Abstract
BACKGROUND
Corticosteroids are used to induce remission in auto-immune hepatitis. They are not universally effective; therefore, alternative treatments are needed. In this study Cysclosporine-A has been compared with prednisolone as an alternative treatment in a randomized controlled trial. This paper is an interim analysis of an ongoing clinical trial.
METHODS
Sixteen years and older consenting patients were enrolled. Group-A received prednisolone and group-B cyclosporine-A according to a preset protocol and followed at regular intervals for 48 weeks. Final assessment was done at week 48. Primary outcome was response rate as defined below. “Complete response” was defined as achieving AST and ALT in the normal range and absence of any clinical signs of deterioration, and partial response was defined as a decrease in AST and ALT by less than half of their original values but not to within normal limit. Non-responding ones at week eight were switched to the other arm.
RESULTS
Thirty-nine patients were enrolled (24 group-A, 9 male). Mean AST and ALT at baseline were higher in group-B, but other variables were comparable. At week 12, 34.8% and 64.3% of group-A and B had achieved AST and ALT in the normal range (less than 40 IU/L) respectively ( p=0.081). Corresponding figures at week 48 were 50.0% and 47.6% ( p=0.62 & 0.48 respectively). At week 12, 86.9% and 85.7% of patients had AST and ALT levels less than twice upper normal limit in groups-A and B respectively ( p=0.54 & 0.42).Corresponding figures at week 48 were 90.0% for both groups. There was one treatment failure in group-B which did not respond to prednisolone either. Serious adverse events (death and liver transplantation) occurred in group-A only. Serum creatinine did not change during the study period in either group.
CONCLUSION
According to our data, Cyclosporine-A is as effective as prednisolone for induction of remission in AIH. Adverse events and serious adverse events were more common with prednisolone.
Keywords: Auto-immune hepatitis, Cyclosporine-A, Prednisolone, Randomized controlled trial, Remission, Adverse effect
INTRODUCTION
Autoimmune hepatitis (AIH) is a chronic and relapsing disease mostly affecting young women. The disease is diagnosed with elevated liver enzymes, elevated serum gamma-globulin levels, presence of autoantibodies in serum, absence of viral, metabolic, and toxic causes (including alcohol), and compatible liver histologic findings in the appropriate clinical setting .1Not being a common disease, it is a relentless condition and can lead to cirrhosis within months to years if not treated appropriately.2 Mainstays of treatment are glucocorticoids with or without azathioprine. About 30% of patients treated as such either develop intolerable adverse effects or are refractory to treatment.2 Until recently, it was believed that 10-40% of those whose disease is controlled with treatment, achieve sustained remission (remission off therapy) and in the rest the disease is kept in check, but treatment cannot be discontinued.3 Recent evidence suggests that even a higher proportion of patients (almost all) may need long term maintenance therapy.4 Considering the side-effects of glucocorticoids and the fact that a significant proportion of patients do not achieve remission, other therapeutic options are needed. Several alternatives have been reported in the literature including budesonide,3 cyclosporine,5- 6 mycophenolate mofetil,7 and tacrolimus.8 Except for budesonide, other agents have not been studied in randomized controlled trials (RCT) and their efficacy has been shown either in case series or anecdotal case reports. Cyclosporine, a calcineurin inhibitor in clinical use since late 70s, has been shown to be effective for induction of remission in AIH both as salvage therapy as well as in treatment naïve patients .5,6,9-13Besides, it has an acceptable safety profile, especially for short and medium term use. 14, 15In this study, we aimed to assess safety and efficacy of cyclosporine-A (CsA) in direct comparison to the current standard treatment for AIH, i.e. oral prednisolone.
MATERIALS AND METHODS
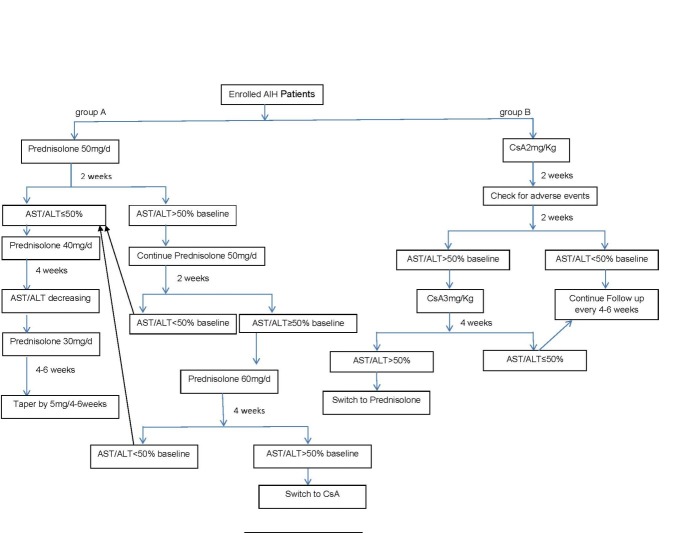
Treatment naïve AIH patients, sixteen years of age and older, were eligible to be enrolled in this single center RCT if they had none of the exclusion criteria (see below). Thirty six patients needed to be enrolled in each arm of this study. AIH was diagnosed if the patient had at least 4 weeks of elevated liver enzymes, increased serum gamma-globulins, and tested positive for any of the following at a titer ≥1/40: antinuclear antibody (ANA), antismooth muscle antibody (ASMA), or anti-liver kidney microsomal antibody type-I. They were also required to test negative for hepatitis B surface antigen, immunoglobulin-M anti-hepatitis-A antibody and a third generation ELISA anti-hepatitis C antibody. Wilson’s disease was assessed by checking serum ceruloplasmin and copper and in equivocal cases 24 hour urine copper. Hemochromatosis was sought by serum iron/total iron binding capacity and serum ferritin. Alpha-1 antitrypsin deficiency was checked directly. A percutaneous liver biopsy was done whenever possible either at presentation or at the first possible occasion given the patient consented for that. The biopsies were scored according to the modified hepatitis activity index.16 Patients were then labeled as having probable or definite AIH according to the simplified criteria for diagnosing AIH.17 Patients were excluded if they were treated before, were not willing or able to participate, had decompensated cirrhosis (as manifested by presence of ascites, esophageal varices, and or hepatic encephalopathy), those with alcohol use, alcoholic liver disease and drug induced liver disease, had uncontrolled hypertension or diabetes mellitus, or concomitant neoplastic disease (as defined by harboring untreated malignancy, or treated within the previous five years). The patients were randomized according to a computer generated random table to either receive prednisolone or CsA. Allocation concealment was central, i.e. after the diagnosis of AIH was secured and the patient was considered eligible and consented to participate in the study, a central person who was not involved in any stage of patient care was contacted and after providing the patients’ identity and information, she opened a sealed envelope and informed the responsible physician to which group was the patient allocated. Considering the gross changes in facies which occur with use of prednisolone, it was not possible to blind the in charge physicians regarding the study medication. The patients were then either started on prednisolone 50 mg/day (group-A) or CsA 2mg/kg body weight in two divided doses administered orally 12 hours apart (group-B). The patients were followed in two weeks and their medication adjusted according to a preset protocol (Figure1). The second follow-up was at week four and thereafter at 4-6 weeks intervals. The patients underwent a detailed interview looking for adverse events of the medications and a thorough physical examination including blood pressure measurement in the sitting position after five minutes of rest at each follow-up. The following lab data were obtained at each visit: aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), total bilirubin (TB), direct bilirubin (DB), complete blood count (CBC), prothrombin time (PT) and international normalized ratio (INR), serum creatinine, triglycerides (TG), cholesterol (TC), high density lipoprotein (HDL), low density lipoprotein (LDL), and fasting blood sugar (FBS). Azathioprine at a dose of 1mg/kg was added to the regimen when prednisolone was tapered to 20mg/day or at month eight after starting CsA. Then it was pushed up to 2mg/kg by 25-50mg increments at successive visits if feasible. CsA was continued for 48 weeks and prednisolone was tapered as shown in Figure-1. Worsening AST/ALT/TB/INR, clinical deterioration (as manifested by appearance of hepatic encephalopathy or jaundice), and a decrease in AST & ALT by less than half of baseline values at week 8 (Figure1) were considered as “treatment failure”. In case of treatment failure at week 8, the patient was switched to the other arm, i.e. if was receiving prednisolone he/she was switched to CsA and vice versa. Final assessment was done at week 48. “Complete response” was defined as achieving AST and ALT in the normal range and absence of any clinical signs of deterioration, and partial response was defined as a decrease in AST and ALT by less than half of their original values but not to within normal limit. Cyclosporine level was measured in some patients but not in all. All patients signed an informed consent. The study protocol conformed to the guidelines of the 1975 Helsinki declaration for medical research involving human subjects, was approved by the institutional board review of the digestive disease research center of Tehran University of Medical Sciences (IRB-00001641) and registered at “www.clinicaltrials.gov” (NCT01170351).
Fig.1.
Study algorithm, AIH: Autoimmune hepatitis, CsA: Cyclosporine-A, AST: Aspartate transaminase, ALT: Alanine transaminase
Categorical variables are summarized as numbers and percentages and continuous variables as means +/- standard deviations (SD) or medians and ranges where appropriate. Independent sample t-test and Fisher’s exact test were used to compare means between groups; chi-square was used to compare categorical variables, and multiple logistic regression to assess predictability of response to treatment. A p-value of less than 0.05 was considered as statistically significant.
RESULTS
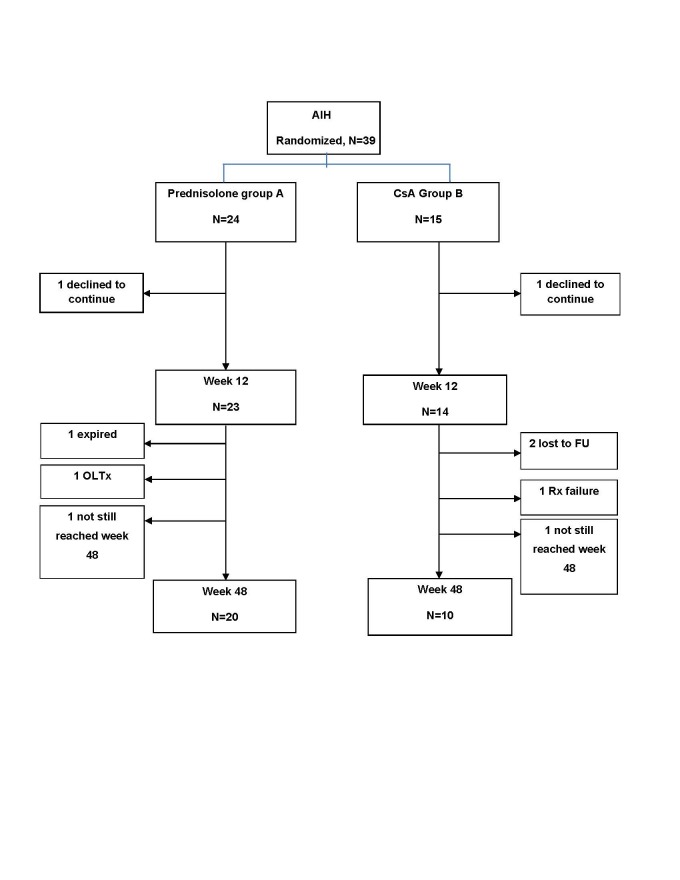
Thirty-nine patients have been enrolled so far (the study is still recruiting). Thirty-seven (9 males) have reached week 48, and two week 12 of the study (study flow-chart is shown in Figure 2). Twenty-four received prednisolone (7 males, 29.2%) and fifteen CsA (2 males, 13.3%). Table 1 shows demographic and some baseline characteristics of the patients including histologic cirrhosis, their characterization according to the simplified AIH criteria, and autoantibody profiles. There were more ANA positive patients in group-B and there was a trend for more histologic cirrhosis in this group as well ( table 1). Baseline, week 12, and week 48 variables are shown in Table-2 . As shown there, mean pretreatment AST and ALT were higher in group-B than group-A (p =0.006, and 0.019 respectively). Other baseline variables were comparable between the two groups ( Tables-1 & 2). At week 12, two patients (one in each group) had declined to continue receiving care at this center (Figure 2). Of the 23 evaluable patients in group-A at week 12, 8 had complete response (34.8%, ITT: 33.3%) and twenty had achieved (86.9%, intention to treat (ITT): 83.3%) AST &amp;amp;amp;amp;amp; ALT levels <2x UNL. Of the 14 evaluable patients in group-B at this time, 9 had complete response (64.3%, ITT: 60.0%) and 12 had achieved AST &amp;amp;amp;amp;amp; ALT levels <2x UNL (85.7%, ITT: 80.0%). There was a trend for more patients in group-B to have complete response (p=0.08) at this stage. The two groups were similar in achieving AST &amp;amp;amp;amp;amp; ALT levels < 2x UNL on both per-protocol (p =1.00) and ITT analysis at this stage. At week 48, 12 of 20 (60.0%, ITT: 50.0%) available patients in group-A and 7 of 10 (70.0%, ITT: 46.7%) in group-B had complete response respectively (p =0.90 &amp;amp;amp;amp;amp; 1) and 18 of 20 (90.0%, ITT: 75.0%) patients in group A and 9 of 10 (90.0%, ITT: 60.0%) in group B had achieved AST &amp;amp;amp;amp;amp; ALT levels <2xUNL (p =1.00 &amp;amp;amp;amp;amp; 0.70 for per protocol and ITT analyses respectively) (Table 3). Two patients in group-B who had normal AST and ALT at week 12 were lost to follow up at week 48. One of them for whom week 24 data were available, had persistently normal AST and ALT at that time. Two patients in group-A and one in group-B had ALT slightly above two times UNL. One patient in group-A had received a liver transplantation at week 24 because of hepatic encephalopathy and prolongation of INR, and one died of fulminant abdominal sepsis at week 32 despite being in complete biochemical remission. One patient in this group had a relapse when the dose of prednisolone reached 10 mg per day despite being in complete remission at week 12. In group-B, one patient, a 53 years old lady, had rising bilirubin and INR with minimal change in AST/ALT levels from baseline at week 4 and was switched to prednisolone (treatment failure). Her condition deteriorated progressively and became febrile. She was listed for urgent liver transplantation, but before getting a liver allograft died of progressive hepatic encephalopathy and liver failure despite intensive supportive care.
Fig.2.
Study flow-chart, AIH: Autoimmune hepatitis, CsA: Cyclosporine-A, FU: Follow-up, OLTx: Orthotopic liver transplantation, Rx: Treatment
Table 1. Demographic & Baseline Characteristics .
| Variable | Prednisolone | CsA |
| Age (years, mean ± SD) | 33.6 ± 12.7 | 30.0 ± 10.3 |
| Gender (Female %) | 70.8% | 86.7% |
| ANA positive* | 9 | 10 |
| ASMA positive* | 18 | 8 |
| LKM positive* | 2 | 0 |
|
Gammaglobulin
( g/dl, mean ± SD) |
3.7 ±1.5 | 3.6 ± 1.6 |
| Liver Biopsy available (N) | 14 | 14 |
| Cirrhosis according to liver biopsy (N)$ | 6 | 11 |
| Definite AIH (N)# | 14 | 12 |
| Probable AIH (N)# | 8 | 1 |
Table 2. Baseline, week 12, and end of study biochemical values .
| Variable | Baseline | 12th Week | 48th Week | ||||||
|
A
Mean (+/-SD) |
B
Mean (+/-SD) |
P-value |
A
Mean (+/-SD) |
B
Mean (+/-SD) |
P- value |
A
Mean (+/-SD) |
B
Mean (+/-SD) |
P-value |
|
|
AST
(U/L) |
450.8 (374.4) |
857.9 (501.4) |
0.006 |
40.00 (17.7) |
47.00 (40.1) |
0.548 |
49.1 (66.6) |
38.0 (33.0) |
0.625 |
|
ALT
(U/L) |
415.0 (294.4) |
667.3 (340.2) |
0.019 |
47.3 (25.9) |
38.9 (38.0) |
0.426 |
49.5 (99.2) |
26.6 (26.6) |
0.482 |
|
ALP
(U/L) |
410.5 (333.0) |
305.9 (212.6) |
0.296 |
166.0 (88.3) |
214.4 (104.0) |
0.198 |
210.1 (149.3) |
166.7 (53.3) |
0.467 |
|
*GGT
(IU/L) |
136.6 (120.7) |
118.8 (96.5) |
0.692 |
92.8 (145.3) |
56.6 (41.2) |
0.599 |
95.5 (123.3) |
32.4 (12.7) |
0.335 |
| Gamma Globulin (g/dl) |
3.7 (1.5) |
3.6 (1.6) |
0.863 |
1.9 (0.8) |
2.1 (0.6) |
0.495 |
1.5 (0.6) |
2.1 (1.0) |
0.126 |
| Creatinine (mg/dl) |
0.81 (0.27) |
0.73 (0.21) |
0.383 |
0.84 (0.15) |
1.00 (0.20) |
0.016 |
0.77 (0.15) |
0.86 (0.17) |
0.216 |
| Direct bilirubin (mg/dl) / |
5.7 (6.9) |
5.3 (7.5) |
0.887 |
0.5 (0.5) |
0.8 (0.7) |
0.343 |
0.5 (0.4) |
0.4 (0.2) |
0.683 |
|
Total bilirubin
(mg/dl) |
8.5 (9.7) |
8.8 (9.8) |
0.940 |
1.7 (2.5 ) |
1.7 (0.9) |
0.994 |
1.3 (1.2) |
1.3 (0.3) |
0.882 |
|
**FBS
(mg/dl) |
80.3 (10.8) |
76.7 (16.0) |
0.707 |
90.0 (15.6) |
85.90 (13.3) |
0.614 |
88.8 (13.1) |
92.0 (8.0) |
0.683 |
|
***ESR
(mm/1st hour) |
64.3 (49.4) |
33.8 (35.3) |
0.275 |
49.5 (24.9) |
34.00 (27.0) |
0.466 |
58.5 (46.3) |
22.7 (12.9) |
0.258 |
| ****WBC (1000/mm3) |
4.4 (1.8) |
6.8 (2.9) |
0.411 |
9.3 (4.3) |
7.3 (2.8) |
0.137 |
7.4 (4.5) |
6.1 (2.8) |
0.455 |
|
Hemoglobin
(gm/dl) |
12.6 (1.8) |
12.2 (1.0) |
0.464 |
12.7 (1.7) |
12.3 (1.2) |
0.466 |
13.1 (1.6) |
13.0 (1.3) |
0.863 |
|
Platelet
(1000/mm3) |
553.2 (24) |
176 (60.8) |
0.446 |
193.6 (81.0) |
187.0 (47.9) |
0.795 |
194.7 (86.0) |
172.2 (41.4) |
0.465 |
|
Prothrombin Time
(seconds) |
16.0 (2.7) |
16.5 (4.0) |
0.555 |
14.3 (2.6) |
14.0 (1.6) |
0.686 |
13.6 (1.2) |
13.3 (0.70) |
0.553 |
| INR |
1.5 (0.5) |
1.6 (0.5) |
0.626 |
1.3 (0.5) |
1.2 (0.2) |
0.720 |
1.2 (0.3) |
1.1 (0.2) |
0.412 |
*Gamma-glutamyl transferase, **Fasting Blood Sugar, ***Erythrocyte Sedimentation Rate, **** White Blood Cell
Table 3. Complete and Partial response proportions .
| Week 12 | Week 48 | |||||
| Group A | Group B | P value | Group A | Group B | P value | |
| *Complete Response | 8 (33.3%) | 9 (60%) | 0.08 | 12 (50%) | 7 (46.7%) | 1.0 |
| **Partial Response | 3 (12.5%) | 3 (20%) | 0.65 | 1 (4.2%) | 2 (13.3%) | 0.54 |
*Complete response was defined as AST & ALT levels ≤ 40.
**Partial response was defined as a decrease in AST and ALT by less than half of their original values but not to within normal limit.
Mean serum creatinine in group-A at baseline and at week 48 were 0.81+/-0.27 mg/dl and 0.77 +/- 0.15mg/dl respectively (p =0.25). In group-B, there was a rise in serum creatinine from 0.73 +/- 0.21 mg/dl at baseline to 1.00+/-0.20 mg/dl at week 12 (p =0.01) but with continuing treatment it decreased to 0.86 +/- 0.17 mg/dl at week 48. The final (week 48) mean serum creatinine for patients in this group was not statistically different with their baseline values (p =0.15). Baseline and post-treatment values were comparable between groups as well. Mean gamma globulin level at baseline in group A was 3.7 +/- 1.5 g/dl and in group B 3.6 +/- 1.6 g/dl (p =0.86). Post-treatment mean gamma globulin levels decreased to 1.5 +/- 0.6 g/dl and 2.1 +/- 1.0 g/dl in groups A and B respectively (p=0.24). Sex and baseline AST, ALT, Alkaline phosphatase, TB, gamma globulin, platelet count, and liver biopsy findings (grade and stage according to the modified histologic activity index) were used in a multivariable regression model to see whether any baseline factor can predict response to treatment at week twelve. None of these baseline variables could predict response to treatment in our patients.
DISCUSSION
AIH is an important and treatable cause of liver damage in which corticosteroids are the current mainstay of therapy. The efficacy of prednisolone with or without azathioprine over placebo in improving outcomes for AIH has been shown in controlled trials.18, 19 At least 30% of patients treated with corticosteroids either do not respond to treatment or are urged to discontinue treatment because of intolerable adverse effects.2 Alternative treatments are not well described in the literature. A recent randomized, controlled, phase-IIb study has compared budesonide with prednisone for induction of remission in 203 AIH patients.3 In that particular study, response was defined as “normalization of biochemical tests and absence of steroid specific side-effects”. The investigators reported a 47.0% response rate in the budesonide group and 18.4% response rate in the prednisone group (p<0.001). Biochemical response (normalization of AST & ALT) was reported in 60.0 vs. 38.8% of patients in the two groups respectively (p=0.001). When less than twice upper normal limit for AST and ALT were considered as response, then the two groups were almost similar (89% vs. 81%, p=NS). All patients received azathioprine. The group treated with prednisone followed two different protocols, one a rapid prednisone tapering and another slower prednisone tapering. Specific points have not been reported for the two groups separately and they have been treated as the same in the reported analysis. Therefore, we do not know whether the two prednisone protocols differed in efficacy in this study. The authors concluded that budesonide at a dose of 3mg thrice daily is effective for induction of remission in AIH with less side effects than prednisone. Despite these results, prednisone (or prednisolone) is still considered the mainstay of treatment by most authorities. Cyclosporine has been used as salvage therapy in patients unresponsive to prednisone as well as in treatment-naïve patients.5,9-12 Alvarez et al. tried a 7 months course of CsA for induction of remission in 32 pediatric patients with AIH, two of whom did not continue treatment (one was noncompliant, and the other developed liver failure).13 Twenty-five of these 32 children had normal levels of transaminases at month six of treatment. In a group of 15 French pediatric patients (8 treatment-naïve), Debray et al. reported good response to CsA.20 The treatment naïve patients and the 5 with relapse had normal AST and ALT within 6 months of treatment with CsA. In another two non-remitting patients, addition of CsA to prednisone and azathioprine improved INR values. Our group has reported on 19 AIH patients treated with low dose CsA for 6 months, 10 of whom were treatment-naïve.5 Four patients did not complete the study. CsA led to both histological and biochemical improvement in all patients completing the study. There are several other anecdotal reports of using CsA successfully in AIH patients refractory to corticosteroids.6,9-12
Hereby we report the results of a single center RCT comparing prednisolone and CsA for induction of remission in AIH. At 3 months, 33.6%-86.9% of the prednisolone treated group and 60.0%-85.7% of the CsA treated group achieved complete response (p= 0.081, ITT analysis), despite the fact that baseline transaminases were higher in the CsA-treated group. On intention to treat analysis at this time 86.9% of patients in group-A and 85.7% of patients in group-B had achieved AST & ALT levels <2x UNL. It has been suggested that achieving normal levels of ALT with treatment is more predictable of a durable response and better long term outcomes than the previously defined criterion of achieving levels < 2x UNL,1, 21 although this has been challenged recently.4 Similar numbers of patients, i.e. almost half of patients in both groups, had achieved normal ALT levels (50.0 vs. 46.7% for groups A and B respectively, p=NS, ITT analysis) at week 48. Whether achieving this level of ALT earlier in the course of treatment may translate into better outcomes, remains to be understood. Hypertension and renal failure, the two most important adverse effects with CsA necessitating dose reduction or drug discontinuation were similar between the two groups. Serious adverse events (death due to sepsis and liver transplantation) were seen only in the prednisolone group. There was one treatment-failure in the CsA group. This patient did not respond to prednisolone either and followed a fulminant course. Frequency of other adverse effects was higher in the corticosteroid treated group. Therefore, according to our data, CsA seems to be at least as effective as prednisolone for induction of remission in treatment naïve AIH patients with a probably more favorable and more tolerable adverse event profile.
A shortcoming in our study is the relatively small number of patients. Although the number is small relative to the multicenter RCT of Manns et al.3 comparing budesonide and corticosteroids, but is comparable to other studies assessing treatment effects in AIH.22 Therefore, considering the relatively low incidence of AIH, this may be unavoidable in single center studies on AIH. Besides, this study is still ongoing and more patients have been enrolled since the writing of this paper. The other drawback in interpretation of our data may be the uneven drop off rates between the two groups. There was one patient who declined to follow in the prednislone-treated group (4.1%) while in the CsA group one patient declined to follow and two were lost to follow-up summing up to 20.0%. This may affect the interpretation of data in favor of CsA on the per-protocol analysis, but as the intention to treat analysis showed similar results for both groups and was in line with the per-protocol analysis, this uneven drop-off rate seems not to have affected our findings significantly. To the best of our knowledge, this is the first RCT comparing CsA and prednisolone head to head for induction of remission in AIH and one of the few RCTs on treatment of AIH. According to our data, CsA is at least as effective as prednisolone for this purpose with possible less side-effects. In addition, achieving normal ALT level earlier in the course of treatment with CsA is a new finding which remains to be verified and, if so, clarified whether it may end up in meaningfully better long term outcomes.
CONFLICT OF INTEREST
The authors declare no conflict of interest related to this work.
Please cite this paper as:
Nasseri-Moghaddam S, Nikfam S, Karimian S, Khashayar P, Malekzadeh R. Cyclosporine- A versus prednisolone for induction of remission in auto-immune hepatitis: Interim analysis report of a randomized controlled trial. Middle East J Dig Dis 2013;5:193-200.
References
- 1.Manns MP, Czaja AJ, Gorham JD, Krawitt EL, Mieli-Vergani G, Vergani D. Diagnosis and management of autoimmune hepatitis. Hepatology. 2010;51:2193–2213. doi: 10.1002/hep.23584. [DOI] [PubMed] [Google Scholar]
- 2.Czaja AJ. Progress in the diagnosis and treatment of autoimmune hepatitis. Minerva Med. 2008;99:549–68. [PubMed] [Google Scholar]
- 3.Manns MP, Woynarowski M, Kreisel W, Lurie Y, Rust C, Zuckerman E. et al. Budesonide induces remission more effectively than prednisone in a controlled trial of patients with autoimmune hepatitis. Gastroenterology. 2010;139:1198–206. doi: 10.1053/j.gastro.2010.06.046. [DOI] [PubMed] [Google Scholar]
- 4.van Gerven NM, Verwer BJ, Witte BI, van Hoek B, Coenraad MJ, van Erpecum KJ. et al. Relapse is almost universal after withdrawal of immunosuppressive medication in patients with autoimmune hepatitis in remission. J Hepatol. 2013;58:141–7. doi: 10.1016/j.jhep.2012.09.009. [DOI] [PubMed] [Google Scholar]
- 5.Malekzadeh R, Nasseri-moghaddam S, Kaviani MJ, Taheri H, Kamalian N, Sotoudeh M. Cyclosporin A is a promising alternative to corticosteroids in autoimmune hepatitis. Dig Dis Sci. 2001;46:1321–7. doi: 10.1023/a:1010683817344. [DOI] [PubMed] [Google Scholar]
- 6.Fernandes NF, Redeker AG, Vierling JM, Villamil FG, Fong TL. Cyclosporine therapy in patients with steroid resistant autoimmune hepatitis. Am J Gastroenterol. 1999;94:241–8. doi: 10.1111/j.1572-0241.1999.00807.x. [DOI] [PubMed] [Google Scholar]
- 7.Hennes EM, Oo YH, Schramm C, Denzer U, Buggisch P, Wiegard C. et al. Mycophenolate mofetil as second line therapy in autoimmune hepatitis. Am J Gastroenterology. 2008;103:3063–706. doi: 10.1111/j.1572-0241.2008.02180.x. [DOI] [PubMed] [Google Scholar]
- 8.Van Thiel Van Thiel.DH, WrightH WrightH, CarrollP CarrollP, Abu-Elmagd K, Rodriguez-Rilo H, McMichael J. et al. Tacrolimus: A potential new treatment for autoimmune chronic active hepatitis; results of an open-label preliminary trial. Am J Gastroenterology. 1995;90:771–6. [PMC free article] [PubMed] [Google Scholar]
- 9.Jackson LD, Song E. Cyclosporine in the treatment of corticosteroid resistant autoimmune chronic active hepatitis. Gut. 1995:459–61. doi: 10.1136/gut.36.3.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hyams JS, Ballow M, Leichtner AM. Cyclosporine treatment of autoimmune chronic active hepatitis. Gastroenterology. 1987;93:890–3. doi: 10.1016/0016-5085(87)90454-9. [DOI] [PubMed] [Google Scholar]
- 11.Mistilis SP, Vickers CR, Darroch MH, McCarthy SW. Cyclosporin, a new treatment for autoimmune chronic active hepatitis. Med J Aust. 1985;143:463–5. doi: 10.5694/j.1326-5377.1985.tb123140.x. [DOI] [PubMed] [Google Scholar]
- 12.Sherman KE, Narkewicz M, Pinto PC. Cyclosporine in the management of corticosteroid-resistant type I autoimmune chronic active hepatitis. J Hepatol. 1994;21:1040–7. doi: 10.1016/s0168-8278(05)80615-4. [DOI] [PubMed] [Google Scholar]
- 13.Alvarez F, Ciocca M, Cañero-Velasco C, Ramonet M, de Davila MT, Cuarterolo M. et al. Short-term Cyclosporine induces a remission of autoimmune hepatitis in children. J Hepatol. 1999;30:222–7. doi: 10.1016/s0168-8278(99)80065-8. [DOI] [PubMed] [Google Scholar]
- 14.Sciveres M, Caprai S, Palla G, Ughi C, Maggiore G. Effectiveness and safety of cyclosporine as therapy for autoimmune diseases of the liver in children and adolescents. Aliment Pharmacol Ther. 2004;19:209–17. doi: 10.1046/j.1365-2036.2003.01754.x. [DOI] [PubMed] [Google Scholar]
- 15.Feutren G, Mihatsch MJ. Feutren G, Mihatsch MJRisk factors for cyclosporine-induced nephropathy in patients with autoimmune diseasesInternational Kidney Biopsy Registry of Cyclosporine in Autoimmune Diseases. N Engl J Med. 1992;326:1654–60. doi: 10.1056/NEJM199206183262502. [DOI] [PubMed] [Google Scholar]
- 16.Ishak K, Baptista A, Bianchi L, Callea F, De Groote J, Gudat F. et al. Histological grading and staging of chronic hepatitis. J Hepatol. 1995;22:696–9. doi: 10.1016/0168-8278(95)80226-6. [DOI] [PubMed] [Google Scholar]
- 17.Hennes EM, Zeniya M, Czaja AJ, Parés A, Dalekos GN, Krawitt EL. et al. Simplified criteria for the diagnosis of autoimmune hepatitis. Hepatology. 2008;48:169–76. doi: 10.1002/hep.22322. [DOI] [PubMed] [Google Scholar]
- 18.Murray-Lyon IM. Immunosuppressive therapy for chronic liver disease. J R Coll Physicians Lond. 1973;8:37–44. [PMC free article] [PubMed] [Google Scholar]
- 19.Stellon AJ, Hegarty JE, Portmann B, Williams R. Randomised controlled trial of azathioprine withdrawal in autoimmune chronic active hepatitis. Lancet. 1985;1:668–70. doi: 10.1016/s0140-6736(85)91329-7. [DOI] [PubMed] [Google Scholar]
- 20.Debray D, Maggiore G, Girardet JP, Mallet E, Bernard O. Efficacy of cyclosporin A in children with type 2 autoimmune hepatitis. J Pediatr. 1999;135:111–4. doi: 10.1016/s0022-3476(99)70339-2. [DOI] [PubMed] [Google Scholar]
- 21.Czaja AJ, Menon KV, Carpenter HA. Sustained remission after corticosteroid therapy for type 1 autoimmune hepatitis: a retrospective analysis. Hepatology. 2002;35:890–7. doi: 10.1053/jhep.2002.32485. [DOI] [PubMed] [Google Scholar]
- 22.Johnson PJ, McFarlane IG, Williams R. Azathioprine for long-term maintenance of remission in autoimmune hepatitis. N Engl J Med. 1995;333:958–63. doi: 10.1056/NEJM199510123331502. [DOI] [PubMed] [Google Scholar]