Abstract
Seventy-five percent of spiraling healthcare costs can be attributed to chronic diseases, making prevention and management of chronic conditions one of our highest healthcare priorities, especially as we organize for patient-centered medical homes. Collaborative patient self-management in primary care has been repeatedly demonstrated to be efficacious in reducing both symptoms and increasing quality of life, yet there is no consensus on what, how, when, and by whom self-management programs are best implemented. In this article, we argue that self-management interventions effectively span the continuum of prevention and disease management. Self-management interventions rest on a foundation of five core actions: 1) activate motivation to change; 2) apply domain-specific information from education and self-monitoring; 3) develop skills; 4) acquire environmental resources; and 5) build social support. A range of delivery vehicles, including group interventions, primary care providers, and advanced wireless technology, are described and evaluated in terms of diffusion and cost-containment goals.
Keywords: self-management, self-regulation, chronic illness, chronic disease, interventions, prevention
Healthcare costs in the United States are reaching unprecedented heights. In 2009, healthcare spending was $2.5 trillion, $8,086 per person, or 17.6% of the GDP (California Health Care Foundation, 2011), making it the second highest in the world. Despite this huge financial investment, the life expectancy of people in the United States ranks last on a list of 11 wealthy nations and ranks fiftieth in the world. The average life expectancy for American men is 76.05 years compared to 78.89 years for Canadian men (CIA World Factbook, 2012 estimates). In 2005, 43.8% of the U.S. civilian, non-institutionalized population had one or more chronic conditions (Paez et al., 2009), the economic burden of chronic illness is currently 78% of total health care spending (Bodenheimer et al., 2009), and 96% of Medicare annual spending is for chronic conditions (Foote, 2009). It is estimated that by 2023, there will be a 42% increase in cases of 7 major chronic diseases, costing $4.2 trillion in treatment costs and lost economic output (DeVol et al., 2007).
Lifestyle change is the key to reducing the human and financial burden of chronic disease by preventing disease and delaying advancement of disease. Four risk factors directly contribute to the prevalence and severity of chronic illnesses, as well as their prevention: unhealthy diet, physical inactivity, tobacco use, and alcohol abuse (World Health Organization, 2009). These lifestyle factors—primarily determined by the individual and often not addressed by medical providers—were the leading cause of death in 2000, with tobacco causing 18.1% of deaths, poor diet and physical inactivity causing 16.1%, and alcohol consumption causing 3.5% of all deaths. The significance of patients’ behavioral choices places patient self-management at the center of chronic care and prevention models (Bodenheimer et al., 2002a; Bodenheimer et al., 2002b).
Self-management as the Gold Standard of Chronic Care
The World Health Organization (WHO)’s “best practice” strategy for chronic conditions is to “educate and support patients to manage their own conditions as much as possible” (Epping-Jordan et al., 2001, p. 947). The outcome of a self-management approach is a change in the physician-patient relationship: the patient becomes an active, informed, collaborative participant in healthcare decision-making; assumes responsibility for engaging in health-promoting behaviors and relationships; and develops competence for problem-solving and proactively addressing predictable challenges of the disease (Bodenheimer et al., 2002a; Creer et al., 2004). Chronic illness self-management and healthy lifestyle promotion for the prevention of disease require similar cognitive processes to transform intentions into behavior: capacity for self-evaluation; self-managed action with task- and time-specific, outcome-focused goal setting (Purdie & McCrindle, 2002); active patient involvement; and ongoing planning (Glasgow et al., 2004).
The outcome of self-management interventions (SMI) is that patients have the skills to monitor markers of health and disease, make decisions to modify their own behavior, and develop individualized goal-setting and action plans. Chodosh and colleagues (2005) defined self-management as having a minimum of two components—self-monitoring and decision-making. Creer and colleagues (2004) described it as using “the capacity for self-evaluation and self-managed action” (p. 723). Furthermore, because chronic diseases often limit patients’ aspirations (e.g., diabetes may stop a rock climber from achieving his goals), patients must accept their losses, find new goals to engage in (Rasmussen, Wrosch, Scheier, & Carver, 2006), and develop competence in coping with failure, known as relapse-management (Schwarzer, 2001).
Two comprehensive meta-analyses of SMI studies using randomized trials (Chodosh et al., 2005; Newman et al., 2004) found positive outcomes for patients with diabetes, hypertension, arthritis, and asthma. Three reviews that included studies with nonrandomized methodologies (Barlow et al., 2002; Nolte et al., 2007; Warsi et al., 2004) reported benefits for patients with asthma, arthritis, diabetes, and hypertension. Multiple literature reviews, as well as primary source data, show consensus about a set of common factors for effective self-management (Creer et al., 2004; Fisher et al., 2005; Jerant et al., 2005; Lorig & Holman, 2003; Swendeman et al., 2009).
Necessary Elements for Effective Self-Management
The essential elements for successful self-management are organized within five categories: 1) activate motivation for change, 2) apply information from education and self-monitoring, 3) develop skills, 4) acquire environmental resources, and 5) build social support. These same elements can contribute to successful health maintenance of people who are currently healthy, and thus should be viewed as essential elements of prevention as well as chronic disease management.
Element 1: Activate Motivation for Change
Every SMI program includes educational components or intervention techniques (e.g., Motivational Interviewing) to create motivation to engage in health-promoting behaviors, usually by enhancing individuals’ beliefs in their ability to achieve desired change (self-efficacy). To be activated to change, the individual needs to accept responsibility for lifestyle change and experience a sense of empowerment (Anderson, 1995; Aujoulat et al., 2007), counteracting feelings of being a victim of the illness by feeling in control of their disease. The patient needs to resolve inter-goal conflict (Karoly et al., 2005) and value health and longevity more than the gratifications of the current lifestyle, which may include the pleasures of enjoying rich food, alcohol, cigarettes, and sedentary forms of recreation. Cultural roles and beliefs must be recognized to motivate change: for example, women in many cultures are socialized to value the needs of family members above their own, and will not serve healthier meals (e.g., reduced fat, smaller portions) to the whole family, unless they frame that choice as “protection” rather than “deprivation”. Motivation to change is likely to be stronger for disease management than for prevention (Glasgow et al., 2004).
The Stages of Change model (Prochaska & DiClemente, 1983) applies to self-management commitment (Cassidy, 1999) and describes how an individual is more likely to change behavior when an intervention matches their stage of change. Participants in a self-management treatment for chronic pain were less likely to complete the program if they were judged to be in the pre-contemplation or contemplation stage, rather than the preparation or action stage (Kerns & Rosenberg, 2000). Motivational Interviewing (Miller & Rollnick, 1991), a technique based on the Stages of Change model, has been used in health settings to move patients to a commitment to change (Britt et al., 2004; Knight et al., 2006).
Maintenance of change over time needs to receive as much focus as initial behavior change (Bellg, 2003). Setbacks arise from unexpected declines in health despite adherence to health-promoting plans and need to be framed as challenges to overcome, rather than reasons to relapse to unhealthy behaviors. Cognitive interventions are helpful at this stage, as people are best prepared for setbacks when they have internalized thought patterns that protect against disengagement (Schwarzer, 2001).
Element 2: Apply Information
Education is an essential component of all SMI: patients need information about the disease as well as the general chronic care model. Another source of information is self-observation and self-recording on a regular, established schedule, enabling the patient to modify medication and behaviors without direct supervision of a healthcare professional (Creer et al., 2004). Self-monitoring is comprised of two processes: 1) awareness of bodily symptoms, sensations, daily activities, and cognitive processes and 2) measurements, recordings, and observations that provide information for independent action or consultation with care providers (Wilde & Garvin, 2007). Self-management is enhanced when patients can gather information about the status of their physical health by monitoring specific markers of biological functioning on a daily basis. For example, glycemic level monitored through blood testing is the marker for diabetes; blood pressure and heart rate are markers for cardiovascular conditions; and airflow is the marker for asthma and chronic obstructive pulmonary disease. Self-monitoring of psychological states such as depressed mood, anxiety, and fatigue is another essential component of self-regulation (Maes & Gebhardt, 2000), as psychological states can often impact physical health and adherence to medical regimens. The creation of methods for patients to monitor biomarkers of stress is an important future goal, especially for people living with HIV (Uchino et al., 1996).
Element 3: Skill Development
Patients need skills to manage their specific disease, maintain health-promoting behaviors, and use behavior change technology for problem-solving and creation of action plans. Effective communication and collaboration with health care providers is a common outcome goal in health interventions and a necessary skill for self-management (Barlow et al., 2002; Gifford & Sengupta, 1999; Lorig & Holman, 2003; Skinner et al., 2006). Ideally, patients are empowered to manage their health and their disease through a “partnership” (Bodenheimer, Lorig, Holman, & Grumbach, 2002) in which patients and practitioners enter into collaborative problem-solving and agree upon mutually negotiated goals (Clark et al., 2004). Disease-specific skills include appropriate use of devices (e.g., an inhaler for asthma; a blood pressure monitor for cardiovascular disease), adherence to complicated medication programs, and the ability to interpret new symptoms (Gifford & Sengupta, 1999). Generic health-related skills include those needed for initiating and maintaining lifestyle changes and dealing with psychological distress (Wright et al., 2003). Specific skills for overcoming obstacles and challenges will vary depending on the individual person and their health status. Five of the most important skills are:
Problem-solving. Frequently cited as the most important skill, problem-solving is a prerequisite for successful self-management, and can be viewed as a mediator of improved health outcomes (Glasgow et al., 2004). Problem-solving is typically described as a series of steps: identifying the problem, setting a goal, generating possible solutions, selecting and implementing an action plan, and readjusting plans until goals are reached. Information-seeking skills are part of every phase in problem-solving.
Self-monitoring. Self-monitoring motivates and maintains behavior change by promoting self-efficacy, increasing awareness, and monitoring progress. Self-monitoring has been used effectively to change dietary behaviors through use of food-tracking instruments (Mossavar-Rahmani, 2004).
Stress management and emotional regulation. Coping with negative emotions is a major task for people living with chronic illness (Charmaz, 1991). Because depression is a condition that impedes successful self-management (Bayliss et al., 2007; Hibbard et al., 2007), skills for preventing and managing dysphoric moods are extremely beneficial.
Coping with lapses and setbacks. To cope effectively with lapses, and prevent them from becoming excuses to revert to unhealthy behavior patterns, individuals must have cognitive strategies for framing their behavior as a predictable part of the learning curve, rather than failure (Witkiewitz & Marlatt, 2004). For long-term lifestyle change, individuals need to learn methods to enhance self-control by focusing attention on their goals (Metcalfe & Mischel, 1999), activate problem-solving processes to deal with competing goals (Leventhal & Mora, 2005), and develop appropriate self-talk for stages before, during, and after the challenging situation (“stress inoculation”) (Karoly et al., 2005).
Communicating assertively. Communication skills are needed in a variety of situations to maximize support, confront obstacles, and minimize negative social influences. Situations requiring assertiveness skills include examples such as saying “no” to unhealthy social behaviors, making specific dietary requests at a restaurant, and asking for help from family members.
Element 4: Environmental Resources
“Resource utilization” includes securing medication, supplies, and educational resources (Nagelkerk et al., 2006). The best efforts of self-management will be defeated if there is no access to the necessary supplies from the environment, or if the environment contains pernicious elements. For example, if people living with HIV lack access to antiretroviral drugs, their illness becomes acute and terminal rather than a chronic disease that can be effectively self-managed. Environmental barriers to obtaining resources (e.g., transportation issues) and maintaining healthy behaviors (e.g., safe settings for exercise) require action plans to overcome them. Part of self-management is to be aware of, and take concerted action to avoid, environmental settings that derail goal attainment, and to use problem-solving skills to develop and achieve action plans.
Recommendations for environmental structuring (Kitsantas, 2000) and principles of environmental control (Bodenheimer et al., 2002a) are included in many SMI. In behavior modification, environment is relevant as a source of situational cues and a potent reinforcer for both positive and negative behaviors. The self-managing patient is advised to create a home environment with stimuli for healthy behaviors (e.g., fruits and vegetables in the refrigerator; treadmill in front of the television), take steps to remove stimuli for problematic behavior (e.g., junk food, alcohol), and seek healthy environments while avoiding unhealthy environments (e.g., gyms vs. fast food restaurants).
Element 5: Social Support
The current approach to SMI may over-emphasize individual variables such as self-efficacy and neglect the importance of social factors (Taylor & Bury, 2007). Supportive relationships have been identified as an important component in interventions to promote healthy behaviors (Bull et al., 2006), and good self-managers have extensive support networks (MacDonald et al., 2008). Health-promoting decisions can be both supported or impeded by family and friends. Negative influences can come from well-intentioned people, as when they reinforce a “sick role” instead of supporting the autonomy and empowerment of the patient. Cultural norms and values need to be addressed to ensure optimum social support (Karoly et al., 2005; Steed et al., 2005). Health providers are an essential source of encouragement, positive reinforcement, and emotional support.
Development of Self-Management Competence
Competence in self-management occurs over time, in phases. Interventions should be targeted to the appropriate stage (Hibbard et al., 2007) and meet patients at their current level of information and skills (Skinner et al., 2006). Figure 1 presents a three-phase model that shows the patient’s journey to a healthy lifestyle. This figure simplifies the process of developing competence, which is best viewed as iterative and cyclical, as the patient makes progress on sub-goals at different rates, and will inevitably experience new challenges over time, requiring a return to the education phase. However, this model illustrates the different outcomes—knowledge, attitudes, and skills—at different stages in the trajectory of choosing healthy behaviors, and may help determine the best delivery vehicle for self-management interventions.
Figure 1.
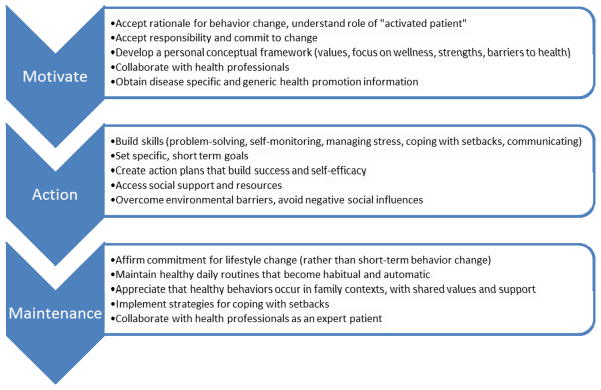
Patient process of developing self-management.
Delivery Vehicles for Self-Management Interventions
The diffusion of interventions that promote successful self-management is essential for improving health-related quality of life, reducing health care costs over time, and preventing the progression of chronic conditions. In this section, we review delivery vehicles with varying levels of expense and professional involvement for promoting self-management. As new delivery vehicles are developed, interventions can be tailored to the cultural and social needs of individuals, wider dissemination will be possible, and gaps in one form of delivery will be filled by the services of another.
Primary Care Settings
The Chronic Care Model (CCM) requires a “major rethinking” of primary care practice, with non-physician personnel trained to support patient self-management, and new, collaborative roles for health care professionals (Bodenheimer et al. 2002b, p. 1776). While behavioral interventions in the medical office are effective and interventions introduced by a physician garner greater participation (Glasgow et al., 1997), patients participate in clinical decisions only 9% of the time, pointing to the need for physicians to learn skills for group facilitation, problem-solving, goal-setting, and cognitive-behavioral techniques (Newman et al., 2004).
Group Interventions
There are many successful evidence-based group interventions for chronic illness that promote behavioral self-management, yet these interventions are not broadly diffused in the United States, are typically delivered only for a few diseases (e.g., diabetes), and are usually implemented only when diseases become chronic and debilitating rather than as strategies of primary and secondary prevention. Group interventions are often led by peers (expert patients who have received special training) or health professionals, such as nurses and dieticians (Steed et al., 2005).
There are drawbacks to relying on standardized self-management group interventions as the sole means of developing competent patients. Critics of the U.K. Expert Patients Programme complain that it lacks flexibility and individualization because of requirements to adhere to rigid protocols (MacDonald et al., 2008), and should be better integrated with primary care and attend more to social factors (Taylor & Bury, 2007). Lack of participation and attrition are barriers to success (Clark et al., 2004; Steed et al. 2005), especially when support for the maintenance of behavior change is not provided. Group programs rarely provide support for the maintenance phase (the final stage in the Stages of Change model), with the exception of a program offering a booster session a few months after completion (Clifford et al., 1991; Steed et al., 2005).
Books, Manuals, and Other Media
Books, audio tapes, video tapes, CD-ROMs, and self-directed manuals can be effective ways to promote self-management (Janevic et al., 2003), with or without an in-person intervention, depending on the personal preferences, age, and education level of the audience. For example, Self-directed Behavior (Watson & Tharp, 2007) teaches how to design step-by-step self-change interventions, integrates empirically-validated approaches, and provides guidelines for how to revise plans when strategies are unsuccessful.
Disease Management Companies
Commercial disease management companies offer financial savings and improved health, generally through increased use of care managers rather than the systematic teaching of skills of self-management (Crosson & Madvig, 2004). American Healthways, the largest disease management company, provides a program with health coaching, patient education, and phone calls to patients, including a “virtual health coach” project that integrates information therapy and behavior change interventions based on the Stages of Change model (American Healthways, 2005).
Individual or Family Coaching Sessions
Another delivery vehicle is individual coaching with a health educator or counselor (alone or in combination with other methods), or one-on-one sessions and individual follow-ups as a supplement to the core program. Individual coaching or counseling provides added motivation, time to address an individual’s specific barriers or challenges, opportunities to include family members as social support, and enhancement of an individual’s capacity for self-control.
Technological Innovations
Websites, e-mail, and touch-screen computers offer tools for self-management (Glasgow et al., 1997; Lorig, 2005; Tate et al., 2001). One example is Diabetes Prevention Source (DPS) Health’s Behavior Change Suite™, a web-based platform that incorporates key behavior change strategies in an online, interactive experience that includes goal-setting, self-monitoring, visual feedback on progress over time, automated reminders, physical activity monitoring with integrated accelerometers, virtual real-time or message-based coaching, interactive lessons, streaming audio and video, and social support and networking features. Interactive voice response (IVR), mobile phones, and text messages also facilitate self-monitoring through data collection, monitoring and reporting of blood glucose level (for diabetics), positive reinforcement messages, and reminders for medication and behavior adherence (Bardone et al., 2000; Fjeldsoe, 2009). Mobile personal sensing (MPS) offers the opportunity for broadly accessible, privacy-preserving “ecological momentary assessment (EMA),” the self-report of symptoms, behaviors, feelings, and cognitions the moment that they occur (Moscowitz & Young, 2006). MPS is moving towards technology for biosensors to monitor physiological health data, collect information for activity classification, and provide notifications for problematic contexts and locations (Ramanathan et al., 2009). EMA has been used for self-monitoring to change behaviors related to drug and alcohol use, coping with chronic pain, and stress and cardiovascular reactivity (Freedman et al., 2006).
Conclusion
There has been little consensus on the components of SMIs that are most effective, but our five-element, three-phase framework can be used to evaluate currently-implemented SMIs, identify gaps in interventions, and identify each patient’s strengths and deficits for personalized interventions. Self-management is applicable to primary prevention for healthy people as well as the health and lifestyle improvement of the chronically ill.
Self-management approaches are more economical than traditional disease management because professional roles can be filled by health educators and counselors rather than physicians. Moreover, they can take place in community settings, on the Internet, or by phone to lessen the demand on over-burdened medical settings (Newman et al., 2004). It is predicted that patients who adhere to healthy lifestyles and habitually use self-management tools will be protected from the severe symptoms that require hospital utilization, a major contributor to high healthcare costs. Currently, no long-term follow-up research has been completed to demonstrate that SMI significantly reduces healthcare costs over an extended period of time.
Shifting responsibility of some aspects of health management to patients requires a change in how provider and patient roles are viewed by both. However, self-management does not mean the exclusion of health professionals. Rather, it represents a new model of active collaboration between “expert patients” and patient-centered practitioners. Self-management interventions will result in decreased burden on the overall health care system and will support more optimized utilization of limited resources. Additionally, it will increase patient investment in, and responsibility for, their own care. Health care professionals will have roles in both education of self-management methods and ongoing support for maintenance of results. Personnel with less education, such as Community Health Workers (CHW), will be necessary for providing adjuctive support, basic follow-up and feedback, positive reinforcement, and accountability. Primary care providers can support patient self-management by encouraging patients to become more informed, active, and responsible in the provider-patient relationship, and by trusting patients to be experts on their symptoms and health condition, particularly the longer the patient has coped with the condition.
Health policy changes will be necessary to make self-management a priority and provide funds for training, employment, technology development, and dissemination. Most importantly, there should be a termination of the sharp division between prevention and disease management that is evident in the structure and missions of different government agencies. As a solution to the staggering costs of chronic disease, DeVol and colleagues (2007) propose incentives for prevention and early intervention through private-public partnerships, and a national commitment to promote health and wellness.
A “family wellness” model offers the best potential for sustained improvement in global health, by going beyond a disease management model to instill healthy behaviors at the level of the family as primary prevention (Rotheram-Borus et al., 2009). Ultimately, the solution to the high human and financial cost of chronic illnesses will be health-promoting families, with each member, regardless of health status, committed to eating well, keeping active, avoiding alcohol and tobacco, and embracing stress-reducing behaviors through the self-management of healthy living.
References
- American Healthways. Center for Information Therapy to jointly develop Ix best practices. The Free Library: 2005. 2005 Nov 1; Retrieved August 03, 2009 from http://www.thefreelibrary.com/AmericanHealthways.
- Anderson RM. Patient empowerment and the traditional medical model: A case of irreconcilable differences? Diabetes Care. 1995;18:412–415. doi: 10.2337/diacare.18.3.412. [DOI] [PubMed] [Google Scholar]
- Aujoulat I, d’Hoore W, Deccache A. Patient empowerment in theory and practice: Polysemy or cacophony? Patient Education and Counseling. 2007;66(1):13–20. doi: 10.1016/j.pec.2006.09.008. [DOI] [PubMed] [Google Scholar]
- Bardone AM, Krahn DD, Goodman BM, Searles JS. Using interactive voice response technology and timeline follow-back methodology in studying binge eating and drinking behavior: Different answers to different forms of the same question? Addictive Behaviors. 2000;25:1–11. doi: 10.1016/s0306-4603(99)00031-3. [DOI] [PubMed] [Google Scholar]
- Barlow JH, Wright CC, Sheasby JE, Turner AP, Hainsworth JM. Self-management approaches for people with chronic conditions: a review. Patient Education and Counseling. 2002;48:177–187. doi: 10.1016/s0738-3991(02)00032-0. [DOI] [PubMed] [Google Scholar]
- Bayliss EA, Ellis JL, Steiner JF. Barriers to self-management and quality-of-life outcomes in seniors with mutimorbidities. Annals of Family Medicine. 2007;5:395–402. doi: 10.1370/afm.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellg AJ. Maintenance of Health Behavior Change in Preventive Cardiology: Internalization and Self-Regulation of New Behaviors. Behavior Modification. 2003;27:103–131. doi: 10.1177/0145445502238696. [DOI] [PubMed] [Google Scholar]
- Bodenheimer T, Chen E, Bennett HD. Confronting the growing burden of chronic disease: Can the U.S. health care workforce do the job? Health Affairs. 2009;28(1):64–74. doi: 10.1377/hlthaff.28.1.64. [DOI] [PubMed] [Google Scholar]
- Bodenheimer T, Lorig K, Holman H, Grumbach K. Patient self-management of chronic disease in primary care. Journal of the American Medical Association. 2002a;288(19):2469–2475. doi: 10.1001/jama.288.19.2469. [DOI] [PubMed] [Google Scholar]
- Bodenheimer T, Wagner EH, Grumbach K. Improving primary care for patients with chronic illness. Journal of the American Medical Association. 2002b;288(14):1775–1779. doi: 10.1001/jama.288.14.1775. [DOI] [PubMed] [Google Scholar]
- Britt E, Hudson SM, Blampied NM. Motivational interviewing in health settings: a review. Patient Education and Counseling. 2004;53:147–155. doi: 10.1016/S0738-3991(03)00141-1. [DOI] [PubMed] [Google Scholar]
- Bull S, Eakin E, Reevers M, Riley K. Multi-level support for physical activity and healthy eating. Journal of Advanced Nursing. 2006;54(5):585–593. doi: 10.1111/j.1365-2648.2006.03861.x. [DOI] [PubMed] [Google Scholar]
- California Health Care Foundation. California Health Care Almanac: Health Care Costs 101. 2011 May; Retrieved on March 5, 2011 from http://www.chcf.org/~/media/MEDIA%20LIBRARY%20Files/PDF/H/PDF%20HealthCareCosts11.pdf.
- Cassidy CA. Using the transtheoretical model to facilitate behavior change in patients with chronic illness. Journal of the American Academy of Nurse Practitioners. 1999;11:281–287. doi: 10.1111/j.1745-7599.1999.tb00578.x. [DOI] [PubMed] [Google Scholar]
- Central Intelligence Agency. The World Factbook. Updated February. 2012;15:2012. Retrieved March 5, 2012, from https://www.cia.gov/library/publications/the-world-factbook/rankorder/2102rank.html. [Google Scholar]
- Charmaz K. Good days, bad days: The self in chronic illness and time. New Brunswick, NJ: Rutgers University Press; 1991. [Google Scholar]
- Chodosh J, Morton SC, Mojica W, Maglione M, Suttorp MJ, Hilton L, Rhodes S, Shekelle P. Meta-analysis: chronic disease self-management programs for older adults. Annals of Internal Medicine. 2005;143:427–438. doi: 10.7326/0003-4819-143-6-200509200-00007. [DOI] [PubMed] [Google Scholar]
- Clark M, Hampson SE, Avery L, Simpson R. Effects of a brief tailored intervention on the process and predictors of lifestyle behaviour change in patients with type 2 diabetes. Psychology, Health & Medicine. 2004;9(4):440–449. [Google Scholar]
- Clifford PA, Tan SY, Gorsuch RL. Efficacy of a self-directed behavioral health change program: Weight, body composition, cardiovascular fitness, blood pressure, health risk, and psychosocial mediating variables. Journal of Behavioral Medicine. 1991;14:303–323. doi: 10.1007/BF00845457. [DOI] [PubMed] [Google Scholar]
- Creer TL, Holroyd KA, Glasgow RE, Smith TW. Health Psychology (chapter 15) In: Lambert MJ, editor. Bergin and Garfield’s Handbook of Psychotherapy and Behavior Change. 5. New York: John Wiley & Sons; 2004. pp. 697–742. [Google Scholar]
- Crosson FJ, Madvig P. Does population management of chronic disease lead to lower costs of care? Health Affairs. 2004;23(6):76–78. doi: 10.1377/hlthaff.23.6.76. [DOI] [PubMed] [Google Scholar]
- DeVol R, Bedroussian A, Charuworn A, Chatterjee A. An unhealthy America: the economic burden of chronic disease -- Charting a new course to save lives and increase productivity and economic growth. Santa Monica, CA: Milken Institute; 2007. [Google Scholar]
- Epping-Jordan J, Bengoa R, Kawar R, Sabaté E. The challenge of chronic conditions: WHO responds. British Medical Journal. 2001;323:947–8. doi: 10.1136/bmj.323.7319.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher EB, Brownson CA, O’Toole ML, Shetty G, Anwuri VV, Glasgow RE. Ecological approaches to self-management: the case of diabetes. American Journal of Public Health. 2005;95(9):1523–1535. doi: 10.2105/AJPH.2005.066084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fjeldsoe BS, Marshall AL, Miller YD. Behavior change interventions delivered by mobile telephone short-message service. American Journal of Preventive Medicine. 2009;36(2):165–173. doi: 10.1016/j.amepre.2008.09.040. [DOI] [PubMed] [Google Scholar]
- Foote SM. Next steps: How can Medicare accelerate the pace of improving chronic care? Health Affairs. 2009;28:99–102. doi: 10.1377/hlthaff.28.1.99. [DOI] [PubMed] [Google Scholar]
- Freedman MJ, Lester KM, McNamara C, Milby JB, Schumacher JE. Cell phones for ecological momentary assessment with cocaine-addicted homeless patients in treatment. Journal of Substance Abuse Treatment. 2006;30(2):105–111. doi: 10.1016/j.jsat.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Gifford AL, Sengupta S. Self-management health education for chronic HIV infection. AIDS Care. 1999;11(1):115–30. doi: 10.1080/09540129948243. [DOI] [PubMed] [Google Scholar]
- Glasgow RE, LaChance PA, Toobert DJ, Brown J, Hampson SE, Riddle MC. Long-term effects and costs of brief behavioural dietary interventions for patients with diabetes delivered from the medical office. Patient Education and Counseling. 1997;32:175–184. doi: 10.1016/s0738-3991(97)00039-6. [DOI] [PubMed] [Google Scholar]
- Glasgow RE, Toobert DJ, Barrera M, Strycker LA. Assessment of problem-solving: A key to successful diabetes self-management. Journal of Behavioral Medicine. 2004;27(5):477–490. doi: 10.1023/b:jobm.0000047611.81027.71. [DOI] [PubMed] [Google Scholar]
- Hibbard JH, Mahoney ER, Stock R, Tusler M. Self-management and health care utilization: Do increases in patient activation result in improved self-management behaviors? Health Services Research. 2007;42(4):1443–1463. doi: 10.1111/j.1475-6773.2006.00669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janevic MR, Janz NK, Dodge JA, Lin X, Pan W, Sinco BR, Clark NM. The role of choice in health education intervention trials: a review and case study. Social Science and Medicine. 2003;56:1581–1594. doi: 10.1016/s0277-9536(02)00158-2. [DOI] [PubMed] [Google Scholar]
- Jerant AF, von Friederichs-Fitzwater MM, Moore M. Patients’ perceived barriers to active self-management of chronic conditions. Patient Education and Counseling. 2005;57:300–307. doi: 10.1016/j.pec.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Karoly P, Boekaerts M, Maes S. Toward consensus in the psychology of self-regulation: How far have we come? How far do we have yet to travel? Applied Psychology: An International Review. 2005;54(2):300–311. [Google Scholar]
- Kerns RD, Rosenberg R. Predicting responses to self-management treatments for chronic pain: application of the pain stages of change model. Pain. 2000;84:49–55. doi: 10.1016/S0304-3959(99)00184-0. [DOI] [PubMed] [Google Scholar]
- Kitsantas A. The role of self-regulation strategies and self-efficacy perceptions in successful weight loss maintenance. Psychology & Health. 2000;15(6):811–820. [Google Scholar]
- Knight KM, McGowan L, Dickens C, Bundy C. A systematic review of motivational interviewing in physical health care settings. British Journal of Health Psychology. 2006;11(2):319–32. doi: 10.1348/135910705X52516. [DOI] [PubMed] [Google Scholar]
- Leventhal H, Mora PA. Is there a science of the processes underlying health and illness behaviors? A comment on Maes and Karoly. Applied Psychology: An International Review. 2005;54(2):255–266. [Google Scholar]
- Lorig K. What are the barriers to healthcare systems using a biopsychosocial approach and how might they be overcome? In: White P, editor. Biopsychosocial medicine: an integrated approach to understanding illness. New York: Oxford University Press; 2005. pp. 201–215. [Google Scholar]
- Lorig K, Holman H. Self-management education: History, definition, outcomes and mechanisms. Annals of Behavioral Medicine. 2003;26(1):1–7. doi: 10.1207/S15324796ABM2601_01. [DOI] [PubMed] [Google Scholar]
- MacDonald W, Rogers A, Blakeman T, Bower P. Practice nurses and the facilitation of self-management in primary care. Journal of Advanced Nursing. 2008;62(2):191–199. doi: 10.1111/j.1365-2648.2007.04585.x. [DOI] [PubMed] [Google Scholar]
- Maes S, Gebhardt W. Self-regulation and health behavior: The health behavior goal model. In: Boekaerts M, Pintrich PR, Zeidner M, editors. Handbook of self-regulation. Burlington, MA: Elsevier Academic Press; 2000. pp. 343–368. [Google Scholar]
- Metcalfe J, Mischel W. A hot/cool-system analysis of delay of gratification: Dynamics of willpower. Psychological Review. 1999;106:3–19. doi: 10.1037/0033-295x.106.1.3. [DOI] [PubMed] [Google Scholar]
- Miller WR, Rollnick S. Motivational Interviewing: Preparing People to Change Addictive Behavior. New York: Guilford Press; 1991. [Google Scholar]
- Moscowitz DS, Young SN. Ecological momentary assessment: what it is and why it is a method of the future in clinical psychopharmacology. Journal of Psychiatry and Neuroscience. 2006;31(1):13–20. [PMC free article] [PubMed] [Google Scholar]
- Mossavar-Rahmani Y, Henry H, Rodabough R, Bragg C, Brewer A, Freed T, et al. Additional self-monitoring tools in the dietary modification component of the Women’s Health Initiative. Journal of the American Dietetic Association. 2004;104(1):76–85. doi: 10.1016/j.jada.2003.10.017. [DOI] [PubMed] [Google Scholar]
- Nagelkerk J, Reick K, Meengs L. Perceived barriers and effective strategies to diabetes self-management. Journal of Advanced Nursing. 2006;54(2):151–158. doi: 10.1111/j.1365-2648.2006.03799.x. [DOI] [PubMed] [Google Scholar]
- Newman S, Steed L, Mulligan K. Self-management interventions for chronic illness. Lancet. 2004;364:1523–1537. doi: 10.1016/S0140-6736(04)17277-2. [DOI] [PubMed] [Google Scholar]
- Nolte S, Elsworth GR, Sinclair AJ, Osborne RH. The extent and breadth of benefits from participating in chronic disease self-management courses: A national patient-reported outcomes survey. Patient Education and Counseling. 2007;65(3):351–360. doi: 10.1016/j.pec.2006.08.016. [DOI] [PubMed] [Google Scholar]
- Paez KA, Zhao L, Hwang W. Rising out-of-pocket spending for chronic conditions: A ten-year trend. Health Affairs. 2009;28(1):15–25. doi: 10.1377/hlthaff.28.1.15. [DOI] [PubMed] [Google Scholar]
- Prochaska JO, DiClemente CC. Stages and processes of self-change of smoking: Toward an integrative model of change. Journal of Consulting and Clinical Psychology. 1983;51:390–395. doi: 10.1037//0022-006x.51.3.390. [DOI] [PubMed] [Google Scholar]
- Purdie N, McCrindle A. Self-regulation, self-efficacy and health behavior change in older adults. Educational Gerontology. 2002;28(5):379–400. [Google Scholar]
- Ramanathan N, Burke J, Cenizal CJ, Estrin D, Ryder J, Rotheram-Borus MJ, Samanta V, Swendeman D. Improving Personal and Environmental Health Decision Making with Mobile Personal Sensing (May 12, 2009) Center for Embedded Network Sensing; 2009. Posters. Paper 430. Available at: http://repositories.cdlib.org/cens/Posters/430. Pretrieved June 23, 2009. [Google Scholar]
- Rasmussen HN, Wrosch C, Scheier MF, Carver CS. Self-regulation processes and health: The importance of optimism and goal adjustment. Journal of Personality. 2006;74(6):1721–1747. doi: 10.1111/j.1467-6494.2006.00426.x. [DOI] [PubMed] [Google Scholar]
- Rotheram-Borus MJ, Swendeman D, Flannery D. Family wellness, not HIV prevention. AIDS and Behavior. 2009;13:409–413. doi: 10.1007/s10461-008-9515-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzer R. Social-cognitive factors in changing health-related behaviors. Current Directions in Psychological Science. 2001;10(2):47–51. [Google Scholar]
- Skinner TC, Carey ME, Cradock S, Daly H, Davies MJ, Doherty Y, Heller S, Khunti K, Oliver L. Diabetes education and self-management for ongoing and newly diagnosed (DESMOND): Process modeling of pilot study. Patient Education and Counseling. 2006;64:369–377. doi: 10.1016/j.pec.2006.04.007. [DOI] [PubMed] [Google Scholar]
- Steed L, Lankester J, Barnard M, Earle K, Hurel S, Newman S. Evaluation of the UCL Diabetes Self-Management Programme (UCL-DSMP): a randomized controlled trial. Journal of Health Psychology. 2005;10:261–275. doi: 10.1177/1359105305049775. [DOI] [PubMed] [Google Scholar]
- Swendeman D, Ingram BL, Rotheram-Borus MJ. Common elements in self-management of HIV and other chronic illnesses: An integrative framework. AIDS Care. 2009;21(10):1321–1334. doi: 10.1080/09540120902803158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tate DF, Wing RR, Winett RA. Using internet technology to deliver a behavioral weight loss program. Journal of the American Medical Association. 2001;285:1172–1177. doi: 10.1001/jama.285.9.1172. [DOI] [PubMed] [Google Scholar]
- Taylor D, Bury M. Chronic illness, expert patients and care transition. Sociology of Health and Illness. 2007;29:27–45. doi: 10.1111/j.1467-9566.2007.00516.x. [DOI] [PubMed] [Google Scholar]
- Taylor D, Bury M. Chronic illness, expert patients, and care transition. Sociology of Health and Illness. 2007;29(1):27–45. doi: 10.1111/j.1467-9566.2007.00516.x. [DOI] [PubMed] [Google Scholar]
- Uchino BM, Cacioppo JT, Kiecolt-Glaser JK. The relationship between social support and physiological process: A review with emphasis on underlying mechanisms and implications for health. Psychological Bulletin. 1996;119:488–531. doi: 10.1037/0033-2909.119.3.488. [DOI] [PubMed] [Google Scholar]
- Warsi A, Wang PS, LaValley MP, Avorn J, Solomon DH. Self-management education programs in chronic disease: a systematic review and methodological critique of the literature. Archives of Internal Medicine. 2004;164:1641–1649. doi: 10.1001/archinte.164.15.1641. [DOI] [PubMed] [Google Scholar]
- Watson DL, Tharp RG. Self-directed behavior. 9. Belmont, CA: Wadsworth Publishing Company; 2007. [Google Scholar]
- Wilde MH, Garvin S. A concept analysis of self-monitoring. Journal of Advanced Nursing. 2007;57(3):339–350. doi: 10.1111/j.1365-2648.2006.04089.x. [DOI] [PubMed] [Google Scholar]
- Witkiewitz K, Marlatt GA. Relapse prevention for alcohol and drug problems: That was zen, this is tao. American Psychologist. 2004;59:224–235. doi: 10.1037/0003-066X.59.4.224. [DOI] [PubMed] [Google Scholar]
- World Health Organization. 2008–2013 Action Plan for the Global Strategy for the Prevention and Control of Noncommunicable Diseases. 2009 Retrieved July 14, 2009 from http://www.who.int/chp/action/en/
- Wright CC, Barlow JH, Turner AP, Bancroft GV. Self-management training for people with chronic disease: An exploratory study. British Journal of Health Psychology. 2003;8(4):465–476. doi: 10.1348/135910703770238310. [DOI] [PubMed] [Google Scholar]


