Summary
Cytochrome bd is a terminal quinol:O2 oxidoreductase of respiratory chains of many bacteria. It contains three hemes, b558, b595, and d. The role of heme b595 remains obscure. A CO photolysis/recombination study of the membranes of Escherichia coli containing either wild type cytochrome bd or inactive E445A mutant was performed using nanosecond absorption spectroscopy. We compared photoinduced changes of heme d-CO complex in one-electron-reduced, two-electron-reduced, and fully-reduced states of cytochromes bd. The line shape of spectra of photodissociation of one-electron-reduced and two-electron-reduced enzymes is strikingly different from that of the fully-reduced enzyme. The difference demonstrates that in the fully-reduced enzyme photolysis of CO from heme d perturbs ferrous heme b595 causing loss of an absorption band centered at 435 nm, thus supporting interactions between heme b595 and heme d in the di-heme oxygen-reducing site, in agreement with previous works. Photolyzed CO recombines with the fully-reduced enzyme monoexponentially with τ ~12 µs, whereas recombination of CO with one-electron-reduced cytochrome bd shows three kinetic phases, with τ ~14 ns, 14 µs, and 280 µs. The spectra of the absorption changes associated with these components are different in line shape. The 14 ns phase, absent in the fully-reduced enzyme, reflects geminate recombination of CO with part of heme d. The 14 µs component reflects bimolecular recombination of CO with heme d and electron backflow from heme d to hemes b in ~4% of the enzyme population. The final, 280 µs component, reflects return of the electron from hemes b to heme d and bimolecular recombination of CO in that population. The fact that even in the two-electron-reduced enzyme, a nanosecond geminate recombination is observed, suggests that namely the redox state of heme b595, and not that of heme b558, controls the pathway(s) by which CO migrates between heme d and the medium.
Keywords: respiration, chlorin, cytochrome, ligand binding, gas molecule, photobiology
1. Introduction
Cytochrome bd is a terminal oxidase of aerobic respiratory chains of many bacteria [1–3]. It catalyzes electron transfer from quinol to molecular oxygen (to produce water) [4, 5] and couples this exergonic reaction to the generation of a membrane potential [6–10].
Apart from energy conservation, cytochrome bd endows bacteria with a number of specific physiological functions. Cytochrome bd facilitates both pathogenic and commensal bacteria to colonize oxygen-poor environments [11–14], serves as an oxygen scavenger and inhibits degradation of O2-sensitive enzymes [15], increases virulence and survival in host mammalian cells [16, 17] of pathogens, enhances bacterial tolerance to nitrosative stress [18–23], supports disulfide bond formation upon protein folding [24] and may contribute to mechanisms of detoxification of hydrogen peroxide in the bacterial cell [25].
Cytochrome bd is not a member of the well-known family of heme-copper oxidases. Neither of its two subunits (CydA and CydB) shows sequence homology to any subunit of heme-copper family members [26, 27]. In contrast to heme-copper oxidases, cytochrome bd is not a proton pump and does not contain copper in the active site [5, 28]. It contains only hemes as redox-cofactors which are heme b558, heme b595 and heme d, with stoichiometry 1:1:1 per enzyme molecule.
The roles of the three hemes in cytochrome bd are different. The low-spin hexacoordinate heme b558 is the electron entry site, it directly accepts electrons from quinol [29, 30]. The high-spin and likely pentacoordinate heme d is the site where binding, activation and further reduction of O2 by four electrons to H2O occurs. This chlorin cofactor is likely responsible for the remarkably high affinity of the enzyme for oxygen leading to formation of a stable oxygenated complex [31, 32]. The high-spin pentacoordinate heme b595 apparently accepts electrons from heme b558 to deliver them to heme d [33, 34] but the issue as to whether this is its only role remains unanswered. A number of observations indicate that heme b595 and heme d can form a common di-heme site for the oxygen reduction [8, 9, 35–43]. Nevertheless, no significant redox interactions between hemes d and b595 can be observed [44]. It has also been proposed that heme b595 may serve as a second oxygen-binding site [45, 46].
It was shown that the interaction of heme d with ligands differs in the fully reduced (R) enzyme (all the three hemes are reduced) and the one-electron-reduced “mixed-valence” (MV1) enzyme (heme d is reduced, heme b558 and heme b595 are oxidized). In particular, it was found that:
In the MV1 CO-bound isolated WT cytochrome bd from E. coli, upon photodissociation of CO from heme d, a significant part of photodissociated CO (~50–70%) does not leave the protein but recombines with heme d within a few hundred ps. In contrast, for the enzyme in the R state under the same conditions, no such heme d-CO geminate recombination is observed [39, 41]. In addition, this ultrafast spectroscopy study also showed that the spectra of CO dissociation from the R and MV1 forms of the WT isolated cytochrome bd on a picosecond time scale are different in line shape, pointing to the interaction between the close-lying hemes d and b595 [39, 41]. The possible presence of later processes prior to bimolecular CO recombination has not been investigated so far.
The apparent rate constants for thermal (spontaneous) dissociation of NO and CO from the protein are much higher for the R cytochrome bd from E.coli than in case of the enzyme in the MV1 state [19].
In the reaction of the R cytochrome bd from Azotobacter vinelandii with oxygen, the rate of O2 binding depends linearly on the oxygen concentration up to the air level. On the contrary, when the enzyme is in the MV1 state, the rate of O2 binding is hyperbolic, thus revealing a saturation behavior. It was proposed that in case of the MV1 cytochrome bd, the enzyme in equilibrium exists in the two different conformations, but only one of which can bind oxygen. When in the “closed” conformation, cytochrome bd provides no access for O2 to heme d2+, whereas in the “open” conformation, oxygen binds easily. The R enzyme is always in the open conformation [32]. Thus the redox state of one or both of hemes b modulates ligand binding properties to heme d.
In the present work, we performed a systematic nanosecond study of the E. coli membranes containing cytochrome bd by varying the number of electrons in the bd oxidase. We used both the WT cytochrome bd and the E445A mutant of subunit I (CydA) that is catalytically inactive [47] and cannot be completely reduced even with excess dithionite [8]. This unique property of the mutant allowed us to generate not only the R and MV1 redox states but also the two-electron-reduced (MV2) state of cytochrome bd which is impossible to generate in the WT and has remained uncharacterized in the previous transient absorption spectroscopy studies. Here we were able to compare in detail the photoinduced absorption changes in various redox states of the enzyme on time scales that were not investigated previously and obtain new information about the heme-heme and heme-CO interactions.
2. Materials and methods
2.1. Chemicals
Carbon monoxide was from Air Liquide; sodium dithionite was from Merck. Other basic chemicals and biochemicals were from Sigma-Aldrich, Merck, and Fluka.
2.2. Strains and plasmids
E. coli strain GO105 (cyd AB::kan, cyo, recA) devoid of cytochrome bo3 and cytochrome bd quinol oxidases [48] was used as the host strain for expressing both the wild type and E445A mutant cytochrome bd from a plasmid. In both cases, plasmid pTK1 containing the whole operon encoding cytochrome bd and the ampicillin-resistance gene was introduced into the strain [47].
2.3. Cell growth and membrane preparation
The WT cells of E. coli were grown aerobically as reported in [39]; the E445A mutant cells were grown anaerobically as described in [47]. To obtain the E. coli membranes, both the WT and E445A mutant cells, washed twice with 5 mM sodium phosphate (pH 7.5), 0.17 M NaCl, and a few grains of solid 4-(2-aminoethyl)-benzenesulfonyl fluoride, were suspended in 20 mM Tris(hydroxymethyl)-aminomethan/HCl (pH 8.3), 0.5 mM ethylenediaminetetraacetate, 5 mM MgSO4, 15 mM benzamidine, 1 mM dl-dithiothreitol, 0.5 mg/L leupeptin, and a few grains of solid Deoxyribonuclease I and 4-(2-aminoethyl)-benzenesulfonyl fluoride; then the suspension was passed twice by 30-mL portions through a French press. Intact and partially broken cells were removed by centrifugation at 17,600×g for 5 min at 4 °C. The membranes were pelleted (125,000×g, 4 hrs, 4 °C), frozen in liquid nitrogen, and stored at −80 °C.
2.4. Sample preparation
All measurements were performed in 50 mM N-(2-hydroxyethyl)piperazine-N’-(2-ethanesulfonate)/50 mM 2-(N-cyclohexylamino)-ethanesulfonate (pH 8.0), and 0.5 mM ethylenediaminetetraacetate in a home-made optical cell of 2.5 mm pathway at room temperature. Cytochrome bd concentrations in the WT and E445A mutant membranes in the samples were 7.3 µM and 1 µM, respectively. The optical cell was first purged with argon and the sample was flowed into the cell under argon pressure. Experiments were carried out with the three stable states of carbon monoxide-bound cytochromes bd: (a) dithionite-reduced wild type (WT R-CO, b5582+b5952+d2+-CO) and (b) dithionite-reduced mutant (E445A MV2-CO, b5582+b5953+d2+-CO) were obtained by bubbling the sample, pre-reduced with 50–100 mM sodium dithionite for 30 min, with 100% CO; (c) one-electron-reduced wild type (WT MV1-CO, b5583+b5953+d2+-CO) was prepared by purging the as isolated membrane-bound cytochrome bd (which is mainly a one-electron-reduced oxy species, b5583+b5953+d2+-O2) with argon gas and then by replacing argon with 100% CO. To check the redox and ligation status of cytochrome bd, static absorption spectra of the samples were recorded before and after the measurements with the use of a dual pathway spectrophotometer described in [49].
2.5. Enzyme concentration
The cytochrome bd content in the E. coli membranes was judged from the heme d concentration. In the WT membranes, the heme d concentration was determined from the dithionite-reduced-minus-‘air-oxidized’ difference absorption spectra using Δε628-607 of 10.8 mM−1cm−1 [38] and from the (CO-bound/dithionite-reduced)-minus-(dithionite-reduced) difference spectra using Δε643-623 = 13.2 mM−1cm−1 [10]. In the E445A mutant membranes, the heme d concentration was determined from the (CO-bound/dithionite-reduced)-minus-(dithionite-reduced) difference spectra using Δε643-623 = 11.1 mM−1cm−1 that corresponds to Δε628-670 of 25 mM−1cm−1 for the dithionite-reduced absolute absorption spectra of the isolated enzyme [8].
2.6. Nanosecond spectroscopy
The photoinduced absorption changes in the membranes were measured with a home-built nanosecond spectrophotometer described in [50]. The flash (excitation at 640 nm, near the α band of heme d [44, 51, 52], 5 ns fwhm) was provided by a Nd:Yag pumped dye laser. The absorption changes were probed at discrete wavelengths and delay times after the exciting flash by flashes provided by an optical parametric oscillator pumped by the third harmonic of a Nd:Yag (5 ns fwhm).
2.7. Data analysis
Origin 7 (OriginLab Corporation) was used for data manipulation and presentation.
3. Results
3.1. Recombination of CO with the WT cytochrome bd in the R state
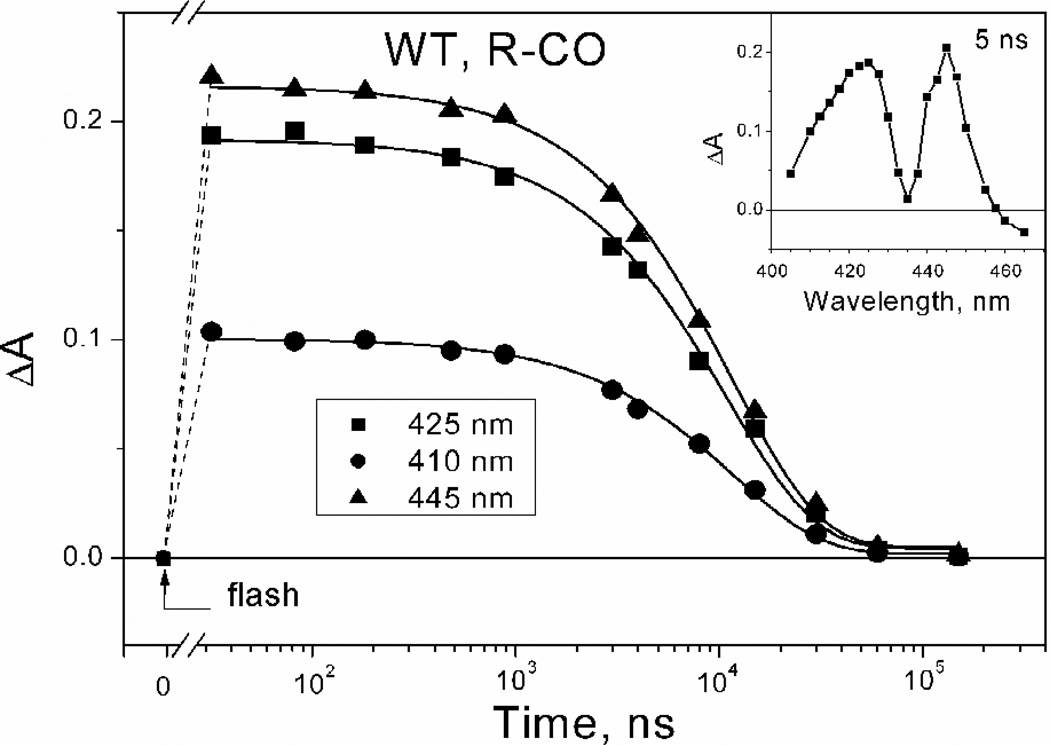
CO recombines with the WT cytochrome bd in the R state monoexponentially with τ ~ 12 µs at 100% CO, as evidenced from the kinetics of flash-induced absorption changes at selected wavelengths (Fig. 1, main panel). This value corresponds to a second-order rate constant of CO recombination to heme d of ~ 8×107 M−1s−1, in line with the previously reported values for the isolated enzyme from E. coli [53] and A. vinelandii [40, 54]. A difference transient absorption spectrum recorded 5 ns after the flash has a reversed ‘W’ shape (Fig. 1, inset). Such peculiar W-shape is consistently observed as well in static Soret difference absorption spectra of CO binding to the enzyme in the R state [38, 40, 52, 55, 56].
Fig. 1.
Absorption changes measured after photodissociation of CO from the E. coli membranes containing the WT cytochrome bd in the R-CO state. Main panel: Kinetics of absorption changes at selected wavelengths during CO recombination. The kinetic data points (symbols) are shown with their best fits to single exponentials (solid lines) yielding τ ~ 12 µs. Arrow indicates the moment of laser flash. Inset: Transient absorption spectrum at a delay time of 5 ns. For conditions, see the Materials and methods section.
We have previously shown that CO does not recombine with the isolated fully reduced enzyme in the time scale up to 300 ps [39]. Recent experiments (M.H.V. & V.B.B., unpublished results) have extended this range up to 4 ns. Altogether our experiments show that only bimolecular recombination occurs after dissociation of the heme d-CO bond in the R-CO state.
3.2. Recombination of CO with the WT cytochrome bd in the MV1 state
Flash-induced absorption changes of the WT cytochrome bd in the MV1-CO state were monitored under the same conditions. Fig. 2A shows the transient spectra at delay times of 5 ns, 200 ns, and 60 µs. It can be seen that the 5 ns-spectrum of the WT MV1-CO enzyme (Fig. 2A) is clearly different in line shape from its counterpart of the WT R-CO enzyme (Fig. 1, inset). The difference between the 5 ns R-CO and MV1-CO transient spectra is mainly a bleaching at 435 nm (Fig. 2D), in agreement with earlier measurements on the picoseconds time scale [41]. As shown in Fig. 2B, the spectral evolution in the WT MV1 enzyme is multiphasic. It can be fitted with three exponential phases with time constants of ~14 ns, 14 µs (10–15 µs in different experiments), and 280 µs (140–290 µs in different experiments). This is in contrast to a single (12 µs) phase observed for the WT R enzyme (Fig. 1). The spectra of the absorption changes associated with the three components are different in line shape. The spectrum of the 14 ns component, absent in the WT R cytochrome bd, reflects the decay of an induced absorption with a maximum at 435 nm (Fig. 2C), which we assign to geminate recombination of CO with part of WT MV1 enzyme. The spectral characteristics of this component are similar to that of the ~100 ps phase [39] also attributed to geminate recombination. The spectrum of the absorption changes associated with the 14 µs component shows a maximum at 420 nm with a shoulder around 430 nm (Fig. 2C). This component is not homogeneous and likely reflects at least two different processes - bimolecular recombination of CO with heme d on the microsecond time scale and the photolysis-induced electron transfer (backflow) from heme d to hemes b in a small fraction of the enzyme molecules (see Discussion).
Fig. 2.
Absorption changes measured after photodissociation of CO from the E. coli membranes containing the WT cytochrome bd in the MV1-CO state. (A) Transient absorption spectra at delay times of 5 ns, 200 ns, and 60 µs. (B) Kinetics of absorption changes at selected wavelengths during CO recombination. The kinetic data points (symbols) are shown with their best fits (solid lines). The kinetics at 435 nm is fitted to the sum of three exponentials yielding τ ~ 14 ns, 14 µs, and 280 µs. 417.5 nm has been selected as the wavelength isosbestic for the 14 ns and 280 µs components, therefore for that kinetics a one-exponential fit with τ ~ 14 µs is sufficient. Arrow indicates the moment of laser flash. (C) Spectra of the absorption changes associated with the 14-ns, 14-µs, and 280-µs components. The spectrum of the 14-ns phase is calculated as the difference between the transient spectra measured at delay times of 5 ns and 200 ns. The spectrum of the 280-µs phase is the spectrum measured 60 µs after the flash. The amplitude of the latter has been divided by e−60/280 to correct for the decay at 60 µs. The spectrum of the 14-µs phase is calculated as the difference between the transient spectrum measured at a delay time of 200 ns and the spectrum of the 280-µs phase. (D) Difference between R-CO and MV1-CO transient spectra at a delay time of 5 ns. The spectra are normalized at 445 nm (spectrum MV1-CO multiplied by 7.5) as the spectral properties at this wavelength are independent of the oxidation state of the b-hemes [41].
The spectrum of the absorption changes associated with the 280 µs component has a maximum at 438 nm and a minimum at 422 nm (Fig. 2C), and may be attributed to reversal of the electron backflow and bimolecular recombination in this enzyme fraction. According to modeling (not shown), the 280 µs component reflects re-reduction of ~3.8% of heme d, with the electron simultaneously returning from heme b595 and heme b558 in the proportion of ~ 70%/30%, respectively. Such relative contributions of the hemes b to the reversed electron transfer are consistent with those observed recently with the isolated enzyme at 1% CO [10].
3.3. Recombination of CO with the E445A mutant cytochrome bd in the MV2 state
Flash-induced absorption changes of the E445A mutant cytochrome bd in the MV2-CO state (Fig. 3) are generally similar to those observed with the WT MV1 cytochrome bd but also markedly different from the WT R cytochrome bd. Fig. 3A shows the corresponding transient spectra at 5 ns, 200 ns, and 1.5 ms. The 5 ns spectrum of the E445A MV2-CO enzyme (Fig. 3A) resembles that of the WT MV1-CO enzyme (Fig. 2A). It is worth mentioning that the 5 ns difference spectra for WT MV1-CO and E445A MV2-CO have comparable amplitudes (within a factor of 2), though the concentrations of WT and E445A differ considerably (7.3 µM vs. 1.0 µM). A few explanations of such difference which do not exclude each other can be considered. First, in MV1-CO only part of heme d is reduced (initially as oxy ferrous heme d complex), whereas in MV2-CO all heme d population is in the ferrous state. Therefore, after replacement of O2 with CO, the actual heme d-CO concentration in MV1-CO should be substantially lower than 7.3 µM because neither ferryl nor oxidized heme d species being also present in the as-prepared MV1 state can react with CO. Second, MV1-CO and MV2-CO may differ in a fraction that undergoes geminate recombination of CO and heme d at earlier (subnanosecond) times. If in the former case the enzyme population involved in subnanosecond recombination is larger, the amplitude at a delay time of 5 ns will become smaller. Third, the quantum yield of photodissociation of CO from heme d in E445A MV2-CO may be larger than that in WT MV1-CO.
Fig. 3.
Absorption changes measured after photodissociation of CO from the E. coli membranes containing the E445A mutant cytochrome bd in the MV2-CO state. (A) Transient absorption spectra at delay times of 5 ns, 200 ns, and 1.5 ms. (B) Kinetics of absorption changes at selected wavelengths during CO recombination. The kinetic data points (symbols) are shown with their reasonable fits to the sum of two exponentials (solid lines) yielding τ ~ 14 ns, and 42 µs. Approximation with three exponentials does not improve the fit significantly. Arrow indicates the moment of laser flash. (C) Difference between transient absorption spectra of the WT cytochrome bd in the R-CO state and the E445A mutant cytochrome bd in the MV2-CO state at a delay time of 5 ns (normalized at 445 nm). (D) Comparison of the spectra of the absorption changes associated with the nanosecond components for the WT cytochrome bd in the MV1-CO state and the E445A mutant cytochrome bd in the MV2-CO state (normalized on the maximum). The latter spectrum is a difference between the MV2-CO E445A transient spectra at delay times of 5 ns and 200 ns.
The kinetics of CO recombination at selected wavelengths for the MV2-CO state (Fig. 3B, symbols) can be reasonably fitted with two exponentials (Fig. 3B, solid lines) with time constants of ~14 ns, and 42 µs (38–42 µs in different experiments). Using three exponentials does not improve fit significantly. These changes can mainly reflect geminate and bimolecular recombination of CO with heme d on the nanosecond and microsecond time scales, respectively. Remarkably, the 280 µs component observed in the MV1 state is absent. This implies that under these conditions back electron transfer from heme d to heme b595 requires heme b558 to be in the oxidized state.
The spectrum of the bimolecular recombination component differs somewhat from the nanosecond component. This may be due to interaction of dissociated CO with the hemes while it is sequestered close to the active site.
CO recombination to the E445A MV2 enzyme (Fig.3B) appeared to be about 3-fold slower than that to the WT MV1 cytochrome bd (Fig. 2B). Two possible explanations can be suggested. First, the mutation could affect an access channel for ligand transfer between the bulk phase and heme d. The existence of such channel(s) in cytochrome bd has been proposed earlier [19, 32, 39]. Second, the mutation could decrease the affinity of ferrous heme d for CO.
4. Discussion
Earlier, recombination of CO with the dithionite-reduced E. coli membranes containing the WT cytochrome bd was studied on the micro/millisecond time scale at the 532 nm excitation [57]. These membranes were treated with detergent. Treatment of membrane-bound cytochrome bd with detergent can markedly attenuate scattering in the near UV typical of the native membranes that allowed resolving flash-induced absorption changes in the Soret. However, treatment of cytochrome bd with detergent can lead to appearance of a denatured fraction of heme b reacting with CO [2, 56]. Such a heme b-CO complex can be easily photolyzed at the 532 nm excitation that resulted in additional slower phases of CO recombination with heme b [40, 54, 57], significantly complicating interpretation of the data [57]. In the present experiments, native membranes of E. coli, devoid of such an undesired reaction, were used. The use of a specific set-up in the photolysis experiments allows us to monitor absorption changes with a very high resolution even in the near UV region, starting from the time of 5 ns (see the Materials and methods section). Upon selective excitation of the α band of heme d (at 640 nm), CO is photolyzed only from heme d.
In the present study we showed that at the 640 nm excitation the flash-induced absorption changes for the E. coli membranes containing the WT cytochrome bd in the R-CO and MV1-CO states under the same conditions differ in (i) line shape of the transient spectra, (ii) number of recombination phases, (iii) amplitude of the response.
(i) As previously observed for the picosecond CO photodissociation spectra of the isolated WT enzyme [41], the transient spectra at 5 ns of the WT membranes containing cytochrome bd in the R and MV1 states are clearly different in line shape. The WT R spectrum has a reversed W-shape with a minimum at 435 nm (Fig. 1, inset) whereas the main feature of that of the WT MV1 is the maximum at 435 nm (Fig. 2A). The difference between the normalized R and MV1 spectra is the bleaching at 435 nm (Fig. 2D) [41]. An explanation suggested in [39, 41] for the picosecond spectra is that in the R enzyme photolysis of CO from heme d perturbs ferrous heme b595 causing loss of an absorption band centered at 435 nm. The data of nanosecond spectroscopy support this conclusion and further substantiate the assignment of the bleaching at 435 nm (Fig. 2D) to the interaction between heme d and heme b595.
(ii) One of the main results of this work is that the dynamics of the flash-induced absorption changes for WT cytochrome bd is strikingly different in the R-CO and MV1-CO states. In R-CO, there is a single phase of bimolecular recombination with τ ~ 12 µs at 1 atm CO (Fig. 1). In contrast, in MV1-CO, there are three phases of recombination with time constants of ~14 ns, 14 µs, and 280 µs (Fig. 2B) plus a picosecond phase of geminate recombination (τ~70–200 ps) observed earlier [39]. Thus, there are totally four phases in MV1-CO.
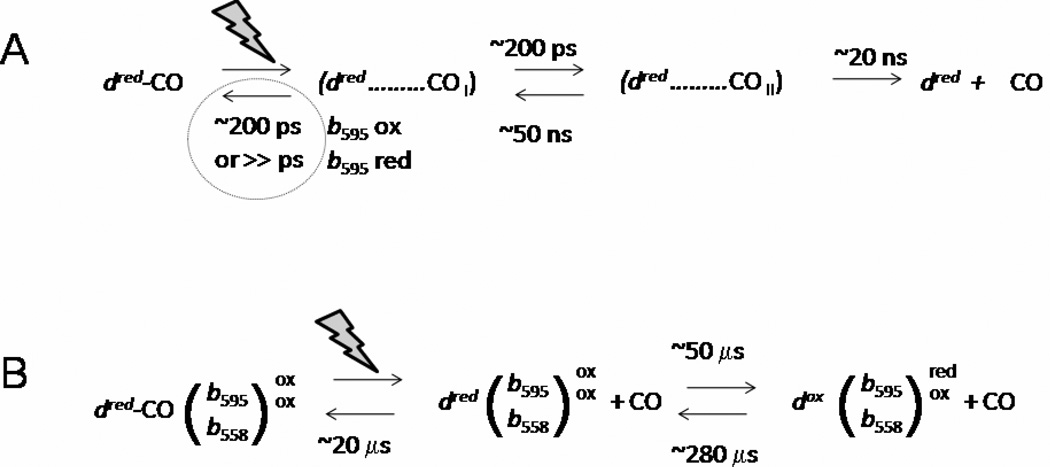
The line shape of the spectra associated with the three phases of recombination in MV1-CO is different. The spectrum of the 14 ns component, absent in the R enzyme, is the induced absorption with a maximum at 435 nm (Fig. 2C), very similar to the ps MV1-CO spectrum [41]. This similarity allows us to suggest that the 14 ns component reflects a second phase of geminate recombination of CO with heme d in part of MV1, following the ~100 ps phase [39]. The presence of at least two phases of CO geminate recombination indicates the presence of several distinct configurations of non-bound CO within the protein moiety. As discussed previously [39], the absence of geminate recombination in the fully reduced enzyme implies that the redox state of heme b595 controls the pathways of unbound CO. Our present finding of a second geminate rebinding phase also only in MV complexes indicates that all geminate rebinding to heme d is influenced by heme b595, suggesting that the nanosecond rebinding occurs via the conformation from which picosecond rebinding occurs (Fig. 4A), in agreement with the proposed proximity of hemes d and b595.
Fig. 4.
(A) Minimal scheme of geminate recombination phases of CO starting from the MV1-CO and R-CO states. Two different configurations of dissociated CO in the protein (dred……COi i=I, II) are required to explain the two geminate recombination phases. The ratio of forward and backward rates from these configurations is roughly estimated from the amplitudes of the phases; the sums of the rates correspond to the experimentally observed rates. The state (dred + CO) denotes a state where CO has escaped from the protein. In this minimal scheme, if heme b595 is reduced prior to dissociation of the heme d-CO bond, geminate recombination from the (dred……COI) state does not compete efficiently with population of the (dred……COII) state. (B) Minimal scheme of bimolecular CO recombination and electron transfer starting from the MV1-CO. The first dissociation step comprises all steps in scheme (A). The ratio of forward and backward rates from the CO dissociated dred is roughly estimated from the amplitudes of the phases; the sums of the rates correspond to the experimentally observed rates.
Based on transmembrane electron transfer kinetics observed in previous electrogenicity studies (Fig. 7, trace 3 in Ref. [7]), and of our modeling of the 280 µs phase, we propose that the 14 µs and 280 µs heme d – CO recombination phases also reflect backflow (14 µs phase) and reverse backflow (280 µs phase) between heme d and the b hemes. Fig. 4B depicts a scheme encompassing the ensemble of competing reactions on the microsecond time scale that summarizes our interpretation of the data, as discussed below.
In this view, the 14 µs component consists of at least two different processes: (a) bimolecular recombination of CO with the remaining ferrous unligated heme d on the microsecond time scale, and (b) the electron backflow from heme d to hemes b in ~ 3.8% of the enzyme molecules (see Fig. 4B).
The conclusion that the 14 µs component includes recombination of CO with heme d that is bimolecular rather than geminate is based on the data of Junemann et al. [58] who first observed such recombination with the A. vinelandii cytochrome bd in the MV1 state monitoring the heme d α-band. They showed that the rate of recombination increases linearly with the CO concentration, hence the recombination is indeed bimolecular. Their observation that the second-order rate constant of recombination for the MV1 enzyme (1×108 M−1s−1) is only slightly slower than that for the R enzyme (1.5×108 M−1s−1) [58] is also in agreement with our data.
Another factor that contributes to this phase is electron redistribution from heme d to hemes b in ~ 3.8% of the MV1 cytochrome bd induced by photolysis. This is in agreement with the spectrum of the next, 280 µs component, showing a maximum at 438 nm and a minimum at 422 nm (Fig. 2C), that reflects return of the electron from the hemes b to heme d. It is worth to mention that, even under optimum conditions, the amplitude of the electron backflow is quite small due to the large redox potential difference between d and b hemes. Furthermore, since there is a competition between CO recombination and backflow [7], the backflow could previously be detected by micro/millisecond absorption spectroscopy only at low CO concentrations [7, 58]. At 1% CO, ~ 11% of heme d is oxidized following CO photolysis and the electron simultaneously moves to hemes b558 and b595 [10] and then returns back as CO recombines. At 100% CO, no internal electron redistribution in the MV1 cytochrome bd has been detected by absorption spectroscopy hitherto. The reverse electron flow was found to be associated with the generation of membrane potential [7]. The finding that the signal-to-noise ratio of electrometric traces is superior to that of absorbance traces allowed us to observe electrometric backflow transients even at high CO concentrations [7]. At 100% CO, the electron backflow is present but its amplitude decreases to be of about one-fourth of that at 1% CO (see Fig. 7 of [7]), i.e. around 3% of heme d can be oxidized following CO photolysis provided this value is 11% at 1% CO. The electrometric backflow response decays with time constant of about 360 µs [7] that is in rough agreement with the 280 µs phase observed in this work. Owing to the fact that extinction of hemes b in the Soret is much larger than that of heme d [44] and due to a very high sensitivity of the technique used, we are now able to observe spectrophotometrically flash-induced internal electron redistribution and its relaxation even at 100% CO.
It should be noted that the 280 µs component is not observed in the MV2-CO enzyme (Fig. 3). This agrees with our conclusion about the origin of the microsecond phases in the MV1-CO cytochrome bd because flash-induced electron redistribution between hemes d and b558 is not possible in the MV2-CO state (both hemes are reduced in this state). Electron backflow from heme d to heme b595 in the E445A MV2-CO enzyme is apparently also negligible under these conditions. As such electron transfer does occur to a significant extent starting from the MV1-CO state, this finding implies that the redox state of heme b558 influences the redox potential difference between hemes d and b595. This reasoning is consistent with the report of negative redox interactions between hemes b558 and b595 [44]. The fact that the difference between the normalized spectra of the 14 µs phase for MV1-CO and the 42 µs phase for MV2-CO (not shown) is similar to the reversed spectrum of the 280 µs component further supports such conclusion.
It has to be noted that the lack of heme b595 reduction in photolyzed MV2-CO state seems to contrast with a report of Belevich et al. [8] where such reduction was observed. The experimental conditions of that work were different: it was carried out with the isolated detergent-solubilized enzyme, at 1% CO, the kinetics of the electron transfer was not resolved [8]. Two possible explanations of such apparent discrepancy can be suggested. First, due to the mutation, the rate of electron transfer between the hemes may become slower in the E445A enzyme as compared to the WT enzyme. Since, as mentioned above, there must be a competition between CO recombination and backflow, at 100% CO used in this study, the photolysis-induced electron transfer from heme d to heme b595 may be indeed negligible by that reason. Second, the midpoint potential values for WT cytochrome bd from E. coli can significantly depend on the nature of membrane environment such as detergent used for the enzyme solubilization [59]. The latter has never been tested with the E445A mutant cytochrome bd. It is possible that in the mutant cytochrome bd of the bacterial membranes (this work) ΔEm between heme d and heme b595 is larger (i.e., Em of b595 is lower) than that in the isolated detergent-solubilized enzyme [8] that in the former case would make the electron backflow thermodynamically unfavorable to occur. The combination of these two reasons cannot also be excluded.
(iii) R-CO and MV1-CO WT cytochromes bd differ in the yield of the observed photolysis of CO from ferrous heme d at 5 ns. Although recorded under the same enzyme concentration and other experimental conditions, a normalization factor of ~7.5 is required for the 5 ns WT MV1-CO transient spectrum to match that of R-CO, provided the extinction coefficients of R-CO and MV1-CO at 445 nm are virtually identical [41]. Since in the “as prepared” state of WT cytochrome bd used to generate MV1-CO, only about 70% of heme d is in the oxy form (MV1-O2), the actual factor is ~5.2. This means that in case of WT MV1 cytochrome bd, no more than ~20% of ferrous heme d can be observed as recombining with CO through a bimolecular mechanism on the microsecond time scale. This is in full agreement with the picosecond studies where in at least half of the photolyzed WT MV1-CO purified enzyme, subnanosecond geminate recombination of CO and heme d occurs [39] and the quantum yield of photodissociation of CO from heme d was found to be ~3-fold diminished in the presence of oxidized hemes b [41].
In all earlier studies where ligand-reaction pattern was compared for WT cytochrome bd in R and MV1 states and the differences were observed [19, 32, 39, 41], the redox state of which of the two b hemes modulates ligand binding/dissociation properties of the heme d active site, was not established. This could be heme b595, or heme b558, or both. In this work we used the E445A mutant cytochrome bd in which heme b595 remains in the ferric state even in the presence of a strong reductant. This unique possibility to have two-electron-reduced mixed-valence enzyme (MV2) was used to answer this question. First, we found that the line shape of the photodissociation spectrum (at a delay time of 5 ns) of the E445A mutant in the MV2-CO state (Fig. 3A) is similar to that of the WT cytochrome bd in the MV1-CO state (Fig. 2A) but strikingly different from that of the WT enzyme in the R state (Fig. 1, inset). In contrast to the latter spectrum, it does not display the sharp bleaching feature at 435 nm as clearly demonstrates the difference between the 5 ns transient spectra of the WT R and E445A MV2 cytochromes bd (Fig. 3C). This difference is also reminiscent of that between the picosecond transient spectra of the R and MV1 purified enzymes under isotropic conditions [41]. Hence we can conclude that dissociation of CO from heme d perturbs the Soret band of heme b595 but not that of heme b558. The data thus further support interactions between high-spin protoporphyrin b595 and chlorin d in the di-heme oxygen-reducing site, in agreement with previous works [8, 9, 36–43].
Second, in the case of E445A MV2-CO, apart from a microsecond phase of bimolecular recombination, there is also an additional phase of CO recombination on the nanosecond time sale with τ ~ 14 ns (Fig. 3), absent in the WT R cytochrome bd. The fact that the spectrum of the 14 ns component of E445A MV2-CO is very similar in line shape to that of WT MV1-CO (Fig. 3D), supports the conclusion that also in E445A MV2-CO cytochrome bd geminate recombination of CO with heme d on the nanosecond time scale occurs. Thus, the redox state of heme b595 controls the pathway(s) by which CO migrates between heme d and the medium. In light of this finding we can also suggest that the same holds true for O2 [32] and NO [19].
Acknowledgements
This work was supported by the Russian Foundation for Basic Research (to V.B.B., grant 08-04-00093). V.B.B. was recipient of a FEBS short-term fellowship. We thank Dr. Pierre Joliot (Paris) and Dr. Alexander Konstantinov (Moscow) for their interest and stimulating discussions of this work.
Abbreviations
- WT
wild type
- R
fully reduced (three-electron-reduced) species (b5582+b5952+d2+)
- MV1
one-electron-reduced “mixed-valence” species (b5583+b5953+d2+)
- MV2
two-electron-reduced “mixed-valence” species (b5582+b5953+d2+)
- fwhm
full width at half-maximum
- τ
time constant, reciprocal of rate constant, t1/e
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Poole RK, Cook GM. Redundancy of aerobic respiratory chains in bacteria? Routes, reasons and regulation. Adv. Microb. Physiol. 2000;43:165–224. doi: 10.1016/s0065-2911(00)43005-5. [DOI] [PubMed] [Google Scholar]
- 2.Junemann S. Cytochrome bd terminal oxidase. Biochim. Biophys. Acta. 1997;1321:107–127. doi: 10.1016/s0005-2728(97)00046-7. [DOI] [PubMed] [Google Scholar]
- 3.Borisov VB. Cytochrome bd: structure and properties. Biochemistry (Moscow) 1996;61:565–574. (translated from Biokhimiya (in Russian) (1996), 61, 786–799). [PubMed] [Google Scholar]
- 4.Tsubaki M, Hori H, Mogi T. Probing molecular structure of dioxygen reduction site of bacterial quinol oxidases through ligand binding to the redox metal centers. J. Inorg. Biochem. 2000;82:19–25. doi: 10.1016/s0162-0134(00)00140-9. [DOI] [PubMed] [Google Scholar]
- 5.Borisov VB, Verkhovsky MI. Oxygen as acceptor [Chapter 3.2.7] In: Böck A, Curtiss R III, Kaper JB, Neidhardt FC, Nyström T, Rudd KE, Squires CL, editors. EcoSal - Escherichia coli and Salmonella: cellular and molecular biology. Washington, DC: ASM Press; 2009. < http://www.ecosal.org>. [Google Scholar]
- 6.Puustinen A, Finel M, Haltia T, Gennis RB, Wikstrom M. Properties of the two terminal oxidases of Escherichia coli. Biochemistry. 1991;30:3936–3942. doi: 10.1021/bi00230a019. [DOI] [PubMed] [Google Scholar]
- 7.Jasaitis A, Borisov VB, Belevich NP, Morgan JE, Konstantinov AA, Verkhovsky MI. Electrogenic reactions of cytochrome bd. Biochemistry. 2000;39:13800–13809. doi: 10.1021/bi001165n. [DOI] [PubMed] [Google Scholar]
- 8.Belevich I, Borisov VB, Zhang J, Yang K, Konstantinov AA, Gennis RB, Verkhovsky MI. Time-resolved electrometric and optical studies on cytochrome bd suggest a mechanism of electron-proton coupling in the di-heme active site. Proc. Natl. Acad. Sci. USA. 2005;102:3657–3662. doi: 10.1073/pnas.0405683102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Belevich I, Borisov VB, Verkhovsky MI. Discovery of the true peroxy intermediate in the catalytic cycle of terminal oxidases by real-time measurement. J. Biol. Chem. 2007;282:28514–28519. doi: 10.1074/jbc.M705562200. [DOI] [PubMed] [Google Scholar]
- 10.Borisov VB, Belevich I, Bloch DA, Mogi T, Verkhovsky MI. Glutamate 107 in subunit I of cytochrome bd from Escherichia coli is part of a transmembrane intraprotein pathway conducting protons from the cytoplasm to the heme b595/heme d active site. Biochemistry. 2008;47:7907–7914. doi: 10.1021/bi800435a. [DOI] [PubMed] [Google Scholar]
- 11.Baughn AD, Malamy MH. The strict anaerobe Bacteroides fragilis grows in and benefits from nanomolar concentrations of oxygen. Nature. 2004;427:441–444. doi: 10.1038/nature02285. [DOI] [PubMed] [Google Scholar]
- 12.Shi L, Sohaskey CD, Kana BD, Dawes S, North RJ, Mizrahi V, Gennaro ML. Changes in energy metabolism of Mycobacterium tuberculosis in mouse lung and under in vitro conditions affecting aerobic respiration. Proc. Natl. Acad. Sci. USA. 2005;102:15629–15634. doi: 10.1073/pnas.0507850102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Loisel-Meyer S, Jimenez de Bagues MP, Kohler S, Liautard JP, Jubier-Maurin V. Differential use of the two high-oxygen-affinity terminal oxidases of Brucella suis for in vitro and intramacrophagic multiplication. Infect. Immun. 2005;73:7768–7771. doi: 10.1128/IAI.73.11.7768-7771.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jones SA, Chowdhury FZ, Fabich AJ, Anderson A, Schreiner DM, House AL, Autieri SM, Leatham MP, Lins JJ, Jorgensen M, Cohen PS, Conway T. Respiration of Escherichia coli in the mouse intestine. Infect. Immun. 2007;75:4891–4899. doi: 10.1128/IAI.00484-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hill S, Viollet S, Smith AT, Anthony C. Roles for enteric d-type cytochrome oxidase in N2 fixation and microaerobiosis. J. Bacteriol. 1990;172:2071–2078. doi: 10.1128/jb.172.4.2071-2078.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Way SS, Sallustio S, Magliozzo RS, Goldberg MB. Impact of either elevated or decreased levels of cytochrome bd expression on Shigella flexneri virulence. J. Bacteriol. 1999;181:1229–1237. doi: 10.1128/jb.181.4.1229-1237.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Endley S, McMurray D, Ficht TA. Interruption of the cydB locus in Brucella abortus attenuates intracellular survival and virulence in the mouse model of infection. J. Bacteriol. 2001;183:2454–2462. doi: 10.1128/JB.183.8.2454-2462.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Borisov VB, Forte E, Konstantinov AA, Poole RK, Sarti P, Giuffre A. Interaction of the bacterial terminal oxidase cytochrome bd with nitric oxide. FEBS Lett. 2004;576:201–204. doi: 10.1016/j.febslet.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 19.Borisov VB, Forte E, Sarti P, Brunori M, Konstantinov AA, Giuffre A. Redox control of fast ligand dissociation from Escherichia coli cytochrome bd. Biochem. Biophys. Res. Commun. 2007;355:97–102. doi: 10.1016/j.bbrc.2007.01.118. [DOI] [PubMed] [Google Scholar]
- 20.Mason MG, Shepherd M, Nicholls P, Dobbin PS, Dodsworth KS, Poole RK, Cooper CE. Cytochrome bd confers nitric oxide resistance to Escherichia coli. Nat. Chem. Biol. 2009;5:94–96. doi: 10.1038/nchembio.135. [DOI] [PubMed] [Google Scholar]
- 21.Forte E, Borisov VB, Konstantinov AA, Brunori M, Giuffre A, Sarti P. Cytochrome bd a key oxidase in bacterial survival and tolerance to nitrosative stress. Ital. J. Biochem. 2007;56:265–269. [PubMed] [Google Scholar]
- 22.Borisov VB, Forte E, Sarti P, Brunori M, Konstantinov AA, Giuffre A. Nitric oxide reacts with the ferryl-oxo catalytic intermediate of the CuB-lacking cytochrome bd terminal oxidase. FEBS Lett. 2006;580:4823–4826. doi: 10.1016/j.febslet.2006.07.072. [DOI] [PubMed] [Google Scholar]
- 23.Borisov VB, Forte E, Giuffre A, Konstantinov A, Sarti P. Reaction of nitric oxide with the oxidized di-heme and heme-copper oxygen-reducing centers of terminal oxidases: Different reaction pathways and end-products. J. Inorg. Biochem. 2009;103:1185–1187. doi: 10.1016/j.jinorgbio.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 24.Bader M, Muse W, Ballou DP, Gassner C, Bardwell JCA. Oxidative protein folding is driven by the electron transport system. Cell. 1999;98:217–227. doi: 10.1016/s0092-8674(00)81016-8. [DOI] [PubMed] [Google Scholar]
- 25.Borisov VB, Davletshin AI, Konstantinov AA. Peroxidase activity of cytochrome bd from Escherichia coli. Biochemistry (Moscow) 2010;75:428–436. doi: 10.1134/s000629791004005x. (translated from Biokhimiya (in Russian) (2010), 75, 520–530). [DOI] [PubMed] [Google Scholar]
- 26.Green GN, Fang H, Lin R-J, Newton G, Mather M, Georgiou CD, Gennis RB. The nucleotide sequence of the cyd locus encoding the two subunits of the cytochrome d terminal oxidase complex of Escherichia coli. J. Biol. Chem. 1988;263:13138–13143. [PubMed] [Google Scholar]
- 27.Poole RK. Oxygen reactions with bacterial oxidases and globins: binding, reduction and regulation. Anthonie van Leeuwenhoek. 1994;65:289–310. doi: 10.1007/BF00872215. [DOI] [PubMed] [Google Scholar]
- 28.Mogi T, Tsubaki M, Hori H, Miyoshi H, Nakamura H, Anraku Y. Two terminal quinol oxidase families in Escherichia coli: variations on molecular machinery for dioxygen reduction. J. Biochem. Mol. Biol. Biophys. 1998;2:79–110. [Google Scholar]
- 29.Junemann S, Wrigglesworth JM. Antimycin inhibition of the cytochrome bd complex from Azotobacter vinelandii indicates the presence of a branched electron transfer pathway for the oxidation of ubiquinol. FEBS Lett. 1994;345:198–202. doi: 10.1016/0014-5793(94)00372-6. [DOI] [PubMed] [Google Scholar]
- 30.Spinner F, Cheesman MR, Thomson AJ, Kaysser T, Gennis RB, Peng Q, Peterson J. The haem b558 component of the cytochrome bd quinol oxidase complex from Escherichia coli has histidine-methionine axial ligation. Biochem. J. 1995;308:641–644. doi: 10.1042/bj3080641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Belevich I, Borisov VB, Konstantinov AA, Verkhovsky MI. Oxygenated complex of cytochrome bd from Escherichia coli: stability and photolability. FEBS Lett. 2005;579:4567–4570. doi: 10.1016/j.febslet.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 32.Belevich I, Borisov VB, Bloch DA, Konstantinov AA, Verkhovsky MI. Cytochrome bd from Azotobacter vinelandii: evidence for high-affinity oxygen binding. Biochemistry. 2007;46:11177–11184. doi: 10.1021/bi700862u. [DOI] [PubMed] [Google Scholar]
- 33.Poole RK, Williams HD. Proposal that the function of the membrane-bound cytochrome a1-like haemoprotein (cytochrome b-595) in Escherichia coli is a direct electron donation to cytochrome d. FEBS Lett. 1987;217:49–52. doi: 10.1016/0014-5793(87)81240-1. [DOI] [PubMed] [Google Scholar]
- 34.Kobayashi K, Tagawa S, Mogi T. Electron transfer process in cytochrome bd-type ubiquinol oxidase from Escherichia coli revealed by pulse radiolysis. Biochemistry. 1999;38:5913–5917. doi: 10.1021/bi982088n. [DOI] [PubMed] [Google Scholar]
- 35.Krasnoselskaya I, Arutjunjan AM, Smirnova I, Gennis R, Konstantinov AA. Cyanidereactive sites in cytochrome bd complex from E. coli. FEBS Lett. 1993;327:279–283. doi: 10.1016/0014-5793(93)81004-j. [DOI] [PubMed] [Google Scholar]
- 36.Hill JJ, Alben JO, Gennis RB. Spectroscopic evidence for a heme-heme binuclear center in the cytochrome bd ubiquinol oxidase from Escherichia coli. Proc. Natl. Acad. Sci. USA. 1993;90:5863–5867. doi: 10.1073/pnas.90.12.5863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tsubaki M, Hori H, Mogi T, Anraku Y. Cyanide-binding site of bd-type ubiquinol oxidase from Escherichia coli. J. Biol. Chem. 1995;270:28565–28569. doi: 10.1074/jbc.270.48.28565. [DOI] [PubMed] [Google Scholar]
- 38.Borisov V, Arutyunyan AM, Osborne JP, Gennis RB, Konstantinov AA. Magnetic circular dichroism used to examine the interaction of Escherichia coli cytochrome bd with ligands. Biochemistry. 1999;38:740–750. doi: 10.1021/bi981908t. [DOI] [PubMed] [Google Scholar]
- 39.Vos MH, Borisov VB, Liebl U, Martin J-L, Konstantinov AA. Femtosecond resolution of ligand-heme interactions in the high-affinity quinol oxidase bd: A di-heme active site? Proc. Natl. Acad. Sci. USA. 2000;97:1554–1559. doi: 10.1073/pnas.030528197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Borisov VB, Sedelnikova SE, Poole RK, Konstantinov AA. Interaction of cytochrome bd with carbon monoxide at low and room temperatures: evidence that only a small fraction of heme b595 reacts with CO. J. Biol. Chem. 2001;276:22095–22099. doi: 10.1074/jbc.M011542200. [DOI] [PubMed] [Google Scholar]
- 41.Borisov VB, Liebl U, Rappaport F, Martin J-L, Zhang J, Gennis, R.B, Konstantinov AA, Vos MH. Interactions between heme d and heme b595 in quinol oxidase bd from Escherichia coli: a photoselection study using femtosecond spectroscopy. Biochemistry. 2002;41:1654–1662. doi: 10.1021/bi0158019. [DOI] [PubMed] [Google Scholar]
- 42.Hori H, Tsubaki M, Mogi T, Anraku Y. EPR study of NO complex of bd-type ubiquinol oxidase from Escherichia coli. J. Biol. Chem. 1996;271:9254–9258. doi: 10.1074/jbc.271.16.9254. [DOI] [PubMed] [Google Scholar]
- 43.Arutyunyan AM, Borisov VB, Novoderezhkin VI, Ghaim J, Zhang J, Gennis RB, Konstantinov AA. Strong excitonic interactions in the oxygen-reducing site of bd-type oxidase: the Fe-to-Fe distance between hemes d and b595 is 10 A. Biochemistry. 2008;47:1752–1759. doi: 10.1021/bi701884g. [DOI] [PubMed] [Google Scholar]
- 44.Bloch DA, Borisov VB, Mogi T, Verkhovsky MI. Heme/heme redox interaction and resolution of individual optical absorption spectra of the hemes in cytochrome bd from Escherichia coli. Biochim. Biophys. Acta. 2009;1787:1246–1253. doi: 10.1016/j.bbabio.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 45.Rothery RA, Houston AM, Ingledew WJ. The respiratory chain of anaerobically grown Escherichia coli: Reactions with nitrite and oxygen. J. Gen. Microbiol. 1987;133:3247–3255. doi: 10.1099/00221287-133-11-3247. [DOI] [PubMed] [Google Scholar]
- 46.D'mello R, Hill S, Poole RK. The Cytochrome bd quinol oxidase in Escherichia coli has an extremely high oxygen affinity and two-oxygen-binding haems: implicaitons for regulation of activity in vivo by oxygen inihibition. Microbiology. 1996;142:755–763. doi: 10.1099/00221287-142-4-755. [DOI] [PubMed] [Google Scholar]
- 47.Zhang J, Hellwig P, Osborne JP, Huang HW, Moenne-Loccoz P, Konstantinov AA, Gennis RB. Site-directed mutation of the highly conserved region near the Q-loop of the cytochrome bd quinol oxidase from Escherichia coli specifically perturbs heme b595. Biochemistry. 2001;40:8548–8556. doi: 10.1021/bi010469m. [DOI] [PubMed] [Google Scholar]
- 48.Kaysser TM, Ghaim JB, Georgiou C, Gennis RB. Methionine-393 is an axial ligand of the heme b558 component of the cytochrome bd ubiquinol oxidase from Escherichia coli. Biochemistry. 1995;34:13491–13501. doi: 10.1021/bi00041a029. [DOI] [PubMed] [Google Scholar]
- 49.Joliot P, Beal D, Frilley B. Une nouvelle methode spectrophotometrique destinee a l'etude des reactions photosynthetiques. Journal de chimie physique. 1980;77:209–216. [Google Scholar]
- 50.Beal D, Rappaport F, Joliot P. A new high-sensitivity 10-ns time-resolution spectrophotometric technique adapted to in vivo analysis of the photosynthetic apparatus. Rev. Sci. Instrum. 1999;70:202–207. [Google Scholar]
- 51.Koland JG, Miller MJ, Gennis RB. Potentiometric analysis of the purified cytochrome d terminal oxidase complex from Escherichia coli. Biochemistry. 1984;23:1051–1056. [Google Scholar]
- 52.Lorence RM, Koland JG, Gennis RB. Coulometric and spectroscopic analysis of the purified cytochrome d complex of Escherichia coli: Evidence for the identification of "cytochrome a1" as cytochrome b595. Biochemistry. 1986;25:2314–2321. doi: 10.1021/bi00357a003. [DOI] [PubMed] [Google Scholar]
- 53.Hill BC, Hill JJ, Gennis RB. The room temperature reaction of carbon monoxide and oxygen with the cytochrome bd quinol oxidase from Escherichia coli. Biochemistry. 1994;33:15110–15115. doi: 10.1021/bi00254a021. [DOI] [PubMed] [Google Scholar]
- 54.Junemann S, Rich PR, Wrigglesworth JM. CO flash photolysis of cytochrome bd from Azotobacter vinelandii. Biochem. Soc. Trans. 1995;23:157S. doi: 10.1042/bst023157s. [DOI] [PubMed] [Google Scholar]
- 55.Junemann S, Wrigglesworth JM. Cytochrome bd oxidase from Azotobacter vinelandii. Purification and quantitation of ligand binding to the oxygen reduction site. J. Biol. Chem. 1995;270:16213–16220. doi: 10.1074/jbc.270.27.16213. [DOI] [PubMed] [Google Scholar]
- 56.Borisov VB. Interaction of bd-type quinol oxidase from Escherichia coli and carbon monoxide: Heme d binds CO with high affinity. Biochemistry (Moscow) 2008;73:14–22. doi: 10.1134/s0006297908010021. (translated from Biokhimiya (in Russian) (2008), 73, 18–28). [DOI] [PubMed] [Google Scholar]
- 57.Muntyan MS, Bloch DA, Drachev LA, Skulachev VP. Kinetics of CO binding to putative Na+-motive oxidases of the o-type from Bacillus FTU and of the d-type from Escherichia coli. FEBS Lett. 1993;327:347–350. doi: 10.1016/0014-5793(93)81018-u. [DOI] [PubMed] [Google Scholar]
- 58.Junemann S, Wrigglesworth JM, Rich PR. Effects of decyl-aurachin D and reversed electron transfer in cytochrome bd. Biochemistry. 1997;36:9323–9331. doi: 10.1021/bi970055m. [DOI] [PubMed] [Google Scholar]
- 59.Lorence RM, Miller MJ, Borochov A, Faiman-Weinberg R, Gennis RB. Effects of pH and detergent on the kinetic and electrochemical properties of the purified cytochrome d terminal oxidase complex of Escherichia coli. Biochim. Biophys. Acta. 1984;790:148–153. doi: 10.1016/0167-4838(84)90218-8. [DOI] [PubMed] [Google Scholar]