Abstract
Purpose
Tuberculosis (TB) is a major infectious disease and is responsible for two million deaths annually. For the identification and quantitation of Mycobacterium tuberculosis (M. tuberculosis), a causative agent of TB, a sandwich enzyme-linked immunosorbent assay (ELISA) against the MPT64 protein of M. tuberculosis, an antigen marker of the M. tuberculosis complex, was developed.
Materials and Methods
The MPT64 protein was expressed, and anti-MPT64 monoclonal antibodies were prepared. A sandwich ELISA was established using recombinant MPT64 protein and anti-MPT64 monoclonal antibodies. The sandwich MPT64 ELISA was evaluated using reference and clinical mycobacterial strains.
Results
The sandwich MPT64 ELISA detected MPT64 protein from 2.1 ng/mL to 250 ng/mL (equivalent to 1.7×104 CFU/mL and 2.0×106 CFU/mL). All 389 clinical M. tuberculosis isolates tested positive in the sandwich MPT64 ELISA (sensitivity, 100%), and the assay showed no cross reactivity to any tested nontuberculous mycobacterial strain (specificity, 100%).
Conclusion
The sandwich MPT64 ELISA is a highly sensitive and quantitative test for MPT64 protein, which can identify M. tuberculosis.
Keywords: Mycobacterium tuberculosis, MPT64 protein, sandwich ELISA
INTRODUCTION
Tuberculosis (TB) is a major infectious disease that infects one-third of the world's population. Mycobacterium tuberculosis (M. tuberculosis), a cause of TB, infects 30 million people every year and causes 8 million new TB cases and 3 million deaths annually.1,2 The situation has been worsened by the appearance of multi-drug or extended drug-resistant M. tuberculosis strains, as well as by the combination with human immunodeficiency virus infection.3
Diagnosis of TB relies mainly on microbiological tests, such as smear microscopy and culturing of M. tuberculosis. Smear microscopy is a classical and fast diagnostic method, which detects acid-fast bacilli using Ziehl-Neelsen stain, but its sensitivity is low.4,5 Culturing of mycobacteria is the gold standard for diagnosing TB; however, it takes three to six weeks to form colonies.6,7,8 Many molecular methods have been developed to identify M. tuberculosis; however, they are used in only a limited number of diagnostic laboratories due to their high costs.9,10,11
Therefore, it is essential to develop a simple and rapid assay, which can identify M. tuberculosis and differentiate M. tuberculosis from nontuberculous mycobacteria (NTM) in cases of contamination by fast-growing NTM.2,11 Moreover, it is necessary to quantitate M. tuberculosis to monitor the therapeutic effects of antimycobacterial drugs.
The MPT64 antigen is a major secretory protein of M. tuberculosis, and has been shown to differentiate the M. tuberculosis complex from NTM.12 An immunochromatographic assay targeting MPT64 antigen (MPT64 ICA) was developed and is a very simple and rapid test for identifying M. tuberculosis.13 However, MPT64 ICA requires more sensitivity to detect M. tuberculosis in cultured specimens, and is not useful for assessing M. tuberculosis bacilli.13,14 Recently, Liu, et al.15 established sandwich enzyme-linked immunosorbent assay (ELISA) against MPT64 using polyclonal antibody, but its detection level was not high.
Therefore, in this study, in order to develop a highly sensitive and quantitative assay for M. tuberculosis, we established a sandwich ELISA for the MPT64 protein of M. tuberculosis using expressed MPT64 protein and prepared anti-MPT64 monoclonal antibodies, which can quantify the amount of MPT64 protein and differentiate M. tuberculosis from other mycobacteria. The sensitivity and specificity of this assay were evaluated using reference and clinical mycobacterial strains.
MATERIALS AND METHODS
Bacterial strains and growth conditions
M. tuberculosis H37Rv (American Type Culture Collection) was used as a reference strain, and was also used for cloning of the MPT64 protein. Five reference strains of M. tuberculosis, 46 NTM reference strains, 389 clinical M. tuberculosis isolates, and 64 clinical NTM isolates, including 12 M. abscessus isolates, 25 M. avium isolates, and 27 M. intracellulare isolates, were used for this study (Table 1). Of the clinical isolates, 231 clinical M. tuberculosis isolates grown on 3% Ogawa medium (Asan Pharmaceutical., Seoul, Korea) and 158 clinical M. tuberculosis strains grown in the BacT/ALERT Automated System (BioMérieux, Durham, France) were used in this study. All clinical NTM isolates were grown on 3% Ogawa medium. All clinical isolates were identified by Ziehl-Neelsen staining, the AdvanSure TB/NTM real-time PCR kit (LG life science, Seoul, Korea), and REBA Myco-ID® (M&D, Wonju, Korea).
Table 1.
List of Mycobacterial Strains
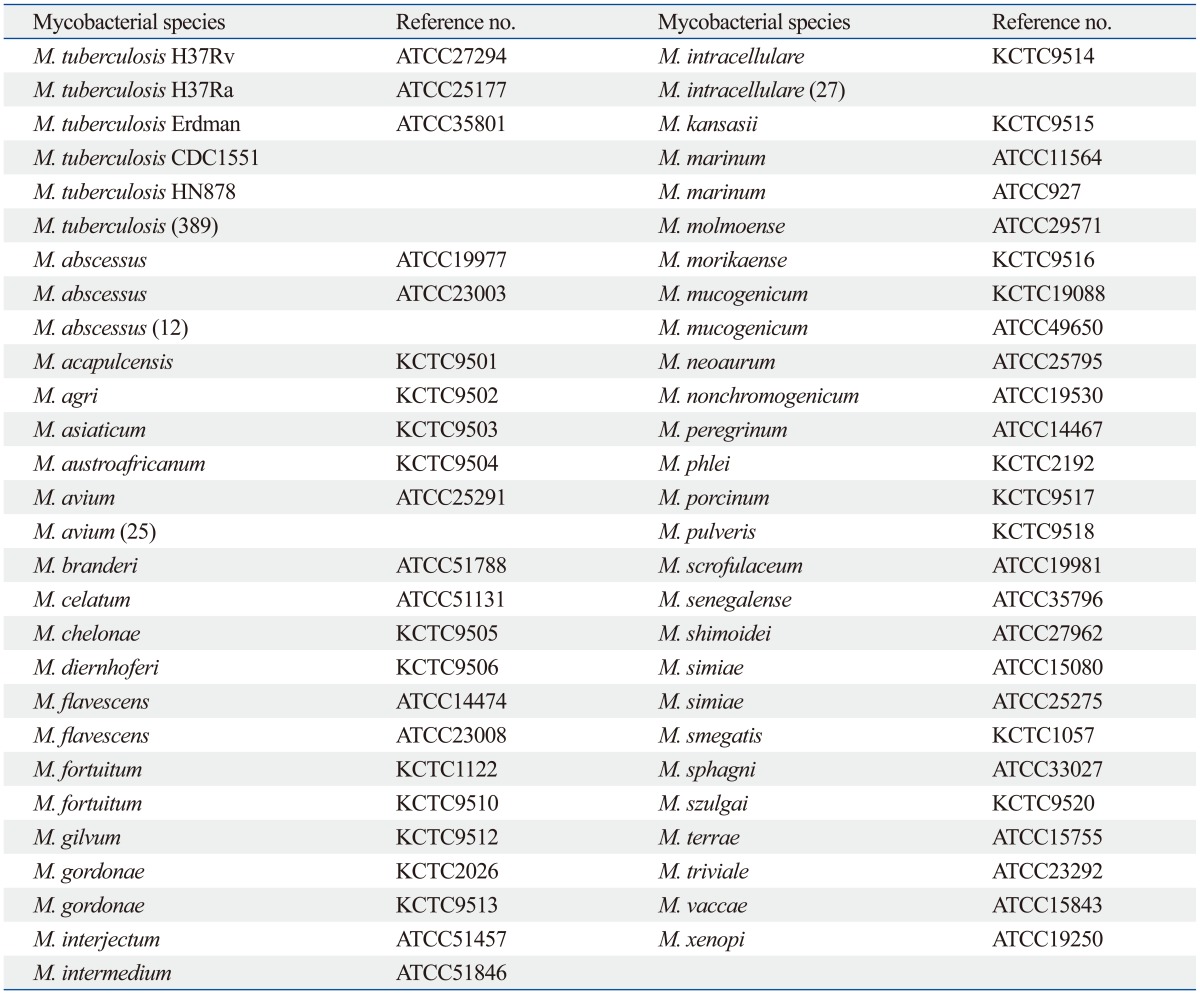
ATCC, American Type Culture Collection; KCTC, Korean Collection for Type Culture.
( ): Number of strains.
PCR amplification and cloning of mpt64 of M. tuberculosis
The mpt64 gene was amplified by PCR using oligonucleotide primers designed to include an EcoRI restriction enzyme site at the 5' end and an XbaI restriction enzyme site at the 3' end. The sequences of the primers were 5'-GAA TTC GCG CCC AAG ACC TAC TGC GAG-3' (EcoRI/MPT64-F) and 5'-TCT AGA CTA GGC CAG CAT CGA GTC GAT C-3' (XbaI/MPT64-R). The amplified mpt64 gene was ligated into the pT7 Blue vector (Novagen, Darmstadt, Germany), and their sequences were confirmed.
Expression and purification of recombinant MPT64
The mpt64 gene was ligated into the pMAL-p2x expression vector (New England Biolabs, Beverly, MA, USA), and MPT64 protein was expressed using E. coli TB-1 (Invitrogen, San Diego, CA, USA). The recombinant MPT64 protein was purified using affinity chromatography with an amylose resin column (New England Biolabs) and analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and a Western blot assay using mouse polyclonal anti-M. tuberculosis antibody, which was kindly provided by Prof. S.N. Cho (Yonsei University, Seoul, Korea).
Production of anti-MPT64 monoclonal antibodies
Ten eight-week-old female BALB/c mice (Orient Bio, Seongnam, Korea) were immunized intraperitoneally (i.p.) three times at two-week intervals with 40 µg of recombinant MPT64 protein emulsified in incomplete Freund's adjuvant (Sigma-Aldrich Co., St. Louis, MO, USA). Spleen cells were isolated and fused with SP2/0 myeloma cells at a ratio of 5:1 in the presence of polyethylene glycol 1500 (Roche Diagnostics GmbH, Mannheim, Germany). The hybridomas were selected in HAT medium (hypoxanthine-aminopterin-thymidine medium) and screened by measuring their binding activity to recombinant MPT64 protein by indirect ELISA. Highly reactive hybridomas were enriched in ascetic fluid from BALB/c mice pretreated with 1.0 mL of Pristance (Aldrich, Milwaukee, WI, USA), and the immunoglobulins were purified by chromatography on a protein G-Sepharose 4B flow (Amersham Bioscience, Piscataway, NJ, USA).
Sandwich enzyme-linked immunosorbent assay for MPT64 protein
Initially, anti-MPT64 monoclonal antibodies were screened for their reactivity to recombinant MPT64 protein, and highly reactive anti-MPT64 monoclonal antibodies were tested for their suitability for the sandwich ELISA. The optimum dilutions of these reagents were selected by checkerboard titration. Next, the sandwich ELISA was performed as follows: briefly, 96-well microtiter plates (Nunc, Roskilde, Denmark) were coated with anti-MPT64 monoclonal antibody in a suitable concentration and incubated at 4℃ overnight. After blocking with non-fat dry milk, recombinant MPT64 protein in phosphate buffered saline (PBS) was added and incubated for 2 h at 37℃. Subsequently, wells were washed four times and incubated with other horseradish peroxidase (HRP)-conjugated anti-MPT64 monoclonal antibodies for 1 h at 37℃. Finally, after six washes, 3,3',5,5'-Tetramethylbenzidine (TMB) substrate was added to the wells, the plates were incubated for 20 min in the dark, and absorbance was read at 450 nm after stopping the reaction with 2.5 N H2SO4. For generation of a standard curve, 1.0 µg/mL to 1000 µg/mL of recombinant MPT64 protein was used in the sandwich ELISA. The detection limit of the assay was defined as the mean value of blank plus three times its standard deviation.
Evaluation of MPT64 sandwich ELISA using clinical Mycobacterium isolates
To determine the detection limit of the MPT64 sandwich ELISA, a series of diluted culture suspensions of M. tuberculosis H37Rv were applied to the MPT64 sandwich ELISA, which was counted by inoculation onto Middlebrook 7H10 agar (Difco, Detroit, MI, USA) plates. To determine the sensitivity and specificity of the tests, 389 clinical M. tuberculosis isolates, five M. tuberculosis reference strains, 46 NTM reference strains, and 64 clinical NTM isolates were tested (Table 1). Three or four colonies from 3% Ogawa medium were collected and suspended in 200 µL of PBS, and then 100 µL of the suspension was used as a sample. The samples were classified as positive when the absorbance value was greater than or equal to twice the absorbance value of the negative control.
Statistical analysis
Statistical analysis was performed using Microsoft® Office Excel (2010) and the GraphPad Prism (version 4.0) program.
RESULTS
Cloning and expression of the mpt64 gene
The gene coding for the MPT64 protein was amplified from the M. tuberculosis H37Rv genome by PCR, and the sequence was confirmed (data not shown). The expressed recombinant MPT64 protein has a molecular weight of approximately 65 kDa and is reactive with the mouse polyclonal anti-M. tuberculosis antibody (Fig. 1).
Fig. 1.
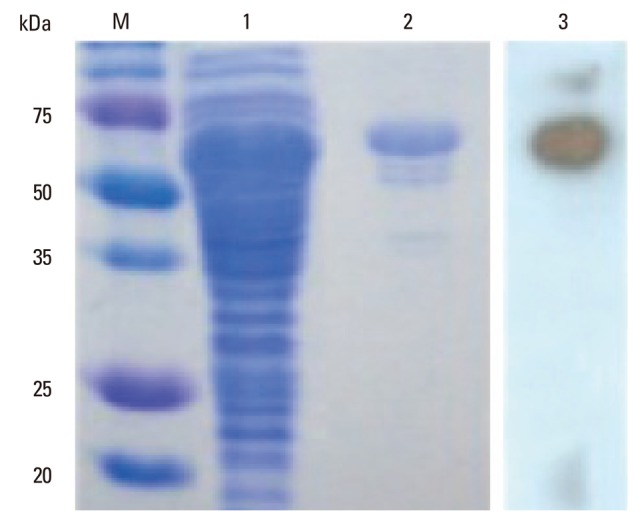
Analysis of expression of the MPT64 protein in TB-1 cells. TB-1 cells were inducted with 1 mM IPTG for 4 h, and the cell lysates were analysed by SDS-PAGE, after which the gel was stained with Coomassie blue (lanes 1 to 2) or analysed by Western blot with a mouse polyclonal anti-M. tuberculosis antibody (lane 3). Lane M: pre-stained protein molecular weight markers. Lane 1: cell lysate of TB-1 cells. Lane 2: purified recombinant MPT64. Lane 3: Western blot analysis with anti-MPB64 antibody. TB, tuberculosis; SDS-PAGE, sodium dodecyl sulfate-polyacrylamide gel electrophoresis; IPTG, isopropyl β-D-1-thiogalactopyranoside.
Characterization of anti-MPT64 protein monoclonal antibodies
Prepared hybridomas were screened for their ability to secrete monoclonal antibodies against the MPT64 protein. Two antibodies (1A4 and 2E9) from screened hybridomas showed the highest reactivity in the immunoassay (data not shown). The immunoglobulin isotype of both antibodies belongs to the IgG1 subclass having the lambda light chain (data not shown).
Establishment of a sandwich ELISA for MPT64 protein
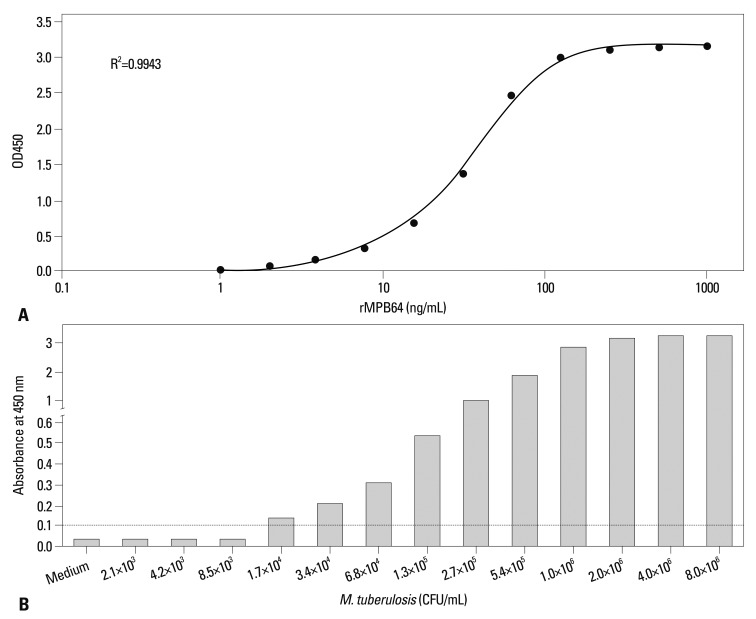
A sandwich ELISA for the MPT64 protein was established using a 1A4 monoclonal antibody as a capture antibody for the MPT64 protein and HRP-conjugated 2E9 monoclonal antibody as a detection antibody for MPT64 protein, which produced minimal background, as well as the highest sensitivity and accuracy for the quantification of the MPT64 protein. The optimal concentrations of the capture antibody 1A4 and detection antibody 2E9 were 2 µg/mL and 4 µg/mL, respectively, and the secondary antibody was diluted to 1:20000. A typical calibration curve for quantification of the MPT64 protein was generated by plotting the concentration of recombinant MPT64 protein versus the absorbance, which yielded a coefficient of determination (R2) of 0.9943 (Fig. 2A). The lower limit of detection of this assay was 2.1 ng/mL, equivalent to an absorbance value of 0.102, which was the mean signal of the blank plus three times its standard deviation, and this assay was linear over the range of 3.8 to 250 ng/mL. Therefore, this assay might be a highly sensitive assay to quantify the MPT64 protein of M. tuberculosis.
Fig. 2.
Calibration curve of the developed sandwich ELISA for MPT64 protein (A), and detection limits of the assay (B). The calibration curve was obtained from the mean value of six sandwich ELISAs with the anti-MPT64 antibodies Mab-1A4 and Mab-2E9 under optimized conditions (A). Culture supernatants of M. tuberculosis H37Rv in Middlebrook 7H9 broth with enrichment were serially diluted and applied to the test. The cut-off of the MPT64 sandwich ELISA is indicated by a dotted line. ELISA, enzyme-linked immunosorbent assay; CFU, colony-forming unit; OD, optical density.
Detection limit of MPT64 sandwich ELISA and MPT64 ICA
To evaluate the ability of the MPT64 sandwich ELISA to detect secreted MPT64 protein from growing M. tuberculosis, the sequential culture supernatants of M. tuberculosis H37Rv were tested. The MPT64 sandwich ELISA detected levels from 1.7×104 CFU/mL of M. tuberculosis as positive, based on a cut-off value of 0.102, which is equivalent to 2.1 ng/mL (Fig. 2B). The upper limit of detection of the MPT64 sandwich ELISA was 2.0×106 CFU/mL (OD450=3.15), which is equivalent to 253 ng/mL. The MPT64 sandwich ELISA could quantitatively measure the concentration of MPT64 protein in the culture supernatant, which was correlatively related to the numbers of M. tuberculosis.
Sensitivity and specificity of the MPT64 sandwich ELISA
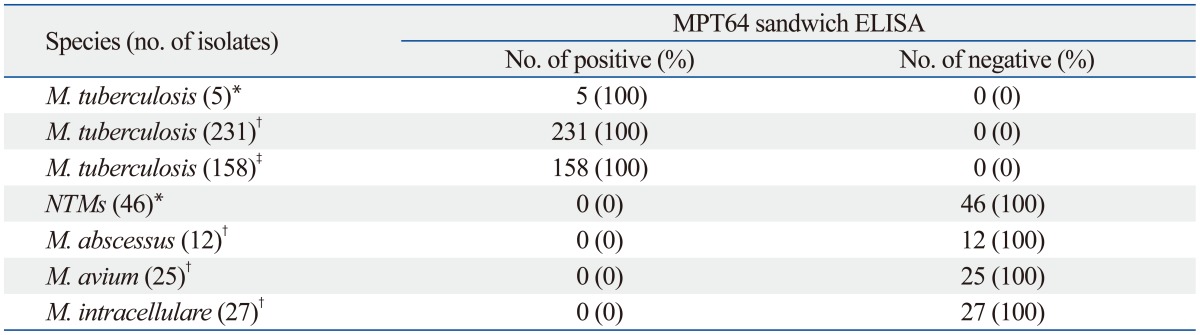
The sensitivity and specificity of these tests were evaluated using 51 reference strains of M. tuberculosis and NTMs, 389 clinical M. tuberculosis isolates, and 64 clinical NTM isolates, including 12 clinical M. abscessus isolates, 25 clinical M. avium isolates, and 27 clinical M. intracellulare isolates (Table 2). In the MPT64 sandwich ELISA, all five reference M. tuberculosis strains and all 389 clinical M. tuberculosis isolates, including 231 M. tuberculosis isolates grown on 3% Ogawa media and 158 M. tuberculosis grown in the liquid culture system, were detected as positive, equating to a sensitivity of 100%. All NTM strains, including 46 reference NTM strains (39 species) and 64 clinical NTM isolates (3 species), generated negative results in the MPT64 sandwich ELISA.
Table 2.
Comparison of the Results Obtained by the MPT64 Sandwich ELISA and the Lmmunochromatographic TB Ag MPT64 Rapid Test for M. tuberculosis and Other Nontuberculous Mycobacterial Strains
NTM, nontuberculous mycobacteria; ELISA, enzyme-linked immunosorbent assay; TB, tuberculosis.
*Reference mycobacterial strains.
†Clinical isolates grown on 3% Ogawa medium.
‡Clinical isolates grown in the BacT/ALERT Automated System.
DISCUSSION
In this study, we developed a highly sensitive and quantitative sandwich ELISA for the MPT64 protein of M. tuberculosis using anti-MPT64 monoclonal antibodies. This MPT64 sandwich ELISA could measure the quantity of secreted MPT64 protein from M. tuberculosis, which differentiates M. tuberculosis from NTM. The lower limit of detection of the MPT64 sandwich ELISA was 2.1 ng/mL, and this assay could detect 1.7×104 CFU/mL of M. tuberculosis. Recently, Liu, et al.15 developed an MPT64 ELISA using polyclonal antibodies, and its lower limit of detection was 10 ng/mL, which is approximately five times higher than ours. A possible explanation for this is that the anti-MPT64 monoclonal antibodies used for the sandwich ELISA might have a higher affinity than polyclonal antibodies. The MPT64 ICA could detect 1×105 CFU/mL of M. tuberculosis as positive.13,14 This suggests that the MPT64 sandwich ELISA might be a more sensitive assay than MPT64 ICA for the detection of MPT64 protein. The sensitivity and specificity of the MPT64 sandwich ELISA were both 100%. These results seem to be comparable to or more sensitive than MPT64 ICA, which has a reported sensitivity of 92-99% and a specificity of 97-100%.12,13,15 This indicates that the MPT64 protein is highly specific for the M. tuberculosis complex and is therefore a comparably more sensitive target analyte.16 In addition, the MPT64 sandwich ELISA has an advantage over the MPT64 ICA, in that the MPT64 sandwich ELISA is able to quantify the MPT64 protein of M. tuberculosis, which is significantly correlated with the count of M. tuberculosis (R2=0.998, p<0.001). This would be very useful for measuring counts of M. tuberculosis, because it takes three to six weeks for enumeration of M. tuberculosis and also could contribute to the monitoring of the therapeutic effects of anti-mycobacterial drugs, as well as to the more rapid screening of anti-mycobacterial drug candidates.
In conclusion, a MPT64 sandwich ELISA, developed using recombinant MPT64 protein and anti-MPT64 monoclonal antibodies, was shown to be a highly sensitive and specific assay for the identification of M. tuberculosis via detection of the MPT64 protein, and may be a quantitative assay for measuring the MPT64 protein of M. tuberculosis.
ACKNOWLEDGEMENTS
This work was supported by a grant of the Korean Health Technology R&D, Ministry of Health & Welfare, Republic of Korea [HI10C1708 (A101750), SNC], by a grant of the Korea Food and Drug Administration, Health & Welfare, Republic of Korea (BYJ, 13Fre04) and Biotech Laboratory of Standard Diagnostics, Republic of Korea.
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Raviglione MC, Snider DE, Jr, Kochi A. Global epidemiology of tuberculosis. Morbidity and mortality of a worldwide epidemic. JAMA. 1995;273:220–226. [PubMed] [Google Scholar]
- 2.Morris K. Global tuberculosis control amid the world economic crisis. Lancet Infect Dis. 2009;9:144–145. doi: 10.1016/s1473-3099(09)70030-1. [DOI] [PubMed] [Google Scholar]
- 3.Snider DE, Jr, Castro KG. The global threat of drug-resistant tuberculosis. N Engl J Med. 1998;338:1689–1690. doi: 10.1056/NEJM199806043382309. [DOI] [PubMed] [Google Scholar]
- 4.Jenkins SG. Evaluation of new technology in the clinical microbiology laboratory. Diagn Microbiol Infect Dis. 1995;23:53–60. doi: 10.1016/0732-8893(95)00154-9. [DOI] [PubMed] [Google Scholar]
- 5.Thongraung W, Chongsuvivatwong V, Pungrassamee P. Multilevel factors affecting tuberculosis diagnosis and initial treatment. J Eval Clin Pract. 2008;14:378–384. doi: 10.1111/j.1365-2753.2007.00871.x. [DOI] [PubMed] [Google Scholar]
- 6.Chakravorty S, Sen MK, Tyagi JS. Diagnosis of extrapulmonary tuberculosis by smear, culture, and PCR using universal sample processing technology. J Clin Microbiol. 2005;43:4357–4362. doi: 10.1128/JCM.43.9.4357-4362.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hillemann D, Rüsch-Gerdes S, Richter E. Application of the Capilia TB assay for culture confirmation of Mycobacterium tuberculosis complex isolates. Int J Tuberc Lung Dis. 2005;9:1409–1411. [PubMed] [Google Scholar]
- 8.Rodrigues C, Vadwai V. Tuberculosis: laboratory diagnosis. Clin Lab Med. 2012;32:111–127. doi: 10.1016/j.cll.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 9.Cho SN, Brennan PJ. Tuberculosis: diagnostics. Tuberculosis (Edinb) 2007;87(Suppl 1):S14–S17. doi: 10.1016/j.tube.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 10.Pathak D, Chakravorty S, Hanif M, Tyagi JS. Lysis of tubercle bacilli in fresh and stored sputum specimens: implications for diagnosing tuberculosis in stored and paucibacillary specimens by PCR. BMC Microbiol. 2007;7:83. doi: 10.1186/1471-2180-7-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Magana-Arachchi D, Perera J, Gamage S, Chandrasekharan V. Low cost in-house PCR for the routine diagnosis of extra-pulmonary tuberculosis. Int J Tuberc Lung Dis. 2008;12:275–280. [PubMed] [Google Scholar]
- 12.Brent AJ, Mugo D, Musyimi R, Mutiso A, Morpeth S, Levin M, et al. Performance of the MGIT TBc identification test and meta-analysis of MPT64 assays for identification of the Mycobacterium tuberculosis complex in liquid culture. J Clin Microbiol. 2011;49:4343–4346. doi: 10.1128/JCM.05995-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Park MY, Kim YJ, Hwang SH, Kim HH, Lee EY, Jeong SH, et al. Evaluation of an immunochromatographic assay kit for rapid identification of Mycobacterium tuberculosis complex in clinical isolates. J Clin Microbiol. 2009;47:481–484. doi: 10.1128/JCM.01253-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ang CF, Cajucom MA, Kim Y, Bang H, Lee H, Cho SN, et al. Evaluation of a rapid assay for identification of Mycobacterium tuberculosis grown in solid and liquid media. Int J Tuberc Lung Dis. 2011;15:1475–1477. doi: 10.5588/ijtld.10.0709. [DOI] [PubMed] [Google Scholar]
- 15.Liu Z, Zhu C, Yang H, Hu H, Feng Y, Qin L, et al. Clinical value of ELISA-MPT64 for the diagnosis of tuberculous pleurisy. Curr Microbiol. 2012;65:313–318. doi: 10.1007/s00284-012-0157-9. [DOI] [PubMed] [Google Scholar]
- 16.Hasegawa N, Miura T, Ishii K, Yamaguchi K, Lindner TH, Merritt S, et al. New simple and rapid test for culture confirmation of Mycobacterium tuberculosis complex: a multicenter study. J Clin Microbiol. 2002;40:908–912. doi: 10.1128/JCM.40.3.908-912.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]