Abstract
HIV-1 Nef is a critical AIDS progression factor yet underexplored target for antiretroviral drug discovery. A recent high-throughput screen for pharmacological inhibitors of Nef-dependent Src-family kinase activation identified a diphenylpyrazolodiazene hit compound with submicromolar potency in HIV-1 replication assays against a broad range of primary Nef variants. This compound, known as ‘B9’, binds directly to Nef and inhibits is dimerization in cells as a possible mechanism of action. Here were synthesized a diverse set of B9 analogs and identified structural features essential to antiretroviral activity. Chemical modifications to each of the three rings present in the parent compound were identified that did not compromise antiviral action. These analogs will guide the development of next-generation compounds with appropriate pharmacological profiles for assessment of antiretroviral activity in vivo.
Keywords: HIV-1, HIV Nef, Nef inhibitors, antiretroviral drug discovery
HIV/AIDS remains as a persistent global public health problem. According to UNAIDS, more than 2.5 million people have died of HIV-related causes worldwide since the pandemic began in 1981 and 34 million are currently living with the virus. The advent of potent combination antiretroviral therapy in the mid-1990s changed the course of the HIV epidemic from a life-threatening illness to a chronic condition.1 These drugs target HIV-1 enzymes critical to the viral life cycle as well as fusion of the virus to the host cell. Although antiretroviral drug cocktails have increased the life expectancy of infected individuals, they do not clear the virus and require lifelong administration. However, the rise of multidrug-resistant strains of HIV-1,2 together with uncertain vaccine prospects,3 underscore the urgent need for new antiretroviral drugs with mechanisms of action complementary to existing agents.1
The HIV-1 genome shares three genes with other retroviruses (Gag, Pol, and Env) and also encodes proteins essential for regulation of viral transcription (Tat) and RNA splicing (Rev). Currently approved antiretroviral drugs target products of the HIV-1 Pol and Env genes, including reverse transcriptase, integrase, protease as well as the gp41 envelope glycoprotein essential for host cell fusion with the virus.1 HIV-1 also encodes four unique accessory proteins (Nef, Vif, Vpu, and Vpr) which combat host cell restriction factors, promote viral growth and represent alternative targets for antiretroviral drug discovery.4 Of these, HIV-1 Nef is a particularly attractive drug target because of its roles in HIV-1 infectivity, viral replication and immune escape of HIV-infected cells.5 Early studies established that Nef is required for high-titer replication of SIV and disease progression in non-human primates.6 Remarkably, targeted expression of Nef alone in the CD4+ cell compartment of transgenic mice is sufficient to cause a severe AIDS-like syndrome, supporting the singular importance of this viral protein in HIV-1 pathogenesis.7 Conversely, strains of HIV-1 with defective Nef alleles have been isolated from a subset of patients with long-term, non-progressive HIV infection, implicating Nef as a critical virulence factor for human AIDS.8–10 Taken together, these findings make a strong case for Nef as a valid target for antiretroviral drug discovery.11
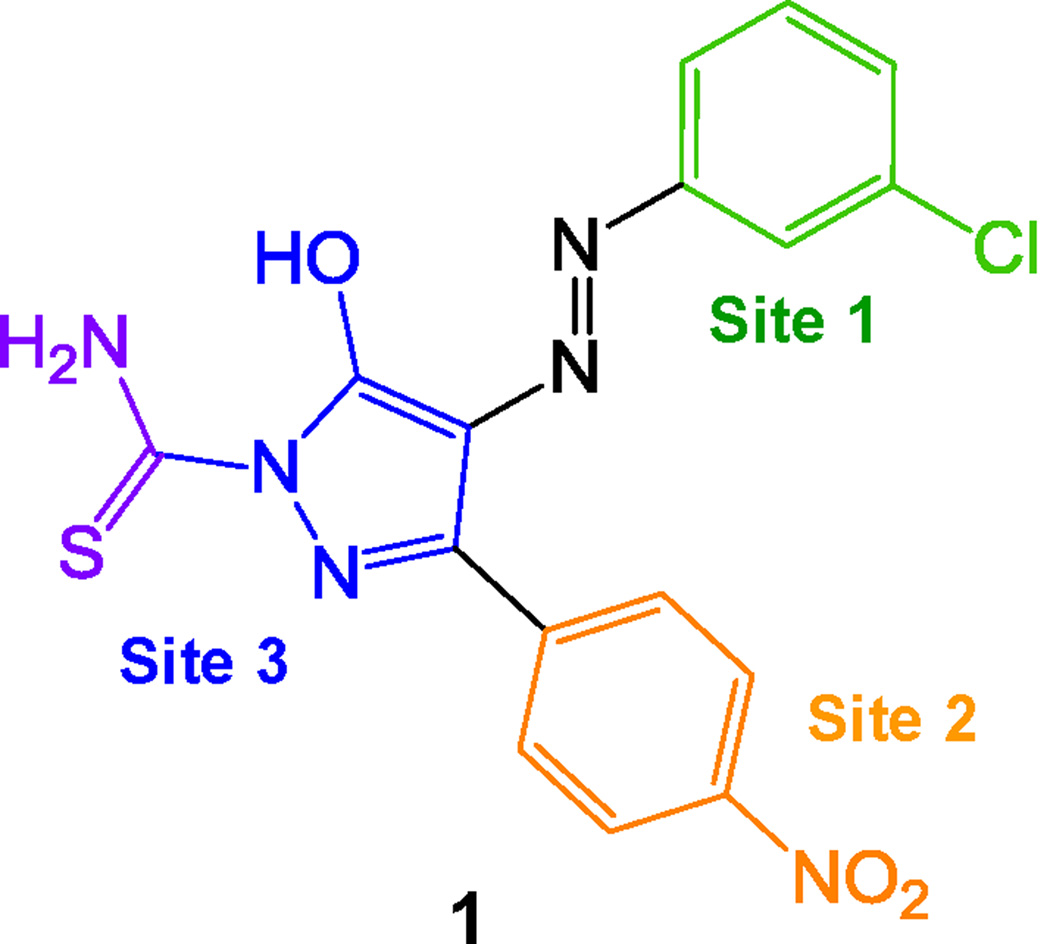
Drug discovery campaigns targeting Nef have been hampered by the lack of a screening assay. Nef does not exhibit any known enzymatic or biochemical activity amenable to HTS assay design. To circumvent this problem, Emert-Sedlak et al. developed an indirect assay linking Nef to the activation of Hck, a Src-family kinase and host cell effector protein for Nef.12;13 This kinase-coupled Nef assay was then automated, enabling HTS of a large and diverse chemical library of more than 220,000 compounds.13 Four compound classes emerged from this screen that potently inhibited HIV-1 infectivity and replication in multiple cell lines. In particular, a unique diphenylpyrazolodiazene scaffold (1) was identified that blocked Nef-dependent enhancement of HIV-1 replication with submicromolar potency. Furthermore, this compound was shown to bind directly to recombinant purified Nef protein by surface plasmon resonance, and to inhibit Nef dimerization in a cell-based fluorescence complementation assay. Other work has shown that dimerization is critical to many Nef functions in the context of HIV infection,14;15 supporting the idea that Nef dimerization blockers may represent a new class of antiretroviral therapies. In this report, we describe the synthesis and virological evaluation of analogs that are focused on systematic modifications to Sites 1–3 in this compound (Figure 1).
Figure 1. Structure of the diphenylpyrazolodiazene Nef antagonist B9 [1] with analog modification sites indicated.
Colors of each modification site match the bars in the antiviral data set shown in Figure 2.
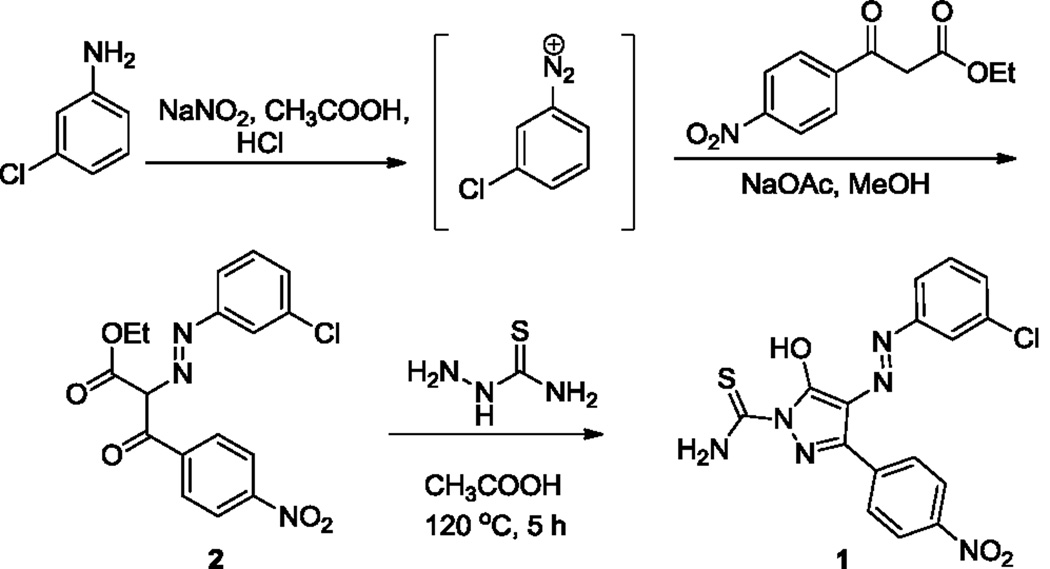
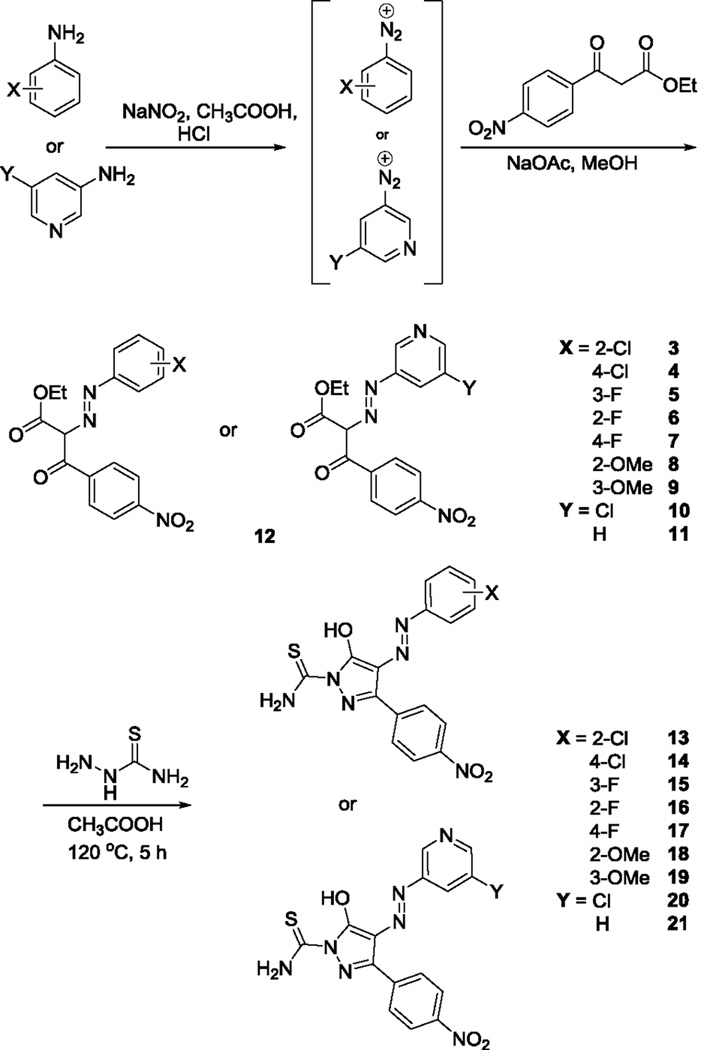
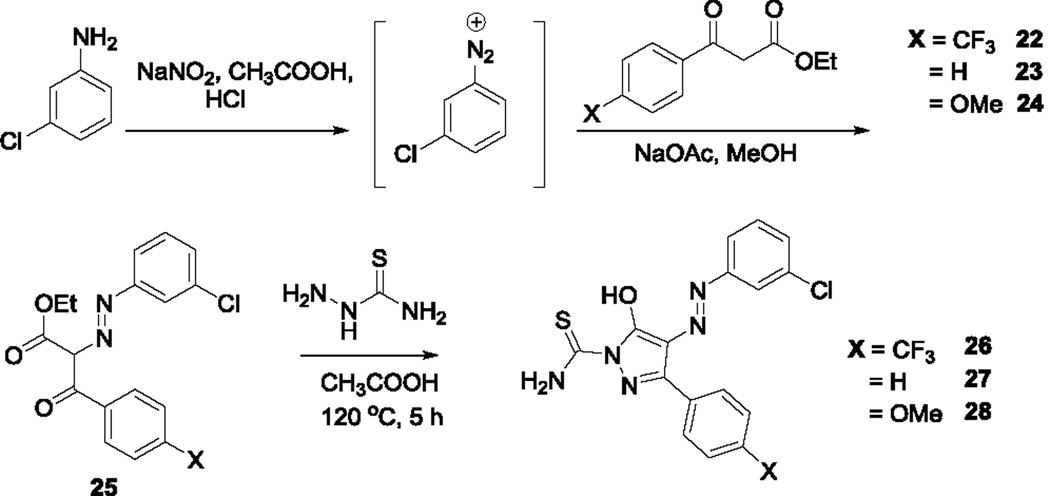
The re-synthesis of the diphenylpyrazolodiazene hit compound 1 was accomplished in three steps following a literature protocol.16 In this procedure, 3-chloroaniline was diazotized and treated with ethyl 4-nitrobenzoylacetate to give the oxopropanoate intermediate 2, which was then treated with thiosemicarbazide to give 1 (Scheme 1). For Site 1 SAR, substituted anilines 3–9 and pyridines 10–11 were treated with ethyl 4-nitrobenzoylacetate to give the oxopropanoate intermediates 12, which were then treated with thiosemicarbazide to give compounds 13–21 (Scheme 2).
Scheme 1.
Re-synthesis of hit compound, B9 [compound 1].
Scheme 2.
Synthesis of B9 Site 1 precursors 3–11 and analogs 13–21.
To assess the anti-HIV activity of the Site 1 analogs, we used the TZM-bl reporter cell line, in which the HIV-1 LTR is linked to the transcription of luciferase.17 This system provides a convenient assay for initial events in the viral life cycle including attachment, entry, integration, and viral transcription, and is often used to assess viral infectivity. Compound 1 has been previously shown to inhibit Nef-dependent enhancement of HIV-1 infectivity in TZM-bl cells with an IC50 value in the single-digit micromolar range.13
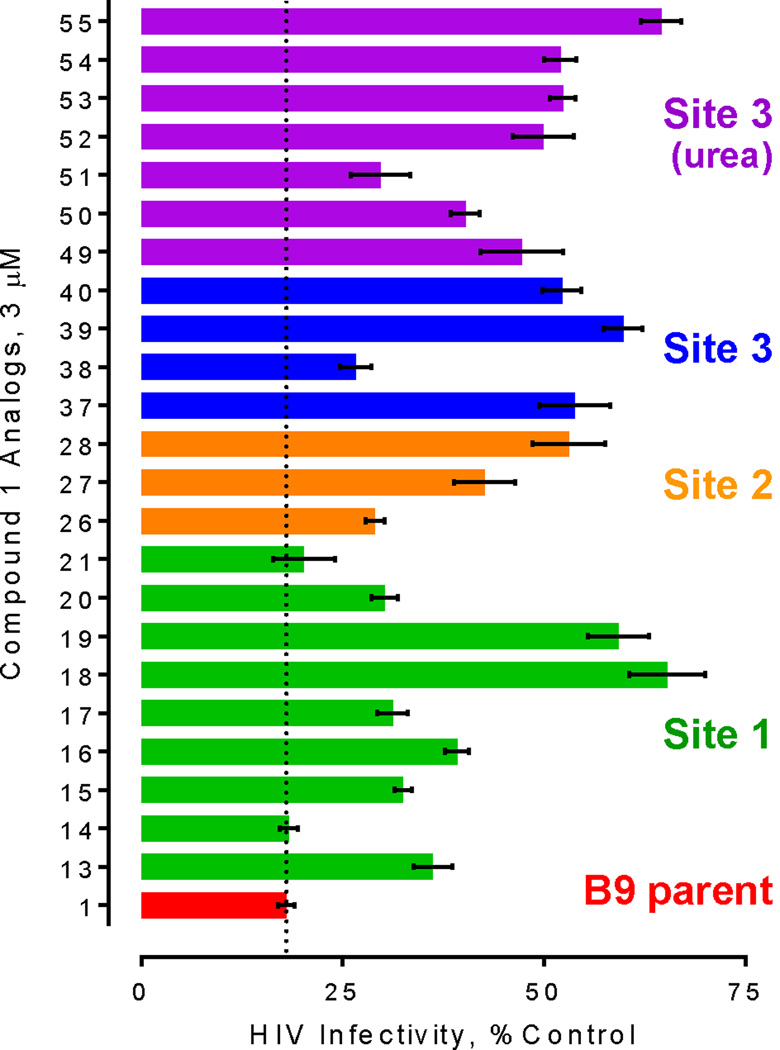
Compound 1 as well as analogs 13–21 were assayed for their impact on Nef-dependent enhancement of HIV-1 infectivity, and the results are shown in Figure 2. These results show that the position of chloro group is important for full activity, with the 4-chloro analog 14 equipotent to that of compound 1, while the 2-chloro analog 13 was less potent. Substitution of fluorine at site 1 positions 2, 3, or 4 (15–17) also compromised activity relative to compound 1. Substitution of the halogen with an electron-donating group (O-Me) at position 2 or 3 (18, 19) resulted in a substantial loss of antiviral activity. Finally, pyridine substitution at Site 1 did not compromise activity, while addition of a chloro group at position 5 of the pyridine ring reduced activity somewhat (20, 21).
Figure 2.
Assessment of Nef inhibitor action on Nef-dependent enhancement of antiviral activity in the TZM-bl HIV infectivity assay. Compounds (3 µM) were added to cultures of TZMbl reporter cells followed by infection with wild-type HIV-1 (NL4-3 strain) in 96-well plates. Viral infectivity was assessed as luciferase activity 48 h later as described13. Data were corrected for luciferase activity observed with cells infected with Nef-defective HIV, and are expressed mean percent of control values from untreated cells ± SEM. Nef enhances HIV infectivity by 2- to 3- fold in this assay13. Activity for compound 1 (red bar) is highlighted as a reference control (dotted line).
Modifications to Site 2 involved substituting the 4-nitro group with electron-donating and withdrawing substituents (Scheme 3). Replacement of the nitro group with a trifluoromethyl group (26) maintained antiviral activity (Figure 2). On the other hand, complete removal of the nitro group (27) or replacement with an O-methyl moiety (28) resulted in a significant loss of activity. These results show that the nitro group can be effectively replaced by a trifluormethyl substituent.
Scheme 3.
Synthesis of Site 2 precursors 22–24 and analogs 26–28.
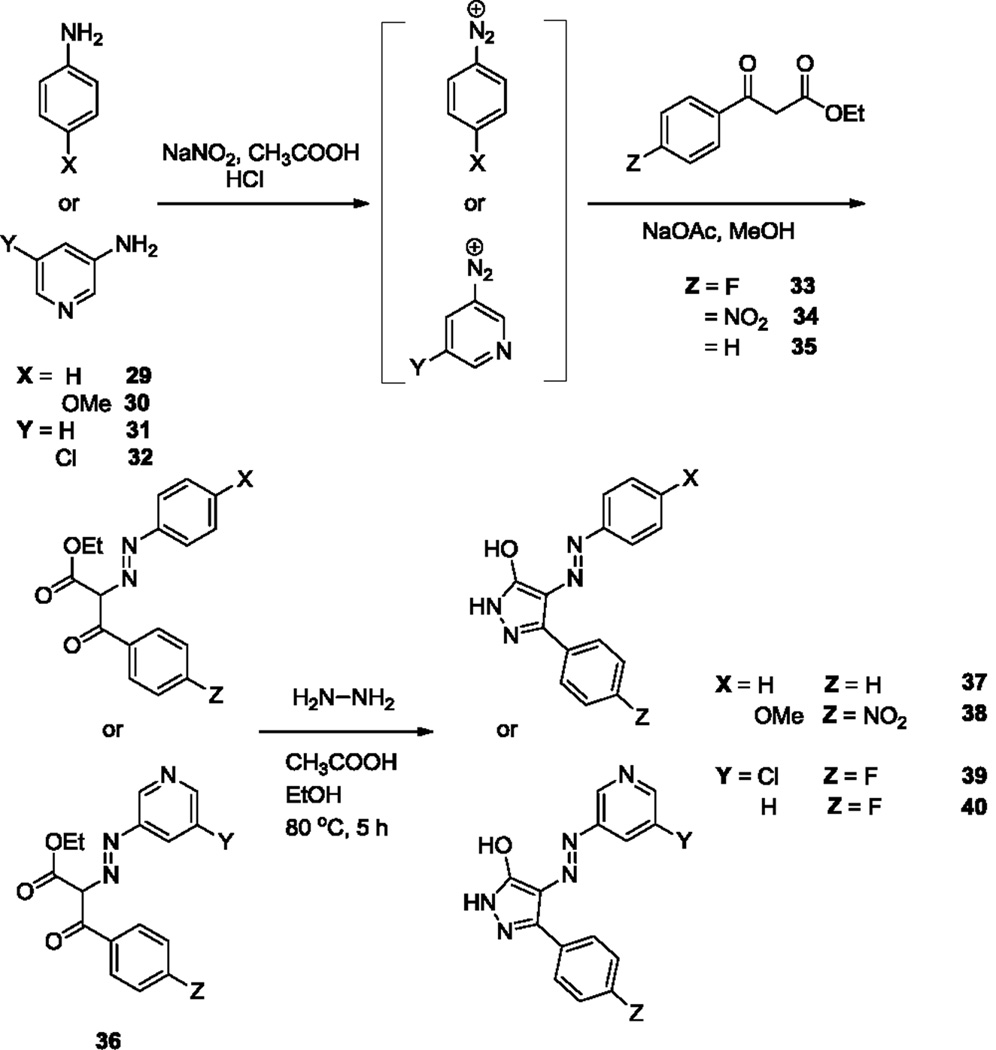
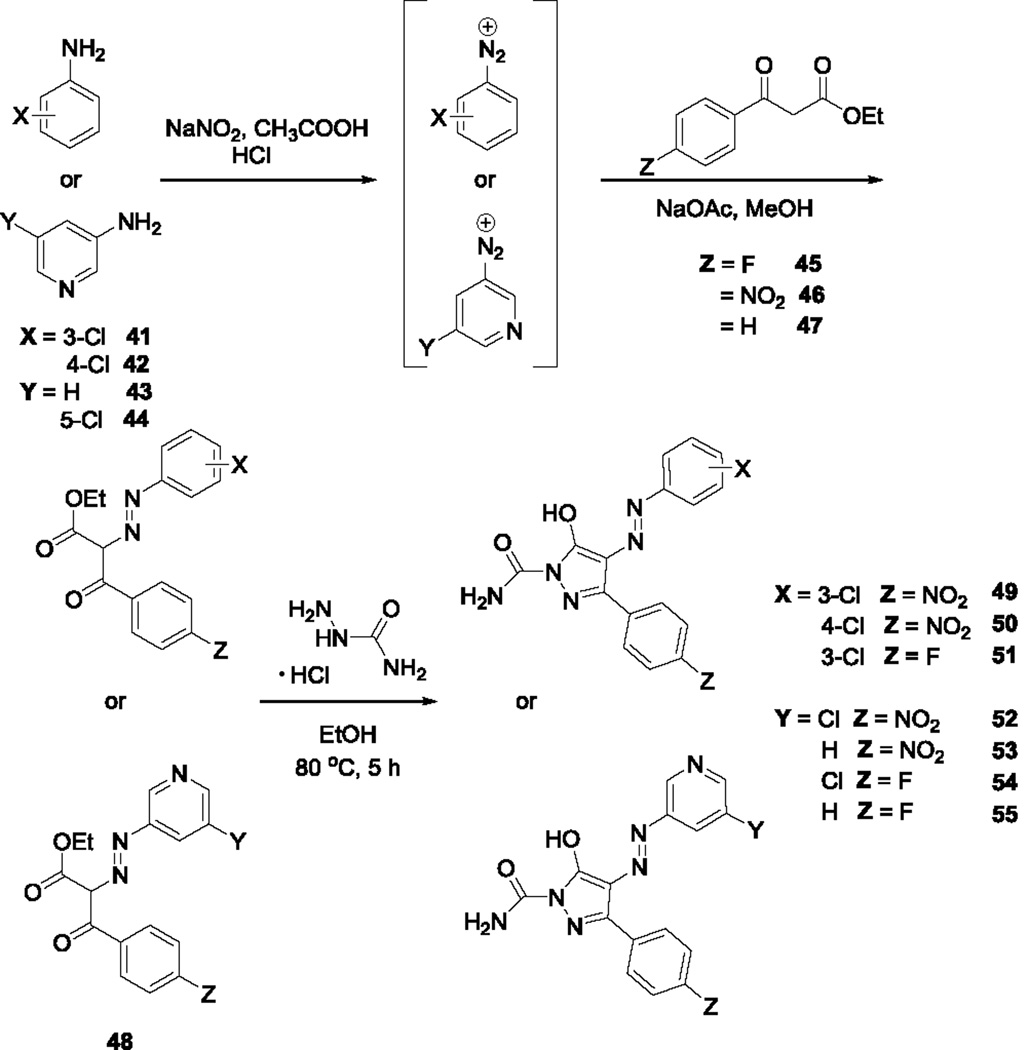
To determine if the thiourea moiety is necessary for activity, Site 3 modifications were made that removed the thiourea moiety completely (37–40, Scheme 4) or substituted it with urea18 (49–55, Scheme 5). Of the analogs lacking the thiourea, only compound 38 exhibited substantial antiviral effects, suggesting that substitution at this position on the pyrazole ring is important for activity. Replacement of the thiourea in the original structure with urea (49) resulted in loss of activity, suggesting that the sulfur atom plays a key role in antiretroviral activity. Previous docking studies with compound 1 positioned the thiourea moiety between conserved Nef residues Gln104, Gln107, and Asn 126 in the Nef dimer interface. Modifications at this position, in the absence of other changes, may impact this mode of interaction with the target protein. Of the remaining urea analogs, only compound 51 retained appreciable antiviral activity, suggesting that substitution of a fluorine atom for the nitro group in the original structure partially compensates for the loss of the thiourea. None of the analogs tested exhibited toxicity at the assay concentration in TZM-bl reporter cells, as determined by Cell Titer Blue cell viability assay (data not shown).
Scheme 4.
Synthesis of Site 3 analogs, 37–40.
Scheme 5.
Synthesis of Site 3 urea analogs, 49–55.
In summary, we present the first structure-activity study of a recently described pharmacological antagonist of HIV-1 Nef, a critical virulence factor for AIDS. Overall, these studies show that chemical modifications can be made to all three rings in the original diphenylpyrazolodiazene scaffold without appreciable loss of activity. Several functional groups that may represent pharmacological liabilities in vivo were successfully replaced, including the site 2 nitro group and the thiourea on site 3. Both of these groups are replaced in compound 51 (with fluorine and urea, respectively) without appreciable loss of antiviral activity, suggesting that these moieties are not essential for inhibition of Nef function. Taken together, these results will guide the development of next-generation compounds with appropriate pharmacological profiles for assessment of Nef-directed antiretroviral activity in vivo. In particular, the SAR developed for sites 1–3 as reported here will be useful in the design of analogs in which the diazene joining sites 1 and 3 is replaced with carbon-based linkers.
Supplementary Material
Acknowledgments
This study was supported by National Institutes of Health grants AI57083 and AI102704 (to T.E.S.) and by the Commonwealth of Pennsylvania (SAP 4100054875 to the University of Pittsburgh Drug Discovery Institute).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplementary data Supplementary data associated with this article can be found in the online version.
References
- 1.Ghosh RK, Ghosh SM, Chawla S. Expert.Opin.Pharmacother. 2011;12:31. doi: 10.1517/14656566.2010.509345. [DOI] [PubMed] [Google Scholar]
- 2.Michaud V, Bar-Magen T, Turgeon J, Flockhart D, Desta Z, Wainberg MA. Pharmacol.Rev. 2012;64:803. doi: 10.1124/pr.111.005553. [DOI] [PubMed] [Google Scholar]
- 3.Kwong PD, Mascola JR, Nabel GJ. J Int.AIDS Soc. 2012;15:17407. doi: 10.7448/IAS.15.2.17407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Malim MH, Emerman M. Cell Host Microbe. 2008;3:388. doi: 10.1016/j.chom.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 5.Foster JL, Garcia JV. Retrovirology. 2008;5:84. doi: 10.1186/1742-4690-5-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kestler H, Ringler DJ, Mori K, Panicali DL, Sehgal PK, Daniel MD, Desrosiers RC. Cell. 1991;65:651. doi: 10.1016/0092-8674(91)90097-i. [DOI] [PubMed] [Google Scholar]
- 7.Jolicoeur P. Curr.HIV.Res. 2011;9:524. doi: 10.2174/157016211798842062. [DOI] [PubMed] [Google Scholar]
- 8.Mariani R, Kirchhoff F, Greenough TC, Sullivan JL, Desrosiers RC, Skowronski J. J.Virol. 1996;70:7752. doi: 10.1128/jvi.70.11.7752-7764.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kirchhoff F, Greenough TC, Brettler DB, Sullivan JL, Desrosiers RC. N.Engl.J.Med. 1995;332:228. doi: 10.1056/NEJM199501263320405. [DOI] [PubMed] [Google Scholar]
- 10.Zou W, Denton PW, Watkins RL, Krisko JF, Nochi T, Foster JL, Garcia JV. Retrovirology. 2012;9:44. doi: 10.1186/1742-4690-9-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smithgall TE, Thomas G. Drug Discov Today: Technol. 2013;10:523. doi: 10.1016/j.ddtec.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Emert-Sedlak L, Kodama T, Lerner EC, Dai W, Foster C, Day BW, Lazo JS, Smithgall TE. ACS Chem.Biol. 2009;4:939. doi: 10.1021/cb900195c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Emert-Sedlak LA, Narute P, Shu ST, Poe JA, Shi H, Yanamala N, Alvarado JJ, Lazo JS, Yeh JI, Johnston PA, Smithgall TE. Chem.Biol. 2013;20:82. doi: 10.1016/j.chembiol.2012.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Poe JA, Smithgall TE. J.Mol.Biol. 2009;394:329. doi: 10.1016/j.jmb.2009.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu LX, Heveker N, Fackler OT, Arold S, Le Gall S, Janvier K, Peterlin BM, Dumas C, Schwartz O, Benichou S, Benarous R. J.Virol. 2000;74:5310. doi: 10.1128/jvi.74.11.5310-5319.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kalluraya B, Rahiman AM, Banji D. Arch.Pharm.(Weinheim) 2001;334:263. doi: 10.1002/1521-4184(200109)334:8/9<263::aid-ardp263>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 17.Gervaix A, West D, Leoni LM, Richman DD, Wong-Staal F, Corbeil J. Proc.Natl.Acad.Sci.U.S.A. 1997;94:4653. doi: 10.1073/pnas.94.9.4653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garg HG, Singh PP. J Pharm.Sci. 1970;59:876. doi: 10.1002/jps.2600590641. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.