Abstract
Objectives
This study assesses practice variation of secondary prevention medication prescription among coronary artery disease (CAD) patients treated in outpatient practices participating in the NCDR® PINNACLE Registry®.
Background
Among patients with CAD, secondary prevention with a combination of beta-blockers, angiotensin converting enzyme inhibitors/angiotensin receptor blockers, and statins reduces cardiac mortality and myocardial infarction (MI). Accordingly, every CAD patient should receive the combination of these medications for which they are eligible. However, little is known about current prescription patterns of these medications and the variation in use among outpatient cardiology clinics.
Methods
Using data from NCDR® PINNACLE Registry®, a national outpatient cardiology practice registry, we assessed medication prescription patterns among eligible CAD patients between July 2008 and December 2010. Overall rates of prescription and variation by practice were calculated, adjusting for patient characteristics.
Results
Among 156,145 CAD patients in 58 practices, 103,830 (66.5%) were prescribed the optimal combination of medications for which they were eligible. The median rate of optimal combined prescription by practice was 73.5% and varied from 28.8% to 100%. After adjustment for patient factors, the practice median rate ratio for prescription was 1.25 (95% CI 1.2,1.32), indicating a 25% likelihood that 2 random practices would differ in treating identical CAD patients.
Conclusions
Among a national registry of CAD patients treated in outpatient cardiology practices, over one-third of patients failed to receive their optimal combination of secondary prevention medications. Significant variation was observed across practices, even after adjusting for patient characteristics, suggesting that quality improvement efforts may be needed to support more uniform practice.
Keywords: CAD, Outpatient Practice, Secondary Prevention
INTRODUCTION
Among patients with coronary artery disease (CAD), secondary prevention with a combination of anti-platelets, beta-blockers (BB), angiotensin converting enzyme inhibitors (ACEI)/angiotensin receptor blockers (ARB), and statins reduces cardiac mortality and myocardial infarction (MI) (1). Accordingly, clinical guidelines and performance measures call for the prescription of these medications to all eligible patients (1, 2). However, the translation of these recommendations to clinical practice is poorly understood (3-5).
One important factor in optimal secondary prevention may be the outpatient cardiology practice where CAD patients are treated. Prevention efforts are a major focus of care in this setting, and longitudinal therapeutic relationships are often established between the cardiac clinician and patient. Therefore, the opportunity and motivation to provide optimal secondary prevention in outpatient cardiology practices is strong. However, little is known about secondary prevention medication prescription patterns among CAD patients in the outpatient setting. Understanding these patterns and any variations in care can help identify higher performing practices, whose techniques for delivering optimal care can be better understood and potentially generalized to all practices.
To understand outpatient practice patterns, we analyzed data from the NCDR® PINNACLE Registry®, the largest outpatient quality improvement registry of patients treated in ambulatory cardiology clinics in the U.S. We characterized practice patterns and variation in secondary prevention medication prescription rates and assessed the impact of practice site on the optimal prescription of these medications after accounting for patient factors.
METHODS
Data Source
Our analysis used data from the NCDR PINNACLE Registry. PINNACLE is the first national, prospective, outpatient-based cardiac quality improvement registry of patients seen in cardiology practices in the United States (6,7). The American College of Cardiology Foundation (ACCF) launched the registry in 2008. Participating practices collect patient data at the point of care for each outpatient visit. Patient data include demographics, co-morbidities, symptoms, vital signs, medications, contraindications to medications, and laboratory values. Data elements are collected either by PINNACLE paper-based case report forms or export of a practice's electronic health record (EHR) to comprehensively capture the requisite data elements for PINNACLE program participation (6). Data collection is standardized through written definitions, uniform data entry and transmission requirements, and data quality checks.
Study Population and Patient Eligibility
For our study, we assessed PINNACLE patient and practice data collected during index clinic visits of CAD patients between July 2008 and December 2010. CAD was defined by the treating clinician, and included prior history of MI, percutaneous coronary intervention (PCI), or coronary artery bypass grafting (CABG). We defined patient eligibility for three secondary prevention medication classes – BB, ACEI/ARB, and statins - in accordance with the 2011 ACCF/American Heart Association Task Force on Performance Measures and American Medical Association-Physician Consortium for Performance Improvement (2). We elected to not measure anti-platelet use since over-the-counter medications such as aspirin are incompletely captured in practice EHRs and the PINNACLE registry. Eligibility included BB therapy for those with either a prior MI or a left ventricular ejection fraction (LVEF)<40%, ACEI/ARB therapy for those with either diabetes mellitus (DM) or a LVEF<40%, and statin therapy in those with an LDL≥100 mg/dL. Although this criterion for statin use has been expanded to include patients with all levels of LDL, we elected to use the more restrictive definition to conform to the 2011 performance measures guidelines. In addition, we elected to use the 2011 performance measure for BB use, though recent guidelines indicate that its use is optional for those patients who had a remote (>3 years) uncomplicated MI. Patients with documented medical, patient, or system reasons for not being prescribed any of the studied medication classes were excluded from analyses for that particular class and clinical encounter. If there were no documented reasons for not prescribing a medication, the patient was considered eligible for receiving the medication. We also excluded data from practices that had fewer than 10 eligible CAD patients, since sample sizes this small cannot be sufficiently modeled in regression analyses. For each medication class, we also excluded those practices with treatment rates of 0%, since this likely represented inadequate documentation of medication prescription rather than actual failures of treatment.
Outcomes
We analyzed combined and individual prescription rates of the three medication classes among eligible patients at the patient and practice level (2). Evidence of prescription was collected from the practice case report form or EHR. Combined prescription rates were calculated as the percentage of fulfilled “quality opportunities”, using the method defined by the Joint Commission (8). These were calculated by dividing the number of medications prescribed over the number of medications for which the individual patient was eligible. Thus, a patient eligible for two of the three medication classes who received both prescriptions would have a combined rate of 100%, while a patient eligible for all three medication classes who received two prescriptions would have a combined rate of 67%. Optimal combined prescription was defined as fulfilling 100% of prescription opportunities.
Our primary analyses were based on the first encounter of each patient, since this represents the first opportunity that the clinician has to prescribe the optimal combination of medications. However, we recognize that many clinicians choose to prescribe these medications sequentially and over time, rather than in a single visit, in order to avoid side effects or other complications of therapy. Therefore, we conducted a sensitivity analysis of the outcome to account for prescription of the medication class at any visit over the year following the index visit. Since the last date for an index clinic visit included in our cohort was December 2010, all patients had at least one year of elapsed time after their index visit. This approach can cause some variation in the number of eligible patients compared to the first encounter analysis, since patients can become eligible or ineligible for different therapies at visits after their index visit (e.g. new diagnosis of DM or development of statin-induced myopathy).
Covariates
Patient factors that could affect prescription patterns were identified based on prior studies and clinical reasoning (5,6,9). These included demographics (age, gender, insurance payer) and clinical characteristics (dyslipidemia, hypertension (HTN), DM, current smoker, peripheral arterial disease (PAD), atrial fibrillation or flutter (AF), history of stroke or transient ischemic attack (TIA), MI within the prior year, angina, CABG within the prior year, PCI within the prior year, and heart failure (HF)). These variables were collected for description and inclusion in our regression models as covariates.
Statistical Analysis
After specifying our study cohort, we grouped patients by receipt of optimal combined medication prescription and compared their characteristics using t-tests for continuous variables and chi-square tests for categorical variables. Next, median practice rates of optimal combined and individual medication prescription were determined for each practice and examined with descriptive plots. Practice characteristics, stratified by practice prescription rates above and below the median combined rate of medication prescription, were compared using t-tests for continuous variables and chi-square tests for categorical variables.
To control for differential distribution of patient factors among practices, multivariable regression models for optimal combined and individual prescription rates were constructed. Practice was modeled as a random, rather than fixed, effect. Hierarchical modeling techniques were used to account for clustering of patients by practice. Variation in prescription rates by practice site was calculated using a median rate ratio (MRR). The MRR estimates the differences between prescription patterns for identical patients between two randomly selected practices. It can be interpreted as the likelihood ratio that the same patient would receive a different combination of prescriptions at two different practices in our cohort (10,11). An MRR of 1.0 indicates that no variation exists between practices. Thus, the MRR is always ≥1. For example, a MRR of 1.5 indicates a 50% likelihood that a similar patient would receive different prescriptions at two different practices. It provides an estimate of the effect size of the practice on the outcome, much as the odds ratio estimates the effect size of patient factors on the outcome (11,12). Based on prior literature, a MRR >1.2 indicates clinically significant variation (13).
Significant (>5%) missing data rates in our cohort included 18.5% for smoking status, 5.7% for CABG within the prior year, 5.7% for PCI within the prior year, 5.6% for MI within the prior year, and 5.4% for insurance status. In order to avoid case-wise deletion of those cases with missing data points, we modeled a separate “unknown” category for each of these data elements and included those categories in our analysis.
Secondary Analyses
We conducted several secondary analyses to further explore our primary findings. First, we hypothesized that practices with greater proportions of CAD patients would have higher optimal prescription rates, potentially due to improved familiarity and expertise in CAD treatment. Therefore, we entered the practice proportion of CAD patients in our regression models. Second, we hypothesized that participation in the PINNACLE registry would also lead to higher prescription rates, since the registry is a quality improvement initiative that provides quarterly performance reports to participating practices. Accordingly, we analyzed the subset of practices that submit data to PINNACLE using EHRs (n=29) since these practices had both pre-PINNACLE and post-PINNACLE participation data for analysis. We compared prescription rates among these practices before and after PINNACLE participation, as defined by the date when the practice entered into its contract with PINNACLE. Third, we hypothesized that practices with longer participation in PINNACLE, and thus more information about their prescription rate performance and opportunities to improve it, would have higher prescription rates. Thus, we included PINNACLE participation in our models, measured as days from the date of the contractual agreement between the practice and PINNACLE. Fourth, we recognized that better prescription rates among practices might actually represent better documentation, rather than truly better performance. Therefore, in line with prior studies, we measured the correlation between practice performance rates and documentation of contraindications to medications (13). If better performance were due to better documentation, then we would expect higher correlation between performance rates and documented medication exclusions. Conversely, the absence of any correlation would suggest that better performance is unlikely to be attributable to better documentation. Fifth, we hypothesized that larger practices may have different rates of prescription performance than smaller practices. Thus, we conducted a correlation analysis between practice size and prescription performance. We also included practice size as a covariate in our primary regression model.
All analyses were performed using the SAS version 9.1 (SAS Institute, Cary, NC). The authors had full access to the data and take full responsibility for the integrity of the data.
RESULTS
Combined prescription rates
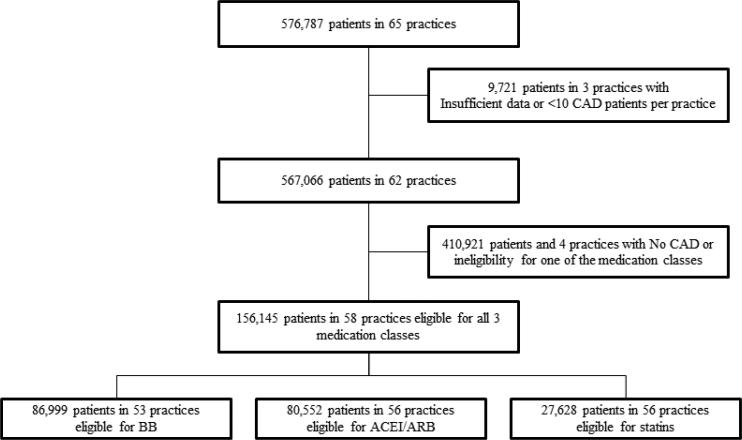
Between 2008 and 2010, the PINNACLE registry contained data on 576,787 patients seen in 65 practices. After exclusion of patients without CAD (see Figure 1) and practices with <10 eligible CAD patients, our final study cohort of patients eligible for all 3 medication types was 156,145 treated in 58 practices.
Figure 1. Study Cohort Creation.
Study cohort creation identifying PINNACLE patients with CAD and eligibility for secondary prevention medication prescription
Among this cohort, 103,830 (66.5%) patients had their optimal combination of medications prescribed at their index clinic visit. This percentage increased to 69.7% (121662/174586) after accounting for all visits among eligible patients occurring within the year following the index visit. These patients, as compared to those who were not prescribed their optimal combination of medications, were older, more likely male, and more likely insured (Table 1). They had higher rates of dyslipidemia, HTN, PAD, and prior MI; but lower rates of DM, stroke, and PCI.
Table 1.
Patient Characteristics by Optimal Combined Prescriptions of Secondary Prevention Medications
| Optimal Combination of anti-platelet, BB, ACEI/ARB, and Statin N=156,145 |
|||
|---|---|---|---|
| Yes n = 103830 (66.5%) | No n =52315 (33.5%) | P-Value | |
| Age | 68.0 +/− 11.9 | 67.4 +/−13.4 | <0.001 |
| Male | 65797 (63.5%) | 29347 (56.2%) | <0.001 |
| Race | <0.001 | ||
| White | 41095 (39.6%) | 19161 (36.6%) | |
| Black | 4694 (4.5%) | 2330 (4.5%) | |
| Other | 406 (0.4%) | 204 (0.4%) | |
| Missing | 57635 (55.5%) | 30620 (58.5%) | |
| Insurance | <0.001 | ||
| None | 3810 (3.7%) | 2106 (4.0%) | |
| Private | 54056 (52.1%) | 30401 (58.1%) | |
| Public | 38751 (37.3%) | 16782 (32.1%) | |
| Unknown | 7213 (6.9%) | 3026 (5.8%) | |
| Current Tobacco Use | <0.001 | ||
| Yes | 15016 (14.5%) | 7449 (14.2%) | |
| No | 74344 (71.6%) | 36225 (69.2%) | |
| Unknown | 14470 (13.9%) | 8641 (16.5%) | |
| Heart Failure | 39387 (37.9%) | 17747 (33.9%) | <0.001 |
| Dyslipidemia | 84539 (81.4%) | 38283 (73.2%) | <0.001 |
| Diabetes | 45978 (44.3%) | 25702 (49.1%) | <0.001 |
| Hypertension | 85954 (82.8%) | 39886 (76.3%) | <0.001 |
| PAD | 20007 (19.3%) | 8811 (16.8%) | <0.001 |
| Prior Stroke/TIA | 15000 (14.4%) | 7641 (14.6%) | 0.397 |
| Angina | 14747 (14.2%) | 5932 (11.4%) | <0.001 |
| Atrial Fibrillation | 18184 (17.5%) | 9359 (17.9%) | 0.065 |
| PCI within 12 months | <0.001 | ||
| Yes | 22489 (21.7%) | 9246 (17.7%) | |
| No | 75137 (72.4%) | 39475 (75.5%) | |
| Unknown | 6204 (6.0%) | 3594 (6.9%) | |
| CABG within 12 months | <0.001 | ||
| Yes | 14213 (13.7%) | 9579 (18.3%) | |
| No | 83360 (80.3%) | 39128 (74.8%) | |
| Unknown | 6257 (6.0%) | 3608 (6.9%) | |
| MI within 12 months | <0.001 | ||
| Yes | 24986 (24.1%) | 14351 (27.4%) | |
| No | 73117 (70.4%) | 34462 (65.9%) | |
| Unknown | 5727 (5.5%) | 3502 (6.7%) | |
*PAD: Peripheral Arterial Disease
†TIA: Transient Ischemic Attack
‡PCI: Percutaneous Coronary Intervention
§CABG: Coronary Artery Bypass Graft
∥MI: Myocardial Infarction
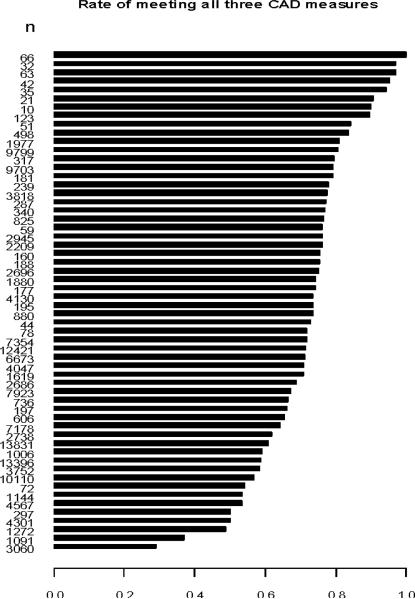
Among the 58 practices in our cohort, the median practice prescription rate of the optimal combination of medications was 73.5%. Rates varied from 28.8% to 100% (Figure 2). After including all visits among eligible patients in the year following the index visit, the median prescription rate increased to 75.1% and ranged from 46.1% to 100%. Between practices above and below the median prescription rate, number and type of providers in a practice, length of practice's PINNACLE participation, and geographical location of the practice were similar (Table 2).
Figure 2. Optimal Combined Prescriptions of Secondary Prevention Medications by Practice.
Among the 58 practices in our study cohort, rates of optimal combined prescription of secondary prevention medications ranges from 28.8% to 100%. The median rate of prescription was 73.5%.
Table 2.
Practice Characteristics by Median Rates of Optimal Combined Prescription of Secondary Prevention Medications
| Practice Characteristics by Aggregate Prescription of CAD Medications | ||||
|---|---|---|---|---|
| CAD Meds Fulfillment Rate Above Median | CAD Meds Fulfillment Rate Below Median | P-value | ||
| Practice Characteristic | n = 29 | n = 29 | ||
| Number MDs (Median (IQR)), | 2.0 (1.0, 11.0) | 11.0 (3.0, 21.0) | 0.170 | |
| Number of NPs (Median (IQR)) | 0.0 (0.0, 0.0) | 0.0 (0.0, 0.0) | 0.858 | |
| Number of providers (Median (IQR)) | 2.0 (1.0, 14.0) | 13.0 (3.0, 27.0) | 0.229 | |
| Months of PINNACLE registry participation, Median (IQR) | 21.0 (12.0, 24.0) | 12.0 (12.0, 21.0) | 0.067 | |
| Practice location, n (%) | Urban | 24 (82.8%) | 27 (93.1%) | 0.170 |
| Rural | 5 (17.2%) | 2 (6.9%) | ||
After adjustment for patient factors, significant practice variation in optimal prescribing patterns of combined medications was present (MRR 1.25 (95% CI 1.2, 1.32)). After adjustment, patient factors significantly associated with higher rates of optimal prescription were male gender, dyslipidemia, HTN, PAD, PCI performed within the prior year, and heart failure (Table 3). Patient factors significantly associated with lower prescription rates were prior stroke/TIA, MI occurrence within the prior year, and DM.
Table 3.
Practice and Patient Factors Associated with Optimal Combined Prescriptions of Secondary Prevention Medications Following Multivariable Adjustment
| Optimal Combination of anti-platelet, BB, ACEI/ARB, and Statin | |
|---|---|
| Rate Ratio (95% CI) | |
| Practice Median Rate Ratio | 1.25 (1.20, 1.32)* |
| Age: 65 -< 75 | 1.02 (1.00, 1.04) |
| Age: >= 75 | 0.99 (0.96, 1.02) |
| Male | 1.09 (1.06, 1.11)* |
| Insurance: Private | 1.01 (0.96, 1.05) |
| Insurance: Public | 1.02 (0.99, 1.06) |
| Insurance: Unknown | 1.04 (0.96, 1.12) |
| A-Fib | 0.99 (0.96, 1.02) |
| Dyslipidemia | 1.15 (1.08, 1.23)* |
| Hypertension | 1.16 (1.10, 1.21)* |
| Diabetes Mellitus | 0.89 (0.86, 0.92)* |
| Current Smoker: Yes | 1.01 (0.99, 1.02) |
| Current Smoker: Unknown | 0.96 (0.92, 1.00) |
| PAD | 1.07 (1.02, 1.12)* |
| Prior Stroke/TIA | 0.96 (0.94, 0.97)* |
| MI w/in 12 Months: Yes | 0.94 (0.90, 0.97)* |
| MI w/in 12 Months: Unknown | 0.84 (0.68, 1.03) |
| Angina | 1.02 (0.99, 1.06) |
| CABG w/in 12 Months: Yes | 0.90 (0.78, 1.04) |
| CABG w/in 12 months: Unknown | 0.85 (0.76, 0.94)* |
| PCI w/in 12 Months: Yes | 1.08 (1.05, 1.11)* |
| PCI w/in 12 Months: Unknown | 0.99 (0.91, 1.08) |
| Heart Failure | 1.05 (1.02, 1.08)* |
P-value <0.05
†PAD: Peripheral Arterial Disease
‡TIA: Transient Ischemic Attack
§MI: Myocardial Infarction
∥CABG: Coronary Artery Bypass Graft
¶PCI: Percutaneous Coronary Intervention
Individual medication prescription rates
Among patients eligible for individual medication classes, beta-blockers were prescribed in 73.3% (63,800/86,999), ACEI/ARBs in 69.4% (55,933/80,552), and statins in 68.2% (18,833/27,628). After inclusion of all visits among eligible patients occurring within the year following the index visit, these rates increased to 77.3%, 72.3%, and 72.5%, respectively. Unadjusted patient factors associated with prescription are listed in Supplemental Tables 1-3.
Among practices, the median prescription rate of beta-blockers was 78.4% (range 35.2-100%), ACEI/ARBs 75.5% (range 39.1-100%), and statins 73.3% (range 10.7-100%) (Supplemental Figures 1-3). After inclusion of all visits among eligible patients occurring within the year following the index visit, these values increased to 79.4% (range 46.2-100%) for beta-blockers, 78.1% (range 45.4-100%) for ACEI/ARBs, and 77.7% (range 41.8-100%) for statins.
After adjustment, practice was significantly associated with prescription of all medication classes. Adjusted MRRs were 1.22 (95% CI 1.18, 1.28) for beta-blockers, 1.19 (95% CI 1.16, 1.26) for ACEI/ARBs, and 1.31 (95% CI 1.24, 1.44) for statins (Supplemental Table 4). Adjusted patient characteristics associated with individual medications are also listed in Supplemental Table 4.
Secondary analyses
We conducted five secondary analyses. First, we assessed the impact of the proportion of patients with CAD within a practice on practice prescription patterns. The median proportion of patients with CAD among practices was 58.8%. After adjustment, practices with a greater proportion of CAD patients did not have significantly better prescribing patterns than practices with a lower proportion of CAD patients. Second, we compared optimal combined prescription rates before PINNACLE participation to rates after participation among the 29 PINNACLE practices with data available before and after participation in PINNACLE. After adjustment, practices were 14% less likely to prescribe the optimal combination of medications in eligible patients prior to, compared to after, PINNACLE participation (HR 0.86, 95% CI 0.75, 0.98). Thus, PINNACLE participation among this subset of practices appears to significantly contribute to documented practice prescription patterns of optimal medications. Third, we assessed the impact of length of participation in PINNACLE on prescription patterns. The median length of PINNACLE participation was 13.5 months. After adjustment, length of participation was not associated with an increased likelihood of aggregate medication prescription. Fourth, we compared correlations between documentation rates of medication contraindications and prescription rates. The correlation for beta-blockers was 0.35 (p-value 0.01), ACEI/ARBs 0.09 (p-value 0.5), and statins 0.2 (p-value 0.18). The only significant correlation occurred among beta-blockers, suggesting that better documentation may partially explain some the beta-blocker prescription patterns. However, among the other two medication classes, better documentation does not appear to play a meaningful role in prescription performance. Fifth, we assessed the impact of practice size on prescription patterns. The correlation between the two variables was - 0.04 (p-value = 0.79), indicating no significant relationship. After adjustment, practice size was not associated with an increased likelihood of combined medication prescription (OR 0.99 95% CI 0.99, 1.0).
DISCUSSION
Among U.S. cardiology practices participating in the NCDR PINNACLE registry, we found that over one-third of patients with CAD were not prescribed an optimal combination of BB, ACEI/ARBs, and statins. In addition, practice prescription patterns varied widely. After adjustment for patient factors, there was 25% likelihood that different practices had different prescription patterns for similar patients. Collectively, our findings suggest that prescription of evidence-based CAD therapies is neither optimal nor uniform and offer insights into opportunities to further improve care among outpatients with CAD.
Prior studies of secondary prevention prescribing practices in the ambulatory setting have illustrated substantial, but improving, gaps in care. Substantial improvements have been made in prescription of these medications at the point of hospital discharge, but similar gains in the ambulatory setting - where the vast majority of CAD prevention efforts occur – were slower to follow (14,15). Stafford and colleagues, in a 2001 survey of outpatient medical practices, found that rates of aspirin and beta-blocker use among CAD patients were well below 50% (5). Several years later, the International Reduction of Atherothrombosis for Continued Health (REACH) registry collected data on ambulatory care patterns among patients with or at risk for cardiovascular disease (3,9,16)., Among U.S. patients, 77% of patients were prescribed anti-platelets, 51.8% beta-blockers, 49.5% ACEIs, and 76.9% statins (9). Follow-up assessments four years later found no significant change in rates (16). Most recently, Chan and colleagues, using 2008-09 data from the PINNACLE registry, found higher rates of secondary prevention medication prescription among CAD patients (6). In their analysis, 84.9% of patients were receiving anti-platelets, 86.4% beta-blockers, 72.4% ACEI/ARBs, and 84.3% lipid-lowering agents.
Our PINNACLE study demonstrated lower rates of medication prescription than those noted by Chan and colleagues, potentially due to the inclusion of a larger and more heterogeneous patient and practice population. We also focused on the combined prescription of all eligible medications to patients, rather than just individual classes, as a more accurate reflection of optimal preventive care and maximal risk reduction. Accordingly, there may be merit to using a “quality opportunities” paradigm in both the treatment and performance measurement of CAD patients.
We found substantial variation in prescription patterns by practice site. Although overall rates of secondary prevention prescription have improved with time, the role of the outpatient practice setting on these rates has not previously been characterized. A prior study from a U.S. cohort in the REACH registry found that practice characteristics, such as specialty type or geographic location, could significantly affect prescription rates (3). The initial data from PINNACLE also suggested heterogeneity in practice patterns by demonstrating substantial variation in compliance rates (from 13 to 97%) for various cardiac performance measures (6). However, neither of these studies directly measured or compared prescription pattern variation by practice site. Understanding this variation is an integral piece of quality assessment, since effective quality improvement efforts require understanding and targeting individual practices.
Our analysis also directly explored the association of outpatient practice in current prescribing patterns among PINNACLE registry participants using MRRs. Our findings suggest that patients may receive sub-optimal care solely due to the practice where they choose to seek care. These gaps are even more striking when one considers that the voluntary participants in the PINNACLE registry likely represent cardiology practices that are particularly focused on providing high quality care. Although it is encouraging that PINNACLE participation appeared to increase the rates of optimal prescription, concerning variation in practice still exists. Examining primary care practices – where a majority of CAD patients receive preventative care -may demonstrate even larger variations in care. As a result, our analysis both illustrates and likely underestimates the true amount of variation in US outpatient practice.
The reasons behind this overall practice variation in combined and individual medication prescription are unclear. Incomplete documentation of medication contraindications or incorrect classification of patient eligibility for therapies may be a partial answer, though our secondary analysis examining correlations between prescription rates and contraindication notations suggests that this is not a major contributor. Other sources of practice variation may lie in differing clinician opinions about the efficacy of therapies, an unawareness of current recommendations, competing interests during the clinical encounter, clinical inertia, or practice patterns that can lead to omissions of therapies (17,18). For example, cardiologists may rely on a patient's primary care provider to prescribe the appropriate medications. Another fruitful area of inquiry may be examination of prescribing patterns among multi-site practices, as factors such as referral patterns within practices and shared documentation protocols have also have an effect on performance. Although our current cohort is too small to explore these patterns, future analyses of PINNACLE as it continues to grow would be instructive. Further investigation is warranted to understand these and other factors that contribute to both high and low performing practices. As with other clinical care processes that rely on systems and some degree of standardization (such as rapid door-to-balloon times in acute MI), the study of high performing practices may suggest needed improvements for low performing practices (19).
Our findings should be interpreted in light of several considerations. First, as discussed above, some of our findings may be due to incomplete documentation of medication contraindications or incorrect classification of medication eligibility, though our secondary analyses suggest this is not a significant concern. In addition, as noted in our methods section, there may be reasonable and appropriate heterogeneity of practice around individual medication use, such as the optional use of BB in patients with a remote history of an MI. Second, our findings are derived from a registry of cardiology practices, which may not represent the broader medical practice of cardiac prevention. However, as mentioned earlier, we would expect actual rates in broader practice to be, if anything, worse. Third, we had limited data on practice characteristics, and thus were unable to provide extensive information on both high and low performing practices. Finally, we did not explore the lifestyle factors (e.g. smoking cessation, weight loss) or the use of cardiac rehabilitation that are also part of the CAD guidelines and performance measures.
In conclusion, we found that more than one-third of CAD patients seen in cardiology practices participating in the PINNACLE registry were not prescribed their optimal combination of prevention medications. In addition, we found significant variation in prescription patterns by practice, resulting in substantial differences in how similar patients were treated at different practices. Further investigation into reasons behind these practice-level differences is needed to provide the right care to the right patient, regardless where they seek that care.
Supplementary Material
Acknowledgements
The authors acknowledge Katherine Fagan for editorial support.
Disclosures:
Drs. Maddox and Bradley are supported by Career Development Award grants from the U.S. Department of Veterans Affairs Health Services Research and Development division.
Dr. Chan is supported by a Career Development Grant Award (K23HL102224) from the National Heart Lung and Blood Institute.
Dr. Spertus discloses that he has received a contract from the ACCF to analyze the PINNACLE registry. He also has grant support from the NIH, AHA, Lilly, Genentech and EvaHeart. He has served as a consultant to United Healthcare, St. Jude Medical, Amgen and Genentech.
Dr. Bhatt discloses the following relationships - Advisory Board: Medscape Cardiology; Board of Directors: Boston VA Research Institute, Society of Chest Pain Centers; Chair: American Heart Association Get With The Guidelines Science Subcommittee; Honoraria: American College of Cardiology (Editor, Clinical Trials, Cardiosource), Duke Clinical Research Institute (clinical trial steering committees), Slack Publications (Chief Medical Editor, Cardiology Today Intervention), WebMD (CME steering committees); Other: Senior Associate Editor, Journal of Invasive Cardiology; Research Grants: Amarin, AstraZeneca, Bristol-Myers Squibb, Eisai, Ethicon, Medtronic, Sanofi Aventis, The Medicines Company; Unfunded Research: FlowCo, PLx Pharma, Takeda.
Dr. Peterson is supported by grant K08 HS019814-01 from the Agency for Healthcare Research and Quality.
Disclaimer:
This research was supported by the American College of Cardiology Foundation's National Cardiovascular Data Registry (NCDR). The views expressed in this manuscript represent those of the author(s), and do not necessarily represent the official views of the NCDR or its associated professional societies identified at www.ncdr.com. They also do not necessarily represent the official views of the U.S. Department of Veterans Affairs.
Partners and Sponsors:
PINNACLE Registry® is an initiative of the American College of Cardiology Foundation. Bristol-Myers Squibb and Pfizer Inc. are Founding Sponsors of the PINNACLE Registry.
Abbreviations List
- 1. ACCF
American College of Cardiology Foundation
- 2. EHR
Electronic Health Record
- 3. MRR
Median Rate Ratio
- 4. REACH
Reduction of Atherothrombosis for Continued Health
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Smith SC, Jr, Benjamin EJ, Bonow RO, et al. AHA/ACCF Secondary Prevention and Risk Reduction Therapy for Patients with Coronary and other Atherosclerotic Vascular Disease: 2011 update: a guideline from the American Heart Association and American College of Cardiology Foundation. Circulation. 2011;124(22):2458–2473. doi: 10.1161/CIR.0b013e318235eb4d. [DOI] [PubMed] [Google Scholar]
- 2.Drozda J, Jr, Messer JV, Spertus J, et al. ACCF/AHA/AMA-PCPI 2011 performance measures for adults with coronary artery disease and hypertension: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Performance Measures and the American Medical Association-Physician Consortium for Performance Improvement. J. Am. Coll. Cardiol. 2011;58(3):316–336. doi: 10.1016/j.jacc.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 3.Kumar A, Fonarow GC, Eagle KA, et al. Regional and practice variation in adherence to guideline recommendations for secondary and primary prevention among outpatients with atherothrombosis or risk factors in the United States: a report from the REACH Registry. Crit Pathw Cardiol. 2009;8(3):104–111. doi: 10.1097/HPC.0b013e3181b8395d. [DOI] [PubMed] [Google Scholar]
- 4.McGlynn EA, Asch SM, Adams J, et al. The quality of health care delivered to adults in the United States. N. Engl. J. Med. 2003;348(26):2635–2645. doi: 10.1056/NEJMsa022615. [DOI] [PubMed] [Google Scholar]
- 5.Stafford RS, Radley DC. The underutilization of cardiac medications of proven benefit, 1990 to 2002. J. Am. Coll. Cardiol. 2003;41(1):56–61. doi: 10.1016/s0735-1097(02)02670-0. [DOI] [PubMed] [Google Scholar]
- 6.Chan PS, Oetgen WJ, Buchanan D, et al. Cardiac performance measure compliance in outpatients: the American College of Cardiology and National Cardiovascular Data Registry's PINNACLE (Practice Innovation And Clinical Excellence) program. J. Am. Coll. Cardiol. 2010;56(1):8–14. doi: 10.1016/j.jacc.2010.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chan PS, Oetgen WJ, Spertus JA. The Improving Continuous Cardiac Care (IC(3)) program and outpatient quality improvement. Am. J. Med. 2010;123(3):217–219. doi: 10.1016/j.amjmed.2009.09.019. [DOI] [PubMed] [Google Scholar]
- 8.Kahn CN, 3rd, Ault T, Isenstein H, Potetz L, Van Gelder S. Snapshot of hospital quality reporting and pay-for-performance under Medicare. Health Aff (Millwood) 2006;25(1):148–162. doi: 10.1377/hlthaff.25.1.148. [DOI] [PubMed] [Google Scholar]
- 9.Bhatt DL, Steg PG, Ohman EM, et al. International prevalence, recognition, and treatment of cardiovascular risk factors in outpatients with atherothrombosis. JAMA. 2006;295(2):180–189. doi: 10.1001/jama.295.2.180. [DOI] [PubMed] [Google Scholar]
- 10.Larsen K, Petersen JH, Budtz-Jørgensen E, Endahl L. Interpreting parameters in the logistic regression model with random effects. Biometrics. 2000;56(3):909–914. doi: 10.1111/j.0006-341x.2000.00909.x. [DOI] [PubMed] [Google Scholar]
- 11.Larsen K, Merlo J. Appropriate assessment of neighborhood effects on individual health: integrating random and fixed effects in multilevel logistic regression. Am. J. Epidemiol. 2005;161(1):81–88. doi: 10.1093/aje/kwi017. [DOI] [PubMed] [Google Scholar]
- 12.Goldstein H BW, Rasbash J. Partitioning Variation in Multilevel Models. Understanding Statistics.Vol 1. 4. 2002:223. [Google Scholar]
- 13.Chan PS, Maddox TM, Tang F, Spinler S, Spertus JA. Practice-level variation in warfarin use among outpatients with atrial fibrillation (from the NCDR PINNACLE program). Am. J. Cardiol. 2011;108(8):1136–1140. doi: 10.1016/j.amjcard.2011.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jollis JG, DeLong ER, Peterson ED, et al. Outcome of acute myocardial infarction according to the specialty of the admitting physician. N. Engl. J. Med. 1996;335(25):1880–1887. doi: 10.1056/NEJM199612193352505. [DOI] [PubMed] [Google Scholar]
- 15.Peterson ED, Shah BR, Parsons L, et al. Trends in quality of care for patients with acute myocardial infarction in the National Registry of Myocardial Infarction from 1990 to 2006. Am. Heart J. 2008;156(6):1045–1055. doi: 10.1016/j.ahj.2008.07.028. [DOI] [PubMed] [Google Scholar]
- 16.Bhatt DL, Eagle KA, Ohman EM, et al. Comparative determinants of 4-year cardiovascular event rates in stable outpatients at risk of or with atherothrombosis. JAMA. 2010;304(12):1350–1357. doi: 10.1001/jama.2010.1322. [DOI] [PubMed] [Google Scholar]
- 17.Parchman ML, Pugh JA, Romero RL, Bowers KW. Competing demands or clinical inertia: the case of elevated glycosylated hemoglobin. Ann Fam Med. 2007;5(3):196–201. doi: 10.1370/afm.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ho PM, Magid DJ, Shetterly SM, et al. Importance of therapy intensification and medication nonadherence for blood pressure control in patients with coronary disease. Arch. Intern. Med. 2008;168(3):271–276. doi: 10.1001/archinternmed.2007.72. [DOI] [PubMed] [Google Scholar]
- 19.Bradley EH, Curry LA, Webster TR, et al. Achieving rapid door-to-balloon times: how top hospitals improve complex clinical systems. Circulation. 2006;113(8):1079–1085. doi: 10.1161/CIRCULATIONAHA.105.590133. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.