Abstract
Opium is one of the oldest herbal medicines currently used as an analgesic, sedative and antidiarrheal treatment. The effects of opium are principally mediated by the μ-, κ- and δ-opioid receptors. Opioid substances consist of all natural and synthetic alkaloids that are derived from opium. Most of their effects on gastrointestinal motility and secretion result from suppression of neural activity. Inhibition of gastric emptying, increase in sphincter tone, changes in motor patterns, and blockage of peristalsis result from opioid use. Common adverse effects of opioid administration include sedation, dizziness, nausea, vomiting, constipation, dependency and tolerance, and respiratory depression. The most common adverse effect of opioid use is constipation. Although stool softeners are frequently used to decrease opioid-induced bowel dysfunction, however they are not efficacious. Possibly, the use of specific opioid receptor antagonists is a more suitable approach. Opioid antagonists, both central and peripheral, could affect gastrointestinal function and visceromotor sensitivity, which suggests an important role for endogenous opioid peptides in the control of gastrointestinal physiology. Underlying diseases or medications known to influence the central nervous system (CNS) often accelerate the opioid’s adverse effects. However, changing the opioid and/or route of administration could also decrease their adverse effects. Appropriate patient selection, patient education and discussion regarding potential adverse effects may assist physicians in maximizing the effectiveness of opioids, while reducing the number and severity of adverse effects.
Keywords: Opium, Analgesic, Opioid, Gastrointestinal motility
INTRODUCTION
Opioid substances comprise all natural and synthetic alkaloid derivatives of opium. Opium is one of the oldest medicines in use as an analgesic and sedative. The root of the word opium is derived from “opos”, a Greek word which means a liquid collected from the opium poppy.1
Opium has been clinically used for many years. Original documents regarding the use of opium drugs have been found as early as 3000 BC. In the first century, for the first time, the anesthetic and sedative effects of opiates have been described in “De Materia Medica”. Because of its euphoric effects, opioid substances have been used in different cultures and religions. There are numerous documents regarding opioid use in traditional Iranian medicine.1, 2 Morphine, the first opium alkaloid was introduced in 1805 by Friedrich Wilhelm Serturner. Later, different alkaloid components have been introduced of which all have analgesic effects on the central or peripheral nervous systems. These analgesic effects are due to decreased perception and reaction to pain, and increased pain tolerance.1
The alimentary canal might be has the largest neurological network after central nervous system (CNS). This neurological network is called the enteric nervous system (ENS). This nervous system has a vital role in all activities of the digestive system, of which it’s activities are performed by different receptors and substances. The most important substances include acetylcholine, vasoactive intestinal polypeptides, nitric oxide, tachykinins, adenosine triphosphate, opioid peptides, and neuropeptide Y,5-hydroxy trypticon.1-3
Because of the frequent use of opium and its prevalence of gastrointestinal adverse effects, in this review article we discuss the physiology of opioids and clinical aspects that relate to the adverse effects of opium use on the alimentary tract.
OPIOID RECEPTORS
Pharmacologically, the opiates activate specific receptors along the nervous system. There are three major receptor subtypes, μ, κ, and δ, of which subtype μ seems to be more common. The δ receptor is another opiate receptor that does not influence mesenteric excitatory motor neurons. Recently a new receptor, the opioid receptor–like 1 (ORL-1) or nociceptin/orphanin (FQ) has been isolated. The activation or blockage of opioid receptors allows for important changes in the gastrointestinal tract’s clinical presentation.4 The receptors bind to opioids that originate from plants, either synthetic or endogenous (dermophins and deltorphins), and amphibian skin.
In 1975, for the first time, Hughes and Kosterlitz isolated an endogenous opioid receptor antagonist (leucine–enkephalin,methionine–enkephalin).5,6 These researchers discovered the interaction between these substances and the ENS. In addition, the gut is not a unique site for opioid receptor antagonist function; these antagonists may additionally function via their receptors in the brain.7 Opiate-induced effects possibly have a greater association due to their concentration in the ENS rather than the CNS.8, 9
The opioid receptors belong to the family of metabotropin membrane receptors that act by binding to the GI/GO sites of the G-protein. Opioid receptors decrease intracellular cyclic adenosinenmonophosphate( cAMP) by inhibiting adenylate cyclase. Transduction pathways that include potassium channel activation, membrane hyperpolarization and inhibition of calcium channels are involved in this mechanism.10,11 However, the action of opioid receptor–like-1 and opioids nociceptin/orphanin (ORL1-N/OFQ s )different. The ORL-1 receptor is expressed by reduction of calcium channels and cAMP activity, however potassium channel activation is also seen.12
Effects of opioid agonists on the alimentary tract
As mentioned earlier, the antagonistic effect of opioids occur via opioid receptors in which the μ receptor participates more in their clinical effects. Opioid receptors in the brain and gastrointestinal tract affect pain regulation. Opioids possibly influence both the inhibitory and excitatory neural system of alimentary muscles.11, 13
Most opioid effects on gastrointestinal motility and secretion occur via suppression of neural activity (Table1). The inhibitory effects of morphine on intestinal peristalsis in guinea pigs has been demonstrated by Trendelenberg in 1917. Later studies have shown that morphine-like medicines reduce the frequency of peristaltic waves and maximal ejection pressure. Inhibition of the acetylcholine and non-adrenergic non-cholinergic (NANC) neurotransmitter release of ENS might be involved in this phenomen.13, 14
Table 1. Opioid receptors and their functions.
| Receptor | Clinical effects | Location | |
|
µ |
Analgesia Changes smooth muscle tone Sedation Mood alteration Nausea/vomiting |
Mesenteric plexus Brain Spinal cord Sub-mucosal plexus |
|
| δ |
Decreases colonic transit time |
Mesenteric plexus Brain |
|
|
κ |
Central analgesia Decreases colonic transit time Visceral nociception antagonist |
Mesenteric plexus Brain Spinal cord |
According to a recent investigation, κ receptors have an important effect on pain management. Stimulation of κ receptors in animals has shown an increase in pain threshold and a reverse in peritoneal irritation that induced ileus and visceral pain.15-17 The antinociceptive effects of κ receptor agonists such as fedotozine and asimadoline are observed at the terminal ends of afferent pelvic nerve fibers.18-20 Because of the association between these receptors with enteric and ENS neurons, it has been proposed that peripheral receptor agonists might be useful in managing chronic visceral pain. Fedotozine has been shown to reduce irritable bowel syndrome (IBS) symptoms by increase the threshold of perception.20, 21 In addition, studies on asimadoline in humans have shown that this agent caused a reduced sensation of gastric and colonic distention.22 The effects of these types of medications on the ENS with regards to chronic or acute use are not same. Both in vivo and in vitro studies have shown that chronic exposure of these agents causes reduced neuronal sensitivity.14 Naloxan, an opioid antagonist, elevates both neuronal excitability and pain threshold the ENS.23
Gut
Most opioid agonists, including morphine, cause decreases in gastric motility in humans that are independent of their routes of administration.14, 24,25 These agents cause increased contraction of the antrum and pylorus along with decreased resting tone in the musculature of the gastric reservoir. Their contraction is mediated by activation of both inhibitory and excitatory motor neurons which consequently re-establishes contractility tone. Stimulation of μ receptors enables the opioid to decrease gastric motility. In contrast, opioids cause increased tonic contractions of the antral muscles and upper duodenum.26, 27
Intestine
In the intestine, opioid agonists block peristaltic reflex by stimulation of receptors along the ENS in addition to the inhibition of nicotinic post-synaptic receptors.28,29 Opioid agonists cause increased resting tone in circular(spherical) muscles of the small and large intestine along with enhanced rhythmic contractions.30 Opioids, as morphine, in addition to their effects on the ENS, also act on the spinal cord and brain to suppress intestinal motility.31 On the other hand, opioids have inhibitory effects on motor neurons at the level of the submucosal plexus, causing suppression of the secretion and liquidity of intestinal contents resulting in the formation of hard stools. Administration of opioids inside CNS has the same effect by causing an elevation in sympathetic activities of ENS.32, 33
Colon
Delayed colonic transit due to opioids causes prolonged contract time of the colon, which in turn enhances liquid absorption. Studies have suggested that all types of opioid receptors have this effect.34 Colonic transit time in the cecum and ascending colon in addition to the frequency of defecation are decreased by morphine–like derivatives. Several studies have suggested that these substances affect anorectal function which increases pain threshold volumes for minimal perception and the sensation of defecation.34, 35 Musial has demonstrated that loperamid increased internal anal sphincter tone and improved continence mechanisms.36
Biliary system
Opioids also impact the biliary system and sphincter of Oddi. Administration of opioids results in relaxation of the sphincter of Oddi, subsequently resulting in biliary stasis.1, 34,37 On the other hand, opioid peptides cause decreased gallbladder contractions that have been induced by cholecystokinin (CCK)which in turn assists with bile saturation and cholelithiosis.38-40
Immune function
The effects of opioids during inflammation on nociception, motility and secretion are increased,39-41 which could be attributed to changes in permeability and integrity of the perineurium that results in easier access to opioid agonists.42,43 In a study on mice that lacked μ receptors, it was observed that these mice were more susceptible to induced colitis which emphasized the effects of this receptor on intestinal inflammation.44,45 In addition, this might be related to up-regulation of μ and κ receptors.46 Endogenous opioids also influence immune cells, enhance cytokine production and T-cell proliferation.43.45
Visceral Sensation
Opiates have been shown to reduce somatic and visceral pain sensation. Their effects possibly act via either the central (spinocerebellar) or peripheral (spinal) nervous systems, which influence the perception of visceral pain.47 Morphine-like derivatives act by increasing the nociceptive threshold via μ receptors primarily in CNS.48 Meanwhile, κ receptor agonists such as fedotozine could increase the threshold of the discomfort sensation associated with gastric and colonic distention in IBS patients and healthy volunteers.21,49 The same result was observed with asimadoline (a κ receptor agonist that does not cross the blood brain barrier) administration in healthy subjects. Possibly, the κ agonist acted on the peripheral afferent pathway.50, 51
Secretion
Opioids such as lopramid and diphenoxylate are usually used as antidiarrhea drugs because of their anti-secretory properties. The mechanism of morphine-like derivatives on gastrointestinal secretions is mainly indirect; possibly they act on receptors located on the mesenteric plexus and sub-mucosa13, 22which induct serotonin secretion. Serotonin consequently stimulates noradrenalin release which has inhibitory effects on enterocyte secretion by activation of alpha-2-adrenoceptors. Data have revealed that endogenous opiates are involved in gastric acid regulation. Morphine-like derivatives could decrease bicarbonate, fluid and electrolyte secretions in the intestine. They also reduce inflammatory fluid secretion during cholecystitis which contributes to pain relief. In addition, opioids primarily increase fluid absorption by delaying colonic transit.13, 52,53
ADVERSE EFFECTS OF OPIOID USE
Common side effects of opioid medicines are dependency, tolerance, constipation, sedation, dizziness, vomiting, and respiratory depression. These effects sometimes cause discontinuation of the medication. Although constipation and nausea are considered the two main complications of opioids that rarely develop tolerances against them, other less common side effects include delayed gastric emptying, hyperalgesia, immunologic effects and hormonal dysfunction (Tables 2and 3). Flushing, pruritus and anaphylaxis are also adverse effects of opioid use and are the result of histamine release that involves the μ and κ receptors.54 Some studies have reported that fentanyl and its analogs do not cause histamine release.54, 55
Table 2. The effects of opioids on numbers of hormones .
| Hormone | Effect | Symptom |
|
Testosterone |
Decrease |
Decreased libido, energy, erectile dysfunction |
| Estrogen | Decrease |
Sexual dysfunction Reduced bone mineral density and osteoporosis |
| Cortisol | Decrease | Hormonal alteration |
| LH | Decrease |
Decreased androgen level Hypomenorrhea Amenorrhea |
| Gonadotropin Releasing Hormone | Decrease |
Decreased androgen level Hormones levels |
Table 3. Immunological effects of opioids.
| Receptor | Effects |
| µ |
↓ NK cell activity ↓ Macrophage activities ↓T-cell proliferation, NO release |
| Δ |
↑ NK cell activity ↓ Plaque-forming cell |
| δ, antagonists | ↓ Delayed hypersensitivity reaction |
| Κ | ↓ Humoral immunity |
| κ antagonists |
↑ Plaque-forming cell ↓ Humoral immunity |
Immunologic Effects
Opioids affect the immune system by decreasing resistance to bacterial infections, which are mostly due to exogenous opioids. It has been demonstrated that exogenous opioid administration, either acute or chronic, suppresses antibody and cellular immune responses. Mechanisms of this suppression involve the central and peripheral nervous systems(CNS&PNS) where the hypothalamic–pituitary–adrenal axis and autonomic nervous system are involved.17,56,57
Constipation
Constipation occurs in approximately 90% of patients who take opioids and can occur with a single dose.58, 59Long-term constipation causes substantial morbidity with reduced quality of life in patients. The μ receptors are possibly more involved in this phenomenon.34, 59 Studies have shown that intestinal motility is not influenced by morphine administration within the spinal cord.13,59
Although it is not completely clear whether the effect of opioids develop by stimulation of the central or peripheral nervous system, however it is generally accepted that morphine and its extracted drugs change autonomic outflow via the CNS18,19,59and by stimulating opioid receptors within the ENS. Unlike other opioid complications, constipation is not tolerated overtime, necessitating monitoring and treatment. The severity of constipation can be evaluated by the patient assessment constipation system and patient assessment of constipation quality of life questionnaire (PAC-SYM and PAC–QOL), among others.21 Based on studies of patients who suffer from cancer pain, the transdermal patch fentanyl may cause less constipation compared to morphine. Fentanyl is a lipophilic substance; transdermal fentanyl absorption is dependent on body weight and body fat mass.60-62 To manage constipation, in addition to changing the narcotic drug and treatment with anti-constipation medications (Table 4), the administration of opioid receptor antagonists is important. Currently, methylnaltrexone and alvimopan are under clinical trials. Both are μ receptor antagonists whose efficacies have not been completely proven (Table 5).
Table 4. Novel medications for the treatment of opioid-induced constipation.
| Name | Class | Efficacy | Clinical effects |
| Naloxone |
Non-selective opioid antagonist |
Reverses opiate-induced delay in orocecal and colonic transit. |
Naloxone PR formulation prevents OIC in patients who received PR oxycodone. |
| Methylnaltrexone | Opioid antagonist | Reverses effects of opiates in health and of chronic methadone treatment on orocecal transit; no effect on small intestinal or colonic transit delayed by codeine (30 mg q.i.d.) in opiate-naive healthy subjects. | S.c. methylnaltrexone (0.15 mg/kg) on alternate days was effective in inducing laxation in patients with advanced illness. |
| Alvimopan | PAMORA |
8 mg oral dose accelerated colonic transit and Reversed the effects of codeine in opiate-naive healthy volunteers who received codeine, 30 mg q.i.d. |
0.5 mg Alvimopan dose efficacious in treating OIC; rare instances of ischemic heart disease |
| Tapentadol |
Narcotic analgesic plus norepinephrine reuptake inhibitor |
ND |
Tapentadol ER 100-250 mg b.i.d. equally effective for moderate to severe chronic steoarthritis-related knee pain compared to oxycodone HCl (CR) 20 – 50 mg b.i.d., given daily with less bowel dysfunction symptoms |
| NKTR-118 |
PAMORA; PEGylated naloxol conjugate |
Normalized morphine-induced delay in orocecal transit. |
25 and 50 mg NKTR-118 had increased numbers of SBM during the first week and during 4 weeks of treatment of OIC patients |
| TD-1211 | PAMORA | ND | 5 and 10 mg/day TD-1211 increased average SBM/week over 2 weeks in OIC patients |
CR, Controlled release; ER, Extended release; ND, Not done; OIC, Opiate-induced constipation; PAMORA, Peripherally-restricted –opioid receptor antagonist; PEG, Polyethylene glycol; PR, Prolonged release; SBM, Spontaneous bowel movement; s.c., Subcutaneous administration
Table 5. Antiemetics based on recommendations by the American College of Physicians.
| Drug | Cause of nausea | Dose |
| Prochlorperazine | Initiation of opioid therapy | 10 mg orally or 25 mg rectally, 2 or 3 times daily |
| Haloperidol | Stimulation of chemoreceptor | 1.5–5 mg, orally 3–4 times daily; 2–10 mg |
| Intramuscularly, 2 or 3 times daily | ||
| Prochlorperazine | Trigger zone by chemotherapy | 10 mg orally or 25 mg rectally, 2 or 3 times daily |
| Methotrimeprazine |
2–6.25 mg intramuscularly, 3 times daily; 6–25 mg, over 24 h |
|
| Scopolamine (transdermal patch) | Vertigo | Apply 1 patch every 2–3 days |
| Meclizine |
50 mg orally or 25–50 mg intramuscularly, 3 times daily |
|
| Metoclopramide | Delayed gastric emptying | 10–20 mg, 2–3 times daily; 1–3 mg h intravenously |
| Octreotide | Bowel obstruction | 50–100 lg subcutaneously 2 or 3 times daily or 300 lg |
| During 24hours subcutaneously | ||
| Ondansetron | Multiple causes, refractory | 4–8 mg orally, 2 or 3 times daily |
| Dexamethasone | 2–4 mg orally, 2 or 3 times daily |
Nausea and Vomiting
Opioid administration induces nausea and vomiting, which in turn could lead to the development of patient dissatisfaction with treatment. In this situation, other etiologies should be excluded. Mechanisms of this side effect involve both central and peripheral nervous system . Opioids mainly stimulate the chemoreceptor trigger zone (CTZ), inhibit gut motility and stimulate the vestibular apparatus.63,64 Studies show that the neurokinin-1 (NK-1) and serotonin receptor in post rema area (is a medullary part at the inferoposterior of the fourth ventricle that controls vomiting)may be involved in opioid-induce emesis.65 In this situation, new medications such as palonosetron (serotonin receptor antagonist) and aprepitant (NK-1 antagonist) might be beneficial.66,67 Switching from one opioid to another in addition to the use of sustained-release opioids might assist patients. Adding a prokinetic agent may also be of benefit to patients. The American College of Physicians recommendations for ill and terminal patients are illustrated in Table 5.
Narcotic Bowel Syndrome (NBS)
Narcotic bowel syndrome (NBS) is a subset of opioid–induced bowel dysfunctions accompanied by chronic, frequent abdominal pain that worsens by taking or escalating the dose of opioids. NBS symptoms include abdominal pain, intermittent vomiting, weight loss and occasional ileus-like symptoms.68, 69 These symptoms cause the patient to take more narcotics, however after a short period of relief the pain returns and is stronger, which causes the patient to take additional narcotics. This condition causes a vicious cycle. Patients, who for first time have received high doses of narcotics, tend to report this problem.68 The key to diagnosing NBS is chronic use or escalating dose of narcotics that lead to continued or worsening problems for the patient. This problem has been initially reported two decades ago in the United States, after which other countries also observed cases of NBS.70 Treatment of NBS involves early diagnosis of symptoms, with effective psychological consultations and gradual withdrawal of the narcotics during a specific program. Clonidin, an alpha adrenergic agonist, is indicated for this problem.71, 72 Clonidin works at the alpha-2-receptors in the CNS and gut wall.73 Lofexidine, another alpha-2-adrenergic agonist with less side effects is more suitable for outpatient programs.72,74
Pruritus
The rate of pruritus due to opioids ranges from 2% to 10%.75 Histamine release appears to be the mechanism for developing pruritus. Management of pruritus includes antihistamines, switching opiates, changing the dose of opioides and supportive treatment.75-77
Central Nervous System (CNS)
CNS adverse effects from opioids are primarily sedation and decreased cognition. Although some patients need additional treatment for these effects, most often they are transient. Sedation and decreased cognition usually present during the onset of opioid treatment or with increasing doses. A psycho-stimulant might be used for their management. Administration of antipsychotics could be considered in cognitive side effect.78, 79
Tolerance and Dependency
Tolerance and dependency are common side effects of opioid treatment that are primary or acquired.80 Tolerance and dependency primarily have a genetic origin and could be present from the first dose. Acquired tolerance and dependency is divided into pharmacokinetic, pharmacodynamic and learned categories. Repeated administration leads to pharmacokinetic tolerance, whereas pharmaco-dynamic tolerance results from decreased effectiveness of the drug over time. Learned tolerance is results in decreased effectiveness by incorporation use of the opioid medication. Although the mechanisms of tolerance are not unclear some studies have indicated an increased neuropeptide activity such as the calcitonin gene-related peptide (CGRP) and substance P.80-82
Effect of Opioid Use on the Alimentary Tract
Over 300 analogues of endogenous or exogenous opioid peptides are designed to act on opioid receptors. Numerous research has focused on the provision of new molecules that do not pass the blood-brain barrier that just have peripheral effects. Morphine and morphine-like medications work mostly via the opioid μ receptor, which is responsible for the majority of opioid effects in the brain and periphery. Opioid analogues have unwanted effects on bowel dysfunction such as severe constipation, hard stools, incomplete evacuation, straining, bloating and gastro-esophageal reflux. However many of these medications might be used to manage excessive exertion such as fistula or stomy output. Two popular opiates frequently used are loperamide and diphenoxylate.
Loperamide and Diphenoxylate
Loperamide is a derivate of meperidin which inhibits calcium channels and calmodulin in the intestine’s smooth muscles, as well as inhibiting fluid secretion in the colon. Loperamide does not pass the blood-brain barrier. Oral loperamide absorption can be increased by raising the pH in the gut. Therefore gastric hypersecretion theoretically decreases the clinical effects of loperamide. Inhibiting P-glycoprotein, an efflux pump in the intestine, would increase the oral absorption of loperamide. P-glycoprotein is found in different organs such as the brain and kidneys, thus it’s inhibition by different medicines could result in the passage of loperamide to the brain and CNS.83 The elements with inhibitory effects on P-glycoprotein and substances which metabolize cytochrome P450 2C9(CYP2C9) and cytochrome P450 3A4(CYP3A4) can enhance the systemic effects of loperamide. Concurrent use of itraconazole and gemfibrozil increase plasma levels by increasing absorption, and decrease loperamide elimination.83-86
Diphenoxylate is a derivate of meperidin which at low doses leads to constipation; at high doses it causes systemic effects such as euphoria. Excessive use of this medication could lead to cardiovascular problems due to its anticholinergic effects. Diphenoxylate is rapidly absorbed following oral administration.87
Esophageal Motility
Some studies shows that morphine-like drugs decrease lower esophageal sphincter relaxation, resulting in fewer reflux episodes along with a reduction in the time of contact between decreased pH liquids and the esophageal membrane. The therapeutic use of opioids for gastro esophageal reflux has yet to be established.88 , 89
Dyspepsia
Various forms of dyspepsia are related to neuromuscular and sensory abnormalities. Visceral hypersensitivity in response to different stimulation could be considered an important mechanism of dyspepsia.90 κ receptor agonists have been shown to influence visceral perception without changing gastric emptying. Drugs such as fedotozine could be effective in eliminating bloating, abdominal pain and post-prandial fullness in functional dyspepsia.91 In different studies, asimadoline a selective κ receptor agonist, did not favorite on dyspepsia in one study this drug overall did not significantly alter maximum-tolerated volume, symptoms post nutrient challenge or symptoms over 8 weeks in functional dyspepsia.51,92
Irritable Bowel Syndrome (IBS)
IBS is a complex, symptom-based diagnostic problem in which abdominal discomfort, bloating and disturbed defecation are present. Although the etiologies of IBS remain unclear, however a combination of sensory and motor changes to the alimentary tract have been proposed.93 μ receptor agonists such as morphine, loperamide, diphonexilate, and mepridin have decreasing effects on circular and longitudinal muscles of the intestine. Of these, loperamide has not effects on CNS.93, 94 Currently, loperamide is mainly used to treat diarrhea-predominant IBS. Loperamide decreases the frequency, urgency and pain in IBS patients. Tremebutin has an affinity for μ, δ and κ receptors without affecting colonic motility, and decrease post-prandial motor activity in IBS patients. Tremebutin also accelerates bowel transit time in patients who have constipation. Asimadoline, a selective κ receptor agonist, is also used in this manner.94, 95
In constipation-predominant IBS, μ receptor antagonists have been evaluated based on the hypothesis that endogenous opiates might influence motility disorders. Fedotozine, a κ receptor agonist, increases the threshold of perception in the colon and might be useful for IBS patients.96 In one study, low dose naltreoxone has been shown to improve pain and general feeling in IBS patients. However this result was not confirmed in a case control study.93 There have not been enough clinical trials to establish the efficacy of opiates antagonist in IBS.
Diarrhea
Worldwide, the accepted treatments for chronic and acute diarrhea are loperamide and diphenoxylate, in which loperamide is preferred. Mostly these drugs are not used for either palliative or control of acute diarrhea. Opiates are commonly used in short bowel syndrome. These agents act by stimulating the μ receptor by slowing colonic transit. They also have anti-secretory effects.86, 97,98 However, enkephalin, an endogenous opiate present its antisecretory effects by stimulate sigma receptor in basolateral membrane of enterocyte via inhibition of adenylate cyclase.99 It is degraded by enkephalinase. Racecadotril, an antagonist for enkephalinase favorably affects both the frequency and weight of stool in children and adults and is comparable with loperamide.86,98 Codeine has an antidiarrheal effect of which approximately 10% of this medication is metabolized into morphine by the CYP2D6 enzyme.100 Other systemic opiates may be used to treat diarrhea when the patient can not tolerate or respond to the usual agents.
Liver Impairment
Hepatic failure affects the metabolism of a majority of opioid drugs; the activity of some of these drugs is dependent upon hepatic biotransformation to active metabolites. Narcotics possibly have a role in development or even aggravation of hepatic encephalopathy, therefore cautious use and careful monitoring are warranted.101,102
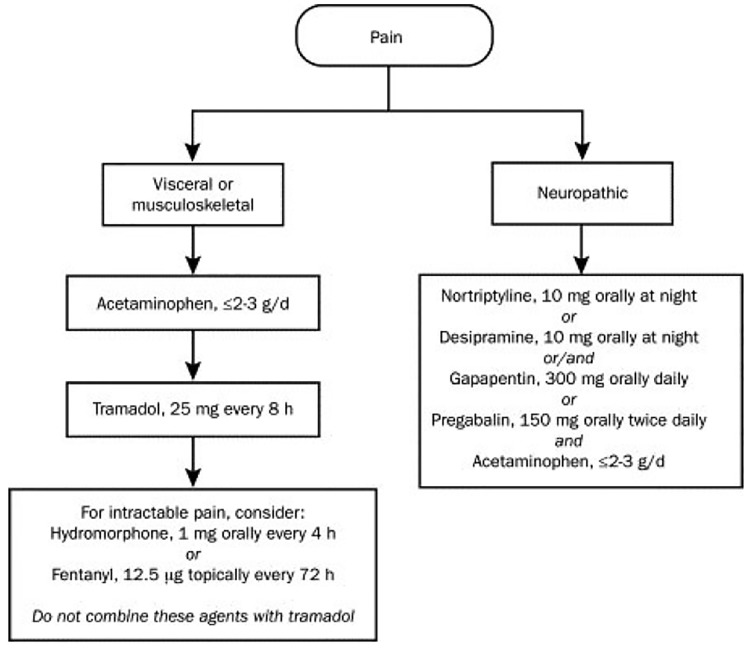
On the other hand, hepatic failure may reduce the analgesic effects of these medications. In addition, clearance of opioids (morphine, oxycodone, tramadol and alfentanil) might be impaired during hepatic failure. According to some studies the bioavailability of a number of opiates such as morphine, hydromorphone and oxycodone are enhanced during hepatic failure and adjustment of doses or longer intervals must be considered. Also the elimination of toxic metabolites decreases during liver failure, increasing the risk of toxicity of some narcotics such as pethidine (meperidine) that has a toxic metabolite(Figure 1). Administration of these agents should be performed with caution or otherwise avoided. Hepatic disease is not influence on pharmacokinetic of the phenylpiperidine family (fentanyl).101-103
Fig.1.
Pain management in liver failure
CONCLUSION
Opioid medications are widely used in the management of acute and chronic pain. As with other medications there are potential adverse effects. Serious adverse effects usually develop in patients that use these medications for extended periods. Education of patients and health service staff along with the correct prescription and proper consideration of adverse effects, particularly at the onset, as well as changing the dose of medication could assist in the management of common and uncommon adverse effects
CONFLICT OF INTEREST
The authors declare no conflict of interest related to this work.
Please cite this paper as:
Khansari MR, Sohrabi MR, Zamani Farhad. The Useage of Opioids and their Adverse Effects in Gastrointestinal Practice: A Review. Middle East J Dig Dis 2013;5:5-16.
References
- 1.Brownstein MJ. A brief history of opiates, opioid peptides, and opioid receptors. Proc Natl Acad Sci U S A. 1993;90:5391–3. doi: 10.1073/pnas.90.12.5391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benyhe S. Morphine: new aspects in the study of an ancient compound. Life Sci. 1994;55:969–79. doi: 10.1016/0024-3205(94)00631-8. [DOI] [PubMed] [Google Scholar]
- 3.Chamouard P, Klein A, Martin E, Adloff M, Angel F. Regulatory role of enteric kappa opioid receptors in human colonic motility. Life Sci. 1993;53:1149–56. doi: 10.1016/0024-3205(93)90551-d. [DOI] [PubMed] [Google Scholar]
- 4.Chiou LC, Liao YY, Fan PC, Kuo PH, Wang CH, Riemer C. et al. Nociceptin/orphanin FQ peptide receptors: pharmacology and clinical implications. Curr Drug Targets. 2007;8:117–35. doi: 10.2174/138945007779315605. [DOI] [PubMed] [Google Scholar]
- 5.Hughes J, Kosterlitz HW, Smith TW. The distribution of methionine-enkephalin and leucine-enkephalin in the brain and peripheral tissues. Br J Pharmacol. 1977;61:639–47. doi: 10.1111/j.1476-5381.1977.tb07557.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hughes J, Smith TW, Kosterlitz HW, Fothergill LA, Morgan BA, Morris HR. Identification of two related pentapeptides from the brain with potent opiate agonist activity. Nature. 1975;258:577–80. doi: 10.1038/258577a0. [DOI] [PubMed] [Google Scholar]
- 7.Manara L, Bianchetti A. The central and peripheral influences of opioids on gastrointestinal propulsion. Annu Rev Pharmacol Toxicol. 1985;25:249–73. doi: 10.1146/annurev.pa.25.040185.001341. [DOI] [PubMed] [Google Scholar]
- 8.Foss JF. A review of the potential role of methylnaltrexone in opioid bowel dysfunction. Am J Surg. 2001;182:19S–26S. doi: 10.1016/s0002-9610(01)00783-8. [DOI] [PubMed] [Google Scholar]
- 9.Kurz A, Sessler DI. Opioid-induced bowel dysfunction: pathophysiology and potential new therapies. Drugs. 2003;63:649–71. doi: 10.2165/00003495-200363070-00003. [DOI] [PubMed] [Google Scholar]
- 10.Nagy I, Rang HP. Rang, Similarities and differences between the responses of rat sensory neurons to noxious heat and capsaicin. J Neurosci. 1999;19:10647–55. doi: 10.1523/JNEUROSCI.19-24-10647.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wood JD, Galligan JJ. Galligan, Function of opioids in the enteric nervous system. Neurogastroenterol Motil. 2004;16:17–28. doi: 10.1111/j.1743-3150.2004.00554.x. [DOI] [PubMed] [Google Scholar]
- 12.Calo’ G, Guerrini R, Rizzi A, Salvadori S, Regoli D. Pharmacology of nociceptin and its receptor: a novel therapeutic target. Br J Pharmacol. 2000;129:1261–83. doi: 10.1038/sj.bjp.0703219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Luca A, Coupar IM. Insights into opioid action in the intestinal tract. Pharmacol Ther. 1996;69:103–15. doi: 10.1016/0163-7258(95)02053-5. [DOI] [PubMed] [Google Scholar]
- 14.Holzer P. Opioids and opioid receptors in the enteric nervous system: from a problem in opioid analgesia to a possible new prokinetic therapy in humans. Neurosci Lett. 2004;361:192–5. doi: 10.1016/j.neulet.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 15.Harada Y, Nishioka K, Kitahata LM, Kishikawa K, Collins JG. Visceral antinociceptive effects of spinal clonidine combined with morphine, [D-Pen2, D-Pen5] enkephalin, or U50,488H. Anesthesiology. 1995;83:344–52. doi: 10.1097/00000542-199508000-00015. [DOI] [PubMed] [Google Scholar]
- 16.Gebhart GF, Su X, Joshi S, Ozaki N, Sengupta JN. Peripheral opioid modulation of visceral pain. Ann N Y Acad Sci. 2000;909:41–50. doi: 10.1111/j.1749-6632.2000.tb06675.x. [DOI] [PubMed] [Google Scholar]
- 17.Peterson PK, Molitor TW, Chao CC. The opioid-cytokine connection. J Neuroimmunol. 1998;83:63–9. doi: 10.1016/s0165-5728(97)00222-1. [DOI] [PubMed] [Google Scholar]
- 18.Su X, Sengupta JN, Gebhart GF. Effects of kappa opioid receptor-selective agonists on responses of pelvic nerve afferents to noxious colorectal distension. J Neurophysiol. 1997;78:1003–12. doi: 10.1152/jn.1997.78.2.1003. [DOI] [PubMed] [Google Scholar]
- 19.Su X, Joshi SK, Kardos S, Gebhart GF. Sodium chann el blocking actions of the kappa-opioid receptor agonist U50,488 contribute to its visceral antinociceptive effects. J Neurophysiol. 2002;87:1271–9. doi: 10.1152/jn.00624.2001. [DOI] [PubMed] [Google Scholar]
- 20.Gebhart GF.J.J. Bonica Lecture--2000: Physiology, pathophysiology, and pharmacology of visceral pain. Reg Anesth Pain Med. 2000;25:632–8. doi: 10.1053/rapm.2000.18187. [DOI] [PubMed] [Google Scholar]
- 21.Delvaux M, Louvel D, Lagier E, Scherrer B, Abitbol JL, Frexinos J. The kappa agonist fedotozine relieves hypersensitivity to colonic distention in patients with irritable bowel syndrome. Gastroenterology. 1999;116:38–45. doi: 10.1016/s0016-5085(99)70226-x. [DOI] [PubMed] [Google Scholar]
- 22.Delgado-Aros S, Chial HJ, Camilleri M, Szarka LA, Weber FT, Jacob J. et al. Effects of a kappa-opioid agonist, asimadoline, on satiation and GI motor and sensory functions in humans. Am J Physiol Gastrointest Liver Physiol. 2003;284:G558–66. doi: 10.1152/ajpgi.00360.2002. [DOI] [PubMed] [Google Scholar]
- 23.North RA. Effects of morphine on myenteric plexus neurones. Neuropharmacology. 1976;15:719–21. doi: 10.1016/0028-3908(76)90043-5. [DOI] [PubMed] [Google Scholar]
- 24.Yuan CS, Foss JF, O’Connor M, Roizen MF, Moss J. Effects of low-dose morphine on gastric emptying in healthy volunteers. J Clin Pharmacol. 1998;38:1017–20. doi: 10.1177/009127009803801105. [DOI] [PubMed] [Google Scholar]
- 25.Lydon AM, Cooke T, Duggan F, Shorten GD. Delayed postoperative gastric emptying following intrathecal morphine and intrathecal bupivacaine. Can J Anaesth. 1999;46:544–9. doi: 10.1007/BF03013544. [DOI] [PubMed] [Google Scholar]
- 26.Thörn SE, Wickbom G, Philipson L, Leissner P, Wattwil M. Myoelectric activity in the stomach and duodenum after epidural administration of morphine or bupivacaine. Acta Anaesthesiol Scand. 1996;40:773–8. doi: 10.1111/j.1399-6576.1996.tb04531.x. [DOI] [PubMed] [Google Scholar]
- 27.Brock C, Olesen SS, Olesen AE, Frøkjaer JB, Andresen T, Drewes AM. Opioid-induced bowel dysfunction: pathophysiology and management. Drugs. 2012;72:1847–65. doi: 10.2165/11634970-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 28.Sarna SK, Otterson MF. Small intestinal amyogenesia and dysmyogenesia induced by morphine and loperamide. Am J Physiol. 1990;258:G282–9. doi: 10.1152/ajpgi.1990.258.2.G282. [DOI] [PubMed] [Google Scholar]
- 29.Cherubini E, North RA. Mu and kappa opioids inhibit transmitter release by different mechanisms. Proc Natl Acad Sci U S A. 1985;82:1860–3. doi: 10.1073/pnas.82.6.1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Frantzides CT, Cowles V, Salaymeh B, Tekin E, Condon RE. Morphine effects on human colonic myoelectric activity in the postoperative period. Am J Surg. 1992;163:144–8. doi: 10.1016/0002-9610(92)90267-u. [DOI] [PubMed] [Google Scholar]
- 31.Wong CL. Central and peripheral inhibitory effects of morphine on intestinal transit in mice. Methods Find Exp Clin Pharmacol. 1986;8:479–83. [PubMed] [Google Scholar]
- 32.Chuang TK, Killam KF Jr, Chuang LF, Kung HF, Sheng WS, Chao CC. et al. Mu opioid receptor gene expression in immune cells. Biochem Biophys Res Commun. 1995;216:922–30. doi: 10.1006/bbrc.1995.2709. [DOI] [PubMed] [Google Scholar]
- 33.Kvam TM, Baar C, Rakvåg TT, Kaasa S, Krokan HE, Skorpen F. Genetic analysis of the murine mu opioid receptor: increased complexity of Oprm gene splicing. J Mol Med (Berl) 2004;82:250–5. doi: 10.1007/s00109-003-0514-z. [DOI] [PubMed] [Google Scholar]
- 34.Sternini C, Patierno S, Selmer IS, Kirchgessner A. The opioid system in the gastrointestinal tract. Neurogastroenterol Motil. 2004;16:3–16. doi: 10.1111/j.1743-3150.2004.00553.x. [DOI] [PubMed] [Google Scholar]
- 35.Göke M, Ewe K, Donner K, Meyer zum Büschenfelde KH. Influence of loperamide and loperamide oxide on the anal sphincter A manometric study. Dis Colon Rectum. 1992;35:857–61. doi: 10.1007/BF02047873. [DOI] [PubMed] [Google Scholar]
- 36.Musial F, Enck P, Kalveram KT, Erckenbrecht JF. The effect of loperamide on anorectal function in normal healthy men. J Clin Gastroenterol. 1992;15:321–4. doi: 10.1097/00004836-199212000-00011. [DOI] [PubMed] [Google Scholar]
- 37.Flancbaum L, Alden SM. Morphine cholescintigraphy. Surg Gynecol Obstet. 1990;171:227–32. [PubMed] [Google Scholar]
- 38.Vasquez TE, Rimkus DS, Hass MG, Larosa DI. Efficacy of morphine sulfate-augmented hepatobiliary imaging in acute cholecystitis. J Nucl Med Technol. 2000;28:153–5. [PubMed] [Google Scholar]
- 39.Mani AR, Moore KP. New insights into the role of endogenous opioids in the pathogenesis of gastrointestinal and liver disease. Gut. 2009;58:893–5. doi: 10.1136/gut.2007.141648. [DOI] [PubMed] [Google Scholar]
- 40.Antonijevic I, Mousa SA, Schäfer M, Stein C. Perineurial defect and peripheral opioid analgesia in inflammation. J Neurosci. 1995;15:165–72. doi: 10.1523/JNEUROSCI.15-01-00165.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brack A, Labuz D, Schiltz A, Rittner HL, Machelska H, Schäfer M. et al. Tissue monocytes/macrophages in inflammation: hyperalgesia versus opioid-mediated peripheral antinociception. Anesthesiology. 2004;101:204–11. doi: 10.1097/00000542-200407000-00031. [DOI] [PubMed] [Google Scholar]
- 42.Stein C. The control of pain in peripheral tissue by opioids. N Engl J Med. 1995;332:1685–90. doi: 10.1056/NEJM199506223322506. [DOI] [PubMed] [Google Scholar]
- 43.Sacerdote P, Franchi S, Panerai AE. Non-Analgesic Effects of Opioids: Mechanisms and Potential Clinical Relevance of Opioid-Induced Immunodepression. Curr Pharm Des. 2012;18:6034–42. doi: 10.2174/138161212803582496. [DOI] [PubMed] [Google Scholar]
- 44.Philippe D, Dubuquoy L, Groux H, Brun V, Chuoï-Mariot MT, Gaveriaux-Ruff C. et al. Anti-inflammatory properties of the mu opioid receptor support its use in the treatment of colon inflammation. J Clin Invest. 2003;111:1329–38. doi: 10.1172/JCI16750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Philippe D, Chakass D, Thuru X, Zerbib P, Tsicopoulos A, Geboes K. et al. Mu opioid receptor expression is increased in inflammatory bowel diseases: implications for homeostatic intestinal inflammation. Gut. 2006;55:815–23. doi: 10.1136/gut.2005.080887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jiménez N, Puig MM, Pol O. Antiexudative effects of opioids and expression of kappa- and delta-opioid receptors during intestinal inflammation in mice: involvement of nitric oxide. J Pharmacol Exp Ther. 2006;316:261–70. doi: 10.1124/jpet.105.091991. [DOI] [PubMed] [Google Scholar]
- 47.Wilder-Smith CH, Hill L, Osler W, O’Keefe S. Effect of tramadol and morphine on pain and gastrointestinal motor function in patients with chronic pancreatitis. Dig Dis Sci. 1999;44:1107–16. doi: 10.1023/a:1026607703352. [DOI] [PubMed] [Google Scholar]
- 48.De Schepper HU, Cremonini F, Park MI, Camilleri M. Opioids and the gut: pharmacology and current clinical experience. Neurogastroenterol Motil. 2004;16:383–94. doi: 10.1111/j.1365-2982.2004.00513.x. [DOI] [PubMed] [Google Scholar]
- 49.Mangel AW, Fehnel SE. Design of treatment trials in irritable bowel syndrome: opioid agonists and atypical benzodiazepine antagonists. Neurogastroenterol Motil. 2008;20:1086–93. doi: 10.1111/j.1365-2982.2008.01198.x. [DOI] [PubMed] [Google Scholar]
- 50.Delgado-Aros S, Chial HJ, Cremonini F, Ferber I, McKinzie S, Burton DD. et al. Effects of asimadoline, a kappa-opioid agonist, on satiation and postprandial symptoms in health. Aliment Pharmacol Ther. 2003;18:507–14. doi: 10.1046/j.1365-2036.2003.01670.x. [DOI] [PubMed] [Google Scholar]
- 51.Talley NJ, Choung RS, Camilleri M, Dierkhising RA, Zinsmeister AR. Asimadoline, a kappa-opioid agonist, and satiation in functional dyspepsia. Aliment Pharmacol Ther. 2008;27:1122–31. doi: 10.1111/j.1365-2036.2008.03676.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Turvill J, Farthing M. Enkephalins and enkephalinase inhibitors in intestinal fluid and electrolyte transport. Eur J Gastroenterol Hepatol. 1997;9:877–80. doi: 10.1097/00042737-199709000-00010. [DOI] [PubMed] [Google Scholar]
- 53.Wade PR, Palmer JM, McKenney S, Kenigs V, Chevalier K, Moore BA. et al. Modulation of gastrointestinal function by MuDelta, a mixed micro opioid receptor agonist/ micro opioid receptor antagonist. Br J Pharmacol. 2012;167:1111–1125. doi: 10.1111/j.1476-5381.2012.02068.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bigliardi-Qi M, Gaveriaux-Ruff C, Pfaltz K, Bady P, Baumann T, Rufli T. et al. Deletion of mu- and kappa-opioid receptors in mice changes epidermal hypertrophy, density of peripheral nerve endings, and itch behavior. J Invest Dermatol. 2007;127:1479–88. doi: 10.1038/sj.jid.5700661. [DOI] [PubMed] [Google Scholar]
- 55.Bowdle TA. Adverse effects of opioid agonists and agonist-antagonists in anaesthesia. Drug Saf. 1998;19:173–89. doi: 10.2165/00002018-199819030-00002. [DOI] [PubMed] [Google Scholar]
- 56.Sharp BM, Roy S, Bidlack JM. Evidence for opioid receptors on cells involved in host defense and the immune system. J Neuroimmunol. 1998;83:45–56. [PubMed] [Google Scholar]
- 57.McCarthy L, Wetzel M, Sliker JK, Eisenstein TK, Rogers TJ. Opioids, opioid receptors, and the immune response. Drug Alcohol Depend. 2001;62:111–23. doi: 10.1016/s0376-8716(00)00181-2. [DOI] [PubMed] [Google Scholar]
- 58.Harada Y, Nishioka K, Kitahata LM, Kishikawa K, Collins JG. Visceral antinociceptive effects of spinal clonidine combined with morphine, [D-Pen2, D-Pen5] enkephalin, or U50,488H. Anesthesiology. 1995;83:344–52. doi: 10.1097/00000542-199508000-00015. [DOI] [PubMed] [Google Scholar]
- 59.Gebhart GF, Su X, Joshi S, Ozaki N, Sengupta JN. Peripheral opioid modulation of visceral pain. Ann N Y Acad Sci. 2000;909:41–50. doi: 10.1111/j.1749-6632.2000.tb06675.x. [DOI] [PubMed] [Google Scholar]
- 60.Ahmedzai S, Brooks D. Transdermal fentanyl versus sustained-release oral morphine in cancer pain: preference, efficacy, and quality of lifeThe TTS-Fentanyl Comparative Trial Group. J Pain Symptom Manage. 1997;13:254–61. doi: 10.1016/s0885-3924(97)00082-1. [DOI] [PubMed] [Google Scholar]
- 61.Weschules DJ, Bain KT, Reifsnyder J, McMath JA, Kupperman DE, Gallagher RM. et al. Toward evidence-based prescribing at end of life: a comparative analysis of sustained-release morphine, oxycodone, and transdermal fentanyl, with pain, constipation, and caregiver interaction outcomes in hospice patients. Pain Med. 2006;7:320–9. doi: 10.1111/j.1526-4637.2006.00184.x. [DOI] [PubMed] [Google Scholar]
- 62.Pergolizzi J, Böger RH, Budd K, Dahan A, Erdine S, Hans G. et al. Opioids and the management of chronic severe pain in the elderly: consensus statement of an International Expert Panel with focus on the six clinically most often used World Health Organization Step III opioids (buprenorphine, fentanyl, hydromorphone, methadone, morphine, oxycodone) Pain Pract. 2008;8:287–313. doi: 10.1111/j.1533-2500.2008.00204.x. [DOI] [PubMed] [Google Scholar]
- 63.Holden JE, Jeong Y, Forrest JM. The endogenous opioid system and clinical pain management. AACN Clin Issues. 2005;16:291–301. doi: 10.1097/00044067-200507000-00003. [DOI] [PubMed] [Google Scholar]
- 64.Raynor K, Kong H, Chen Y, Yasuda K, Yu L, Bell GI. et al. Pharmacological characterization of the cloned kappa-, delta-, and mu-opioid receptors. Mol Pharmacol. 1994;45:330–4. [PubMed] [Google Scholar]
- 65.Saito R, Takano Y, Kamiya HO. Roles of substance P and NK(1) receptor in the brainstem in the development of emesis. J Pharmacol Sci. 2003;91:87–94. doi: 10.1254/jphs.91.87. [DOI] [PubMed] [Google Scholar]
- 66. Walsh SL, Heilig M, Nuzzo PA, Henderson P, Lofwall MR.Effects of the NK(1) antagonist, aprepitant, on response to oral and intranasal oxycodone in prescription opioid abusers. Addict Biol 2012. [DOI] [PMC free article] [PubMed]
- 67.Jordan K, Jahn F, Jahn P, Behlendorf T, Stein A, Ruessel J. et al. The NK-1 receptor-antagonist aprepitant in high-dose chemotherapy (high-dose melphalan and high-dose T-ICE: paclitaxel, ifosfamide, carboplatin, etoposide): efficacy and safety of a triple antiemetic combination. Bone Marrow Transplant. 2011;46:784–9. doi: 10.1038/bmt.2010.205. [DOI] [PubMed] [Google Scholar]
- 68.Choung RS, Locke GR, Zinsmeister AR, Schleck CD, Talley NJ. Opioid bowel dysfunction and narcotic bowel syndrome: a population-based study. Am J Gastroenterol. 2009;104:1199–204. doi: 10.1038/ajg.2009.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Drossman DA, Morris CB, Edwards H, Wrennall CE, Weinland SR, Aderoju AO. et al. Diagnosis, characterization, and 3-month outcome after detoxification of 39 patients with narcotic bowel syndrome. Am J Gastroenterol. 2012;107:1426–40. doi: 10.1038/ajg.2012.142. [DOI] [PubMed] [Google Scholar]
- 70.Wong V, Sobala G, Losowsky M. A case of narcotic bowel syndrome successfully treated with clonidine. Postgrad Med J. 1994;70:138–40. doi: 10.1136/pgmj.70.820.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Devi G. Management of drug and alcohol withdrawal. N Engl J Med. 2003;349:405–7. doi: 10.1056/NEJM200307243490420. [DOI] [PubMed] [Google Scholar]
- 72.Fudala PJ, Bridge TP, Herbert S, Williford WO, Chiang CN, Jones K. et al. Office-based treatment of opiate addiction with a sublingual-tablet formulation of buprenorphine and naloxone. N Engl J Med. 2003;349:949–58. doi: 10.1056/NEJMoa022164. [DOI] [PubMed] [Google Scholar]
- 73.Ma H, Tang J, White PF, Wender RH, Leverone T, Quon R. et al. The effect of clonidine on gastrointestinal side effects associated with ultra-rapid opioid detoxification. Anesth Analg. 2003;96:1409–12. doi: 10.1213/01.ANE.0000060451.82578.3A. [DOI] [PubMed] [Google Scholar]
- 74.Day E, Strang J. Outpatient versus inpatient opioid detoxification: a randomized controlled trial. J Subst Abuse Treat. 2011;40:56–66. doi: 10.1016/j.jsat.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 75.Frost J, Spigset O. Opioid-induced pruritus. Tidsskr Nor Laegeforen. 2012;132:2180–1. doi: 10.4045/tidsskr.12.0539. [DOI] [PubMed] [Google Scholar]
- 76.Ständer S, Weisshaar E. Medical treatment of pruritus. Expert Opin Emerg Drugs. 2012;17:335–45. doi: 10.1517/14728214.2012.711316. [DOI] [PubMed] [Google Scholar]
- 77.Nalamachu SR. Opioid rotation in clinical practice. Adv Ther. 2012;29:849–63. doi: 10.1007/s12325-012-0051-7. [DOI] [PubMed] [Google Scholar]
- 78.Furlan AD, Sandoval JA, Mailis-Gagnon A, Tunks E. Opioids for chronic noncancer pain: a meta-analysis of effectiveness and side effects. CMAJ. 2006;174:1589–94. doi: 10.1503/cmaj.051528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Christo PJ. Opioid effectiveness and side effects in chronic pain. Anesthesiol Clin North America. 2003;21:699–713. doi: 10.1016/s0889-8537(03)00087-7. [DOI] [PubMed] [Google Scholar]
- 80.Praveen KT, Law F, O’Shea J, Melichar J. Opioid dependence. Am Fam Physician. 2012;86:565–6. [PubMed] [Google Scholar]
- 81.Courty P, Authier N. Pain in patients with opiates dependence. Presse Med. 2012;41:1221–5. doi: 10.1016/j.lpm.2012.07.030. [DOI] [PubMed] [Google Scholar]
- 82.Juurlink DN, Dhalla IA. Dependence and Addiction During Chronic Opioid Therapy. J Med Toxicol. 2012;8:393–9. doi: 10.1007/s13181-012-0269-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Skarke C, Jarrar M, Schmidt H, Kauert G, Langer M, Geisslinger G. et al. Effects of ABCB1 (multidrug resistance transporter) gene mutations on disposition and central nervous effects of loperamide in healthy volunteers. Pharmacogenetics. 2003;13:651–60. doi: 10.1097/00008571-200311000-00001. [DOI] [PubMed] [Google Scholar]
- 84.Niemi M, Tornio A, Pasanen MK, Fredrikson H, Neuvonen PJ, Backman JT. Itraconazole, gemfibrozil and their combination markedly raise the plasma concentrations of loperamide. Eur J Clin Pharmacol. 2006;62:463–72. doi: 10.1007/s00228-006-0133-z. [DOI] [PubMed] [Google Scholar]
- 85.Tornio A, Neuvonen PJ, Backman JT. The CYP2C8 inhibitor gemfibrozil does not increase the plasma concentrations of zopiclone. Eur J Clin Pharmacol. 2006;62:645–51. doi: 10.1007/s00228-006-0155-6. [DOI] [PubMed] [Google Scholar]
- 86.Baker DE. Loperamide: a pharmacological review. Rev Gastroenterol Disord. 2007;7:S11–8. [PubMed] [Google Scholar]
- 87.Awouters F, Niemegeers CJ, Janssen PA. Pharmacology of antidiarrheal drugs. Annu Rev Pharmacol Toxicol. 1983;23:279–301. doi: 10.1146/annurev.pa.23.040183.001431. [DOI] [PubMed] [Google Scholar]
- 88.Penagini R, Bianchi PA. Effect of morphine on gastroesophageal reflux and transient lower esophageal sphincter relaxation. Gastroenterology. 1997;113:409–14. doi: 10.1053/gast.1997.v113.pm9247457. [DOI] [PubMed] [Google Scholar]
- 89.Penagini R, Allocca M, Cantù P, Mangano M, Savojardo D, Carmagnola S. et al. Relationship between motor function of the proximal stomach and transient lower oesophageal sphincter relaxation after morphine. Gut. 2004;53:1227–31. doi: 10.1136/gut.2003.035246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gisbert JP, Calvet Calvo X, Ferrándiz Santos J, Mascort Roca JJ, Alonso-Coello P, Marzo Castillejo M. Managing of the patient with dyspepsia Clinical Practice Guideline Update 2011. Aten Primaria. 2012;44:728–33. doi: 10.1016/j.aprim.2012.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Talley NJ. Nonulcer dyspepsia: current approaches to diagnosis and management. Am Fam Physician. 1993;47:1407–16. [PubMed] [Google Scholar]
- 92.Camilleri M. Novel pharmacology: asimadoline, a kappa-opioid agonist, and visceral sensation. Neurogastroenterol Motil. 2008;20:971–9. doi: 10.1111/j.1365-2982.2008.01183.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kariv R, Tiomny E, Grenshpon R, Dekel R, Waisman G, Ringel Y. et al. Low-dose naltreoxone for the treatment of irritable bowel syndrome: a pilot study. Dig Dis Sci. 2006;51:2128–33. doi: 10.1007/s10620-006-9289-8. [DOI] [PubMed] [Google Scholar]
- 94.Corazziari E. Role of opioid ligands in the irritable bowel syndrome. Can J Gastroenterol. 1999;13:71A–75A. doi: 10.1155/1999/598659. [DOI] [PubMed] [Google Scholar]
- 95.Mangel AW, Bornstein JD, Hamm LR, Buda J, Wang J, Irish W. et al. Clinical trial: asimadoline in the treatment of patients with irritable bowel syndrome. Aliment Pharmacol Ther. 2008;28:239–49. doi: 10.1111/j.1365-2036.2008.03730.x. [DOI] [PubMed] [Google Scholar]
- 96.Dapoigny M, Abitbol JL, Fraitag B. Efficacy of peripheral kappa agonist fedotozine versus placebo in treatment of irritable bowel syndrome. A multicenter dose-response study. Dig Dis Sci. 1995;40:2244–9. doi: 10.1007/BF02209014. [DOI] [PubMed] [Google Scholar]
- 97.Menees S, Saad R, Chey WD. Agents that act luminally to treat diarrhoea and constipation. Nat Rev Gastroenterol Hepatol. 2012;9:661–74. doi: 10.1038/nrgastro.2012.162. [DOI] [PubMed] [Google Scholar]
- 98.Sellin JH. A practical approach to treating patients with chronic diarrhea. Rev Gastroenterol Disord. 2007;7:S19–26. [PubMed] [Google Scholar]
- 99.Farthing MJ. Farthing MJIntroductionEnkephalinase inhibition: a rational approach to antisecretory therapy for acute diarrhoea. Aliment Pharmacol Ther. 1999;13:1–2. [PubMed] [Google Scholar]
- 100.Caraco Y. Genes and the response to drugs. N Engl J Med. 2004;351:2867–9. doi: 10.1056/NEJMe048278. [DOI] [PubMed] [Google Scholar]
- 101.Chandok N, Watt KD. Pain management in the cirrhotic patient: the clinical challenge. Mayo Clin Proc. 2010;85:451–8. doi: 10.4065/mcp.2009.0534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Rossi S, Assis DN, Awsare M, Brunner M, Skole K, Rai J. et al. Use of over-the-counter analgesics in patients with chronic liver disease: physicians’ recommendations. Drug Saf. 2008;31:261–70. doi: 10.2165/00002018-200831030-00007. [DOI] [PubMed] [Google Scholar]
- 103.Delcò F, Tchambaz L, Schlienger R, Drewe J, Krähenbühl S. Dose adjustment in patients with liver disease. Drug Saf. 2005;28:529–45. doi: 10.2165/00002018-200528060-00005. [DOI] [PubMed] [Google Scholar]