Abstract
Object
Difficulty with step initiation, called ‘start hesitation,’ is related to bradykinesia of gait and an early hallmark of freezing of gait in Parkinson’s disease (PD). This study investigated the effects of deep brain stimulation (DBS) and levodopa on step initiation in 29 patients with PD before and six months after DBS surgery randomized to either the bilateral subthalamic nucleus (STN) or globus pallidus internus (GPi).
Methods
We measured the amplitude and duration of anticipatory postural adjustments (APAs), the feed-forward postural preparation that precedes the onset of voluntary step initiation, based on center of pressure displacements on a forceplate. We also measured the length and velocity of the first step from kinematic motion analysis. The subjects were a cohort from a large, multi-center, double-blinded, clinical trial randomized to DBS in either bilateral STN (15 subjects) or bilateral GPi (14 subjects). Twenty-eight elderly healthy control subjects were tested and nine PD control subjects, who met criteria for DBS, were tested at baseline and six months later. Differences in step initiation were investigated in two conditions before surgery (Off/On levodopa) and in four conditions after surgery (Off/On levodopa combined with Off/On DBS).
Results
The PD subjects had smaller amplitudes and longer durations of APAs compared to healthy control subjects in all conditions. Before surgery, APAs improved with levodopa. After surgery, the APAs were significantly worse than in the best treatment state before surgery and responsiveness to levodopa decreased. No differences were detected between STN and GPi groups. Comparison with PD control subjects who did not have surgery confirmed that deterioration of step preparation was not related to disease progression.
Step length and velocity were smaller in PD-DBS group than control group in all conditions. Before surgery, levodopa improved both length and velocity of the first step. Both step length and velocity were unchanged in the best treatment state before surgery (DOPA condition) as compared with after surgery (DBS+DOPA), with only the step velocity in STN group getting worse after surgery.
Conclusions
Six months of DBS in the STN or GPi impaired the anticipatory postural preparation for step initiation, the opposite effect as levodopa. Step execution was not as disrupted as postural preparation by DBS, suggesting independent motor pathways for preparation and execution of gait. Although turning the stimulators on after surgery had an added benefit with levodopa on postural preparation to step, comparison pre- and post-surgery suggests that either the surgery itself or six months of continuous stimulation may result in alteration of circuits or plastic changes that impair step initiation.
Keywords: Parkinson’s disease, Deep Brain Stimulation, Anticipatory Postural Adjustments, Posture control, Step initiation
INTRODUCTION
Step initiation is impaired in people with Parkinson’s Disease (PD), that results in the clinically-observed impairments of start-hesitation and freezing of gait5,9. Studies have shown that problems with step initiation in PD are often due to a reduced size of the postural preparation for single limb support36, 19.
The feed-forward, postural preparation that precedes the onset of voluntary movements, such as voluntary step initiation, is referred to as anticipatory postural adjustments (APAs)5, 6,11, 26,31. Preparation for a voluntary step involves displacement of the center of pressure (CoP, i.e. the application point of the resultant ground reaction force) backward and laterally toward the swing limb that results in movement of the body center of mass forward and over the stance limb5, 31. APAs are thought to involve separate neural circuitry from voluntary movements, with coupling between the postural preparation and voluntary step occurring in the medullary reticular formation that receives projections from the basal ganglia and is affected by PD40, 41. Also, APAs likely involve the supplementary motor area, which is suppressed in patients with PD18,31, 22. The small APA amplitudes in patients with PD have been shown to significantly increase with levodopa36, but the effects of deep brain stimulation (DBS) on step initiation is unclear9,28.
In the present study, we characterized both APAs prior to self-initiated steps and the steps themselves in 29 PD subjects before functional neurosurgery and six months after electrodes were implanted. Target DBS sites were randomized to either the subthalamic nucleus (STN) or the globus pallidus internus (GPi) as part of a VA/NINDS multicenter, double-blind, clinical trial comparing the STN and GPi target sites for DBS44.
Deep brain stimulation provides remarkable benefits and it generally improves the same symptoms improved by levodopa in Parkinson’s disease2, 25, 38. Stimulation of the STN or GPi reduces bradykinesia, rigidity, tremor and some gait difficulties34. However, recently we found that DBS surgery impairs automatic postural responses to external perturbations, which are also not improved with levodopa40. Impairment of automatic postural responses after DBS surgery is consistent with the observation that the number of falls may increase after DBS surgery, especially after electrodes are implanted in STN16. Also, our recent meta-analysis showed that the PIGD sub-score (postural instability and gait disorder) of the motor UPDRS continues to worsen 5 years after DBS surgery, especially in STN, despite minimal long-term deterioration of the cardinal signs in the UPDRS43.
In the present study, we investigated whether DBS has the same effect as levodopa in improving step initiation in Parkinson’s disease5, 36. We evaluated step initiation in both the On and Off levodopa conditions both before surgery, and six months after surgery when the short-term effects of surgery have stabilized8. The aims of this study were to: 1) determine the effects of DBS on step initiation both On and Off levodopa and 2) compare the effects of DBS in STN and GPi on step initiation.
METHODS
Subjects
Twenty-nine subjects with idiopathic PD (61.3 ± 7.7 years) who received DBS surgery participated in this study; 22 of these subjects were a smaller cohort from a large, multi-center clinical trial of DBS44 and were recruited from the Seattle and Portland sites. The subjects (DBS PD) were randomized into one of two DBS surgical sites: globus pallidus internus (GPi, N=14) or subthalamic nucleus (STN, N=15).
There were two control groups: 28 healthy control subjects (CTRL, 62.4 ± 7.4 years) and nine PD control subjects (PD-C, 60.3 ± 7.8 years; disease duration 11.6 ± 6.3 years), who met the criteria for DBS surgery. CTRL subjects completed one test session with 4 sets of trials to match the four post-surgery conditions of the PD subjects, All subjects gave informed consent in accordance with Oregon Health & Science University and the Veterans Administration Medical Center Institutional Review Board regulations for human subject studies. Table 1 summarizes the clinical characteristics of the DBS PD subjects when entering the study.
TABLE 1.
Personal and clinical data of subjects with Parkinson’s disease (means and standard deviations)
| Site | N of subjects | Gender | Disease Duration (years) | Age (years) | Hoehn & Yahr | Levodopa-Equivalent dose (mg/day)
|
|
|---|---|---|---|---|---|---|---|
| Pre-surgery | Post-surgery | ||||||
| GPi | 14 | 13M,1F | 12.9 ±10.17 | 61.1 ±8.4 | OFF 3.5±0.9 ON 2.9±0.7 |
1305.9 ± 667.4. | 1097.3 ± 361.7 |
| STN | 15 | 11M, 4F | 11.9 ±4.8 | 61.4 ±5.5 | OFF 3.2±0.7 ON 2.3±0.6 |
1313.1 ± 670.2 | 950.6 ± 512.1* |
Values are expressed as the means ± standard deviations.
p=0.02 (pre – post surgery Levodopa equivalent dose)
Surgerical Procedure
The PD subjects were randomly assigned (following a double-blind method) into either STN (N=15) or GPi (N=14) groups; the PD subjects and experimenters remained unaware of the stimulation site for the duration of the multi-center study. The surgical procedure was done by a neurosurgeon (author KB) with extensive experience in DBS surgeries. The PD subjects underwent a bilateral surgical implantation of DBS electrodes (Medtronic, 3387), that were inserted bilaterally through two pre-coronal sulcus burr holes. Intra-operative microelectrode recordings were performed to confirm target localization, and corrections to the implant site were made accordingly. One week after electrode implantation, a single- (Medtronic, Soletra) or dual-channel (Medtronic, Kinetra) internal pulse generator (IPG) was surgically implanted in the infra-clavicular area. MRI’s were available for 19 of the subjects. For STN, the target contact was the second contact (contact 1), and for GPI, the target contact was the most distal contact (contact 0). The mean distance (mm) of the target contacts (± SD), relative to the anterior commissure-posterior commissure midpoint (mid-commissural point [MCP]) was: STN (x= 11.24±1.45, y= −4.01±1.09, z= −4.86±1.25) and GPi (x= 20.33±1.36, y= −3.4±1.6, z= −4.04±1.15).
After the DBS stimulator was turned on, a movement disorders neurologist (author PH) periodically adjusted the IPG parameters to empirically achieve optimized PD symptom control and an absence of marked side effects, as well as made appropriate changes in the PD pharmacologic therapy. The mean amplitude of the DBS was 3.35 V (range 2.2 to 4.4 V), with 70% of the subjects having a 90 μm pulse width (five subjects at 60, one subject at 120, and two subjects at 150 μm), and at a rate of 185 Hz for 77% of the subjects (the rest of the subjects were divided between 130 and 150 Hz).
Experimental conditions and procedures
Before surgery (pre-surgery condition), subjects with PD were tested in two conditions in the following order: 1) OFF condition: the practical off, anti-parkinson medication state with a medication washout of at least 12 hours and 2) DOPA condition: on levodopa medication, at least an hour after taking their usual dose. The DBS PD subjects were tested again six months after surgery (post-surgery condition) in 4 different conditions in the following order:
DBS condition: only the DBS stimulators turned on after a 12-hour levodopa washout;
OFF condition: the DBS stimulators were turned off at least 30 minutes before testing;
DOPA condition: subjects were tested one hour after taking levodopa medication in their usual dose with the stimulators still off;
DBS+DOPA condition: subjects were tested at least 30 minutes after DBS stimulators were switched on again while still in the on medication.
At the start of each trial, the subjects stood with each foot on separate side-by side force plates. They were instructed to voluntarily take 2 steps forward, starting with the right foot. The steps were self-initiated at the subjects’ normal, comfortable pace. Three trials of step initiation were acquired, starting with feet parallel approximately 25 cm apart. Initial stance was consistent across trials by having subjects stand within tracings of their foot outlines on the force-plates and by coaching subjects to maintain their habitual anterior-posterior and medio-lateral CoP position as monitored by the experimenters on an oscilloscope.
To check for any practice or fatigue effects due to repeated trials, the Control group completed one test session with four sets of trials to match the four postsurgery conditions of the subjects in the PD-DBS group. The PD-C group was tested (on and off medication) at baseline and 6 months later to determine if changes in step initiation were related to disease progression.
Clinical evaluation of the Motor Section (III) of the Unified Parkinson’s Disease Rating Scale (UPDRS)14 and Hoehn and Yahr scale21 were administered immediately before each test condition.
Data acquisition and analysis
The APA phase of step initiation was measured by the lateral CoP excursion toward the initial swing limb. APA magnitude was measured from the baseline to the peak lateral CoP (Peak-CoP). The APA duration was measured as the time from the onset of the first measurable change in lateral CoP (APA start) to time of foot-off (e.g., the instant the vertical forces detected that the initial swing limb left the force plate). Four vertical force sensors under each force-plate were used to calculate the position of the total body CoP. Data from the force-platform were acquired at 480 Hz and were low-pass filtered at 50 Hz.
The step initiation phase was characterized by the Length and Velocity of the first step measured via reflective markers placed on the right lateral malleolus that were detected by infrared cameras (Motion Analysis, Inc., Santa Rosa, CA). Velocity was computed as the ratio between step length and step duration. Kinematic data were acquired at 60 Hz. The median of three step initiation trials was used to represent performance in each condition. For details on data acquisition and APAs parameters, see Rocchi et al.36.
Statistical analyses
To calculate the statistical significance of the comparisons made from our dataset, a repeated measure ANOVA was used, after checking for normal distribution of the data. The tests were performed on all the APA and step measures. The statistical test was implemented according to the specific comparisons of interest:
Comparison with healthy control subjects: comparison between the DBS PD subjects and healthy control subjects was performed with a repeated measure ANOVA, using group (DBS/control) as a between factor.
STN-GPi differences: a preliminary evaluation was performed to detect possible differences between subjects with DBS in STN or GPi. To this aim, site was the between factor and treatment condition was the within factor.
-
Effect of treatment. To detect differences due to surgery procedure (pre/post-surgery conditions) and due to different treatment conditions the ANOVA was performed separately on STN and GPi groups considering only within factor(s), to evaluate:
Pre-surgery responsiveness to levodopa treatment (within factor: treatment, considering only pre-surgery data);
Post-surgery responsiveness to levodopa, to DBS and to their interaction (within factor: treatment, considering only post-surgery data).
-
Pre/post-surgery comparisons including the Off states and best treatment state, namely DOPA pre-surgery and DBS+DOPA post-surgery (within factors: surgery and treatment)
In 2) and 3), A post-hoc multiple comparison test (all-pairwise by Fisher’s LSD Test) was performed to detect pairs of means with statistically significant differences.
-
Control subjects: the repeated measures ANOVA was also used to assess changes between baseline and six months post-surgery in the PD-C group, and over repeated sessions within a day in the healthy control group.
All statistical analyses were made using NCSS Software (Hintze 2000).
RESULTS
Anticipatory postural adjustment phase
In the healthy control group, the APA magnitude and duration were not significantly different across the four sequential sessions. For this reason, the healthy control group mean (± SEM) of the first experimental session was used in all figures.
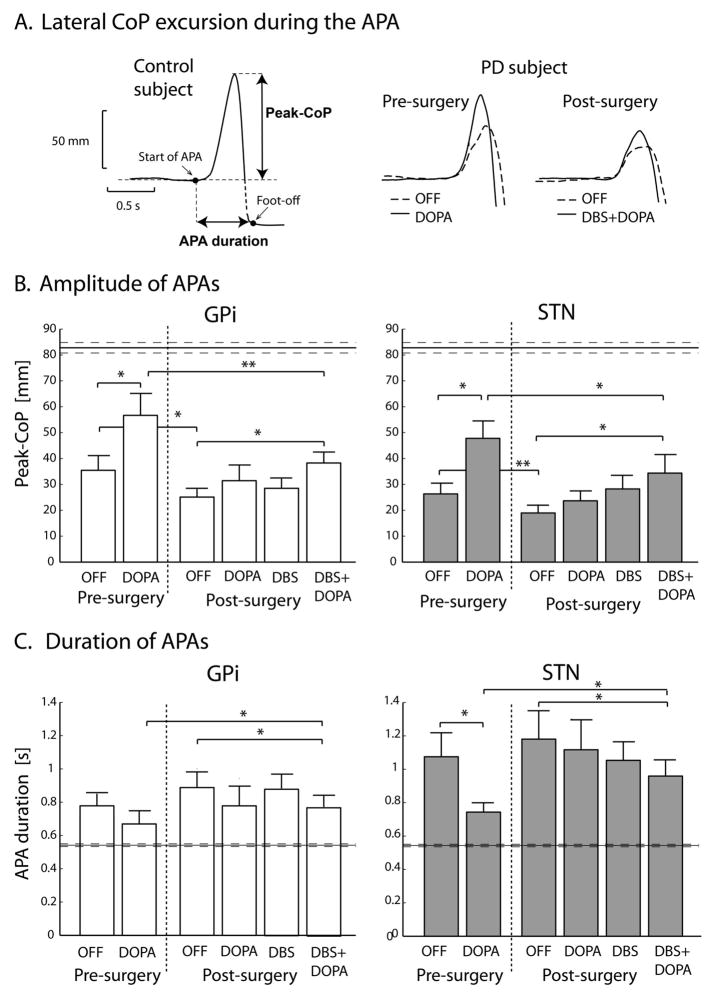
The APA magnitude was smaller and the APA duration was longer in the DBS PD group than in healthy control group (p<0.01) in both the On and Off conditions before surgery and in all four post-surgery conditions. Representative APAs (lateral CoP excursion) during step initiation are illustrated in Figure 1A. for a control and PD subject.
Figure 1.
Anticipatory postural adjustments (APA). A) Representative time-series of lateral CoP excursion. B) Bar graph demonstrating Peak-CoP, i.e. peak of lateral CoP excursion during APA. C) Bar graph showing APA duration, i.e. the time necessary to take the first step from APA onset. Data are represented as means ± standard errors of the means. Horizontal lines in B and C represent values for healthy control group.
* p<0.05; ** p<0.005.
The STN and GPi groups did not differ in response of APA variables across conditions. The differences found were between treatment conditions as detailed below and shown in Figure 1B and 1C.
Anticipatory postural adjustment magnitude
1) Before surgery, the Peak-CoP was significantly increased by levodopa (p<0.01) in both STN and GPi groups, although significantly smaller for PD subjects than for the healthy control subjects. These results were consistent with our previous study36. 2) After surgery, the DOPA, DBS and DBS+DOPA conditions had larger Peak-CoP values compared to the OFF condition for both STN and GPi groups, but only the DBS+DOPA condition reached significance (p<0.05 for both groups; Figure 1B). 3) The Peak-CoP in the post-surgery OFF condition was less than in the pre-surgery OFF condition. Comparison of the best-treatment states before and after surgery showed that Peak-CoP in the post-surgery DBS+DOPA condition was significantly less than the pre-surgery DOPA condition both for STN and GPi groups (see Figure 1B). In fact, after surgery, Peak-CoP was no longer increased by levodopa in either group.
Anticipatory postural adjustment duration
1) Before surgery, levodopa decreased the APA-duration closer to normal values but only in the STN group (p<0.5). While this is consistent with levodopa effects in previous studies36, the GPi group did not have a significant levodopa effect, perhaps because APA-duration in the Off condition was already close to the control group level. 2) After surgery, the best treatment condition (DBS+DOPA) improved (shortened) APA-duration compared to the post-surgery Off condition (p<0.05 in both STN and GPi groups). 3) The APA-duration in the Off condition after surgery was longer than pre-surgery, but did not reach statistical significance. The APA-duration was better (shorter) in the pre-surgery DOPA condition compared to the post-surgery DBS+DOPA condition (best-treatment states; p<0.05 for both groups, see Figure 1C).
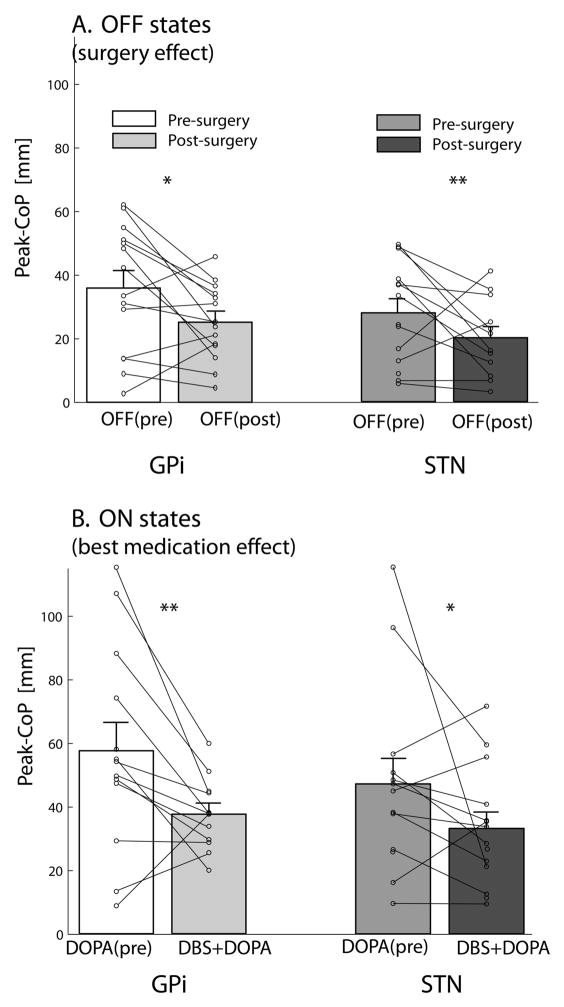
To better illustrate the changes related to the surgery effect (pre-surgery OFF vs post-surgery OFF) and the best-treatment effect (pre-surgery DOPA vs post-surgery DBS+DOPA), we plotted the individual subject values along with the group means (Figure 2). Note that the peak APA magnitude reduction after surgery in the OFF conditions, for both STN and GPi groups, was statistically significant (p<0.05 in GPi and p<0.005 in STN, post-hoc multiple comparison test) and was consistent for all but three patients in the GPi group, and for all but two patients in the STN group (Figure 2A). Similarly, the comparison between best treatment ON conditions (Figure 2B) showed DBS+DOPA was not as effective as DOPA pre-surgery in improving the APA size (p<0.005 in GPi and p<0.05 in STN, post-hoc multiple comparison test). The worsening of APA amplitude after surgery was true for all but two subjects in the GPi, and for all but three subjects in the STN group.
Figure 2.
Anticipatory postural adjustment amplitude post-surgery compared to pre-surgery. A: Graph demonstrating OFF states to investigate surgery effect; B Graph showing the ON levodopa states to compare best medical management states before and after surgery. Means ± standard errors of the means and single subject values are represented.
* p<0.05; ** p<0.005.
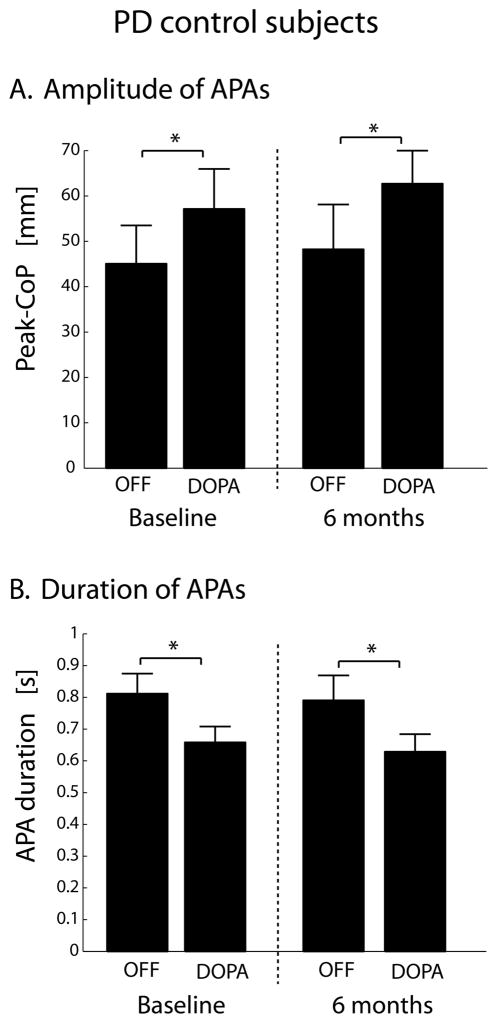
The Peak-CoP and APA durations the PD-C group was similar to DBS PD subjects before surgery, both when evaluated at baseline and during the follow-up, six months later (Figure 3). In both cases, the Peak-CoP magnitude increased and APA-duration decreased in the DOPA condition compared to the OFF condition (p<0.05). No differences in these two measures were found between baseline and six month follow-up testing.
Figure 3.
Bar graphs showing APA amplitude (A) and APA duration (B) in the PD control group, evaluated at baseline and 6 months later.
* p<0.05.
The Stepping phase
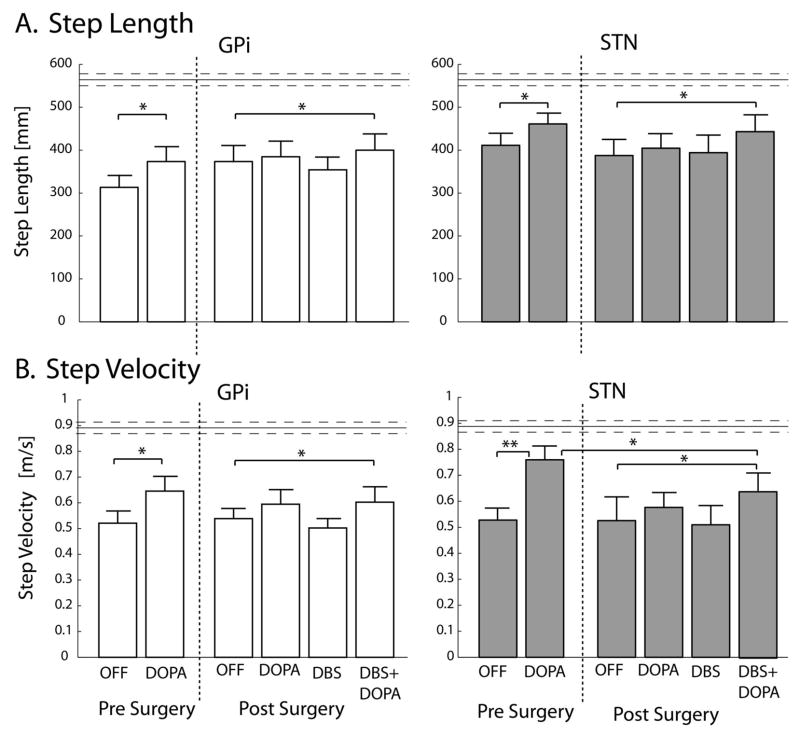
The repeated measure ANOVA did not detect differences between the STN and GPi groups, in terms of surgery effect or response to treatments, for both kinematic variables (Step Length and Velocity). Parkinson’s disease reduced the length (p<0.01) and velocity (p<0.01) of the first step compared to healthy control subjects. In addition: 1) Before surgery, levodopa improved the stepping phase, since both length and velocity of the first step increased in the DOPA condition compared to OFF condition (Length: p< 0.05; Velocity; p<0.05 for GPi, p< 0.005 in STN group). 2) After surgery, for both STN and GPi groups, Length and Velocity in DBS+DOPA condition increased compared to the OFF condition (p<0.05). 3) For GPi subjects, Length and Velocity in the DBS+DOPA condition were restored to similar level as the DOPA pre-surgery condition. For STN group, although Length was restored to DOPA pre-surgery level, Velocity remained lower than in the DOPA pre-surgery condition (p<0.05).
Clinical evaluation
Before surgery, clinical signs, as measured by the UPDRS III, were greatly ameliorated in the DOPA condition compared to the OFF condition (p<0.001). After surgery, the results confirmed the reduction of clinical signs with therapies compared to the OFF condition (p<0.001), for both the STN and GPi groups. In fact, the administration of levodopa, in the patients’ usual dose with activation of the stimulator resulted in a UPDRS III score comparable to the DOPA condition before surgery. Values and more detailed stats of UPDRS III in the six tested conditions are presented in Table 2.
TABLE 2.
UPDRS III – Motor Score pre and post-surgery (mean ± std) and statistics
| DBS Site | Pre-surgery | Post-surgery | ||||
|---|---|---|---|---|---|---|
| OFF | ON | OFF | DBS | DOPA | DBS+DOPA | |
| GPi | 50.9±18.2 | 28.5±14.4 | 47.1±17.0 | 34.5±18.4 | 31.6±12.8 | 22.8±12.7 |
| STN | 49.0±11.9 | 21.2±11.0 | 51.1±20.9 | 33.5±11.5 | 35.2±15.1 | 20.6±8.4 |
Values expressed as the means ± standard deviations.
Pre-surgery: OFF vs DOPA: p<0.001
Post-surgery: OFF vs DOPA or DBS: p<0.01
OFF vs DBS+DOPA: p<0.001
DBS or DOPA vs DBS+DOPA: p<0.01
The PD-C subjects had a comparable UPDRS III, with 45.5±16 and 22.9±8.9 at baseline and 45.9±12.8 and 24.2±9.6 after 6 months (off and on levodopa respectively). The levodopa equivalent dose was 1234±450 at baseline and 1144±326 after 6 months, values comparable to DBS PD subjects before surgery (see Table 1).
DISCUSSION
Our results showed that six months of DBS can impair step initiation. Prior to surgery, the subjects with PD showed smaller than normal lateral weight shifts associated with APAs in preparation for a step as well as smaller and slower steps, consistent with the literature5, 36. Also, consistent with the literature, APA amplitude, step size and velocity were improved with levodopa before surgery5, 36, 34. However, six months after surgery, the size of the postural preparation was smaller, duration of step initiation was longer and the pre-surgery improvement with levodopa was not restored.
The APAs were impaired by DBS surgery similarly in both the GPi and STN groups. In contrast, automatic postural responses to external perturbations in the same subjects were impaired by DBS surgery in the STN group, but not the GPi, group40. Similarly, jaw movements in the same subjects were impaired by DBS surgery only in the STN group, but not the GPi group34. Thus, DBS in STN impaired APAs, automatic postural responses and jaw movements, whereas DBS in GPi impaired APAs but did not change automatic postural responses or jaw movements in the same subjects. These results suggest that STN plays a similar role for these axial motor behaviors, whereas GPi circuitry may play different roles. Two previous studies investigated effects of DBS in STN on step initiation28, 38. Both these studies concluded that DBS in the STN improved step initiation. However, it is important to note that these studies considered only post-surgery data. We found similar, small improvements in APAs in the DBS+DOPA condition after surgery, compared to the Off condition after surgery, as shown in Figure 1. Nevertheless, APA amplitudes and durations were worse in the DBS+DOPA condition than in the DOPA condition before surgery. To our knowledge, this is the first study comparing movement initiation pre- and post- DBS surgery. Surprisingly, we found levodopa and DBS produced different effects on step initiation. In fact, whereas levodopa improved APAs for step initiation, DBS in either STN or GPi impaired them. Previous studies showing that the effects of DBS on motor tasks are similar to the effects of levodopa are consistent with a simple circuitry model of PD1, 12. This model suggests that DBS and dopamine both inhibit excessive, tonic basal ganglia outflow to thalamocortical and brainstem circuits. In contrast, the positive effects of levodopa and negative effects of DBS on APAs for step initiation in our study suggest that DBS may have some non-dopaminergic effects. In fact, the poor responsiveness of APAs to levodopa after surgery, compared to before surgery suggests that the DBS surgery, itself, impairs the action of levodopa to act on APAs.
A poor APAs response to levodopa after surgery is unlikely due to reduction of levodopa. In our subjects, the levodopa equivalent dose was slightly decreased after compared to before surgery, with a significant change (p=0.02) only in the STN group, consistent with the literature5. However, a correlation between the decline in APA peak and change in levodopa dose before and after surgery was not significant (p=0.2 in GPi group, p=0.75 in STN group). Although we cannot know the reason for the reduced levodopa effect after surgery, it is possible that stabilization of abnormal basal ganglia network oscillations with DBS may in some way limit the effects of levodopa on specific postural tasks such as step initiation that depends on interaction of PPN and other locomotor brainstem areas and the supplementary motor area32. Although the physiological mechanisms responsible for DBS effects are unknown, long-term plastic changes of synapses and circuitry have been observed due to chronic DBS25, 4.
We do not know whether impairment of step preparation after DBS surgery is related to a lesion effect or to six months of continuous, high-frequency stimulation in the basal ganglia, resulting in long-lasting alterations of circuits that are important for postural preparation, even when the stimulators were turned off. Future studies should compare step initiation before surgery and after surgery, but before the stimulators have been turned on, to differentiate between a surgery procedure effects versus a chronic stimulation effect.
Our comparison with a PD control group who did not have surgery dispels the likelihood that worsening of step initiation after DBS surgery is due to progression of disease across six months. In fact, the PD control subjects had better step initiation with levodopa medication both at baseline and 6 months later unlike the subjects with DBS surgery, who only improved with levodopa before surgery.
Interestingly, movement execution was not as disrupted by DBS as postural preparation prior to movement. Specifically, step length was the same in the best treatment states before and after surgery in both GPi and STN groups and step velocity was the same before and after surgery in the GPi group. The different effects of DBS on postural preparation and stepping suggest independent motor pathways for preparation and execution of movement4. Studies recording neural activity in the reticular formation of the brainstem of cats showed different neurons active during the APAs and movement phase4. In addition, recent studies showed that APAs may be triggered independently of the step by an acoustic startle30, 34. In addition, lesions isolated to the supplementary motor area resulted in loss of APAs prior to arm movements, which were not affected by the lesion 18, 31.
From a clinical viewpoint, the motor UPDRS was the same before and after surgery, in the best-treatment conditions (DOPA and DBS+DOPA), consistent with the literature16. The UPDRS appears not to be a sensitive measure of anticipatory postural adjustments prior to step initiation, so additional clinical tests of balance should be considered4. The lack of quantitative improvement in postural preparation for a step after DBS surgery is consistent with clinical studies suggesting that DBS may not be as effective a treatment for axial signs in patients with PD as it is for tremor, rigidity and bradykinesia 2, 5, 24. In fact, the VA/NINDS multi-center clinical trial comparing the STN and GPi also showed an increase in injurious falls after DBS surgery, especially for the STN group, although both groups otherwise benefited from surgery with fewer on/off fluctuations, 12, 44.
One limitation of our study was differences in the STN/GPi groups at baseline for APA-duration and magnitude. This bias was not correlated with diversity in clinical signs and appeared even though DBS PD subjects were randomized to STN and GPi surgery sites. Although the subjects sample-size included in the present study is quite large, when compared to analogous previous works 4, 18, 21, 28, 38 a limitation of our study is still represented by the limited number of subjects. However, our study also has the advantages of considering both pre- and post-surgery conditions and both STN and GPi sites, whereas previous DBS studies examining step initiation and gait only included post-surgery conditions for one DBS site. In addition, it is noteworthy that we evaluated performance in several different axial tasks such as speech34, postural responses40 as well as anticipatory postural adjustments prior to step initiation. A comprehensive evaluation of DBS-PD subjects, with a variety of motor tasks will allow a better understanding of the effects of DBS and of the role of the basal ganglia in axial motor control.
Our study highlights the importance of comparing motor behavior pre- and post-surgery to determine the effects of DBS on patients with PD. Despite the many benefits of DBS for patients with PD, our study points to potential postural problems associated with initiating walking that may worsen start-hesitation or freezing after surgery30.
Figure 4.
Bar graphs showing kinematics of the first step: A) Step Length, B) Step Velocity. Data are represented as means ± standard errors of the means. Horizontal lines represent values for healthy control group.
* p<0.05; ** p<0.005.
Acknowledgments
We acknowledge the assistance of Triana Nagel for subject scheduling & data collection; Marilee Stephens and Lesley Silar for help with data collection, and Dr Ali Samii for referring patients for study from the Seattle location. MRI images to verify electrode placement were available courtesy of the VA CSP Study #468, “A comparison of best medical therapy and deep brain stimulation of subthalamic nucleus and globus pallidus for the treatment of Parkinson’s disease”. This research was supported by the National Institute on Aging grants AG19706 and AG006457.
References
- 1.Albin RL, Young AB, Penney JB. The functional anatomy of disorders of the basal ganglia. Trends Neurosci. 1995;18:63–64. [PubMed] [Google Scholar]
- 2.Anderson VC, Burchiel KJ, Hogarth P, Favre J, Hammerstad JP. Pallidal vs subthalamic nucleus deep brain stimulation in Parkinson disease. Archives of Neurology. 2005;62:554–560. doi: 10.1001/archneur.62.4.554. [DOI] [PubMed] [Google Scholar]
- 3.Bejjani BP, Gervais D, Arnulf I, Papadopoulos S, Demeret S, Bonnet AM, et al. Axial parkinsonian symptoms can be improved: the role of levodopa and bilateral subthalamic stimulation. J Neurol Neurosurg Psychiatry. 2000;68:595–600. doi: 10.1136/jnnp.68.5.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beuter A, Modolo J. Delayed and lasting effects of deep brain stimulation on locomotion in Parkinson’s disease. Chaos. 2009;19:026114. doi: 10.1063/1.3127585. [DOI] [PubMed] [Google Scholar]
- 5.Bloem BR, Hausdorff JM, Visser JE, Giladi N. Falls and freezing of gait in Parkinson’s disease: a review of two interconnected, episodic phenomena. Mov Disord. 2004;19:871–84. doi: 10.1002/mds.20115. [DOI] [PubMed] [Google Scholar]
- 6.Bouisset S, Richardson J, Zattara M. Do anticipatory postural adjustments occurring in different segments of the postural chain follow the same organisational rule for different task movement velocities, independently of the inertial load value? Exp Brain Res. 2000;132:79–86. doi: 10.1007/s002219900228. [DOI] [PubMed] [Google Scholar]
- 7.Bronstein JM, Tagliati M, Alterman RL, Lozano AM, Volkmann J, Stefani A, et al. Deep brain stimulation for Parkinson disease: an expert consensus and review of key issues. Arch Neurol. 2011;68:165–171. doi: 10.1001/archneurol.2010.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burchiel KJ, Anderson VC, Favre J, Hammerstad JP. Comparison of pallidal and subthalamic nucleus deep brain stimulation for advanced Parkinson’s disease: results of a randomized, blinded pilot study. Neurosurgery. 1999;45:1375–1384. doi: 10.1097/00006123-199912000-00024. [DOI] [PubMed] [Google Scholar]
- 9.Burleigh-Jacobs A, Horak FB, Nutt JG, Obeso JA. Step initiation in Parkinson’s disease: influence of levodopa and external sensory triggers. Mov Disord. 1997;12:206–215. doi: 10.1002/mds.870120211. [DOI] [PubMed] [Google Scholar]
- 10.Crenna P, Carpinella I, Rabuffetti M, Rizzone M, Lopiano L, Lanotte M, Ferrarin M. Impact of subthalamic nucleus stimulation on the initiation of gait in Parkinson’s disease. Exp Brain Res. 2006;172:519–532. doi: 10.1007/s00221-006-0360-7. [DOI] [PubMed] [Google Scholar]
- 11.Couillandre A, Breniere Y, Maton B. Is human gait initiation program affected by a reduction of the postural basis? Neurosci Lett. 2000;285:150–154. doi: 10.1016/s0304-3940(00)01015-6. [DOI] [PubMed] [Google Scholar]
- 12.DeLong MR. Primate models of movement disorders of basal ganglia origin. Trends Neurosci. 1990;13:281–285. doi: 10.1016/0166-2236(90)90110-v. [DOI] [PubMed] [Google Scholar]
- 13.Drew T, Prentice S, Schepens B. Cortical and brainstem control of locomotion. Prog Brain Res. 2004;143:251–261. doi: 10.1016/S0079-6123(03)43025-2. [DOI] [PubMed] [Google Scholar]
- 14.Fahn S, Elton RL . UPDRS Development Committee. Unified Parkinson’s Disease Rating Scale. In: Fahn S, Marsden CD, Calne DB, Goldstein M, editors. Recent Developments in Parkinson’s Disease. Florham Park, NJ: Macmillan; 1987. pp. 153–163. [Google Scholar]
- 15.Ferrarin M, Rizzone M, Bergamasco B, Lanotte M, Recalcati M, Pedotti A, et al. Effects of bilateral subthalamic stimulation on gait kinematics and kinetics in Parkinson’s disease. Exp Brain Res. 2005;160:517–527. doi: 10.1007/s00221-004-2036-5. [DOI] [PubMed] [Google Scholar]
- 16.Follett KA, Weaver FM, Stern M, Hur K, Harris CL, Luo P, et al. Pallidal versus subthalamic deep-brain stimulation for Parkinson’s disease. N Engl J. 2010;362:2077–2091. doi: 10.1056/NEJMoa0907083. [DOI] [PubMed] [Google Scholar]
- 17.Franchignoni F, Horak F, Godi M, Nardone A, Giordano A. Using psychometric techniques to improve the Balance Evaluation Systems Test: the mini-BESTest. J Rehabil Med. 2010;42:323–331. doi: 10.2340/16501977-0537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gurfinkel VS, Elner AM. Participation of the secondary motor area of the frontal lobe of the brain in organizing postural components of human voluntary movement. Neirofiziologiia. 1988;20:7–15. [PubMed] [Google Scholar]
- 19.Halliday SE, Winter DA, Frank JS, Patla AE, Prince F. The initiation of gait in young, elderly, and Parkinson’s disease subjects. Gait Posture. 1998;8:8–14. doi: 10.1016/s0966-6362(98)00020-4. [DOI] [PubMed] [Google Scholar]
- 20.Hausdorff JM, Gruendlinger L, Scollins L, O’Herron S, Tarsy D. Deep brain stimulation effects on gait variability in Parkinson’s disease. Mov Disord. 2009;24:1688–1692. doi: 10.1002/mds.22554. [DOI] [PubMed] [Google Scholar]
- 21.Hoehn M, Yahr M. Parkinsonism: onset, progression and mortality. Neurology. 1967;17:427–442. doi: 10.1212/wnl.17.5.427. [DOI] [PubMed] [Google Scholar]
- 22.Jacobs JV, Lou JS, Kraakevik JA, Horak FB. The supplementary motor area contributes to the timing of the anticipatory postural adjustment during step initiation in participants with and without Parkinson’s disease. Neuroscience. 2009;164:877–885. doi: 10.1016/j.neuroscience.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johnsen EL, Mogensen PH, Sunde NA, Østergaard K. Improved asymmetry of gait in Parkinson’s disease with DBS: gait and postural instability in Parkinson’s disease treated with bilateral deep brain stimulation in the subthalamic nucleus. Mov Disord. 2009;24:590–597. doi: 10.1002/mds.22419. [DOI] [PubMed] [Google Scholar]
- 24.Kleiner-Fisman G, Fisman DN, Sime E, Saint-Cyr JA, Lozano AM, Lang AE. Long-term follow up of bilateral deep brain stimulation of the subthalamic nucleus in patients with advanced Parkinson disease. J Neurosurg. 2003;99:489–495. doi: 10.3171/jns.2003.99.3.0489. [DOI] [PubMed] [Google Scholar]
- 25.Krack P, Batir A, Van Blercom N, Chabardes S, Fraix V, Ardouin C, et al. Five-year follow-up of bilateral stimulati on of the subthalamic nucleus in advanced Parkinson’s disease. New England Journal of Medicine. 2003;349:1925–1934. doi: 10.1056/NEJMoa035275. [DOI] [PubMed] [Google Scholar]
- 26.Lepers R, Breniere Y. The role of anticipatory postural adjustments and gravity in gait initiation. Exp Brain Res. 1995;107:118–124. doi: 10.1007/BF00228023. [DOI] [PubMed] [Google Scholar]
- 27.Li XH, Wang JY, Gao G, Chang JY, Woodward DJ, Luo F. High-frequency stimulation of the subthalamic nucleus restores neural and behavioral functions during reaction time task in a rat model of Parkinson’s disease. J Neurosci Res. 2010;88:1510–1521. doi: 10.1002/jnr.22313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu W, McIntire K, Kim SH, Zhang J, Dascalos S, Lyons KE, Pahwa R. Bilateral subthalamic stimulation improves gait initiation in patients with Parkinson’s disease. Gait Posture. 2006;23:492–498. doi: 10.1016/j.gaitpost.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 29.Lubik S, Fogel W, Tronnier V, Krause M, König J, Jost WH. Gait analysis in patients with advanced Parkinson disease: different or additive effects on gait induced by levodopa and chronic STN stimulation. J Neural Transm. 2006;113:163–173. doi: 10.1007/s00702-005-0310-8. [DOI] [PubMed] [Google Scholar]
- 30.MacKinnon CD, Bissig D, Chiusano J, Miller E, Rudnick L, Jager C, et al. Preparation of anticipatory postural adjustments prior to stepping. J Neurophysiol. 2007;97:4368–4379. doi: 10.1152/jn.01136.2006. [DOI] [PubMed] [Google Scholar]
- 31.Massion J. Movement, posture and equilibrium: interaction and coordination. Prog Neurobiol. 1992;38:35–56. doi: 10.1016/0301-0082(92)90034-c. [DOI] [PubMed] [Google Scholar]
- 32.Moran RJ, Mallet N, Litvak V, Dolan RJ, Magill PJ, Friston KJ, et al. Alterations in brain connectivity underlying beta oscillations in Parkinsonism. PLoS Comput Biol. 2011;7(8):e1002124. doi: 10.1371/journal.pcbi.1002124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moreau C, Defebvre L, Destée A, Bleuse S, Clement F, Blatt JL, et al. STN-DBS frequency effects on freezing of gait in advanced Parkinson disease. Neurology. 2008;71:80–84. doi: 10.1212/01.wnl.0000303972.16279.46. [DOI] [PubMed] [Google Scholar]
- 34.Perlmutter JS, Mink JW. Deep Brain Stimulation. Annu Rev Neurosci. 2006;29:229–257. doi: 10.1146/annurev.neuro.29.051605.112824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Robertson LT, St George RJ, Carlson-Kuhta P, Hogarth P, Burchiel KJ, Horak FB. Site of deep brain stimulation and jaw velocity in Parkinson disease. J Neurosurg. 2011;115:985–994. doi: 10.3171/2011.7.JNS102173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rocchi L, Chiari L, Mancini M, Carlson-Kuhta P, Gross A, Horak FB. Step initiation in Parkinson’s disease: influence of initial stance conditions. Neurosci Lett. 2006;406:128–132. doi: 10.1016/j.neulet.2006.07.027. [DOI] [PubMed] [Google Scholar]
- 37.Rochester L, Baker K, Nieuwboer A, Burn D. Targeting dopa-sensitive and dopa-resistant gait dysfunction in Parkinson’s disease: selective responses to internal and external cues. Mov Disord. 2011;26:430–435. doi: 10.1002/mds.23450. [DOI] [PubMed] [Google Scholar]
- 38.Rodriguez-Oroz MC, Obeso JA, Lang AE, Houeto JL, Pollak P, Rehncrona S. Bilateral deep brain stimulation in Parkinson’s disease: a multicentre study with 4 years follow-up. Brain. 2005;128:2240–2249. doi: 10.1093/brain/awh571. [DOI] [PubMed] [Google Scholar]
- 39.Rogers MW, Kennedy R, Palmer S, Pawar M, Reising M, Martinez KM, et al. Postural preparation prior to stepping in patients with Parkinson’s disease. J Neurophysiol. 2011;106:915–924. doi: 10.1152/jn.00005.2010. [DOI] [PubMed] [Google Scholar]
- 40.Schepens B, Drew T. Strategies for the integration of posture and movement during reaching in the cat. J Neurophysiol. 2003;90:3066–86. doi: 10.1152/jn.00339.2003. [DOI] [PubMed] [Google Scholar]
- 41.Schepens B, Stapley P, Drew T. Neurons in the pontomedullary reticular formation signal posture and movement both as an integrated behavior and independently. J Neurophysiol. 2008;100:2235–53. doi: 10.1152/jn.01381.2007. [DOI] [PubMed] [Google Scholar]
- 42.St George RJ, Carlson-Kuhta P, Burchiel KJ, Hogarth P, Frank N, Horak FB. The effect of subthalamic and pallidal deep brain stimulation on postural responses in patients with Parkinson’s disease. J Neurosurgery. doi: 10.3171/2012.2.JNS11847. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.St George RJ, Nutt JG, Burchiel KJ, Horak FB. A meta-regression of the long-term effects of deep brain stimulation on balance and gait in PD. Neurology. 2010;75:1292–1299. doi: 10.1212/WNL.0b013e3181f61329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weaver FM, Follett K, Stern M, Hur K, Harris C, Marks WJ, Jr, et al. Bilateral deep brain stimulation vs best medical therapy for patients with advanced Parkinson disease: a randomized controlled trial. JAMA. 2009;301:63–73. doi: 10.1001/jama.2008.929. [DOI] [PMC free article] [PubMed] [Google Scholar]