor influences contractility of skeletal muscle. FASEB J. 19, 1184–1186 10.1096/fj.04-3333fje [DOI] [PubMed] [Google Scholar]
- Semsarian C., Wu M. J., Ju Y. K., Marciniec T., Yeoh T., Allen D. G., et al. (1999). Skeletal muscle hypertrophy is mediated by a Ca2+-dependent calcineurin signalling pathway. Nature 400, 576–581 10.1038/23054 [DOI] [PubMed] [Google Scholar]
- Tavi P., Westerblad H. (2011). The role of in vivo Ca2+ signals acting on Ca2+-calmodulin-dependent proteins for skeletal muscle plasticity. J. Physiol. 589, 5021–5031 10.1113/jphysiol.2011.212860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson R. J., Jackson M. F., Olah M. E., Rungta R. L., Hines D. J., Beazely M. A., et al. (2008). Activation of pannexin-1 hemichannels augments aberrant bursting in the hippocampus. Science 322, 1555–1559 10.1126/science.1165209 [DOI] [PubMed] [Google Scholar]
- Vanden Abeele F., Bidaux G., Gordienko D., Beck B., Panchin Y. V., Baranova A. V., et al. (2006). Functional implications of calcium permeability of the channel formed by pannexin 1. J. Cell Biol. 174, 535–546 10.1083/jcb.200601115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Dahl G. (2010). SCAM analysis of Panx1 suggests a peculiar pore structure. J. Gen. Physiol. 136, 515–527 10.1085/jgp.201010440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Jackson D. G., Dahl G. (2013). The food dye FD&C Blue No. 1 is a selective inhibitor of the ATP release channel Panx1. J. Gen. Physiol. 141, 649-656 10.1085/jgp.201310966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woehrle T., Yip L., Manohar M., Sumi Y., Yao Y., Chen Y., et al. (2010). Hypertonic stress regulates T cell function via pannexin-1 hemichannels and P2X receptors. J. Leukoc. Biol. 88, 1181–1189 10.1189/jlb.0410211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhi G., Ryder J. W., Huang J., Ding P., Chen Y., Zhao Y., et al. (2005). Myosin light chain kinase and myosin phosphorylation effect frequency-dependent potentiation of skeletal muscle contraction. Proc. Natl. Acad. Sci. U.S.A. 102, 17519–17524 10.1073/pnas.0506846102 [DOI] [PMC free article] [PubMed] [Google Scholar]