Summary
Toxoplasma gondii proliferates within host cell vacuoles where the parasite relies on host carbon and nutrients for replication. To assess how T. gondii utilizes these resources, we mapped the carbon metabolism pathways in intracellular and egressed parasite stages. We determined that intracellular T. gondii stages actively catabolize host glucose via a canonical, oxidative tricarboxylic acid (TCA) cycle, a mitochondrial pathway in which organic molecules are broken down to generate energy. These stages also catabolize glutamine via the TCA cycle and an unanticipated γ-aminobutyric acid (GABA) shunt, which generates GABA and additional molecules that enter the TCA cycle. Chemically inhibiting the TCA cycle completely prevents intracellular parasite replication. Parasites lacking the GABA shunt exhibit attenuated growth and are unable to sustain motility under nutrient-limited conditions, suggesting that GABA functions as a short-term energy reserve. Thus, T. gondii tachyzoites have metabolic flexibility that likely allows the parasite to infect diverse cell types.
Introduction
Toxoplasma gondii is an obligate intracellular protozoan parasite of warm-blooded animals and may chronically infect one third of the World’s human population (Weiss and Dubey, 2009). While the majority of T. gondii infections are asymptomatic, severe disease and death can occur in prenatal infections and in immunocompromised individuals (Weiss and Dubey, 2009). Acute infections are mediated by the replicative tachyzoite stages that develop from ingested oocysts or tissue cysts and rapidly disseminate to extra-intestinal tissues. While tachyzoites are effectively cleared in immunocompetent individuals, a small proportion of these parasites differentiate to bradyzoites that can form tissue cysts in the brain, liver and muscle and sustain long-lived chronic infections (Dubey et al., 1998). Bradyzoites can spontaneously differentiate back to tachyzoites, leading to disease activation in immunocompromised patients.
Tachyzoites are capable of invading and proliferating in virtually any nucleated cell in mammalian hosts. In common with other intracellular pathogens, T. gondii tachyzoites must salvage carbon sources and other essential nutrients from their host cell. T. gondii tachyzoites reside within a unique parasitophorous vacuole in infected host cells that is surrounded by membrane that is freely permeable to many host metabolites (Schwab et al., 1994). Despite having access to a variety of carbon sources, T. gondii tachyzoites are generally thought to primarily utilize glucose as carbon source and to be dependent on glycolysis for ATP synthesis (Al-Anouti et al., 2004; Saliba and Kirk, 2001; Pomel et al., 2008; Starnes et al., 2009; Polonais and Soldati-Favre, 2010). While these stages encode all of the genes for a complete tricarboxylic acid cycle and a mitochondrial respiratory chain, they lack a mitochondrial isoform of pyruvate dehydrogenase (PDH) that normally links glycolysis with the TCA cycle via conversion of pyruvate to acetyl-CoA (Fleige et al., 2007; Mullin et al., 2006; Brooks et al., 2010; Nair et al., 2011; Saito et al., 2008; Crawford et al., 2006; Saliba and Kirk, 2001; Pomel et al., 2008; Starnes et al., 2009; Polonais and Soldati-Favre, 2010). The precise role of the TCA cycle in glucose catabolism, if any, therefore remains poorly understood. The possibility that T. gondii tachyzoites could catabolize other carbon sources in the mitochondrion has recently been suggested by the finding that genetic disruption of parasite glucose uptake had little effect on intracellular parasite growth in vitro and virulence in animal models (Blume et al., 2009). Egressed tachyzoites lacking the glucose transporter TgGT1/TgST2 exhibited restored gliding motility in the presence of glutamine, suggesting that these parasites can utilize this amino acid in response to glucose starvation (Blume et al., 2009). Glutamine uptake and catabolism via an unusual bifurcated TCA cycle has been reported in asexual intraerythroctic stages of the related apicomplexan parasite, Plasmodium falciparum (Olszewski et al., 2010), although the functional significance of mitochondrial TCA cycle enzymes in these stages remains undefined (Hino et al., 2012). In T. gondii, there is conflicting data as to whether the TCA cycle and glutaminolysis is important for ATP generation in intracellular tachyzoites under normal growth conditions. While an active respiratory chain is essential for normal intracellular replication (Seeber et al., 2008; Vercesi et al., 1998; Srivastava et al., 1997; Lin et al., 2011), genetic deletion of the TCA cycle enzyme, succinyl-CoA synthetase had only a modest effect on tachyzoite growth in vitro (Fleige et al., 2008).
Here we have used a combination of metabolite profiling and stable isotope labelling approaches to reassess T. gondii central carbon metabolism in intracellular parasites and in egressed tachyzoites. Egressed tachyzoites enter a growth-arrested state but maintain an active gliding motility that is thought to be powered by glycolytic ATP synthesis (Pomel et al., 2008). Intriguingly, egressed tachyzoites can remain motile for 1–2 hours in the absence of carbon sources, suggesting that they may utilize an as yet uncharacterized reserve material (Lin et al., 2011). We find that both intracellular and egressed tachyzoites constitutively catabolize glucose and glutamine under normal growth conditions., Pyruvate was found to be converted to acetyl-CoA and fully catabolized in a complete TCA cycle, despite the absence of an annotated mitochondrial PDH, while glutamine enters the TCA cycle via either α-ketoglutarate or a previously unrecognized γ-aminobutyric acid (GABA) shunt. Furthermore we show that a functional TCA cycle is essential for intracellular replication while the GABA shunt may allow accumulation of energy reserves. These findings provide important insights into T. gondii central carbon metabolism that may underlie the extraordinary capacity of these parasites to replicate within a wide variety of host cells.
Results
Extracellular tachyzoites accumulate high levels of γ-aminobutyrate (GABA)
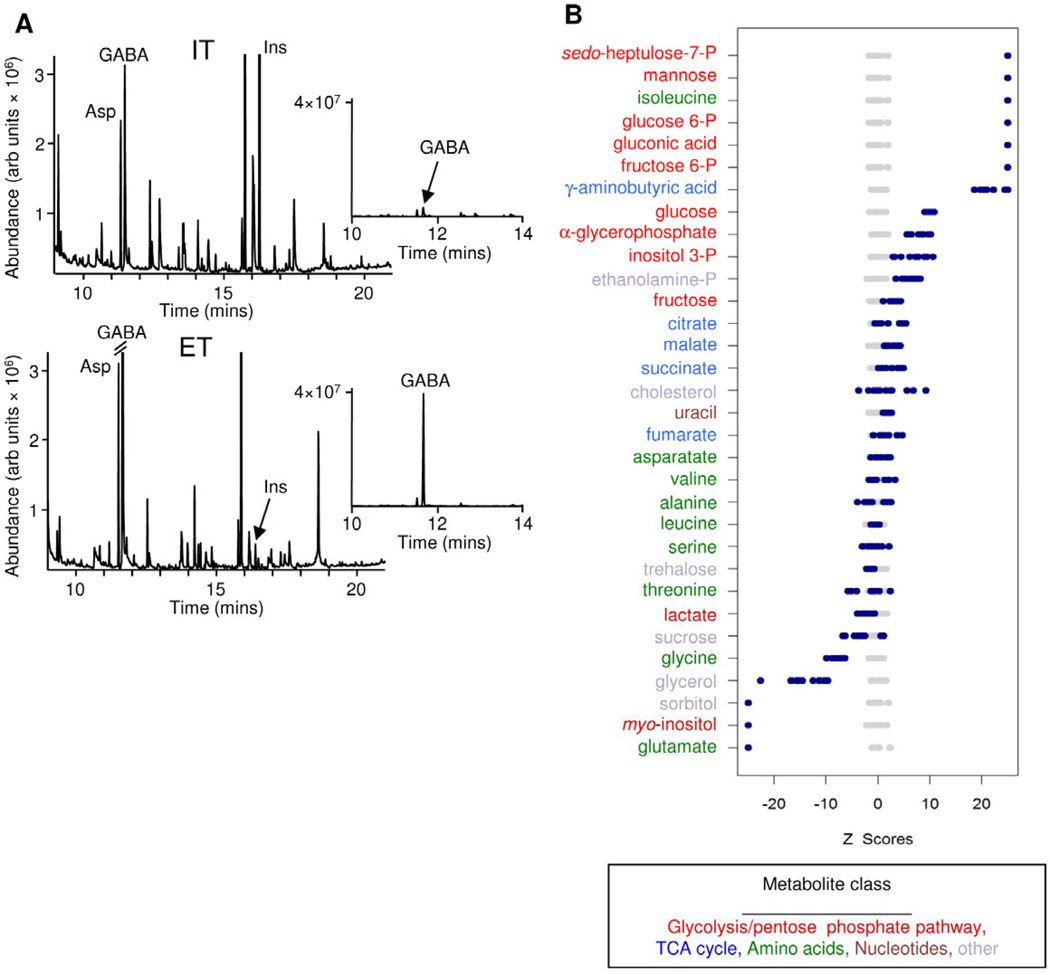
Polar metabolite levels in intracellular and egressed tachyzoite stages of T. gondii RH strain were determined by gas chromatography-mass spectrometry (GC-MS) (Figure 1A, S1). More than 200 metabolite peaks were detected and the two stages were robustly separated by principal component analysis (Figure S1). Significant differences were observed in the normalized intracellular levels of many intermediates in central carbon metabolism (Figure 1B). Specifically, several intermediates involved in glycolysis, the pentose phosphate pathway and inositol metabolism were highly elevated in extracellular tachyzoites (z-score >20), suggesting that glucose uptake increases following tachyzoite egress and/or that fluxes through the lower section of glycolysis decrease. In contrast, similar levels of TCA cycle intermediates were detected in both stages (Figure 1B), indicating the operation of a partial or complete TCA cycle throughout development. The levels of proteinogenic amino acids were generally similar in both stages with the exception of isoleucine and glutamate, which were significantly (z-score >20) increased or decreased in extracellular tachyzoites, respectively (Figure 1B). Unexpectedly, both tachyzoite stages contained high levels of the non-proteinogenic amino acid, γ-aminobutyric acid (GABA) (Figures 1A insert, 1B). GABA was the major amino acid in both stages and levels increased dramatically (20-fold) following tachyzoite egress (0.3 ± 0.06 nmol to 7.3 ± 0.7 nmol)/108 cell equivalents) (Figure 1A, B). GABA levels in uninfected host cells and in the culture medium were >100-fold lower than in the parasite extracts (data not shown). Collectively, these metabolite profiling studies indicate that both tachyzoite stages have an active mitochondrial metabolism that includes a partial or complete TCA cycle and a putative GABA shunt.
Figure 1. Metabolomic analysis of intracellular and extracellular tachyzoites.
(A) GC-MS chromatograms of polar metabolite extracts from intracellular (IT) and extracellular (ET) tachyzoites (2 × 108 cell equivalents). Inserts shows a section of these chromatograms (10 to 14 min) with 10-fold expansion of the y-scale to show changes in γ-aminobutyric acid (GABA) levels in the two stages. Peaks corresponding to aspartate (Asp) and myo -inositol (Ins) that are either unchanged or decreased in extracellular tachyzoites are indicated. (B) Z-score plots of selected metabolites. Plotted z-scores show the mean and standard deviations of individual metabolites in replicate analyses following normalization to the intracellular tachyzoite sample set. Grey circles refer to metabolite levels in intracellular tachyzoites (which typically cluster within 5 s.d. of the mean) while blue circles refer to metabolite levels in extracellular tachyzoites. Metabolite levels that deviate from the mean by >5 s.d. are considered significant (Note that the z-score plots are truncated at 25 s.d. for clarity). See also Figure S1.
Intracellular and extracellular tachyzoites catabolize glucose in a complete TCA cycle
To further define the metabolic capacity of T. gondii tachyzoites, infected HFF and egressed tachyzoites were metabolically labelled with 13C-U-glucose. 13C-U-glucose is internalized by infected HFFs and rapidly assimilated by intracellular tachyzoites, supporting the notion that these stages have direct access to host metabolites (Ramakrishnan et al., 2011). Label was incorporated into a range of glycolytic and pentose phosphate pathway intermediates, as well as the major long chain fatty acids of cellular phospholipids (Figure 2A). We have recently shown that the labelling of fatty acid with 13C-glucose reflects the activity of the apicoplast FASII fatty acid synthase that utilizes acetyl-CoA derived from glycolytic intermediates (Ramakrishnan et al., 2011). Label derived from 13C-glucose was also incorporated into glycolytic and pentose phosphate pathway intermediates in egressed tachyzoites (Figure 2A). However, in contrast to the situation in intracellular tachyzoites, negligible labelling of fatty acids was observed in egressed tachyzoites (Figure 2A), indicating that de novo fatty acid synthesis is repressed in this non-proliferating stage.
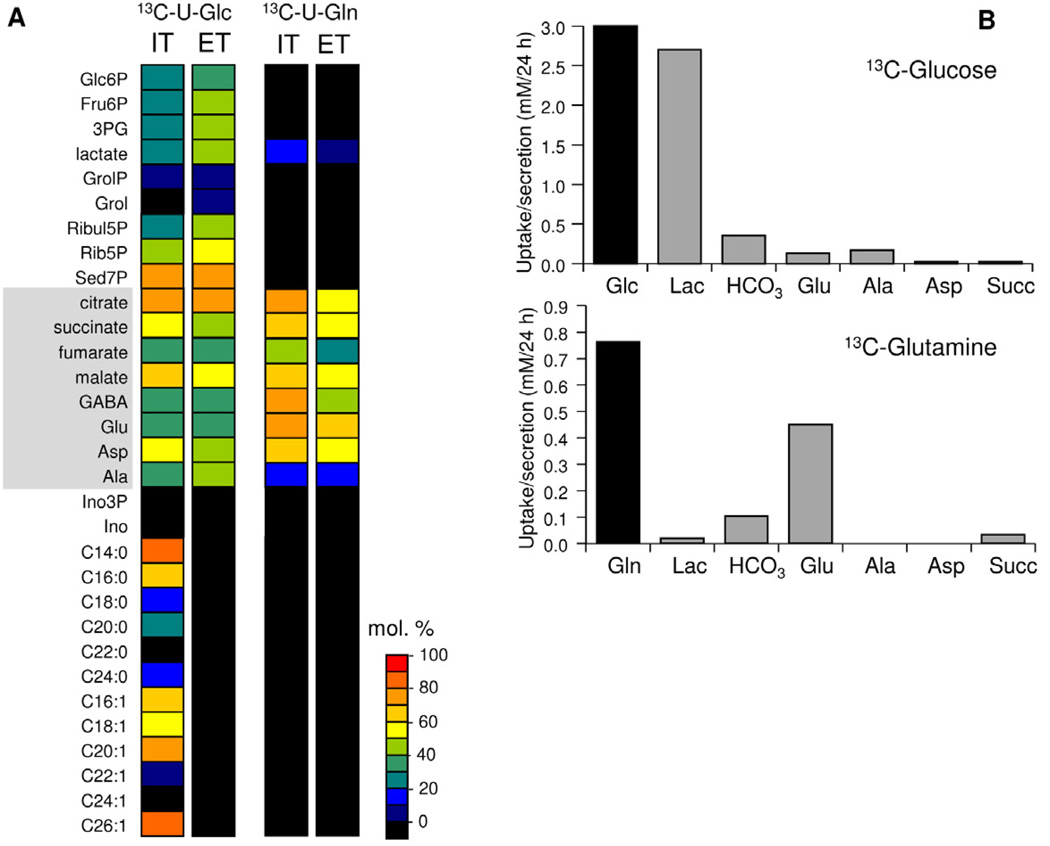
Figure 2. T. gondii tachyzoites catabolize glucose in a complete TCA cycle.
(A) Infected HFF or egressed tachyzoites (ET) were suspended in medium containing either 13C-U-glucose or 13C-U-glutamine for 4 hr. Intracellular tachyzoites (IT) were isolated from host material prior to metabolite extraction. Incorporation of 13C into selected polar metabolites and fatty acids (derived from total lipid extracts) was quantified by GC-MS and levels (mol percent containing one or more 13C carbons) after correction for natural abundance are represented by heat plots. (B) Egressed tachyzoites were incubated in full medium containing either 13C-U-glucose (upper panel) or 13C-U-glutamine (lower panel) in place of naturally labelled glucose or glutamine, respectively. Culture medium was collected at 6, 12 and 24 hr and analysed by 13C-NMR. Rates of utilization of each carbon source are shown in black, while rate of secretion of lactate (Lac), CO2 (detected as H13CO3), glutamate (Glu), alanine (Ala), aspartate (Asp) and succinate (Suc) are shown in grey. See also Figure S2.
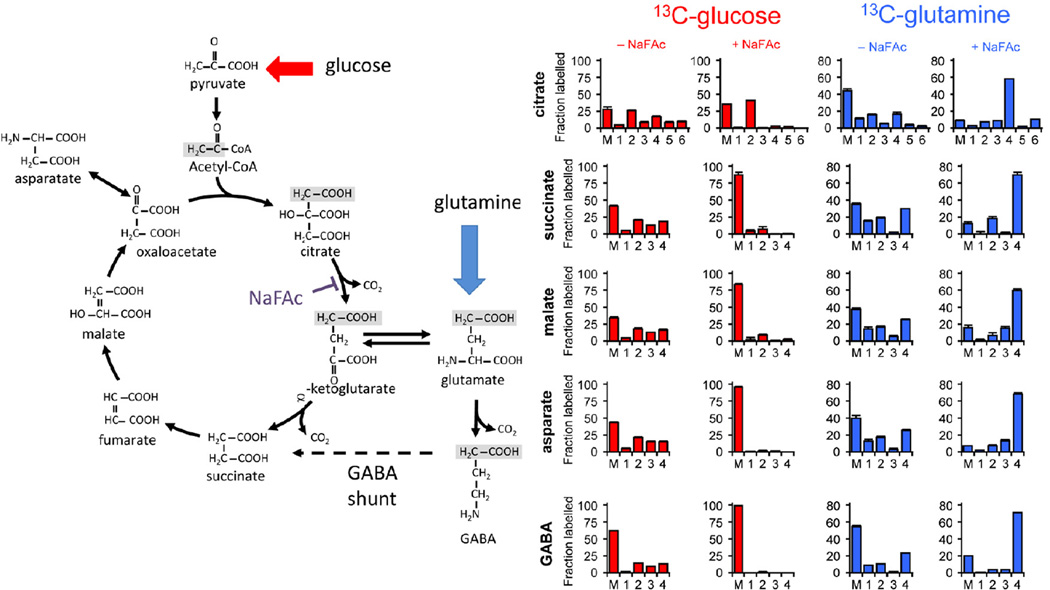
Unexpectedly, label derived from 13C-U-glucose was efficiently incorporated into TCA cycle intermediates and interconnected amino acids, such as glutamate and aspartate, in both developmental stages (Figure 2A). The major mass isotopomers of citrate in 13C-glucose-fed tachyzoites contained two, four or six labelled carbons, indicating the operation of a canonical TCA cycle (Figure 3). Similarly, the presence of +1 and +3 isotopomers could arise through the various decarboxylation reactions in the TCA cycle (consistent with production of H13CO3 (Figure 2B)) or via the direct conversion of 13C3-pyruvate/13C3-phosphoenolpyruvate (PEP) to 13C3-oxaloacetate by the anaplerotic enzymes, pyruvate carboxylase or PEP carboxykinase. To distinguish between these possibilities, tachyzoites were labelled with 13C-U-glucose in the presence of sodium fluoroacetate (NaFAc), a potent inhibitor of the TCA cycle enzyme aconitase (Saunders et al., 2011). Treatment of tachyzoites with NaFAc resulted in a 160-fold increase in citrate and aconitate levels, consistent with effective inhibition of the aconitase reaction (Figure S3). The accumulated citrate was almost exclusively labelled with +2 labelled carbons, while labelling of other TCA cycle intermediates was greatly reduced (Figure 3). GABA was similarly labelled to other TCA cycle intermediates (Figure 3) suggesting that the carbon backbone of this amino acid is derived in part from TCA cycle α-ketoglutarate and de novo synthesized glutamate. Consistent with this conclusion, the labelling of GABA in 13C-glucose-fed parasites was completely inhibited by NaFAc (Figure 3). Together, these findings demonstrate that glycolytic end-products can be fully catabolized in a canonical TCA cycle in T. gondii tachyzoites and that this cycle can also be used to generate amino acids such as aspartate, glutamate and GABA.
Figure 3. Complete TCA cycle and GABA shunt in T. gondii tachyzoites.
Left panel: The diagram represents a reconstruction of a complete TCA cycle and GABA shunt in T. gondii tachyzoites inferred from isotopomer analysis. Acetyl-CoA generated from 13C-glucose is used to synthesize citrate. Grey boxes indicate the fate of carbons in the incoming acetyl group in early intermediates of the TCA cycle. Uniformly labelled citrate is generated through multiple rounds through the cycle. Inputs of 5-carbon and 4-carbon skeletons from glutamate or GABA comprise the major anaplerotic influxes. NaFAc leads to inhibition of the TCA enzyme, aconitase. Right panel: Abundance of different TCA cycle isotopomers after labelling of egressed tachyzoites with 13C-U-glucose (red graphs) or 13C-glutamine (blue graphs) for 4 hr. Parasites were treated with or without NaFAc at the initiation of labelling. The numbers on the x-axis indicate the number of labelled carbons in each metabolite. The y-axis indicates the fractional abundance of each mass isotopomer. Data are represented as mean +/− SEM, where n = 5. See also Figure S3.
Intracellular and egressed tachyzoites co-utilize glutamine in addition to glucose
Egressed tachyzoites can switch to using glutamine as a carbon source when glucose up-take is inhibited (Blume et al., 2009). To investigate whether glutamine is used constitutively under normal growth conditions, infected HFF and egressed tachyzoites were cultivated in glucose-replete medium containing 13C-U-glutamine. All of the TCA cycle intermediates were strongly labelled with generation of +2 and +4 isotopomers (Figure 3). Addition of NaFAc resulted in loss of +2 isotopomers and enrichment in +4 isotopomers in all labelled intermediates, consistent with entry at α-ketoglutarate and a block prior to citrate synthesis (Figure 3). GABA exhibited the same isotopomer labelling pattern as other TCA cycle intermediates indicating that this metabolite can be derived from exogenous glutamine as well as de novo-synthesized glutamate, and the potential operation of a GABA shunt (Figure 3A). 13C-glutamine-fed tachyzoites also exhibited low but significant levels of labelling in lactate and alanine, reflecting the decarboxylation of oxaloacetic acid or malate to pyruvate (Figure 2A).
To further investigate the carbon source preference of egressed tachyzoites, these parasites were also labelled with 13C-acetate, 13C-leucine or 13C-glycerol. With the exception of 13C-acetate (maximum labelling of citrate, 10%), negligible labelling of parasite metabolites was detected (Figure S2). These data suggest that egressed tachyzoites primarily utilize glucose and glutamine under nutrient-replete conditions. They also provide additional evidence that the acetyl-CoA used in the TCA cycle is not derived from the single, cytoplasmically located acetyl-CoA synthetase (Mazumdar and Striepen, 2007).
The TCA cycle is required for intracellular growth of T. gondii tachyzoites
Quantitative 13C-NMR analysis of the culture supernatant of 13C-U-glucose-fed egressed tachyzoites revealed that nearly all (95%) of the internalized 13C-glucose was catabolized to lactate and H13CO3 (a proxy for CO2) or secreted as succinate, glutamate, alanine and aspartate (Figure 2B) indicating minimal incorporation of glucose into biomass in these non-dividing stages. 13C-glutamine uptake was approximately 25% the rate of glucose uptake (Figure 2B) and was predominantly catabolized to H13CO3 and glutamate (Figure 2B). Assuming that the bulk of the H13CO3 production is associated with the TCA cycle and that the yield of ATP from glycolysis and oxidative phosphorylation is 2 and 36 mole/mole of glucose catabolized, respectively, these data suggest that egressed tachyzoites derive the bulk (>80%) of their ATP energy needs from the mitochondrial catabolism of glucose and glutamine.
To assess whether the TCA cycle is also important in intracellular stages, HFF were infected with freshly egressed tachyzoites in the presence of NaFAc. HFF have a highly glycolytic metabolism (Lemons et al., 2010) and NaFAc has no effect on HFF growth or their susceptibility to parasite infection (Figure S4). However, NaFAc completely inhibited plaque formation when added prior to infection or 2 hr after infection (Figure 4C), suggesting that a complete TCA cycle activity is required for parasite growth in these host cells. Significantly, growth inhibition was not reversed by addition of 13 mM glutamine (Figure 4D), suggesting that a defect in aconitase activity cannot be rescued solely by the supply of down-stream TCA cycle metabolites.
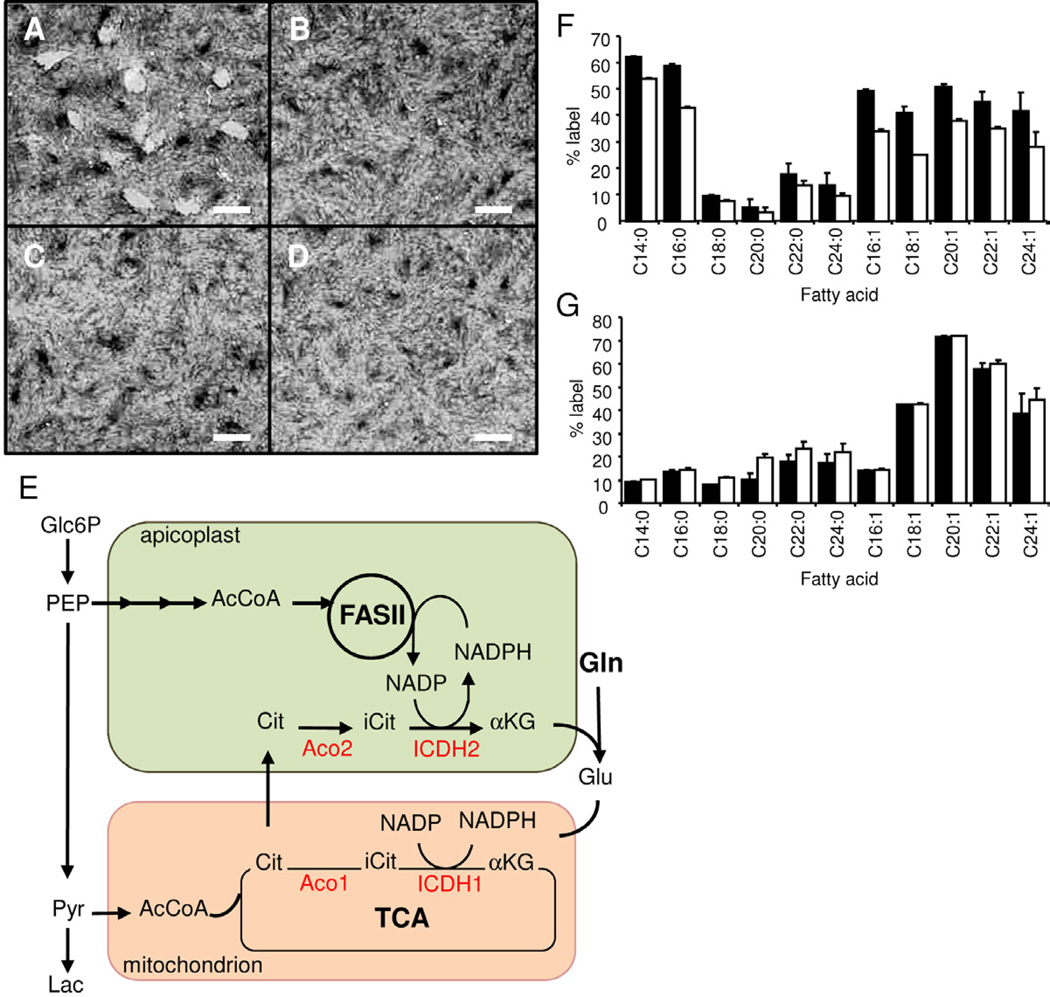
Figure 4. T. gondii tachyzoites require a functional TCA cycle for intracellular growth.
(A–D) HFF were infected with freshly-egressed tachyzoites (103) in the presence or absence of 0.2 mM NaFAc. (A) Infection in the absence of NaFAc. (B & C) Tachyzoites were pre-treated with NaFAc 4 hr prior to infection and infected cultures maintained in medium containing NaFAc. In (C), the medium was supplemented with an additional 13 mM glutamine. (D) NaFAc was added 2 hr after initiation of infection. The appearance of plaques was monitored by crystal violet staining after 6 days. Bars represent 15 mm. (E) Schematic reconstruction of the proposed metabolic pathways and compartmentalisation of glycolysis, the TCA cycle and FASII fatty acid biosynthesis. (F) 13C-glucose and (G) 13C-acetate incorporation into tachyzoite fatty acids. Nomenclature Cx:y is shown where x is the number of carbons and y is the number of double bonds in the fatty acid chain. Error bars indicate standard deviation, where n = 6. Abbreviations: AcCoA, acetyl-CoA; αKG, α-ketoglutarate; Cit, citrate; G6P, glucose 6-phosphate; Glu, glutamate; Gln, glutamine; GABA, γ-aminobutyric acid; iCit, isocitrate; Lac, lactate; OAA, oxaloacetic acid; PEP, phosphoenolpyruvate; Pyr, pyruvate; Aco, aconitase; ICDH, isocitrate dehydrogenase. See also Figure S4.
Inhibition of the TCA cycle results in partial inhibition of de novo fatty acid biosynthesis in the apicoplast
T. gondii express apicoplast targeted isoforms of aconitase and an NADP+-dependent isocitrate dehydrogenase (Pino et al., 2007). These enzymes could in principle convert mitochondrial-derived citrate to α-ketoglutarate, providing this organelle with a source of NADPH for FASII-fatty acid biosynthesis (Figure 4E). To investigate whether disruption of citrate synthesis impacts on apicoplast FASII synthesis, intracellular tachyzoites were labelled with 13C-glucose in the presence or absence of NaFAc. Addition of NaFAc resulted in a reproducible inhibition of FASII-dependent fatty acid biosynthesis (~20%), as measured by incorporation of 13C-U-glucose into long chain fatty acids (Ramakrishnan et al., 2011) (Figure 4F). Inhibition of the FASII pathway was not due to a global deficiency in ATP synthesis or accumulation of inhibitory fluoroacetate-CoA, as NaFAc treatment did not significantly affect the incorporation of 13C-acetate into total fatty acids (Figure 4G). The latter primarily reflects fatty acid biosynthesis by the ER-located fatty acid elongase system (Ramakrishnan et al., 2011). These data support the presence of a shunt in which mitochondrial citrate is transported to the apicoplast and converted to α-ketoglutarate with regeneration of NADPH (Figure 4E). The incomplete inhibition of FASII-fatty acid biosynthesis in the presence of NaFAc likely reflects the presence of other mechanisms for regenerating NADPH in the apicoplast (Brooks et al., 2010).
Identification of enzymes involved in GABA synthesis
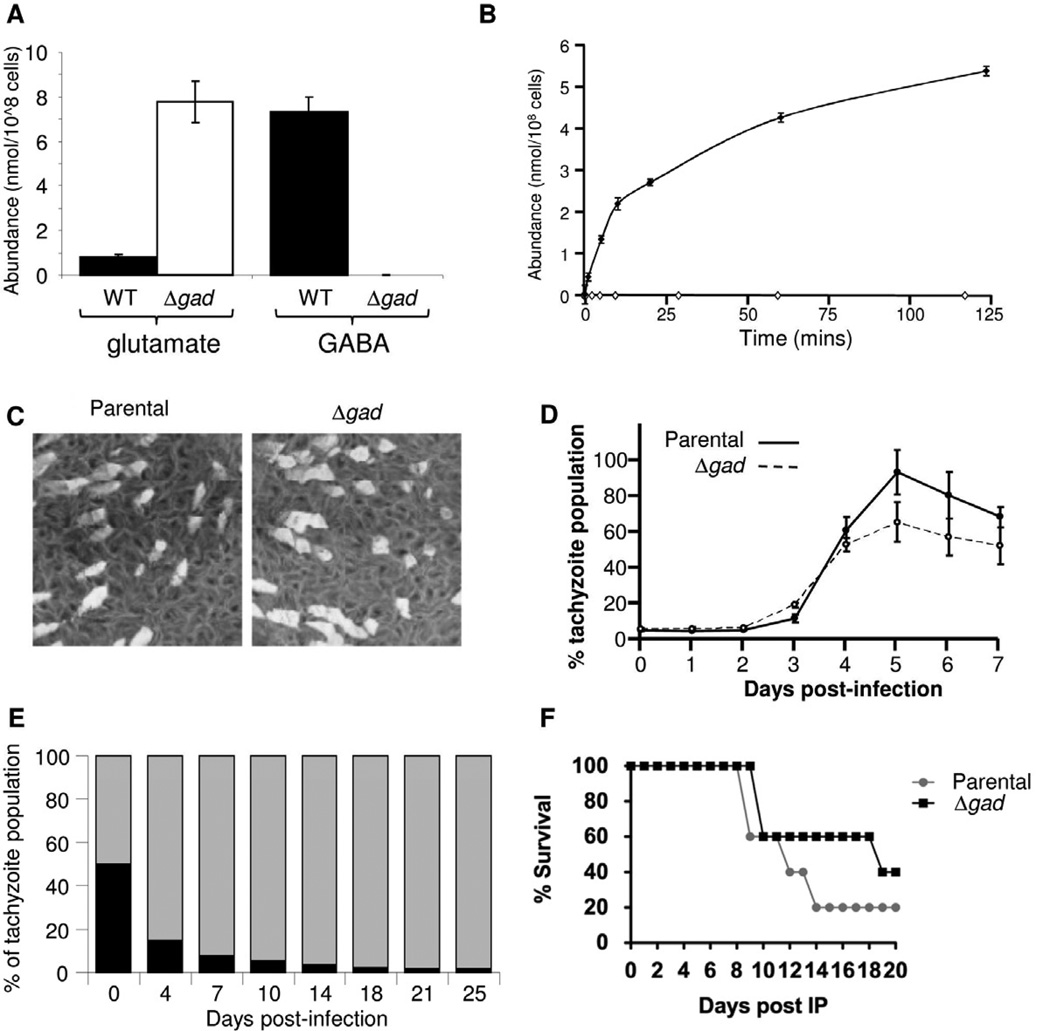
The T. gondii and P. falciparum genomes encode a putative lysine decarboxylase (TGME49_080700 and PFD0285c, respectively; Table S1) which shares conserved domains with members of the bacterial amino acid decarboxylase superfamily (Cook et al., 2007) that include glutamate decarboxylase (GAD, EC 4.1.1.15), the first enzyme in the GABA shunt. However, cadaverine, the product of lysine decarboxylation, was not detectable in our metabolomic analyses, raising the possibility that these genes encode the glutamate decarboxylase. To functionally characterize the putative TgGAD gene, a null mutant was generated in the RHΔKu80 parasite line (Huynh and Carruthers, 2009) (Figure S5). A TgGAD deletion clone (Δgad) was identified by PCR (Figure S5) and successful ablation of the gene verified by Southern blot analysis (Figure S5). The Δgad tachyzoites lacked detectable levels of GABA and contained highly elevated levels of glutamate (Figure 5A), along with significant changes in abundance of a number of other metabolites involved in central carbon metabolism (Figure S6). Cell lysates of Δgad, also lacked GAD activity, as measured by incubation of parasite cell lysates with 13C-glutamate and analysis of the products by GC-MS (Figure 5B). Intriguingly, GAD activity in wild type parasites was only observed when lysates were supplemented with ATP (Figure S6). In the absence of ATP, the major product synthesized was α-ketoglutarate. No GABA production was observed in cell lysates prepared from the Δgad knockout line (Figure 5A,B). These studies confirm that TGME49_080700 encodes the only functional TgGAD and that this enzyme is directly or indirectly activated by ATP.
Figure 5. Loss of GAD results in complete ablation of GABA biosynthesis and partial attenuation of parasite propagation.
(A) Intracellular GABA and glutamate levels in parental and Δgad tachyzoites. (B) Cell-free extracts of T. gondii parental (filled circles) and Δgad tachyzoites (open circles) were incubated with 10 mM 13C-U-glutamate in the presence of 1 mM ATP. The synthesis of GABA at indicated time points was measured by GC-MS. Error bars represent standard deviation, where n = 3. (C) Parental and Δgad parasite lines are both capable of generating plaques in HFF monolayers. (D) Fluorescence growth assays showing similar growth rates (no significant difference) for parental (solid line, closed circles), and Δgad (dotted line, open squares) strains. Each data point represents the mean of 6 wells and the error bars indicate standard deviation where n = 6. (E) Equal numbers of wild type parasites (grey bar) expressing the dTomato fluorescent protein and Δgad mutant tachyzoites (black bar) were used to infect HFF. The recovery of fluorescent and non-fluorescent parasites was determined by FACS analysis of 2 × 106 cells at indicated days. The Δgad parasite line was rapidly outcompeted by the WT line. (F) Swiss Webster mice were infected with Type-I parental and Δgad tachyzoites (10 parasites/mouse) and mouse survival observed over 20 days. See also Figure S5.
The T. gondii genomes also contain putative genes for other enzymes in the GABA shunt. These include the glutamate transamidase (GDC, EC 2.6.1.19), a succinic-semialdehyde dehydrogenase (SSDH, EC 1.2.1.16) that was localized to the mitochondrion (Fig S5) and a GABA transporter (Table S1). Homologs for most of these genes were also identified in the genomes of Plasmodium falciparum and Neospora caninum (Table S1), consistent with the operation of a similar pathway in these parasites.
The GABA shunt impacts on tachyzoite fitness in vivo and in vitro but is not essential
Both the parental and Δgad mutant lines induced similar sized plaques in HFF monolayers when assayed independently (Figure 5C,D). However, the Δgad mutant displayed a clear attenuation of growth in a competition infection experiment with its parental strain (Figure 5E). This selective advantage was also evident in infections in vivo, where BALB/c mice infected with wild type succumb to infection faster than those infected with the mutant (Figure 5F). The TgGAD gene was also deleted in the less virulent type II Prugniaud strain (Fox et al., 2009). The PruΔKu80Δgad mutant lacked the capacity to synthesize GABA, but was able to form plaques in complete growth medium in vitro (Figure S5) and able to differentiate to bradyzoites in an in vitro assay (Fux et al., 2007) (Figure S5). As with RHΔgad, this mutant exhibited a modest reduction in virulence in mice compared to the parental strain based on survival, time to death or weight loss (Figure S5). These data suggest that the GABA shunt increases parasite fitness, but that this pathway is not essential for survival.
The GABA shunt is required to maintain tachyzoite motility under nutrient limited conditions
T. gondii tachyzoites are able to sustain normal motility and virulence for at least one hour after egress in the absence of exogenous carbon sources, suggesting that they may have a short term energy reserve (Lin et al., 2011). To investigate whether GABA is catabolized under nutrient limiting conditions, egressed tachyzoites were suspended in PBS with or without exogenous carbon sources (Figure 6A). Intracellular GABA levels were maintained at very high levels when suspended in PBS containing exogenous glutamine. In contrast GABA levels were rapidly depleted when tachyzoites were suspended in PBS or PBS containing glucose (Figure 6A). GABA levels were further depleted by the addition of 2-deoxyglucose, an inhibitor of glycolysis (Figure 6A). These results suggest that the large intracellular pool of GABA in egressed tachyzoites is utilized when either carbon or amino acid sources are limiting.
Figure 6. GABA can be used to sustain tachyzoite motility under nutrient limited conditions.
(A) Wild type (RH) tachyzoites were suspended in medium containing glucose and/or glutamine as carbon source, PBS or PBS containing 2-deoxyglucose (DOG). Intracellular GABA levels were measured at the indicated time points over 4 hr. GABA was rapidly depleted in the absence of glutamine. Error bars indicate standard deviation, where n = 2 and results are representative of 4 biological replicates. (B) Parental and Δgad tachyzoites were suspended in medium lacking carbon sources and allowed to glide on poly-L-lysine-coated cover slips after addition of 2 µM ionophore to stimulate motility. Parasites were fixed and the resulting gliding trails were visualized using α-SAG1 antibodies. The Δgad mutant displays a clear defect in gliding motility under these conditions. (C) Parental and Δgad tachyzoites were incubated in HBSS-HEPES with or without NaFAc, and different carbon sources, as indicated. The average trail length/parasite was documented in 10 fields (>100 parasites). Counts are of representative frames from two independent experiments. Data are represented as mean +/− SEM. See also Figure S6.
Tachyzoites are dependent on gliding motility to reach and invade new host cells. To determine whether the TCA cycle and GABA shunt are required for gliding motility, egressed tachyzoites of the wild type and Δgad strains were suspended in PBS containing NaFAc or different carbon sources and motility monitored by fluorescence microscopy. Consistent with previous reports, wild type parasites retained active gliding motility in the absence of exogenous carbon sources for several hours (Figure 6B, C) (Lin et al., 2011). Parasite motility was completely blocked by NaFAc-treatment, although motility in the presence of NaFAc was largely restored by addition of glutamine (Figure 6C), suggesting that operation of a partial TCA cycle is sufficient to sustain the energy needs of parasite gliding. Strikingly, the Δgad mutant had a comparable defect in gliding motility to that of wild type parasites treated with NaFAc (Figure 6C). The motility of the Δgad mutant was largely restored by addition of glutamine or GABA, although in contrast to the situation in wild type parasites, addition of exogenous glutamine did not restore motility when the Δgad mutant was treated with NaFAc (Figure 6C). Collectively, these data suggest that the GABA shunt plays a key role in regulating the catabolism of glutamine/glutamate in the TCA cycle and that this pathway cannot be by-passed by the direct conversion of glutamate to α-ketoglutarate under the conditions tested.
Discussion
Metabolite profiling and stable isotope labelling have been used to identify pathways of carbon metabolism in intracellular and egressed T. gondii tachyzoite stages. We show that these parasite stages constitutively utilize both glucose and glutamine as major carbon sources and that the energy metabolism of these stages is dependent on mitochondrial metabolism and oxidative phosphorylation. The highly efficient and flexible energy metabolism of T. gondii tachyzoites may underlie the extraordinary capacity of these parasites to proliferate within a wide range of host cells.
Previous studies have suggested that intracellular tachyzoites are primarily dependent on glucose uptake and glycolysis for ATP synthesis. Free glucose is present at relatively high concentrations in the cytosol of mammalian cells (1–6 mM) (Behjousiar et al., 2012) and is likely to be passively transported across the limiting membrane of the parasitophorous vacuole. 13C-glucose internalized by infected HFF was rapidly internalized by intracellular tachyzoites and incorporated into intermediates in the glycolytic and pentose phosphate pathways. Unexpectedly, intermediates in the TCA cycle were also labelled with 13C-glucose, and contained an isotopomer fingerprint consistent with the operation of a canonical oxidative TCA cycle. Chemical inhibition of the parasite aconitase with NaFAc effectively blocked tachyzoite growth in HFF monolayers indicating that the mitochondrial catabolism of glucose is essential for growth of intracellular tachyzoites. This phenotype is more severe than that observed following the inducible down-regulation of the TCA cycle enzyme, succinyl-CoA synthetase (Fleige et al., 2008), which is now explained by the presence of the GABA shunt that by-passes the steps catalysed by α-ketoglutarate dehydrogenase and succinyl-CoA synthetase (Figure 7). The primary function of this cyclical TCA cycle is the generation of reducing equivalents for oxidative phosphorylation, as genetic or chemical inhibition of the T. gondii mitochondrial respiratory chain, including the unusual Type II NADH dehydrogenases inhibits intracellular growth (Lin et al., 2009; Lin et al., 2011). However, as shown here and proposed elsewhere, the TCA cycle may also generate anabolic precursors for fatty acid and heme biosynthesis (Mazumdar et al., 2006; Ramakrishnan et al., 2011).
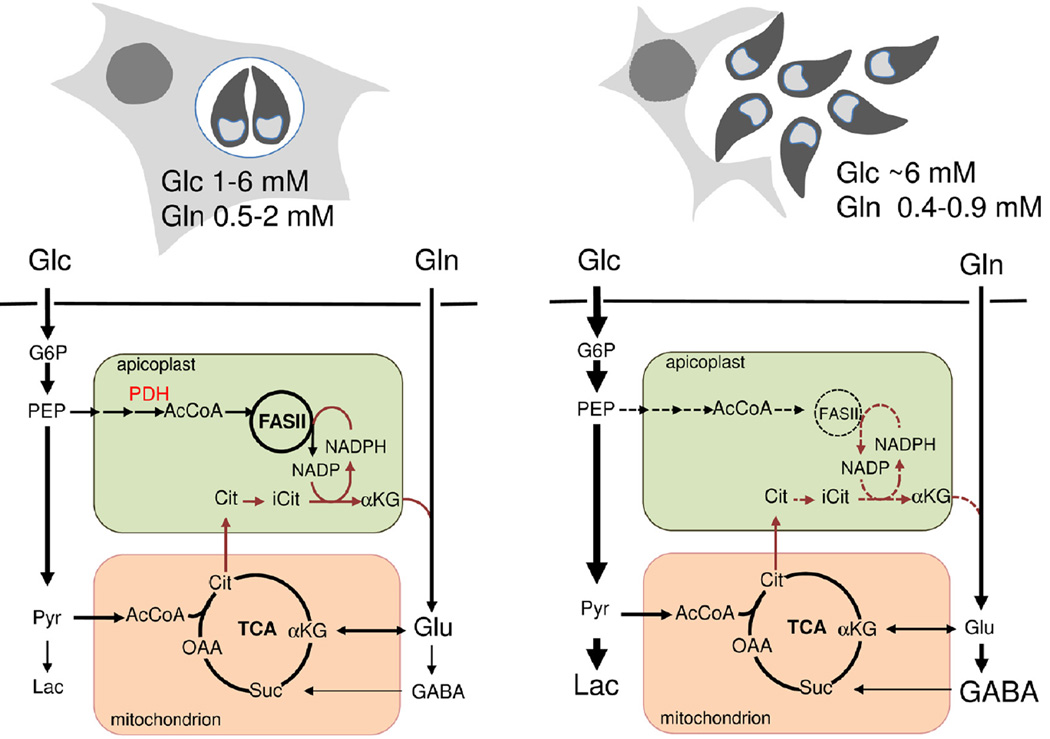
Figure 7. Role of the TCA cycle in intracellular and egressed T. gondii tachyzoites.
Intracellular tachyzoites co-utilize glucose and glutamine scavenged from the host and both carbon sources are catabolized in mitochondria via a canonical oxidative TCA cycle (left panel). Glucose is also used to fuel the FASII-dependent pathway of fatty acid biosynthesis in the apicoplast. Inhibition of tachyzoite aconitase (comprising mitochondrial and apicoplast isoforms) results in a reduction of FASII biosynthesis, providing evidence that a citrate shunt between the mitochondrion and apicoplast (brown lines) is at least partially required for regeneration of reducing equivalents for apicoplast fatty acid synthesis. Tachyzoites are likely exposed to elevated glucose levels following host cell egress (right panel). However, these stages continue to co-utilize glutamine and are dependent on operation of the TCA cycle for most of their ATP synthesis and normal gliding motility. The GABA shunt identified in this study involves at least three enzymes and a putative mitochondrial transporter (steps not shown). GABA accumulated under glutamine-replete conditions may function as a short-term energy reserve under nutrient limiting conditions. For Abbreviations see Figure 3.
The mechanism by which pyruvate is converted to acetyl-CoA remains to be established as T. gondii and other apicomplexan parasites lack a mitochondrial PDH complex (Seeber et al., 2008; Polonais and Soldati-Favre, 2010; Danne et al., 2012). In principle, acetyl-CoA generated by the apicoplast PDH could be transported to the mitochondrion. However, organellar membranes are impermeable to acetyl-CoA and inter-organellar transport typically involves a carnitine/acetyl-carnitine shuttle, for which there is no precedent in the case of plastid membranes. Moreover, exogenous 13C-acetate was only minimally incorporated into TCA cycle intermediates indicating that acetyl-CoA generated by the single cytoplasmic acetyl-CoA synthetase (Mazumdar and Striepen, 2007) is not a major source of mitochondrial acetyl-CoA. Alternatively, pyruvate could be converted to acetyl-CoA by the mitochondrial-localized branched chain α-keto acid dehydrogenase (BCKDH) complex (Polonais and Soldati-Favre, 2010), which in some cases can utilize pyruvate as substrate (Heath et al., 2007; Pettit et al., 1978). In support of this hypothesis, all members of the Apicomplexa retain the subunits of the BCKDH complex, even when other enzymes required for catabolism of branched chain amino acids have been lost (Polonais and Soldati-Favre, 2010). We also found little evidence for catabolism of branched chain amino acids in T. gondii, further supporting the possibility that the BCKDH is primarily used for mitochondrial pyruvate catabolism. The establishment of an alternative mechanism for coupling glycolysis with TCA cycle appears to have evolved prior to the divergence of apicomplexa from related protist groups and the evolution of obligate parasitic life style (Danne et al., 2012).
The finding that intracellular tachyzoites co-utilize glutamine under glucose-replete conditions further highlighted the metabolic flexibility of these parasites and the potential importance of mitochondrial metabolism. Glutamine is present at high concentrations (0.5–2 mM) in the cytosol of mammalian cells (Behjousiar et al., 2012) and the uptake and catabolism of this amino acid by intracellular tachyzoites appears to be sufficient to sustain oxidative phosphorylation in the absence of glucose uptake (Blume et al., 2009). Glutaminolysis could also be required to top up TCA cycle intermediates exported from the mitochondrion for anabolic processes. In particular, we provide evidence for a citrate shunt involving apicoplast-located isoforms of aconitase and the NADP-dependent isocitrate dehydrogenase (Denton et al., 1996; Pino et al., 2007). This shunt could regenerate apicoplast pools of NADPH that are required for FASII-dependent fatty acid biosynthesis in intracellular tachyzoites. However, other mechanisms for regenerating NADPH in the apicoplast must also exist, as inhibition of citrate synthesis only partially blocked fatty acid synthesis (Fast et al., 2001).
Anabolic pathways, such as fatty acid biosynthesis, are repressed in egressed tachyzoites and most of the glucose and glutamine taken up by this stage is used to generate ATP for gliding motility and host cell invasion. 13C-NMR analysis of the secreted end-products of egressed tachyzoites indicated that glucose is primarily catabolized to lactate, while approximately 20% is oxidized in the TCA cycle. Given the relative yield of ATP from glycolysis and oxidative phosphorylation, these data suggest that oxidative phosphorylation could accounts for >90% of ATP synthesis in egressed tachyzoites. Consistent with this conclusion, chemical inhibition of aconitase resulted in a marked decrease in gliding motility, which was largely restored by addition of exogenous glutamine. While previous studies have highlighted the role of glycolysis in supplying ATP for motility (Pomel et al., 2008; Lin et al., 2011), disruption or inhibition of glycolytic enzymes would also lead to reduced TCA cycle flux. Thus, TCA cycle fluxes appear to be important for both intracellular and egressed tachyzoites.
The identification of a functional GABA shunt in T. gondii tachyzoites highlighted the utility of metabolomic approaches for discovering parasite metabolic pathways that are not anticipated from genome annotations. Genes encoding a putative glutamate decarboxylase, a glutamate deaminase, a succinic semialdehyde dehydrogenase, and a GABA transporter were subsequently identified in the T. gondii genomes. Bioinformatic analyses indicate that this shunt is present in some other apicomplexa, including P. falciparum (Figure S5, Table S1), consistent with the reported presence of GABA in asexual red blood cell stages (Teng et al., 2009). T. gondii mutant strains lacking GAD exhibited reduced fitness both in vitro, in competition with the parental strain, and in vivo, in murine infections. The relatively modest growth phenotype of the Δgad mutant is reminiscent of the mild growth phenotype of T. gondii glucose transporter mutants (Blume et al., 2009), and underlies the remarkable flexibility of these parasites to adapt to altered fluxes in central carbon metabolism. This robust flexibility would allow tachyzoites to adapt to major fluctuations in nutrient supply (during for example, tachyzoite egress) without the need for transcriptional adjustment. A central role of allosteric or metabolic regulation of TCA cycle fluxes was suggested by the finding that GAD activity was strongly activated when cell lysates were charged with ATP. The conversion of glutamate to GABA under ATP-replete conditions may be required to prevent overproduction of NAD(P)H and leakage of reactive oxygen species from the mitochondrial respiratory chain (Wellen and Thompson, 2010; Murphy, 2009). GABA accumulated under these conditions could subsequently be used as a short-term energy reserve material. The presence of such an internal reserve has been posited based on the ability of egressed tachyzoites to retain full motility, ATP levels and invasiveness for at least one hour in the absence of any exogenous carbon sources (Lin et al., 2011). We show that the accumulated GABA is utilized under these conditions and that the Δgad mutant has a severe motility defect in the absence of carbon sources. Interestingly, glutamine did not restore the motility of the Δgad mutant in the presence of NaFAc, suggesting that an active GABA shunt may be required to maintain a high flux through a partial TCA cycle. The GABA shunt may thus have an important role in regulating carbon metabolism under both nutrient replete and starvation conditions.
Finally, the finding that non-proliferating tachyzoites stages can both synthesize GABA and utilize exogenous GABA is of interest given the strong tropism that these parasites display for the central nervous system during chronic infections. While our current studies suggest that GABA is not actively secreted, it is possible that release of GABA from dead or slow-growing bradyzoites stages could underlie the changes in behaviour, mood and mental health of T. gondii-infected humans and mice (Webster, 2007; Lamberton et al., 2008; Webster and McConkey, 2010).
Experimental Procedures
Parasite culture and construction of mutants
T. gondii RH (wild type), RHΔKu80 and RHΔKu80Δgad, PruΔKu80, and PruΔKu80Δgad tachyzoites were maintained by passage through human foreskin fibroblasts (HFF) or in hTERT-BJ1 (Clontech) cells in Dulbecco’s modified Eagle’s medium supplemented with 5% (for Type I) and 10% (Type II) fetal bovine serum (FBS, Invitrogen) at 37°C with 5% CO2 (Mazumdar et al., 2006). Parasite cloning and plaque assays were performed in HFF. Mutant lines lacking the TGME49_080700 (GAD) locus, and lines stably expressing RFP, were generated as described in Supplemental Experimental Procedures. Bradyzoite differentiation was performed by growth in alkaline pH as previously described (Fux et al., 2007). The phylogenetic analysis of GABA genes is described in supplementary Experimental Procedures.
Metabolite extraction of T. gondii tachyzoites
Confluent HFF cultures in 175 cm2 flasks were infected with freshly-egressed tachyzoites and harvested when >90% of the HFF contained > 64 tachyzoites. Host cell and parasite metabolism was quenched by placing culture flasks on ice and the overlying medium removed by aspiration. Host cells were washed twice with ice-cold PBS, pH 7.4, scraped into 5 mL ice cold PBS and lyzed by passage through a syringe needle. Released tachyzoites were pelleted by centrifugation (4,000 rpm, 25 min, 0°C), and washed in ice-cold PBS prior to extraction. Extracellular tachyzoites were obtained after 60–80% of infected host cells.had lyzed. Egressed tachyzoites were transferred to a 50mL centrifuge tube and metabolically quenched by immersion of the tube in a dry ice/ethanol bath (Saunders et al., 2011). Parasite pellets were washed three times with ice-cold PBS prior to metabolite extraction.
Intracellular tachyzoites and egressed tachyzoites (2 × 108 cell equivalents) were extracted in chloroform/methanol/water (1:3:1 v/v containing 1 nmol scyllo-inositol as internal standard) for 20 min at 60°C. Polar and apolar metabolites were separated by phase partitioning and polar metabolites derivitized by methoximation and trimethylsilylation (TMS) and analysed by GC-MS as previously described (Saunders et al., 2011). Apolar metabolites were derivitized by MethPrep II (Grace) and analysed by GC-MS (Supplemental Experimental Procedures). Culture supernatants (540 mL) from 13C-glucose/13C-glutamine-fed egressed tachyzoites were diluted with 70µl D2O containing D6-DSS (5 mM), 13C-U-glycerol (2.1 mM), imidazole (2.1 mM) and NaN3 (0.2%) and 13C-spectra collected at 200 MHz using an 800 MHz Bruker-Biospin Avance fitted with a cryoprobe (Saunders et al., 2011) (Supplemental Experimental Procedures). Data were processed with Bruker TOPSPIN 2.0 and spectra assigned by reference to authentic standards. (Supplemental Experimental Procedures).
Stable isotope labelling of T. gondii tachyzoites
For polar metabolites, infected HFF or freshly-egressed tachyzoites were resuspended in DMEM in which the unlabelled glucose or glutamine was replaced with 8 mM 13C-U-glucose or 13C-U-glutamine (Ramakrishnan et al., 2011). Parasites were harvested after 4 hr and metabolites extracted as above. The aconitase inhibitor, sodium fluoroacetate (NaFAc), was added at 2 mM at the same time as the 13C-glucose/13C-glutamine. Changes in the mass isotopomers of key intermediates in central carbon metabolism were assessed by GC-MS analysis (Supplemental Experimental Procedures). The level of labelling of individual metabolites was estimated as the percentage of the metabolite pool containing one or more 13C atoms after correction for natural abundance.
GAD in vitro enzyme assay
Metabolically quenched extracellular tachyzoites were prepared as described above and lyzed in hypotonic buffer (double-distilled water containing 4% EDTA-free protease inhibitors (Roche) with regular sonication (0°C, 10 min), followed by a freeze-thaw in liquid nitrogen. Cell lysates (50 mL, 5 × 107 cells equivalents) were incubated with 50 mL assay buffer (20 mM pyridoxine/0.4% (v/v) β-mercaptoethanol ± 2 mM ATP in PBS (pH 6.8)) containing 10 mM 13C-glutamate at 37°C. The reaction was quenched at indicated times by boiling for 5 min and 13C-labeled polar metabolites were recovered and detected by GC-MS.
Lytic and plaque viability assays
Freshly-egressed tachyzoites (103) were pre-treated with or without 2 mM NaFAc for 4 hr before being used to infect host HFF. After 6 days, HFF were stained with Crystal Violet (Sigma) and cell growth assessed by presence of intact HFF. In variations of the same experiment, NaFAc was added 2 hr post-infection. As a control, HFF were treated with 2 mM NaFAc for 6 days prior to infection. The growth of parental and Δgad lines (Huynh and Carruthers, 2009), expressing a tandem red fluorescent protein (RFP-RFP) transgene was measured using a real-time fluorescence assay (Sheiner et al., 2011). For competition experiments, parental parasites expressing the RFP-RFP transgene were mixed with equal number of non-fluorescent Δgad parasites and used to infect HFF. The number of fluorescent and non-fluorescent parasites was scored by fluorescence-activated cell counting every time the cultures lysed.
Gliding assay
Freshly egressed tachyzoites were 3.0 µm-filter purified and resuspended in Hank’s balanced salt solution supplemented with 20 mM HEPES (HBSS-H). Parasites were layered on poly-L-lysine-coated coverslips and parasites were allowed to adhere for 15 min at RT. The coverslips were overlaid with HBSS-H containing 2 mM ionomycin, a strong inducer of parasite motility, and incubated for 15 min at 37°C. Parasites were fixed with 4% paraformaldehyde/0.005% glutaraldehyde (15min, RT) and trails visualized by immunofluorescence using the α-SAG1 antibody (Dubremetz et al., 1985). For gliding experiments using different carbon sources, HBSS-H was supplemented with the corresponding compound (0.2 mM glutamine, 4.5 mg/ml glucose, 2 mM GABA, 0.2 mM NaFAc) and washes, adherence and induction were performed in the resulting medium.
Nutrient stress analysis
Egressed tachyzoites were preincubated in PBS containing glucose (6mM), glutamine (6 mM) and 0.1% fatty acid free BSA (37 °C, 2 hr) prior to being resuspended in PBS with or without glucose (6 mM), glutamine 6 mM), or 2-deoxyglucose (6 mM). Parasites were sampled at the indicated time points and polar metabolites levels determined by GC-MS.
Supplementary Material
Highlights.
Toxoplasma gondii depends on mitochondrial metabolism for energy generation
Host glucose and glutamine are catabolized via a canonical oxidative TCA cycle
Carbon fluxes are regulated by an unanticipated γ-aminobutyric acid (GABA) shunt
Intracellular GABA pools are used as an energy reserve to sustain motility
Acknowledgements
We thank Dr Maria Doyle and Ms Milica Ng (University of Melbourne) for bioinformatics analysis and Jenny Chambers (University of Melbourne) for 13C-NMR analyses. This work was supported by the National Health and Medical Research Council of Australia (NHMRC) and the National Institutes of Health (NIH AI084415). MJM is a NHMRC Principal Research Fellow. BS is a Georgia Research Alliance Distinguished Investigator and LS was supported in part by a fellowship from the Swiss National Fund.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Al-Anouti F, Tomavo S, Parmley S, Ananvoranich S. The expression of lactate dehydrogenase is important for the cell cycle of Toxoplasma gondii. J Biol Chem. 2004;279:52300–52311. doi: 10.1074/jbc.M409175200. [DOI] [PubMed] [Google Scholar]
- Behjousiar A, Kontoravdi C, Polizzi KM. In situ monitoring of intracellular glucose and glutamine in CHO cell culture. PLoS One. 2012;7:e34512. doi: 10.1371/journal.pone.0034512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blume M, Rodriguez-Contreras D, Landfear S, Fleige T, Soldati-Favre D, Lucius R, Gupta N. Host-derived glucose and its transporter in the obligate intracellular pathogen Toxoplasma gondii are dispensable by glutaminolysis. Proc Natl Acad Sci U S A. 2009;106:12998–13003. doi: 10.1073/pnas.0903831106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks CF, Johnsen H, van Dooren GG, Muthalagi M, Lin SS, Bohne W, Fischer K, Striepen B. The toxoplasma apicoplast phosphate translocator links cytosolic and apicoplast metabolism and is essential for parasite survival. Cell Host Microbe. 2010;7:62–73. doi: 10.1016/j.chom.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook T, Roos D, Morada M, Zhu G, Keithly JS, Feagin JE, Wu G, Yarlett N. Divergent polyamine metabolism in the Apicomplexa. Microbiology. 2007;153:1123–1130. doi: 10.1099/mic.0.2006/001768-0. [DOI] [PubMed] [Google Scholar]
- Crawford MJ, Thomsen-Zieger N, Ray M, Schachtner J, Roos DS, Seeber F. Toxoplasma gondii scavenges host-derived lipoic acid despite its de novo synthesis in the apicoplast. EMBO J. 2006;25:3214–3222. doi: 10.1038/sj.emboj.7601189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danne JC, Gornik SG, Macrae JI, McConville MJ, Waller RF. Alveolate mitochondrial metabolic evolution: dinoflagellates force reassessment of the role of parasitism as a driver of change in apicomplexans. Mol Biol Evol. 2012 doi: 10.1093/molbev/mss205. [DOI] [PubMed] [Google Scholar]
- Denton H, Roberts CW, Alexander J, Thong KW, Coombs GH. Enzymes of energy metabolism in the bradyzoites and tachyzoites of Toxoplasma gondii. FEMS Microbiol Lett. 1996;137:103–108. doi: 10.1111/j.1574-6968.1996.tb08090.x. [DOI] [PubMed] [Google Scholar]
- Dubey JP, Lindsay DS, Speer CA. Structures of Toxoplasma gondii tachyzoites, bradyzoites, and sporozoites and biology and development of tissue cysts. Clin Microbiol Rev. 1998;11:267–299. doi: 10.1128/cmr.11.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubremetz JF, Rodriguez C, Ferreira E. Toxoplasma gondii: redistribution of monoclonal antibodies on tachyzoites during host cell invasion. Exp Parasitol. 1985;59:24–32. doi: 10.1016/0014-4894(85)90053-0. [DOI] [PubMed] [Google Scholar]
- Fast NM, Kissinger JC, Roos DS, Keeling PJ. Nuclear-encoded, plastid-targeted genes suggest a single common origin for apicomplexan and dinoflagellate plastids. Mol Biol Evol. 2001;18:418–426. doi: 10.1093/oxfordjournals.molbev.a003818. [DOI] [PubMed] [Google Scholar]
- Fleige T, Fischer K, Ferguson DJP, Gross U, Bohne W. Carbohydrate metabolism in the Toxoplasma gondii apicoplast: localization of three glycolytic isoenzymes, the single pyruvate dehydrogenase complex, and a plastid phosphate translocator. Eukaryot Cell. 2007;6:984–996. doi: 10.1128/EC.00061-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleige T, Pfaff N, Gross U, Bohne W. Localisation of gluconeogenesis and tricarboxylic acid (TCA)-cycle enzymes and first functional analysis of the TCA cycle in Toxoplasma gondii. Int J Parasitol. 2008;38:1121–1132. doi: 10.1016/j.ijpara.2008.01.007. [DOI] [PubMed] [Google Scholar]
- Fox BA, Ristuccia JG, Gigley JP, Bzik DJ. Efficient gene replacements in Toxoplasma gondii strains deficient for nonhomologous end joining. Eukaryot Cell. 2009;8:520–529. doi: 10.1128/EC.00357-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fux B, Nawas J, Khan A, Gill DB, Su C, Sibley LD. Toxoplasma gondii strains defective in oral transmission are also defective in developmental stage differentiation. Infect Immun. 2007;75:2580–2590. doi: 10.1128/IAI.00085-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath C, Posner MG, Aass HC, Upadhyay A, Scott DJ, Hough DW, Danson MJ. The 2-oxoacid dehydrogenase multi-enzyme complex of the archaeon Thermoplasma acidophilum - recombinant expression, assembly and characterization. FEBS J. 2007;274:5406–5415. doi: 10.1111/j.1742-4658.2007.06067.x. [DOI] [PubMed] [Google Scholar]
- Hino A, Hirai M, Tanaka TQ, Watanabe Y-I, Matsuoka H, Kita K. Critical roles of the mitochondrial complex II in oocyst formation of rodent malaria parasite Plasmodium berghei. J Biochem. 2012;152:259–268. doi: 10.1093/jb/mvs058. [DOI] [PubMed] [Google Scholar]
- Huynh M-H, Carruthers VB. Tagging of endogenous genes in a Toxoplasma gondii strain lacking Ku80. Eukaryot Cell. 2009;8:530–539. doi: 10.1128/EC.00358-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamberton PHL, Donnelly CA, Webster JP. Specificity of the Toxoplasma gondii-altered behaviour to definitive versus non-definitive host predation risk. Parasitology. 2008;135:1143–1150. doi: 10.1017/S0031182008004666. [DOI] [PubMed] [Google Scholar]
- Lemons JMS, Feng X-J, Bennett BD, Legesse-Miller A, Johnson EL, Raitman I, Pollina EA, Rabitz HA, Rabinowitz JD, Coller HA. Quiescent fibroblasts exhibit high metabolic activity. PLoS Biol. 2010;8:e1000514. doi: 10.1371/journal.pbio.1000514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin SS, Blume M, von Ahsen N, Gross U, Bohne W. Extracellular Toxoplasma gondii tachyzoites do not require carbon source uptake for ATP maintenance, gliding motility and invasion in the first hour of their extracellular life. Int J Parasitol. 2011;41:835–841. doi: 10.1016/j.ijpara.2011.03.005. [DOI] [PubMed] [Google Scholar]
- Lin SS, Gross U, Bohne W. Type II NADH dehydrogenase inhibitor 1-hydroxy-2-dodecyl-4(1H)quinolone leads to collapse of mitochondrial inner-membrane potential and ATP depletion in Toxoplasma gondii. Eukaryot Cell. 2009;8:877–887. doi: 10.1128/EC.00381-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin SS, Gross U, Bohne W. Two internal type II NADH dehydrogenases of Toxoplasma gondii are both required for optimal tachyzoite growth. Mol Microbiol. 2011;82:209–221. doi: 10.1111/j.1365-2958.2011.07807.x. [DOI] [PubMed] [Google Scholar]
- Mazumdar J, H Wilson E, Masek K, A Hunter C, Striepen B. Apicoplast fatty acid synthesis is essential for organelle biogenesis and parasite survival in Toxoplasma gondii. Proc Natl Acad Sci U S A. 2006;103:13192–13197. doi: 10.1073/pnas.0603391103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullin KA, Lim L, Ralph SA, Spurck TP, Handman E, McFadden GI. Membrane transporters in the relict plastid of malaria parasites. Proc Natl Acad Sci U S A. 2006;103:9572–9577. doi: 10.1073/pnas.0602293103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy MP. How mitochondria produce reactive oxygen species. Biochem J. 2009;417:1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair SC, Brooks CF, Goodman CD, Strurm A, McFadden GI, Sundriyal S, Anglin JL, Song Y, Moreno SNJ, Striepen B. Apicoplast isoprenoid precursor synthesis and the molecular basis of fosmidomycin resistance in Toxoplasma gondii. J Exp Med. 2011;208:1547–1559. doi: 10.1084/jem.20110039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olszewski KL, Mather MW, Morrisey JM, Garcia BA, Vaidya AB, Rabinowitz JD, Llinás M. Branched tricarboxylic acid metabolism in Plasmodium falciparum. Nature. 2010;466:774–778. doi: 10.1038/nature09301. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Pettit FH, Yeaman SJ, Reed LJ. Purification and characterization of branched chain alpha-keto acid dehydrogenase complex of bovine kidney. Proc Natl Acad Sci U S A. 1978;75:4881–4885. doi: 10.1073/pnas.75.10.4881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pino P, Foth BJ, Kwok L-Y, Sheiner L, Schepers R, Soldati T, Soldati-Favre D. Dual targeting of antioxidant and metabolic enzymes to the mitochondrion and the apicoplast of Toxoplasma gondii. PLoS Pathog. 2007;3:e115. doi: 10.1371/journal.ppat.0030115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polonais V, Soldati-Favre D. Versatility in the acquisition of energy and carbon sources by the Apicomplexa. Biol Cell. 2010;102:435–445. doi: 10.1042/BC20100005. [DOI] [PubMed] [Google Scholar]
- Pomel S, Luk FCY, Beckers CJM. Host cell egress and invasion induce marked relocations of glycolytic enzymes in Toxoplasma gondii tachyzoites. PLoS Pathog. 2008;4:e1000188. doi: 10.1371/journal.ppat.1000188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramakrishnan S, Docampo MD, Macrae JI, Pujol FM, Brooks CF, van Dooren GG, Hiltunen JK, Kastaniotis AJ, McConville MJ, Striepen B. The apicoplast and endoplasmic reticulum cooperate in fatty acid biosynthesis in the apicomplexan parasite Toxoplasma gondii. J Biol Chem. 2011;287:4957–4971. doi: 10.1074/jbc.M111.310144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito T, Nishi M, Lim MI, Wu B, Maeda T, Hashimoto H, Takeuchi T, Roos DS, Asai T. A novel GDP-dependent pyruvate kinase isozyme from Toxoplasma gondii localizes to both the apicoplast and the mitochondrion. J Biol Chem. 2008;283:14041–14052. doi: 10.1074/jbc.M709015200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saliba KJ, Kirk K. Nutrient acquisition by intracellular apicomplexan parasites: staying in for dinner. Int J Parasitol. 2001;31:1321–1330. doi: 10.1016/s0020-7519(01)00258-2. [DOI] [PubMed] [Google Scholar]
- Saunders EC, Ng WW, Chamber JM, Ng M, Naderer T, Kroemer JO, Likic VA, McConville MJ. Isoptopomer profiling of Leishmania mexicana promastigotes reveals important roles for succinate fermentation and aspartate uptake in TCA cycle anaplerosis, glutamate synthesis and growth. J Biol Chem. 2011;286:27706–27717. doi: 10.1074/jbc.M110.213553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeber F, Limenitakis J, Soldati-Favre D. Apicomplexan mitochondrial metabolism: a story of gains, losses and retentions. Trends Parasitol. 2008;24:468–478. doi: 10.1016/j.pt.2008.07.004. [DOI] [PubMed] [Google Scholar]
- Sheiner L, Demerly JL, Poulsen N, Beatty WL, Lucas O, Behnke MS, White MW, Striepen B. A systematic screen to discover and analyze apicoplast proteins identifies a conserved and essential protein import factor. PLoS Pathog. 2011;7:e1002392. doi: 10.1371/journal.ppat.1002392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava IK, Rottenberg H, Vaidya AB. Atovaquone, a broad spectrum antiparasitic drug, collapses mitochondrial membrane potential in a malarial parasite. Journal of Biological Chemistry. 1997;272:3961. doi: 10.1074/jbc.272.7.3961. [DOI] [PubMed] [Google Scholar]
- Starnes GL, Coincon M, Sygusch J, Sibley LD. Aldolase is essential for energy production and bridging adhesin-actin cytoskeletal interactions during parasite invasion of host cells. Cell Host Microbe. 2009;5:353–364. doi: 10.1016/j.chom.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng R, Junankar PR, Bubb WA, Rae C, Mercier P, Kirk K. Metabolite profiling of the intraerythrocytic malaria parasite Plasmodium falciparum by (1)H NMR spectroscopy. NMR Biomed. 2009;22:292–302. doi: 10.1002/nbm.1323. [DOI] [PubMed] [Google Scholar]
- Vercesi AE, Rodrigues CO, Uyemura SA, Zhong L, Moreno SN. Respiration and oxidative phosphorylation in the apicomplexan parasite Toxoplasma gondii. J Biol Chem. 1998;273:31040–31047. doi: 10.1074/jbc.273.47.31040. [DOI] [PubMed] [Google Scholar]
- Webster JP. The effect of Toxoplasma gondii on animal behavior: playing cat and mouse. Schizophr Bull. 2007;33:752–756. doi: 10.1093/schbul/sbl073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster JP, McConkey GA. Toxoplasma gondii-altered host behaviour: clues as to mechanism of action. Folia Parasitol (Praha) 2010;57:95–104. doi: 10.14411/fp.2010.012. [DOI] [PubMed] [Google Scholar]
- Weiss LM, Dubey JP. Toxoplasmosis: A history of clinical observations. Int J Parasitol. 2009;39:895–901. doi: 10.1016/j.ijpara.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellen KE, Thompson CB. Cellular metabolic stress: considering how cells respond to nutrient excess. Mol Cell. 2010;40:323–332. doi: 10.1016/j.molcel.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.