Abstract
Mutations in TAR DNA-binding protein 43 (TDP-43) are associated with familial forms of amyotrophic lateral sclerosis and frontotemporal lobar degeneration. Although recent studies have revealed that mutant TDP-43 in neuronal and glial cells is toxic, how mutant TDP-43 causes primarily neuronal degeneration in an age-dependent manner remains unclear. Using adeno-associated virus (AAV) that expresses mutant TDP-43 (M337V) ubiquitously, we found that mutant TDP-43 accumulates preferentially in neuronal cells in the postnatal mouse brain. We then ubiquitously or selectively expressed mutant TDP-43 in neuronal and glial cells in the striatum of adult mouse brains via stereotaxic injection of AAV vectors and found that it also preferentially accumulates in neuronal cells. Expression of mutant TDP-43 in neurons in the striatum causes more severe degeneration, earlier death and more robust symptoms in mice than expression of mutant TDP-43 in glial cells; however, aging increases the expression of mutant TDP-43 in glial cells, and expression of mutant TDP-43 in older mice caused earlier onset of phenotypes and more severe neuropathology than that in younger mice. Although expression of mutant TDP-43 in glial cells via stereotaxic injection does not lead to robust neurological phenotypes, systemic inhibition of the proteasome activity via MG132 in postnatal mice could exacerbate glial TDP-43-mediated toxicity and cause mice to die earlier. Consistently, this inhibition increases the expression of mutant TDP-43 in glial cells in mouse brains. Thus, the differential accumulation of mutant TDP-43 in neuronal versus glial cells contributes to the preferential toxicity of mutant TDP-43 in neuronal cells and age-dependent pathology.
INTRODUCTION
The accumulation of misfolded proteins in neurons is a common neuropathological feature of neurodegenerative diseases, including Alzheimer's disease, Parkinson's disease, Huntington's disease, amyotrophic lateral sclerosis (ALS) and frontotemporal lobar degeneration (FTLD). The major component of inclusions in the brains of patients with ALS and FTLD is found to be TAR DNA-binding protein of 43 kDa (TDP-43) (1–3), and autosomal dominant missense mutations in the TDP-43 gene have been identified in patients with ALS (4,5). TDP-43, a nuclear protein of 414 amino acids, belongs to the heterogeneous ribonucleoprotein family and is involved in gene transcription, splicing and nuclear body functions (6,7). Loss of TDP-43 causes early embryonic lethality in mice (8,9), suggesting that TDP-43 is essential for early development.
TDP-43 mutation-mediated pathology may involve both loss- and gain-of-function mechanisms (10). The fact that overexpression of wild-type TDP-43 in rodents can lead to a variety of neurodegenerative phenotypes (11,12) suggests that the accumulation of TDP-43 is critical for the development of neuropathology. Mutations in TDP-43 may facilitate this accumulation, therefore leading to neuropathology. In support of this idea, accumulation of TDP-43 is age-dependent and leads to neuronal degeneration in an age-dependent manner. Based on the gain of toxic function of TDP-43, overexpression of TDP-43 has been widely used to generate a variety of animal models for investigating disease pathogenesis. For example, the overexpression of mutant TDP-43 in glial cells can also result in severe neurological phenotypes in animal models (13,14).
Glial cells are essential for the normal function and survival of neuronal cells in the brain, and glial cell dysfunction is involved in neurodegenerative diseases (15). Nonetheless, most neurodegenerative diseases, including ALS, preferentially affect neuronal cells. Given the toxicity of mutant TDP-43 in both neuronal and glial cells, we need to determine why TDP-43 preferentially affects neuronal cells and how TDP-43 in glial cells contributes to disease progression. Understanding this would also help unravel the pathogenesis of various neurodegenerative diseases commonly caused by the accumulation of misfolded proteins.
The relative contributions of neuronal and glial TDP-43 to disease have not been rigorously compared, perhaps because expression of transgenic mutant proteins from early embryonic stages and in various types of cells in animals makes it difficult to assess cell type-specific effects of mutant TDP-43 in adults. To circumvent this difficulty, we used stereotaxic injection to selectively express mutant TDP-43 in neurons and astrocytes in the mouse brain striatum. We found that mutant TDP-43 preferentially accumulates in neuronal cells and causes neuropathology, however, aging promotes the accumulation of TDP-43 in astrocytes, and reducing TDP-43 degradation by inhibiting proteasome activity enhances the toxicity of glial TDP-43 and phenotype severity. Our findings suggest that the preferential accumulation of TDP-43 in neuronal cells causes neuronal vulnerability, and aging-related glial dysfunction also plays an important role in disease progression.
RESULTS
Expression of mutant TDP-43 in different types of cultured cells
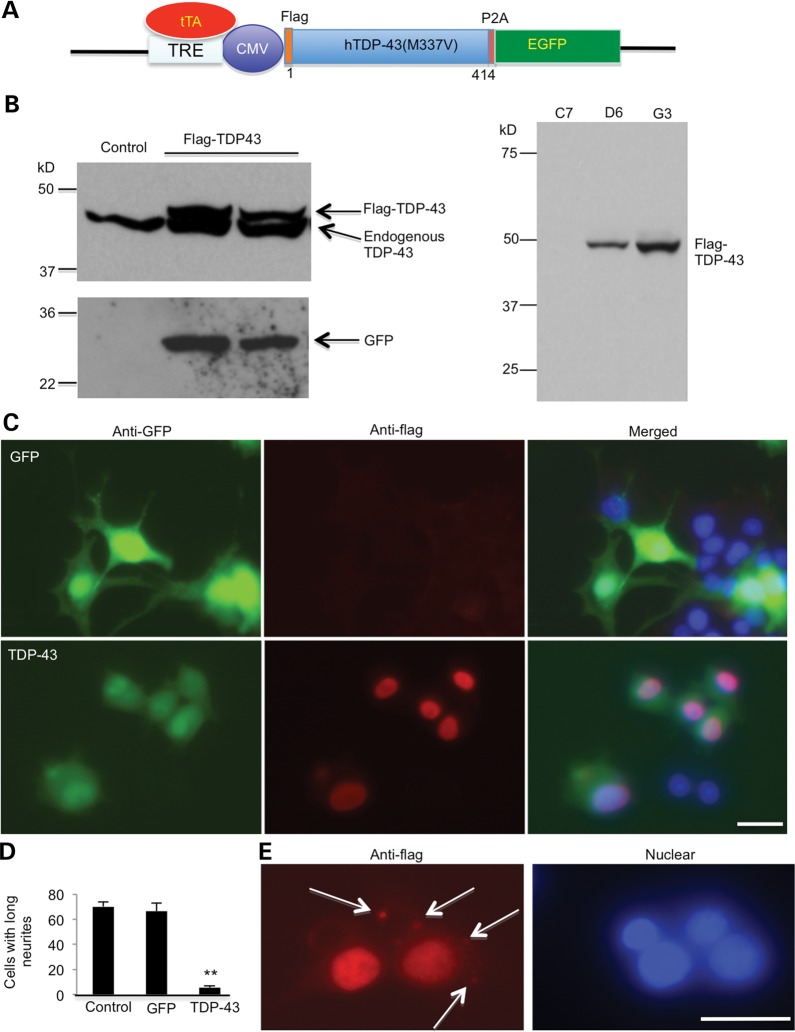
Owing to the cytotoxicity of mutant TDP-43, we developed PC12 cell lines in which the expression of human TDP-43 (M337V) is inducible under the control of the tetracycline-responsive element (TRE). pTRE-hTDP-43 and pTRE-GFP vectors were constructed (Fig. 1A) and transfected into the Tet-off PC12 cells. Transfected cells were then selected with Hygromycin B and G418. After several selections, we established a few cell lines expressing GFP or TDP-43. For further studies, we used stable cell line clone C7, which expresses GFP, as a control, and D6 and G3, which express mutant TDP-43. The expression of mutant TDP-43 in these cells was induced by adding a tetracycline analog, doxycycline (Dox), and verified by western blotting with anti-GFP or anti-TDP43 (Fig. 1B, left panel) and anti-Flag (Fig. 1B, right panel). After adding 100 ng/ml NGF to induce neurite outgrowth in PC12 cells, the C7 (GFP control) cells developed long neurites, but the D6 (hTDP-43) cells did not (Fig. 1C). Counting PC12 cells showed a greater number of GFP-transfected cells with neurites than TDP-43-transfected PC12 cells (Fig. 1D), indicating that mutant TDP-43 is toxic to neuronal cells and inhibits their neurite outgrowth. In addition, when cultured cells were under metabolic stress without glucose in culture medium for 2 days, mutant TDP-43 formed a few small aggregates that appear to be localized outside the nucleus (Fig. 1E), consistent with the cytoplasmic mislocalization of mutant TDP-43 seen in the brains of patients.
Figure 1.
Expression of mutant TDP-43 suppresses neurite outgrowth of PC12 cells. (A) Schematic drawing of the TDP-43 (M337V) in the Tet-Off vector. Mutant human TDP-43 (M337V) is tagged with Flag and linked to EGFP via 2A oligopeptides (P2A), which are self-cleaved to separate TDP-43 from EGFP when expressed in cells. Flag-TDP-43 is expressed in the absence of doxycycline. CMV, cytomegalovirus promoter. (B) Western blotting with anti-TDP-43 or anti-GFP antibodies revealing the expression of transfected Flag-TDP-43 and GFP in stably transfected PC12 cell lines (D6, G3) (left panel). Anti-flag antibody also verified the expression of transfected TDP-43 (right panel). Cells were examined after removing doxycycline for 6 days. (C) Double fluorescent immunostaining of PC12 cells (G3 clone) showing neurites in GFP control, but not in flag-TDP-43-transfected cells in response to NGF treatment. Scale bar: 10 μm. (D) Quantification of PC12 cells containing neurites longer than two cell body diameters (n = 300 each group). *P < 0.05. (E) In some flag-TDP-43-transfected cells under metabolic stress without glucose in culture medium for 2 days, cytoplasmic aggregates (arrows) are seen.
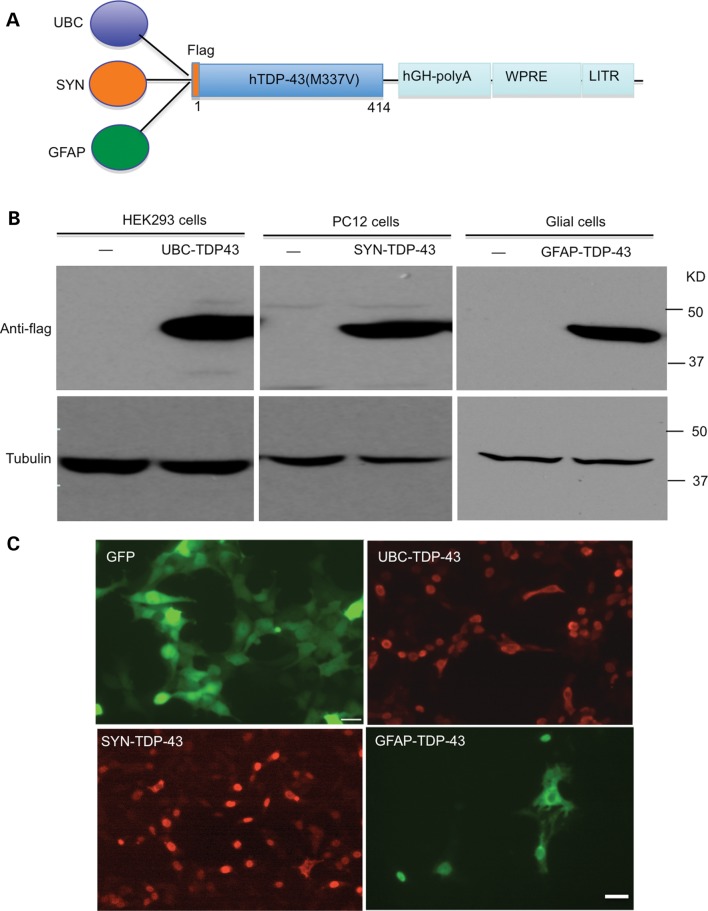
To compare the cytotoxicity of mutant TDP-43 in neuronal and glial cells in the brain, we constructed three adeno-associated virus (AAV) 9 vectors that can express mutant TDP-43 in the mouse brain under the control of the ubiquitin promoter, neuronal promoter (synapsin-1) (16) or 2-kb glial fibrillary acidic protein (GFAP) promoter (17) (Fig. 2A). Because overexpression of wild-type TDP-43 also causes cytotoxicity (11,12), overexpression of wild-type TDP-43 may not be a good control for us to compare cell type-specific toxicity of mutant TDP43. Thus, we used GFP as a control and therefore also generated GFP control vectors. We first verified the expression of TDP-43 by these different promoters in different cell lines. TDP-43 under the ubiquitin promoter (UBC-TDP-43) was expressed in HEK293 cells, TDP-43 under the synapsin-1 promoter (SYN-TDP-43) was expressed in PC12 cells and TDP-43 under the GFAP promoter (GFAP-TDP-43) was expressed in primary glial cells. The expression of mutant TDP-43 was verified by western blotting (Fig. 2B) and immunocytochemical (Fig. 2C) analysis. The results confirmed that these promoters could drive the expression of mutant TDP-43 in different types of cells.
Figure 2.
Expression of mutant TDP-43 in neuronal and glial cell lines. (A) Schematic diagrams of AAV9 viral vectors that contain human ubiquitin promoter (UBC), human synapsin-1 promoter (SYN) or human GFAP promoter. WPRE, woodchuck hepatitis virus post-transcriptional control element. (B) Western blotting with anti-flag detecting the expression of TDP-43 in HEK293, PC12 and cultured primary astrocyte cells. (–): non-infected cells. (C) Anti-GFP and anti-flag immunostaining also verified the expression of UBC-GFP control, UBC-TDP-43, SYN-TDP-43 and GFAP-TDP-43 viruses in different cell types. Scale bar: 10 μm.
Preferential mutant TDP-43 toxicity in neuronal cells
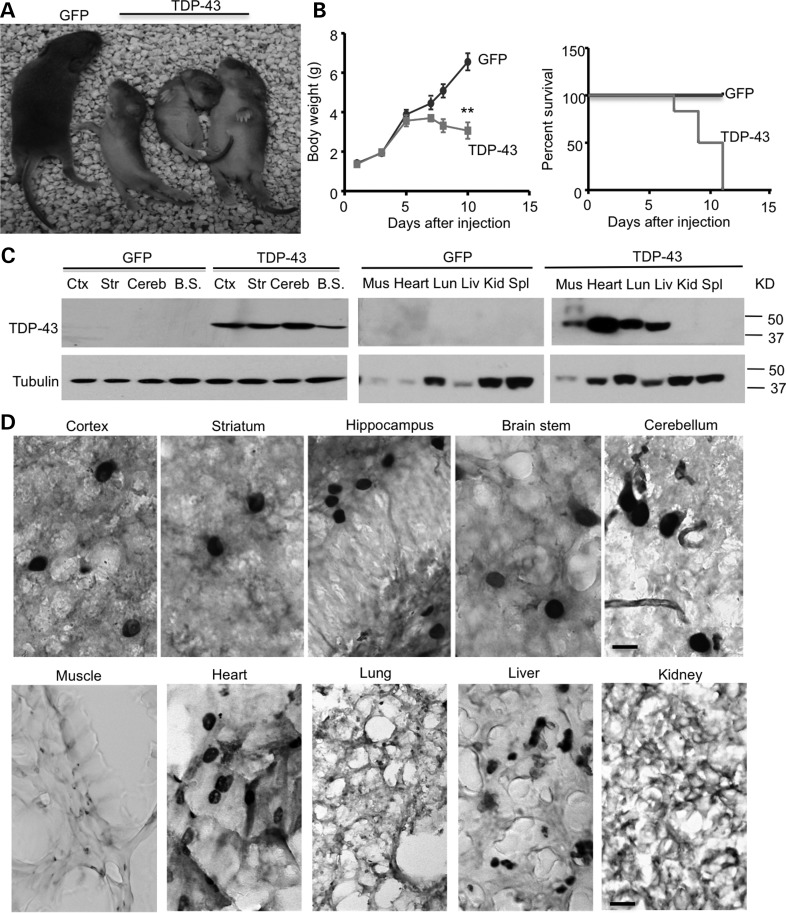
Having generated adenoviral vectors to express mutant TDP-43 in different types of cells, we wanted to know how TDP-43 is distributed in the mouse brain. We first examined its distribution in postnatal mouse brains by intravenous injection of UBC-TDP-43 vector into P1 mouse pups (n = 6), as the intravenous delivery of AAV9 vectors would allow the transgene to cross the blood–brain barrier and target neurons and astrocytes in the brain (18,19). Seven days after injection of UBC-TDP-43, the mice displayed progressive paralysis. We found that injection of adenoviral TDP-43 under the ubiquitin promoter could significantly reduce the body weight gain and increased mortality of newborn mouse pups compared with the control group that was injected with adenoviral GFP (Fig. 3A–B). All mice that were injected with AAV-TDP-43 died 11 days after i.v. injections. Using western blotting, we verified the expression of mutant TDP-43 in the brain and peripheral tissues (Fig. 3C). Immunohistochemical analysis demonstrated the distribution of mutant TDP-43 in various brain regions, including the cortex, hippocampus and striatum (Fig. 3D). Importantly, high-magnification micrographs revealed that mutant TDP-43 is enriched in the nuclei of neuronal cells, but not in glial and other types of cells (Fig. 3D). In peripheral tissues, more TDP-43 also accumulates in the nuclei of cells in the heart and liver than in the muscle, lung and kidney (Fig. 3D).
Figure 3.
Expression of UBC-TDP-43 in postnatal-day-12 mice injected with AAV-UBC-TDP-43 at postnatal day 1. (A) The neonatal P1 mouse pups were intravenously (i.v) injected with 1.2 × 1010 vg of AAV9-UBC-GFP or UBC-TDP-43. At P12, mutant TDP-43 expression reduced the size of mice compared with UBC-GFP-injected mice. (B) Body weight (left panel) and survival rate (Kaplan–Meier plot, right panel) assays showing that mutant TDP-43 reduced body weight gain and caused early death of mice (n = 6 for each group). **P < 0.01. (C) Western blotting with anti-flag showing that the intravenous injection of AAV9-UBC-TDP-43 at P1 leads to the widespread expression of UBC-TDP-43 in the brain and peripheral tissues in mice at P12. Ctx, cortex; Str, striatum; Cereb, cerebellum; B.S., brain stem; Mus, muscle; Lun, lung; Liv, liver; Kid, kidney; Spl, spleen. (D) TDP-43 immunostaining of the brain and peripheral tissues of UBC-TDP-43-injected mice at P12. Note that mutant TDP-43 is enriched in neuronal cells in the brain and also in the nuclei of cells in heart and liver. Scale bar: 10 μm.
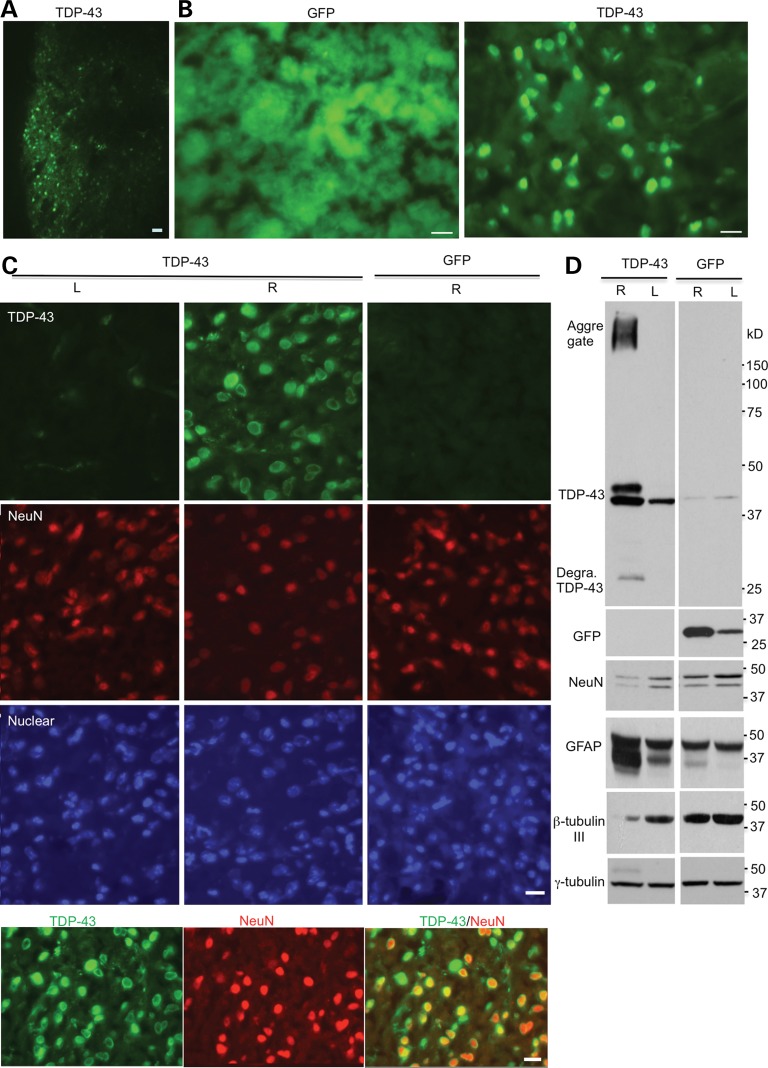
The mortality of mouse pups after being injected with viral TDP-43 could be due to its toxicity in peripheral tissues. To further define the selective neurotoxicity of TDP-43 and to examine whether mutant TDP-43 also preferentially accumulates in neuronal cells in adult mice, we used stereotaxic injection to deliver mutant TDP-43 into the striatum, a region that is relatively small for injection and is important for movement coordination such that we can assess the toxicity and behavioral phenotypes caused by mutant TDP-43 in the mouse brain. Also, neuronal and glial TDP-43 inclusions are present in the striatum of ALS patients (20). We carried out unilateral injection of AAV vectors in the right side of the striatum (R) and first verified the expression of GFP or TDP-43 in the injected striatum of mice via immunofluorescent staining (Fig. 4A). Immunostaining with anti-TDP-43 clearly demonstrated that mutant TDP-43 is abundantly distributed in the nuclei of neuronal cells, but not in glial cells, although TDP-43 was expressed under the control of the ubiquitin promoter (Fig. 4B). Importantly, the expression of mutant TDP-43 reduced the density of neuronal cell numbers compared with the non-injected region or adenoviral GFP-injected region. To further verify that TDP-43 toxicity is not due to adenoviral expression, we performed immunocytochemistry (Fig. 4C) and western blotting (Fig. 4D) and found that only overexpression of TDP-43, but not GFP, via AAV vector in 18-month-old mice could reduce β-tubulin-III, a neuronal marker protein, and increase GFAP, an astrocyte-specific marker protein that is increased in response to neuronal injury. As we used an antibody that can react with the internal region of TDP43, it is difficult to define whether the small band is N- or C-terminal TDP-43 (Fig. 4D). Also, the antibody reacts weakly with endogenous mouse TDP-43 such that the band seen in the ‘L’ sample is likely due to expression of the limited injected viruses that were diffuse from the injected ‘R’ side.
Figure 4.
Stereotaxic injection of AAV-UBC-TDP-43 viruses into the striatum of mice. (A, B) Mice at 3 months of age were injected with UBC-GFP or UBC-TDP-43 (2.5 × 1010 vg) into the right side of their striatum. After 15 days, immunofluorescent staining showed the expression of GFP or TDP-43 in the injected striatum. Scale bars: 10 μm. (C) High-magnification (40×) micrographs showing the expression of TDP-43 in neuronal nuclei with a reduced number of NeuN-positive cells in the right striatum (R), but not in the non-injected striatum (L) or UBC-GFP-injected right (R) side of the striatum. Colocalization of NeuN and TDP-43 is seen in the merged image (low panel). Scale bars: 10 μm. (D) Western blotting confirming the expression of UBC-TDP-43 or UBC-GFP in the injected right (R) side of the striatum in 18-month-old mice. Degra, degraded. Note that there are decreased levels of NeuN as well as β-tubulin-III and an increased level of GFAP in the presence of TDP-43 compared with the non-injected or UBC-GFP-injected striatum.
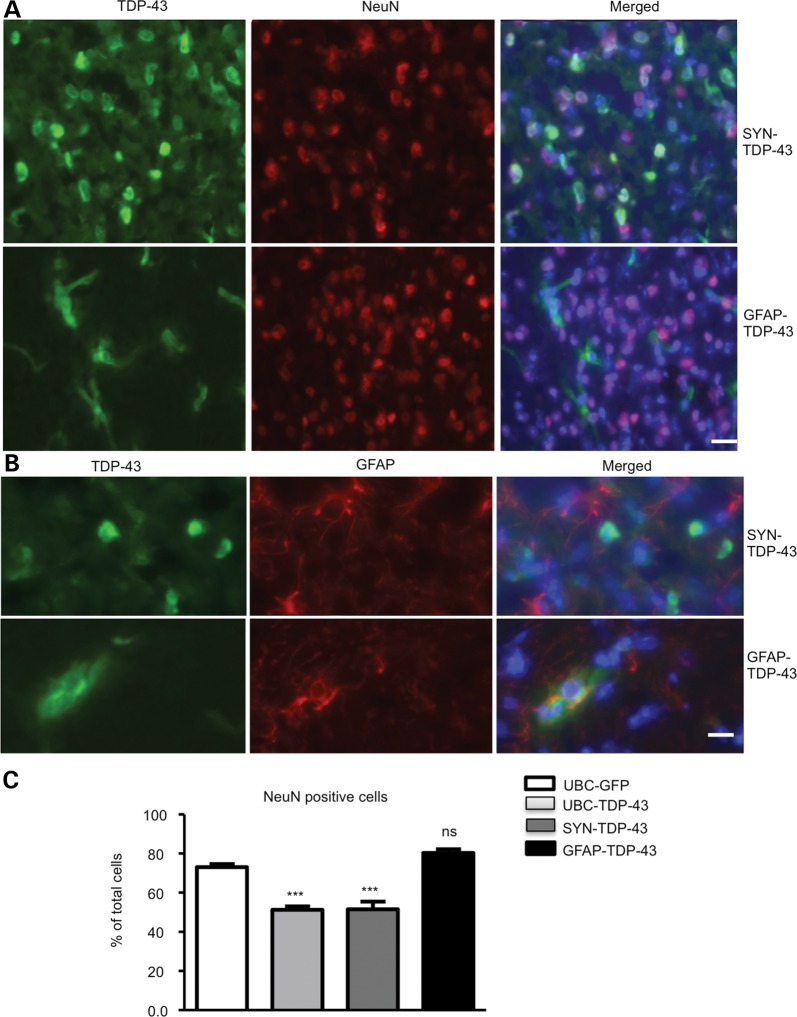
Because it was important to compare TDP-43 toxicity in neuronal and glial cells, we delivered adenoviral SYN-TDP-43 and GFAP-TDP-43 into the mouse striatum (n = 6 per group) via stereotaxic injection to express TDP-43 in neuronal and glial cells, respectively. Immunofluorescent staining confirmed that SYN-TDP-43 and GFAP-TDP-43 were selectively expressed in neurons and astrocytes (Fig. 5A). High-magnification micrographs also show the selective expression of SYN-TDP-43 in NeuN-positive cells and GFAP-TDP-43 in GFAP-positive astrocytes (Fig. 5B). Counting the numbers of NeuN- and GFAP-positive cells revealed a reduced number of NeuN-positive cells in the SYN-TDP-43-injected region, whereas the GFAP-TDP-43-injected brain region did not show loss of NeuN-positive cells (Fig. 5C), suggesting neuronal cells are more vulnerable than glial cells to mutant TDP-43.
Figure 5.
Stereotaxic injection of SYN-TDP-43 and GFAP-TDP-43 viruses into the striatum of mice. (A) Double immunofluorescent staining showing the expression of SYN-TDP-43 and GFAP-TDP-43 in the striatum of mice at 3 months of age after being injected for 15 days. Scale bar: 10 μm. (B) High-magnification micrographs showing the selective expression of SYN-TDP-43 and GFAP-TDP-43 in NeuN-positive neurons and GFAP-positive astrocytes, respectively. Scale bar: 10 μm. (C) Counting NeuN-positive and GFAP-positive cells showing that UBC-TDP-43 and SYN-TDP-43 reduced the number of NeuN-positive cells. Expression of GFAP-TDP-43 in astrocytes did not significantly alter the numbers of NeuN-positive cells. ***P < 0.001 compared with UBC-GFP-injected striatum.
Neuronal expression of mutant TDP-43 causes more severe phenotypes
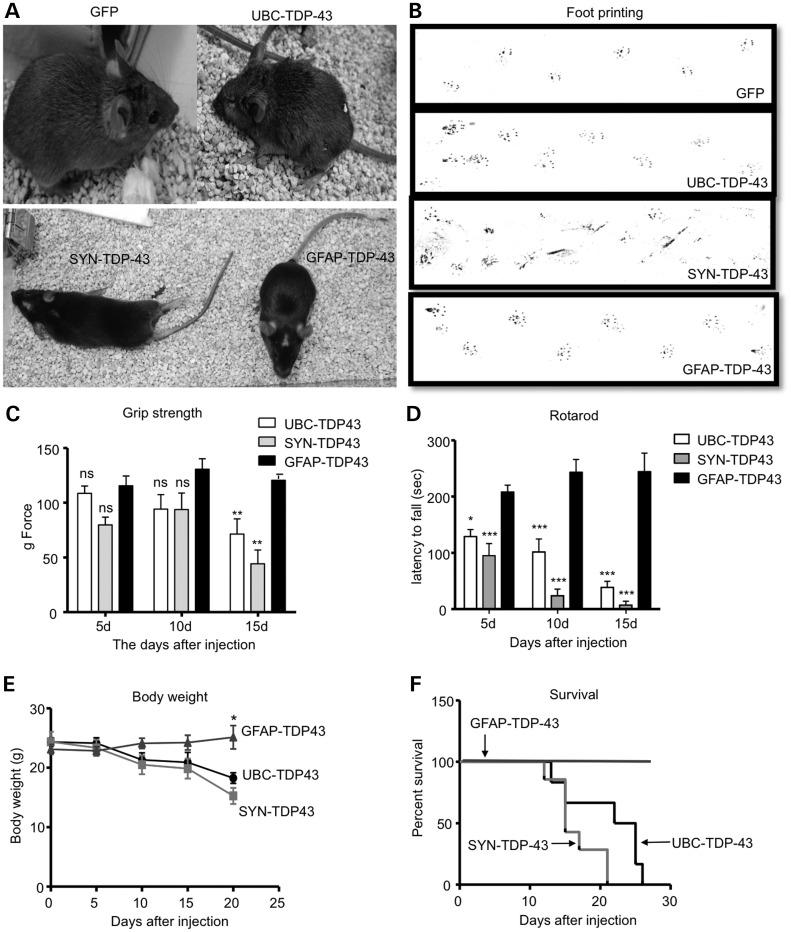
If neuronal cells are more sensitive to mutant TDP-43, we should observe more severe phenotypes in mice injected with SYN-TDP-43 than with GFAP-TDP-43. We therefore compared behavioral phenotypes of mice that were injected with UBC-TDP-43, SYN-TDP-43 or GFAP-TDP-43. Mice expressing UBC-TDP-43 or SYN-TDP-43 on the right side of their striatum appeared to be smaller, with hunchback appearance or kyphosis, and they had movement difficulty compared with GFP- or GFAP-TDP-43-injected mice (Fig. 6A). Because the striatum controls body movement and coordination, we tested these mice using rotarod and footprinting assays. Mice injected with UBC-TDP-43 or SYN-TDP-43 showed abnormal gait (Fig. 6B). The grip strength assay revealed significant weakness of the legs in SYN-TDP-43-injected mice as compared with UBC-TDP-43- or GFP-injected mice (Fig. 6C), suggesting that SYN-TDP-43 produced more toxicity in neurons that control leg grip strength. Consistently, the rotarod test showed more severe impairment of motor function of SYN-TDP-43-injected mice than UBC-TDP-43-injected mice (Fig. 6D). In addition, these mice started to lose body weight (Fig. 6E) and die (Fig. 6F) at Day 12 after virus injection. Importantly, GFAP-TDP-43-injected mice did not show the severe phenotypes seen in UBC-TDP-43- and SYN-TDP-43-injected mice. Thus, the expression of mutant TDP-43 in neuronal cells caused much more severe phenotypes than in glial cells.
Figure 6.
Expression of mutant TDP-43 in neuronal cells in the striatum of mice causes more severe phenotypes than those in glial cells. (A) Mice that were injected with UBC-TDP-43 or SYN-TDP-43 into their striatum for 15 days are smaller in size and show paralysis compared with UBC-GFP- or GFAP-TDP-43-injected mice. (B) The foot-printing assay (front paws in red and hind paws in blue) revealing gait abnormalities in UBC-TDP-43- and SYN-TDP-43-injected mice compared with UBC-GFP- and GFAP-TDP-43-injected mice. (C, D) Motor function impairment of UBC-TDP-43- and SYN-TDP-43-injected mice, which was revealed by hind limb grip strength (C) and rotarod performance (D) assays. Two-way (genotype × time) ANOVA revealed statistic significance (**P < 0.01, ***P < 0.001). (E, F) Body weight (E) and survival (F) assays showing that expression of mutant TDP-43 in neuronal cells caused a greater reduction in body weight and survival in UBC-TDP-43- or SYN-TDP-43-injected mice compared with GFAP-TDP-43-injected mice (n = 6 per group). In C–F, *P < 0.05, **P < 0.01, ***P < 0.001 compared with GFAP-TDP-43 group.
Aging promotes the accumulation of mutant TDP-43 in glial cells and exacerbates the neurological phenotypes of mutant TDP-43
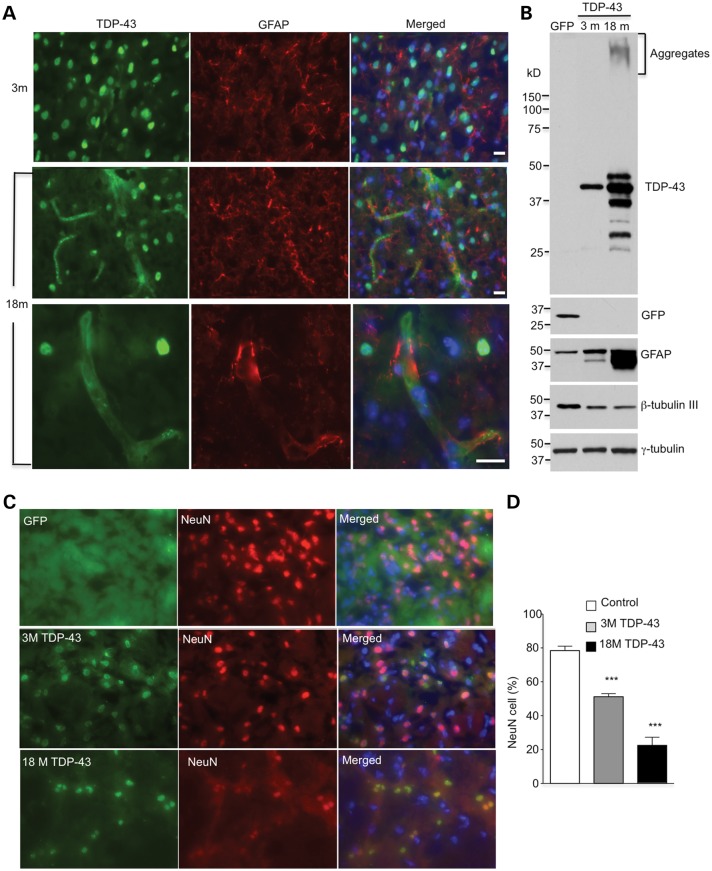
We have found that mutant TDP-43, when expressed under the ubiquitin promoter, preferentially accumulates in neuronal cells in the brains of mice at 3 months of age. As mutant TDP-43 mediates pathology in patients in an age-dependent manner, we wondered whether aging could influence the distribution and expression of mutant TDP-43 in different types of cells in the brain. To explore this, we injected UBC-TDP-43 into the striatum of old mice at 18 months of age. Immunocytochemical staining showed that mutant TDP-43 was distributed not only in NeuN-positive neurons but also in GFAP-positive astrocytes in the older mouse brain (Fig. 7A). Western blotting analysis confirmed that there was an increase in the levels of mutant TDP-43 and that it formed aggregates in the injected striatum in 18-month-old mice compared with the young mice at 3 months of age (Fig. 7B). This age-dependent increase is consistent with the fact that aging can promote the accumulation of misfolded proteins in the brain. The western blotting result also showed an increased level of GFAP, which supports the distribution of mutant TDP-43 in astrocytes (Fig. 7B). Double immunofluorescent staining and examining the number of NeuN-positive cells in the striatum revealed a greater reduction of neuronal cells in older mice that were injected with mutant TDP-43 (Fig. 7C, D).
Figure 7.
Aging promotes the expression of UBC-TDP-43 in glial cells and exacerbates neuronal degeneration. (A) Double immunostaining of the striatum of mice at 3 and 18 months of age showing that more GFAP-positive cells express TDP-43 in 18-month-old mice after injection of AAV9-UBC-TDP-43 for 15 days. The bottom panel shows high-magnification graphs in which TDP-43 is expressed in GFAP-positive astrocytes. Scale bar: 10 μm. (B) Western blotting also revealing the increased levels of TDP-43 and GFAP in the injected striatum of an 18-month-old mouse compared with that in a 3-month-old mouse. Neuronal marker protein, β-tubulin-III, is also decreased. (C) Double immunostaining of the striatum of mice at 3 and 18 months of age showing a decrease in NeuN-positive cells (% of total cells) after UBC-TDP-43 had been injected for 15 days. Scale bar: 10 μm. (D) Quantitative assessment of NeuN-positive cells showing fewer NeuN cells in 18-month-old mice than in 3-month-old mice (n = 600 cells each group). ***P < 0.001 compared with the non-injected control striatum.
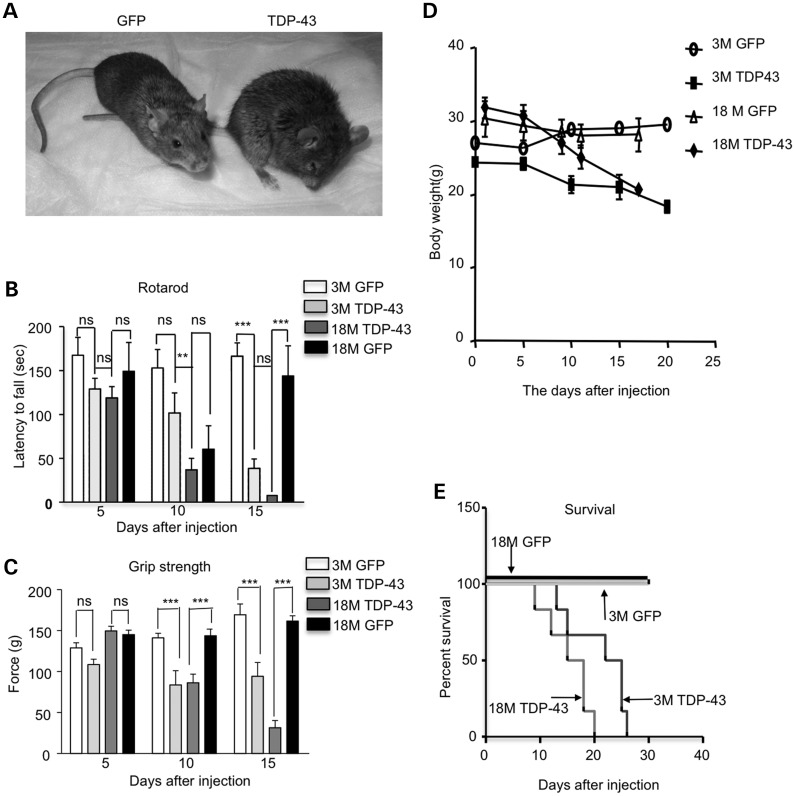
Consistent with neurodegeneration worsening with age, the older mice at 18 months had a hunchback appearance (Fig. 8A). Their motor function, as assessed by rotarod performance and hind limb grip strength assays, was more severely affected than younger mice at 3 months of age (Fig. 8B, C). These old mice also lost more weight (Fig. 8D) and died sooner (Fig. 8E) than the younger mice at 3 months of age after injection of UBC-TDP-43. As a control, viral GFP was also injected into the striatum of young and old mice, and these control mice lived normally without body weight loss or motor function deficits. Thus, aging specifically exacerbates the pathology caused by mutant TDP-43.
Figure 8.
Aging increases the severity of phenotypes of UBC-TDP-43-injected mice. (A) Pictures of 18-month-old mice that were injected with UBC-GFP or UBC-TDP-43. (B, C) Rotarod performance (B) and hind limb grip strength (C) tests showing more severe motor impairment in 18-month-old mice than 3-month-old mice at 10 days after stereotaxic injection of AAV-UBC-TDP-43 (n = 6 per group). Two-way (genotype × time) ANOVA revealed statistic significance (**P < 0.01, ***P < 0.001). (D, E) Mice at 18 months of age also showed reduced body weight (D) and earlier death (E) than 3-month-old mice after their striata were injected with AAV-UBC-TDP-43 virus. The control is AAV-UBC-GFP-injected mice (n = 6 per group).
Inhibiting proteasome activity enhances glial TDP-43 toxicity
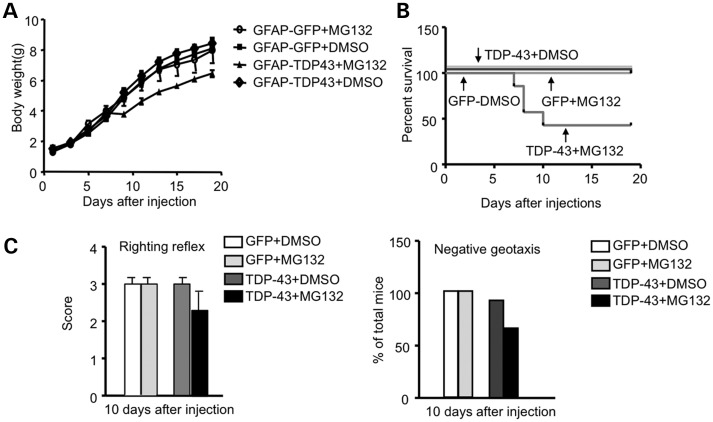
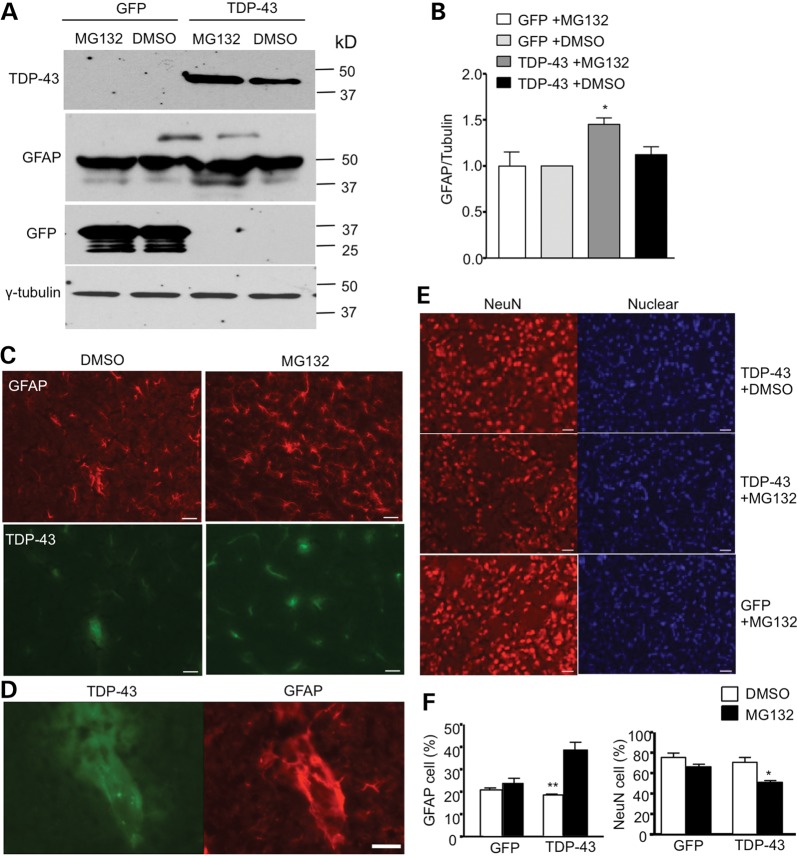
Our research has shown that aging increases the level of mutant TDP-43 in glial cells, and recent studies found that overexpression of mutant TDP-43 in glial cells can cause neurological symptoms in transgenic mice (13,14). Given that the ubiquitin–proteasome activity in brain tissue declines with age (21–23), we wondered whether inhibiting the proteasome function could increase the level of TDP-43 in glial cells and exacerbate the glial toxicity of mutant TDP-43. MG132, a proteasome inhibitor, is known to inhibit brain proteasome activity when subcutaneously administered into newborn mice during the first 10 days of life, before the blood–brain barrier is completely established (24). We therefore subcutaneously injected MG132 (5 mg/kg) or control vehicle (DMSO) into P1 mouse pups that had been intraventricularly injected with viral GFP or TDP-43 under the GFAP promoter. These mouse pups were then treated with MG132 for 20 days and examined for growth, survival and behaviors. MG132 treatment reduces the body weight gain of mouse pups injected with TDP-43 virus (Fig. 9A). This treatment also caused ∼50% of TDP-43-injected pups to die during postnatal days 7–10 (Fig. 9B). Increasing TDP-43 levels in glial cells in this postnatal period by inhibiting proteasome activity may have a particularly great impact on the postnatal development of mice. We also found that MG132 treatment affected the righting reflex and negative geotaxis of mouse pups injected with TDP-43, but not the control GFP virus (Fig. 9C). Consistently, MG132 treatment also increased the level of TDP-43 in the injected mouse brain (Fig. 10A–B). The GFAP level was elevated in TDP-43 virus-injected mouse brain (Fig. 10A), which was also supported by immunocytochemical staining (Fig. 10C) and the colocalization of TDP-43 and GFAP in reactive astrocytes (Fig. 10D). The overexpression of mutant TDP-43 in astrocytes was recently found to cause motor neuron degeneration in a non-cell-autonomous manner (14). Similarly, there was a decrease in NeuN-positive cells in the striatum when glial TDP-43 expression was increased by inhibiting the proteasome function via MG132 (Fig. 10E). We saw more GFAP-positive cells in the striatum of mice treated with MG132 (Fig. 10F), supporting the idea that reactive astrocytes were increased. Taken together, our studies suggest that increased mutant TDP-43 levels in astrocytes can promote neuronal toxicity, even when neuronal cells do not express mutant TDP-43.
Figure 9.
Inhibition of proteasome activity of postnatal mice enhances glial expression of TDP-43 and toxicity. (A) Changes in body weight of mice injected with AAV GFAP-TDP-43 or GFAP-GFP at P1, which was expressed under the control of GFAP promoter. The mice were then subcutaneously injected with MG132 (5 mg/kg/day) for 10 days (n = 6 each group). (B) About 50% of GFAP-TDP-43-injected mice treated with MG132 die during postnatal days 7–10. (C) MG132 treatment reduced the righting reflex and negative geotaxis behavior of GFAP-TDP-43 virus-injected pups as compared with AAV GFAP-GFP-injected mouse pups.
Figure 10.
Inhibition of proteasome activity increases the expression of TDP-43 in astrocytes and neuronal toxicity. (A) Western blotting showing that MG132 treatment increased the level of TDP-43 and GFAP in the brain of mouse pups that were injected with AAV GFAP-TDP-43. AAV GFAP-GFP injection served as a control. (B) Quantitative analysis of the relative levels of TDP-43 and GFAP (ratio to tubulin relative to GFP with DMSO group) on western blots. (C–D) Double immunostaining of P20-day-old mouse pups that were intraventricularly injected with AAV GFAP-TDP-43 at P1 and then subcutaneously injected with DMSO or MG132 (5 mg/kg) daily. The brain sections were stained with anti-flag for TDP-43 (green) and anti-GFAP (red) for astrocytes. (E) Immunofluroescent staining with anti-NeuN (red) for neuronal cells and Hoechst dye for the nucleus. Scale bar: 10 μm. (F) The percentage of NeuN- and GFAP-positive cells of total cells in AAV GFAP-TDP-43-injected-mouse pup brains after MG132 treatment.
DISCUSSION
A variety of neurodegenerative diseases, including Alzheimer's disease (AD), Parkinson's disease (PD), Huntington's disease (HD), ALS and FTLD, are caused by misfolded proteins that are ubiquitously expressed; however, the disease proteins preferentially affect neuronal cells, despite their presence in various types of cells, including glial cells, which constitute the majority of cells in the brain. The mechanism behind the selective neuropathology in these neurodegenerative diseases remains elusive, although glial dysfunction is known to play an important role in the neuropathology (15,25). Moreover, it is evident that transgenic mutant proteins in glial cells also cause neurological symptoms in animal models (13,14,26,27). An important question, then, is how the disease proteins in neuronal and glial cells contribute to the development of neurological phenotypes.
We chose TDP-43 as a disease protein to address this question because it is expressed in both neuronal and glial cells in patient brains (28,29). Although the exact mechanisms by which mutant TDP-43 causes disease remain unknown, its severe cytotoxicity allows us to define the relationship between its expression in different types of cells and the associated pathology. Expression of transgenic mutant TDP-43 via neuronal or glial promoters in neuronal or glial cells can cause similar neurological phenotypes in animals (14,30). Because of differences in the expression and distribution of transgenes in different transgenic mouse models, it is difficult to define the relationship between the cell type-specific expression of mutant proteins and phenotypes by comparing these transgenic animal models. In addition, because transgenes are expressed from early developmental stages, distinguishing between the age-dependent effects of mutant proteins in old neurons and their cumulative effects during the aging process is difficult.
Via stereotaxic injection of AAV vectors, we were able to deliver mutant proteins into a defined brain region of mice at different ages. Using different promoters to control the expression of mutant TDP-43, this mutant protein could be selectively expressed in neuronal or glial cells. This approach, however, involves the overexpression of transgenic proteins. Although overexpression of mutant proteins has been widely used to study gain-of-toxic-function mechanisms of misfolded proteins, it requires ruling out artifacts of overexpression by viral vectors. Because overexpression of wild-type TDP-43 is also toxic (11,12), a better control would be to overexpress a non-TDP-43 protein. In our studies, we used AAV-GFP as a control protein to define the specific toxicity of mutant TDP-43. In addition, we performed unilateral injection of viral vectors into one side of the striatum and used the other non-injected side as a control in the same animal to help define the pathology caused by mutant proteins. Finally, we also compared mice injected with the same viral vectors at different ages to uncover aging-related effects on mutant protein toxicity.
Our findings show that mutant TDP-43 preferentially accumulates in neuronal cells versus glial cells. This selectivity does not depend on aging, as administration of mutant TDP-43 under the ubiquitin promoter into postnatal mouse pups at P1 also resulted in the enrichment of mutant proteins in neuronal cells. Thus, there is an intrinsic difference between neuronal and glial cells in dealing with mutant proteins. Because of this difference, mutant proteins are more likely to accumulate in neuronal cells and affect their function. We did not examine the expression of mutant TDP-43 in other types of glial cells, such as microglia, when mutant TDP-43 was expressed under the control of UBC promoter. The abundant expression of mutant TDP-43 in neuronal cells via SYN-TDP-43 injection, however, underscores the preferential toxicity of mutant TDP-43 in neuronal cells. Consistent with this idea, mice expressing mutant TDP-43 in neuronal cells in the striatum show much more robust phenotypes than those expressing mutant TDP-43 in astrocytes.
Our findings support the idea that mutant TDP-43 is preferentially accumulated in neuronal cells. First, under the ubiquitin promoter that drives gene expression ubiquitously in various types of cells, we saw enrichment of mutant TDP-43 in neuronal cells in mouse brain. Second, although mutant TDP-43 is not abundant in astrocytes in young mice at 3 months of age, aging can markedly increase the expression of mutant TDP-43 in astrocytes in 18-month-old mice. Third, inhibiting proteasome function can increase the expression of mutant TDP-43 in astrocytes, and the increased mutant TDP-43 levels can cause some postnatal mice to die 7–10 days after birth, suggesting that an abnormally high level of TDP-43 in astrocytes can affect early development. Although the accumulation of mutant TDP-43 in astrocytes normally occurs with aging, our findings underscore the importance of the glial toxicity of mutant TDP-43. In conjunction with the evidence that glial cells have higher proteasome activity than neuronal cells (23), our findings also indicate that it is the reduced capacity to remove mutant proteins in neuronal cells that leads to the preferential accumulation of misfolded proteins in neurons.
TDP-43 is known to have an important nuclear function in regulating gene expression, but how it causes disease has been a mystery (10,31). Transgenic mice expressing mutant TDP-43 in neuronal and glial cells have revealed that mutant TDP-43 affects neuronal function in autonomous and non-autonomous ways (14,30). For example, mutant TDP-43 in astrocytes can reduce glutamate transporter GLT-1 and increase neurotoxic factor Lcn2 to kill neurons in transgenic mice (14). Our results here demonstrated that unilateral injection of AAV-TDP-43 in the striatum can cause neurodegeneration and significantly affect the important function of this brain region, leading to movement impairment and early death. These findings also suggest that the expression levels of TDP-43 are critical for severe toxicity in neuronal and glial cells. In fact, overexpression of wild-type TDP-43 is also cytotoxic and can cause reactive gliosis, gait abnormalities and early lethality in mice (11,12). Thus, clearance of TDP-43 would be important to prevent its toxicity. As aging can increase the accumulation of mutant TDP-43 by reducing its clearance, improving cellular function, such as through ubiquitin-proteasome activity, should be beneficial for reducing the pathology. Finally, the glial expression of TDP-43 also contributes to the neuropathology, and this contribution is more likely to be associated with aging, which increases the accumulation of mutant TDP-43 in cells. Thus, improving glial cell function could also be an effective means to treat these neurodegenerative diseases.
MATERIAL AND METHODS
Plasmids, antibodies and reagents
The pUAS-hTDP-43-M337V plasmid (32), which encodes human mutant TDP-43 (M337V), was used as a template for PCR to generate flag-hTDP-43-P2A-GFP construct in the expression vector pTRE-Tight (Clontech, USA). The flag-hTDP-43 cDNA was also subcloned into a pAAV-MCS vector (Cell Biolabs) to generate AAV9 (pAAV-Flag-TDP-43) vector. DNAs encoding human ubiquitin promoter (1250 nt), synapsin-1 promoter (560 nt) and GFAP promoter (2210 nt) were isolated via PCR and inserted at Mlu1 and Sac1 restriction sites to form pAAV-UBC-Flag-TDP-43, pAAV-SYN-Flag-TDP-43 and pAAV-GFAP-Flag-TDP-43 vectors. The control AAV-GFP was constructed by PCR and inserted into the vectors containing ubiquitin or GFAP promoter.
The following antibodies were used: mouse monoclonal anti-human TDP-43 (Abnova, clone 2E2-D3), rabbit polyclonal anti-TDP-43 (G400, Cell Signaling), mouse monoclonal anti-Flag M2, (Sigma, Clone M2), mouse monoclonal antibody GFP (GTX628528, GeneTex), rabbit anti-NeuN (ABN78, Millipore), chicken anti-β tubulin-III (AB9354, Millipore), mouse monoclonal anti-γ-tubulin, mouse anti-GFAP (Thermo Scientific) and rabbit polyclonal anti-GFAP (AB5804, Millipore). All secondary antibodies were purchased from Jackson ImmunoResearch Laboratories. Hygromycin B 50 mg/ml (H3274 Sigma), MG132 (C2211, Sigma) and doxycycline (dox) administration (Sigma, St. Louis, MO, USA) were also used in the study.
Cell culture and drug treatment
Human embryonic kidney HEK293 cells were cultured in DMEM/F12 supplemented with 10% FBS, 100 U/ml penicillin, 100 μg/ml streptomycin and 0.25 μg/ml amphotericin B. Tet-off PC12 cells (Clontech) were prepared in DMEM/F12 complete media (10% horse serum, 5% fetal bovine serum and 1% penicillin-streptomycin) at 37°C, 5% CO2. Medium was changed every 2 days. Enriched astrocyte cultures were prepared from 1- to 2-day-old postnatal mouse pups by shaking the 15-day culture plates for 15 min to dissociate any astrocytes (33). Recombinant human NGF (100 ng/mL, R&D Systems) was added into neuronal culture medium to induce neurite growth.
Tet-off PC12 cell lines stably transfected with pTRE-hTDP-43
The tet-off PC12 cell lines were purchased from Clontech (63134, Clontech). To generate the inducible hTDP-43 cell line, pTRE-flag-TDP-43-P2AGFP plasmid and pTK-hyg vector as a selection marker were co-transfected into the tet-off PC12 cell line. Tet-off PC12 cell line transfected with pTRE-GFP and pTK-hyg served as a control. Cell medium were changed for 2 days in DMEM containing 10% horse serum and 5% fetal calf serum and G418 (150 µg/ml, G-5013, Sigma) with Hygromycin B (150 µg/ml, 10687010, Invitrogen). After 8–10 days of culture, positive cell clones were picked up and TDP-43 expression was induced by removing doxycycline hyclate (Dox, D-9891, Sigma). Western blotting was performed to verify the expression of transfected hTDP-43 in the selected cell lines. Two cell lines (hTDP-43-D6 and hTDP-43-G3) were found to express hTDP-43 protein. The positive PC12 cell lines were maintained in DMEM/F12 medium with 10% fetal bovine serum and 5% horse serum, 100 U/ml penicillin, 100 mg/ml G418 (100 µg/ml) and Hygromycin B (100 µg/ml) at 37°C in an atmosphere of 5% CO2 and 95% humidity. To induce neurite extension, 100 ng/ml NGF (N6009, Sigma) was used for the PC12 cells.
Western blot analysis, immunofluorescent microcopy and immunohistochemistry
For western blots, cultured cells or brain tissues were homogenized in RIPA buffer (50 mm Tris, pH 8.0; 150 mm NaCl; 1 mm EDTA, pH 8.0; 1 mm EGTA, pH 8.0; 0.1% SDS; 0.5% DOC and 1% Triton X-100) with 1× protease inhibitors from Sigma (P8340). The cell or tissue lysates were diluted in 1× SDS sample buffer and sonicated for 10s after incubation at 100°C for 5 min. The total lysates (20 μg) were loaded in Tris-glycine gel (Invitrogen) and blotted to a nitrocellulose membrane. The western blots were developed using the ECL prime kit (GE Healthcare). For immunofluorescent staining of cultured cells, cultured PC12 cells grown in 12-well plates were fixed in 4% paraformaldehyde in PBS for 10 min, permeabilized with 0.2% Triton X-100 in PBS for 30 min, blocked with 3% normal donkey serum in 3% BSA/PBS for 1 h and incubated with primary antibodies in 2% normal goat serum in 3% BSA/PBS overnight at 4°C. After several washes with PBS, the cells were incubated with secondary antibodies conjugated with either Alexa-488 or Alexa-594 (Invitrogen). Hoechst dye (0.01 μg/ml) was used to label the nuclei. For immunofluorescent staining of mouse brains, mice were anesthetized with 5% chloral hydrate and perfused with 10 ml 0.9% NaCl, and then with 20 ml of 4% paraformaldehyde in 0.1m PB through the left cardiac ventricle. Mouse brains were then removed and further fixed in the same solution overnight. Mouse brains were cryopreserved with 15 and 30% sucrose before being cut into 10 μm (for immunofluorescence staining) or 40 μm (for immunohistochemistry staining) sections with a cryostat (Leica CM1850) at −20°C. The sections were then stained with antibodies for immunofluorescent labeling or immunohistochemistry staining as described previously (34). Light micrographs were acquired on a Zeiss microscope (Axiovert 200 MOT) with a 63× lens (LD-Achroplan 63×/0.75). The microscope was connected to a digital camera (Hamamatsu Orca-100) linked to a computer with the Openlab software.
Virus production and intraventricular injection of AAV9 virus
The packaging AAV2/9 plasmid was obtained from the vector core at University Pennsylvania. AAV2/9 viruses were packaged and amplified by The Viral Vector Core at Emory University. The titer of the AAV9-UBC-hTDP-43, AAV9-UBC-GFP, AAV9-SYN-hTDP-43, AAV9-GFAP-hTDP-43 and AAV9-GFAP-GFP were 1.4 × 1013, 2.3 × 1013, 1.8 × 1013, 0.8 × 1013 and 0.7 × 1013 vector genomes (vg)/ml, respectively.
Neonatal mouse injections
Animals were housed and maintained in the animal facility at Emory University under specific pathogen-free conditions. All animal procedures were performed according to the NIH guidelines and were approved by the Institutional Animal Care and Use Committee of Emory University. Wild-type C57BL/6 mouse littermates were used to produce P1 mice. The newborn P1 pups were injected with AAV9-UBC-hTDP-43 viruses. The viruses were diluted to 1.2 × 1011 vg/ml in PBS, and 100 μl of solution was subsequently drawn into 31G insulin syringes (BD Ultra-Fine II U-100 Insulin Syringes). The needle was inserted into the superficial temporal vein, and the plunger was manually and slowly pushed. Mice were injected with either AAV9-UBC-GFP virus as a control or AAV9-UBC-hTDP-43 virus (n = 6 mice per group). The pups were carefully cleaned after the injection, rubbed with their original bedding and returned to their original cage with the mother.
Stereotaxic injection of AAV9 virus into the mouse striatum
A total of 2.5 × 1010 vg viruses (AAV9-UBC-hTDP-43, AAV9-SYN-hTDP-43, AAV9-GFAP-hTDP-43, AAV9-UBC-GFP and AAV9-GFAP-GFP as control virus) were stereotaxically injected into the right striatum of mice at different ages (3–18 months, n = 6 per group). The mice were anesthetized by injections of Avertin (0.5 mg/g i.p.); their heads were placed in a Kopf stereotaxic frame (Model 1900) equipped with a digital manipulator, a UMP3-1 Ultra pump and a 10-μl Hamilton microsyringe (Hamilton Co., Reno, NV, USA), and a 33G needle was inserted through a 1-mm drilled hole on the scalp. Injections were made at the following stereotaxic coordinates: 0.6 mm anterior to bregma, 2.0 mm lateral to the midline (left or right side) and 3.3 mm ventral to the dura, with bregma set at zero. The microinjections were carried out at a rate of 0.20 μl/min. The microsyringe was left in place for an additional 10 min before and after each injection. At 15 days after the injection, the striatum was isolated for examining the expression of GFP or hTDP-43 via immunostaining or western blotting.
Animal behavioral analysis
All animal tests were in accordance with NIH guidelines for procedures and were approved by the Institutional Animal Care and Use Committee of Emory University. Mouse body weight was measured after virus injection daily for 15–20 days. To examine gait function, mice were allowed to walk across a paper-lined chamber and into an enclosed box 12 days after viral injection. The hind paws of the mouse were dipped in blue ink, and the front paws were dipped in red ink. After several practice runs, traces of mouse paws were recorded. Rotarod performance was assessed using a rotarod test (Rotamex 4/8, Columbus Instruments International, Columbus, OH, USA). Mice were trained for 10 min on 3 consecutive days with the rotarod speed at 5 rpm, and testing commenced the following 3 days. The speed of the rod was set to 5 rpm and increased by 0.1 rpm/s. Each mouse was subjected to three trials, and the average data of each group were charted. Grip strength measurements for hind limbs were tested for 5 days by using a grip strength meter (Columbus Instruments). The grip strength meter test was performed by allowing the animals to grasp a platform with one hind limb (left or right), followed by pulling the animal until it released the platform. The digital score of the force on the grip strength meter was recorded and used for statistical analysis. The moribund mice were scored as ‘dead’ and were killed, and tissues were collected (n = 6, per group). MG132 (N-(1)-l-leucyl-N-[(1S)-1-formyl-3-methylbutyl]-l-leucinamide, Sigma C2211) is a specific, potent, reversible and cell-permeable proteasome inhibitor (Ki = 4 nM) (Sigma). Mouse pups at postnatal day 1 were administered MG132 by subcutaneous injection (n = 6, per group). The dose of MG132 used was 5 mg/kg/day starting from the 1st day after virus injection. DMSO injection served as a control (n = 6, per group).
Righting reflex and negative geotaxis
The animal was placed face up to turn over to a prone position on its feet within 30 s. Scoring was as follows: four paws were on the floor (3), one or more paws left under the body (2), no successful and vigorous attempts to right (1) and no response (0). For assessing negative geotaxis behavior, the animal was first placed face down on a 30° incline, and then latency to turn 180° was recorded. The maximum time perform the test was 30s.
Quantification of neurite extension and neuronal cells
Fluorescent microscope images were obtained as described earlier. A total of 20 images (total area = 7.22 mm2) were analyzed. The images were analyzed in triplicates, for every image, and cells with neurites longer than 2 cell body diameters were counted. For counting neuronal and glial cells, the percentage of NeuN- or GFAP-positive cells of total cells was obtained by examining more than six non-overlapping fields (20×) in each brain section. More than 600 randomly selected cells from each subject were counted, and the numbers were used for statistical analysis.
Statistical analysis
Statistical significance was assessed using the two-tailed Student's t-test whenever two groups were compared. When analyzing multiple groups, we used one-way ANOVA to determine statistical significance. For mice that were repeatedly subjected to behavioral tests, we analyzed the data using two-way (genotype × time) ANOVA. Data are presented as mean ± SEM. Calculations were performed with GraphPad Prism software (GraphPad Software, Inc.). A P-value of <0.05 was considered statistically significant.
FUNDING
S.Y. was supported in part by the China Scholarship Council. This work was supported by grants from the National Institutes of Health (NS036232, NS041669 and NS045016) and The State Key Laboratory of Molecular Developmental Biology, China.
ACKNOWLEDGEMENTS
We thank Dr Fenbiao Gao for providing TDP-43 plasmid and Cheryl Strauss for critical reading of the manuscript.
Conflict of Interest statement. None declared.
REFERENCES
- 1.Arai T., Hasegawa M., Akiyama H., Ikeda K., Nonaka T., Mori H., Mann D., Tsuchiya K., Yoshida M., Hashizume Y., et al. TDP-43 is a component of ubiquitin-positive tau-negative inclusions in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Biochem. Bioph. Res. Co. 2006;351:602–611. doi: 10.1016/j.bbrc.2006.10.093. [DOI] [PubMed] [Google Scholar]
- 2.Chen-Plotkin A.S., Lee V.M., Trojanowski J.Q. TAR DNA-binding protein 43 in neurodegenerative disease. Nat. Rev. Neurol. 2010;6:211–220. doi: 10.1038/nrneurol.2010.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Neumann M., Sampathu D.M., Kwong L.K., Truax A.C., Micsenyi M.C., Chou T.T., Bruce J., Schuck T., Grossman M., Clark C.M., et al. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science. 2006;314:130–133. doi: 10.1126/science.1134108. [DOI] [PubMed] [Google Scholar]
- 4.Sreedharan J., Blair I.P., Tripathi V.B., Hu X., Vance C., Rogelj B., Ackerley S., Durnall J.C., Williams K.L., Buratti E., et al. TDP-43 mutations in familial and sporadic amyotrophic lateral sclerosis. Science. 2008;319:1668–1672. doi: 10.1126/science.1154584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pesiridis G.S., Lee V.M.Y., Trojanowski J.Q. Mutations in TDP-43 link glycine-rich domain functions to amyotrophic lateral sclerosis. Hum. Mol. Genet. 2009;18:R156–R162. doi: 10.1093/hmg/ddp303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cohen T.J., Lee V.M., Trojanowski J.Q. TDP-43 functions and pathogenic mechanisms implicated in TDP-43 proteinopathies. Trends. Mol. Med. 2011;17:659–667. doi: 10.1016/j.molmed.2011.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lagier-Tourenne C., Cleveland D.W. Rethinking ALS: The FUS about TDP- 43. Cell. 2009;136:1001–1004. doi: 10.1016/j.cell.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sephton C.F., Good S.K., Atkin S., Dewey C.M., Mayer P., Herz J., Yu G. TDP-43 is a developmentally regulated protein essential for early embryonic development (vol 285, pg 6826, 2010) J. Biol. Chem. 2010;285:38740–38740. doi: 10.1074/jbc.M109.061846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu L.S., Cheng W.C., Hou S.C., Yan Y.T., Jiang S.T., Shen C.K.J. TDP-43, a neuro-pathosignature factor, is essential for early mouse embryogenesis. Genesis. 2010;48:56–62. doi: 10.1002/dvg.20584. [DOI] [PubMed] [Google Scholar]
- 10.Lee E.B., Lee V.M.Y., Trojanowski J.Q. Gains or losses: molecular mechanisms of TDP43-mediated neurodegeneration. Nat. Rev. Neurosci. 2012;13:38–50. doi: 10.1038/nrn3121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wils H., Kleinberger G., Janssens J., Pereson S., Joris G., Cuijt I., Smits V., Ceuterick-de Groote C., Van Broeckhoven C., Kumar-Singh S. TDP-43 transgenic mice develop spastic paralysis and neuronal inclusions characteristic of ALS and frontotemporal lobar degeneration. P. Natl. Acad. Sci. U.S.A. 2010;107:3858–3863. doi: 10.1073/pnas.0912417107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu Y.F., Gendron T.F., Zhang Y.J., Lin W.L., D'Alton S., Sheng H., Casey M.C., Tong J.M., Knight J., Yu X., et al. Wild-type human TDP-43 expression causes TDP-43 phosphorylation, mitochondrial aggregation, motor deficits, and early mortality in transgenic mice. J. Neurosci. 2010;30:10851–10859. doi: 10.1523/JNEUROSCI.1630-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Diaper D.C., Adachi Y., Lazarou L., Greenstein M., Simoes F.A., Di Domenico A., Solomon D.A., Lowe S., Alsubaie R., Cheng D., et al. Drosophila TDP-43 dysfunction in glia and muscle cells cause cytological and behavioural phenotypes that characterize ALS and FTLD. Hum. Mol. Genet. 2013;22:3883–3893. doi: 10.1093/hmg/ddt243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tong J., Huang C., Bi F., Wu Q., Huang B., Liu X., Li F., Zhou H., Xia X.G. Expression of ALS-linked TDP-43 mutant in astrocytes causes non-cell- autonomous motor neuron death in rats. EMBO J. 2013;32:1917–1926. doi: 10.1038/emboj.2013.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lobsiger C.S., Leveland D.W. Glial cells as intrinsic components of non- cell-autonomous neurodegenerative disease. Nat. Neurosci. 2007;10:1355–1360. doi: 10.1038/nn1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kugler S., Meyn L., Holzmuller H., Gerhardt E., Isenmann S., Schulz J.B., Bahr M. Neuron-specific expression of therapeutic proteins: evaluation of different cellular promoters in recombinant adenoviral vectors. Mol. Cell. Neurosci. 2001;17:78–96. doi: 10.1006/mcne.2000.0929. [DOI] [PubMed] [Google Scholar]
- 17.Gong Y.H., Parsadanian A.S., Andreeva A., Snider W.D., Elliott J.L. Restricted expression of G86R Cu/Zn superoxide dismutase in astrocytes results in astrocytosis but does not cause motoneuron degeneration. J. Neurosci. 2000;20:660–665. doi: 10.1523/JNEUROSCI.20-02-00660.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Foust K.D., Nurre E., Montgomery C.L., Hernandez A., Chan C.M., Kaspar B.K. Intravascular AAV9 preferentially targets neonatal neurons and adult astrocytes. Nat. Biotechnol. 2009;27:59–65. doi: 10.1038/nbt.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miyake N., Miyake K., Yamamoto M., Hirai Y., Shimada T. Global gene transfer into the CNS across the BBB after neonatal systemic delivery of single-stranded AAV vectors. Brain. Res. 2011;1389:19–26. doi: 10.1016/j.brainres.2011.03.014. [DOI] [PubMed] [Google Scholar]
- 20.Zhang H., Tan C.F., Mori F., Tanji K., Kakita A., Takahashi H., Wakabayashi K. TDP-43-immunoreactive neuronal and glial inclusions in the neostriatum in amyotrophic lateral sclerosis with and without dementia. Acta. Neuropathol. 2008;115:115–122. doi: 10.1007/s00401-007-0285-7. [DOI] [PubMed] [Google Scholar]
- 21.Keller J.N., Hanni K.B., Markesbery W.R. Possible involvement of proteasome inhibition in aging: implications for oxidative stress. Mech. Ageing. Dev. 2000;113:61–70. doi: 10.1016/s0047-6374(99)00101-3. [DOI] [PubMed] [Google Scholar]
- 22.Necchi D., Lomoio S., Scherini E. Dysfunction of the ubiquitin-proteasome system in the cerebellum of aging Ts65Dn mice. Exp. Neurol. 2011;232:114–118. doi: 10.1016/j.expneurol.2011.08.009. [DOI] [PubMed] [Google Scholar]
- 23.Tydlacka S., Wang C.E., Wang X.J., Li S.H., Li X.J. Differential activities of the ubiquitin-proteasome system in neurons versus glia may account for the preferential accumulation of misfolded proteins in neurons. J. Neurosci. 2008;28:13285–13295. doi: 10.1523/JNEUROSCI.4393-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Romero-Granados R., Fontan-Lozano A., Aguilar-Montilla F.J., Carrion A.M. Postnatal proteasome inhibition induces neurodegeneration and cognitive deficiencies in adult mice: a new model of neurodevelopment syndrome. PloS one. 2011;6:e28927. doi: 10.1371/journal.pone.0028927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garden G.A., La Spada A.R. Intercellular (Mis)communication in neurodegenerative disease. Neuron. 2012;73:886–901. doi: 10.1016/j.neuron.2012.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bradford J., Shin J.Y., Roberts M., Wang C.E., Li X.J., Li S. Expression of mutant huntingtin in mouse brain astrocytes causes age-dependent neurological symptoms. Pro. Natl. Acad. Sci. U.S.A. 2009;106:22480–22485. doi: 10.1073/pnas.0911503106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gu Y.P., Chen Y., Zhang X.F., Li G.W., Wang C.Y., Huang L.Y.M. Neuronal soma-satellite glial cell interactions in sensory ganglia and the participation of purinergic receptors. Neuron Glia. Biol. 2010;6:53–62. doi: 10.1017/S1740925X10000116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Seilhean D., Cazeneuve C., Thuries V., Russaouen O., Millecamps S., Salachas F., Meininger V., LeGuern E., Duyckaerts C. Accumulation of TDP-43 and alpha-actin in an amyotrophic lateral sclerosis patient with the K17I ANG mutation. Acta. Neuropathol. 2009;118:561–573. doi: 10.1007/s00401-009-0545-9. [DOI] [PubMed] [Google Scholar]
- 29.Swarup V., Phaneuf D., Dupre N., Petri S., Strong M., Kriz J., Julien J.P. Deregulation of TDP-43 in amyotrophic lateral sclerosis triggers nuclear factor kappaB-mediated pathogenic pathways. J. Exp. Med. 2011;208:2429–2447. doi: 10.1084/jem.20111313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wegorzewska I., Bell S., Cairns N., Miller T., Baloh R. TDP-43 mutant transgenic mice develop features of ALS and frontotemporal lobar degeneration. Neurology. 2010;74:A98–A98. doi: 10.1073/pnas.0908767106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu Z.S. Does a loss of TDP-43 function cause neurodegeneration? Mol. Neurodegener. 2012;7:27. doi: 10.1186/1750-1326-7-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lu Y., Ferris J., Gao F.B. Frontotemporal dementia and amyotrophic lateral sclerosis-associated disease protein TDP-43 promotes dendritic branching. Mol. Brain. 2009;2:30. doi: 10.1186/1756-6606-2-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shin J.Y., Fang Z.H., Yu Z.X., Wang C.E., Li S.H., Li X.J. Expression of mutant huntingtin in glial cells contributes to neuronal excitotoxicity. J. Cell. Biol. 2005;171:1001–1012. doi: 10.1083/jcb.200508072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang D., Wang C.E., Zhao B., Li W., Ouyang Z., Liu Z., Yang H., Fan P., O'Neill A., Gu W., et al. Expression of Huntington's disease protein results in apoptotic neurons in the brains of cloned transgenic pigs. Hum. Mol. Genet. 2010;19:3983–3994. doi: 10.1093/hmg/ddq313. [DOI] [PMC free article] [PubMed] [Google Scholar]