Abstract
BACKGROUND
A considerable portion of gastrointestinal malignancies undergoes surgery without curative resection. This study was conducted to assess surgical outcome in patients with gastrointestinal cancers.
METHODS
We reviewed individuals with esophagus, stomach, colon and rectum cancers admitted for surgical treatment after initial preoperative evaluations. Surgical outcome, stage of tumors and 1 and 5 years survival rate were assessed and analyzed.
RESULTS
Two hundred and fifty five patients with esophagus, stomach and colorectal malignancies, who were admitted for surgical resection, were reviewed. Two hundred and twenty two patients were underwent surgery but tumor was not resected in 41 cases (18.6%). Based on pathological assessment, stage of tumors was III or IV in 108 individuals (48.9%). The proportion of tumor with advanced stage was significantly higher in patients with gastroesophageal cancers than those with colorectal malignancies (62.6% versus 31.6%), p<0.0001). The proportion of non-resectable tumor was also significantly higher in patients with esophageal and gastric cancers ( p=0.0001). Palliative surgery was done in 26.1% of patients treated by surgical resection. The proportion of palliative surgery was significantly lower in patients with gastric cancer ( p=0.001). 1 and 5-year survival were significantly longer in colorectal cancer and those with curative surgery ( p=0.001). Survival of patients with palliative resection was the same as patient without tumor resection.
CONCLUSION
Despite preoperative evaluations, there are still a considerable proportion of patients who are diagnosed as inoperable during surgery. Further researches seem to be necessary in order to provide more precise preoperative staging. Screening programs should also be considered for GI cancers in high-risk areas. It seems that palliative resection would not improve survival of patients with advanced GI malignancies.
Keywords: Alimentary tract, Malignancy, Surgical outcome
INTRODUCTION
Alimentary tract malignancies account for a substantial proportion of global cancer death. Malignancies of esophagus, stomach, colon and rectum are the most common diagnosed malignancies of the alimentary tract with variable incidence and mortality worldwide. In 2012, new cases of esophagus, stomach, and colorectal cancers in the world were estimated about 481645, 988602 and 1235108 respectively.1 The estimated death due to esophageal, stomach and colorectal cancer were approximately 406533, 737419, and 609051 respectively worldwide in 2012 .1 The burden of alimentary tract malignancies is enormous in developing countries like Iran where the incidence of gastroesophageal malignancies and cancer-related mortality of colorectal malignancies are rising .1- 3Reports from Iran have shown that patients with stomach cancer have a poor survival rate even when compared with esophageal cancer.4 One of the most important determinants of longer survival has been early diagnosis and being treated with a curative approach.5
Several studies have shown that many cases of alimentary tract malignancies are diagnosed in advanced stages that prohibit curative surgical resection.6- 8 Moreover, there are even patients who undergo surgery but preoperative plan of resection cannot be performed since the tumors are more advanced than presumed. The literature lacks a comprehensive report regarding the latter group, so we retrospectively assessed surgical outcome and stage of tumors in patients who had planned to undergo a curative surgery.
MATERIALS AND METHODS
We retrospectively reviewed the data of all patients admitted to the surgery department of Shariati hospital affiliated to Tehran University of Medical Sciences, with the diagnosis of esophageal, gastric or colorectal cancers for elective cancer resection from January 2008 to October 2010. Patients with tumor recurrence after previous surgery and those with planned palliative surgery were excluded. All included patients had preoperative evaluations based on the surgery department preoperative assessment protocol ( table 1).
Table 1. Protocol of Preoperative Evaluations of Patients Based on Tumor Type .
| Cancer Type | Chest X Ray | Upper GI series or Barium enema | Endoscopy | EUS | CT scan | MRI |
| Esophageal | √ | √ | √ | √ | Chest/abdomen | |
| Gastric | √ | √ | √ | √* | Abdomen/pelvis | |
| Colorectal | √ | √ | √** | Abdomen/pelvis | √*** |
* EUS was performed in selected patients with proposed locally advanced lesions in CT scan
** In rectal cancers
*** used in selected patients with equivocal rectal EUS
EUS: Endoscopic ultrasonography
MRI: Magnetic Resonance Imaging
Neoadjuvant chemotherapy or chemoradiotherapy had been considered for patients with locally advanced rectal cancer spreading through the rectum wall and patients with esophagus and cardia cancer spreading through the wall or multiple lymph nodes involvements. Patients with suspected advanced disease underwent laparoscopy in the beginning of surgery.
Surgical tumor resection was considered curative once complete resection of tumor with planned negative margins (proximal, distal, and radial) and adequate lymphadenectomy were achieved. Palliative resection was defined as surgical resections just to alleviate symptoms, including recurrent or massive bleeding or luminal obstruction, when curative surgery was not achievable.
Stage of tumors was determined according to the American Joint Committee on Cancer (AJCC) staging systems, 6th edition.9 Pathological reports, surgical findings, and preoperative imaging studies were used to determine stage of tumors. In a prospective manner, we called and closely visited our patients for follow up.
Data was analyzed using SPSS version 17 (SPSS, Chicago, Illinois, USA). Chi-square test was used for analysis of findings and comparisons. Survival data were analyzed using Kaplan–Meier method and Cox proportional hazard models. P-values of less than 0.05 were considered as significant. In survival analysis, we excluded cases with mortality in 30 days after surgery (mostly due to postoperative complication). Patients with lost to follow up were compared with those remained in follow-ups in terms of demographic data to ensure that lost patients have not had any particular pattern.
RESULTS
Two hundred and fifty five patients admitted to the surgery ward for elective surgery. Complete preoperative evaluations were done for all of them ( table 1). Thirty four patients (13.3%) discharged prior to operation due to underlying severe medical co-morbidities or lack of consent for surgery (Figure 1).
Figure 1.
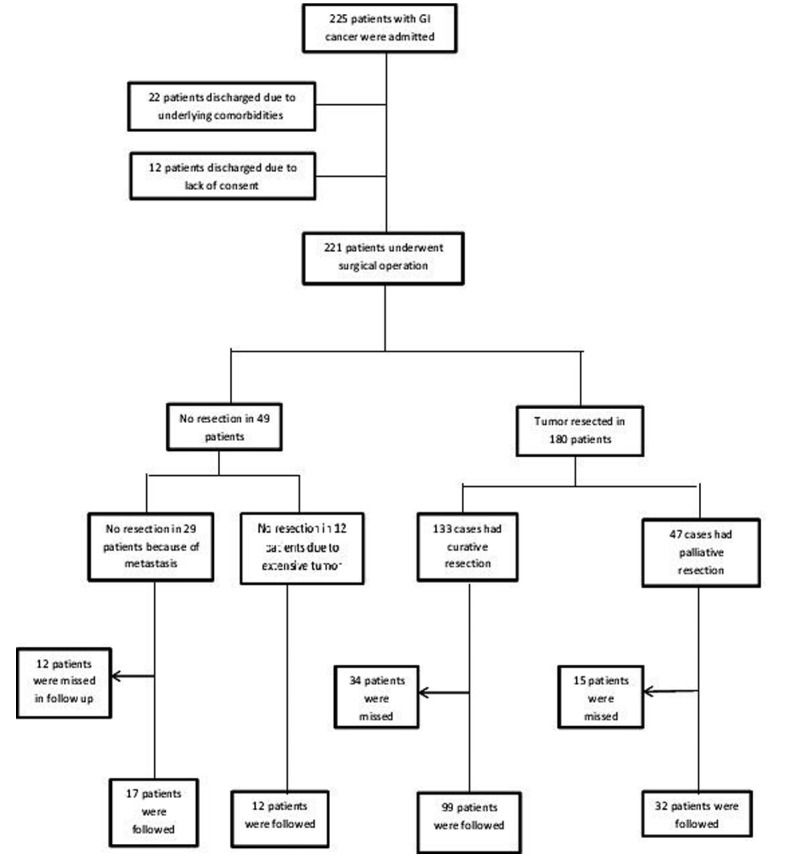
Outcome of patients with alimentary tract malignancies who were admitted in the surgery ward of Shariati hospital.
Amongst 221 patients who underwent surgery, 71 cases (32.1%) were women and 150 cases were men (67.9%) with mean (SD) age of 56.5(SD=14.5) years (range: 15-84 years). 25, 98 and 98 patients were diagnosed with esophageal, gastric and colorectal cancer, respectively. The 30-day mortality occurred in 22 (12%) patients. This included 6 (33%) esophagus, 7 (3.8%) gastric, and 9 (4.9%) colorectal malignancies. 30-day mortality was not significantly different in patients with curative or palliative resection. Resection was not achievable in 41 patients (18.5%) either due to metastasis or peritoneal seeding or locally advanced unresectable tumors (Figure 1). Surgical resection was performed in 180 cases (81.4%) that were curative in 73.9%, and palliative in 26.1% of patients (Figure 1). Table 2 shows data categorized by tumor type. The proportion of patients who did not undergone surgery and discharged was significantly higher in patients with esophageal malignancies than those with gastric and colorectal cancer (28.6% versus 9.3% and 12.5%, p=0.013). The proportion of unresectable tumor was significantly lower in patients with colorectal cancer than those with esophageal and gastric malignancies (5.4% versus 17.1% and 26.8%, p =0.0001). Palliative surgeries were performed in 10.2%, 31.4% and 22.3% of patients with gastric, esophageal and colorectal cancers, respectively (p =0.001).
Table 2. Consequences Of Admission And Surgical Treatment In Patients With Gastrointestinal Malignancies According To Tumor Type .
| Esophageal CA (N=35) | Gastric CA (N=108) |
Colorectal CA
N=112) |
P Value | |
| Admission outcome | 0.013 | |||
| Not operated | 10(28.6%) | 10(9.3%) | 14(12.5%) | |
| Operated | 25(71.4%) | 98(90.7%) | 98(87.5%) | |
| Cause of not being operated | 0.479 | |||
| Due to severe comorbidities | 8(80%) | 6( 60%) | 8( 57.1%) | |
| Due to lack of consent | 2(20%) | 4( 40%) | 6( 42.0%) | |
| Resectability during surgery | 0.0001 | |||
| Not resected | 6(24%) | 29( 29.6%) | 6( 6.1%) | |
| Resected | 19(76%) | 69( 70.4%) | 92( 93.9%) | |
| Cause of not being resected | 0.885 | |||
| Due to metastasis and/or seeding | 4(66.7%) | 21( 72.4%) | 4( 66.7%) | |
| Due to extensive tumor | 2(33.3%) | 8( 27.6%) | 2( 33.3%) | |
| Type of resection | 0.001 | |||
| Curative | 8(42.1%) | 58( 84.1%) | 67( 72.8%) | |
| Palliative | 11(57.9%) | 11( 15.9%) | 25( 27.2%) |
Overall 18 (8.1%), 95 (43%), 55 (24.9%) and 53 (24%) of patients were diagnosed in stages I, II, III and IV, respectively ( table 3). The percentage of advanced stages (III or IV) was significantly higher in gastroesophageal than colorectal malignancies (62.6% versus 31.6%, p < 0.0001). We found that Stage III and IV, were further than stage I and II in esophageal and gastric cancer, but in colorectal cancer, these proportion were reversed (p <0.001).
Table 3. TNM Staging And Grading Of Cancers In Operated Patients According To AJCC Staging System .
|
Esophageal CA
(n=25) |
Gastric CA
(n=95) |
Colorectal CA
(n=98) |
|
| T | |||
| Tx | 6(24%) | 24(24.5%) | 4(4.1%) |
| T0 | 0(0%) | 0(0%) | 0(0%) |
| T1 | 0(0%) | 3(3.1%) | 0(0%) |
| T2 | 3(12%) | 21(17.9%) | 11(11.2%) |
| T3 | 13(42%) | 40(21.4%) | 74(75.5%) |
| T4 | 3(12%) | 10(10.2%) | 9(9.2%) |
| N | |||
| Nx | 6(24%) | 24(24.5%) | 6(6.1%) |
| N0 | 7(28%) | 38(38.8%) | 72(73.5%) |
| N1 | 12(48%) | 26(26.5%) | 13(13.3%) |
| N2 | N/A | 7(7.1%) | 7(7.1%) |
| N3 | N/A | 3(3.1%) | N/A |
| M | |||
| Mx | 0(0%) | 0(0%) | 0(0%) |
| M0 | 21(84%) | 76(77.6%) | 79(80.6%) |
| M1 | 4(16%) | 22(22.4%) | 19(19.4%) |
| Stage* | |||
| 1 | 0(0%) | 11(11.2%) | 7(7.1%) |
| 2 | 7(28%) | 28(28.6%) | 60(61.2%) |
| 3 | 14(42%) | 29(29.6%) | 12(12.2%) |
| 4 | 4(16%) | 30(30.6%) | 19(19.4%) |
* P value < 0.0001
N/A = not applicable
TNM: Tumor, node, metastasis
AJCC: American Joint Committee on Cancer
Seventy five individuals (29.1%) were lost to post surgery follow-up for survival because of changing in address or migration, So data for 183 cases were used in survival analysis. 1 and 5 year survival rate for esophagus, gastric and colorectal cancers is reported in table 4. In this study, no significant difference was observed between male and female in 3 groups in terms of survival (p = 0.135). The survival of patients with age age ≤ 60, and age > 60 was not different (p =0.103). Curative surgery was performed in 133 cases, 8 patients (42.1%) in esophagus, 58 patients (84.1%) in gastric and 67 patients (72.8%) in colorectal groups (p <0.001). Survival rate of colorectal cancer was higher than gastric and esophagus cancer (p =.0.009). Also survival of patients with curative surgery was more than those with palliative surgery or surgery without tumor resection (p <0.001) ( table 5). By adjusting other variables (age, sex, stage, cancer type, surgery outcome), the risk of death for patients who had palliative surgery was almost three times higher than curative surgery (hazard ratio=0.32; CI95%= 0.21-0.6; p <0.001) while Survival of patients with palliative surgery were the same as those who had surgery without tumor resection (p =0.756) ( table 5).
Table 4. 1 and 5 Year Survival Time For GI Cancers .
| Cancer type | 1-year survival | 5-year survival | Median |
| Esophagus | 0.60 | 0.44 | 41(7-75) |
| Gastric | 0.60 | 0.31 | 17(7-32) |
| Colorectal | 0.80 | 0.45 | 57(41-75) |
| Total | 0.70 | 0.38 | 41(26-57) |
Table 5. Simple and multiple survival analysis for GI cancers (we applied mean and its confidence interval since median was not available for some strata) .
| Log rank Mean (Confidence interval) | P Value | Multi variable COX Hazard ratio (CI) | P Value | |
| Age* |
0.103 |
1.47 (0.9-2.3) | 0.101 | |
| <60 year | 31.45(27.0-35.9) | |||
| ≥60 year | 27.73(22.7-32.7) | |||
| Sex | 0.839 | 1.10 (0.7-1.8) | 0.703 | |
| Male | 29.87(25.7-34.0) | |||
| Female | 29.30(23.6-34.9) | |||
| Cancer type$ | 0.009 | |||
| Esophagus | 28.33(17.9-38.8) | 0.96 (0.4-2.1) | 0.924 | |
| Gastric | 23.74(18.6-28.9) | 2.0 (1.1-3.5) | 0.016 | |
| Colorectal | 34.75(30.2-39.3) | 1 | - | |
| Surgery# | <0.001 | |||
| Non-resectable | 14.75(7.4-22.1) | 1.12 (0.5-2.3) | 0.756 | |
| Curative | 35.54(31.7-39.3) | 0.32 (0.2-0.6) | <0.001 | |
| Pallative | 23.00(15.8-30.2) | |||
| Stage | 0.087 | |||
| I. II | 36.01(31.0-41.1) | 0.82 (0.5-1.3) | 0.424 | |
| III, IV | 23.78(19.8-27.7) | 1 | - |
* Age < 60 as reference
$ Colorectal as reference
#palliative as reference)
We compared patients with lost to follow up with those completed follow ups in terms of demographic data. There were no significant differences in Gender (p =1), age (p =0.447), cancer type (p =0.06), and surgery outcome (p =0.581) between patients that missed and who completed follow up.
DISCUSSION
Malignancies of esophagus, stomach, colon and rectum are the most common gastrointestinal (GI) cancers and cause a considerable proportion of malignant disease mortality worldwide. These tumors mainly present when tumor causes luminal obstruction or hemorrhage. Diagnosis of GI cancers requires strong clinical suspicion in primary care followed by prompt endoscopic evaluation and biopsy. Early detection of these cancers is crucial since complete surgical resection is the mainstay of therapy. Although surgical resection may have beneficial effects on long term survival in advanced stages of GI cancers, however the best surgical results have reported in early stages.10- 12
We found high proportion of advanced cancers in this group of patients who were scheduled for curative resection. Previous reports had also shown that majority of GI tumors were diagnosed in advanced stages. Enzinger et al. reported that more than half of patients diagnosed with esophageal cancer had unresectable tumor or distant metastasis. In addition, 40 to 54 percent of those undergone surgical resection were in stage III.13 In another study, more than 50 percent of gastric cancers were diagnosed at stages III or IV.14 Another study showed that in colon and rectum cancers about 22 and 24 percent colon of resected tumors were in stages III and IV, respectively.15
There is no comprehensive report about patients with non-resectable GI cancers who undergo surgery. We showed that in one-fifth of patients with alimentary tract tumors who undergone surgery, the tumor left unresected because of local advancement and/or metastasis. These surgical findings were not distinguishable in preceding imaging studies, so staging laparoscopy is proposed in evaluation of many malignancies, e.g. gastroesophageal cancers 16 but it is still invasive and pose risks of anesthesia to the patients. In approximately one quarter of patients, only palliative resection of the tumor was possible, suggesting that curative approaches are not feasible in a considerable number of patients in the time of surgery.
The proportion of cases who discharged without surgical treatment (not operated cases) was significantly higher in esophageal cancer. This finding can be explained by higher risk of surgery due to underlying comorbidities, and need for an acceptable cardiopulmonary function. We found a lower rate of premature termination of surgery (laparotomy or laparoscopy without resection) in colorectal malignancies. This can be due largely to accepted protocol of resecting metastatic colorectal cancer and also due in part to less crucial structures involved by colorectal tumors. Less palliative surgical resection was done in patients with stomach cancer, due to less obstructive symptoms, and no benefit from resection of metastatic gastric cancer.
Aghcheli et al. showed that median survival was 7 months in Esophageal squamous cell carcinoma and age at diagnosis was reversed with survival but ultimately the association was disappeared with adjustment for treatment, that is similar to our result .5 In another study patients with surgery and/or chemotherapy treatment had significant longer survival than without such treatments.4 Bashash et al. compared 1-year survival in Canada (British Columbia) and Iran (Ardabil). They found that for gastric cancer the 1-year survival rate was 48% and 21% and for esophageal cancer was 33% and 17% in and in Canada and Iran respectively.17
We showed 1 year survival for esophageal cancer was 44 % and for gastric cancer 36 %. One study showed that median survival for palliative resection was higher than those whose tumor has not been resected in colorectal cancers. They compared in terms of age, gender, preoperative presence of obstruction and tumor stage.18 Mafune KI et al. were indicated palliative surgery in some cases esophageal cancer is effective as a treatment.19 Also review article of Swan et al. illustrated that palliative resection remains a controversial topic in points of gastric cancer.20 Despite these articles our analysis revealed that there is not any difference in terms of survival between “palliative resection” and “surgery without tumor resection” even after adjustment for cancer type and tumor stage.
It seems that targeting and evaluating the factors that cause delay in diagnosis and treatment may partly improve the above-mentioned problems. One factor that leads to late diagnosis of GI cancer is the absence of symptoms in low stage of tumors. Therefore, screening of individuals with greater risks of developing GI malignancies is crucial for early detection of the tumor. Experiences in Japan demonstrated that early endoscopic screening of patients with stomach cancer has led to diagnosis of 40-60% of tumors in an early stage. Consequently, the mortality rate of the disease has declined in this country.21 The similar approach has been recommended for colorectal cancer. Screening with occult blood test and flexible sigmoidoscopy or colonoscopy has led to finding of cases with low stages lesions and curative resection approaches .22,23Repeated endoscopic evaluations in patients with Barrett's esophagus have shown similar results in diagnosis of esophageal adenocarcinoma in low stages.24 Recently, it has been shown that cytosponge test is a valid non-invasive method for detecting precursor lesions of esophageal adenocarcinoma (barrette's lesions), suggesting it as a suitable screening program to be used in primary care setting .25
Other factors leading to late diagnosis of GI cancers include lack of public information, hesitancy in visiting family physicians, and delayed referrals to specialists. Public education and awareness, immediate referral of suspected patients instead of administrating inappropriate medications, prompt diagnostic investigations, and following up with endoscopic procedures and biopsy have been suggested for improvement of these pitfalls.6, 8
The limitation of this study was that we could not enroll patients with apparent metastasis or extensive disease diagnosed by imaging studies, because they are directly referred to oncologists for nonsurgical therapies. In fact, the proportion of patients with unresectable GI cancer is actually greater than what we observed in this study. So, it is recommended to carry out more studies to cover all diagnosed malignancies managed both in outpatient and inpatient services. Another limitation of this study is low number of patients with esophageal malignancies. A large number of colorectal and gastric resections have been performed in our center but esophageal surgeries were in moderate volume. It may affect the 30 days mortality, recurrence and survival rate. Also this may affect the comparisons between different cancer types in this report. Further studies with larger sample size are required.
In conclusion, we found that half of alimentary tract malignancies are diagnosed at advanced stages. Despite preoperative evaluations, there are still a considerable proportion of patients who are diagnosed as inoperable during surgery. Further studies are necessary in order to provide more precise preoperative staging.
CONFLICT OF INTEREST
The author declares no conflict of interest related to this work.
Please cite this paper as:
Soroush AR. Surgical Outcome in Patients with Gastrointestinal Malignancies; A Report from a Large Referral Hospital, 2008-2010. Middle East J Dig Dis 2013;5:201-8.
References
- 1. Ferlay J, Shin HR, Bray F, Forman D, Mathers C , Parkin DM. GLOBOCAN 2008 v1.2, Cancer Incidence and Mortality Worldwide: IARC Cancer Base No. 12.
- 2.Jafari N, Abolhassani F, Naghavi M, Pourmalek F, Moradi Lakeh M, Kazemeini H. et al. National Burden of Disease and Study in Iran. Iranian J Publ Health. 2009;38:71–3. doi: 10.1186/1478-7954-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Etemadi A, Sadjadi A, Semnani S, Nouraie SM, Khademi H, Bahadori M. Cancer Registry in Iran: a Brief Overview. Arch Iran Med. 2008;11:577–80. [PubMed] [Google Scholar]
- 4.Aghcheli K, Marjani HA, Nasrollahzadeh D, Islami F, Shakeri R, Sotoudeh M. et al. Prognostic Factors for Esophageal Squamous Cell Carcinoma—A Population-Based Study in Golestan Province, Iran, a High Incidence Area. PLoS One. 2011;6:e22152. doi: 10.1371/journal.pone.0022152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Samadi F, Babaei M, Yazdanbod A, Fallah M, Nouraie M, Nasrollahzadeh D. et al. et al. Survival Rate of Gastric and Esophageal Cancersin Ardabil Province, North-West of Iran. Arch Iran Med. 2007;10:32–7. [PubMed] [Google Scholar]
- 6.Magdalena E, Maria R, Elena C, Joan L, Amador R, Salvador P. et al. Factors influencing delay in the diagnosis of colorectal cancer: a study protocol. BMC Cancer. 2007;7 doi: 10.1186/1471-2407-7-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martin IG, Young S, Sue-Ling H, Johnston D. Delays in the diagnosis of oesophagogastric cancer: a consecutive case series. Br Med J. 1997;314:467–70. doi: 10.1136/bmj.314.7079.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mikulin T, Hardcastle JD. Gastric cancer--delay in diagnosis and its causes. Eur J Cancer Clin Oncol. 1987;23:1683–90. doi: 10.1016/0277-5379(87)90450-0. [DOI] [PubMed] [Google Scholar]
- 9.Greene FL. AJCC cancer staging manual. Springer Verlag. 2002 [Google Scholar]
- 10.Budisin NI, Majdevac IZ, Budisin ES, Manic D, Patrnogic A, Radovanovic Z. Surgery for patients with gastric cancer in the terminal stage of the illness-TNM stage IV. J BUON. 2009;14:593–603. [PubMed] [Google Scholar]
- 11.Izbicki JR, Hosch SB, Knoefel WT, Passlick B, Bloechle C, Broelsch CE. Extended resections are beneficial for patients with locally advanced colorectal cancer. Dis Colon Rectum. 1995;38:1251–6. doi: 10.1007/BF02049148. [DOI] [PubMed] [Google Scholar]
- 12.Mariette C, Taillier G, Van Seuningen I, Triboulet JP. Factors affecting postoperative course and survival after en bloc resection for esophageal carcinoma. Ann Thorac Surg. 2004;78:1177–83. doi: 10.1016/j.athoracsur.2004.02.068. [DOI] [PubMed] [Google Scholar]
- 13.Enzinger PC, Mayer RJ. Esophageal cancer. N Engl J Med. 2003;349:2241–52. doi: 10.1056/NEJMra035010. [DOI] [PubMed] [Google Scholar]
- 14.Wilkinson NW, Howe J, Gay G, Patel-Parekh L, Scott-Conner C, Donohue J. Differences in the pattern of presentation and treatment of proximal and distal gastric cancer: results of the 2001 gastric patient care evaluation. Ann Surg Oncol. 2008;15:1644–50. doi: 10.1245/s10434-008-9877-2. [DOI] [PubMed] [Google Scholar]
- 15.Smith JAE, King PM, Lane RHS, Thompson MR. Evidence of the effect of ‘specialization’on the management, surgical outcome and survival from colorectal cancer in Wessex. Br J Surg. 2003;90:583–92. doi: 10.1002/bjs.4085. [DOI] [PubMed] [Google Scholar]
- 16.Kaiser GM, Sotiropoulos GC, Frühauf NR, Stavrou GA, Peitgen K, Pöttgen C. et al. Value of staging laparoscopy for multimodal therapy planning in esophago-gastric cancer. Int Surg. 2007;92:128–32. [PubMed] [Google Scholar]
- 17.Bashash M, Yavari P, Hislop TG, Shah A, Sadjadi A, Babaei M. et al. Comparison of two diverse populations, British Columbia, Canada, and Ardabil, Iran, indicates several variables associated with gastric and esophageal cancer survival. J Gastrointest Cancer. 2011;42:40–5. doi: 10.1007/s12029-010-9228-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baham A, RentschM RentschM, Pullmann K, Mantouvalou L, Spatz H. et al. Survival benefit in patients after palliative resection vs non-resection colon cancer surgery. World J Gastroenteral. 2006 Nov7;12(41):6634–8. doi: 10.3748/wjg.v12.i41.6634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mafune KI, Tanaka Y, Takubo K. Autopsy findings in patients with esophageal carcinoma: comparison between resection and nonresection groups. J Surg Oncol. 2000;74:196–200. doi: 10.1002/1096-9098(200007)74:3<196::aid-jso7>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 20.Swan R, Miner TJ. Current role of surgical therapy in gastric cancer. Word J Gastroenterol. 2006;12:372–9. doi: 10.3748/wjg.v12.i3.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maruyama K, Okabayashi K, Kinoshita T. Progress in gastric cancer surgery in Japan and its limits of radicality. World J Surg. 1987;11:418–25. doi: 10.1007/BF01655804. [DOI] [PubMed] [Google Scholar]
- 22.Towler BP, Irwig L, Glasziou P, Weller D, Kewenter J. Screening for colorectal cancer using the faecal occult blood test, Hemoccult (Cochrane Review) Cochrane Database Syst Rev. 2000;(2):CD001216. doi: 10.1002/14651858.CD001216. [DOI] [PubMed] [Google Scholar]
- 23.Inadomi JM, Sonnenberg A. The impact of colorectal cancer screening on life expectancy. Gastrointest Endosc. 2000;51:517–23. doi: 10.1016/s0016-5107(00)70282-3. [DOI] [PubMed] [Google Scholar]
- 24.Macdonald CE, Wicks AC, Playford RJ. Ten years’ experience of screening patients with Barrett’s esophagus in a university teaching hospital. Gut. 1997;41:303. doi: 10.1136/gut.41.3.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kadri SR, Lao-Sirieix P, O'Donovan M, Debiram I, Das M, Blazeby JM. et al. Acceptability and accuracy of a non-endoscopic screening test for Barrett's oesophagus in primary care: cohort study. BMJ. 2010;341:c4372. doi: 10.1136/bmj.c4372. [DOI] [PMC free article] [PubMed] [Google Scholar]


