Abstract
Hepatocellular carcinoma (HCC) represents one of the most common neoplasms worldwide. Liver trasnplantation (LT) is the treatment of choice for selected group of patients with HCC.
LT is actually a consolidated therapeutic option for HCC because it cures both tumor and underlying cirrhosis.
In 1996, the publication of a pivotal prospective study on less than 50 patients, transplanted for HCC under predefined criteria (single HCC ≤ 5 cm or 3 HCC ≤ 3 cm each), the so called “Milan criteria”, showed a 4-year survival of 75%. However, the indication of LT for HCC treatment has evolved over recent years.
The possibility of an extension of Milan criteria as indication for LT is already a debated issue.
Living donor LT (LDLT) is an alternative option if waiting list is long and offers the possibility of a LT after a short time.
In this review, the current indications and results of liver transplantion for HCC have been dsicusssed.
Keywords: Liver transplantation, Hepatocellular carcinoma, Indications, Down staging, Outcomes
INTRODUCTION :
Hepatocellular Carcinoma (HCC), is the most common primary malignant liver tumor in adults and a major cause of morbidity and mortality. HCC is associated with hepatitis B and hepatitis C viruses, Wilson`s disease, Hemochromatosis, α1-antitrypsin deficiency, alcoholic cirrhosis, primary biliary cirrhosis and primary sclerosing cholangitis. These medical conditions eventually end up with cirrhosis which is risk factor for HCC.1 HCC is responsible for more than one million deaths per year and the incident is 2.5 per 100,000 in United States which is on the rise.2
Liver transplantation (LT) as a treatment for HCC falls back to late 80s and early 90s. 1, 3 -7 Early studies showed a poor outcome in term of survival and high tumor recurrence.8, 9 This was mainly due to unselectively listing the patients for LT.10 Controversy about the role of transplantation in the treatment of HCC made Bismuth et al. 11 to perform a study and compare the 3 year survival in 60 patients who underwent resection and 60 patients who received LT. They found out the overall survival rate was the same in both groups and interestingly the rate of recurrence was lower in transplantation group. LT in the patients who had up to two nodules with maximum diameter of 30 mm yield a better results than resection.11 Later in 1996 a landmark study by Mazzaferro et al.12 lead to development of enrolling criteria commonly known as Milan Criteria (MC). MC along with other criteria which will be addressed in this article, are designed to selectively enroll patients for LT and reduce the risk of mortality. Before reviewing these criteria, it is seems necessary to mention these points:
1. In addition to size and number of tumors, some other known predictors of HCC recurrence after LT are: tumor differentiation, vascular invasion and extra-hepatic invasion.13 So while in current guidelines Tumor biopsy is not an absolute requirements for diagnosis of HCC 1, 14 pathological examination can help to predict tumor recurrence and overall survival.1
2. In fact, tumor biological behavior can be used as a predictor for both tumor progression and recurrence. Patients with poor tumor biology who have a greater chance of tumor recurrence can be dropped-out from the waiting list because of progression beyond the pre-defined eligibility criteria so they will not be counted in the outcome of LT and tumor-free survival can have a falsely higher rate. So this is probable that there be a bias in the result of any LT with pre-defined criteria which does not include pre-transplant tumor biopsy and biological behavior.1
Interestingly, some studies showed that even tumor biomarkers and blood parameters have a role in predicting tumor recurrence and overall survival rate.1 These include molecular biomarkers such as: Epithelial cell adhesion molecule (EpCAM) , some micro-RNA such as miR-26 and some genes in hepatic tissue which are not involved in tumor.1, 15 Blood parameters such as PIVKA-II and α-fetoprotein (AFP) have been used in some eligibility criteria.1, 16, 17 However it is important to know that some other studies show that measuring blood parameters such as AFP lacks adequate sensitivity and specificity to diagnose and follow HCC.14, 18, 19
3. Diagnosis of HCC should be based on imaging and/or tumor biopsy. 14 Among the imaging procedures some such as contrast-enhanced ultrasonography may result in false-positive HCC in patients with cholangiocarcinoma 14 so use of contrast-enhanced CT or MRI is more validated.
4. It is important to note that after LT, patients generally receive Immunosuppressive medications which can increase the risk of developing new malignancies and thus decrease the disease-free period. However this decrease in disease-free period must not necessarily be considered as a marker for survival rate.1
Review of criteria for transplanting HCC patients (Figure 1)
Fig. 1.
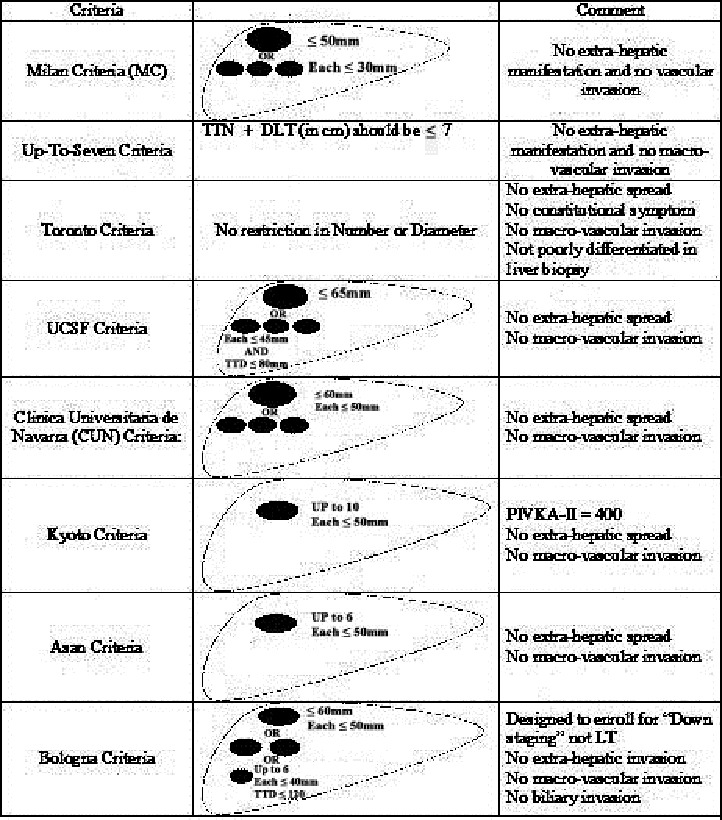
Different Criteria for LT in HCC patients
1. Milan Criteria (MC): First introduced by Mazzaferro et al.12, MC Has been used as an enrolling criteria and predictor for tumor recurrence after LT. These criteria predict a low incidence of recurrence (about 10%) for transplant patients who have solitary tumor ≤ 50 mm in diameter or up to 3 tumors each with diameter ≤ 30 mm, with no extra-hepatic manifestation and no vascular invasion. Several studies have showed the efficacy of these criteria. While these criteria were (and still are) very useful to determine the outcome of LT in patients with HCC, as time passed concerns raised that MC may be too restrictive 20 and may eliminate many patients from the waiting list who may benefit from LT. Several studies have shown that some patients with HCC outside these criteria may have a chance to benefit from LT too.21 It must be also noted that imaging has limitations such as missing very small lesions and being inable to determine tumor biological behavior.20 There are several studies which compare MC with newly developed criteria.10, 16, 20-26
2. Up-To-Seven Criteria: This criteria is derived from another study by Mazzaferro et al. They performed a retrospective study on patients with HCC exceeding the MC who had underwent LT in another centers .21Data from 1556 patients who underwent LT in a period of 10 months in 36 Centers were collected. Among them 1112 patients (71%) exceeded MC and 444 patients (28%) had HCC within MC. 21 Up-to-seven criteria is defined as the sum of the number of the tumors and diameter of the largest tumor (in cm) not exceeding seven. Also there must be no macro-vascular or extra-hepatic invasion.21 In this study, 5 years survival rate for patients with HCC within MC was 73.3% while it was 53.6% for patients with HCC beyond MC. However 5 years survival rate for patients beyond MC but with no macro-vascular invasion who were within the Up-to-seven criteria was 71.2%. Some of the findings of this study are listed in Table 1 .
Table1. Effect of criteria above Milan Criteria on outcome of Liver transplantation.
3.Toronto Criteria: In a study by Dubay et al. 20 all patients with radiological diagnosis of HCC between 1996 to 2008 who had gone under LT were evaluated. Prior to 2004 patients were mostly selected for LT, by MC or individually by surgeons` decision. From 2004 they used the so-called extended “Toronto Criteria” to select patients with HCC beyond MC. According to these Criteria patients could become candidate for LT if there was no extra hepatic spread, no systemic or constitutional symptom directly related to HCC, no macro-vascular invasion on imaging and the dominant lesion was not poorly differentiated on biopsy. These criteria had no restriction in tumor size or number.20 A group of 189 patients with HCC within MC were compared to 105 patients with HCC beyond MC. From the patients with HCC beyond MC,5 had even more advanced disease which was above Toronto Criteria but were not excluded from the list to prevent any bias. After pathology review they found out that Imaging underestimated 30% of the patients within MC and overestimated 23% of the patients beyond MC. The overall 5-year survival was 72% and five year disease-free survival was 68%. Interestingly, there was no significant difference between Patients within MC and beyond MC in terms of overall survival and disease-free survival. So they concluded that not only imaging is not a reliable tool to include or exclude patients from waiting list for LT, but patients with HCC above restricted MC can also benefit from the same survival rate from liver transplantation.20
4.UCSF Criteria: It was first described by Yao et al.10 They prospectively analyzed the outcome of 70 patients with cirrhosis and HCC who underwent LT between 1988-2000. Eleven patients had modified pathological stage pT1 HCC defined as 1 nodule ≤ 19 mm and 35 patients had stage pT2 HCC defined as 1 nodule between 20-50 mm or 2-3 nodules all ≤30 mm. Eighteen patients had modified pathological stage T3 HCC defined as 1 nodule > 50 mm or 2-3 nodules, at least one > 30 mm. Among them 3 patients had solitary tumor with mean diameter 62 mm and 15 patients had multiple (up to 3) tumors with a mean diameter of 41 mm. Six patients had modified pathological stage pT4, four had more than 4 nodules (pT4a) and two had invasion to main portal vein (pT4b).
According to this study, 5 year survival rate of patients with pT1 or pT2 HCC (72.4%) was similar to the rates reported by studies which used MC. patients with stage pT3 had a 5 year survival rate of 74.1% so they suggested that survival rate comparable to patients within MC can be achieved in patients with up to modified stage pT3 HCC who have the later known as UCSF criteria: no extra-hepatic or macro-vascular spread and a solitary tumor with diameter ≤ 65 mm or up to 3 tumors each with diameter ≤ 45 mm but with total tumor diameter (TTD) ≤ 80 mm. However patients with stage pT3 above these criteria and pT4 had a significant worse 1 year survival comparing to lower stages.10 This study was further validated by the same authors 27 and others.28
5.Clinica Universitaria de Navarra (CUN) Criteria: First introduced by Herrero et al. 29 in 2001 after they published their own experience for LT in Patients with HCC. CUN criteria included: solitary nodule with diameter ≤ 60 mm or 2-3 nodules with up to 50 mm diameter. Later in 2008 they updated the results of their previous series of LT.30 Like most of the other criteria, they used imaging to make decision for patient enrollment. Patients with any extra hepatic spread and macro-vascular invasion in imaging were excluded.
Survival rate was compared between patients with HCC who underwent LT (n=85) and patients without HCC who received LT (n=178). A comparison of patients within MC (47) to patients within CUN Criteria (24) was performed. Survival rate and tumor recurrence were not significantly different between these two groups. Five and ten year survival rates were 70% and 43% for patients within MC and 73% and 56% for patients beyond MC.
This group concluded that MC is too restrictive as patients transplanted according to their expanded criteria had comparable survival rates with patients within MC.30
6.Kyoto Criteria: Outcome of Living donor liver transplant (LDLT) in 125 patients with HCC was analyzed in a study by Ito et al.16 According to pre-transplant imaging 70 patients were within MC and 55 were not. They had previously shown that recurrence rate after LDLT in patients with HCC beyond MC are significantly higher than patients within MC. 31 They performed this study to determine the optimal expanded criteria. Their inclusion criteria for LDLT were no extra-hepatic or macro-vascular invasion. Also tumors should be unsuitable for resection or ablation therapy.16 Ninety four patients had received other treatments such as chemoembolization, hepatic resection and etc. before being listed for LDLT. At the beginning of the study there was no restriction on the number or size of the tumor. At the end of the study 88 patients remained alive and 37 died of tumor-related or unrelated (mostly infections) causes. They also evaluated serum α-fetoprotein and protein Induced by Vitamin K absence or antagonist-II ( PIVKA-II) pre-operatively. The overall survival rate was 68.3% with no significant difference between those within MC or beyond MC. Analyzing data in terms of tumor number showed that patients with 4-10 tumors had no significant difference in recurrence rate as compared to patients with ≤ 3 tumors. But patients with more than 11 tumors had a significantly higher recurrence rate. In terms of tumor size, patients with tumors > 50 mm had significantly higher recurrence rate comparing to those with smaller tumors. Analyzing the final data showed that ≥ 11 tumors, tumor diameter > 50 mm and PIVKA-II > 400 are associated with higher tumor recurrence rate. So the Kyoto Criteria, includes: ≤ 10 tumors, all ≤ 50 mm in diameter and PIVKA-II ≤ 400.16
7.Asan Criteria: It was first introduced by Lee et al.25 when they analyzed the outcome of 221 HCC patients who underwent LDLT in a single center. They were trying to simultaneously expand the enrolling criteria while not increasing the recurrence rate. Their criteria was based on explant pathology and included: ≤ 6 tumors with maximum tumor diameter of ≤ 50 mm and no extra-hepatic or macro-vascular invasion. They named this criteria the Asan criteria.
They classified patients according to MC and UCSF criteria on the basis of imaging findings and explant pathology before and after LT respectively. Also after defining their own criteria, patients were classified on the basis of imaging and pathology according to Asan criteria. One hundred sixty four patients were within MC while 4.5% and 10% more patients were within UCSF and ASAN criteria respectively. They compared the overall 5 year survival rate between those who met MC and those within ASAN criteria which were 76% and 76.3% respectively. While the 5-year survival was 44.5% for patients exceeding MC and 18.9% for patients exceeding ASAN criteria. About 13.6% of patients within MC and 9.1% of the patients beyond MC but within ASAN criteria had tumor recurrence in 3 years, while the recurrence was 73.6% beyond ASAN criteria.25
8.Bologna Criteria: Ravaioli et al. 32performed a study on patients with HCC but in contrast to the previously mentioned studies they did not aim to expand MC. Instead they enrolled patients beyond MC to “down staging” protocols and consequently enrolled the ones who met MC for LT. Their criteria for down-staging was: One nodule ≤ 60 mm or 2 nodules ≤ 50 mm or less than 6 nodules ≤ 40 mm, sum of the diameters ≤ 120 mm and no macro-vascular, biliary or extra-hepatic invasion.32 We will discuss this article later in “down staging” discussion.
The concept of “Metro ticket”
A quick look at different criteria shows that outcomes continuously worsen with further increasing the size or number of the tumor. Hence the “Metro ticket” concept was introduced: “the longer the distance, the higher the price”.1 After a study was presented by Mazzaferro et al. in 2006, a web-based survey for data collection of patients with HCC who received LT was proposed. This website contains a software named “Metro ticket calculator” which can predict a 3 year or 5 year survival rate of the patients based on characteristic of their HCC.21
Living donor liver transplantation (LDLT) for HCC
The biggest challenge in organ transplantation is organ shortage. 33 On the other hand by expanding the enrolling criteria the number of patients eligible for LT is increasing which can increase the waiting time and subsequently dropout rate. LDLT can be a solution for these challenges. When considering LDLT, the first additional risk which comes to mind is donor morbidity or mortality.1 Since 2002, the numbers of LDLT has decreased in US.33 This could be due to less income during post-operative recovery and expenses which might not be paid by insurance companies.33 Interestingly, while there is a trend for expanding the eligibility criteria for LDLT, the main enrolling criteria including MC and UCSF are originally derived from experiences with DDLT.
As a general rule, similar eligibility criteria are used for both DDLT and LDLT. Patients on waiting list for DDLT may experience a longer period of waiting between listing and LT so patients who have poor tumor biological behavior are more likely to be dropped out because of tumor progression. Patients on the waiting list for LDLT may not experience such a waiting time and even patients with poor tumor biological behavior may receive LT and subsequently show increased rate of recurrence and worse outcome.1
For years it was controversial whether early graft regeneration after LDLT can have any adverse effects on the recurrence of HCC or not.34 Hwang et al. 34 tried to assess applicability of selection criteria for DDLT to LDLT. They performed a multicenter analysis of the outcome of 312 HCC patients who underwent DDLT or LDLT from 1992-2002 in Korea. There were no gross differences of tumor characteristic between patients in these two groups. HCC recurrence rate was 18% in patients receiving DDLT and 15.5% in patients receiving LDLT. There were no statistical differences in recurrence rate between these two groups. The overall 3 year survival rate was 61.1% after DDLT and 73.2% after LDLT. This study was further validated by Di Sandro et al. 35
In a study by Yao et al.36 among the 168 patients with HCC who received LT, 26 patients (15.5%) received LDLT. Among the patients who received LDLT, univariate analysis showed 1 and 3.5 years recurrence-free survival rates of 87.5% and 56.2% respectively while the 1 and 5 years recurrence-free survival rate were 93.1% and 88.9% respectively among patients who received DDLT. But when tumor stage was factored in analysis, the 19 patients who received LDLT and did not exceed pT3A had a 1 and 3 years survival rate similar to those who received DDLT.36
Other therapeutic interventions for HCC
Current therapies for HCC can be divided into two groups: surgical interventions including LT and liver resection (LR) and non-surgical modalities which are generally known as loco-regional therapies. Some of the loco-regional therapies for HCC include trans-arterial chemoembolization (TACE), ethanol injection (EI) and RFA. These treatments are usually performed for patients within Child B or C category who are not selected for LR.2, 37
Liver resection and loco-regional therapies may be used either as “primary”, “bridging” or “down-staging” therapy depending on the tumor size, number and other tumor characteristics.
Role of Radiofrequency ablation: This procedure can not only be used percutaneously, but in patients with compensated liver function can also be used laparoscopically or during laparotomy.36 Many studies have showed the efficacy of RFA in treatment of small HCCs. However almost all these studies were designed for “bridging therapy” or “down staging therapy” and have not approached it as a curative treatment.1
Livraghi et al.38 showed that RFA can be the treatment of choice for patients with single tumor ≤ 20 mm, even when surgical resection is possible, due to lower complication, lower cost and similar survival rate.38
In another study by Lu et al. 39 24 patients with 47 HCC nodules underwent RFA before LT. Thirty five tumors had diameter less than 30 mm and 29/35 (83%) of them were successfully treated. Overall 35/47 nodules (74%) were successfully treated. Mean maximal diameter for nodules successfully treated and unsuccessfully treated were 20 mm and 31 mm respectively. RFA has recently gained more attention and popularity as it may cause complete tumor necrosis with fewer treatment sessions as compared to ethanol injection.36
Role of Trans arterial chemo embolization (TACE): There is good evidence indicating that TACE can be used for management of unresectable HCC 40 but it is important to note that it cannot be used as a primary curative procedure.1 In fact its main role is in “bridging” or “down staging” therapy.
Although in some studies TACE has been shown to prolong the survival rate in patients who are not candidates for curative treatment, 41 there are others which do not show any survival benefit 42 which could be related to the fact that they only included patients younger than 65 years old.41 So Cohen et al. 41 evaluated the outcome of 102 patients with HCC who underwent TACE between 2001 to 2010. All patients who received TACE as sole treatment were included. They followed the patients until 2012. From 102 patients, 38 (37.2%) were younger than 65 years old, 41 (40.2%) between 65 and 75 and 23 (22.5%) ≥ 75 years old. Data analysis showed no difference in survival among older and younger patients. There were no differences in post-procedure complications. They also observed that there was no significant difference between these 3 groups regarding increase in creatinine level after procedure which could reflect contrast-induced nephropathy. They concluded that age is not a risk factor for adverse effects after TACE.41
Role of Liver resection (LR): As a primary treatment, LR has the advantage of no waiting list. It also has lower operation-related morbidity and mortality, shorter recovery time and no requirement for immunosuppression.1, 2, 43, 44 However several factors should be evaluated before making a patient a candidate for LR. These factors include anatomical position such as existence of bi-lobar tumor, central positioning, proximity to major vessels and medical situations such as portal hypertension and Child`s classification for liver cirrhosis. All these problems restrict the use of LR.1 In a study by Ishizawa et al. it was shown that measurement of portal pressure is a useful tool for both selection of the patients for LR and prediction of outcome. However they concluded than even with existence of portal hypertension, LR can be performed.45
In a study by Shabahang et al. the outcome of patients with liver cirrhosis who received LT (n=65) were compared to those who underwent LR(n=44).2 The length of operation was much less in the LR group. Seventy percent of the LR group were extubated in the operation room and the majority of the patients who were admitted in ICU were discharged after one night. But all patients in the LT group spent at least 3 nights in ICU. While the LR group had more advanced tumors, There was no significant difference in overall survival between the two groups.2
Some studies suggest that for patients with HCC who do not have cirrhosis, LR is the treatment of choice vs. LT.2, 46 Patients in Child’s category B or C usually are not good candidates for LR as advanced cirrhosis can increase complications such as infection or hemorrhage.2, 47 But patients in Child’s A category can be candidate for either LT or LR .2 There are many studies comparing LT and LR in this group.2, 43, 48, 49
It must be considered that LR can also be used as a bridging therapy before LT.1 Furthermore, it seems reasonable that patients who are at early stages of HCC first undergo LR and then, if recurrence occurs, LT. As such they will receive a potentially curative treatment while avoiding the waiting list of LT and allowing patients who are at actual need for LT to receive the graft more rapidly.1
Bridging and down-staging therapy:
“Bridging therapy” is defined as any intervention aimed at maintaining the tumor within the pre-defined eligibility criteria while the patient is waiting for LT. This can reduce the wait list dropout.1 The overall dropout from the waiting list for liver transplantation is about 22%. With bridging therapy this can be increased to 75% after 18 months in waiting list.50-52 However, centers using expanded criteria vs. MC, can enroll more patients for LT. This can result in increasing the number of candidates for LT and subsequently increasing the wait time and inevitably increasing dropout.51
One early experience of TACE for bridging therapy was reported by Spreafico.53 In this study 21 patients had 33 HCC nodules and after TACE 12/33 nodules (36%) had more than 90% necrosis defined as complete response. Harnois et al.54 introduced TACE as a procedure with minimal systemic toxicity which has antitumor effect and the ability to minimize tumor progression before LT. All of the 27 patients in this study had less than 3 tumors and each ≤ 50 mm. There was no extra-hepatic spread or vascular invasion. Twenty four patients completed the TACE protocol and received LT. The 2 years disease-free survival was 84%.54
Frangakis et al.51 performed a study on 87 patients with HCC who were listed for LT from 2001-2008. All patients were within MC. Their primary aim was to compare waiting list dropout between patients who receive TACE (n=35) and the ones who do not receive it before transplantation (n=52). The risk of dropping off the waiting list was 15% for the non-TACE group and 3% for the TACE group.51
Some earlier experiences with pre-transplant bridging therapy did not have favorable results.55 In some of them the authors believed that pre-transplant TACE not only does not increase survival rate but also may put patients at increased risk for complication.56
Oldhafer et al. 56 compared 21 patients with HCC who received pre-transplant TACE (group 1) with 21 patients who did not (group 2). They reported no difference in survival rate between two groups. Although 14/21 patients in the TACE group had marked tumor necrosis (> 50%) but as these patients had no benefit in survival compared to the other group and also seemed to be at higher risk of developing complications, the authors concluded that bridging therapy with TACE might be not as effective as it is claimed to be.
Similarly, Perez Saborido et al.55 performed a study on 46 patients undergoing LT for HCC. They divided patients into 2 groups: group 1 who underwent pre-transplant TACE (n=18, 39.1%) and group 2 who did not receive TACE (n=28, 60.9%). The recurrence rate, 3 and 5 year survival rate and 5 year disease-free survival rate were not significantly different between the 2 groups. They finally concluded that although TACE is a safe procedure, it does not significantly improve tumor recurrence or survival rate.55
The use of multimodal pre-transplant therapies are probably more likely to help subjects than single modality.1 Yao et al.36 performed a study on 168 patients with HCC who received LT. Twenty six patients (15.5%) received LDLT. Decision for Pre-transplant loco-regional therapy was made on a case by case basis. Seventy three patients (43.5%) received one or more TACE session. Fifteen patients (8.9%) received ablation including percutaneous ethanol injection (PEI), RFA, percutaneous acetic acid injection and open cryoablation. Fifteen patients (8.9%) received combined TACE and ablation therapy (either percutaneous RFA or PEI). Sixty five patients (38.7%) received no specific treatment before LT. The 5 years recurrence-free survival rate was not significantly different between the patients with pT1, pT2 and pT3A. But the 5 years recurrence-free survival rate in patients with pT3B and pT4 was significantly different than lower stages. In fact the 5 years recurrence-free survival rate was 93.9% for 141 patients who were within pre-defined (UCSF) expanded criteria (pT1, pT2 and pT3A) and 42.6% for the patients who exceeded these criteria (pT3B and pT4). They also compared the survival rate between patients who received pre-transplant bridging therapy and the ones who did not. As the patients with pT1 had no recurrence, the impact of bridging therapy was not compared in pT1 subgroups. Among the 90 patients with pT2, There were no difference in 5 year recurrence-free survival rate in 56 patients who received loco regional therapy and 34 patients who did not receive any treatment before LT (p=0.8). Among the 36 patients with pT3, The 5 year recurrence-free survival rate in patients who received loco regional therapy was significantly better than the patients who did not receive any treatment before LT.36 The authors concluded that pre-transplant loco regional therapy results in increased survival after LT.
Cillo et al.13 also reported the results of their study on 100 patients with HCC including 60 within MC and 40 beyond MC. When patients had acceptable liver function, they underwent a laparoscopic or laparotomy treatment procedure including LR, ethanol injection, radio frequency ablation or TACE. Among the patients on the waiting list, 83 received at least one treatment and the other 17 did not receive therapy due to hepatic impairment or other reasons. Patients who were beyond MC received more number of procedures per patient. During the study 68 patients received LT, 12 were removed from the waiting list (death, poorly differentiated tumor, macrovascular invasion) and 20 were still on the waiting list. The rate of LT was higher in patients beyond MC (78%) comparing to patients within MC (62%). Overall 5/40 patients (12.5%) beyond MC and 7/60 patients (11.7%) within MC were dropped out while on the waiting list. The survival rate after 3 and 5 years were 85% and 79% in patients beyond MC and 69% and 69% for the ones within MC. They also divided the patients beyond MC in two groups: Patients who were beyond MC but within UCSF criteria (UCSF IN=16) and the ones who were also beyond UCSF criteria (UCSF OUT=24). Table 2 shows the outcome of the comparison between these two groups (Table 2 ).
Table 2. Outcomes of HCC patients within and beyond UCSF criteria .
| Variables | UCSF IN (n=16) | UCSF OUT (n=24) |
| Number of LT | 13 (81%) | 18 (75%) |
| Number of drop out | 2 (12%) | 3 (12%) |
| 3 years survival rate | 85% | 85% |
| 5 years survival rate | 85% | 76% |
So they concluded that even the expanded UCSF criteria is not a significant variable for rate of LT, drop out and survival.
“Down-staging” is defined as any intervention to bring the tumor which is beyond the pre-defined eligibility criteria, down to be within those criteria and therefore making the patient a candidate for LT.1 While several articles show the effectiveness of therapies such as RFA, ethanol injection and trans-arterial chemoembolization as a “bridging” therapy, the effectiveness of these interventions are not very clear for “down staging”.57
Before patient undergo down-staging several factors should be assessed including a predefined period of stability. This minimum period of stability ensures that patients with tumors with unfavorable biological behavior who are at increased risk of recurrence after LT are excluded from the list of down-staging.1 In fact the success of down staging a tumor to predefined eligibility criteria may reflect a more favorable tumor biology and subsequently lower recurrence rate.50
For a considerable period of time regional ablation therapy and TACE were only used for HCC patients who were not candidate for either LR or LT. Nowadays these treatments are also being used for down staging before LT.
Chapman et al. performed a study to evaluate the outcome of down-staging of HCC patients with TACE.57 Of the 76 patients who were candidate for down-staging, 27 patients (35.5%) had partial response (defined as 30% decrease in the sums of longest tumor`s diameters), 27 (35.5%) had progressive disease (defined as 20% increase in the sums of longest tumor diameters) and 22 (29%) had stable disease (defined as everything between partial response and progressive disease). Only 23.7% of the patients who were candidate for down-staging were successfully down-staged to MC and became candidate for LT. But among those with partial response, 18 out of 27 had tumor “down staging” to stage II to be eligible to enroll for LT under MC.57 This rate is significantly different from the rate reported by Ravaioli et al.32 In contrast to the study by Chapman, patients could be beyond MC but had to have single tumor ≤ 60 mm or 2 tumors ≤ 50 mm or less than 6 tumors ≤ 40 mm and sum of the diameters ≤ 120 mm. Macro-vascular, biliary or extra-hepatic invasion were excluded. The patients had to meet MC after down staging to be candidate for LT. Again in contrast to the study by Chapman, they used several techniques for down staging including: TACE, LR and RFA depending on the anatomical location of the tumor and liver function. From 2003 to 2006, 177 patients were considered for LT. However the success rate of down staging was reported to be 90%. Only 5/48 patients could not complete the down staging protocol (died) mainly because of tumor progression. But the rate of exclusion for tumor progression before LT was significantly higher in down staging group (13/48, 27.1%) comparing to Milan group (15/129, 11.6%). The rate of LT was 88/129 (68%) in Milan group and 32/48 (67%) in down staging group. 17 out of 120 patients who received LT had tumor recurrence (14%). The 3 years recurrence-free survival rate was 83% and 75% in Milan group and down staging group respectively and 3 years disease-free survival rate was 71% in both.32
Jang et al. 50 also performed a study on patients with HCC beyond MC. They used TACE for down staging. Among their 386 patients, 160 (41.5%) could be successfully down staged to MC. In fact 90 patients (56.3%) had complete necrosis of all tumors after TACE and 70 patients while still having viable tumor had marked tumor regression “down” to MC. Of the 160 patients who were successfully down staged, 37 patients received LT (including 30 LDLT and 7 DDLT) and 8 patients underwent LR. Among the remaining 115 patients in the waiting list, 82 patients were dropped off the list (mainly because of tumor progression) and 33 were still on the LT list at the time of publishing the article. The overall 2 and 5 years post-transplant survival rate were 70.3% and 54.6% respectively and the 2 and 5 years recurrence-free survival rate were 71.8% and 66.3%. Multivariate analysis showed that increased AFP beyond 100 ng/mL, tumor diameter ≥ 70 mm and lack of complete necrosis by TACE are predictors for tumor recurrence. Patients who had none of these risk factors had up to 6 years recurrence-free survival rate of 87.5%.50
CONCLUSION
Liver transplantation is now considered the best treatment option for HCC and cirrhosis for patients who fulfill the eligibility criteria. In addition to the size or number of tumor. Ttumor biological behavior is an important predictor for tumor progression and post-transplant survival and recurrence. Therefore, attempts to develop new expanded eligibility criteria which can also reduce unnecessary exclusions of potential candidates seem to be reasonable.
CONFLICT OF INTEREST
The authors declare no conflict of interest related to this work.
Please cite this paper as:
Saidi RF, Hejazi Kenari SK. Liver Transplantation for Hepatocellular Carcinoma: Past,Present and Future. Middle East J Dig Dis 2013;5:181-92.
References
- 1.Maggs JRL, Suddle AR, Aluvihare V, Heneghan MA. Systematic review: the role of liver transplantation in the management of hepatocellular carcinoma. Aliment Pharmacol Ther. 2012;35:1113–34. doi: 10.1111/j.1365-2036.2012.05072.x. [DOI] [PubMed] [Google Scholar]
- 2.Maggs JRL, Suddle AR, Aluvihare V, Heneghan MA. Systematic review: the role of liver transplantation in the management of hepatocellular carcinoma. Aliment Pharmacol Ther. 2012;35:1113–34. doi: 10.1111/j.1365-2036.2012.05072.x. [DOI] [PubMed] [Google Scholar]
- 3.Haug CE, Jenkins RL, Rohrer RJ, Auchincloss H, Delmonico FL, Freeman RB. et al. Liver transplantation for primary hepatic cancer. Transplantation. 1992;53:376–82. doi: 10.1097/00007890-199202010-00021. [DOI] [PubMed] [Google Scholar]
- 4.Selby R, Kadry Z, Carr B, Tzakis A, Madariaga JR, Iwatsuki S. Liver transplantation for hepatocellular carcinoma. World J Surg. 1995;19:53–8. doi: 10.1007/BF00316980. [DOI] [PubMed] [Google Scholar]
- 5.Yokoyama I, Todo S, Iwatsuki S, Starzl TE. Liver transplantation in the treatment of primary liver cancer. Hepatogastroenterology. 1990;37:188–93. [PMC free article] [PubMed] [Google Scholar]
- 6.Onaca N, Davis GL, Jennings LW, Goldstein RM, Klintmalm GB. Improved results of transplantation for hepatocellular carcinoma: a report from the International Registry of Hepatic Tumors in Liver Transplantation. Liver Transpl. 2009;15:574–80. doi: 10.1002/lt.21738. [DOI] [PubMed] [Google Scholar]
- 7.Pichlmayr R, Weimann A, Steinhoff G, Ringe B. Liver transplantation for hepatocellular carcinoma: clinical results and future aspects. Cancer Chemother Pharmacol. 1992;31 Suppl:S157–61. doi: 10.1007/BF00687127. [DOI] [PubMed] [Google Scholar]
- 8.Penn I. Hepatic transplantation for primary and metastatic cancers of the liver. Surgery. 1991;110:72634. discussion 734.35. [PubMed] [Google Scholar]
- 9.Pichlmayr R, Weimann A, Ringe B. Indications for liver transplantation in hepatobiliary malignancy. Hepatology. 1994;20:33S–40S. doi: 10.1016/0270-9139(94)90271-2. [DOI] [PubMed] [Google Scholar]
- 10.Yao FY, Ferrell L, Bass NM, Watson JJ, Bacchetti P, Venook A. et al. Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology. 2001;33:1394–403. doi: 10.1053/jhep.2001.24563. [DOI] [PubMed] [Google Scholar]
- 11.Bismuth H, Chiche L, Adam R, Castaing D, Diamond T, Dennison A. Liver resection versus transplantation for hepatocellular carcinoma in cirrhotic patients. Ann Surg. 1993;218:145–51. doi: 10.1097/00000658-199308000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F. et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693–9. doi: 10.1056/NEJM199603143341104. [DOI] [PubMed] [Google Scholar]
- 13.Cillo U, Vitale A, Grigoletto F, Gringeri E, D’Amico F, Valmasoni M. et al. Intention-to-treat analysis of liver transplantation in selected, aggressively treated HCC patients exceeding the Milan criteria. Am J Transplant. 2007;7:972–81. doi: 10.1111/j.1600-6143.2006.01719.x. [DOI] [PubMed] [Google Scholar]
- 14.Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–2. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Llovet JM, Paradis V, Kudo M, Zucman-Rossi J. Tissue biomarkers as predictors of outcome and selection of transplant candidates with hepatocellular carcinoma. Liver Transpl. 2011;17 Suppl 2:S67–71. doi: 10.1002/lt.22340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ito T, Takada Y, Ueda M, Haga H, Maetani Y, Oike F. et al. Expansion of selection criteria for patients with hepatocellular carcinoma in living donor liver transplantation. Liver Transplant Soc. 2007;13:1637–44. doi: 10.1002/lt.21281. [DOI] [PubMed] [Google Scholar]
- 17.Kwon CHD, Kim DJ, Han YS, Park JB, Choi GS, Kim SJ. et al. HCC in living donor liver transplantation: can we expand the Milan criteria? Dig Dis. 2007;25:313–9. doi: 10.1159/000106911. [DOI] [PubMed] [Google Scholar]
- 18.Singal A, Volk ML, Waljee A, Salgia R, Higgins P, Rogers MAM. et al. Meta-analysis: surveillance with ultrasound for early-stage hepatocellular carcinoma in patients with cirrhosis. Aliment Pharmacol Ther. 2009;30:37–47. doi: 10.1111/j.1365-2036.2009.04014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lok AS, Sterling RK, Everhart JE, Wright EC, Hoefs JC, Di Bisceglie AM. et al. Des-gamma-carboxy prothrombin and alpha-fetoprotein as biomarkers for the early detection of hepatocellular carcinoma. Gastroenterology. 2010;138:493–502. doi: 10.1053/j.gastro.2009.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.DuBay D, Sandroussi C, Sandhu L, Cleary S, Guba M, Cattral MS. et al. Liver transplantation for advanced hepatocellular carcinoma using poor tumor differentiation on biopsy as an exclusion criterion. Ann Surg. 2011;253:166–72. doi: 10.1097/sla.0b013e31820508f1. [DOI] [PubMed] [Google Scholar]
- 21.Mazzaferro V, Llovet JM, Miceli R, Bhoori S, Schiavo M, Mariani L. et al. Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: a retrospective, exploratory analysis. Lancet Oncol. 2009;10:35–43. doi: 10.1016/S1470-2045(08)70284-5. [DOI] [PubMed] [Google Scholar]
- 22.Sugawara Y, Tamura S, Makuuchi M. Living donor liver transplantation for hepatocellular carcinoma: Tokyo University series. Dig Dis. 2007;25:310–2. doi: 10.1159/000106910. [DOI] [PubMed] [Google Scholar]
- 23.Jonas S, Mittler J, Pascher A, Schumacher G, Theruvath T, Benckert C. et al. Living donor liver transplantation of the right lobe for hepatocellular carcinoma in cirrhosis in a European center. Liver Transpl. 2007;13:896–903. doi: 10.1002/lt.21189. [DOI] [PubMed] [Google Scholar]
- 24.Takada Y, Ito T, Ueda M, Sakamoto S, Haga H, Maetani Y. et al. Living donor liver transplantation for patients with HCC exceeding the Milan criteria: a proposal of expanded criteria. Dig Dis. 2007;25:299–302. doi: 10.1159/000106908. [DOI] [PubMed] [Google Scholar]
- 25.Lee S-G, Hwang S, Moon DB, Ahn CS, Kim KH, Sung KB. et al. Expanded indication criteria of living donor liver transplantation for hepatocellular carcinoma at one large-volume center. Liver Transpl. 2008;14:935–45. doi: 10.1002/lt.21445. [DOI] [PubMed] [Google Scholar]
- 26.Zheng SS, Xu X, Wu J, Chen J, Wang W-L, Zhang M. et al. Liver transplantation for hepatocellular carcinoma: Hangzhou experiences. Transplantation. 2008;85:1726–32. doi: 10.1097/TP.0b013e31816b67e4. [DOI] [PubMed] [Google Scholar]
- 27.Yao FY, Xiao L, Bass NM, Kerlan R, Ascher NL, Roberts JP. Liver transplantation for hepatocellular carcinoma: validation of the UCSF-expanded criteria based on preoperative imaging. Am J Transplant. 2007;7:2587–96. doi: 10.1111/j.1600-6143.2007.01965.x. [DOI] [PubMed] [Google Scholar]
- 28.Duffy JP, Vardanian A, Benjamin E, Watson M, Farmer DG, Ghobrial RM. et al. Liver transplantation criteria for hepatocellular carcinoma should be expanded: a 22-year experience with 467 patients at UCLA. Ann Surg. 2007;246:502–9; discussion 509. doi: 10.1097/SLA.0b013e318148c704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Herrero JI, Sangro B, Quiroga J, Pardo F, Herraiz M, Cienfuegos JA. et al. Influence of tumor characteristics on the outcome of liver transplantation among patients with liver cirrhosis and hepatocellular carcinoma. Liver Transpl. 2001;7:631–6. doi: 10.1053/jlts.2001.25458. [DOI] [PubMed] [Google Scholar]
- 30.Herrero JI, Sangro B, Pardo F, Quiroga J, Iñarrairaegui M, Rotellar F. et al. Liver transplantation in patients with hepatocellular carcinoma across Milan criteria. Liver Transpl. 2008;14:272–8. doi: 10.1002/lt.21368. [DOI] [PubMed] [Google Scholar]
- 31.Takada Y, Ueda M, Ito T, Sakamoto S, Haga H, Maetani Y. et al. Living donor liver transplantation as a second-line therapeutic strategy for patients with hepatocellular carcinoma. Liver Transpl Am J Transplant. 2006;12:912–9. doi: 10.1002/lt.20642. [DOI] [PubMed] [Google Scholar]
- 32.Ravaioli M, Grazi GL, Piscaglia F, Trevisani F, Cescon M, Ercolani G. et al. Liver transplantation for hepatocellular carcinoma: results of down-staging in patients initially outside the Milan selection criteria. Am J Transplant. 2008;8:2547–57. doi: 10.1111/j.1600-6143.2008.02409.x. [DOI] [PubMed] [Google Scholar]
- 33.Saidi RF. Current status of liver transplantation. Arch Iran Med. 2012;15:772–6. [PubMed] [Google Scholar]
- 34.Hwang S, Lee S-G, Joh J-W, Suh K-S, Kim D-G. Liver transplantation for adult patients with hepatocellular carcinoma in Korea: comparison between cadaveric donor and living donor liver transplantations. Liver Transpl. 2005;11:1265–72. doi: 10.1002/lt.20549. [DOI] [PubMed] [Google Scholar]
- 35.Di Sandro S, Slim AO, Giacomoni A, Lauterio A, Mangoni I, Aseni P. et al. Living donor liver transplantation for hepatocellular carcinoma: long-term results compared with deceased donor liver transplantation. Transplant Proc. 2009;41:1283–5. doi: 10.1016/j.transproceed.2009.03.022. [DOI] [PubMed] [Google Scholar]
- 36.Yao FY, Kinkhabwala M, LaBerge JM, Bass NM, Brown R Jr, Kerlan R. et al. The impact of pre-operative loco-regional therapy on outcome after liver transplantation for hepatocellular carcinoma. Am J Transplant. 2005;5:795–804. doi: 10.1111/j.1600-6143.2005.00750.x. [DOI] [PubMed] [Google Scholar]
- 37.Hanazaki K, Kajikawa S, Shimozawa N, Mihara M, Shimada K, Hiraguri M. et al. Survival and recurrence after hepatic resection of 386 consecutive patients with hepatocellular carcinoma. J Am Coll Surg. 2000;191:381–8. doi: 10.1016/s1072-7515(00)00700-6. [DOI] [PubMed] [Google Scholar]
- 38.Livraghi T, Meloni F, Di Stasi M, Rolle E, Solbiati L, Tinelli C. et al. Sustained complete response and complications rates after radiofrequency ablation of very early hepatocellular carcinoma in cirrhosis: Is resection still the treatment of choice? Hepatology. 2008;47:82–9. doi: 10.1002/hep.21933. [DOI] [PubMed] [Google Scholar]
- 39.Lu DSK, Yu NC, Raman SS, Limanond P, Lassman C, Murray K. et al. Radiofrequency ablation of hepatocellular carcinoma: treatment success as defined by histologic examination of the explanted liver. Radiology. 2005;234:954–60. doi: 10.1148/radiol.2343040153. [DOI] [PubMed] [Google Scholar]
- 40.Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: Chemoembolization improves survival. Hepatology. 2003;37:429–42. doi: 10.1053/jhep.2003.50047. [DOI] [PubMed] [Google Scholar]
- 41.Cohen MJ, Bloom AI, Barak O, Klimov A, Nesher T, Shouval D. et al. Trans-arterial chemo-embolization is safe and effective for very elderly patients with hepatocellular carcinoma. World J Gastroenterol. 2013;19:2521–8. doi: 10.3748/wjg.v19.i16.2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Oliveri RS, Wetterslev J, Gluud C. Transarterial (chemo)embolisation for unresectable hepatocellular carcinoma. Cochrane Database Syst Rev Online 2011:CD004787. [DOI] [PubMed]
- 43.Yamamoto J, Iwatsuki S, Kosuge T, Dvorchik I, Shimada K, Marsh JW. et al. Should hepatomas be treated with hepatic resection or transplantation? Cancer. 1999;86:1151–8. doi: 10.1002/(sici)1097-0142(19991001)86:7<1151::aid-cncr8>3.0.co;2-v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Margarit C, Escartín A, Castells L, Vargas V, Allende E, Bilbao I. Resection for hepatocellular carcinoma is a good option in Child-Turcotte-Pugh class A patients with cirrhosis who are eligible for liver transplantation. Liver Transpl. 2005;11:1242–51. doi: 10.1002/lt.20398. [DOI] [PubMed] [Google Scholar]
- 45.Ishizawa T, Hasegawa K, Aoki T, Takahashi M, Inoue Y, Sano K. et al. Neither multiple tumors nor portal hypertension are surgical contraindications for hepatocellular carcinoma. Gastroenterology. 2008;134:1908–16. doi: 10.1053/j.gastro.2008.02.091. [DOI] [PubMed] [Google Scholar]
- 46.El-Gazzaz G, Wong W, El-Hadary MK, Gunson BK, Mirza DF, Mayer AD. et al. Outcome of liver resection and transplantation for fibrolamellar hepatocellular carcinoma. Transpl Int. 2000;13 Suppl 1:S406–9. doi: 10.1007/s001470050372. [DOI] [PubMed] [Google Scholar]
- 47.Farges O, Malassagne B, Flejou JF, Balzan S, Sauvanet A, Belghiti J. Risk of major liver resection in patients with underlying chronic liver disease: a reappraisal. Ann Surg. 1999;229:210–5. doi: 10.1097/00000658-199902000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Baccarani U, Isola M, Adani GL, Benzoni E, Avellini C, Lorenzin D. et al. Superiority of transplantation versus resection for the treatment of small hepatocellular carcinoma. Transpl Int. 2008;21:247–54. doi: 10.1111/j.1432-2277.2007.00597.x. [DOI] [PubMed] [Google Scholar]
- 49.Otto G, Heuschen U, Hofmann WJ, Krumm HG, Hinz U, Herfarth C. Is transplantation really superior to resection in the treatment of small hepatocellular carcinoma? Transplant Proc. 1997;29:489–91. doi: 10.1016/s0041-1345(96)00220-5. [DOI] [PubMed] [Google Scholar]
- 50.Jang JW, You CR, Kim CW, Bae SH, Yoon SK, Yoo YK. et al. Benefit of downsizing hepatocellular carcinoma in a liver transplant population. Aliment Pharmacol Ther. 2010;31:415–23. doi: 10.1111/j.1365-2036.2009.04167.x. [DOI] [PubMed] [Google Scholar]
- 51.Frangakis C, Geschwind J-F, Kim D, Chen Y, Koteish A, Hong K. et al. Chemoembolization decreases drop-off risk of hepatocellular carcinoma patients on the liver transplant list. Cardiovasc Intervent Radiol. 2011;34:1254–61. doi: 10.1007/s00270-010-0077-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wiesner RH, Freeman RB, Mulligan DC. Liver transplantation for hepatocellular cancer: the impact of the MELD allocation policy. Gastroenterology. 2004;127(5 Suppl 1):S261–7. doi: 10.1053/j.gastro.2004.09.040. [DOI] [PubMed] [Google Scholar]
- 53.Spreafico C, Marchianò A, Regalia E, Frigerio LF, Garbagnati F, Andreola S. et al. Chemoembolization of hepatocellular carcinoma in patients who undergo liver transplantation. Radiology. 1994;192:687–90. doi: 10.1148/radiology.192.3.8058934. [DOI] [PubMed] [Google Scholar]
- 54.Harnois DM, Steers J, Andrews JC, Rubin JC, Pitot HC, Burgart L. et al. Preoperative hepatic artery chemoembolization followed by orthotopic liver transplantation for hepatocellular carcinoma. Liver Transpl. 1999;5:192–9. doi: 10.1002/lt.500050307. [DOI] [PubMed] [Google Scholar]
- 55.Pérez Saborido B, Meneu JC, Moreno E, García I, Moreno A, Fundora Y. Is transarterial chemoembolization necessary before liver transplantation for hepatocellular carcinoma? Am J Surg. 2005;190:383–7. doi: 10.1016/j.amjsurg.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 56.Oldhafer KJ, Chavan A, Frühauf NR, Flemming P, Schlitt HJ, Kubicka S. et al. Arterial chemoembolization before liver transplantation in patients with hepatocellular carcinoma: marked tumor necrosis, but no survival benefit? J Hepatol. 1998;29:953–9. doi: 10.1016/s0168-8278(98)80123-2. [DOI] [PubMed] [Google Scholar]
- 57.Chapman WC, Majella Doyle MB, Stuart JE, Vachharajani N, Crippin JS, Anderson CD. et al. Outcomes of neoadjuvant transarterial chemoembolization to downstage hepatocellular carcinoma before liver transplantation. Ann Surg. 2008;248:617–25. doi: 10.1097/SLA.0b013e31818a07d4. [DOI] [PubMed] [Google Scholar]
- 58.Befeler AS, Hayashi PH, Di Bisceglie AM. Liver transplantation for hepatocellular carcinoma. Gastroenterology. 2005;128:1752–64. doi: 10.1053/j.gastro.2005.03.033. [DOI] [PubMed] [Google Scholar]


