Highlights
-
•
A dL lesion is not repaired as effectively as an AP site.
-
•
The repair of a cluster with dL and 8-oxodGuo lesions is compromised.
-
•
Delayed repair of the cluster leads to an increase in mutation frequency.
Keywords: Clustered DNA damage; Base excision repair; Mutation; Oxidized abasic sites; 2-Deoxyribonolactone; 8-Oxo-7,8-dihydro-2′-deoxyguanosine
Abbreviations: 8-oxodGuo, 8-oxo-7,8-dihydro-2′-deoxyguanosine; dL, 2-deoxyribonolactone; AP, abasic site; THF, tetrahydrofuran; SP, short patch; LP, long patch
Abstract
A signature of ionizing radiation is the induction of DNA clustered damaged sites. Non-double strand break (DSB) clustered damage has been shown to compromise the base excision repair pathway, extending the lifetimes of the lesions within the cluster, compared to isolated lesions. This increases the likelihood the lesions persist to replication and thus increasing the mutagenic potential of the lesions within the cluster. Lesions formed by ionizing radiation include 8-oxo-7,8-dihydro-2′-deoxyguanosine (8-oxodGuo) and 2-deoxyribonolactone (dL). dL poses an additional challenge to the cell as it is not repaired by the short-patch base excision repair pathway. Here we show recalcitrant dL repair is reflected in mutations observed when DNA containing it and a proximal 8-oxodGuo is replicated in Escherichia coli. 8-oxodGuo in close proximity to dL on the opposing DNA strand results in an enhanced frequency of mutation of the lesions within the cluster and a 20 base sequence flanking the clustered damage site in an E. coli based plasmid assay. In vitro repair of a dL lesion is reduced when compared to the repair of an abasic (AP) site and a tetrahydrofuran (THF), and this is due mainly to a reduction in the activity of polymerase β, leading to retarded FEN1 and ligase 1 activities. This study has given insights in to the biological effects of clusters containing dL.
1. Introduction
Lesions induced by ionizing radiation are chemically the same as isolated lesions produced during oxygen metabolism by the cell. However, the spatial distribution of DNA lesions induced by ionizing radiation is much different than that caused by oxygen metabolism. When ionizing radiation deposits its energy heterogeneously within a localized area of a few nanometres in the nucleus of a cell, direct ionization and formation of radicals within the DNA occurs. Some of these lesions are formed in clusters, comprising two or more individual lesions within one or two helical turns of the DNA helix, induced by passage of a single radiation track. The ability of ionizing radiation to produce clustered DNA damage sites, against a background plethora of endogenous damage, is thought to contribute to the biological effects of ionizing radiation. In contrast to radiation, normal endogenous processes are thought to form clustered damage sites at low levels [1,2]. It has been proposed that the complexity and yield of radiation-induced clustered DNA damage increases with increasing ionization density of the radiation [3,4]. Consistent with this hypothesis, biological effects of ionizing radiation, such as lethality, mutation induction and irrepairability of double strand breaks (DSB), increase with increasing ionizing density of radiation [5,6]. Furthermore, the yield of non-DSB clusters is about four to eight times that of prompt DSB with γ-irradiation in mammalian cells [7–10] and only a small sub-class of these non-DSB clustered damaged sites are converted at early times into DSB post-irradiation [11].
The base excision repair (BER) pathway (reviewed in [12]) has evolved to repair small DNA lesions, such as those produced by endogenous oxygen species, and is also largely responsible for the removal of many lesions induced by ionizing radiation. The BER pathway is a multi-step process involving the sequential action of several proteins. The short or long patch BER sub-pathways are initiated by the removal of the damaged base by a lesion specific glycosylase. This is followed by incision of the resulting abasic (AP) site by a lyase activity associated with the glycosylase (bi-functional glycosylases) or by an AP endonuclease. Short patch (SP) BER involves the addition of one nucleotide by DNA polymerase β [13], followed by joining of the DNA ends by DNA ligase III/XRCC1 [14]. Long patch (LP) BER is orchestrated by DNA polymerase β/δ, proliferating cell nuclear antigen (PCNA), flap endonuclease (FEN1) and ligase 1 [15,16] and involves the incorporation of two to six nucleotides into the repair gap [15,17,18]. How the SP or LP BER pathways are selected is still not fully understood but factors that influence the choice of pathway include the type of lesion, the local concentration of BER proteins, ATP concentration, the stage of cell cycle and whether a cell is differentiated (reviewed in [19]). Some lesions, such as reduced or oxidized AP sites, are resistant to the lyase activity of polymerase β and therefore have to be processed by the LP-BER pathway [20].
DNA clustered damage sites containing a mixture of AP sites, 8-oxo-7,8-dihydro-2′-deoxyguanosine (8-oxodGuo), 5,6-dihydrothymine (DHT) and thymine glycol (Tg) lesions can compromise the efficiency of the BER pathway and as a consequence, the mutation frequencies of lesions in clusters are higher than those of isolated lesions (reviewed in [21]). The generality of the importance of the type of lesions, inter-lesion distance, and relative orientation of lesions within a cluster, determined following cluster processing in Escherichia coli [22–25], has also been confirmed in mammalian cells [26,27] and yeast cells [28]. In addition, certain clustered damaged sites have a greater dependence for the LP-BER pathway than isolated lesions [29].
AP sites can arise either from oxidative stress and ionizing radiation or from the removal of a base lesion by a DNA glycosylase. As AP sites are non-coding they have the potential to cause transversions due to the preferential incorporation of adenine opposite an AP site in replication and repair [30]. Ionizing radiation and oxidative stress also produce various oxidized AP sites, including 2-deoxyribonolactone (dL) which account for about 10% of total 2-deoxyribose oxidation [31]. Whereas AP sites are generally repaired by SP-BER, dL utilizes LP-BER for their repair. An 8-oxodGuo lesion poses an additional repair challenge to the cell as it can exist in two conformations (anti- and syn-) resulting in base pairing with either cytosine or adenine [32]. The latter leads to a G:C to T:A transversion.
The aim of this study was to gain an understanding of how a clustered DNA damage site containing an obligate substrate for the LP-BER pathway is processed. To date, synthetic models of clustered damage sites have generally contained lesions which are processed mainly by the SP-BER pathway when formed in DNA as individual lesions. Specific oligonucleotides with bistranded clusters containing dL and 8-oxodGuo lesions, separated by up to five bases in different orientations were synthesized and subjected to in vitro BER assays to study the efficiency of repair of the dL lesion. In addition the clustered DNA damaged sites were placed in a plasmid context and the mutagenic/cytotoxic potential of the cluster was investigated in an E. coli based assay.
2. Materials and methods
2.1. Substrate oligonucleotides
The single stranded dL containing oligonucleotides were synthesized from ones containing a photochemical precursor, as described previously [33] (Supplementary Fig. 1). The 8-oxodGuo, THF and the uracil containing oligonucleotides were purchased PAGE purified from Eurogentec (Seraing, Belgium). The sequences of the double stranded oligonucleotides following hybridization and conversion of either the uracil site into an AP site or the dL precursor (nitroindole, NI) into a dL lesion are presented in Table 1. The nomenclature of the relative positions of the two lesions in the clustered DNA damage site was developed by David-Cordonnier et al [34]. A positive or negative number has been assigned to each residue in the double stranded oligonucleotides in Table 1. This number refers to the separation, in base pairs, of one lesion located 5′ (positive number) or 3′ (negative number) to the second lesion on the opposing strand.
Table 1.
Sequence of double-stranded oligonucleotides.
| Position | Sequence |
|---|---|
| −5 | 5′-ctcttagtcaggaaXatgtctctatgctgggagcaaaggc |
| 3′-gagaatcagtccttataca8agatacgaccctcgtttccg | |
| −1 | 5′-ctcttagtcaggaatatgXctctatgctgggagcaaaggc |
| 3′-gagaatcagtccttataca8agatacgaccctcgtttccg | |
| +1 | 5′-ctcttagtcaggaatatgtcXctatgctgggagcaaaggc |
| 3′-gagaatcagtccttataca8agatacgaccctcgtttccg | |
| +5 | 5′-ctcttagtcaggaatatgtctctaXgctgggagcaaaggc |
| 3′-gagaatcagtccttataca8agatacgaccctcgtttccg | |
| Control 1 | 5′-ctcttagtcaggaatatgtctctatgctgggagcaaaggc |
| 3′-gagaatcagtccttataca8agatacgaccctcgtttccg | |
| Control 2 | 5′-ctcttagtcaggaatatgtctctatgctgggagcaaaggc |
| 3′-gagaatcagtccttatacaXagatacgaccctcgtttccg | |
| Control 3 | 5′-ctcttagtcaggaatatgtctctatgctgggagcaaaggc |
| 3′-gagaatcagtccttatacaYagatacgaccctcgtttccg | |
| Control 4 | 5′-ctcttagtcaggaatatgtcZctatgctgggagcaaaggc |
| 3′-gagaatcagtccttatacagagatacgaccctcgtttccg |
8, 8-oxodGuo; X, dL (following conversion from the photolabile precursor, NI, as described in Section 2); Y, AP site (following conversion from uracil as described in Section 2); Z, THF. −1, −5, position on the complementary strand of 8-oxodGuo 3′ from the dL lesion; +1, +5, position on the complementary strand of 8-oxodGuo 5′ from the dL lesion. Control 1, control 2, control 3 and control 4 are the control oligonucleotides with 8-oxodGuo, dL, an AP site, or THF present as single lesions, respectively.
2.2. Preparation of 5′-32P-end labeled oligonucleotides
Oligonucleotide (0.2 μg) was 5′-32P-end-labeled using 10 U of T4 polynucleotide kinase (Invitrogen, Paisley, UK) with 25 μCi [γ-32P]ATP (6000 Ci/mmol, 10 mCi/ml, Perkin Elmer, Boston, USA) in 20 μl of buffer (70 mM Tris-HCl pH 7.6, 10 mM MgCl2, 100 mM KCl, 1 mM β-2-mercaptoethanol) for 30 min at 37 °C. Unincorporated labeled nucleotides were removed from the labeled oligonucleotides by centrifugation through a Quick Spin G-50 Sephadex column (GE Healthcare, Little Chalfont, UK) following the manufacturer's instructions.
2.3. Preparation of 3′-32P-end labeled oligonucleotides
Oligonucleotides (0.2 μg) were 3′-32P-end labeled using 15 U of terminal deoxynucleotidyl transferase (TdT) (Invitrogen, Paisley, UK), 30 μCi [α-32P]dATP (5000 Ci/mmol, 10 mCi/ml, Perkin Elmer), incubated in 40 μl of TdT buffer (100 mM potassium cacodylate pH 7.2, 2 mM CoCl2, 200 μM 1, 4-dithiothreitol (DTT)) for 30 min at 37 °C. The reaction was stopped by a subsequent incubation for 10 min at 70 °C and then unincorporated labeled nucleotides were removed by centrifugation through a Quick Spin G-25 Sephadex column (GE Healthcare, Little Chalfont, UK) following the manufacturer's instructions.
2.4. Hybridization of oligonucleotides
The 5′- or 3′-32P-labeled oligonucleotides were hybridized with a 2-fold excess of the non-radiolabeled complementary strand. Efficient annealing of the oligonucleotides was verified on a 12% native polyacrylamide gel.
2.5. Preparation of an AP site
The purified double-stranded oligonucleotides which contained a uracil residue were treated with 1 U of uracil-DNA-glycosylase (UDG) (Invitrogen, Paisley, UK) in 100 μl of buffer (10 mM Tris-HCl pH 7.5, 50 mM NaCl, 1 mM EDTA) for 30 min at 37 °C to produce an AP site. The AP site containing oligonucleotides were stable at 4 °C for at least two weeks.
2.6. Preparation of a dL lesion
The purified double stranded oligonucleotide (10,000 cpm/μl, 16 fmol) containing the photolabile nucleoside precursor (NI) was placed in a glass vial (which eliminates wavelengths <300 nm) and exposed to a 50 W tungsten halogen light source for 2 h. Wavelengths <315 nm and >∼900 nm were eliminated with a heat absorbing filter made from 3 mm KG1 toughened SCHOTT glass (UQG Optics, Cambridge, UK). This transmission spectrum of the filter was confirmed spectrophotometrically. Conversion of the precursor to dL was confirmed by treating an aliquot with NaOH (0.1 M) at 37 °C for 30 min, which cleaves DNA containing the lactone, followed by neutralization with HCl (0.1 M). Conversion to dL was typically > 85%, and was verified on a denaturing polyacrylamide gel (Supplementary Fig. 2). The dL containing oligonucleotides were stored at 4 °C and used within 48 h of preparation.
2.7. Reconstitution of long patch BER with purified proteins
For analysis of APE1, polymerase β and ligase 1 activities, 5′-32P-end labeled double stranded oligonucleotides (10,000 cpm, 16 fmol) were incubated with 3 ng APE1; 3 ng APE1 and 2 ng polymerase β; 3 ng APE1, 2 ng polymerase β, 10 ng FEN1 and 20 ng ligase 1, respectively. For analysis of FEN1, 32P-3′-end labeled double stranded oligonucleotides (10,000 cpm, 16 fmol) were incubated with 3 ng APE1, 2 ng polymerase β and 10 ng FEN1. Reactions were carried out in a 10 μl reaction solution (80 mM HEPES pH 7.9, 10 mM MgCl2, 2 mM DTT, 200 μM EDTA, 4 mM ATP, 800 μg/ml BSA, 40 μM each of dATP, dTTP, dGTP and dCTP) at 37 °C for 0, 5, 15 and 30 min. The reactions were stopped by the addition of 10 μl denaturing stop solution (98% formamide, 2 mM EDTA, 0.025% bromophenol blue, 0.025% xylene cyanol) and then subjected to electrophoresis on a 20% denaturing polyacrylamide gel containing 8 M urea in 1× TBE (89 mM Tris–HCl, 89 mM boric acid, 2 mM EDTA pH 8.3) at a constant power of 85 W. The bands in the gel were quantified as described above. The errors represent standard errors of the mean from at least three experiments.
2.8. Escherichia coli strains
Isogenic strains CC104 (wild type), BH540 (fpg::KanR), BH980 (mutY::KanR) and BH990 (fpg::KanR mutY::KanR) were kind gifts from Dr. S. Boiteux (CNRS, France). Isogenic strain CC104 (Nth::KanR) was a kind gift from Dr. S. Yonei (Kyoto University, Japan).
2.9. Determination of mutation frequency
The mutation frequency arising from the clustered DNA damaged site was determined as described previously [24]. Briefly, the double stranded oligonucleotides were phosphorylated at their 5′ termini with 10 U of T4 polynucleotide kinase (Invitrogen, Paisley, UK) and 25 mM ATP in 20 μl of buffer (70 mM Tris–HCl pH 7.6, 10 mM MgCl2, 100 mM KCl, 1 mM β-2-mercaptoethanol) for 30 min at 37 °C. pUC18 plasmid DNA was linearized with SmaI followed by treatment with 20 U calf intestinal phosphatase (New England Biolabs, Massachusetts, USA) for 15 min at 37 °C, followed by a second incubation for 45 min at 55 °C. The plasmid DNA was then gel purified using the Qiaquick gel purification system (Qiagen, Hilden, Germany) and the concentration determined spectrophotometrically. Phosphorylated double-stranded oligonucleotide (5 pmol) was ligated into 200 fmol linearized pUC18 DNA with 400 U T4 ligase (New England Biolabs, MA, USA) in 20 μl buffer (50 mM Tris–HCl pH 7.5, 10 mM MgCl2, 10 mM DTT, 1 mM ATP, 25 μg/ml BSA) for 16 h at 16 °C, followed by dialysis using 0.025 μm nitrocellulose filters (Millipore, Massachusetts, USA). The ligation product containing a dL precursor was subjected to photolysis as described above before 50 ng of the ligation product was transformed into 60 μl electro-competent bacteria using a Bio-Rad (California, USA) E. coli pulser set at 1.8 mV. Transformants were either selected in 5 ml LB broth containing 100 μg/ml ampicillin or on LB agar plates containing 100 μg/ml ampicillin for 16 h at 37 °C. Plasmid DNA was retrieved from 1.5 ml of a mini culture either from the gross transformant culture or grown from a single bacterial colony using a Quiakit-spin mini-prep kit (Qiagen, Hilden, Germany). DNA eluate (15 μl) was digested with Bsma1 and the samples were electrophoresed on a 1% agarose gel at 4 V/cm. Following electrophoresis the gel images were captured under UV light using a CCD camera and then analyzed using Quantity One software (Bio-Rad, California, USA). If the restriction site within the cloned oligonucleotide is correctly repaired then there will be four Bsma1 restriction sites leading to fragments lengths of 1352 bp, 765 bp, 403 bp and 206 bp. In contrast, only three Bsma1 restriction sites will be present, leading to fragment lengths of 1755 bp, 765 bp, and 206 bp if repair of the cloned oligonucleotide resulted in the induction of a mutation. The mutation frequency is expressed as the intensity of the 1755 bp fragment as percentage of the total intensities of the 1755 bp, 1352 bp and 403 bp fragments.
2.10. Sequence analysis of mutated plasmid DNA
Single bacterial colonies grown on LB agar plates containing 100 μg/ml ampicillin were picked at random and incubated for 16 h in 5 ml LB broth containing 100 μg/ml ampicillin at 37 °C. The plasmid DNA was retrieved and digested with Bsma1, as described above. DNA preparations, identified to contain an induced mutation within the cloned oligonucleotide, were sequenced by Source Bioscience Life Sciences (Nottingham, UK) with the following forward and reverse primers, 5′-d(CTTCGCTATTACGCCAGCTG) and 5′-d(GGCACGACAGGTTTCCCGACTGGA), respectively. The primers amplify sequence across the site of damage in the cloned oligonucleotide. The sequencing data was analyzed using FinchTV software.
3. Results
3.1. Induction of mutations in E. coli
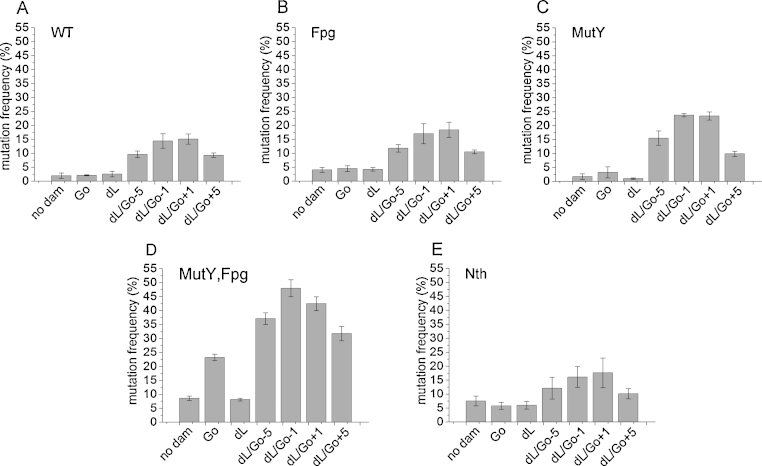
An E. coli based in vivo plasmid assay [24] was used to assess the mutagenic potential of the bistranded clustered damaged sites containing dL/8-oxodGuo at different relative positions (Table 1, oligonucleotides 1–5 and controls 1 and 2; Fig. 1). No decrease in numbers of E. coli colonies relative to plasmid containing a single dL or a single 8-oxodGuo lesion was observed for any of the clustered damaged sites, showing that few if any of the clusters had been converted in to DSB. The mutation frequency of plasmid DNA carrying a single isolated dL lesion in E. coli is the same as that of a no-damage control in WT, fpg null, mutY null, mutY:fpg null and nth null backgrounds (Fig. 1). In general, the mutY:fpg null and nth null E. coli produced mutation frequencies that increase 3–4 fold compared to that of WT E. coli. Similarly, a plasmid carrying a single isolated 8-oxodGuo lesion gives the same mutation frequency as that of the no-damage control in WT, fpg null, mutY null and nth null E. coli but the mutation frequency increases by about 2.5 fold in a mutY:fpg null background compared to the plasmid with no lesions (Fig. 1). When an 8-oxodGuo lesion is placed close to the dL lesion on the opposing DNA strand, the mutation frequency increases in the WT and mutant backgrounds tested. The extent of increase in mutation frequency above that of dL as an isolated lesion varies with the genetic background of E. coli. In WT and mutY:fpg null E. coli the mutation frequency of the clustered lesions increases up to 5 fold of that of an isolated dL lesion (Fig. 1A and D), in fpg null E. coli up to 4 fold (Fig. 1B), mutY null E. coli up to 10 fold (Fig. 1C) and Nth null E. coli up to 3 fold (Fig. 1E). The actual mutation frequency of the clustered lesions at position −1 in mutY:fpg null E. coli is 47%, compared to 8% for an isolated dL lesion, whereas these figures are 14% for the clustered lesions compared to 3% for an isolated dL in WT E. coli.
Fig. 1.
Mutation frequency of 8-oxodGuo, Go and dL in E. coli when present as single lesions or together as a DNA clustered damaged site. (A) WT E. coli. (B) fpg deficient E. coli. (C) mutY deficient E. coli. (D) mutY and fpg deficient E. coli. (E) Nth deficient E. coli. Error bars show standard deviation from at least three experiments.
Mutations arising from the clustered DNA damaged sites containing dL and 8-oxodGuo lesions at position ±1 in fpg null and fpg/mutY null E. coli were characterized by sequencing (Table 2). As there is a higher mutation frequency in the mutant backgrounds, the chance of selecting a colony containing a mutation is increased so that fewer colonies would have to be screened and sequenced. It was found that the most abundant mutation (>70%; Table 2) is a G:C to T:A transversion, most probably arising from the mis-incorporation of adenine opposite an 8-oxodGuo lesion. At a lower frequency, a number of point mutations and deletions of one or two bases, and in one case a deletion of 10 bases, were detected on either the dL or 8-oxodGuo containing DNA strands (detailed in Table 2). About a third of these additional lesions were in the same clone as a G:C to T:A transversion and interestingly the majority are 3′ to the dL or 5′ to the 8-oxodGuo lesions.
Table 2.
Mutations arising from the clustered DNA damaged sites containing dL and 8-oxodGuo lesions at position ±1 in mutY null and fpg/mutY null E. coli.
| Cluster |
Mutation frequencya |
Type of mutation |
Frequency type of mutation occursb |
|---|---|---|---|
| mutY null E. coli | |||
| dL/8-oxodGuo-1 | 15.6% (20/128) | G:C to T:A transversion | 60% (12/20) |
| G:C to T:A transversion + A to T transversion 5 bases 5′ to 8-oxodGuo | 5% (1/20) | ||
| G:C to T:A transversion + ΔT 7 bases 3′ to 8-oxodGuo | 5% (1/20) | ||
| Δ8-oxodGuo | 10% (2/20) | ||
| Δ8-oxodGuo + ΔC, 2 bases 3′ to 8-oxodGuo | 5% (1/20) | ||
| 8-oxodGuo to C transversion | 5% (1/20) | ||
| ΔT 4 bases 5′ to dL | 5% (1/20) | ||
| ΔA 8 bases 5′ to 8-oxodGuo | 5% (1/20) | ||
| dL/8-oxodGuo + 1 | 29.4% (20/68) | G:C to T:A transversion | 50% (15/20) |
| G:C to T:A transversion + ΔT 4 bases 3′ to 8-oxodGuo | 5% (1/20) | ||
| G:C to T:A transversion + ΔG 7 bases 5′ to 8-oxodGuo | 5% (1/20) | ||
| G:C to T:A transversion + ΔC 8 bases 3′ to 8-oxodGuo | 5% (1/20) | ||
| G:C to T:A transversion + T to C transition 2 bases 3′ to dL | 5% (1/20) | ||
| G:C to T:A transversion + G to T transversion 14 bases 5′ to dL | 5% (1/20) | ||
| Δ8-oxodGuo | 10% (2/20) | ||
| Δ8-oxodGuo + ΔAT 3 bases 3′ to 8-oxodGuo | 5% (1/20) | ||
| Δ8-oxodGuo + G to T transversion 14 bases 5′ to dL, ΔA 5 bases 5′ to dL | 5% (1/20) | ||
| 10 bp deletion at dL | 5% (1/20) | ||
|
fpg null/mutY null E. coli | |||
| dL/8-oxodGuo-1 | 58.5% (24/41) | G:C to T:A transversion | 79.2% (19/24) |
| G:C to T:A transversion + A to C transversion 1 bases 3′ to 8-oxodGuo + G to A 7 bases 5′ 8-oxodGuo | 4.2% (1/24) | ||
| G:C to T:A transversion + ΔT, 6 bases 3′ to 8-oxodGuo | 4.2% (1/24) | ||
| G to T transversion 12 bases 5′ to dL | 4.2% (1/24) | ||
| T to C transition 11 bases 5′ to dL | 4.2% (1/24) | ||
| ΔA 6 bases 5′ to dL + ΔA 13 bases 5′ to dL | 4.2% (1/24) | ||
| dL/8-oxodGuo + 1 | 50.9% (27/53) | G:C to T:A transversion | 70.4% (19/27) |
| 8-oxodGuo deletion | 11.1% (3/27) | ||
| Δ8-oxodGuo + ΔA 3 bases 3′ to 8-oxodGuo | 3.7% (1/27) | ||
| ΔdL + ΔT 6 bases 5′ to dL | 3.7% (1/27) | ||
| ΔC 2 bases 3′ to 8-oxodGuo | 3.7% (1/27) | ||
| ΔGT 13 bases 5′ to dL | 3.7% (1/27) | ||
| A to C transversion 1 bases 3′ to 8-oxodGuo | 3.7% (1/27) | ||
Mutation frequency was calculated by dividing the number of individual mutations found in the oligonucleotide sequence ligated in to pUC19 plasmid by the total number of oligonucleotide sequences sequenced.
The frequency a mutation occurred was calculated by dividing the number of times a mutation was seen by the total number of mutated oligonucleotide sequences.
3.2. Reconstitution of the LP-BER pathway in vitro
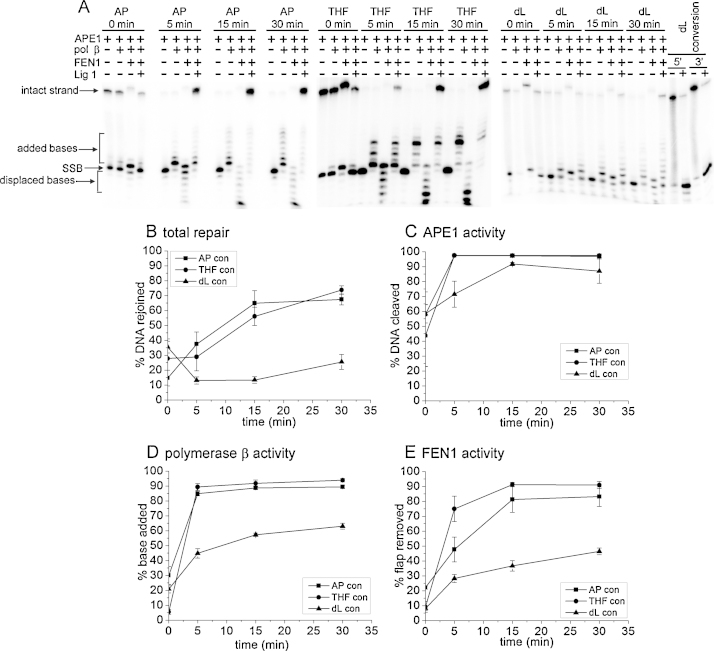
To further understand the events leading to increased mutation frequencies of the clustered sites in E. coli, the repair of the clusters was investigated by reconstituting LP-BER in vitro using purified BER proteins. First the repair of dL in isolation was studied. Repair of the dL lesion was confirmed to proceed via the long-patch pathway as repair of the dL lesion was not seen in the absence of FEN1 (Supplementary Fig. 3). Fig. 2 shows the repair of dL compared to that of an AP site and THF, in the context of the control oligonucleotides shown in Table 1 (controls 2–4). Fig. 2A is a phospohorimaging scan showing the products of repair as repair proceeds down the BER pathway for an AP site, a THF and a dL lesion. The level of overall repair is shown graphically in Fig. 2B, and it can be seen the total repair is reduced by 4.2–3.7 (p ≤ 0.02) for a dL lesion when compared to the repair of an AP site and a THF lesion. Assessing the activity of each of the BER proteins in the pathway, it can be seen that the initial rate (over the first 5 min) of APE1 is 3–4 times lower for the cleavage of a dL lesion, compared to that for an AP site and a THF lesion (p = 0.04) but there is no difference in the rate of APE1 cleavage by 15 min (Fig. 2C). This result is in agreement with an earlier study by Xu and colleagues [35]. For an AP site and a THF lesion the subsequent steps of BER: repair synthesis by polymerase β; flap removal by FEN1 and ligation by ligase 1 to complete repair, proceed efficiently (Fig. 2B, D and E). In contrast, for a dL lesion, activity of polymerase β over 30 min of the repair assay is reduced 1.4 and 2.1 fold compared to that of an AP site and a THF lesion, respectively (p = 0.001) (Fig. 2D). Further, the number of bases incorporated into the repair patch for a dL lesion is only 1–2, compared with up to 5 for a THF lesion. The first base incorporated in to the repair gap by polymerase β is dependent on the complementary sequence. For the control oligonucleotides it is G for the AP site and dL and T for THF, and for the oligonucleotides containing a dL/8-oxodGuo cluster it is T (see Table 1). Thus it appears as if the level of repair synthesis by polymerase β is reduced for the repair of a dL lesion. The low level of repair synthesis results in reduced levels of substrate for FEN1 and ligase 1, consequently the product arising from FEN1 activity is reduced by 1.6–2.1 (p ≤ 0.006) when compared to the activity during the repair of an AP site and a THF lesion (Fig. 2E). Total efficiency of repair may be reduced even more (4.2–3.7 fold (p ≤ 0.02)) than the activities of polymerase β and FEN1 as the SSB end created by cleavage of the dL lesion will persist, as strand displacement by polymerase β is minimal and the SSB end is not a substrate for ligation.
Fig. 2.
Repair an AP site (■), a THF lesion (•) and a dL lesion (▴) by purified LP-BER proteins. (A) Phosphorimaging scan showing the products of the repair assay. LP-BER was reconstituted with purified proteins, adding in one protein at a time to see all steps of repair. For dL conversion (–) and (+) denote treatment with NaOH to determine if the dL conversion was successful (see Section 2). 5′ and 3′ refer to 32P-labeling of the 5′ or 3′ terminus. (B) Total repair as measured by restitution of the intact strand. Reaction contains APE1, polymerase β, FEN1 and ligase1. (C) APE1 activity as measured by cleavage of the intact strand to create a SSB. Reaction contains APE1 only. (D) Polymerase β activity as measured by incorporation of bases. Reaction contains APE1 and polymerase β. (E) FEN1 activity as measured by removal of the DNA flap created by strand displacement. Reaction contains APE1, polymerase β and FEN1. Error bars show standard error of the mean from three experiments.
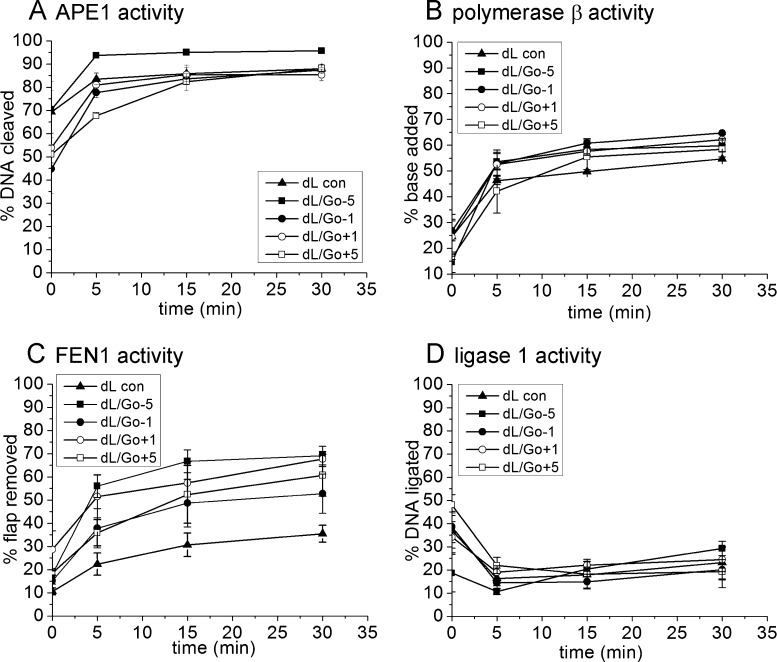
The presence of 8-oxodGuo (Table 1, oligonucleotides 1–5) has no effect on the level of overall repair or on the activities of APE1 and polymerase β, for the repair of the dL lesion, most probably reflecting the repair of the dL being so poor even in the absence of 8-oxodGuo (Fig. 3). However, the activity of FEN1 is increased when 8-oxodGuo is in position −5 or +1 (p ≤ 0.004) relative to the dL lesion.
Fig. 3.
Repair of a dL lesion in the absence (▴) of 8 oxodGuo (Go) and in the presence of 8-oxodGuo (Go) in position −5 (■), −1 (•), +1 (○) and +5 (□). (A) APE1 activity as measured by cleavage of the intact strand. Reaction contains APE1 only. (B) Polymerase β activity as measured by incorporation of bases. Reaction contains APE1 and polymerase β. (C) FEN1 activity as measured by removal of the DNA flap. Reaction contains APE1, polymerase β and FEN1. (D) Ligase 1 activity as measured by restitution of the intact strand. Reaction contains APE1, polymerase β, FEN1 and ligase1. Error bars show standard error of the mean from three experiments.
4. Discussion
dL is an oxidized AP site that can be induced endogenously or by ionizing radiation. We have confirmed that, unlike an AP site, dL cannot be repaired by SP-BER but is an obligate substrate for LP-BER (Supplementary Fig. 3). The level of repair of the dL lesion is approximately 35% that of an AP site in the in vitro reconstituted repair assay used (Fig. 2). A previous study reported that oxidized AP sites are less efficiently incised by APE1 [20,36]. However, we have shown in our in vitro repair assay that the level of APE1 incision of a dL lesion is only reduced over the first 5 min of a 30 min time course, compared to the level of incision of an AP site and a THF lesion. However, the overall repair of a dL lesion is reduced compared to the repair of an AP site and THF lesion (Fig. 2). It was found that an 8-oxodGuo lesion in close proximity to a dL lesion in a bistranded clustered damage site does not affect the repair of the dL lesion in the in vitro repair assay (Fig. 3). In contrast to the inefficient repair of a dL lesion, seen as restitution of the full length oligonucleotide, the efficiency of repair of a THF lesion, a synthetic analogue of a reduced AP site which is also an obligate LP-BER substrate, is similar to that of an AP site (Fig. 2). We therefore propose that the synthetic THF lesion is a poor model for a sugar lesion such as dL, produced either endogenously or by ionizing radiation.
A major difference between the repair of a dL lesion and the repair of a THF lesion is the size of the repair patch. With a THF lesion polymerase β adds 5 bases before ligation takes place. However, repair intermediates accumulate following incision of a dL lesion when just one base has been added to the repair gap, thus very little repair synthesis occurs. No cross-linking was seen between the dL lesion and polymerase β (data not shown), in contrast to a previous report in which a much higher polymerase β concentrations were used [37,38]. Since dL is an obligate LP-BER substrate, ligation cannot take place if only one base has been added to the repair gap as the non-ligatable SSB terminus, resulting from APE1 incision of the dL lesion, will remain. The inefficient strand displacement during the repair of a dL lesion could account for the low activity of FEN1, the subsequent step of the LP-BER pathway, as there would be low levels of substrate produced for this protein. Consequently, ligase 1 activity would also be low as low levels of ligatable substrate would be produced.
In contrast with earlier studies investigating the repair of an AP site or SSB opposing an 8-oxodGuo lesion [29,39], when an 8-oxodGuo lesion is placed on the opposing DNA strand to the dL lesion, the level of repair of the oxidized abasic site is unaffected. This may reflect the low level of repair of the dL when present as an isolated lesion. Although the repair of the 8-oxodGuo lesion was not investigated, it has previously been shown that there is a hierarchy of repair of lesions within a cluster such that AP sites and the resulting SSB are repaired before any base lesions are excised, thus minimizing the formation of DSB [29,40,41]. We confirmed with the dL clusters in the E. coli plasmid based assay that DSB are not formed during processing.
Using the E. coli plasmid based mutation assay, the mutation frequency arising from bistranded clusters containing dL and 8-oxodGuo lesions in close proximity was found to be higher than that arising from either an isolated dL or 8-oxodGuo lesion (Fig. 1), even though 8-oxodGuo did not influence the repair of the dL lesion in the in vitro BER assay. For instance, the mutation frequency arising from the clustered damaged site increases by about 5 fold in mutY/fpg deficient E. coli (Fig. 1). It is possible that a dL lesion is more efficiently repaired in vivo than in vitro, and/or the plasmid based assay in E. coli is more sensitive than the BER assay to detect an influence of 8-oxodGuo on the repair of a dL lesion. It has been shown in vitro that the glycosylases Fpg and Nth may form a crosslink with the β-elimination product of dL [38]. These enzymes were not present in the reconstituted BER assays but formation of a crosslink could contribute to lengthening of the lifetime of dL. The results of the E. coli based assay suggest that the repair of lesions within the clustered damaged site is retarded, lengthening the lifetime of the lesions within the cluster and thus increasing the probability that the clustered lesions are present during replication. The mutation frequencies of dL/8-oxodGuo clusters in a WT and fpg null background were found to be higher compared to U/8-oxodGuo clusters (10–20% compared to 3–15%, respectively) [24] and may reflect the lower efficiency of repair of a dL lesion compared with that for an AP site in the in vitro BER assay.
In vitro repair assays showed that an 8-oxodGuo lesion retards the repair of an AP site but the repair of the 8-oxodGuo lesion is not attempted until the resulting SSB is repaired, as shown by the absence of a DSB [39]. Therefore the lifetime of both the AP site and the 8-oxodGuo lesions are extended, increasing the chances that these lesions will be present at replication. Since the results of the mutation assay with dL/8-oxodGuo clusters are very similar to those of AP site/8-oxodGuo clusters [24], it is probable that the lifetimes of both the dL and 8-oxodGuo lesions are extended also. In contrast to the similar mutation frequencies of AP site/8-oxodGuo and dL/8-oxodGuo bistranded clusters, there is a marked difference in the mutation spectrum. With the AP site/8-oxodGuo cluster the major mutation is a G:C to T:A transversion at the site of the 8-oxodGuo lesion with very little evidence of any other mutations [24]. The major mutation arising from a dL/8-oxodGuo cluster is also a G:C to T:A transversion but in addition a number of single base deletions and substitutions affecting both the dL and 8-oxodGuo containing strands were seen (Table 2), however only a 10 bp deletion targeted the dL lesion directly. AP sites and their oxidation products are non-coding lesions with adenine being preferentially inserted opposite them [30]. The dL lesion is opposite an adenine so if this nucleotide was preferentially incorporated in to the repair gap, no mutation would arise. Approximately a third of the base deletions and substitutions are on the same oligonucleotide sequence as a G:C to T:A transversion. This phenomenon may be attributable to the dL lesion as similar modifications were not seen with AP site/8-oxodGuo, DHT/8-oxodGuo or Tg/8-oxodGuo clusters [24,42,43]. Interestingly the majority of the mutations that are not G:C to A:T transversions are 3′ to the dL or 5′ to the 8-oxodGuo lesions; the reasons for this are as yet not known. It is proposed that due to the increased lifetime of the SSB resulting from incision of the AP site or dL, the 8-oxodGuo lesion remains during replication and thus adenine is incorrectly inserted opposite 8-oxodGuo. The in vitro BER assay showed that strand displacement, following incision of a dL lesion, is inefficient, thus LP-BER would also be predicted to be inefficient. It could be that other, more error prone, repair mechanisms are being utilized by E. coli that result in deletions.
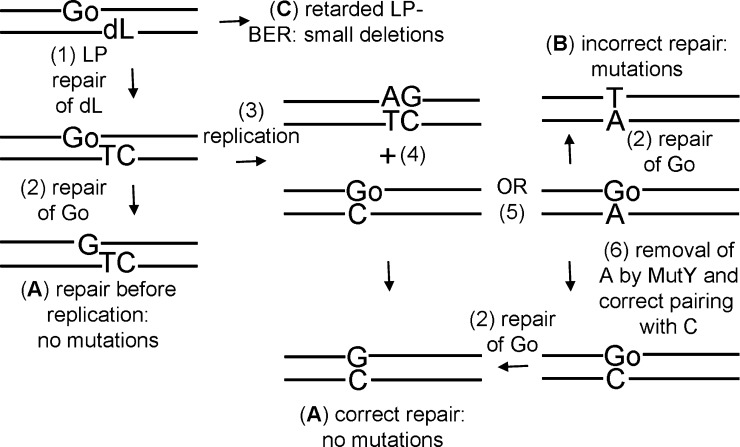
In summary, it has been demonstrated that the dL lesion is not repaired as effectively as other ionizing radiation induced lesions and that of the two BER sub-pathways this lesion can only be repaired by LP-BER. When the dL lesion is in a cluster with 8-oxodGuo the repair of the lesions is compromised resulting in an increase in mutation induction but no DSB formation, as illustrated in the schematic in Fig. 4. This study has given insights in to the biological effects of ionizing radiation and how it can lead to mutations, and potentially contribute to the formation of cancer.
Fig. 4.
Schematic representation of repair of dL/8-oxodGuo clusters in E. coli. The two lesions within the cluster are repaired sequentially, thus avoiding the formation of a DSB. First the dL lesion is repaired by long patch BER, incorporating thymine and cytosine in to the repair patch (1). Then 8-oxodGuo (Go) is repaired (2), resulting in correct repair of the clustered DNA damaged site with no formation of mutations (A). As the lifetime of the lesions within the cluster is extended, replication could occur before repair takes place (3). 8-oxodGuo (Go) can exist in two forms and can base pair with cytosine (4) or adenine (5). Base pairing with cytosine results in non-mutagenic repair of the clustered DNA damaged site (A). An incorrectly base paired adenine can be removed by mutY, allowing correct base pairing to take place (6). If the incorrectly base paired adenine is not removed then repair of 8-oxodGuo (Go) results in a G:C to T:A transversion (C). The existence of other mutations in addition to G:C to T:A transversions demonstrates that retarded LP-BER of dL could lead to small deletions (C).
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Acknowledgments
We thank Mr. Vandan Shah for synthesizing the oligonucleotides containing NI. MMG is grateful to the National Institute of General Medical Science (GM-063028) for support. The work of S.C., P. ON, and M.E.L. was supported by the Medical Research Council, UK, grant MC_PC_12001.
Appendix A. Supplementary data
The following are the supplementary data to this article:
References
- 1.Bennett P.V., Cintron N.S., Gros L., Laval J., Sutherland B.M. Are endogenous clustered DNA damages induced in human cells? Free Radical Biol. Med. 2004;37:488–499. doi: 10.1016/j.freeradbiomed.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 2.Bennett P.V., Cuomo N.L., Paul S., Tafrov S.T., Sutherland B.M. Endogenous DNA damage clusters in human skin, 3-D model, and cultured skin cells. Free Radical Biol. Med. 2005;39:832–839. doi: 10.1016/j.freeradbiomed.2005.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goodhead D.T. Initial events in the cellular effects of ionizing radiations: clustered damage in DNA. Int. J. Radiat. Biol. 1994;65:7–17. doi: 10.1080/09553009414550021. [DOI] [PubMed] [Google Scholar]
- 4.Ward J.F. DNA damage produced by ionizing radiation in mammalian cells: identities, mechanisms of formation, and reparability. Prog. Nucleic Acid Res. Mol. Biol. 1988;35:95–125. doi: 10.1016/s0079-6603(08)60611-x. [DOI] [PubMed] [Google Scholar]
- 5.Blakely E.A., Kronenberg A. Heavy-ion radiobiology: new approaches to delineate mechanisms underlying enhanced biological effectiveness. Radiat. Res. 1998;150:S126–S145. [PubMed] [Google Scholar]
- 6.Goodhead D.T., Thacker J., Cox R. Weiss Lecture. Effects of radiations of different qualities on cells: molecular mechanisms of damage and repair. Int. J. Radiat. Biol. 1993;63:543–556. doi: 10.1080/09553009314450721. [DOI] [PubMed] [Google Scholar]
- 7.Gulston M., Fulford J., Jenner T., de Lara C., O’Neill P. Clustered DNA damage induced by gamma radiation in human fibroblasts (HF19), hamster (V79-4) cells and plasmid DNA is revealed as Fpg and Nth sensitive sites. Nucleic Acids Res. 2002;30:3464–3472. doi: 10.1093/nar/gkf467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jenner T.J., Fulford J., O’Neill P. Contribution of base lesions to radiation-induced clustered DNA damage: implication for models of radiation response. Radiat. Res. 2001;156:590–593. doi: 10.1667/0033-7587(2001)156[0590:cobltr]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 9.Sutherland B.M., Bennett P.V., Sidorkina O., Laval J. Clustered DNA damages induced in isolated DNA and in human cells by low doses of ionizing radiation. PNAS. 2000;97:103–108. doi: 10.1073/pnas.97.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sutherland B.M., Bennett P.V., Sutherland J.C., Laval J. Clustered DNA damages induced by x rays in human cells. Radiat. Res. 2002;157:611–616. doi: 10.1667/0033-7587(2002)157[0611:cddibx]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 11.Gulston M., de Lara C., Jenner T., Davis E., O’Neill P. Processing of clustered DNA damage generates additional double-strand breaks in mammalian cells post-irradiation. Nucleic Acids Res. 2004;32:1602–1609. doi: 10.1093/nar/gkh306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nilsen H., Krokan H.E. Base excision repair in a network of defence and tolerance. Carcinogenesis. 2001;22:987–998. doi: 10.1093/carcin/22.7.987. [DOI] [PubMed] [Google Scholar]
- 13.Singhal R.K., Prasad R., Wilson S.H. DNA polymerase beta conducts the gap-filling step in uracil-initiated base excision repair in a bovine testis nuclear extract. J. Biol. Chem. 1995;270:949–957. doi: 10.1074/jbc.270.2.949. [DOI] [PubMed] [Google Scholar]
- 14.Tomkinson A.E., Chen L., Dong Z., Leppard J.B., Levin D.S., Mackey Z.B., Motycka T.A. Completion of base excision repair by mammalian DNA ligases. Prog. Nucleic Acid Res. Mol. Biol. 2001;68:151–164. doi: 10.1016/s0079-6603(01)68097-8. [DOI] [PubMed] [Google Scholar]
- 15.Matsumoto Y., Kim K., Hurwitz J., Gary R., Levin D.S., Tomkinson A.E., Park M.S. Reconstitution of proliferating cell nuclear antigen-dependent repair of apurinic/apyrimidinic sites with purified human proteins. J. Biol. Chem. 1999;274:33703–33708. doi: 10.1074/jbc.274.47.33703. [DOI] [PubMed] [Google Scholar]
- 16.Pascucci B., Stucki M., Jonsson Z.O., Dogliotti E., Hubscher U. Long patch base excision repair with purified human proteins. DNA ligase I as patch size mediator for DNA polymerases delta and epsilon. J. Biol. Chem. 1999;274:33696–33702. doi: 10.1074/jbc.274.47.33696. [DOI] [PubMed] [Google Scholar]
- 17.Dianov G.L., Prasad R., Wilson S.H., Bohr V.A. Role of DNA polymerase beta in the excision step of long patch mammalian base excision repair. J. Biol. Chem. 1999;274:13741–13743. doi: 10.1074/jbc.274.20.13741. [DOI] [PubMed] [Google Scholar]
- 18.Klungland A., Lindahl T. Second pathway for completion of human DNA base excision-repair: reconstitution with purified proteins and requirement for DNase IV (FEN1) Embo J. 1997;16:3341–3348. doi: 10.1093/emboj/16.11.3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fortini P., Dogliotti E. Base damage and single-strand break repair: mechanisms and functional significance of short- and long-patch repair subpathways. DNA Repair (Amst.) 2007;6:398–409. doi: 10.1016/j.dnarep.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 20.Jacobs A.C., Kreller C.R., Greenberg M.M. Long patch base excision repair compensates for DNA polymerase beta inactivation by the C4′-oxidized abasic site. Biochemistry. 2011;50:136–143. doi: 10.1021/bi1017667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eccles L.J., O’Neill P., Lomax M.E. Delayed repair of radiation induced clustered DNA damage: friend or foe? Mutat. Res. 2011;711:134–141. doi: 10.1016/j.mrfmmm.2010.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cunniffe S.M., Lomax M.E., O’Neill P. An AP site can protect against the mutagenic potential of 8-oxoG when present within a tandem clustered site in E. coli. DNA Repair (Amst.) 2007;6:1839–1849. doi: 10.1016/j.dnarep.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 23.Malyarchuk S., Brame K.L., Youngblood R., Shi R., Harrison L. Two clustered 8-oxo-7,8-dihydroguanine (8-oxodG) lesions increase the point mutation frequency of 8-oxodG, but do not result in double strand breaks or deletions in Escherichia coli. Nucleic Acids Res. 2004;32:5721–5731. doi: 10.1093/nar/gkh911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pearson C.G., Shikazono N., Thacker J., O’Neill P. Enhanced mutagenic potential of 8-oxo-7,8-dihydroguanine when present within a clustered DNA damage site. Nucleic Acids Res. 2004;32:263–270. doi: 10.1093/nar/gkh150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sedletska Y., Radicella J.P., Sage E. Replication fork collapse is a major cause of the high mutation frequency at three-base lesion clusters. Nucleic Acids Res. 2013;41:9339–9348. doi: 10.1093/nar/gkt731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Malyarchuk S., Castore R., Harrison L. DNA repair of clustered lesions in mammalian cells: involvement of non-homologous end-joining. Nucleic Acids Res. 2008;36:4872–4882. doi: 10.1093/nar/gkn450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Malyarchuk S., Castore R., Harrison L. Apex1 can cleave complex clustered DNA lesions in cells. DNA Repair (Amst.) 2009;8:1343–1354. doi: 10.1016/j.dnarep.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kozmin S.G., Sedletska Y., Reynaud-Angelin A., Gasparutto D., Sage E. The formation of double-strand breaks at multiply damaged sites is driven by the kinetics of excision/incision at base damage in eukaryotic cells. Nucleic Acids Res. 2009;37:1767–1777. doi: 10.1093/nar/gkp010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lomax M.E., Cunniffe S., O’Neill P. 8-OxoG retards the activity of the ligase III/XRCC1 complex during the repair of a single-strand break, when present within a clustered DNA damage site. DNA Repair (Amst.) 2004;3:289–299. doi: 10.1016/j.dnarep.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 30.Kunkel T.A., Schaaper R.M., Loeb L.A. Depurination-induced infidelity of deoxyribonucleic acid synthesis with purified deoxyribonucleic acid replication proteins in vitro. Biochemistry. 1983;22:2378–2384. doi: 10.1021/bi00279a012. [DOI] [PubMed] [Google Scholar]
- 31.Chan W., Chen B., Wang L., Taghizadeh K., Demott M.S., Dedon P.C. Quantification of the 2-deoxyribonolactone and nucleoside 5′-aldehyde products of 2-deoxyribose oxidation in DNA and cells by isotope-dilution gas chromatography mass spectrometry: differential effects of gamma-radiation and Fe2+-EDTA. J. Am. Chem. Soc. 2010;132:6145–6153. doi: 10.1021/ja910928n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grollman A.P., Moriya M. Mutagenesis by 8-oxoguanine: an enemy within. Trends Genet. 1993;9:246–249. doi: 10.1016/0168-9525(93)90089-z. [DOI] [PubMed] [Google Scholar]
- 33.Kotera M., Roupioz Y., Defrancq E., Bourdat A.G., Garcia J., Coulombeau C., Lhomme J. The 7-nitroindole nucleoside as a photochemical precursor of 2′-deoxyribonolactone: access to DNA fragments containing this oxidative abasic lesion. Chem. Eur. J. 2000;6:4163–4169. doi: 10.1002/1521-3765(20001117)6:22<4163::aid-chem4163>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 34.David-Cordonnier M.H., Cunniffe S.M., Hickson I.D., O’Neill P. Efficiency of incision of an AP site within clustered DNA damage by the major human AP endonuclease. Biochemistry. 2002;41:634–642. doi: 10.1021/bi011682l. [DOI] [PubMed] [Google Scholar]
- 35.Xu Y.J., DeMott M.S., Hwang J.T., Greenberg M.M., Demple B. Action of human apurinic endonuclease (Ape1) on C1′-oxidized deoxyribose damage in DNA. DNA Repair (Amst.) 2003;2:175–185. doi: 10.1016/s1568-7864(02)00194-5. [DOI] [PubMed] [Google Scholar]
- 36.Demple B., Sung J.S. Molecular and biological roles of Ape1 protein in mammalian base excision repair. DNA Repair (Amst.) 2005;4:1442–1449. doi: 10.1016/j.dnarep.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 37.DeMott M.S., Beyret E., Wong D., Bales B.C., Hwang J.T., Greenberg M.M., Demple B. Covalent trapping of human DNA polymerase beta by the oxidative DNA lesion 2-deoxyribonolactone. J. Biol. Chem. 2002;277:7637–7640. doi: 10.1074/jbc.C100577200. [DOI] [PubMed] [Google Scholar]
- 38.Kroeger K.M., Hashimoto M., Kow Y.W., Greenberg M.M. Cross-linking of 2-deoxyribonolactone and its beta-elimination product by base excision repair enzymes. Biochemistry. 2003;42:2449–2455. doi: 10.1021/bi027168c. [DOI] [PubMed] [Google Scholar]
- 39.Lomax M.E., Cunniffe S., O’Neill P. Efficiency of repair of an abasic site within DNA clustered damage sites by mammalian cell nuclear extracts. Biochemistry. 2004;43:11017–11026. doi: 10.1021/bi049560r. [DOI] [PubMed] [Google Scholar]
- 40.Eccles L.J., Lomax M.E., O’Neill P. Hierarchy of lesion processing governs the repair, double-strand break formation and mutability of three-lesion clustered DNA damage. Nucleic Acids Res. 2010;38:1123–1134. doi: 10.1093/nar/gkp1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Eot-Houllier G., Eon-Marchais S., Gasparutto D., Sage E. Processing of a complex multiply damaged DNA site by human cell extracts and purified repair proteins. Nucleic Acids Res. 2005;33:260–271. doi: 10.1093/nar/gki165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bellon S., Shikazono N., Cunniffe S., Lomax M., O’Neill P. Processing of thymine glycol in a clustered DNA damage site: mutagenic or cytotoxic. Nucleic Acids Res. 2009;37:4430–4440. doi: 10.1093/nar/gkp422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shikazono N., Pearson C., O’Neill P., Thacker J. The roles of specific glycosylases in determining the mutagenic consequences of clustered DNA base damage. Nucleic Acids Res. 2006;34:3722–3730. doi: 10.1093/nar/gkl503. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.