Abstract
Purpose
Diffuse intrinsic pontine gliomas (DIPG) typically progress within 6 months after initial radiation therapy. Usually, progression is accompanied with debilitating symptoms and changes in the MRI appearance.
Methods
We conducted a retrospective chart review of patients with recurrent DIPG at MDAnderson Cancer Center from 1998 to 2010 to asses both, the treatments given and to consider the optimal way to study the benefit of them.
Results
Thirty one patients were identified who were treated with 61 attempts using 26 different individual treatment elements in 31 different regimens. The most frequently used drugs were etoposide (14), bevacizumab nimotuzumab irinotecan and, valproic acid (13 each). Seven patients had repeat radiation to the primary tumor. Response was recorded after 58 treatment attempts and was categorized as 0/7/20/31 for CR/PR/SD/PD, respectively. The median progression free survival after treatment start was 2 months and was found to be correlated to the prior time to progression but not to the number of previous treatment attempts. Among the various treatments, repeat radiation resulted in the highest response rates (4/7), and longest progression free survival. There was evidence suggestive of benefit of classical chemotherapeutic drugs such as cisplatin and temozolomide.
Conclusion
The biology of DIPG appears to change from first diagnosis to progression. Repeat radiotherapy, either alone or combined with other therapies, should be tested in a prospective clinical study. An important factor remains previous time to progression for prediction of treatment benefit.
Keywords: diffuse intrinsic pontine glioma, chemotherapy, radiation, biomathematics
INTRODUCTION
Pediatric diffuse intrinsic pontine gliomas (DIPG) are considered a specific tumor diagnosis based on clinical symptoms and radiographic appearance of the brainstem. The typical MRI appearance of a DIPG is a non-enhancing enlarged pons with homogeneous low signal intensity on T1 and high signal intensity on T2 weighted images. Surgical interventions have not been recommended because complete resection is impossible and any manipulation of the pons is associated with a high risk of severe neurological deficits. In addition, the histological results from pathologic specimens have shown no prognostic relevance. Interestingly, while grade II astrocytic tumors have a relatively benign prognosis in other locations in the brain, the same histology found in the pons is linked a very poor prognosis. DIPG's often shrink in response to conformal radiation therapy of 54-60 Gy with improvement of patients’ symptoms. Unfortunately, the median time to progression is 5-6 months after radiotherapy with a median overall survival rate of less than a year 1-5. Numerous prospective clinical trials have been reported to use chemotherapy prior to6, instead of 7, during 8 or after 9 radiation. Despite some possible minor benefit for small subsets of patients, the dismal median overall survival times indicating the majority of patients remained unchanged, and a successful chemotherapy has not yet been reported.
Recently an increasing number of pediatric clinical studies have included patients with recurrent DIPG's. There have been some phase II studies specifically addressing this population. It is hard to judge the results of these studies as very few data are available to summarize the average course of recurrent glioma or its responsiveness to treatment. Here we summarize the experience of MDAnderson Cancer Center with treatments of recurrent diffuse intrinsic pontine glioma.
METHODS
After obtaining Institutional Review Board approval, a retrospective chart review of all patients treated at MDAnderson Cancer Center for patients with tumor locations in the brain stem. Eligibility criteria for this study include a recurrent/progressive previously treated tumor centered in the pons, with radiographic appearance as described above,. Clinical criteria were not used as formal eligibility criteria, although it turned out that all our DIPG patients also had typical clinical symptoms for a pontine lesion since less than six months. Exclusion criteria included patients in whom the treatment was not clearly documented, while treatments occurring in collaborating institutions were not excluded as long as the patient was a formal patient at MDAnderson and had received some of the care there. The patient medical records, treatment history and all available MRI imaging were reviewed.
Several patients were treated with multiple different regimens for multiple recurrences. To capture these data, the database included one line per documented treatment approach (as opposed to the traditional structure of one line per patient). Calculations were done using the Statistical Package for Social Studies (SPSS, version 16.0). The analysis was aimed as hypothesis generating observational study, not as conformal testing. Event free survival times were defined from date of start treatment to one of the following as the first event: 1)tumor progression on MRI, 2)unquestionable deterioration of clinical symptoms, or 3) death. Potential prognostic factors were analyzed in Kaplan Meier curves, Log Rank tests, and COX regression analyses for combinations of the prognostic factors being considered. As described in the results, only the prior time to progression was found to be a useful factor to predict future progression free survival time. This factor was than used in a regression analysis to calculate the predicted progression free survival of each patient assuming average outcome. The individually observed progression free survival was compared with the predicted defining “EFS-gain” as the difference between the two numbers. Those numbers were used to asses the efficacy of treatments.
Patient groups were defined as having or not having a certain drug as a part of the treatment protocol in order to allow analysis of the effect of individual drug effects since a variety of different treatment concepts and combinations were used in this study population. This analysis was repeated for all drugs that were used more than once. To evaluate individual drugs, cross tables and Chi Square tests were used for response, and non-parametric WILCOXON rank sum tests for EFS-gain. As the patients were treated with various combinations, the different drug comparisons had overlapping patient groups, and every result was re-evaluated for drug combinations to see if it could be caused by a different drug given in combinations.
RESULTS
The database documented 184 treatments in 64 patients with diffuse intrinsic pontine glioma. Excluding first line treatments, and surgical procedures, and those treatments that were started without progressive disease after radiation, 61 treatment approaches in 31 patients met eligibility requirements for further analysis. Should add some demographic data of patients, age, sex, interval from diagnosis,...
The tumors had progressed without treatment in 3 of the 61 treatments, while in the remaining 58 the initial progression occurred while receiving some type of treatment. The first MD Anderson therapy was the third , fourth, fifth, sixth and seventh line strategy in 31, 15, 9, 2 and 4 patients, respectively. The lack of second line treatments and the low number of sixth lines of treatment occurred because maintenance treatments starting after a successful induction were excluded in this analysis which included only treatments starting with progressing tumors. The most frequently used drug was etoposide (14) followed by bevacizumab (13), nimotuzumab (13), irinotecan (13), valproic acid (13), and temozolomide (8), cetuximab (5), rapamycin (3), cis-retinoic acid (2), Cereport (2), cisplatin (2), carboplatin (2) vincristine (2), lomustine (2), temsirolimus (2), the notch inhibitor MK0752 (1), cyclophosphamide (1), ruta6 (1), procarbazine (1), Sorafenib (1), topotecan (1), vinorelbine (1), celecoxib (1), vitamin D (1), and fenofibrate (1). Repeat radiation was used in 9 cases; 7 of which were radiation to the primary irradiated tumor, , while 2 were to metastatic or infiltrative sites not included in the previous radiation field. The treatment protocols included these modalities and drugs in 32 different combinations. For instance, bevacizumab (13), was mostly given in combination with irinotecan (6), but it was also combined with temsirolimus (2), rapamycin (1), radiation (1), valproic acid and etoposide (1), irinotecan and nimotuzumab (1), or radiation and irinotecan (1). The most frequently used protocols were nimotuzumab monotherapy (12) followed by valproic acid/etoposide (9), bevacizumab/irinotecan (5), and cetuximab/irinotecan (3) combinations. All other combinations were used only twice (4) or once (24).
In 58 treatments evaluable for response, there was no complete response (CR=0), 7 partial responses (PR=12.3%), 20 stable diseases (SD=34.5%) and 31 progressive diseases (PD=53.4%) documented at the first time of response evaluation. The response rate was independent of gender, patient age group, if the previous recurrence had occurred on treatment or in a watch and wait period, or how many treatments had been given before. Table 1 shows the responses in relation to the individual drugs given.
Table 1.
| Treatment | N - response | PR | P - PR | N-EFS | mEFS | P-EFS gain |
|---|---|---|---|---|---|---|
| all | 58 | 7 | 61 | 0.172 | ||
| XRT-Primary | 7 | 4 | 0.002 | 7 | 0.326 | 0.017 |
| XRT-MET | 2 | 0 | 0.775 | 2 | 0.036 | 0.099 |
| Bevacizumab | 12 | 2 | 0.438 | 13 | 0.172 | 0.374 |
| Irinotecan | 13 | 2 | 0.486 | 13 | 0.172 | 0.985 |
| Valproic acid | 11 | 2 | 0.388 | 13 | 0.156 | 0.984 |
| Nimotuzumab | 13 | 1 | 0.517 | 13 | 0.186 | 0.494 |
| Oral Etoposide | 10 | 1 | 0.662 | 12 | 0.151 | 0.706 |
| IV etoposide | 2 | 1 | 0.225 | 2 | 0.326 | 0.046 |
| Temozolomide | 8 | 1 | 0.661 | 8 | 0.255 | 0.037 |
| Cetuximab | 5 | 1 | 0.481 | 5 | 0.211 | 0.693 |
| Rapamycin | 3 | 1 | 0.32 | 3 | 0.096 | 0.668 |
| CCNU | 2 | 0 | 0.775 | 2 | 0.096 | 0.633 |
| Cis-retinoic acid | 2 | 0 | 0.775 | 2 | 0.033 | 0.056 |
| Cereport | 2 | 0 | 0.775 | 2 | 0.151 | 0.633 |
| Carboplatin | 2 | 0 | 0.775 | 2 | 0.151 | 0.633 |
| Vincristine | 2 | 0 | 0.775 | 2 | 0.364 | 0.042 |
| Temsirolimus | 2 | 0 | 0.775 | 2 | 0.214 | 0.627 |
| Cisplatin | 2 | 1 | 0.225 | 2 | 0.326 | 0.046 |
Responses and median progression free survival of patients treated for recurrent diffuse intrinsic pontine glioma with various treatment combinations. There were no CRs. The number of patients evaluable for response (n-Response) and the number of partial responses (PR) are shown in the first two columns follows by the P-values of Chi square tests comparing those responses to those of patients receiving other recurrence treatments. No P-value was defined as significant as this is only an observational study. The number of patients included in the event free survival calculation (n-EFS), the median EFS as Kaplan Meier Estimate, are shown in the next two columns. The last column shows the P-value of a Kruskal Wallis test comparing EFS gain of patients treated with the drug to those treated with other drugs as a measure if improved EFS is a relevant finding.
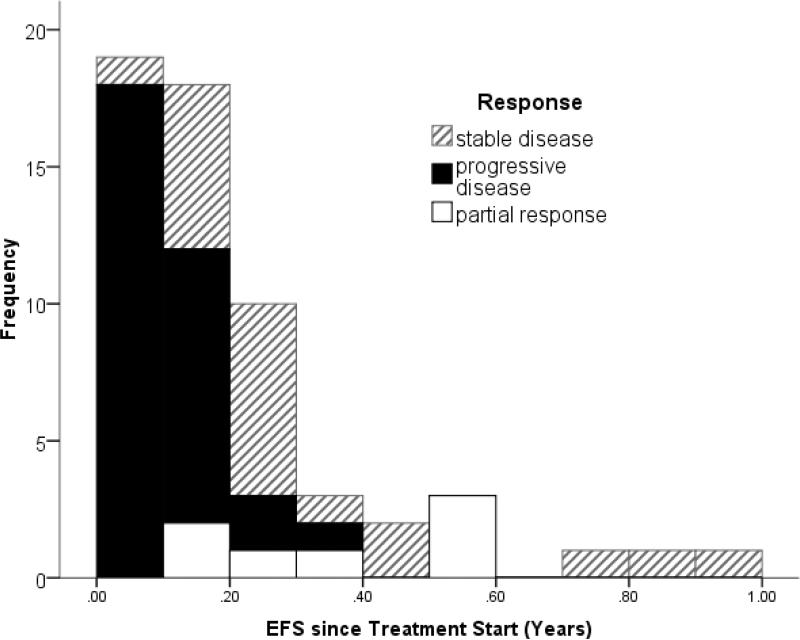
The time to progression was short after most treatments (Fig 1). Four patients had no event recorded of their most recent treatment attempts at the time of the analysis those data were censored at the last date of observation at 0.02 to 0.29 years. The event free survival range for the other 57 was 0.01 to 0.92 years (mean 0.21, median 0.15, left screwed distribution, fig 1). The median event free survival in Kaplan Meier estimates was 0.33 (Standard error SE +0.125), 0.21 (SE + 0.02), and 0.096 (+0.005), for treatments resulting in PR, SD, or PD, respectively (p<0.005 Log Rank Test)
Figure 1.
Progression free survival times in patients treated for recurrent DIPG. The different patterns of the bars indicate if the first evaluation after starting the treatment showed stable disease (hateched) or progressive disease (solid) or partial response (white). Patietns with censored time (event not known yet) are excluded from this figure.
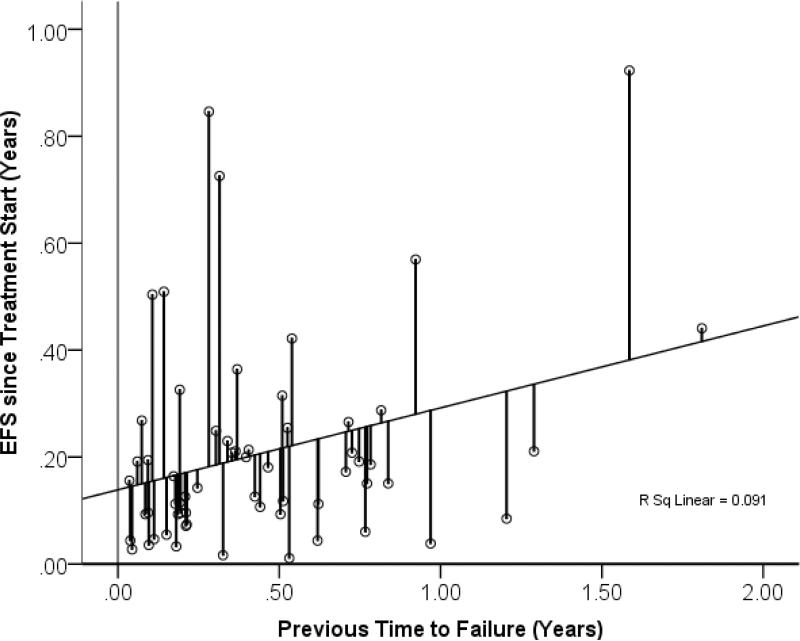
There was a moderate but significant correlation between the progression free survival time after the previous treatment and the event free survival after the current treatment (p=0.026 ANOVA regression analysis). We did not detect any consistent influence of gender, nor of age at diagnosis, or age at treatment start, or time between diagnosis and recurrence, or of the number of previous treatments on event free survival. As only the previous time to progression had a prognostic relevance, the predicted EFS was calculated based only upon that number using linear regression, and the difference between the predicted and the observed EFS was defined as “EFS-gain” and used to measure individual treatment effect.
Among the top 10% of longest EFS gains recorded appeared to be associated with radiation: In detail EFS gain in years with the “best” regimens: Cereport + carboplatin (0.66), nimotuzumab (0.54), cisplatin + temozolomide, valproic acid+ vincristine + etoposide IV (0.53), repeat radiation and rapamycin (0.34), repeat radiation bevacizumab and irinotecan (0.34), and repeat radiation with bevacizumab (0.29). Among the worst 10% of negative event free survival gain numbers recorded, appeared to be more single drug treatments. Those included single drug treatments with nimotuzumab, or MK0752, irinotecan, but also one protocol with a combination of valproic acid + etoposide and another protocol combining vinorelbine, cis-retinoic acid, cyclophosphamide celecoxib vitamin D and fenofibrate. Ranking treatment protocols that were done more than once excluding all the repeat radiation concepts identified the top ranking protocols as: cetuximab + irinotecan (35), Celeport + carboplatinum (34.5), and nimotuzumab monotherapy (31), but those differences did not reach p values suggesting relevance (P=0.29 Kruskat Wallis test).
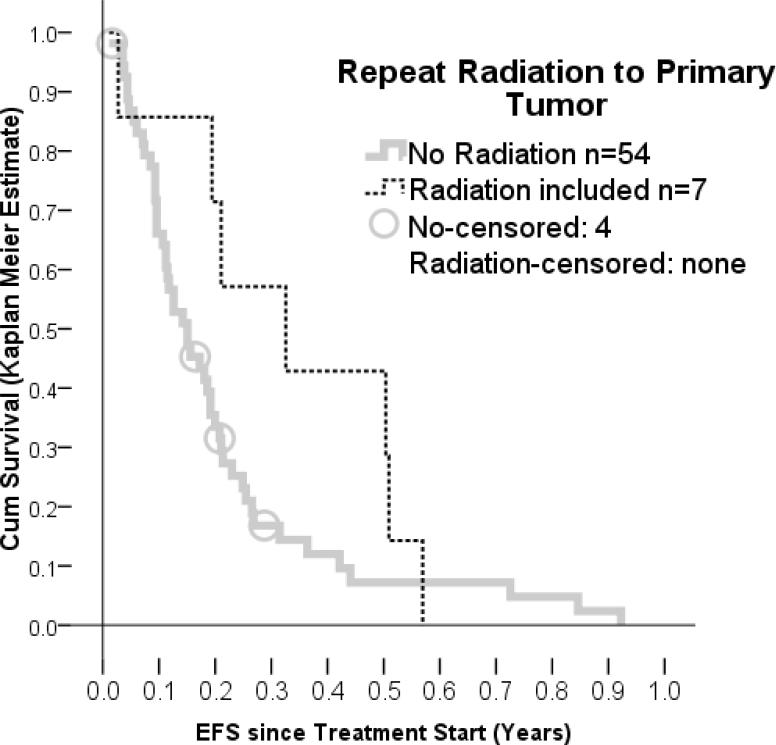
Repeat radiation to the primary tumor resulted in tumor shrinkage in 4 of 7 patients compared to 5 of 52 patients treated with protocols not including radiation to the primary tumor (p= 0.008 Chi Square Test). This treatment gave the most consistent benefit in all the methods that were used for evaluation. Repeat irradiation was also linked to longer EFS; and comparing EFS gain to patients without repeat radiation gave P-values of 0.017, also suggesting positive influence on the disease. These findings were different when radiation was used for metastatic sites, which was done only twice. Those two patients had both progressive disease at the next evaluation and the EFS was shorter (p=0.035 Log Rank Test) than in patients treated differently.
None of the individual drugs therapies resulted in superior response rates. Moderately higher median event free survivals figures were reported for nimotuzumab, intravenous cisplatin, intravenous etoposide, temozolomide, cetuximab and temsirolimus. Among those temozolomide, cisplatin, IV etoposide and vincristine reached P-values below 0.05. These were not independent findings. Temozolomide was given to eight patients and with a p-value of 0.037 might appear most relevant, but it was rarely given as single agent. The drug had been combined with re-irradiation to the primary tumor (2), vincristine (2), cisplatin and intravenous etoposide (2), irinotecan (1), rapamycin (1), and lomustine (1). Cisplatin and intravenous etoposide was used in the same two patients, both of which had previous radiation in their primary treatments but none of the traditional alkylators. When they were treated for recurrence one of them was also treated with vincristine, but none of them had radiation in the recurrence treatment concepts.
Response to protocols containing bevacizumab was evaluable in 12 patients, 2 of which responded, which was not different from the response rate to other treatments. In addition, both of these patients had radiation at the same time. The EFS was not different from the control group regardless which type of analysis was done.
DISCUSSION
This retrospective chart review showed that recurrent DIPG can respond to repeated therapies in 12% of the treatment attempts. The event free survival after a repeated treatment attempt varied between one month and one year. It was surprising to us that neither the number of previous treatment attempts nor the traditional factors such as age and gender had relevant influence on the response or the event free survival time. We judge this as a result of selection: only tumors growing relatively slow can be treated with eight different treatment concepts. These findings suggest that even within the radiographically homogeneous population of diffuse intrinsic pontine glioma, there are still considerable variations between tumors. The only factor found to be related to event free survival was the prior time to failure. The correlation was weak but it was at least in the intuitively right direction: tumors recurring fast tended to recur faster again. Taking this into account for the analysis of treatment success allowed for a more precise comparison of treatment protocols.
The most relevant finding among the different treatments was the tumor responsiveness to radiation, the details of which are described separately (Patel et al 2010). The repeat radiotherapy dose (20 Gy in 10 fractions) was much lower than the initial radiation (54Gy in 30 fractions), which would predict a limited response in recurrent disease; however, the this radiation regimen only takes two weeks of outpatient treatment and clinical symptoms typically improved for a while, making it worth while in the eyes of the patients and their parents. Repeat radiation appears to be safe and may be explored as a component of a combination treatment approach allowing the study of more innovative concepts.
Among the drugs reported were some findings suggestive of beneficial effects for temozolomide, cisplatin, etoposide, nimotuzumab and temsirolimus. This list includes drugs that have been tested as first line treatments in pontine glioma and have not been successful there; however DIPG are not the same tumors when they recur. The histology of the former is often classified as low grade astrocytic tumors, while necrosis and vascular proliferation may be abundant in the latter. Hallmarks of the typical radiographic appearance of the primary tumors are homogeneity and lack of contrast enhancement, while the latter appear heterogeneous sometimes with leptomeningeal metastases and often with pronounced focal contrast enhancement. It is therefore conceivable that the pattern of responsiveness also changes as the tumors recur.
There are obvious limitations to this retrospective chart review. Several patients were not formally enrolled on prospective clinical trials. The patient groups are overlapping between different drugs, and it is therefore difficult to separate the effect of individual agents. However, this problem is not specific for a retrospective chart review, it is even worse when all patients are treated the same not allowing any data to separate effects of individual drugs, making retrospective chart reviews here the superior source of information. There is further strength of such an analysis: All patients were treated in the same institution; there was no “center-effect” that could bias the comparison of patient groups. Also, there were no eligibility criteria which kept specific poor patient population out of the data, a problem increasingly common in prospective cooperative trials. Therefore, the data reflect more the real world of this patient population. Such strength and limitations kept in mind, this was aimed as a hypothesis generating method, as opposed to a conformal hypothesis driven clinical trial.
Conclusion
Recurrent diffuse intrinsic pontine glioma can respond to treatment. The comparison of treatment results should take the individual previous time to recurrence into account. Among a large variety of treatment combinations repeat radiation of the primary tumor was most beneficial. Cisplatinum, etoposide, vincristine, nimotuzumab, cetuximab and tensirolimus warrant further evaluation.
Figure 2.
Event free survival time in recurrent DIPG in relation o previous time to progression in the same patients after the previous treatment attempt. The two number were weakly correlated. The lines indicate the deviation of the observed EFS from the predicted EFS based on the regression analysis. The length of these lines were defined as EFS-gain and used as measure for treatment success. With points far above the regression line suggesting effective treatment., and point below less than average outcomes.
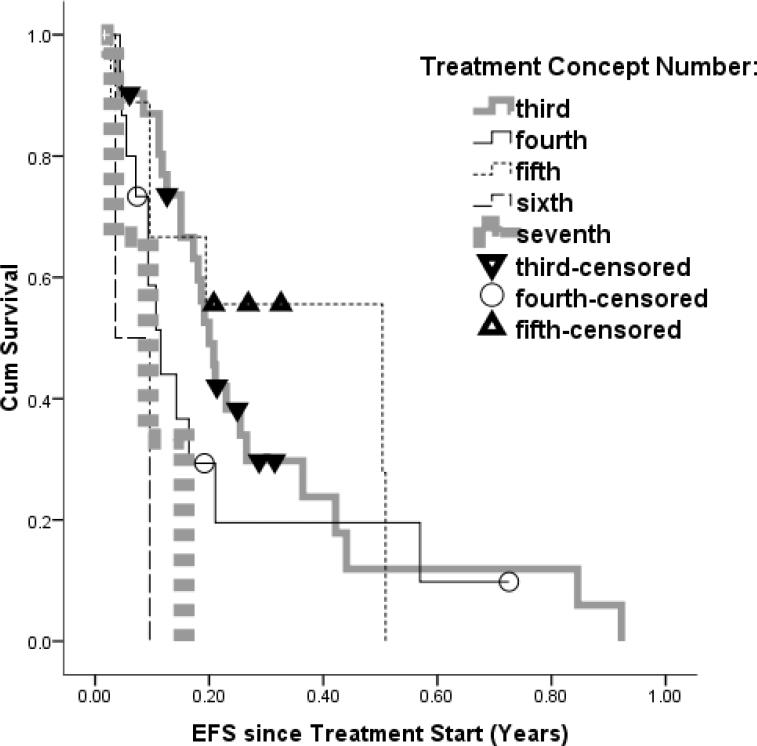
Figure 3.
Kaplan Meier Estimate EFS curves for recurrent DIPG separated by the number of treatments the pateitn had previously. The lines do not separates as assumed, with the fifth line of treatment appearing superior to the second.
Figure 4.
event free survival of patient treated with repeat radiation to the primary tumor in comparison to patients treated with a variety of other protocols.
Acknowledgement
The patient population presented here is overlapping with earlier reports from the same institution as well as several clinical trials in which the patients were enrolled. .
Footnotes
Conflicts of Interest Notification: The authors declare that no potential conflicts of interests exist.
References
- 1.Freeman CR, Krischer JP, Sanford RA, Cohen ME, Burger PC, del Carpio R, et al. Final results of a study of escalating doses of hyperfractionated radiotherapy in brain stem tumors in children: a Pediatric Oncology Group study. Int J Radiat Oncol Biol Phys. 1993;27(2):197–206. doi: 10.1016/0360-3016(93)90228-n. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=8407392. [DOI] [PubMed] [Google Scholar]
- 2.Packer RJ, Boyett JM, Zimmerman RA, Rorke LB, Kaplan AM, Albright AL, et al. Hyperfractionated radiation therapy (72 Gy) for children with brain stem gliomas. A Childrens Cancer Group Phase I/II Trial. Cancer. 1993;72(4):1414–21. doi: 10.1002/1097-0142(19930815)72:4<1414::aid-cncr2820720442>3.0.co;2-c. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=8339232. [DOI] [PubMed] [Google Scholar]
- 3.Farmer JP, Montes JL, Freeman CR, Meagher-Villemure K, Bond MC, O'Gorman AM. Brainstem Gliomas. A 10-year institutional review. Pediatr Neurosurg. 2001;34(4):206–14. doi: 10.1159/000056021. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=11359114. [DOI] [PubMed] [Google Scholar]
- 4.Freeman CR, Farmer JP. Pediatric brain stem gliomas: a review. Int J Radiat Oncol Biol Phys. 1998;40(2):265–71. doi: 10.1016/s0360-3016(97)00572-5. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=9457808. [DOI] [PubMed] [Google Scholar]
- 5.Fangusaro J. Pediatric high-grade gliomas and diffuse intrinsic pontine gliomas. J Child Neurol. 2009;24(11):1409–17. doi: 10.1177/0883073809338960. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=19638636. [DOI] [PubMed] [Google Scholar]
- 6.Wagner S, Reinert C, Schmid HJ, Liebeskind AK, Jorch N, Langler A, et al. High-dose methotrexate prior to simultaneous radiochemotherapy in children with malignant high-grade gliomas. Anticancer Res. 2005;25(3c):2583–7. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=16080497. [PubMed] [Google Scholar]
- 7.Bouffet E, Khelfaoui F, Philip I, Biron P, Brunat-Mentigny M, Philip T. High-dose carmustine for high-grade gliomas in childhood. Cancer Chemother Pharmacol. 1997;39(4):376–9. doi: 10.1007/s002800050586. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=9025780. [DOI] [PubMed] [Google Scholar]
- 8.Wagner S, Warmuth-Metz M, Emser A, Gnekow AK, Strater R, Rutkowski S, et al. Treatment options in childhood pontine gliomas. J Neurooncol. 2006;79(3):281–7. doi: 10.1007/s11060-006-9133-1. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=16598416. [DOI] [PubMed] [Google Scholar]
- 9.Wolff JE, Molenkamp G, Westphal S, Pietsch T, Gnekow A, Kortmann RD, et al. Oral trofosfamide and etoposide in pediatric patients with glioblastoma multiforme. Cancer. 2000;89(10):2131–7. doi: 10.1002/1097-0142(20001115)89:10<2131::aid-cncr14>3.0.co;2-j. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=11066055. [DOI] [PubMed] [Google Scholar]
- 10.Shono T, Tofilon PJ, Bruner JM, Owolabi O, Lang FF. Cyclooxygenase-2 expression in human gliomas: prognostic significance and molecular correlations. Cancer Res. 2001;61(11):4375–81. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=11389063. [PubMed] [Google Scholar]
- 11.Jantke J, Ladehoff M, Kurzel F, Zapf S, Kim E, Giese A. Inhibition of the arachidonic acid metabolism blocks endothelial cell migration and induces apoptosis. Acta Neurochir (Wien) 2004;146(5):483–94. doi: 10.1007/s00701-004-0238-z. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=15118886. [DOI] [PubMed] [Google Scholar]
- 12.Badie B, Schartner JM, Hagar AR, Prabakaran S, Peebles TR, Bartley B, et al. Microglia cyclooxygenase-2 activity in experimental gliomas: possible role in cerebral edema formation. Clin Cancer Res. 2003;9(2):872–7. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=12576462. [PubMed] [Google Scholar]
- 13.Tuettenberg J, Grobholz R, Korn T, Wenz F, Erber R, Vajkoczy P. Continuous low-dose chemotherapy plus inhibition of cyclooxygenase-2 as an antiangiogenic therapy of glioblastoma multiforme. J Cancer Res Clin Oncol. 2005;131(1):31–40. doi: 10.1007/s00432-004-0620-5. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=15565458. [DOI] [PubMed] [Google Scholar]
- 14.Hau P, Kunz-Schughart L, Bogdahn U, Baumgart U, Hirschmann B, Weimann E, et al. Low-dose chemotherapy in combination with COX-2 inhibitors and PPAR-gamma agonists in recurrent high-grade gliomas - a phase II study. Oncology. 2007;73(1-2):21–5. doi: 10.1159/000120028. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=18332649. [DOI] [PubMed] [Google Scholar]
- 15.Erdman SE, Poutahidis T. Cancer inflammation and regulatory T cells. Int J Cancer. 127(4):768–79. doi: 10.1002/ijc.25430. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=20518013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Vleeschouwer S, Fieuws S, Rutkowski S, Van Calenbergh F, Van Loon J, Goffin J, et al. Postoperative adjuvant dendritic cell-based immunotherapy in patients with relapsed glioblastoma multiforme. Clin Cancer Res. 2008;14(10):3098–104. doi: 10.1158/1078-0432.CCR-07-4875. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=18483377. [DOI] [PubMed] [Google Scholar]
- 17.Menard C, Johann D, Lowenthal M, Muanza T, Sproull M, Ross S, et al. Discovering clinical biomarkers of ionizing radiation exposure with serum proteomic analysis. Cancer Res. 2006;66(3):1844–50. doi: 10.1158/0008-5472.CAN-05-3466. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=16452246. [DOI] [PubMed] [Google Scholar]
- 18.Bower JE, Ganz PA, Tao ML, Hu W, Belin TR, Sepah S, et al. Inflammatory biomarkers and fatigue during radiation therapy for breast and prostate cancer. Clin Cancer Res. 2009;15(17):5534–40. doi: 10.1158/1078-0432.CCR-08-2584. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=19706826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu T, Chen J, Lu Y, Wolff JE. Effects of bevacizumab plus irinotecan on response and survival in patients with recurrent malignant glioma: a systematic review and survival-gain analysis. BMC Cancer. 10:252. doi: 10.1186/1471-2407-10-252. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=20525214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wolff JE KR, Wolff B, Pietsch T, Graf N, Peters O, Schmid H, Rutkowski S, Warmuth-Metz M, Kramm CM. High Dose Methotrexate for Pediatric High Grade Glioma – Results of the HIT-GBM-D pilot study. J Neurooncol. 2010 doi: 10.1007/s11060-010-0334-2. Accepted for Publication July 2010. [DOI] [PubMed] [Google Scholar]
- 21.Wolff JE, Classen CF, Wagner S, Kortmann RD, Palla SL, Pietsch T, et al. Subpopulations of malignant gliomas in pediatric patients: analysis of the HIT-GBM database. J Neurooncol. 2008;87(2):155–64. doi: 10.1007/s11060-007-9495-z. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=18209954. [DOI] [PubMed] [Google Scholar]
- 22.Wolff JE, Driever PH, Erdlenbruch B, Kortmann RD, Rutkowski S, Pietsch T, et al. Intensive chemotherapy improves survival in pediatric high-grade glioma after gross total resection: results of the HIT-GBM-C protocol. Cancer. 116(3):705–12. doi: 10.1002/cncr.24730. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=19957326. [DOI] [PubMed] [Google Scholar]
- 23.Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–96. doi: 10.1056/NEJMoa043330. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=15758009. [DOI] [PubMed] [Google Scholar]
- 24.Pollack IF, Hamilton RL, Sobol RW, Nikiforova MN, Nikiforov YE, Lyons- Weiler MA, et al. Mismatch repair deficiency is an uncommon mechanism of alkylator resistance in pediatric malignant gliomas: A report from the children's oncology group. Pediatr Blood Cancer. doi: 10.1002/pbc.22634. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=20589656. [DOI] [PMC free article] [PubMed]
- 25.Pollack IF, Hamilton RL, Burger PC, Brat DJ, Rosenblum MK, Murdoch GH, et al. Akt activation is a common event in pediatric malignant gliomas and a potential adverse prognostic marker: a report from the children's oncology group. J Neurooncol. doi: 10.1007/s11060-010-0297-3. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=20607350. [DOI] [PMC free article] [PubMed]