Abstract
Purpose
Liver resection with colorectal liver metastasis widely accepted and has been considered safe and effective therapeutic option. However, the role of liver resection in breast cancer with liver metastasis is still controversial. Therefore, we reviewed the outcome of liver resection in breast cancer patients with liver metastases in a single hospital experiences.
Materials and Methods
Between January 1991 and December 2006, 2176 patients underwent breast cancer surgery in Gangnam Severance Hospital. Among these patients, 110 cases of liver metastases were observed during follow-up and 13 of these patients received liver resection with potential feasibility to achieve an R0 resection.
Results
The median time interval between initial breast cancer and detection of liver metastasis was 62.5 months (range, 13-121 months). The 1-year and 3-year overall survival rates of the 13 patients with liver resection were 83.1% and 49.2%, respectively. The 1-year and 3-year overall survival rates of patients without extrahepatic metastasis were 83.3% and 66.7% and those of patients with extrahepatic metastasis were 80.0% and 0.0%, respectively (p=0.001).
Conclusion
Liver resection for metastatic breast cancer results in improved patient survival, particularly in patients with solitary liver metastasis and good general condition.
Keywords: Breast cancer, liver metastasis, liver resection, prognos
INTRODUCTION
Because many doctors consider liver metastasis from malignant cancer as a systemic recurrence rather than a local recurrence, their therapeutic aim is only to improve the quality of life using conservative management. However, liver resection in patients with colorectal liver metastasis has now become widely accepted and has been shown to be a safe and effective therapeutic option in several reports.1,2 In contrast to metastasis from colorectal cancer, non-colorectal liver metastasis is only considered for surgical resection in selected cases.
According to the Korea Cancer Center Registry, the rate of breast cancer is rapidly increasing and it ranks sixth in overall cancer incidence and second form of cancer among females in Korea.3 The prognosis of breast cancer has greatly improved as new medical imaging technologies and various chemotherapeutic drugs have been developed. However, liver metastases occur in 15% of breast cancer patients and these patients have a poor prognosis with an average survival period of less than 24 months, even if they receive aggressive systemic and hormone therapy.4,5,6
Recently, liver resection-related mortality rates have largely declined due to improved anesthesia, progression of surgical techniques, and improvements in postoperative managements. In addition, improved chemotherapy agents may reduce the number or size of liver metastases, resulting in favorable conditions before and after surgery.
Some papers has reported that liver resection shows a tendency to improve prognosis in selected patients.7,8,9,10 However, the role of liver resection in breast cancer with liver metastasis is still debatable. Therefore, we reviewed the outcome of patients with breast cancer with liver metastases after liver resection in a single hospital experiences.
MATERIALS AND METHODS
Patients
Between January 1991 and December 2006, 2176 patients underwent breast cancer surgery in Gangnam Severance Hospital, Yonsei University College of Medicine, Seoul, Korea. Among these patients, 110 cases of liver metastasis were observed during follow-up. Of these, 13 patients received liver resection with potential feasibility to achieve an R0 resection. The patients were followed closely until December 30, 2011.
Ambulatory follow-up examinations of all patients were performed every 6 months for 5 years after the surgery and follow-up observation was performed every year subsequently. The following basic examinations were performed every 6 months after surgery: physical examination, radiological examination (chest X-ray, ultrasound, and mammography), and laboratory testing (general blood test, liver function test, and tumor marker test). Abdomen and chest computed tomography, bone scan and PET were routinely performed in all patients to check for systemic metastasis.
Statistical analysis
All statistical analyses were performed using SPSS software, Version 16.0 (SPSS Inc., Chicago, IL, USA). Categorical variables were analyzed using the χ2 or Fisher's exact test, and continuous variables were analyzed using either a Student's t-test or Mann-Whitney U rank tests. A p-value less than 0.05 was considered statistically significant. Survival curves were constructed and compared by the Kaplan-Meier method and the log-rank test, respectively.
RESULTS
Patient characteristics
During the study period, a total of 110 patients were diagnosed with breast cancer with liver metastasis: 91 patients (83%) with multi-organ metastasis, including the liver, and 19 (17%) patients with isolated liver metastasis. Among the 110 patients with liver metastasis, 13 received liver resection with potential feasibility to achieve an R0 resection. The mean age of the 13 patients was 51.0±9.4 years (range 39-64 years) and were all female.
All 13 patients underwent surgery for breast cancer involving either conservative breast surgery or radical mastectomy. The pathology of breast cancer was infiltrating ductal carcinoma in 12 patients (92.3%) and mucinous carcinoma in one patient (7.7%). The histological grade classification of breast cancer was well differentiated in seven patients (53.8%), moderately differentiated in three patients (23.1%), and poorly differentiated in three patients (23.1%). The T stage of breast cancer was T1 in four patients (30.8%), T2 in seven patients (53.8%), T3 in one patient (7.7%), and T4 in one patient (7.7%). The initial N stage of breast cancer was N0 in five patients (38.5%), N1 in two patients (15.3%), N2 in five patients (38.5%), and N3 in one patient (7.7%). The hormone receptor and HER2 status of the breast cancer was available for all patients. Six patients had estrogen (ER) and progesterone (PR) positive tumors, one was ER+/PR-, one was ER-/PR+ and five were ER-/PR-. Five patients were HER2-positive (38.5%). All patients received adjuvant therapy after surgery for breast cancer depending on their hormone receptor status and the stage.
Patients were divided into two groups; solitary liver metastasis and multi-organ metastasis including liver. Single liver metastasis was observed in seven (53.8%) patients, and multi-organ metastasis was detected in six (46.2%) patients. Clinicopathological characteristics of patients in the two groups are listed in Table 1 and 2. The median time interval between initial breast cancer and detection of liver metastasis excluding two patients with synchronous metastasis was 62.5 months (range, 13-121 months).
Table 1.
Clinical Characteristics of the Seven Patients Treated for Solitary Liver Metastasis from Breast Cancer

IDC, infiltrating ductal carcinoma; HG, histologic grade; HR, hormone receptor; RFA, radiofrequency ablation; ER, estrogen; PR, progesterone.
Table 2.
Clinical Characteristics of the Six Patients Treated for Extrahepatic and Liver Metastasis from Breast Cancer
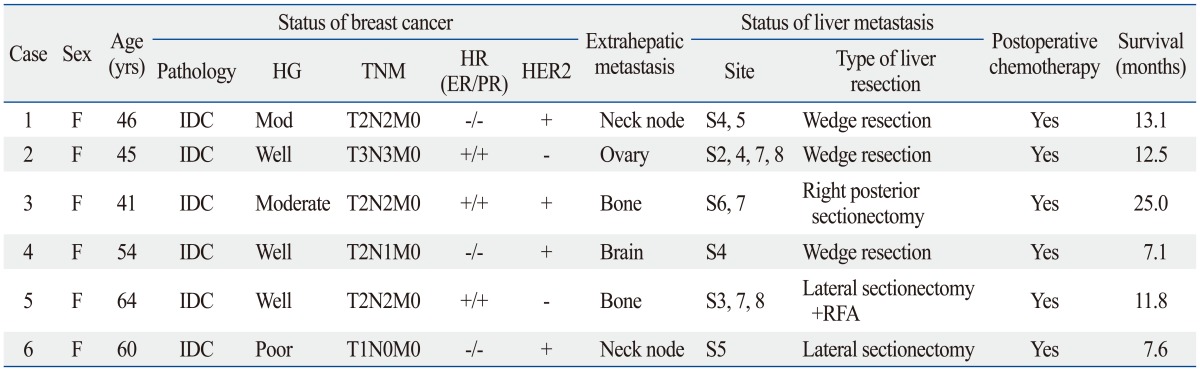
IDC, infiltrating ductal carcinoma; HG, histologic grade; HR, hormone receptor; RFA, radiofrequency ablation; ER, estrogen; PR, progesterone.
Among patients with multi-organ metastasis, two (33.3%) had bone metastasis, two (33.3%) had metastasis in neck nodes, one (16.7%) had brain metastasis, and one (16.7%) had ovarian metastasis.
Liver resection
All patients received adjuvant therapy after surgery for breast cancer depending on their hormone receptor status and tumor stage. Two patients had synchronous metastases--liver metastases at the time of the initial breast cancer diagnosis--and after six cycles of neo-adjuvant therapy, the breast cancer and metastatic liver lesions were both operated upon.
Nine patients (69.2%) received an open liver resection and four patients (30.8%) received laparoscopic liver resection. Major liver resection (three segments or more) was performed in one (7.7%) patient, minor resection (fewer than three segments) in four (30.8%) patients, and wedge resection in eight (61.5%) patients. Liver resection was combined with radiofrequency ablation in two patients.
All patients with extrahepatic metastatic disease were treated with curative intent for their extrahepatic metastatic lesions.
All patients received postoperative adjuvant chemotherapy after liver resection.
Surgical outcomes
The 1-year and 3-year overall survival rates of the 13 patients with liver resection were 83.1% and 49.2%, respectively (Fig. 1). The 1-year and 3-year overall survival rates of patients without extrahepatic metastasis were 83.3% and 66.7% and those of patients with extrahepatic metastasis were 80.0% and 0.0%, respectively (p=0.001) (Fig. 2).
Fig. 1.
Overall survival rate after hepatic resection for breast cancer patients with liver metastasis.
Fig. 2.
Overall survival rate following hepatic resection according to status of extrahepatic metastasis.
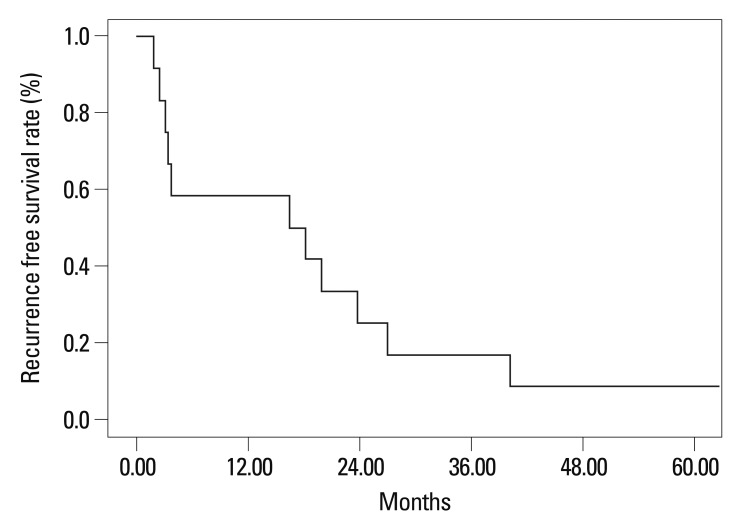
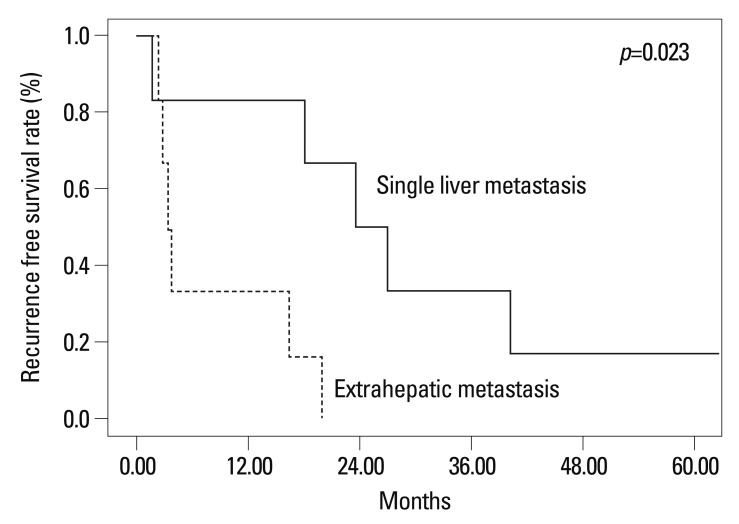
The 1-year and 3-year recurrence free survival rates after liver resection of the 13 patients were 50.0% and 16.7%, respectively (Fig. 3). The 1-year and 3-year recurrence free survival rate after liver resection of patients without extrahepatic metastasis were 83.3% and 33.3% and those patients with extrahepatic metastasis were 33.3% and 0.0%, respectively (p=0.023) (Fig. 4).
Fig. 3.
Recurrence free survival rate after hepatic resection for breast cancer patients with liver metastasis.
Fig. 4.
Recurrence free survival rates following hepatic resection according to status of extrahepatic metastasis.
DISCUSSION
Recurrence rates after breast cancer surgery are 30-40%, and mainly involve bone metastases. After bone and lung metastases, liver metastases represent 40% of total breast cancer recurrence;11 bone metastasis was also the most frequent in this study and liver metastasis ranked third in total recurrences.
Nonetheless, many doctors consider liver metastasis from breast cancer as systemic recurrence. Therefore, their aim is only to improve the quality of the patient's life using alleviation therapy rather than improving survival rates. Recently, patients with liver metastasis from breast cancer have shown improved survival rates due to improvements in anticancer drugs.12,13,14 However, these are not satisfactory long-term survival rates and the average overall survival time is reported to be only 15 months.15
As surgical techniques for liver resection have improved and postoperative morbidity and mortality rates have decreased after operation, attempts of liver resection from breast cancer started in the 1980s. Elias, et al.7 first reported that liver resection in patients with metastatic breast cancer could lengthen patient survival compared with conservative management. Nevertheless, the role of surgical treatment for liver metastasis from breast cancer has not yet been clearly defined.
Many studies have reported that median survival for liver resection in breast cancer ranges between 24 and 60 months and 5-year survival rates are between 21% and 60%.16,17,18,19 In the present study, the average median survival was 57.9 months, and the 3-year survival rate was 59.3%. This represents an improved overall survival rate compared with patients that received systemic chemotherapy treatment.20,21,22
Some reports showed that age, estrogen and HER2 receptor status of the primary tumor, number of metastasis, and resection margins are significant prognostic factors.9,10,17,18,19,23 However, we could not analyze the prognostic factors after liver resection because of the small number of patients in our study.
In this study, the surgical indication of liver metastasis was restricted to cases where an extrahepatic lesion could be controlled by local treatment and no other distant metastases were present. Nonetheless, we demonstrated that the presence of extrahepatic metastasis lesions was a significant prognostic factor after liver resection. In our results, after liver resection of patients with extrahepatic metastasis, the 1- and 3-year survival rates were 60.0% and 20.0%, respectively. Therefore, even when extrahepatic metastasis is present, if the systemic condition of the patient is satisfactory and if cure of the extrahepatic lesion is possible, we think that surgical treatment of the liver lesions should be considered.
It is possible that the treatment outcome of patients with liver metastasis may have been overestimated in this study because it is a retrospective study with a limited number of cases. Nonetheless, although this study contains a small number of patients and has limitations, it suggests that surgery in patients with solitary liver metastasis results in a promising good prognosis.
In conclusion, liver resection for metastatic breast cancer results in improved patient survival, particularly in patients with solitary liver metastasis and good general condition.
Footnotes
The authors have no financial conflicts of interest.
References
- 1.House MG, Ito H, Gönen M, Fong Y, Allen PJ, DeMatteo RP, et al. Survival after hepatic resection for metastatic colorectal cancer: trends in outcomes for 1,600 patients during two decades at a single institution. J Am Coll Surg. 2010;210:744–752. 752–755. doi: 10.1016/j.jamcollsurg.2009.12.040. [DOI] [PubMed] [Google Scholar]
- 2.Morris EJ, Forman D, Thomas JD, Quirke P, Taylor EF, Fairley L, et al. Surgical management and outcomes of colorectal cancer liver metastases. Br J Surg. 2010;97:1110–1118. doi: 10.1002/bjs.7032. [DOI] [PubMed] [Google Scholar]
- 3.Jung KW, Park S, Kong HJ, Won YJ, Lee JY, Seo HG, et al. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2009. Cancer Res Treat. 2012;44:11–24. doi: 10.4143/crt.2012.44.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cutler SJ, Ardyce JA, Taylor SG., 3rd Classification of patients with disseminated cancer of the breast. Cancer. 1969;24:861–869. doi: 10.1002/1097-0142(196911)24:5<861::aid-cncr2820240502>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 5.Jaffe BM, Donegan WL, Watson F, Spratt JS., Jr Factors influencing survival in patients with untreated hepatic metastases. Surg Gynecol Obstet. 1968;127:1–11. [PubMed] [Google Scholar]
- 6.Nadal JM, Jouve M, Mosseri V, Asselain B, Pouillart P. [Metastatic cancer of the breast treated by polychemotherapy: a new prognostic approach] Bull Cancer. 1988;75:757–769. [PubMed] [Google Scholar]
- 7.Elias D, Lasser P, Spielmann M, May-Levin F, el Malt O, Thomas H, et al. Surgical and chemotherapeutic treatment of hepatic metastases from carcinoma of the breast. Surg Gynecol Obstet. 1991;172:461–464. [PubMed] [Google Scholar]
- 8.Goldhirsch A, Gelber RD, Castiglione M. Relapse of breast cancer after adjuvant treatment in premenopausal and perimenopausal women: patterns and prognoses. J Clin Oncol. 1988;6:89–97. doi: 10.1200/JCO.1988.6.1.89. [DOI] [PubMed] [Google Scholar]
- 9.Neri A, Marrelli D, Pedrazzani C, Caruso S, De Stefano A, Mariani F, et al. Prognostic relevance of proliferative activity evaluated by Mib-1 immunostaining in node negative breast cancer. Eur J Surg Oncol. 2008;34:1299–1303. doi: 10.1016/j.ejso.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 10.Konecny GE, Thomssen C, Lück HJ, Untch M, Wang HJ, Kuhn W, et al. Her-2/neu gene amplification and response to paclitaxel in patients with metastatic breast cancer. J Natl Cancer Inst. 2004;96:1141–1151. doi: 10.1093/jnci/djh198. [DOI] [PubMed] [Google Scholar]
- 11.Lee YT. Breast carcinoma: pattern of metastasis at autopsy. J Surg Oncol. 1983;23:175–180. doi: 10.1002/jso.2930230311. [DOI] [PubMed] [Google Scholar]
- 12.Wyld L, Gutteridge E, Pinder SE, James JJ, Chan SY, Cheung KL, et al. Prognostic factors for patients with hepatic metastases from breast cancer. Br J Cancer. 2003;89:284–290. doi: 10.1038/sj.bjc.6601038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perez JE, Machiavelli M, Leone BA, Romero A, Rabinovich MG, Vallejo CT, et al. Bone-only versus visceral-only metastatic pattern in breast cancer: analysis of 150 patients. A GOCS study. Grupo Oncológico Cooperativo del Sur. Am J Clin Oncol. 1990;13:294–298. doi: 10.1097/00000421-199008000-00005. [DOI] [PubMed] [Google Scholar]
- 14.Baur M, Schlappack O, Havelec L, Wrba F, Dittrich C. Prognostic significance of liver metastases as first site of generalisation in patients with breast cancer--a retrospective analysis. Acta Med Austriaca. 2001;28:135–140. doi: 10.1046/j.1563-2571.2001.01033.x. [DOI] [PubMed] [Google Scholar]
- 15.Solomayer EF, Diel IJ, Meyberg GC, Gollan C, Bastert G. Metastatic breast cancer: clinical course, prognosis and therapy related to the first site of metastasis. Breast Cancer Res Treat. 2000;59:271–278. doi: 10.1023/a:1006308619659. [DOI] [PubMed] [Google Scholar]
- 16.Adam R, Aloia T, Krissat J, Bralet MP, Paule B, Giacchetti S, et al. Is liver resection justified for patients with hepatic metastases from breast cancer? Ann Surg. 2006;244:897–907. doi: 10.1097/01.sla.0000246847.02058.1b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thelen A, Benckert C, Jonas S, Lopez-Hänninen E, Sehouli J, Neumann U, et al. Liver resection for metastases from breast cancer. J Surg Oncol. 2008;97:25–29. doi: 10.1002/jso.20911. [DOI] [PubMed] [Google Scholar]
- 18.Hoffmann K, Franz C, Hinz U, Schirmacher P, Herfarth C, Eichbaum M, et al. Liver resection for multimodal treatment of breast cancer metastases: identification of prognostic factors. Ann Surg Oncol. 2010;17:1546–1554. doi: 10.1245/s10434-010-0931-5. [DOI] [PubMed] [Google Scholar]
- 19.Weitz J, Blumgart LH, Fong Y, Jarnagin WR, D'Angelica M, Harrison LE, et al. Partial hepatectomy for metastases from noncolorectal, nonneuroendocrine carcinoma. Ann Surg. 2005;241:269–276. doi: 10.1097/01.sla.0000150244.72285.ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yoshimoto M, Tada T, Saito M, Takahashi K, Uchida Y, Kasumi F. Surgical treatment of hepatic metastases from breast cancer. Breast Cancer Res Treat. 2000;59:177–184. doi: 10.1023/a:1006398401352. [DOI] [PubMed] [Google Scholar]
- 21.Campos SM, Guastalla JP, Subar M, Abreu P, Winer EP, Cameron DA. A comparative study of exemestane versus anastrozole in patients with postmenopausal breast cancer with visceral metastases. Clin Breast Cancer. 2009;9:39–44. doi: 10.3816/CBC.2009.n.007. [DOI] [PubMed] [Google Scholar]
- 22.Crump M, Gluck S, Tu D, Stewart D, Levine M, Kirkbride P, et al. Randomized trial of high-dose chemotherapy with autologous peripheral-blood stem-cell support compared with standard-dose chemotherapy in women with metastatic breast cancer: NCIC MA.16. J Clin Oncol. 2008;26:37–43. doi: 10.1200/JCO.2007.11.8851. [DOI] [PubMed] [Google Scholar]
- 23.Pocard M, Pouillart P, Asselain B, Falcou MC, Salmon RJ. [Hepatic resection for breast cancer metastases: results and prognosis (65 cases)] Ann Chir. 2001;126:413–420. doi: 10.1016/s0003-3944(01)00526-0. [DOI] [PubMed] [Google Scholar]