Abstract
Rationale
Compounds acting on delta opioid receptors (DOR) modulate anxiety-like behaviors, yet the site of action underlying this effect is unknown. DOR mRNA and protein are expressed in the central nucleus of the amygdala, a region that plays an important role in processing fear, stress, and anxiety. We hypothesized that this brain region may contribute to the modulation of anxiety by DOR drugs.
Objective
The present study investigated the role of DOR in the central amygdala in anxiety-like behaviors.
Methods
The selective DOR agonist [D-Pen 2,5]-enkephalin (DPDPE) or antagonist naltrindole was bilaterally microinjected into the central nucleus of the amygdala of adult male Sprague Dawley rats and anxiety-like behaviors were assessed using the elevated plus maze. The effects of DOR agonists on heightened anxiety produced by stress were also investigated.
Results
Rats injected with DPDPE into the central nucleus of the amygdala demonstrated less anxiety-like behavior, as evidenced by significantly greater number of open-arm entries and time spent in the open arms than controls. Naltrindole administered alone did not affect the duration or number of entries onto the open arms; however, naltrindole pre-treatment blocked the anxiolytic effects produced by DPDPE. Systemic administration of the selective DOR agonist, SNC80, or microinjection of DPDPE into the central amygdala prior to a swim stress blocked the anxiogenic effect produced by the swim stress.
Conclusions
These findings provide direct evidence that activation of DOR in the central amygdala reduces anxiety-like behavior and suggest that DOR in this area are important for regulating anxious states.
Keywords: Delta opioid receptor, Central nucleus of the amygdala, Anxiety, Elevated plus maze, Swim stress, DPDPE, Naltrindole, SNC80
Introduction
The delta opioid receptor (DOR) is one of three G-protein coupled opioid receptors (the others being the mu and kappa opioid receptors) found in the central and peripheral nervous systems. Activation of DOR produces or modifies a variety of effects including antinociception (Chiba et al. 1996; Gallantine and Meert 2005), appetitive behaviors (Israel et al. 2005), depressive-like behaviors (Jutkiewicz 2006), locomotion (Perrine et al. 2006), and reward processes (Devine and Wise 1994). Compounds acting on DOR have also been shown to modulate anxiety in preclinical studies. DOR agonists produce anxiolytic-like effects, whereas DOR antagonists produce anxiogenic-like behaviors when tested in several animal models, including the elevated plus maze, light–dark box, and the defensive burying paradigm (Hirata et al. 2007; Nieto et al. 2005; Perrine et al. 2006; Saitoh et al. 2004). For example, decreases in anxiogenic behavior following systemic administration of the selective DOR agonist SNC80 (Nieto et al. 2005; Perrine et al. 2006; Saitoh et al. 2004) and increases in anxiogenic behavior following administration of the selective DOR antagonist naltrindole (Perrine et al. 2006; Saitoh et al. 2005) have been reported. Despite the documented regulation of anxiety by DOR agents, little is known about the neuroanatomy involved in mediating these effects.
Previous studies have demonstrated that DOR compounds can affect stress-induced behaviors as well. Compound RB101, a selective inhibitor of enkephalin catabolizing enzymes, attenuates conditioned suppression of locomotor activity produced by previous mild foot-shock by a DOR-dependent mechanism (Nieto et al. 2005). Likewise, the selective DOR agonist SNC80 attenuates the conditioned suppression of locomotor activity in the fear stress test after initial conditioning to electric foot-shock (Saitoh et al. 2004). Similar findings are reported for the benzodiazepine anxiolytic compound diazepam when tested in comparable foot-shock conditioning paradigms (Fanselow and Helmstetter 1988; Kameyama and Nagasaka 1982), suggesting that the DOR agonists might produce their effects on conditioned fear stress responses due to their anxiolytic properties. These findings are further evidence supporting a role for the DOR in the regulation of anxious behaviors.
The ability of DOR to modulate anxiety in animal models is well documented; still, the anatomical basis for such modulation has not been established. With advancements in the spatial and temporal resolution of brain imaging techniques in humans, the neuroanatomical pathways involved in anxiety disorders, such as panic disorder, post-traumatic stress disorder, and specific phobias, are more clearly defined. The interconnected brain substrates that have been identified include the amygdala, prefrontal cortex, thalamus, and hippocampus (Garakani et al. 2006). The amygdala in particular plays a prominent role in the development of anxious states and responses (for reviews of this subject, see Bishop 2007; Millan 2003). In addition, evidence supports a direct role for the amygdala in mediating the acquisition, consolidation, expression, and reconsolidation of Pavlovian fear conditioning (Rosen 2004). Within the amygdaloid complex, the central nucleus of the amygdala is implicated in the modulation of stress responses and anxiety-like behaviors (Kang et al. 1999). Further, DOR are present within the central amygdala (Sharif and Hughes 1989; Mansour et al. 1995), and neurons in this area are sensitive to direct application of DOR agonists, as shown by electrophysiological recordings (Chieng et al. 2006; Chieng and Christie 2009). Given that DOR are present in the central nucleus of the amygdala (Sharif and Hughes 1989) and that this brain area is involved in regulating anxiety (Kang et al. 1999), the central nucleus of the amygdala might be a site of action whereby compounds acting on DOR produce their effects on anxiety-like behaviors.
The present study examined the effects of local administration of selective DOR agonists and antagonists into the central nucleus of the amygdala on anxiety-like behaviors using the elevated plus maze, a test of anxiety-like behavior in rodents (Pellow et al. 1985). The selective DOR agonist [D-Pen 2,5]-enkephalin (DPDPE) and the selective DOR antagonist naltrindole were administered directly into the central nucleus of the amygdala. DPDPE binds with high affinity to DOR, with approximately 371- and 1,800-fold selectivity for DOR relative to mu and kappa opioid receptors, respectively (Emmerson et al. 1994; Mosberg et al. 1983). Naltrindole binds with high affinity to the DOR, with approximately 100- and 10,000-fold selectivity for DOR relative to mu and kappa opioid receptors, respectively (Fang et al. 1994; Portoghese et al. 1988a, b; Rogers et al. 1990). The objective of this study was to determine if DOR located in the central amygdala are involved in modulating anxiety-like behaviors.
Materials and methods
Animals
Subjects included experimentally naïve male Sprague Dawley rats (Charles Rivers, Wilmington, MA, USA) weighing 275–300 g and approximately 60 days of age at time of arrival in our facility. All rats were placed in groups of two in plastic bins with bedding and housed in an animal room maintained at an ambient temperature of 22±2°C at approximately 50% relative humidity. Lights were on a 12 h light/12 h dark cycle (lights on at 0700 hours), and all behavioral tests were performed between 12:00 and 3:00 p.m. Food and water were available ad libitum throughout the study. Experiments were approved by the Institutional Animal Care and Use Committee of the Temple University. All procedures were conducted in accordance with the “Guide for Care and Use of Laboratory Animals” published by the National Institutes of Health and the ethical guidelines of the International Association for the Study of Pain.
Drugs
The selective DOR agonists, DPDPE (Mosberg et al. 1983) and SNC80 (Bilsky et al. 1995), were supplied by the National Institute on Drug Abuse. DPDPE was dissolved in 0.9% sterile saline and SNC80 in distilled water with the aid of dilute HCl. Naltrindole, a selective DOR antagonist (Portoghese et al. 1988a, b), was supplied by NIDA and dissolved in distilled water. Diazepam was purchased from Sigma (St. Louis, MO, USA) and dissolved in 50% distilled water, 40% ethanol, and 10% propylene glycol.
Surgery
Rats underwent surgery to implant chronic indwelling cannula bilaterally into the central nucleus of the amygdala. Rats were anesthetized with a mixture of ketamine hydrochloride (100 mg/kg, i.p.) and xylazine (10 mg/kg, i.p.). Sterilized stainless steel C313G cannula guides (22 gauge, Plastic One, Roanoke, VA) were implanted bilaterally, terminating 1.0 mm above the central nucleus of the amygdala, using stereotaxic coordinates of 2.3 mm posterior to bregma, 4.1 mm lateral to the midsagittal suture, and 6.4 mm ventral from skull with the incisor bar set 3 mm below the interaural line based on Paxinos and Watson (2007). Cannulas were secured to the skull with dental acrylic and anchoring screws. To maintain cannula patency, a C313DC cannula dummy (Plastic One, Roanoke, VA, USA) of identical length was inserted into the guide cannula immediately after surgery and reinserted between drug injections. Following surgeries, rats were housed individually. Experimental testing began 1 week post-operatively.
Drugs were administered to unrestrained rats in their home cage using a C313I internal cannula (28 gauge; Plastics One, Roanoke, VA, USA) attached to a 10-µl Hamilton syringe using polythene 30 tubing (Fisher Scientific, Pittsburgh, PA, USA). Bilateral injections were given in a volume of 1.0 µl/side delivered over a 60-s period. Following each microinjection, the internal cannula remained in place for an additional 60 s. At the end of the experiment, cannula placements were verified by injection of 1% bromobenzene blue through the cannula and subsequent histological examination.
Apparatus
Elevated plus maze
Anxiety-like behavior was assessed utilizing the elevated plus maze. The test is based on two opposing behavioral tendencies of rodents, the general propensity to explore unfamiliar environments and the general inclination to avoid open spaces (Rodgers and Dalvi 1997; Rodgers and Johnson 1995). Depending on the level of anxiety when tested, the animal will either explore the open areas more (less anxious) or less (more anxious) (Rodgers and Dalvi 1997; Rodgers and Johnson 1995). The elevated plus maze used in this study (Coubourne Instruments, PA) was made of black Plexiglas shaped into a cross that consists of two closed arms and two open arms, all of equal length (45 cm) and width (10 cm). Each closed arm had 30-cm-high walls and each open arm had 0.5-cm-high ledges. The arms extend from a central square platform (5×5 cm) and the maze is elevated 52 cm above the ground. Room lighting was adjusted such that closed arm light levels were maintained at approximately ~160 lux and open arm light levels were maintained at approximately ~200 lux. Each rat was placed in an open arm facing the center of the maze and allowed to freely explore for a period of 5 min while being video recorded. Following testing, rats were returned to their home cages. The maze was cleaned with 30% alcohol and dried after each subject. Amount of time spent in open arms and number of open arm entries were scored by an investigator. The investigator was not blind to the treatment group; however, scoring was confirmed by a second rater blind to drug treatment in selected cases. In these cases, the mean scores of the two raters were statistically compared (intra-rater reliability≥0.93, analyzed by Cronbach's Alpha comparison using SPSS, Chicago, IL, USA). Each rat was only tested once on the elevated plus maze.
Activity measurements
To measure activity, rats were placed in clean plastic bins without bedding and each bin was placed in a metal frame that contained 16 parallel infrared photobeams and receivers (Digiscan DMicro Monitor, Accusan Instruments, Columbus, OH, USA). Photobeam breaks were recorded for ambulatory activity throughout a 75-min session and stored on a computer linked by an interface (Digiscan DMicro, Accusan Instruments). Rats were tested under standard lighting conditions (~450 lux).
Swim stress
Forced swim has been used to induce stress in rodents (Brown et al. 2001; Chaki et al. 2004) and to enhance anxiety state in the elevated plus maze (Korte and DeBoer 2003). Rats were placed into a cylindrical glass tank (46 cm tall×20 cm in diameter) filled to a depth of approximately 30 cm with 25°C water. Rats could swim or float without their hind limbs or tail touching the bottom. After 2 min, rats were removed from the water, dried by the experimenter, and placed back into their home cages.
Experimental procedures
Experiment 1. DPDPE/Diazepam in the elevated plus maze test
One week after cannula implantation, otherwise naive rats were randomly assigned to experimental groups based on dose of drug. At the start of the testing session, the dummy cannula was removed and an internal cannula was inserted. DPDPE (0.05, 0.5, or 1.5 µg/1 µl) or saline was bilaterally microinjected into the central nucleus of the amygdala of each rat while in its home cage. After 60 s, the internal cannula was removed and the dummy cannula was reinserted. Twenty-five minutes after microinjections, rats were placed on the elevated plus maze for 5 min and behaviors were recorded.
The administration of diazepam in a separate set of naïve rats acted as a behavioral control for the elevated plus maze parameters used in this study. Rats were given an injection of diazepam (3 mg/kg, i.p.) or vehicle. Thirty minutes later, rats were placed on the elevated plus maze for 5 min and behaviors were recorded.
Experiment 2. Locomotor activity
Following a 2- to 3-day treatment-free period, cannulated rats used in experiment 1 were placed into the locomotor chambers and allowed to acclimate for 30 min. DPDPE (0.05, 0.5, or 1.5 µg/1 µl) or saline was bilaterally microinjected into the central nucleus of the amygdala, and ambulatory activity was recorded for 45 min.
Experiment 3. Naltrindole antagonism of DPDPE
Seven days after cannula implantation, individually housed naïve rats were randomly assigned to groups based on dose of drug. Distilled water or naltrindole (0.25, 0.5, or 1.5 µg/1 µl) was microinjected bilaterally into the central nucleus of the amygdala. Ten minutes later, rats were administered either saline or DPDPE (0.5 µg/1 µl) bilaterally into the central nucleus of the amygdala. Twenty-five minutes after injection of DPDPE or saline, rats were placed on the elevated plus maze for 5 min and behaviors were recorded.
Experiment 4. SNC80 and DPDPE/swim stress/plus maze test
Naïve rats were injected with SNC80 (1 or 5 mg/kg sc) or its vehicle control (1 ml/kg sc), or bilaterally microinjected with either DPDPE (1.5 µg/1 µl) or saline into the central nucleus of the amygdala. After 25 min, half of the rats in each group were subjected to swim stress, and those rats not exposed to swimming were gently handled and returned to their home cages. Rats exposed to swimming were placed into cylindrical glass tanks filled with 25°C water for 2 min. Rats were dried immediately after swimming and returned to their home cages. Five minutes after swim stress or control handling, rats were placed on the elevated plus maze for 5 min and anxiety-like behaviors were measured.
Statistical analysis
Plus maze test
Percent of time spent in open arms [(total time in open arms/total time) * 100] and percent of open arm entries [(total open arms entries/total entries) * 100] were calculated for each subject given DPDPE and/or naltrindole or diazepam. Percent of time spent in open arms, percent of open arm entries, and numbers of total arm entries for each treatment group were assessed separately using a one-way analysis of variance (ANOVA) followed by post hoc assessments using Tukey's HSD comparisons or by unpaired two-tailed t test for diazepam vs vehicle. Alpha values of P<0.05 were considered statistically significant.
Locomotor activity
Mean ambulatory counts for each treatment group recorded before and after drug administration were assessed separately using a 4×4 and 4×10 repeated measures ANOVA, respectively, with the between-subjects variable of treatment and the within-subjects variable of time. Post hoc assessments were conducted using Tukey's HSD comparisons. Alpha values of P<0.05 were considered statistically significant.
Results
Injection sites
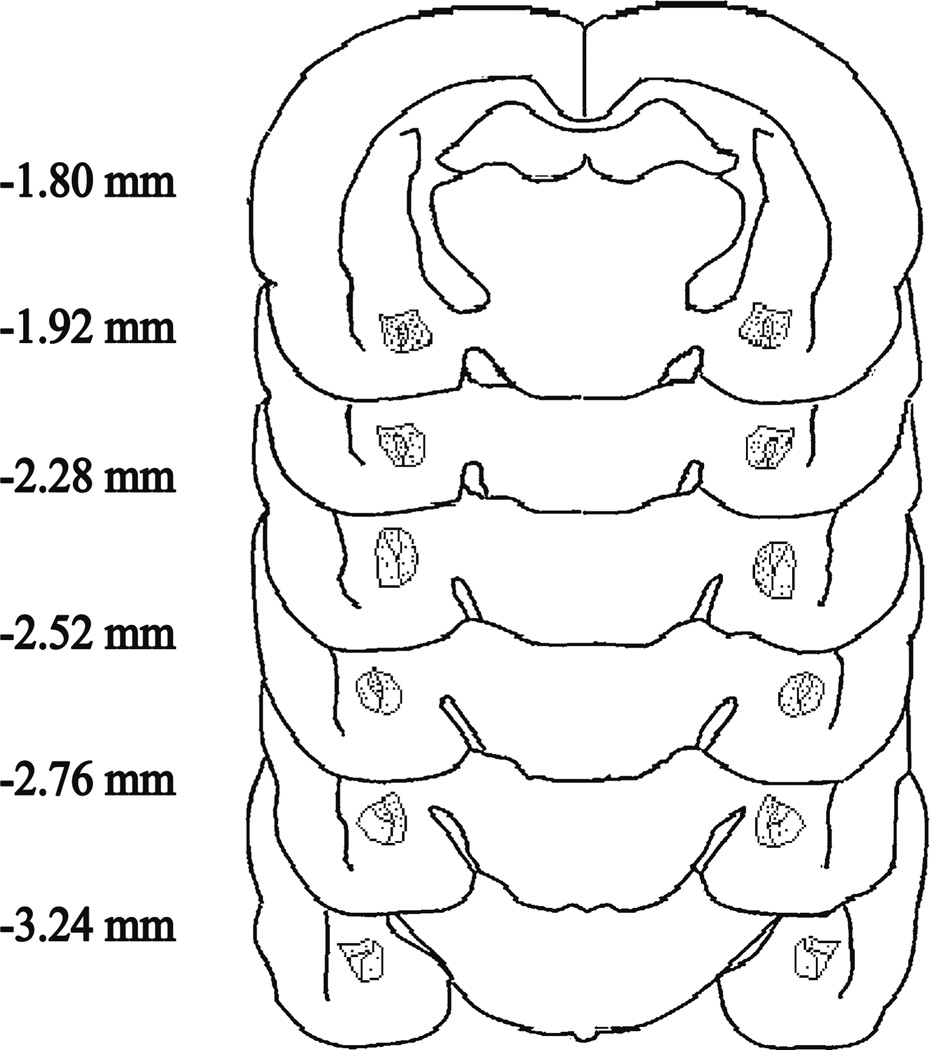
Figure 1 depicts the injection sites plotted on coronal brain sections for rats injected bilaterally with DPDPE (experiments 1, 2, and 4) or naltrindole (experiment 3) or their vehicles. The distance from bregma for each section is indicated in millimeters (Paxinos and Watson 2007) and all individual injection sites are shown. Data from rats with injection sites outside of the central amygdala were removed from the analysis.
Fig. 1.
Injection sites plotted on coronal brain sections for rats injected bilaterally with DPDPE, naltrindole, or vehicle. The distance from bregma for each section is indicated in millimeters (Paxinos and Watson 2007). Each injection site is indicated with a small circle
Experiment 1
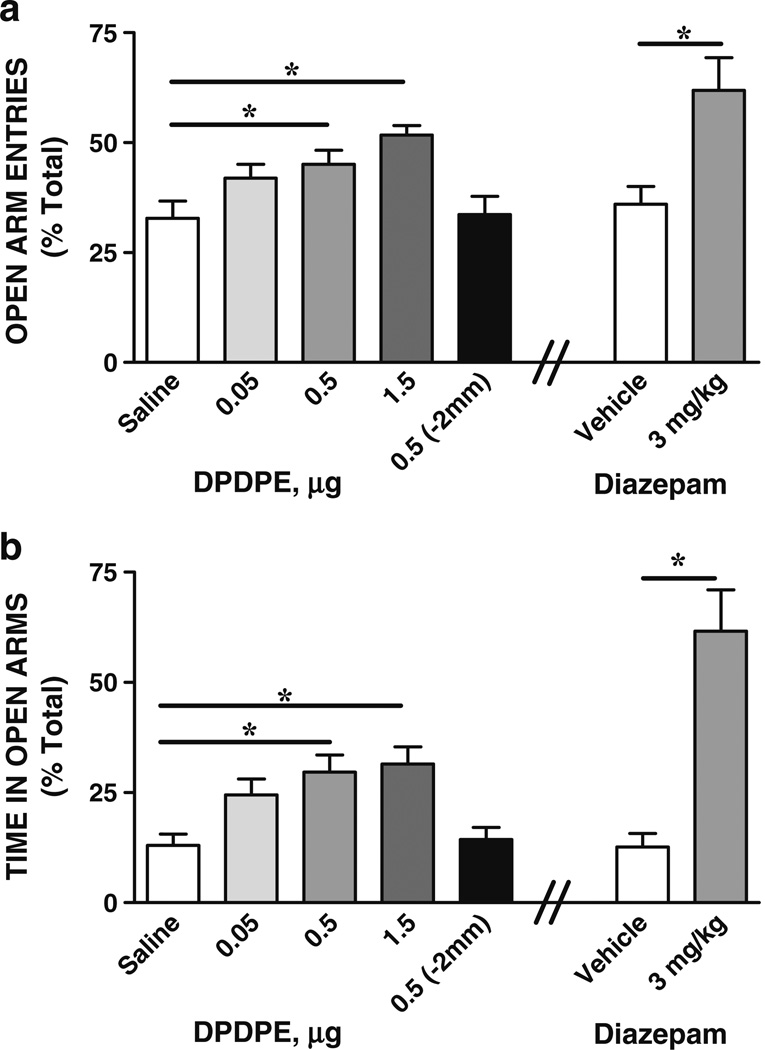
Anxiety-like behavior on the elevated plus maze was measured following microinjection of the DOR agonist DPDPE into the central nucleus of the amygdala or 2 mm dorsal to the central amygdala. Figure 2 illustrates mean (+SEM) percent of open arm entries (Fig. 2a) and time in open arms (Fig. 2b) of rats injected with saline or DPDPE bilaterally into the central nucleus of the amygdala. In a separate set of rats, DPDPE was injected 2 mm dorsal to the central nucleus of the amygdala to assess the brain region specificity of the response, and the behavior of these rats on the plus maze is also shown in Fig. 2a, b. Comparisons of saline and DPDPE treatment groups revealed a significant effect of treatment for time in open arms [F (4, 47)=6.26, P=0.0005] and open arm entries [F (4, 47)=5.31, P=0.001]. Tukey's HSD post-hoc multiple comparisons showed that rats given DPDPE 0.5 µg (N=11) or 1.5 µg (N=9) spent significantly more time in open arms and had significantly more open arm entries than saline controls (N=11). Rats given the lower dose of DPDPE (0.05 µg; N=9) did not differ in amount of open arm entries or time from saline controls or those rats given DPDPE at 0.5 or 1.5 µg. Injection of DPDPE 2 mm dorsal to the central amygdala (N=7) had no significant effect on open arm entries or time in open arms than saline-injected controls (DPDPE/−2 mm vs saline; P>0.05). The effect of the classical anti-anxiety drug diazepam on anxiety-like behaviors was assessed as a positive control for the behavioral procedure. Comparisons of diazepam and its vehicle revealed a significant effect of treatment for open arm entries (unpaired two-tailed t tests, t=−3.1, df=1; P=0.01) and for time in open arms (t=−4.9, df=1; P=0.001) with rats injected with diazepam (3 mg/kg; N=6) spending significantly more time in open arms and having significantly more open arm entries than vehicle controls (N=6) (Fig. 2).
Fig. 2.
Microinjection of DPDPE into the central nucleus of the amygdala reduces anxiety-like behaviors. Percents of open arm entries (a) and time in open arms (b) on the elevated plus maze test following injection of saline or DPDPE bilaterally into the central nucleus of the amygdala are shown. As a behavioral control, the prototypical anti-anxiety drug diazepam (3 mg/kg, ip) and its vehicle were also tested. DPDPE and diazepam increased open arm entries (a) and time in open arms (b), indicating their anxiolytic effects (*P<0.05, 0.5, and 1.5 µg DPDPE vs saline, N=9–11; *P<0.05 diazepam vs vehicle; N=6). To assess site specificity, DPDPE was injected 2 mm dorsal to the central nucleus of the amygdala in a separate control experiment (group DPDPE 0.5 µg/−2 mm). Injection of DPDPE 2 mm dorsal to the central amygdala did not increase open arm entries (a) or time in open arms (b), indicating no anxiolytic effects when DPDPE was injected outside the central amygdala (P>0.05, saline vs DPDPE/2 mm, N=7–9). Data are expressed as means (+SEMs)
Experiment 2
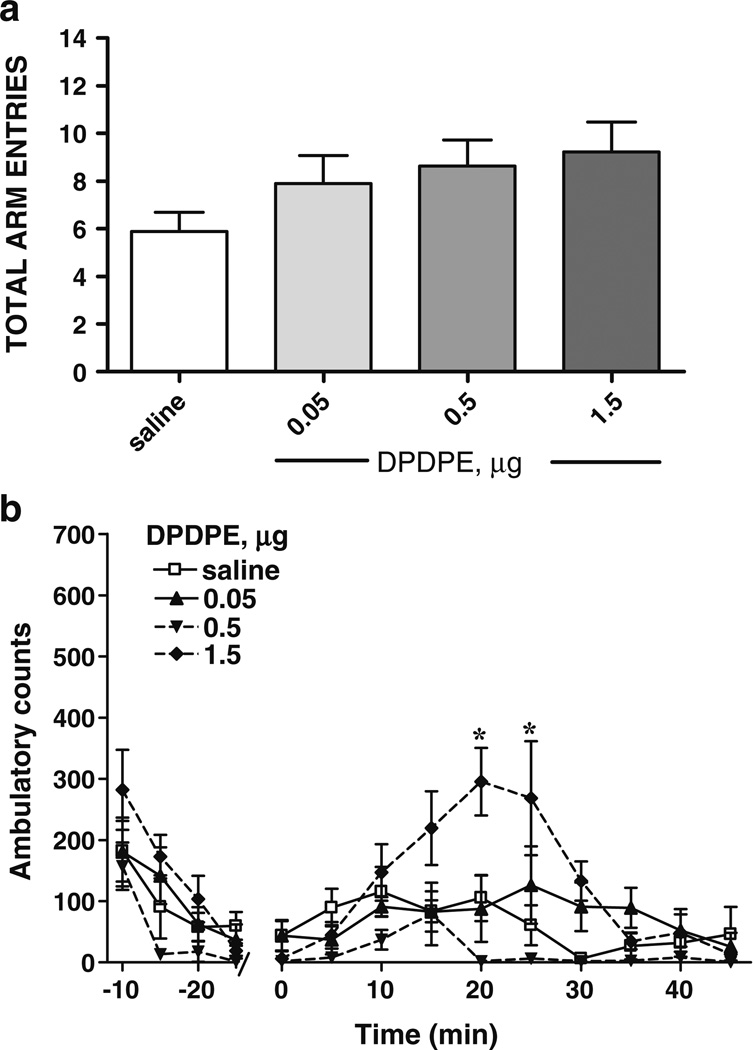
The effect of DPDPE administered into the central nucleus of the amygdala on activity was assessed by two methods, total arm entries on the elevated plus maze and photobeam breaks in an open field. Total arm entries are often used as an indirect measure of ambulatory activity. Figure 3a illustrates mean (+SEM) total arm entries on the plus maze from the behavioral testing recorded in Fig. 2a. Analysis by ANOVA of total arm entries revealed no significant differences between treatment groups [F (3,31)=1.768, P>0.05]. To more directly measure the effects of DPDPE injected into the central amygdala on activity, ambulatory activity was measured in an open field by automated activity monitors. Figure 3b shows means (± SEM) ambulatory counts during the 30 min prior to and the 45 min following bilateral injection of saline or DPDPE into the central nucleus of the amygdala. Over the 30 min prior to injection, comparisons using a repeated measures ANOVA revealed no significant effect of time [F (3, 120)=26.29, P>0.05] or treatment group [F (3, 24)=3.13, P>0.05] for ambulatory counts. Analysis of activity following injection of DPDPE or saline into the central amygdala revealed a significant effect of time [F (17, 408)=26.29, P=0.001] and treatment [F (3, 24)=3.13, P=0.04] for ambulatory activity (Fig. 3b). Tukey's HSD post-hoc multiple comparisons showed that at 20 and 25 min, rats given DPDPE 1.5 µg (N=8) demonstrated more ambulatory activity than those injected with DPDPE 0.05 µg (N=8), DPDPE 0.5 µg (N=4), or saline (N=8). No other comparisons were significant. These results indicate that the lower doses of DPDPE, 0.05 and 0.5 µg, had no effect on activity, whereas the highest dose of DPDPE tested, 1.5 µg, increased ambulatory activity 20–25 min post injection.
Fig. 3.
Activity following intra-central amygdala administration of DPDPE. a The number of total arm entries on the elevated plus maze was not significantly different between rats injected with saline or DPDPE into the central nucleus of the amygdala (P>0.05). b Ambulatory activity as measured by photobeam breaks in an open field is shown for rats injected with saline or DPDPE into the central nucleus of the amygdala. Administration of 1.5 µg DPDPE increased activity, whereas 0.5 and 0.05 µg DPDPE did not (*P<0.05, 1.5 µg DPDPE vs saline controls; N=4–8). Dare are shown as means (±SEM)
Experiment 3
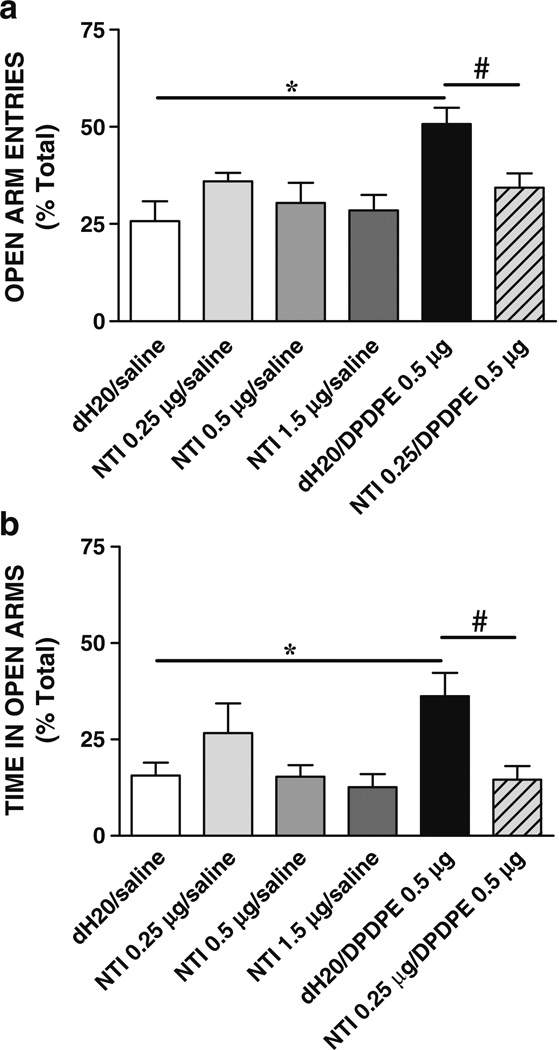
The ability of the selective DOR antagonist, naltrindole, to block the anxiolytic effects of DPDPE was tested in order to ascertain if DPDPE was producing its anxiolytic effects through the DOR. Figure 4 illustrates mean (+ SEM) percent of open arm entries (Fig. 4a) and time in open arms (Fig. 4b) on the plus maze test by rats injected with naltrindole (0.25 µg) or vehicle prior to saline or DPDPE (0.5 µg) bilaterally into the central nucleus of the amygdala. Comparisons using an ANOVA revealed a significant effect of treatment for open arm entries [F (5, 49)=3.77, P=0.02] and for time in open arms [F (5, 49)=7.89, P=0.001]. In contrast, there was no significant effect of treatment on the total number of arm entries [F (5, 49)=1.08, P>0.05] (data not shown). Post-hoc comparisons showed that rats injected with vehicle plus DPDPE (0.5 µg; N=8) spent significantly more time in open arms and had significantly more open arm entries than those rats injected with vehicle plus saline (N=10), replicating the anxiolytic effects of DPDPE shown in the first experiment. Naltrindole (0.25 µg, N=9; 0.5 µg, N=8; 1.5 µg, N=8) injected alone into the central amygdala did not significantly affect time in open arms or open arm entries at the doses tested (vehicle+saline vs naltrindole+saline, P>0.05 all doses). Naltrindole (0.25 µg) administered prior to DPDPE (0.5 µg; N=9) blocked the anxiolytic effects of DPDPE (vehicle/DPDPE vs naltrindole/DPDPE, P<0.05), thus verifying that DPDPE was producing its effects through the DOR.
Fig. 4.
Anxiety-like behaviors following naltrindole and DPDPE administration into the central nucleus of the amygdala. Mean (+SEM) percent of open arm entries (a) and time in open arms b on the elevated plus maze following bilateral administration of vehicle, naltrindole (NTI), DPDPE, and NTI + DPDPE into the central amygdala are shown. The selective DOR antagonist naltrindole significantly blocked the anxiolytic effects of DPDPE, but did not alter anxiety-like behaviors when given alone (*P<0.05, dH2O/DPDPE vs dH2O/saline; #P<0.05 NTI/DPDPE vs dH2O/DPDPE; N=8–10)
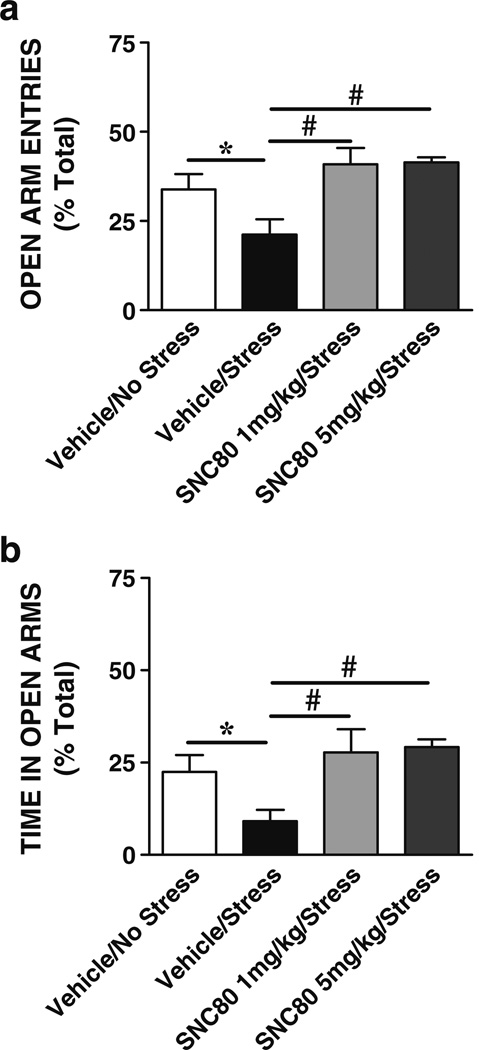
Experiment 4
The effects of DOR agonists on anxiogenic responses produced by a brief swim stressor were tested. Figure 5 illustrates the effects of swim stress with and without SNC80 (1 or 5 mg/kg sc) on the mean (+SEM) percent open arm entries (Fig. 5a) and time in open arms (Fig. 5b) on the plus maze. The ANOVA revealed a significant effect for open arm entries [F (3, 37)=7.56, P=0.0005] and time in open arms [F (3, 37)=6.88, P=0.001], and Tukey's HSD post-hoc multiple comparisons showed that swim stress significantly decreased open arm entries and time in open arms (vehicle+no stress, N=11, vs vehicle+stress, N=11, P<0.05), indicating an increase in anxiety-like behaviors following swim stress. Rats given 1 mg/kg SNC80 (N=8) or 5 mg/kg SNC80 (N=10) prior to stress spent significantly more time in open arms and had significantly more open arm entries than those rats injected with vehicle prior to stress (SNC80+stress vs vehicle+stress, P<0.05). Groups given SNC80 (1 or 5 mg/kg sc) prior to stress did not differ in open arm entries or in time spent in open arms from the control non-stressed group (SNC80+stress vs vehicle+no stress, P>0.05). In a separate comparison, a significant effect was found for total arm entries [F (3, 37)=9.25, P=0.001] with less total entries reported for vehicle+stress than vehicle+no stress and SNC80+stress groups (P<0.05); no other group differences were found (data not shown). These results demonstrate that systemic administration of a DOR agonist can prevent stress-induced anxiogenic behaviors.
Fig. 5.
Effects of stress and SNC80 on anxiety-like behaviors. Mean (+SEM) percent of open arm entries (a) and time in open arms (b) on the elevated plus maze by rats exposed to swim stress after a systemic injection of vehicle or SNC80 (1 mg/kg sc) are shown. Swim stress significantly decreased open arm entries and time in open arms indicating an increase in anxiety-like behaviors (*P<0.05, stress vs no stress controls; N=11). SNC80 significantly reduced the anxiogenic effects produced by swim stress (#P<0.05, SNC80/stress vs vehicle/stress; N=8–10)
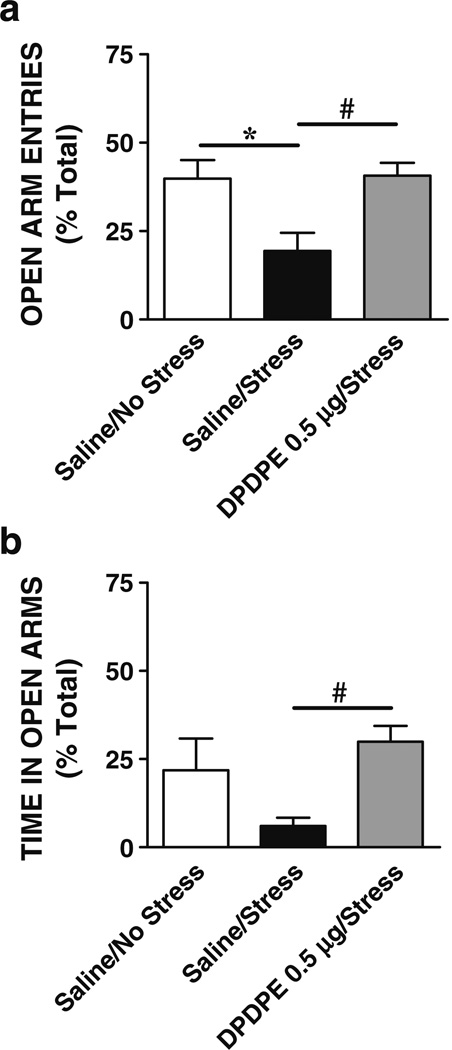
The effects of DPDPE administered directly into the central nucleus of the amygdala on stress-induced anxiety were determined. Figure 6 illustrates the mean (+ SEM) percent of open arm entries (Fig. 6a) and time in open arms (Fig. 6b) on the plus maze by rats bilaterally microinjected into the central nucleus of the amygdala with vehicle or DPDPE prior to swim stress. The ANOVA revealed a significant effect of treatment on open arm entries [F (2, 20)=6.82, P=0.006] or time in open arms [F (2, 20)=5.08, P=0.01]. Tukey's HSD post-hoc multiple comparisons showed that rats injected with DPDPE (0.5 µg; N=8) and exposed to stress spent significantly more time in open arms and had significantly more open arm entries than rats given saline (N=7) prior to stress (DPDPE+stress vs saline+stress, P<0.05), and this group did not differ significantly from rats injected with saline and not stressed (N=7, P>0.05). A separate analysis revealed a significant effect for total arm entries [F (3, 20)=0.16, P=0.003] with less total entries recorded for saline+stress than saline+no stress and DPDPE+stress groups (P<0.01) in agreement with the above data, suggesting that stress reduces general activity on the plus maze.
Fig. 6.
Effects of stress and intra-central amygdala DPDPE on anxiety. Shown are the means (+SEM) percent of open arm entries (a) and time in open arms (b) on the elevated plus maze following injection of vehicle or DPDPE bilaterally into the central nucleus of the amygdala prior to swim stress. DPDPE in the central amygdala blocked the increase in anxiety-like behaviors produced by swim stress (*P<0.05, stress vs no stress controls; #P<0.05, DPDPE/stress vs saline/stress; N=7–8)
Discussion
The aim of this study was to determine the contribution of DOR in the central nucleus of the amygdala in regulating anxiety-like states. In the elevated plus maze test, rats microinjected with the selective DOR agonist DPDPE into the central nucleus of the amygdala spent significantly more time in open arms and had a greater number of open arm entries compared to controls. These results demonstrate that activation of DOR in the central nucleus of the amygdala produces anxiolytic effects. This finding is consistent with previous assessments of systemically administered DOR agonists, which have been shown to reduce anxiety-like behaviors in the elevated plus maze and other tests of anxiety (Hirata et al. 2007; Narita et al. 2006b; Perrine et al. 2006; Saitoh et al. 2004), and extends those findings by identifying an anatomical site of action for this response. The present results also revealed that the DOR agonists SNC80 and DPDPE blocked the anxiogenic effects produced by swim stress. Administration of SNC80 systemically or DPDPE directly into the central nucleus of the amygdala prior to swim stress resulted in significantly more time in open arms and a greater number of open arm entries on the elevated plus maze compared to controls exposed to stress but not given a DOR agonist. Applying a stressor prior to the elevated plus-maze has been used to investigate fear-potentiated behavior that reflects an enhanced state of anxiety vs inherent trait anxiety (Korte and DeBoer 2003). It has been suggested that fear-potentiated behavior in the elevated plus-maze may be useful for investigating the neural mechanisms and potential treatments of anxiety disorders. Taken together, these findings indicate that activation of DOR in the central amygdala can reduce baseline or “trait anxiety” and can also diminish stress-induced or “state anxiety.”
In contrast to the positive modulation of anxiety by DOR agonists in the central amygdala, the present results do not support the central nucleus of the amygdala as the site of the anxiogenic responses produced by DOR antagonists. Administration of naltrindole into the central nucleus of the amygdala did not affect anxiety-like measures, a result contrary to others reported following systemic administration of naltrindole. Previous studies have shown that naltrindole administered systemically at moderate doses (i.e., 3 and 5 mg/kg sc) increases anxiety-like behaviors on the elevated plus maze (Perrine et al. 2006; Saitoh et al. 2004). In addition, mice with a genetic deletion of DOR exhibit heightened anxiety-like behavior when tested in the elevated plus maze and light–dark box (Filliol et al. 2000). Increases in anxiety-like behaviors are also seen in preproenkephalin knockout mice (Konig et al. 1996; Ragnauth et al. 2001), along with increases in freezing in response to fear stimuli (Ragnauth et al. 2001). These prior investigations suggest a tonic influence of endogenous opioid peptides acting on the DOR to dampen baseline anxiety. Investigations assessing the effects of direct administration of naltrindole into specific brain regions on anxious states are limited, yet it has been demonstrated that an injection of naltrindole into the basolateral amygdala produces anxiogenic behavior (Narita et al. 2006a). Thus, it may be that subdivisions of the amygdala contribute differently to the positive and negative regulation of anxiety. Prior research shows that the various nuclei divisions within the amygdala have different roles in encoding stimuli related to emotional valence (Bishop 2007; Millan 2003). The basolateral amygdaloid complex is thought to evaluate and transfer sensory stimuli coding for emotional valence and vigilance to various brain regions and other divisions within the amygdala, whereas the central amygdala is responsible for directing the behavioral, autonomic, and endocrine responses of an anxious state (Bishop 2007; Millan 2003). The results presented herein suggest that, although activation of DOR in the central nucleus of the amygdala can reduce anxiety, blockade of the same receptors does not result in enhanced anxiety-like behaviors. Thus, DOR in different amygdaloid subregions may have different roles in modulating anxiety. Alternatively, it is possible that higher doses of naltrindole might have produced a significant anxiogenic response. Further studies, including full dose–response analyses for naltrindole and other sites of drug injection, are needed to further resolve this issue. In addition, it is possible with the elevated plus maze that a reduction in open arm entries and time is more difficult to detect than an increase in these behaviors. By altering the test conditions to produce lower levels of baseline anxiety (i.e., higher open arm entries and times), anxiogenic effects of naltrindole in the central amygdala might be more apparent. It should be noted, however, that anxiogenic effects of swim stress were detected with this procedure.
Saitoh and colleagues (2005) report that intracerebroventricular (icv) administration of DPDPE does not alter behavior on the elevated plus maze, in contrast to the present findings. Several experimental differences could account for these disparate findings, including the site of injection, dose of DPDPE, time interval between drug administration and anxiety test, and the rat strain used. Saitoh et al. tested anxiety-like behaviors 10 min following icv DPDPE administration, which might not be sufficient time for DPDPE to reach the amygdala in the concentration necessary to be anxiolytic. In the present study, anxiety was measured 25 min after infusion of DPDPE directly into the amydgala and in our preliminary studies, DPDPE did not significantly alter behavior on the plus maze when tested 10 min after infusion (data not included). In addition, Saitoh et al. (2005) used Lewis rats, which, compared to other rat strains, show higher levels of anxiety-related behaviors in the elevated plus-maze, open field, black/white box, and elevated T-maze test of anxiety (Pollier et al. 2000; Ramos et al. 1997, 1998, 2002).
The role of opioid receptors in the central nucleus of the amygdala in the modulation of anxiolytic effects produced by benzodiazepines has been previously investigated. Administration of an HSV-1 vector encoding preproenkephalin into the central nucleus of the amygdala results in local over-expression of enkephalin and potentiates the anxiolytic effects of diazepam (Kang et al. 2000). Systemic administration of naltrindole eliminates the heightened anxiolytic response to low dose diazepam in animals overexpressing enkephalin (Primeaux et al. 2006), suggesting that enkephalin is potentiating the anti-anxiety effects of diazepam through activation of DOR in the central amygdala.
It is thought that stimuli related to states of anxiety induced by stress are encoded differently and have unique brain substrates and underlying neuromechanisms compared to those underlying basal anxiety levels (for a review of this topic, see Bishop 2007; Millan 2003). Given this, the role of DOR in the central nucleus of the amygdala on an induced anxious state was also examined. This assessment examined stress-induced anxiety, which might give insight into the potential use of DOR compounds as therapeutic agents for the treatment of anxiety-related disorders (e.g., anxiety disorder, post-traumatic stress disorder). As described herein, activation of DOR within the central nucleus of the amygdala was found to attenuate stress-induced increases in anxiety. Rats exposed to swim stress and given DPDPE into the central amygdala had more open arm entries and spent more time in open arms compared to controls given saline and exposed to stress prior to plus maze testing. Similar results were found with systemic administration of SNC80. These findings support a role for the DOR in the central nucleus of the amygdala in modulating stress-induced heightened anxiety states. Further studies are needed to fully elucidate the role of DOR in the amygdala on modulating the behavioral responses to stress.
Results presented herein indicate that activation of DOR in the central nucleus of the amygdala can reduce anxiety-like behaviors in the rat. A caveat of the approach used in this study is the potential spread of DPDPE from the site of injection. Because there were no a priori data to indicate that a particular subregion of the central amygdala (i.e., lateral, medial, or capsular division) would be more involved than another, drug was delivered to the entire central amygdala. A control experiment was performed to rule out the possibility that DPDPE diffused up the cannula to act at a site other than the central amygdala to produce its anxiolytic effects. For this control, DPDPE was infused 2 mm dorsal to the central amygdala. Anxiety-like behaviors were not altered when DPDPE was infused dorsal to the central nucleus of the amygdala. Other experiments consisting of administration of very small volumes of DPDPE throughout the entire region would be necessary to define the exact site of action of DPDPE.
Previous studies examining the reliability of the elevated plus maze test have shown that hyper-locomotion can cause an increase in open arm entries, an effect that may contribute to false-positive scoring (for a discussion on this issue, see Wall and Messier 2001; however, also see Perrine et al. 2006). Total arm entries on the plus maze are often used as an indication of changes in activity. Analysis of total arm entries following DPDPE injection into the central nucleus of the amygdala showed that DPDPE did not significantly alter activity on the maze at any of the doses tested, although there was a trend towards a greater number of total arm entries with the highest dose. Open arm entries are reported here as a percent of total arm entries, which controls for general changes in activity levels and reduces the possibility of false-positive responses. Given that systemic administration of DOR agonists can increase motor activity (Perrine et al. 2006), it was of interest to assess more rigorously the effects of DPDPE administered directly into the central nucleus of the amygdala on ambulatory activity. Significant increases in ambulatory behavior measured in the open field were found with the highest dose of DPDPE tested (i.e., 1.5 µg). No differences in activity were found following administration of the low and intermediate doses of DPDPE used in this study. The finding that activation of DOR in the central amygdala can increase locomotor activity has not been previously reported. Taken together, the activity data suggest that the increases in open arm entries and time in open arms following intra-amygdala injection of DPDPE likely reflect a true anxiolytic response. Swim stress, on the other hand, decreased total arm entries, although it selectively reduced open arm entries to a larger extent than closed arm entries. Both SNC80 and DPDPE blocked the reductions in total and open arm entries produced by swim stress.
In conclusion, the findings presented herein indicate that the activation of DOR in the central nucleus of the amygdala reduced both baseline and fear-potentiated anxiety-like behaviors on the elevated plus maze. These findings establish a site of action for the anxiolytic effects of DOR agonists and provide insight into the physiological role of delta opioid system within the central nucleus of the amygdala. Examinations involving other subnuclei of the amygdala are required to fully understand the function of DOR within the amygdala. Nonetheless, the present findings support a positive role for DOR in the central nucleus of the amygdala on dampening levels of baseline and stress-induced anxiety, and suggest that the DOR might be a useful target for the treatment of anxiety-related disorders.
Acknowledgments
The authors would like to thank Mr. Imran Sheikh and Ms. Lauren Harper for their help on the figure preparation and Drs. Khalid Benamar and Jordan Trecki for their technical advice.
Contributor Information
Jovita F. Randall-Thompson, Department of Pharmacology and the Center for Substance Abuse Research, Temple University School of Medicine, 3420 N. Broad Street, Philadelphia, PA 19140, USA
Karen A. Pescatore, Department of Pharmacology and the Center for Substance Abuse Research, Temple University School of Medicine, 3420 N. Broad Street, Philadelphia, PA 19140, USA
Ellen M. Unterwald, Email: Ellen.unterwald@temple.edu, Department of Pharmacology and the Center for Substance Abuse Research, Temple University School of Medicine, 3420 N. Broad Street, Philadelphia, PA 19140, USA; Biology of Addictive Diseases Laboratory, The Rockefeller University, New York, NY 10086, USA.
References
- Bilsky EJ, Calderon SN, Wang T, Bernstein RN, Davis P, Hruby VJ, McNutt RW, Rothman RB, Rice KC, Porreca F. SNC 80, a selective, nonpeptidic and systemically active opioid delta agonist. J Pharmacol Exp Ther. 1995;273:359–366. [PubMed] [Google Scholar]
- Bishop SJ. Neurocognitive mechanisms of anxiety: an integrative account. Trends Cogn Sci. 2007;11:307–316. doi: 10.1016/j.tics.2007.05.008. [DOI] [PubMed] [Google Scholar]
- Brown PL, Hurley C, Repucci N, Drugan RC. Behavioral analysis of stress controllability effects in a new swim stress paradigm. Pharmacol Biochem Behav. 2001;68:263–272. doi: 10.1016/s0091-3057(00)00460-3. [DOI] [PubMed] [Google Scholar]
- Chaki S, Nakazato A, Kennis L, Nakamura M, Mackie C, Sugiura M, Vinken P, Ashton D, Langlois X, Steckler T. Anxiolytic-and antidepressant-like profile of a new CRF1 receptor antagonist, R278995/CRA0450. Eur J Pharmacol. 2004;485:145–158. doi: 10.1016/j.ejphar.2003.11.032. [DOI] [PubMed] [Google Scholar]
- Chiba T, Murase H, et al. Antinociceptive activity of deltorphin analogs in the formalin test. Life Sci. 1996;59(20):1717–1722. doi: 10.1016/s0024-3205(96)00508-5. [DOI] [PubMed] [Google Scholar]
- Chieng B, Christie MJ. Chronic morphine treatment induces functional delta-opioid receptors in amygdala neurons that project to periaqueductal grey. Neuropharmacology. 2009;57:430–437. doi: 10.1016/j.neuropharm.2009.06.034. [DOI] [PubMed] [Google Scholar]
- Chieng B, Christie MJ, Osborne PB. Characterization of neurons in the rat central nucleus of the amygdale: cellular physiology, morphology, and opioid sensitivity. J Comp Neurol. 2006;497:910–927. doi: 10.1002/cne.21025. [DOI] [PubMed] [Google Scholar]
- Devine DP, Wise RA. Self-administration of morphine, DAMGO, and DPDPE into the ventral tegmental area of rats. J Neurosci. 1994;14:1978–1984. doi: 10.1523/JNEUROSCI.14-04-01978.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmerson PJ, Liu MR, Woods JH, Medzihradsky F. Binding affinity and selectivity of opioids at mu, delta and kappa receptors in monkey brain membranes. J Pharmacol Exp Ther. 1994;271:1630–1637. [PubMed] [Google Scholar]
- Fang L, Knapp RJ, Horvath R, Matsunaga TO, Haaseth RC, Hruby VJ, Porreca F, Yamamura HI. Characterization of [3H] naltrindole binding to delta opioid receptors in mouse brain and mouse vas deferens: evidence for delta opioid receptor heterogeneity. J Pharmacol Exp Ther. 1994;268:836–846. [PubMed] [Google Scholar]
- Fanselow MS, Helmstetter FJ. Conditional analgesia, defensive freezing, and benzodiazepines. Behav Neurosci. 1988;102:233–243. doi: 10.1037//0735-7044.102.2.233. [DOI] [PubMed] [Google Scholar]
- Filliol D, Ghozland S, Chluba J, Martin M, Matthes HW, Simonin F, Befort K, Gaveriaux-Ruff C, Dierich A, LeMeur M, Valverde O, Maldonado R, Kieffer BL. Mice deficient for delta- and mu-opioid receptors exhibit opposing alterations of emotional responses. Nat Genet. 2000;25:195–200. doi: 10.1038/76061. [DOI] [PubMed] [Google Scholar]
- Gallantine EL, Meert TF. A comparison of the antinociceptive and adverse effects of the mu-opioid agonist morphine and the delta-opioid agonist SNC80. Basic Clin Pharmacol Toxicol. 2005;97(1):39–51. doi: 10.1111/j.1742-7843.2005.pto_97107.x. [DOI] [PubMed] [Google Scholar]
- Garakani A, Mathew SJ, Charney DS. Neurobiology of anxiety disorders and implications for treatment. Mt Sinai J Med. 2006;73:941–949. [PubMed] [Google Scholar]
- Hirata H, Sonoda S, Agui S, Yoshida M, Ohinata K, Yoshikawa M. Rubiscolin-6, a delta opioid peptide derived from spinach Rubisco, has anxiolytic effect via activating sigma1 and dopamine D1 receptors. Peptides. 2007;28:1998–2003. doi: 10.1016/j.peptides.2007.07.024. [DOI] [PubMed] [Google Scholar]
- Israel Y, Kandov Y, et al. NPY-induced feeding: pharmacological characterization using selective opioid antagonists and antisense probes in rats. Peptides. 2005;26(7):1167–1175. doi: 10.1016/j.peptides.2005.01.017. [DOI] [PubMed] [Google Scholar]
- Jutkiewicz EM. The antidepressant -like effects of delta-opioid receptor agonists. Mol Interv. 2006;6:162–169. doi: 10.1124/mi.6.3.7. [DOI] [PubMed] [Google Scholar]
- Kameyama T, Nagasaka M. The effects of analgesics on quickly-learned conditioned suppression in mice. Neuropharmacology. 1982;21:1283–1289. doi: 10.1016/0028-3908(82)90134-4. [DOI] [PubMed] [Google Scholar]
- Kang W, Wilson SP, Wilson MA. Changes in nociceptive and anxiolytic responses following herpes virus-mediated preproenkephalin overexpression in rat amygdala are naloxone-reversible and transient. Ann N Y Acad Sci. 1999;877:751–755. doi: 10.1111/j.1749-6632.1999.tb09316.x. [DOI] [PubMed] [Google Scholar]
- Kang W, Wilson SP, et al. Overexpression of proenkephalin in the amygdala potentiates the anxiolytic effects of benzodiazepines. Neuropsychopharmacology. 2000;22(1):77–88. doi: 10.1016/S0893-133X(99)00090-1. [DOI] [PubMed] [Google Scholar]
- Konig M, Zimmer AM, Steiner H, Holmes PV, Crawley JN, Brownstein MJ, Zimmer A. Pain responses, anxiety and aggression in mice deficient in pre-proenkephalin. Nature. 1996;383:535–538. doi: 10.1038/383535a0. [DOI] [PubMed] [Google Scholar]
- Korte SM, DeBoer SF. A robust animal model of state anxiety: fear-potentiated behaviour in the elevated plus-maze. Eur J Pharmacol. 2003;463:163–175. doi: 10.1016/s0014-2999(03)01279-2. [DOI] [PubMed] [Google Scholar]
- Mansour A, Fox CA, Akil H, Watson SJ. Opioid-receptor mRNA expression in the rat CNS: anatomical and functional implications. Trends Neurosci. 1995;18:22–29. doi: 10.1016/0166-2236(95)93946-u. [DOI] [PubMed] [Google Scholar]
- Millan MJ. The neurobiology and control of anxious states. Prog Neurobiol. 2003;70:83–244. doi: 10.1016/s0301-0082(03)00087-x. [DOI] [PubMed] [Google Scholar]
- Mosberg HI, Hurst R, Hruby VJ, Gee K, Yamamura HI, Galligan JJ, Burks TF. Bis-penicillamine enkephalins possess highly improved specificity toward delta opioid receptors. Proc Natl Acad Sci U S A. 1983;80:5871–5874. doi: 10.1073/pnas.80.19.5871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narita M, Kaneko C, Miyoshi K, Nagumo Y, Kuzumaki N, Nakajima M, Nanjo K, Matsuzawa K, Yamazaki M, Suzuki T. Chronic pain induces anxiety with concomitant changes in opioidergic function in the amygdala. Neuropsychopharmacology. 2006a;31:739–750. doi: 10.1038/sj.npp.1300858. [DOI] [PubMed] [Google Scholar]
- Narita M, Kuzumaki N, Kaneko C, Tamai E, Khotib J, Miyatake M, Shindo K, Nagumo Y, Tanaka S, Suzuki T. Age-related emotionality is associated with cortical delta-opioid receptor dysfunction-dependent astrogliosis. Neuroscience. 2006b;137:1359–1367. doi: 10.1016/j.neuroscience.2005.10.067. [DOI] [PubMed] [Google Scholar]
- Nieto MM, Guen SL, Kieffer BL, Roques BP, Noble F. Physiological control of emotion-related behaviors by endogenous enkephalins involves essentially the delta opioid receptors. Neuroscience. 2005;135:305–313. doi: 10.1016/j.neuroscience.2005.06.025. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 6th edn. Elsevier, Elsevier; 2007. [Google Scholar]
- Pellow S, Chopin P, File SE, Briley M. Validation of open: closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods. 1985;14:149–167. doi: 10.1016/0165-0270(85)90031-7. [DOI] [PubMed] [Google Scholar]
- Perrine SA, Hoshaw BA, Unterwald EM. Delta opioid receptor ligands modulate anxiety-like behaviors in the rat. Br J Pharmacol. 2006;147:864–872. doi: 10.1038/sj.bjp.0706686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollier F, Sarre S, Aguerre S, Ebinger G, Mormede P, Michotte Y, Chaouloff F. Serotonin reuptake inhibition by citalopram in rat strains differing for their emotionality. Neuropsychopharmacology. 2000;22:64–76. doi: 10.1016/S0893-133X(99)00092-5. [DOI] [PubMed] [Google Scholar]
- Portoghese PS, Sultana M, Nagase H, Takemori AE. Application of the message-address concept in the design of highly potent and selective non-peptide delta opioid receptor antagonists. J Med Chem. 1988a;31:281–282. doi: 10.1021/jm00397a001. [DOI] [PubMed] [Google Scholar]
- Portoghese PS, Sultana M, Takemori AE. Naltrindole, a highly selective and potent non-peptide delta opioid receptor antagonist. Eur J Pharmacol. 1988b;146:185–186. doi: 10.1016/0014-2999(88)90502-x. [DOI] [PubMed] [Google Scholar]
- Primeaux SD, Wilson SP, McDonald AJ, Mascagni F, Wilson MA. The role of delta opioid receptors in the anxiolytic actions of benzodiazepines. Pharmacol Biochem Behav. 2006;85:545–554. doi: 10.1016/j.pbb.2006.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragnauth A, Schuller A, Morgan M, Chan J, Ogawa S, Pintar J, Bodnar RJ, Pfaff DW. Female preproenkephalin-knockout mice display altered emotional responses. Proc Natl Acad Sci U S A. 2001;98:1958–1963. doi: 10.1073/pnas.041598498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos A, Berton O, Mormede P, Chaouloff F. A multiple-test study of anxiety-related behaviours in six inbred rat strains. Behav Brain Res. 1997;85:57–69. doi: 10.1016/s0166-4328(96)00164-7. [DOI] [PubMed] [Google Scholar]
- Ramos A, Mellerin Y, Mormede P, Chaouloff F. A genetic and multifactorial analysis of anxiety-related behaviours in Lewis and SHR intercrosses. Behav Brain Res. 1998;96:195–205. doi: 10.1016/s0166-4328(98)00023-0. [DOI] [PubMed] [Google Scholar]
- Ramos A, Kangerski AL, Basso PF, Da Silva Santos JE, Assreuy J, Vendruscolo LF, Takahashi RN. Evaluation of Lewis and SHR rat strains as a genetic model for the study of anxiety and pain. Behav Brain Res. 2002;129:113–123. doi: 10.1016/s0166-4328(01)00337-0. [DOI] [PubMed] [Google Scholar]
- Rodgers RJ, Dalvi A. Anxiety, defense and the elevated plus-maze. Neurosci Biobehav Rev. 1997;21:801–810. doi: 10.1016/s0149-7634(96)00058-9. [DOI] [PubMed] [Google Scholar]
- Rodgers RJ, Johnson NJ. Factor analysis of spatiotemporal and ethological measures in the murine elevated plus-maze test of anxiety. Pharmacol Biochem Behav. 1995;52:297–303. doi: 10.1016/0091-3057(95)00138-m. [DOI] [PubMed] [Google Scholar]
- Rogers H, Hayes AG, Birch PJ, Traynor JR, Lawrence AJ. The selectivity of the opioid antagonist, naltrindole, for delta-opioid receptors. J Pharm Pharmacol. 1990;42:358–359. doi: 10.1111/j.2042-7158.1990.tb05428.x. [DOI] [PubMed] [Google Scholar]
- Rosen JB. The neurobiology of conditioned and unconditioned fear: a neurobehavioral system analysis of the amygdala. Behav Cogn Neurosci Rev. 2004;3:23–41. doi: 10.1177/1534582304265945. [DOI] [PubMed] [Google Scholar]
- Saitoh A, Kimura Y, Suzuki T, Kawai K, Nagase H, Kamei J. Potential anxiolytic and antidepressant-like activities of SNC80, a selective delta-opioid agonist, in behavioral models in rodents. J Pharmacol Sci. 2004;95:374–380. doi: 10.1254/jphs.fpj04014x. [DOI] [PubMed] [Google Scholar]
- Saitoh A, Yoshikawa Y, Onodera K, Kamei J. Role of delta-opioid receptor subtypes in anxiety-related behaviors in the elevated plus-maze in rats. Psychopharmacology (Berl) 2005;182:327–334. doi: 10.1007/s00213-005-0112-6. [DOI] [PubMed] [Google Scholar]
- Sharif NA, Hughes J. Discrete mapping of brain Mu and delta opioid receptors using selective peptides: quantitative autoradiography, species differences and comparison with kappa receptors. Peptides. 1989;10:499–522. doi: 10.1016/0196-9781(89)90135-6. [DOI] [PubMed] [Google Scholar]
- Wall PM, Messier C. Methodological and conceptual issues in the use of the elevated plus-maze as a psychological measurement instrument of animal anxiety-like behavior. Neurosci Biobehav Rev. 2001;25:275–286. doi: 10.1016/s0149-7634(01)00013-6. [DOI] [PubMed] [Google Scholar]