Abstract
Availability of inorganic nutrients, particularly nitrogen and phosphorous, is often a primary control on crude oil hydrocarbon degradation in marine systems. Many studies have empirically determined optimum levels of inorganic N and P for stimulation of hydrocarbon degradation. Nevertheless, there is a paucity of information on fundamental kinetic parameters for nutrient enhanced crude oil biodegradation that can be used to model the fate of crude oil in bioremediation programmes that use inorganic nutrient addition to stimulate oil biodegradation. Here we report fundamental kinetic parameters (Ks and qmax) for nitrate- and phosphate-stimulated crude oil biodegradation under nutrient limited conditions and with respect to crude oil, under conditions where N and P are not limiting. In the marine sediments studied, crude oil degradation was limited by both N and P availability. In sediments treated with 12.5 mg/g of oil but with no addition of N and P, hydrocarbon degradation rates, assessed on the basis of CO2 production, were 1.10 ± 0.03 μmol CO2/g wet sediment/day which were comparable to rates of CO2 production in sediments to which no oil was added (1.05 ± 0.27 μmol CO2/g wet sediment/day). When inorganic nitrogen was added alone maximum rates of CO2 production measured were 4.25 ± 0.91 μmol CO2/g wet sediment/day. However, when the same levels of inorganic nitrogen were added in the presence of 0.5% P w/w of oil (1.6 μmol P/g wet sediment) maximum rates of measured CO2 production increased more than four-fold to 18.40 ± 1.04 μmol CO2/g wet sediment/day. Ks and qmax estimates for inorganic N (in the form of sodium nitrate) when P was not limiting were 1.99 ± 0.86 μmol/g wet sediment and 16.16 ± 1.28 μmol CO2/g wet sediment/day respectively. The corresponding values for P were 63 ± 95 nmol/g wet sediment and 12.05 ± 1.31 μmol CO2/g wet sediment/day. The qmax values with respect to N and P were not significantly different (P < 0.05). When N and P were not limiting Ks and qmax for crude oil were 4.52 ± 1.51 mg oil/g wet sediment and 16.89 ± 1.25 μmol CO2/g wet sediment/day. At concentrations of inorganic N above 45 μmol/g wet sediment inhibition of CO2 production from hydrocarbon degradation was evident. Analysis of bacterial 16S rRNA genes indicated that Alcanivorax spp. were selected in these marine sediments with increasing inorganic nutrient concentration, whereas Cycloclasticus spp. were more prevalent at lower inorganic nutrient concentrations. These data suggest that simple empirical estimates of the proportion of nutrients added relative to crude oil concentrations may not be sufficient to guarantee successful crude oil bioremediation in oxic beach sediments. The data we present also help define the maximum rates and hence timescales required for bioremediation of beach sediments.
Keywords: oil spill, bioremediation, kinetics, Ks, half saturation constant, maximal rates, Alcanivorax, Cycloclasticus
Introduction
Natural hydrocarbon seeps are quantitatively the largest source of petroleum in marine systems, nevertheless, anthropogenic activities involved in the production transport and use of crude oil and oil products remain important sources of oil pollution (National Research Council, 2003). As a result of the localized release of relatively large quantities of oil, anthropogenic emissions may have effects on local ecosystems that are disproportionate to their contribution to global budgets of hydrocarbons in the sea. The incidence of major oil spills has decreased by 76% from 787 to 190 during the four decades from 1970 to 2010. In terms of volume this corresponds to a 93% decrease and, excluding the Deepwater Horizon blowout, the total quantity of oil spilt during 2010–2011 (13,000 tonnes from 13 recorded spills) was the lowest so far recorded (ITOPF, 2011). Although such statistics indicate that oil spills are generally declining, major accidents like the Deepwater Horizon blowout on 20th April 2010 in the Gulf of Mexico are a stark reminder that accidental oil spills remain an important environment hazard. The Deepwater Horizon accident resulted in the world's largest accidental release of crude oil to the sea, releasing an estimated 4.9 million barrels (780,000 m3) of light crude oil (OSAT-1, 2010). In offshore regions, the Deepwater Horizon spill had substantial impact on coral communities impacted by the plume from the Macondo well (White et al., 2012). In spite of intensive cleanup efforts, a portion of the spilled Macondo oil drifted to shore and remains trapped in coastal sediments. Concentrations of total petroleum hydrocarbon as high as 510 mg g−1 sediment were recorded in the surface 2 cm of heavily polluted marsh sediments even 7 months after the spill (Lin and Mendelssohn, 2012).
Crude oils comprise a complex heterogenous mixture of organic and inorganic compounds and broadly contain four groups of compounds; saturated and aromatic hydrocarbons, resins and asphaltenes (Harayama et al., 1999). Whereas lighter fractions evaporate or are degraded microbially, the heavier and more polar crude oil fractions persist due to their slow degradation rates (Walker et al., 1976). Many hydrocarbon degrading organisms are known (Prince, 2005) and in marine environments a number of specialist hydrocarbon degrading taxa are known (Yakimov et al., 2007). Marine saturated hydrocarbon degrading specialists include Alcanivorax (Yakimov et al., 1998), Planococcus (Engelhardt et al., 2001), Oleiphilus (Golyshin et al., 2002), Oleispira (Yakimov et al., 2003), Thalassolituus (Yakimov et al., 2004). Aromatic hydrocarbon degraders include Cycloclasticus spp. which utilize biphenyl, naphthalene, anthracene, phenanthrene, toluene, and benzoate (Dyksterhouse et al., 1995), and Neptunomonas which can degrade naphthalene, 2-methylnaphthalene and phenanthrene as sole carbon sources, but are unable to use 2,6-dimethylnaphthalene, 1-methylnaphthalene, biphenyl or acenaphthene (Hedlund et al., 1999). The chemical complexity of crude oil thus limits the capacity of a single species to degrade only certain components and the combined efforts of mixed bacterial consortia improve hydrocarbon bioremediation in marine environments (Röling et al., 2002; Dell'Anno et al., 2012). However, artificial microbial consortia cannot substitute for highly complex and dynamic indigenous microbial population essential for complete and efficient hydrocarbon degradation (McKew et al., 2007a).
Marine bacteria from the genera Alcanivorax and Cycloclasticus, have been implicated as key hydrocarbonoclastic agents on a global scale (Maruyama et al., 2003; Cappello et al., 2007). Their abundances and hydrocarbon degradation activity in polluted environments often increases significantly with a concomitant reduction in overall bacterial diversity (MacNaughton et al., 1999; Kasai et al., 2001, 2002a,b; Röling et al., 2002; Cappello et al., 2007; McKew et al., 2007b). A study on bacterial community response in beach sediment impacted by the Deepwater Horizon oil spill demonstrated that Alcanivorax spp. became dominant in polluted sediments and responded rapidly in the early stages following oiling (Kostka et al., 2011; Newton et al., 2013).
A 16S rRNA gene, PCR based denaturing gradient gel electrophoresis (DGGE) analysis and qPCR analysis of microbial population in nutrient amended crude oil treated marine sediment plots revealed an increase in number of Alcanivorax spp. and simultaneous appearance of alkB genes coding for alkane hydroxylase responsible for catabolism of alkanes (Röling et al., 2004; Singh et al., 2011). The success of Alcanivorax spp. as alkane degraders in part lies in their ability to use both branched chain and straight chain alkanes efficiently as sources of carbon and energy (Hara et al., 2003). Importantly, although Alcanivorax borkumensis SK2 genome has been shown to possess high affinity permeases for nitrate and phosphorus (Schneiker et al., 2006) it has been shown that the nitrate transporter ntrB gene and nirB1 for nitrite reductase are down-regulated in the presence of hexadecane by 3.93- and 6.5-fold respectively (Sabirova et al., 2011). Aromatic hydrocarbon degraders also exhibit a strong positive response to nutrient amendments. Abundance of Cycloclasticus spp. in heat treated Arabian light crude oil polluted gravel was shown to increase by 5 orders of magnitude under inorganic nutrient treated conditions and by 2 orders of magnitude under oil contaminated conditions with no nutrients, relative to unoiled sediments without nutrient amendments (Kasai et al., 2002b).
Since Alcanivorax spp and Cycloclasticus spp. do not compete for organic compounds as carbon sources, their initial abundance, metabolic superiority, and growth rate can be very crucial for determining their emergence, activity and ultimate relative abundance in hydrocarbon polluted environments. While these taxa do not compete directly for carbon and energy sources in oil-polluted environments they do compete for electron acceptors and inorganic nutrients and this may dictate the relative degradation of saturated and aromatic hydrocarbons. Indeed nutrient supply has been shown to have differential effects on rates of aliphatic and aromatic hydrocarbon degradation which has been interpreted in the context of resource ratio theory (Smith et al., 1998). Moreover, there is some evidence that inorganic nutrient availability controls selection of different Alcanivorax genotypes (Röling et al., 2002; Head et al., 2006).
Biostimulation efficiently enhances hydrocarbon bioremediation activity (McKew et al., 2007b) and typically saturated hydrocarbon degradation is stimulated initially followed by degradation of aromatic hydrocarbons and polar components respectively (Fusey and Oudot, 1984). In some instances losses of aromatic hydrocarbons before saturated hydrocarbons have been observed (Jones et al., 1983; Cooney et al., 1985). Such differences in hydrocarbon removal patterns could be due to relative growth efficiency of aromatic and aliphatic hydrocarbon degrading organisms under prevailing environmental conditions and their initial abundance. The goal of hydrocarbon bioremediation strategies is to allow degradation activity at maximum rates by providing nutrients in quantities sufficient to support the growth of hydrocarbon degrading organisms and microbial hydrocarbon degradation activity was shown to increase up to 2.5 mg N/L (0.18 mM) beyond which nutrient level does not enhance the rate of degradation (Boufadel et al., 1999). A continuous supply of inorganic nutrient in combination with sand amendments for efficient mass transfer also has been shown to enhance kinetics of microbial growth, and hydrocarbon degradation (Beolchini et al., 2010).
Although biostimulation of hydrocarbon degradation processes has been studied extensively, there has been very limited attempt to systematically understand the kinetics of nutrient enhanced biodegradation of crude oil and to correlate this with the emergence of specific microbial population in hydrocarbon contaminated marine sediments (Röling et al., 2004; Beolchini et al., 2010). The present study therefore focusses on estimation of kinetic parameters for inorganic nutrient-enhanced hydrocarbon degradation and their effect on the microorganisms responsible.
Materials and methods
Sample collection and microcosm set up
Beach sediment samples consisting of fine sand were collected on 6/11/2009 in sterilized glass bottles (Duran) from a site close to St Mary's Island near Whitley Bay, Newcastle upon Tyne, United Kingdom (N 55°04′ 18″, W 01°26′ 59″). Sediment samples were stored at 4°C for a maximum 24 h prior to the start of the experiments. Oil degrading microcosms comprising beach sediment (10 g), North Sea crude oil (125 mg) and different concentrations of inorganic nutrients (sodium nitrate and potassium dihydrogen phosphate) were prepared in triplicate in serum bottles (114 ml capacity). The oil was weighed directly into the serum bottles, the sediment was added and the nutrient solution was pipetted onto the sediment to give the appropriate levels of nutrients (see “Effect of inorganic nutrient concentration” below). The total volume of nutrient solution added was always made up to 250 μl so that all serum bottles received the same amount of liquid. The sediment, oil and nutrient solution were mixed gently with a glass rod and the microcosms were sealed with butyl rubber stoppers and incubated at 24°C in darkness. Microcosms without nutrient amendment, amended with 250 μl of water served as a control. We monitored oxygen content in the headspace simultaneously with CO2 by GC-MS (see below) and in the 6 day incubation period the headspace remained oxic. Our measurements showed that by day 6 the degree of oxygen depletion was 70.1 ± 0.1% (n = 24) of the initial levels. In long term incubations where greater oxygen consumption occurred with increasing oil degradation, the headspace was replaced with air when oxygen dropped below 15% by volume.
Effect of inorganic nutrient concentration on crude oil degradation
Inorganic nutrient treatments were nitrogen alone (0–5% w/w oil), different levels of phosphorus with a constant inorganic nitrogen concentration (0–0.5% P and 3% N w/w oil), or different levels of inorganic nitrogen with constant phosphorus concentration (0–5% N with 0.5% P w/w of oil) all treatments were conducted in triplicate. Control incubations with nutrients and no added oil were also conducted to determine the contribution of indigenous organic carbon to CO2 production.
Effect of crude oil concentration on oil degradation
Microcosms set up as described above were prepared in triplicate with different amounts of oil ranging from 10 to 500 mg of crude oil and 6.25 mg N (44.6 μmole/g sediment) and 0.625 mg P (2.02 μmole/g sediment). This gives a range of N and P levels ranging from 62.5% N and 6.25% P w/w of oil with 10 mg of oil to 1.25% N and 0.125% P w/w of oil with 500 mg of oil. With 125 mg of oil this is equivalent to 5% N and 0.5% P w/w of oil. The effect of crude oil levels on oil biodegradation was also investigated by treatment with different quantities of crude oil (10–500 mg) but a constant ratio of 5% N and 0.5% P w/w of oil. This was conducted because bioremediation treatments often recommend that a particular mass of inorganic nutrients is supplied relative to the amount of oil present (Swannell et al., 1996). In these treatments the absolute concentration of inorganic nutrients therefore increases with the amount of crude oil present Thus, experiments treated with a single level of nutrients contained approximately 45 μmol N/g wet sediment, while those that contained a constant ratio of inorganic nutrients relative to the mass of oil had N concentrations ranging from around 3.5–180 μmol N/g wet sediment. If the same amount of nutrient added to the 500 mg oil treatment was added to 125 mg of oil (as used in all other microcosms) this would equate to 20% N and 2% P w/w of oil.
Estimation of kinetic parameters
Rate data in response to different inorganic nutrient and oil concentrations were fitted to a Monod-type kinetic model (q = qmax *[S]/Ks + [S]) using non-linear regression implemented in SPSS (IBM SPSS Statistics 19.0.0.1). This was used to derive the model parameters, Ks (half saturation constant) and qmax (maximal rates).
Carbon dioxide measurement
Carbon dioxide production as a measure of microbial activity and crude oil degradation was assessed in microcosm headspace samples daily over a period of 6 days using GC-MS. Maximal rates of CO2 production were calculated from the steepest part of the CO2 accumulation curve which typically followed a lag of 3–4 days (Figure S1). Analysis was performed on a Fisons 8060 GC linked to a Fisons MD 800 MS (electron voltage 70 eV, source temperature 200°C, interface temperature 150°C). Hundred micro liter of headspace gas was manually injected via a syringe (SGE Analytical Science) under an atmosphere of N2 gas. Injection through a manifold which is continuously flushed with N2 was used to prevent interference from any ingress of air from the atmosphere during injection. The sample was separated using a HP-PLOT-Q capillary column (30 m × 0.32 mm). Helium was used as the carrier gas (1 ml/min, 65 kPa, split at 100 ml/min; 250°C). Data acquisition, integration and quantification were controlled using Xcalibur 1.2 software. A mixture of gases with 10% CO2 was used as a standard for calibration. Different volumes of standard gas mix were used to produce a calibration curve which was linear over the range of 1–10% CO2. Percent CO2 values were converted to total molar masses for determination of cumulative CO2 production. R2 values for calibration curves ranged from 0.993 to 0.997.
Residual oil extraction and analysis
Petroleum hydrocarbons from the North Sea crude oil treated microcosm sediments were extracted using a mixture of dichloromethane (DCM):methanol (93:7). Prior to extraction a known quantity of squalane was added in to the sediment as surrogate extraction standard. DCM:methanol (20 ml) was added to the sediment in serum bottle microcosms and stored at room temperature overnight. Microcosms were then sonicated for 1 min and the resulting supernatant was transferred into a flask with this extraction procedure being repeated twice more. The solvent containing the extract was passed through an alumina short column (1 cm bed depth) and then rotary evaporated to dryness before being redissolved in DCM. An aliquot of the organic extract in DCM was evaporated to dryness using a stream of nitrogen gas and solvent exchanged into hexane (200 μl). The total solution was added to a 500 mg/3 ml capacity Isolute® C-18 Solid Phase Extraction (SPE) column prewashed with hexane, and eluted with hexane (5 ml). The eluate was transferred into an autosampler vial and made up to 1 ml with hexane, together with a known amount of heptadecylcyclohexane internal standard. This saturated hydrocarbon fraction was analyzed using an Agilent (HP) 5890 Series II gas chromatograph (GC) fitted with a flame-ionization detector (FID). Samples were injected via split-splitless injector (held at 300°C) using an autosampler. The GC was fitted with a 30 × 0.25 mm fused silica capillary column coated with HP-5 phase (0.25 μm). Hydrogen was used as the carrier gas at a flow rate of 2 ml/min. An initial oven temperature of 50°C was held for 2 min and was then heated to 300°C at 5°C/min., where it was held for 20 min. Data were acquired and processed using Thermo LabSystems Atlas software.
DNA extraction
DNA from 500 mg of frozen microcosm sediment was extracted using a FastDNA® SPIN Kit for Soil (MP Biomedicals™) and a ribolyser (Thermo) according to the manufacturer's instruction. The DNA was eluted in sterilized milliQ water (50 μl) and frozen at −20°C prior to further analysis. The remaining 9.5 g of sediment was used for hydrocarbon extraction and analysis.
Primer design
Primers for amplifying 16S rRNA gene fragments from the total bacterial population and Alcanivorax spp. were designed using the probe and PCR primer design software tool Primrose (Ashelford et al., 2002) (Table 1). For Alcanivorax, the forward primer A16SF.493 matched 1606 of 2004048 bacterial sequences in the RDP database release 11, including 1059 of 1108 Alcanivorax sequences. The reverse primer A16SR.659 matched 1144 of 2004048 bacterial sequences in the RDP release 11 including 1058 of 1108 Alcanivorax sequences. The two primers in combination target 1020 of 2004048 bacterial sequences including 1016 of the 1108 Alcanivorax spp. 16S rRNA gene sequences in the database. The inosine-containing primer pair for total bacterial 16S rRNA genes (Gray et al., 2011; Callbeck et al., 2013) targets 855621 of 944469 bacterial sequences with the relevant target region in the RDP database.
Table 1.
Oligonucleotides primers used in qPCR analysis.
| Primer set | Sequence (5′–3′) | Target organisms | Primer location |
|---|---|---|---|
| Al 6S F.493 | CACCGGCTAATTTCGTGC | Alcanivorax | 481–498* |
| Al 6S R.659 | ACCGGAAATTCCACCTCC | Alcanivorax | 647–664* |
| U 1048f | GTGITGCAIGGIIGTCGTCA | Bacteria | 1048–1068** |
| U1371 | ACGTCITCCICICCTTCCTC | Bacteria | 1352–1371** |
Site on 16S rRNA gene of Alcanivorax borkumensis SK2 (S000018396);
Site on E.coli 16S rRNA gene.
PCR-amplification of 16S rRNA genes
Near full length 16S rRNA gene fragments were amplified using primer pair pA and pH (Edward et al., 1988) as described in Röling et al. (2004). For DGGE analysis, 16S rRNA gene fragments were amplified using primers 2 and 3 (Muyzer et al., 1993) as described previously (Röling et al., 2004). All PCR reactions were conducted using a PC Gene thermal cycler.
Agarose gel and denaturing gradient gel electrophoresis (DGGE)
Agarose gel electrophoresis of PCR-amplifed 16S rRNA gene fragments from Alcanivorax spp. was run for 45 min at 80V using a 1.5% (w/v) agarose gel in 1 x TAE buffer. DGGE was conducted at 60°C using a 0.75 mm thick 10% polyacrylamide gel (ratio of acrylamide to bisacrylamide, 37.5:1) with a concentration gradient of 30–55% of denaturant using a Bio-Rad Dcode system. Gels were stained and photographed according to Röling et al. (2004). 100% denaturant comprised 7M Urea and 40% (vol/vol) deionized formamide in TAE buffer. 1 X TAE buffer contained 40 mM Tris-acetate, 1 mM EDTA, pH 8.0). Cloned Alcanivorax sp. 16S rRNA genes amplified using primers 2 and 3 (Muyzer et al., 1993) were used as markers for qualitatively identifying DGGE bands related to Alcanivorax spp. in DGGE profiles of total bacterial population 16S rRNA genes.
16S rRNA gene cloning
DNA extracted from beach sediment as described above, was used to prepare a bacterial 16S rRNA gene clone library from a beach microcosm containing crude oil and treated with 1% N and 0.1% P after 5 days of incubation. The PCR-amplified 16S rRNA gene fragments were cloned with a TOPO® cloning kit (Invitrogen) as per the manufacturer's instructions. The clone libraries were screened for Alcanivorax sp 16S rRNA genes using the primer pairs listed in Table 1. Cloned Alcanivorax 16S rRNA genes were used to prepare standards for qPCR. These primer pairs were also used for detecting the presence of Alcanivorax spp. in microcosm sediments treated with different levels of inorganic nutrients.
Quantitative PCR
Quantitative PCR (qPCR) was used to determine the abundance of bacterial 16S rRNA genes using primer pair U1048f and U1371 (Gray et al., 2011; Callbeck et al., 2013), and abundance of Alcanivorax 16S rRNA genes was quantified using primer pair A16SF.493 and A16SR.659 (Table 1). qPCR was performed in 20 μl of reaction mixture using an iCycler (iQ™5 multicolor, Bio-Rad, Hemel Hempstead, UK) as described in Singh et al. (2011) with the following temperature cycles: one cycle of initial denaturation at 95°C for 7 min followed by 40 cycles of 95°C for 30 s, 61°C for 60 s and 72°C for 40 s. A standard curve for qPCR was prepared by dilution of a PCR-amplified cloned Alcanivorax 16S rRNA gene fragment. Standard curves had R2 values greater than 0.97 and calculated amplification efficiencies ranged from 101 to 113%. The PCR-amplified 16S rRNA gene was gel purified using QIAquick PCR purification kit (Qiagen) and quantified using a nanodrop® ND-1000 spectrophotometer. The number of 16S rRNA gene copies in the undiluted sample was calculated using the formula described by McKew et al. (2007b) and used to prepare a dilution series ranging from 108 to 100 target genes per μl.
Statistical analysis
Two sample t-tests assuming unequal variances, and single factor ANOVA were performed using Microsoft Excel and non-linear regression for estimation of kinetic parameters was conducted using IBM SPSS Statistics 19.0.0.1.
Results
Effect of inorganic nutrient concentration on crude oil degradation
Microcosms treated with N but without P amendment exhibited stimulation of microbial activity. The activity at 0% N and 0% P (1.18 ± 0.15 μmol CO2 produced/g wet sediment/day) was not significantly different from rates of CO2 production in sediments treated with no added oil (1.05 ± 0.27 μmol CO2/g wet sediment/day). Activity when inorganic N alone and oil were present was significantly higher. At 0.1% N and 0% P the rate of CO2 production was 2.01 ± 0.06 μmol CO2/g wet sediment/day (t-test; P = 0.0095) or with 0.5% N and 0% P 3.66 ± 0.05 μmol CO2/g wet sediment/day (t-test; P = 0.000041). Without P treatment, measured oil degrading activity reached a maximum level at 4% N w/w of oil (4.25 ± 0.91 μmol CO2 produced/g wet sediment/day) and at levels of N of 0.5% w/w of oil and above there was no significant difference in the rate of crude oil biodegradation (ANOVA; P = 0.750; Figure 1). These data indicated that crude oil degradation was nitrogen limited, but at levels of nitrogen 0.5% w/w of oil and above another factor became limiting.
Figure 1.
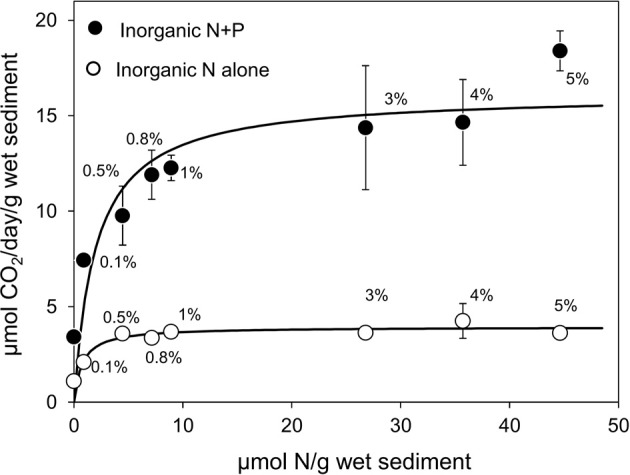
Effect of inorganic N treatment alone (0–5% N w/w of oil—open circles) or inorganic N (0–5% w/w of oil) with constant P (0.5% w/w of oil) treatment (filled circles) on the rate of CO2 production in microcosms containing 10 g beach sediments and 125 mg North Sea crude oil. Data are plotted as micromoles N/g sediment with the equivalent %N w/w of oil annotated next to each data point. Sodium nitrate and potassium dihydrogen phosphate were used as N and P sources. Each data point represents the average value of three replicates. Where error bars are not seen they are smaller than the symbols. Control incubations with nutrients and no added oil were also conducted to determine the contribution of indigenous organic carbon to CO2 production. These typically gave values of 1.05 ± 0.27 μmol CO2/g wet sediment/day (see Figure S1).
Microcosms treated with both inorganic N and P showed an enhancement of oil degradation over and above that seen with nitrogen alone (Figure 1). For example CO2 production in 0.5% N and 0.5% P treated microcosms (9.77 ± 1.54 μmol CO2 produced/g wet sediment/day) was significantly higher than the activity observed with 0.5% N and 0% P (3.66 ± 0.05 μmol CO2 produced/g wet sediment/day) (t-test; P = 0.029). Activity ranged from 3.41 ± 0.25 μmol CO2 produced/g wet sediment/day at 0% N/0.5% P concentration to 18.40 ± 1.04 μmol CO2 produced/g wet sediment/day at 5% N/0.5% P but no significant stimulation of oil degradation was seen at N levels greater than 0.5% w/w of oil when P was not limiting (ANOVA; P = 0.207, Figure 1).
These data were used to estimate half saturation constants and maximal rates using non-linear regression to a Monod-type kinetic model (q = qmax × [S]/Ks + [S]). In sediments with no added P, Ks and qmax for inorganic nitrogen was 0.72 ± 0.32 μmol N/g sediment and 3.93 ± 0.22 μmol CO2 produced/g wet sediment/day. The Ks and qmax for hydrocarbon degradation activity when P was not limiting were 1.99 ± 0.87 μmol N/g wet sediment and 16.16 ± 1.28 μmol CO2/g wet sediment/day respectively (Figure 1). The Ks values for N, with and without P addition, were not statistically significantly different (P > 0.05) whereas qmax when both N and P were provided was significantly (over four times) greater than qmax when only N was provided (P < 0.05).
To systematically determine the level at which P became limiting, microcosms amended with 3% N w/w of oils and different P concentrations were analyzed. With 3% N and 0% P the oil degradation rate was 3.63 ± 0.28 μmol CO2 produced/g wet sediment/day. This was significantly lower (P = 0.04) than rates of CO2 production with 3% N and 0.1% P (14.37 ± 3.24 μmol CO2 produced/g wet sediment/day; Figure 2). With P ranging from 0.1 to 0.5% rates of CO2 production ranged from 10.56 ± 1.21 μmol CO2 produced/g wet sediment/day to 13.05 ± 1.51 μmol CO2 produced/g wet sediment/day and there was no significant difference between the 0.1 and 0.5% P treatments (ANOVA; P = 0.340).
Figure 2.
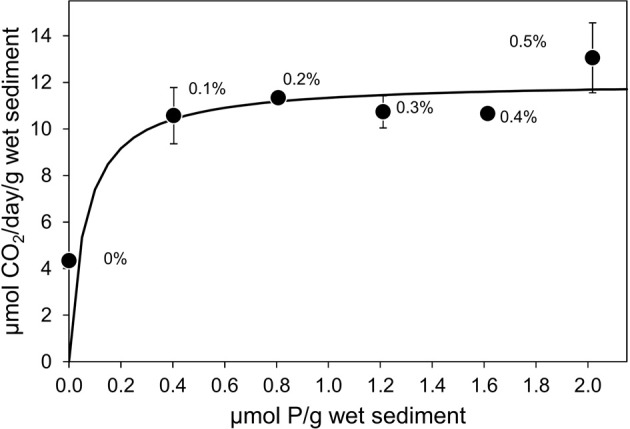
Effect of inorganic phosphorus (0–0.5% P w/w of oil) with constant nitrogen concentration (3% N w/w of oil) on the rate of CO2 production in microcosms containing 10 g beach sediments and 125 mg North Sea crude oil. Data are plotted as micromoles P/g sediment with the equivalent %P w/w of oil annotated next to each data point. Sodium nitrate and potassium dihydrogen phosphate were used as N and P sources. Each data point represents the average value of three replicates. Where error bars are not seen they are smaller than the symbols.
It was thus clear that P limitation of oil degradation was alleviated above 0.1% P w/w of oil. The Ks and qmax values estimated for P were 63 ± 95 nmol/g wet sediment and 12.05 ± 1.31 μmol CO2/g wet sediment/day. The qmax values determined with respect to N (Figure 1) and P (Figure 2) were not statistically significantly different (P > 0.05).
Effect of oil concentration on crude oil biodegradation
Effect of crude oil concentration ranging from 1 mg/g wet sediment to 50 mg/g wet sediment on oil degrading microbial activity was investigated. The microcosms were amended either with a single level of inorganic nutrients irrespective of the amount of oil added (0.625 mg N and 0.0625 mg of P per gram of sediment (equivalent to 5% N and 0.5% P w/w of 125 mg of crude oil) or with a constant ratio of 5% N and 0.5% P w/w of crude oil leading to a range of nutrient levels ranging from 0.05 mg N/0.005 mg P to 2.5 mg N/0.25 mg P per gram of sediment equivalent to 20% N/2% P if in total 125 mg of oil rather than 500 mg of oil was present in the microcosms.
Where a single level of inorganic nutrients was provided, the rate of CO2 evolution increased with increasing quantity of oil between 1 and 20 mg/g sediment (t-test: P = 0.000003; Figure 3). At crude oil concentrations ranging from 20 to 50 mg/g sediment there was no significant difference in the rate of CO2 production with increasing oil concentration (ANOVA: P = 0.08; Figure 3). Thus, up to these levels, equivalent to 5% oil by weight of sediment, crude oil was not auto-inhibitory.
Figure 3.
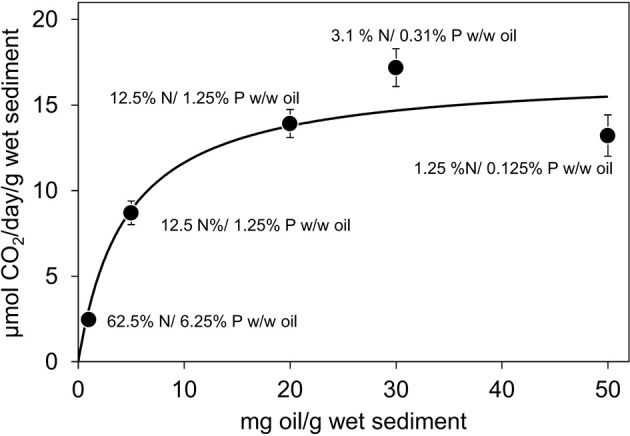
Effect of oil concentration on the rate of CO2 production in microcosm comprising 10 g beach sediments, 6.25 mg N (44.6 μmole/g sediment) and 0.625 mg P (2.02 μmole/g sediment) and 10–500 mg of North Sea crude oil. This gives a range of N and P levels ranging from 62.5% N and 6.25% P w/w of oil with 10 mg of oil to 1.25% N and 0.125% P w/w of oil with 500 mg of oil. With 125 mg of oil this is equivalent to 5% N and 0.5% P w/w of oil. Sodium nitrate and potassium dihydrogen phosphate were used as N and P sources. Each data point represents the average value of three replicates. Where error bars are not seen they are smaller than the symbols.
When a constant ratio of inorganic nutrients was provided with increasing oil concentration, CO2 production rate decreased when oil levels were greater than 12.5 mg oil/g sediment (Figure 4). The CO2 production rate at 20 mg oil/g sediment (10.40 ± 1.35 μmol CO2 produced/g wet sediment/day) was less than the rate at 12.5 mg oil/g sediment (15.59 ± 2.08 μmol CO2 produced/g wet sediment/day) and with 50 mg oil/g sediment the rates dropped further to 0.46 ± 0.04 μmol CO2 produced/g wet sediment/day, equivalent to almost a 97% decrease compared to rate at 12.5 mg oil/g sediment. Differences in CO2 production rates with 12.5 mg oil/g sediment and higher oil concentrations were statistically significant (ANOVA: P = 0.00012). The inhibition of oil degrading activity at a lower oil concentration than that seen when inorganic nutrients were added at a single concentration was most likely due to toxicity of the higher absolute amounts of nutrients present in microcosms containing higher levels of oil. If the microcosm containing 50 mg of oil per gram of sediment is considered, the level of nutrients applied would be equivalent to 20% N and 2% P w/w of oil in a treatment containing 12.5 mg oil/g sediment. Indeed a systematic evaluation of the effect of nutrient concentration ranging from 0 to 20% N with 1/10th the P concentration w/w of 125 mg of oil showed that nutrient levels greater than 7% N/ 0.7% P w/w of oil (equivalent to 62.5 micromoles N and 2.83 micromoles P/g sediment or 226 mM N/10.2 mM P) resulted in a reduction in CO2 production rate from oil degradation (Figure 5).
Figure 4.
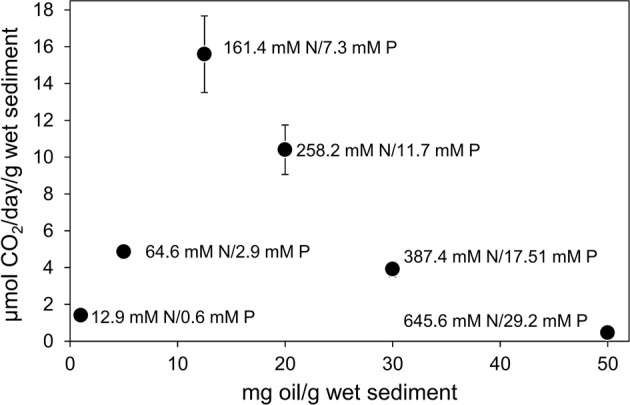
Effect of oil concentration on the rate of CO2 production in microcosm comprising 10 g beach sediment and 10–500 mg North Sea crude oil amended with a constant ratio of 5% N and 0.5% P w/w of oil. Sodium nitrate and potassium dihydrogen phosphate were used as N and P sources. Each point is annotated with the concentration of N and P in millimolar terms, based on the water content of the sediments. Each data point represents the average value of three replicates. Where error bars are not seen they are smaller than the symbols.
Figure 5.
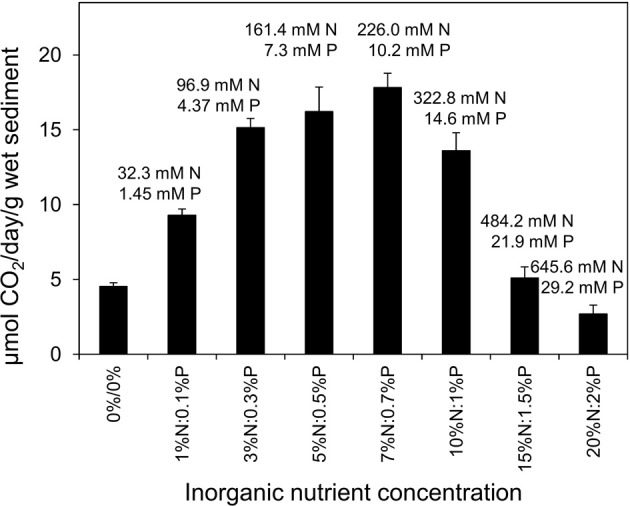
Effect of inorganic N treatment (0–20% w/w of oil) with 1/10th P treatment on the rate of CO2 production in microcosm comprising 10 g beach sediment and 125 mg North Sea crude oil. Sodium nitrate and potassium dihydrogen phosphate were used as N and P sources. For easy cross referencing to Figure 4, each point is annotated with the concentration of N and P in millimolar terms, based on the water content of the sediments. Each data point represents average value of three replicates. One percent N by weight of oil is equivalent to approximately 9 micromoles of N per g sediment and 20% N is equivalent to 178 micromoles of N per g sediment.
Alkane degradation in microcosm incubations
At the end of the 6 day incubation period residual crude oil was extracted from the microcosms treated with 125 mg crude oil and a range of inorganic nutrient concentrations (0%N/0.5%P to 5%N/0.5%P w/w of oil). The saturated hydrocarbon fractions were isolated and the resolved n-alkanes, pristane and phytane were quantified. The nC12 to nC32 alkanes present in the oil comprise approximately 10% by weight of the oil and thus the amount of resolved alkanes at the start of the experiment was about 12,500 μg per microcosm in addition volatile hydrocarbons (nC5 to nC10 and benzene and toluene) comprise around 7000 μg per microcosm. Across all treatments the total amount of alkanes measured (the sum of nC12–nC32) ranged from 9833 ± 1623 to 12168 ± 628 μg per microcosm. This suggests that a moderate amount of alkane degradation occurred over the 6 day incubation. Indeed, there was no statistically significant difference in the total mass of alkanes recovered, irrespective of the inorganic nutrient amendment (ANOVA: P = 0.244). This suggested that the degree of hydrocarbon degradation had been moderate. In addition to losses due to biodegradation some lower molecular weight alkanes may have been lost due to evaporation. Even though the incubations were conducted in sealed serum bottles some evaporative losses may have occurred during sampling the headspace for CO2. A systematic analysis of evaporative loss of volatile alkanes in the headspace demonstrated that flushing the headspace with up to 1800 ml of air removed alkanes up to nC9 to varying degrees, but nC10 was unaffected (Figure S2). Alkanes with lower molecular weight than nC12 were lost during the procedure for purification of the saturated hydrocarbon fraction and are not accounted for in our figures.
A more sensitive way to determine n-alkane degradation is by measuring the ratio of n-alkanes (typically nC17) relative to the concentration of the more slowly degraded branched alkane, pristane. To assess the degree of degradation of alkanes of different molecular weight we determined nC13:pristane, nC17:pristane and nC25:pristane ratios for all of the treatments (Figure 6).
Figure 6.
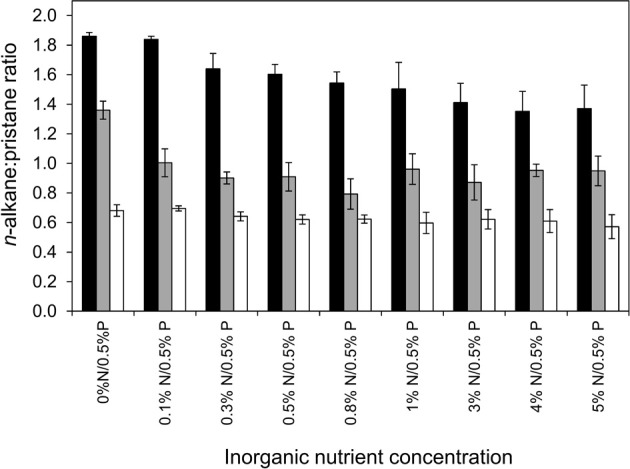
Effect of inorganic N (0–5%) with constant P (0.5%) treatment w/w of oil on the degradation on n-alkane (nC17, nC13, and nC25) to pristane ratios in microcosm comprising 10 g beach sediment and 125 mg North Sea crude oil. Each data point represents average value of three replicates. nC17:pristane black bars, nC13:pristane gray bars, nC25:pristane white bars.
The nC17:pristane ratio of the starting oil was 1.99 ± 0.01. The range of nC17:pristane ratios across treatments was relatively small with a maximum value in the 0%N/0.05%P treatment of 1.86 ± 0.04 and the lowest value measured in the 4%N/0.5%P treatment (1.35 ± 0.23). There were significant differences in the nC17:pristane ratios across all treatments (ANOVA: P = 0.036) resulting from lower values measured in treatments from 0.3% N/0.5%P to 5%N/0.5% (1.35 ± 0.23 to 1.64 ± 0.18) compared to the 0%N/0.5%P and 0.1%N/0.5%P treatments (1.86 ± 0.04 and 1.84 ± 0.04). There were no significant differences in the nC17:pristane ratios between the 0.3%N/0.5%P to 5%N/0.5% treatments (ANOVA: P = 0.589). If nC18:phytane ratios were used the results were essentially the same as obtained with nC17:pristane ratios. A similar pattern was seen with nC13:pristane ratios except that all nutrient treatments greater than 0%N/0.5%P gave nC13:pristane ratios which were statistically indistinguishable (ANOVA: P = 0.815) but were significantly different from the 0%N/0.5%P treatment (P = 0.018). There was no difference in nC25:pristane ratios across all treatments (ANOVA: P = 0.834). Taken together these data indicate that there was a small degree of degradation of n-alkanes and that lower molecular weight alkanes were degraded to a greater degree than higher molecular weight alkanes over the short 6 day time course of the experiments (Figure 6). Estimation of the extent of oil degradation based on a mass balance from the CO2 produced over the 6 day incubation period, indicated that the CO2 generated could account for degradation of 6.19 ± 0.02 to 37.28 ± 2.06% of the total mass of nC5–nC32 alkanes and volatile low molecular weight aromatics (benzene and toluene) initially present, in the 0%N/0.5%P and 5%N/0.5%P treatments respectively. Estimates based on the nC17:pristane ratio were generally similar and ranged from 6.49 to 32.08%. Notwithstanding differences in the volatility of nC13 and pristane, estimates of degradation based on the nC13:pristane ratio indicated a greater degree of degradation with a maximum estimated extent of degradation of 49.85%. Discrepancies in these estimates likely reflect the fact that the CO2 produced integrates degradation of all components of the oil that are being removed whereas the alkane:pristane ratio data provide information on selected compounds which, as a comparison of the nC13:pristane and nC17:pristane suggests, are degraded to different degrees over the time course of the experiment.
Effect of inorganic nutrient amendment on bacterial community composition
Bacterial communities in microcosms treated with crude oil and different levels of inorganic nutrients were characterized by DGGE analysis of PCR-amplified 16S rRNA genes (Figure 7). Following 6 days of incubation a differential response in the bacterial communities to nutrient amendment was observed with bands corresponding to Cycloclasticus sp. being detected at lower nutrient concentrations (0–1% N w/w of oil) and Alcanivorax spp. becoming much more prevalent at higher nutrient concentrations (Figure 7). End-point PCR using Alcanivorax specific primers demonstrated that only 20% of samples (3 out of 15) treated with 0.8%N/0.5%P or less harbored detectable Alcanivorax whereas in samples treated with 1–5%N/0.5%P, 100% of samples (12 out of 12) harbored detectable Alcanivorax 16S rRNA genes. Interestingly all three microcosms treated with 0.5%N/0.5%P contained Alcanivorax 16S rRNA genes detectable by end-point PCR while no samples from 0.8%N/0.5% P-treated microcosms contained detectable Alcanivorax 16S rRNA genes. The selection of Alcanivorax at higher inorganic nutrient concentrations was consistent with the greater alkane degradation observed in these samples.
Figure 7.
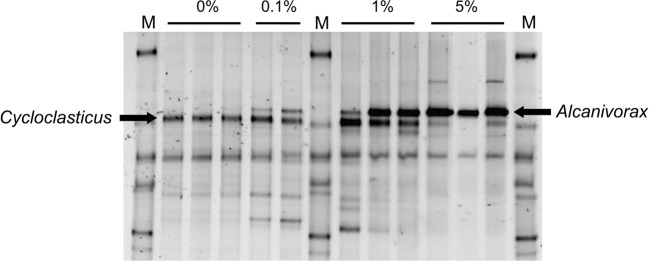
Denaturing gradient gel electrophoresis of 16S rRNA gene fragments from crude oil and nutrient treated beach microcosms incubated for 6 days. Fragments corresponding to Cycloclasticus spp and Alcanivorax spp, are indicated. All microcosms were treated with 0.5%P w/w of oil and the values above the lanes indicate the percentage of inorganic N added relative to the mass of oil.
Quantification of bacterial 16S rRNA genes by qPCR showed a small, but significant increase in total bacterial abundance relative to nutrient levels (ANOVA: P = 0.008). The log bacterial gene abundance was 8.93 ± 0.09/g in sediments treated with 0%N/0.5%P with a maximum value of 10.17 ± 0.18/g sediment treated with 3%N/0.5%P (Figure 8A). The differences were due to higher bacterial 16S rRNA gene abundance in microcosms treated with nutrient concentrations greater than 0.3%N/0.5%P and at all nutrient treatments greater than this, there was no significant difference in total bacterial 16S rRNA gene abundance (ANOVA: P = 0.14).
Figure 8.
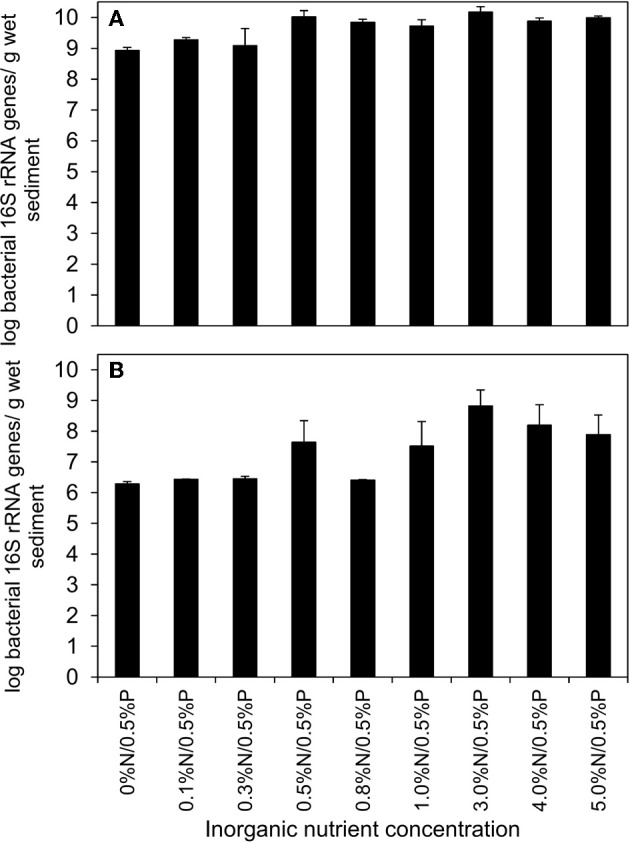
qPCR analysis of total bacterial 16S rRNA genes (A) and Alcanivorax spp. 16S rRNA genes (B) from crude oil and nutrient treated beach microcosms incubated for 6 days. All microcosms were treated with 0.5% P w/w of oil and the values below the columns indicate the percentage of inorganic N added relative to the mass of oil. Each data point represents average value of three replicates.
Log 16S rRNA gene abundance determined using Alcanivorax specific primers ranged from 6.28 ± 0.08/g in sediments treated with 0%N/0.5% P to 8.82 ± 0.52/g, in sediments that received 3% N/0.5% P (Figure 8B). There were significant differences in Alcanivorax 16S rRNA gene abundance (ANOVA: P = 0.014). Alcanivorax 16S rRNA gene abundance in microcosms treated with 0.8% N/0.5% P had anomalously low 16S rRNA gene abundance (log abundance, 6.41 ± 0.02/g sediment) and excluding this value which was not significantly different from Alcanivorax 16S rRNA gene abundance at all nutrient concentrations less than 0.5% N/0.5% P (ANOVA: P = 0.275), showed that at all other nutrient concentrations greater than 0.3% N/0.5% P Alcanivorax genes were significantly more abundant than at lower nutrient levels, while there was no difference in abundance in all treatments greater than 0.3% N/0.5% P (ANOVA: P = 0.671).
Discussion
Kinetics of inorganic nutrient-stimulated crude oil biodegradation
Biostimulation with N and P is an effective method for enhancing the rate of oil bioremediation (Atlas and Bartha, 1972, 1973; Bragg et al., 1994; Venosa et al., 1996; Röling et al., 2002; McKew et al., 2007b; Coulon et al., 2007). Early studies of Atlas and Bartha (1972) were the first to demonstrate inorganic N and P-mediated stimulation of crude oil biodegradation and that both N and P amendment were required for oil degradation. Subsequently the feasibility of using oleophilic fertilizer as biostimulating agents was demonstrated (Atlas and Bartha, 1973). The enhancement of hydrocarbon degradation rate with increasing nitrate concentration (Boufadel et al., 1999), and nitrate plus sand amendments (Beolchini et al., 2010) has also been demonstrated. In the study of Beolchini et al. (2010) the sand enhanced biodegradation of high molecular weight aliphatic hydrocarbons and it was suggested that this was effective because it increased the surface area of the solid/liquid interface in the sediment and increased oxygen diffusion and mass transfer. However, these studies did not attempt to systematically analyze the nutrient enhanced kinetic parameters of crude oil biodegradation. Therefore, the focus of the present study was to evaluate the kinetic parameters for crude oil degradation in relation to N and P treatments.
Oil-stimulated CO2 production rate was used as a proxy for hydrocarbon degradation to determine initial rates in relation to nutrient levels and oil concentration to estimate kinetic parameters that may be useful for modeling crude oil bioremediation programmes for marine beach sediments. N amendment with no added P led to a small but significant stimulation of oil degradation (Figure 1), however when P-limitation was alleviated there was a greater enhancement in the rate of oil degradation up to around 0.8 to 1% N w/w of oil (Figure 1). While qmax values were four-fold higher when both N and P limitation were alleviated compared to alleviation of N limitation alone, Ks values for N were similar irrespective of P provision (0.72 ± 0.32 μmol N/g sediment and 1.99 ± 0.87 μmol N/g wet sediment). The water content of the sediments was determined to be 25.16 ± 0.09% (n = 3) and 250 μl of nutrient solution was added to each sample (i.e., water content of 2.766 ml per microcosm) and on this basis the Ks values were converted to molar concentrations. This gave a Ks value of 2.60 ± 1.16 mM with no P amendment and 7.19 ± 3.14 mM with N and P amendment. This is several orders of magnitude higher than the range of 6.9–122.4 μM reported for heterotrophic bacteria (Reay et al., 1999) and may reflect the fact that the key alkane degraders in the microcosms are Alcanivorax sp. which are known to be stimulated during bioremediation treatments and thus may be better adapted to relatively high inorganic nutrient concentrations. The Ks value for P was much lower at 63 ± 95 nmol/g wet sediment which translates to 227.77 ± 343.46 μM. The mean value obtained is much (orders of magnitude) higher than Ks values typically reported for phosphate utilization by bacteria and aquatic microbial communities which are usually sub micromolar (Vadstein and Olsen, 1989, 0.013–0.247 μM; Schowanek and Verstraete, 1990, 0.17 μM; Cotner and Wetzel, 1992, 0.019–0.225 μM). This may reflect adaptation of these specialist hydrocarbon-degrading taxa to high nutrient concentrations typical of conditions that are used to promote hydrocarbon bioremediation. Many pure cultures of Alcanivorax spp. are available and kinetic analysis with respect to N and P utilization would be highly informative in this regard. It should however, be noted that the error on the estimate of Ks for phosphate is large (±151%) and indicates that there is a statistical probability that Ks has a negative value. This is clearly not possible and one would have to conclude that the lower bound must be a small non-zero value. Putting this statistical incongruity to one side the important point is that it is difficult to draw reliable conclusions about the specific kinetic characteristics of the hydrocarbon degrading organisms with respect to inorganic phosphate from these data. Future studies should focus on analysing the response of hydrocarbon degraders to P at sub micromolar levels.
The occurrence of high affinity permeases for inorganic N and P in the genome of Alcanivorax borkumensis SK2 (Schneiker et al., 2006) seems to contradict the findings of the present study, however, as far as we are aware these have been annotated largely on the basis of sequences from known permeases and there is no direct evidence available regarding the kinetic of these permeases. Moreover organisms may have different sets of permeases with different affinity for inorganic nutrients allowing them to adapt rapidly to a change from nutrient limited to nutrient replete conditions. However, such conclusions would need, to be supported by detailed proteomic analysis of the response of Alcanivorax to growth at different nutrient levels (Sabirova et al., 2006).
Kinetic response of crude oil degrading microbial communities to oil loading
Microbial activity generally increases in proportion to an accessible carbon and energy source. However, high concentrations of hydrocarbons have been shown to inhibit oil biodegradation either by causing nutrient or oxygen limitation or through direct toxicity of volatile hydrocarbons (Fusey and Oudot, 1984; Leahy and Colwell, 1990). Therefore, in addition to assessing the kinetics of hydrocarbon degradation in relation to inorganic nutrient availability we determined the effect of sediment oil loading on biodegradation rates when N and P were not limiting. CO2 evolution rates increased with increasing oil content from 1 to 50 mg crude oil/g sediment (5% oil by weight; Figure 3). This encompasses the range of contamination levels observed following the Exxon Valdez Spill in Prince William Sound, Alaska. The levels of oil in surface sediments in Prince William Sound were highly variable with an average value of 12.2 ± 18.6 mg/g sediment (Bragg et al., 1992). This suggests that effective biodegradation of the labile components of crude oil is likely achievable at oiling levels typically seen in oil spill-affected sediments in the field. Nevertheless, much higher levels of oil contamination (up to 510 mg/g) have been reported following the Deepwater Horizon blow out (Lin and Mendelssohn, 2012). The marsh sediments studied by Lin and Mendelssohn (2012) were from Bay Jimmy within Barataria Bay. Sediments in Barataria bay range from fine sand to coarse silts (most sediment particles in the range 2–5 in the phi scale of Krumbein and Aberdeen, 1937) and Bay Jimmy sediments specifically are organic rich (4.0–16.2% TOC by weight of sediment; Natter et al., 2012). This suggests that oxygen depletion in these sediments may contribute to the relative persistence in these sediments and indeed evidence has been presented that suggests that oiling of these sediments promoted sulfate-reduction (Natter et al., 2012).
The half saturation constant for crude oil in our sediments was estimated to be 5.83 ± 1.46 mg oil/g wet sediment. While this provides a practically useful Ks value for modeling the kinetics of field scale bioremediation it is not straightforward to compare this with literature values for pure compound and/or pure cultures of microorganisms due to the complexity of the mixture of carbon sources and their low water solubility. If the oil masses are converted to moles of carbon (crude oil is typically 84% carbon by weight) this translates into a Ks value of 388 ± 97 μmol C/g sediment. We know from oil chemistry data that only a fraction of the alkanes (nC13–nC17) were being degraded in the system and in the freshly added oil the mass of nC13–nC17 was equivalent to about 3% of the total oil mass, giving a Ks value for the alkanes which were actually being degraded of 11.64 μmol C/g sediment. The low aqueous solubility of alkanes also needs to be considered in this context (e.g., 1.7 nM for hexadecane in seawater at 25°C; Verschueren, 1983). Moreover, aqueous solubility defined under standard physical and chemical conditions may not be truly representative of the situation in situ, where biosurfactants may substantially increase the effective aqueous solubility. Nevertheless, the Ks values reported in terms of mass of sediment would be equivalent to orders of magnitude greater than Ks values reported for hydrocarbon degradation by pure cultures of bacteria which are in the micromolar or even sub-micromolar range. The toluene degrader Cycloclasticus oligotrophicus for example has the lowest known Ks for an organic substrate (0.014 μM; Button et al., 1998).
While the kinetic parameters we have estimated will be useful for modeling the fate of the more labile components of crude oil, as these become degraded more persistent fractions of the oil will be degraded more slowly. To address this, studies of partially degraded and heavier oils will be required. Interestingly, it has been reported that nominally labile crude oil hydrocarbons have persisted in subsurface sediments from Prince William Sound, Alaska, even 16 years after the Exxon Valdez oil spill (Short et al., 2007). This persistence could be explained by anoxia reducing the rates of hydrocarbon degradation, though this was discounted by Short et al. (2007) due to high levels of tidal flushing in the sediments. It was however suggested that nutrient availability may have been a factor in the persistence of these hydrocarbons, and perhaps more importantly the formation of water in oil emulsions (mousse), which reduce the surface area of oil available for microbial attack (Short et al., 2007). Subsequent studies however demonstrated that the long term residual oil was biodegradable and that inorganic nutrients and oxygen stimulated its biodegradation (Venosa et al., 2010). These studies emphasize the need to consider more than just microbiological factors when assessing the fate of spilled oil in the field.
Suppression of bioremediation by high levels of inorganic nutrients
Recommendations for nutrient levels required for crude oil bioremediation are often given in terms of the mass of nutrients required relative to the mass of oil (Röling et al., 2004). Moreover arguments surrounding addition of excessive amounts of nutrients normally relate to avoiding eutrophication of neighboring water bodies (Swannell et al., 1996; Röling et al., 2004). For this reason we determined the response of oil degrading microorganisms to increasing levels of oil in the presence of a constant proportion, but increasing absolute amounts of nutrients. In experiments with increasing oil concentration above 12.5 mg oil/g sediment, marked inhibition of oil degradation was noted (Figure 4). This was shown to be a consequence of toxicity of high levels of nutrients rather than an effect of higher levels of oil (Figure 5). Converting the added nutrients into an aqueous concentration based on the water content of the sediments indicated that inhibition of oil degradation occurred at sodium nitrate and potassium orthophosphate concentrations of 238 mM nitrate and 10.8 mM phosphate or greater. These absolute concentrations are very high (almost 3 orders of magnitude greater than the Ks values determined here), but serve to underline the importance of designing treatment strategies on more than a simple mass balance of oil carbon relative to inorganic nutrient levels. It is possible that a similar effect might result from oxygen depletion at higher oil loadings however in the sediment containing 50 mg oil/g of sediment and 5%/0.5% N/P by mass, oxygen was depleted by only 24.0 ± 2.8% over the incubation period relative to oxygen levels at the start of the experiment. This compares with the treatment with 12.5 mg oil/g of sediment and 5%/0.5% N/P by mass where oxygen was depleted by 31.3 ± 2.8% from the levels at the start of the experiment. Moreover inhibition of oil degradation at 50 mg oil/g of sediment did not occur when lower absolute concentrations of nutrients were provided (Figure 3).
Alkane degradation and growth of Alcanivorax in short term incubations
Biodegradation of saturated and aromatic hydrocarbons in crude oil contaminated environments has been previously demonstrated (Kasai et al., 2002a,b; Röling et al., 2002; Singh et al., 2011). The lack of detectable degradation of total resolved alkanes in our experiments is consistent with the short incubation period of the experiments which were designed to determine initial rates of hydrocarbon degradation, not the full extent of degradation possible. Similar experiments conducted over much longer timescales (30–90 days) typically show complete removal of the resolved alkanes. More detailed analysis of hydrocarbon degradation based on ratios of n-alkanes to pristane demonstrated that there was modest degradation of lower molecular weight alkanes with increasing inorganic nutrient concentrations (Figure 6). This is consistent with the increased rates of CO2 production and increase in abundance of Alcanivorax observed at higher nutrient levels (Figures 1, 8)
Alcanivorax spp. have been found to be globally significant for in situ degradation of straight and branched chain alkanes in marine environments (Dyksterhouse et al., 1995; Yakimov et al., 2005; Head et al., 2006; McKew et al., 2007a,b). A greater decrease in the ratio of nC13: pristane as compared to nC17: pristane and nC25: pristane ratios suggest a preference for degradation of low molecular weight alkanes by the Alcanivorax sp. detected in our experiments.
It is also interesting to note that at low nutrient concentrations Cycloclasticus-like bacteria were prevalent. These are known aromatic hydrocarbon-degrading bacteria with particularly high substrate affinities which contrasts with Alcanivorax which was strongly selected at higher nutrient concentrations (Figure 7) consistent with the high Ks values determined in these experiments. Interestingly despite the significance of inorganic nutrient provision for stimulation of crude oil biodegradation, and the fact that this has been known for several decades, there are relatively few data in the literature on the kinetic parameters for oil degradation with respect to inorganic N or P. This is an important gap in our knowledge of the ecology of hydrocarbon-degrading bacteria and has practical implications for understanding the fate of crude oil in the environment. The present day focus on omics- enabled studies of hydrocarbon-degrading communities would be perfectly complemented by more studies to determine fundamental kinetic and physiological properties of both pure cultures and natural communities of hydrocarbon-degrading bacteria. It will be interesting to note if the high Ks/low affinity kinetics noted in this study are reflected in the kinetic properties of pure cultures and how they are affected by environmental conditions such as temperature. This kind of information will be essential for incorporation of ecological principles such as resource ratio theory into modeling approaches to better understand the fate of spilled oil (Smith et al., 1998). Both theoretical and experimental approaches to understanding the competitiveness of different hydrocarbon-degrading bacteria under different scenarios would be facilitated by such basic knowledge of the physiology of hydrocarbon degrading bacteria (McKew et al., 2007b). This is an attractive proposition given that many of the key players in marine hydrocarbon degradation are available in culture (Yakimov et al., 2007). In addition genome sequences have been determined for a number of these taxa and it is only a matter of time before many more marine obligate hydrocarbon degrading bacterial genomes are sequenced (Schneiker et al., 2006).
Conclusions
The data presented here provide a systematic assessment of key factors that control the biodegradation of crude oil in beach sediments and provide kinetic parameters that can be used in kinetic modeling of beach oil spill bioremediation. Our results not only confirm that crude oil biodegradation in marine beach sediments is sensitive to the level of inorganic N and P nutrient treatments but that the maximum rates of crude oil biodegradation achievable are approximately 16 μmol C/g sediment/day at the incubation temperature of our experiments (24°C). Half saturation constants for N and P in the form of nitrate and phosphate are high compared to values typically seen in pure cultures of heterotrophic bacteria and underline the importance of maintaining high inorganic nutrient concentrations to accelerate hydrocarbon degradation. The half saturation constants also provide fundamental parameters for future kinetic modeling of bioremediation of beached oil spills. The high nutrient levels that promote crude oil biodegradation also select for specialized alkane degrading bacteria from the genus Alcanivorax while selecting against aromatic hydrocarbon degrading bacteria such as Cycloclasticus sp. This suggest that by manipulation of nutrient amendments it may be possible to balance aromatic and aliphatic hydrocarbon degradation, albeit at the expense of lower overall rates of biodegradation due to the lower levels of nutrients required to stimulate aromatic hydrocarbon degrading Cycloclasticus spp. We also demonstrated that nutrient-enhanced bioremediation is effective only up to a point and care should be taken in oil spill bioremediation, not only to protect against eutrophication by avoiding excessive nutrient loading, but also to avoid inhibition of hydrocarbon degradation at high nutrient levels. Similar analyses on a wider range of sediments will establish if the observations we have made in one beach sediment apply broadly across a range of environments and geographical locations.
Author contributions
Ian M. Head, Arvind K. Singh, and Neil D. Gray developed the concept and designed experiments; Arvind K. Singh prepared and analyzed microcosm experiments. Arvind K. Singh, Neil D. Gray, and Angela Sherry conducted headspace gas analysis and microbial community analysis. Arvind K. Singh, Bernard F. J. Bowler, and D. Martin Jones were responsible for and conducted crude oil analysis. Data analysis and interpretation was conducted by Arvind K. Singh, Ian M. Head, Angela Sherry, and Neil D. Gray, Arvind K. Singh, and Ian M. Head wrote the manuscript with critical input from Angela Sherry, Neil D. Gray, and D. Martin Jones.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The financial support from European Commission as Marie Curie Fellowship award to Arvind Singh via the Project BACTOIL (MC–IIF-39431) is gratefully acknowledged. We are also grateful for financial support from the Natural Environment Research Council (Grant NE/E01657X/1 to Ian M. Head, Neil D. Gray, and D. Martin Jones). James Todd is thanked for the supplementary data.
Supplementary material
The Supplementary Material for this article can be found online at: http://www.frontiersin.org/journal/10.3389/fmicb.2014.00160/abstract
Cumulative CO2 production in beach sediment microcosms. Data from microcosms treated with crude oil plus inorganic nitrogen and phosphorus (black circles), inorganic nitrogen and phosphorus but no oil (gray circles) and killed control microcosms treated with formaldehyde (2% final concentration) and crude oil plus inorganic nitrogen and phosphorus (white circles). Oil treated microcosms contained 10 mg crude oil/g sediment and inorganic nutrient amendments equated to 5% N and 0.5% P by weight of the oil.
Effect of headspace flushing on physical removal of volatile saturated hydrocarbons from beach sediment microcosms. Concentrations of the individual compounds (C) after flushing the headspace with different volumes of air are presented relative to initial headspace concentration (C0) of the volatile hydrocarbons.
References
- Ashelford K. E., Weightman A. J., Fry J. C. (2002). PRIMROSE: a computer program for generating and estimating the phylogenetic range of 16S rRNA oligonucleotide probes and primers in conjugation with the RDP-11 database. Nucleic Acids Res. 30, 3481–3489 10.1093/nar/gkf450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atlas R. M., Bartha R. (1972). Degradation and mineralization of petroleum in sea water: limitation by nitrogen and phosphorus. Biotechnol. Bioeng. 14, 309–318 10.1002/bit.260140304 [DOI] [PubMed] [Google Scholar]
- Atlas R. M., Bartha R. (1973). Stimulated biodegradation of oil slicks using oleophilic fertilizers. Environ. Sci. Technol. 7, 538–541 10.1021/es60078a005 [DOI] [PubMed] [Google Scholar]
- Beolchini F., Rocchetti L., Regoli F., Dell'Anno A. (2010). Bioremediation of marine sediments contaminated by hydrocarbons: experimental analysis and kinetic modeling. J. Hazar Mater. 182, 403–407 10.1016/j.jhazmat.2010.06.047 [DOI] [PubMed] [Google Scholar]
- Boufadel M. C., Reeser P., Suidan M. T., Wrenn B. A., Cheng J., Du X., et al. (1999). Optimal nitrate concentration for the biodegradation of n-heptadecane in a variably saturated sand column. Environ. Technol. 20, 191–199 10.1080/0959333200861680822760224 [DOI] [Google Scholar]
- Bragg J. R., Prince R. C., Harner E. J., Atlas R. M. (1994). Effectiveness of bioremediation for the Exxon Valdez oil spill. Nature 368, 413–418 10.1038/368413a01368153 [DOI] [Google Scholar]
- Bragg J. R., Prince R. C., Wilkinson J. B., Atlas R. M. (1992). Bioremediation for Shoreline Cleanup following the 1989 Alaska Oil Spill. (Houston: Exxon Company; ), 94 [Google Scholar]
- Button D. K., Robertson B. R., Lepp P. W., Schmidt T. M. (1998). A small, dilute-cytoplasm, high-affinity, novel bacterium isolated by extinction culture and having kinetic constants compatible with growth at ambient concentrations of dissolved nutrients in seawater. Appl. Environ. Microbiol. 64, 4467–4476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callbeck C. M., Sherry A., Hubert C. R. J., Gray N. D., Voordouw G., Head I. M. (2013). Improving PCR efficiency for accurate quantification of 16S rRNA genes. J. Microbiol. Meth. 93, 148–152 10.1016/j.mimet.2013.03.010 [DOI] [PubMed] [Google Scholar]
- Cappello S., Denaro R., Genovese M., Giuliano L., Yakimov M. M. (2007). Predominant growth of Alcanivorax during experiments on oil spill bioremediation in mesocosms. Microbiol. Res. 162, 185–190 10.1016/j.micres.2006.05.010 [DOI] [PubMed] [Google Scholar]
- Cooney J. J., Silver S. A., Bech E. A. (1985). Factors influencing hydrocarbon degradation in three freshwater lakes. Microb. Ecol. 11, 127–137 10.1007/BF02010485 [DOI] [PubMed] [Google Scholar]
- Cotner J. B., Wetzel R. G. (1992). Uptake of dissolved inorganic and organic phosphorus compounds by phytoplankton and bacterioplankton. Limnol. Oceanogr. 37, 232–243 10.4319/lo.1992.37.2.0232 [DOI] [Google Scholar]
- Coulon F., McKew B. A., Osborn A. M., McGenity T. J., Timmis K. N. (2007). Effect of temperature and biostimulation on oil-degrading microbial communities in temperate estuarine waters. Environ. Microbiol. 9, 177–186 10.1111/j.1462-2920.2006.01126.x [DOI] [PubMed] [Google Scholar]
- Dell'Anno A., Beolchini F., Rocchetti L., Luna G. M., Danovaro R. (2012). High bacterial biodiversity increases degradation performance of hydrocarbons during bioremediation of contaminated harbor marine sediments. Environ. Pollut. 167, 85–92 10.1016/j.envpol.2012.03.043 [DOI] [PubMed] [Google Scholar]
- Dyksterhouse S. E., Gray J. P., Herwig R. P., Lara J. C., Staley J. T. (1995). Cycloclasticus pugetii gen. nov., sp. Nov., an aromatic hydrocarbon degrading-bacterium from marine sediments. Int. J. Syst. Bacteriol. 45, 116–123 10.1099/00207713-45-1-116 [DOI] [PubMed] [Google Scholar]
- Edward U., Rogall T., Blöcker H., Emde M., Böttger E. C. (1988). Isolation and complete nucleotide determination of entire genes. Characterisation of a gene coding for 16S ribosomal RNA. Nucleic Acid Res. 17, 7843–7853 10.1093/nar/17.19.7843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelhardt M. A., Daly K., Swannell R. P. J., Head I. M. (2001). Isolation and characterization of a novel hydrocarbon- degrading, Gram-positive bacterium, isolated from intertidal beach sediment, and description of Planococcus alkanoclasticus sp nov. J. Appl. Microbiol. 90, 237–247 10.1046/j.1365-2672.2001.01241.x [DOI] [PubMed] [Google Scholar]
- Fusey P., Oudot J. (1984). Relative influence of physical removal and biodegrdation in the depuration of petroleum-contaminated Seashore sediments. Mar. Pollut. Bull. 15, 136–141 10.1016/0025-326X(84)90234-0 [DOI] [Google Scholar]
- Golyshin P. N., Chemikova T. N., Abraham W. R., Lunsdorf H., Timmis K. N., Yakimov M. M. (2002). Oleiphilaceae fam nov., to include Oleiphilus messinensis gen. nov., sp. nov., a novel marine bacterium that obligatory utilizes hydrocarbons. Int. J. Syst. Evol. Microbiol. 52, 901–911 10.1099/ijs.0.01890-0 [DOI] [PubMed] [Google Scholar]
- Gray N. D., Sherry A., Grant R. J., Rowan A. K., Hubert C. R. J., Callbeck C. M., et al. (2011). The quantitative significance of Syntrophaceae and syntrophic partnerships in methanogenic degradation of crude oil alkanes. Environ. Microbiol. 13, 2957–2975 10.1111/j.1462-2920.2011.02570.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara A., Syutsubo K., Harayama S. (2003). Alcanivorax which prevails in oil-contaminated seawater exhibits broad substrate specificity for alkane degradation. Environ. Microbiol. 5, 746–753 10.1046/j.1468-2920.2003.00468.x [DOI] [PubMed] [Google Scholar]
- Harayama S., Kishira H., Kasai Y., Syutsubo K. (1999). Petroleum biodegradation in marine environments. J. Mol. Microbiol. Biotechnol. 1, 63–70 [PubMed] [Google Scholar]
- Head I. M., Jones D. M., Röling W. F. M. (2006). Marine microorganisms make a meal of oil. Nat. Rev. Microbiol. 4, 173–182 10.1038/nrmicro1348 [DOI] [PubMed] [Google Scholar]
- Hedlund B. P., Geiselbrecht A. D., Bair T. J., Staley J. T. (1999). Polycyclic aromatic hydrocarbon degradation by a new marine bacterium, Neptunomonas naphthovorans gen. nov., sp. nov. Appl. Environ. Microbiol. 65, 251–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- ITOPF. (2011). Oil Tanker Spill Statistics. The International Tanker Owners Pollution Federation. Available online at: http://www.skuld.com/News/News/ITOPF-Oil-Tanker-Spill-Statistics-2011-released/
- Jones D. M., Douglas A. G., Parkes R. J., Taylor J., Giger W., Schaffner C. (1983). The recognition of biodegraded petroleum-derived aromatic hydrocarbons in recent marine sediments. Mar. Pollut. Bull. 14, 103–108 10.1016/0025-326X(83)90310-7 [DOI] [Google Scholar]
- Kasai Y., Kishira H., Harayama S. (2002b). Bacteria belonging to the genus Cycloclasticus play a primary role in the degradation of aromatic hydrocarbons released in a marine environment. Appl. Environ. Microbiol. 68, 5625–5633 10.1128/AEM.68.11.5625-5633.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasai Y., Kishira H., Sasaki T., Syutsubo K., Watanabe K., Harayama S. (2002a). Predominant growth of Alcanivorax strains in oil contaminated and nutrient-supplemented sea water. Environ. Microbiol. 4, 141–147 10.1046/j.1462-2920.2002.00275.x [DOI] [PubMed] [Google Scholar]
- Kasai Y., Kishira H., Syutsubo K., Harayama S. (2001). Molecular detection of marine bacterial populations on beaches contaminated by the Nakhodka tanker oil-spill accident. Environ. Microbiol. 3, 246–255 10.1046/j.1462-2920.2001.00185.x [DOI] [PubMed] [Google Scholar]
- Kostka J. E., Prakash O., Overholt W. A., Green S. J., Freyer G., Canion A., et al. (2011). Hydrocarbon-degrading bacteria and the bacterial community response in gulf of mexico beach sands impacted by the deepwater horizon oil spill. Appl. Environ. Microbiol. 77, 7962–7974 10.1128/AEM.05402-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krumbein W. C., Aberdeen E. (1937). The sediments of Barataria Bay. J. Sed. Petrol. 7, 3–17 10.1306/D4268F8B-2B26-11D7-8648000102C1865D [DOI] [Google Scholar]
- Leahy J. G., Colwell R. R. (1990). Microbial degradation of hydrocarbons in the environment. Microbiol. Rev. 54, 305–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Q., Mendelssohn I. A. (2012). Impacts and recovery of the Deepwater Horizon oil spill on vegetation structure and function of coastal salt marshes in the northern Gulf of Mexico. Environ. Sci. Technol. 46, 3737–3743 10.1021/es203552p [DOI] [PubMed] [Google Scholar]
- MacNaughton S. J., Stephen J. R., Venosa A. D., Davis G. A., Chang Y. J., White D. C. (1999). Microbial population changes during bioremediation of an experimental oil spill. Appl. Environ. Microbiol. 65, 3566–3574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama A., Ishiwata H., Kitamura K., Sunamura M., Fujita T., Matsuo M., et al. (2003). Dynamics of microbial populations and strong selection for Cycloclasticus pugetii following the Nakhodka oil spill. Microb. Ecol. 46, 442–453 10.1007/s00248-002-3010-z [DOI] [PubMed] [Google Scholar]
- McKew B. A., Coulon F., Osborn A. M., Timmis K. N., McGenity T. J. (2007a). Determining the identity and roles of oil-metabolizing marine bacteria from the Thames estuary, UK. Environ. Microbiol. 9, 165–176 10.1111/j.1462-2920.2006.01125.x [DOI] [PubMed] [Google Scholar]
- McKew B. A., Coulon F., Yakimov M. M., Denaro R., Genovese M., Smith C. J., et al. (2007b). Efficacy of intervention strategies for bioremediation of crude oil in marine ecosystems and effects on indigenous hydrocarbonoclastic bacteria. Environ. Microbiol. 9, 1562–1571 10.1111/j.1462-2920.2007.01277.x [DOI] [PubMed] [Google Scholar]
- Muyzer G., de Waal E. C., Uitterlinden A. G. (1993). Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA. Appl. Environ. Microbiol. 59, 695–700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council. (2003). Oil in the Sea III: Inputs, Fates and Effects. Washington, DC: National Academic Press; [PubMed] [Google Scholar]
- Natter M., Keevan J., Wang Y., Keimowitz A. R., Okeke B. C., Son A., et al. (2012). Level and degradation of deepwater horizon spilled oil in coastal marsh sediments and pore-water. Environ. Sci. Technol. 46, 5744–5755 10.1021/es300058w [DOI] [PubMed] [Google Scholar]
- Newton R. J., Huse S. M., Morrison H. G., Peake C. S., Sogin M. L., McLellan S. L. (2013). Shifts in the microbial community composition of gulf coast beaches following beach oiling. PLoS ONE 8:e74265 10.1371/journal.pone.0074265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- OSAT-1. (2010). Summary Report for Sub-Sea and Sub-Surface Oil and Dispersant Detection: Sampling and Monitoring. Washington, DC: Operational Science Advisory Team (multiagency) [Google Scholar]
- Prince R. C. (2005). “The microbiology of marine oil spill bioremediation,” in Petroleum Microbiology, eds Oliver B., Magot M. (Washington, DC: ASM Press; ), 317–336 [Google Scholar]
- Reay D. S., Nedwell D. B., Priddle J., Ellis-Evans J. C. (1999). Temperature dependence of inorganic nitrogen uptake: reduced affinity for nitrate at suboptimal temperatures in both algae and bacteria. Appl. Environ. Microbiol. 65, 2577–2584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Röling W. F. M., Milner M. G., Jones D. M., Fratepietro F., Swannell R. P. J., Daniel F., et al. (2004). Bacterial community dynamics and hydrocarbon degradation during a field-scale evaluation of bioremediation on a mudflat beach contaminated with buried oil. Appl. Environ. Microbiol. 70, 2603–2613 10.1128/AEM.70.5.2603-2613.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Röling W. F. M., Milner M. G., Jones D. M., Lee K., Daniel F., Swannell R. J. P., et al. (2002). Robust hydrocarbon degradation and dynamics of bacterial communities during nutrient-enriched oil spill bioremediation. Appl. Environ. Microbiol. 68, 5537–5548 10.1128/AEM.68.11.5537-5548.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabirova J. S., Becker A., Lünsdorf H., Nicaud J. M., Timmis K. N., Golyshin P. N. (2011). Transcriptional profiling of the marine oil-degrading bacterium Alcanivorax borkumensis during growth on n-alkanes. FEMS Microbiol. Lett. 319, 160–168 10.1111/j.1574-6968.2011.02279.x [DOI] [PubMed] [Google Scholar]
- Sabirova J. S., Ferrer M., Regenhardt D., Timmis K. N., Golyshin P. N. (2006). Proteomic insights into metabolic adaptations in Alcanivorax borkumensis induced by alkane utilization. J. Bacteriol. 188, 3763–3773 10.1128/JB.00072-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneiker S., Martins dos Santos V. A. P., Bartels D., Bekel T., Brecht M., Buhrmester J., et al. (2006). Genome sequence of the ubiquitous hydrocarbon-degrading marine bacterium Alcanivorax borkumensis. Nat. Biotechnol. 24, 997–1004 10.1038/nbt1232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schowanek D., Verstraete W. (1990). Phosphonate utilization by bacteria in the presence of alternative phosphorus sources. Biodegradation 1, 43–53 10.1007/BF00117050 [DOI] [PubMed] [Google Scholar]
- Short J. W., Irvine G. V., Mann D. H., Maselko J. M., Pella J. J., Lindeberg M. R., et al. (2007). Slightly weathered Exxon Valdez Oil persists in Gulf of Alaska beach sediments after 16 years. Environ. Sci. Technol. 41, 1245–1250 10.1021/es0620033 [DOI] [PubMed] [Google Scholar]
- Singh A. K., Sherry A., Gray N. D., Jones D. M., Röling W. F. M., Head I. M. (2011). Dynamics of Alcanivorax spp. in oil-contaminated intertidal beach sediment undergoing bioremediation, in Applied Microbiology and Molecular Biology in Oilfield Systems, eds Whitby C., Skovhus T. L. (Dordrecht: Springer; ), 199–209 [Google Scholar]
- Smith V. H., Graham D. W., Cleland D. D. (1998). Application of resource-ratio theory to hydrocarbon bioremediation. Environ. Sci. Technol. 32, 3386–3395 10.1021/es9805019 [DOI] [Google Scholar]
- Swannell R. P. J., Lee K., McDonagh M. (1996). Field evaluations of marine oil spill bioremediation. Microbiol. Rev. 60, 342–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vadstein O., Olsen Y. (1989). Chemical compositon and phosphate uptake kinetics of limnetic bacterial communities cultured in chemostats under phosphorus limitation. Limnol. Oceanogr. 34, 939–946 10.4319/lo.1989.34.5.0939 [DOI] [Google Scholar]
- Venosa A. D., Campo P., Suidan M. T. (2010). Biodegradability of lingering crude oil 19 years after the Exxon Valdez oil spill. Environ. Sci. Technol. 44, 7613–7621 10.1021/es101042h [DOI] [PubMed] [Google Scholar]
- Venosa A. D., Suidan M. T., Wrenn B. A., Strohmeier K. L., Haines J. R., Eberhart B. L., et al. (1996). Bioremediation of an experimental oil spill on the shoreline of Delaware Bay. Environ. Sci. Technol. 30, 1764–1775 10.1021/es950754r [DOI] [Google Scholar]
- Verschueren K. (1983). Handbook of Environmental Data of Organic Chemicals, 2nd Edn. New York, NY: Van Nostrand Reinhold Co [Google Scholar]
- Walker J. D., Colwell R. R., Petrakis L. (1976). Biodegradation rates of components of petroleum. Can. J. Microbiol. 22, 1209–1213 10.1139/m76-179 [DOI] [PubMed] [Google Scholar]
- White H. K., Hsing P. Y., Cho W., Shank T. M., Cordes E. E., Quattrini A. M., et al. (2012). Impact of the Deepwater Horizon oil spill on a deep-water coral community in the Gulf of Mexico. Proc. Natl. Acad. Sci. U.S.A. 109, 20303–20308 10.1073/pnas.1118029109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakimov M. M., Denaro R., Genovese M., Cappello S., D'Auria G., Chernikova T. N., et al. (2005). Natural microbial diversity in superficial sediments of Milazzo Harbor (Sicily). and community successions during microcosm enrichment with various hydrocarbons. Environ. Microbiol. 7, 1426–1441 10.1111/j.1462-5822.2005.00829.x [DOI] [PubMed] [Google Scholar]
- Yakimov M. M., Giuliano L., Denaro R., Grisafi E., Chernikova T. N., Abraham W. R., et al. (2004). Thalassolituus oleivorans gen. nov., sp nov., a novel marine bacterium that obligately utilizes hydrocarbons. Int. J. Syst. Evol. Microbiol. 54, 141–148 10.1099/ijs.0.02424-0 [DOI] [PubMed] [Google Scholar]
- Yakimov M. M., Giullano L., Gentile G., Crisafi E., Chemikova T. N., Abraham W. R., et al. (2003). Oleispira Antarctica gen. nov., sp. nov., a novel hydrocarbonoclastic marine bacterium isolated from Antarctic coastal sea water. Int. J. Syst. Evol. Microbiol. 53, 779–785 10.1099/ijs.0.02366-0 [DOI] [PubMed] [Google Scholar]
- Yakimov M. M., Golyshin P. N., Lang S., Moore E. R., Abraham W. R., Lunsdorf H., et al. (1998). Alcanivorax borkumemsis gen. nov., sp. nov., a new hydrocarbon-degrading and surfactant producing marine bacterium. Int. J. Syst. Bacteriol. 48, 339–348 10.1099/00207713-48-2-339 [DOI] [PubMed] [Google Scholar]
- Yakimov M. M., Timmis K. N., Golyshin P. N. (2007). Obligate oil-degrading marine bacteria. Curr. Opin. Biotechnol. 18, 257–266 10.1016/j.copbio.2007.04.006 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Cumulative CO2 production in beach sediment microcosms. Data from microcosms treated with crude oil plus inorganic nitrogen and phosphorus (black circles), inorganic nitrogen and phosphorus but no oil (gray circles) and killed control microcosms treated with formaldehyde (2% final concentration) and crude oil plus inorganic nitrogen and phosphorus (white circles). Oil treated microcosms contained 10 mg crude oil/g sediment and inorganic nutrient amendments equated to 5% N and 0.5% P by weight of the oil.
Effect of headspace flushing on physical removal of volatile saturated hydrocarbons from beach sediment microcosms. Concentrations of the individual compounds (C) after flushing the headspace with different volumes of air are presented relative to initial headspace concentration (C0) of the volatile hydrocarbons.


