Abstract
In addition to their well-known roles in the nervous system, there is increasing recognition that neurotrophins such as brain derived neurotrophic factor (BDNF) as well as their receptors are expressed in peripheral tissues including the lung, and can thus potentially contribute to both normal physiology and pathophysiology of several diseases. The relevance of this family of growth factors lies in emerging clinical data indicating altered neurotrophin levels and function in a range of diseases including neonatal and adult asthma, sinusitis, influenza, and lung cancer. The current review focuses on 1) the importance of BDNF expression and signaling mechanisms in early airway and lung development, critical to both normal neonatal lung function and also its disruption in prematurity and insults such as inflammation and infection; 2) how BDNF, potentially derived from airway nerves modulate neurogenic control of airway tone, a key aspect of airway reflexes as well as dysfunctional responses to allergic inflammation; 3) the emerging idea that local BDNF production by resident airway cells such as epithelium and airway smooth muscle can contribute to normal airway structure and function, and to airway hyperreactivity and remodeling in diseases such as asthma. Furthermore, given its pleiotropic effects in the airway, BDNF may be a novel and appealing therapeutic target.
Keywords: Neurotrophin, Lung, Tropomyosin related kinase, p75, Asthma, Rhinitis, Inflammation, Fibrosis, Bronchopulmonary Dysplasia, Development
1. Introduction
From the time of birth (indeed, even during fetal life) through childhood and adulthood, intrinsic and environmental factors determine the balance between continued lung health vs. the onset and progression of lung diseases. In this regard, development, growth and health of the airways are key towards maintenance of the respiratory function of the lung. Diseases of the airway can stem from factors such as fetal and neonatal developmental abnormalities, exposures to allergens and inflammatory mediators, pollutants, first and secondhand tobacco smoke, and other environmental insults. This results in a wide range of clinically important conditions such as asthma, bronchitis, chronic obstructive pulmonary disease (COPD), fibrosis and cancer, all of which collectively represent a substantial global healthcare and financial burden. Thus, from both clinical and research perspectives, it is important to understand the mechanisms that regulate normal airway development, growth and maintenance, as well as the pathways that contribute to airway disease pathogenesis. Here, unlike in cardiac or liver disease where a few intrinsic cell types are likely involved, the heterogeneity of cell types just in the airway such as the bronchial epithelium, airway smooth muscle, interstitial fibroblasts, resident immune cells, and airway nerves, makes it likely that a number of complex and interactive mechanisms are involved in the pathogenesis of airway disease. Nonetheless, it is possible to speculate that certain mechanisms that are common to different airway diseases do exist and would therefore be important to identify and understand. Recent studies indicate that the family of growth factors called neurotrophins (NTs) that have pleiotropic effects may play such a role in the lung(Aven and Ai, 2013; Braun et al., 2000; Hoyle, 2003; Jacoby, 2004; Lommatzsch et al., 2003; Piedimonte, 2003; Prakash et al., 2010; Renz et al., 2004; Rochlitzer et al., 2006; Taylor-Clark and Undem, 2006; Yao et al., 2006). While the NT family consists of different members, brain-derived neurotrophic factor (BDNF) is emerging as a particularly important player in the lung or airways (Lommatzsch et al., 2003; Prakash et al., 2010; Yao et al., 2006). Of course, data on BDNF expression, its signaling and its physiological or pathophysiological significance are still being explored. However, given the importance of NTs in the brain, the fortuitous increasing recognition that BDNF is a key player in neurological and psychiatric diseases (Allen et al., 2013; Dooley et al., 2013; He et al., 2013; Leal et al., 2013; Nowacka and Obuchowicz, 2013; Numakawa et al., 2013; Park and Poo, 2013; Suliman et al., 2013; Zagrebelsky and Korte, 2013) has led to substantial novel information on complex and elegant aspects of BDNF expression or signaling that may be relevant to airway structure and function. In this article, we review current understanding of the sources and targets of BDNF in the normal airway at different life stages, and the potential contribution of altered BDNF expression and signaling in diseases such as neonatal and adult asthma, COPD, pulmonary fibrosis and cancer. Here, given the limited but emerging information on BDNF in the airways per se, we draw upon information in other cell systems to identify pathways by which BDNF may influence airway structure and function in health and disease, and suggest novel avenues for exploration of BDNF in the airway. Our intent is to stress upon the reader the potential for BDNF as an important physiological and pathophysiological player, perhaps a biomarker and importantly a therapeutic target in airway diseases.
2. Basics of BDNF Expression and Signaling
It is inappropriate not to recognize that much of our knowledge regarding NT signaling comes from studies on the nervous system, beginning more than 60 years ago with the work of individuals such as Rita Levi-Montalcini and Viktor Hamburger on the regrowth of nerves in embryonic limb buds (Blum and Konnerth, 2005; Hennigan et al., 2007; Levi-Montalcini, 1998; Lu et al., 2005; Reichardt, 2006; Teng and Hempstead, 2004). Although a number of pleotropic signaling factors can affect neuronal structure and function and thus act as growth factors, the NT family is classically considered to consist of 4 polypeptides of comparable structure and function: nerve growth factor (NGF), the “original” and best-characterized NT, brain-derived neurotrophic factor (BDNF), neurotrophin-3 (NT3) and neurotrophin-4 (NT4) (Chao et al., 2006; Reichardt, 2006; Skaper, 2008). Consistent with the idea of a nutritive, target-derived factor that promotes growth and survival, NTs including BDNF have been found to regulate neurogenesis, neuronal differentiation, survival, plasticity, and even synaptic transmission and nerve conduction (Chao et al., 2006; He et al., 2013; Kalb, 2005; Leal et al., 2013; Lykissas et al., 2007; Park and Poo, 2013; Suliman et al., 2013; Teng and Hempstead, 2004; Zagrebelsky and Korte, 2013). There is now considerable evidence (and interest) in the idea that NTs, particularly BDNF, are important pathophysiological players in diseases such as depression, schizophrenia, Alzheimer’s disease (Angelucci et al., 2004; Dwivedi, 2009; Numakawa et al., 2013; Saragovi et al., 2009; Schulte-Herbruggen et al., 2008; Shoval and Weizman, 2005), cerebral tumors (Nagatsu et al., 2000; Nagatsu and Sawada, 2005; Thiele et al., 2009), and spinal cord injury repair (Awad et al., 2013; Blesch and Tuszynski, 2002; Dale-Nagle et al., 2010; He et al., 2013; Murray et al., 2002; Pezet and Malcangio, 2004; Ramer, 2012; Sieck and Mantilla, 2009; Skaper et al., 2012; Weishaupt et al., 2012). Here, it is increasingly apparent that BDNF is not just a growth factor, but that BDNF expression and signaling are intricately enmeshed with a number of regulatory pathways including other NTs, sex steroids, glucocorticoids, and inflammation (Babayan and Kramar, 2013; Kapfhammer, 2004; Miranda et al., 1994; Nagatsu and Sawada, 2005; Numakawa et al., 2013; Pluchino et al., 2013; Schaaf et al., 2000; Simpkins et al., 1997; Tabakman et al., 2004). The relevance of many if not all of these pathways lies in their recognized roles in airway diseases such as asthma and COPD (Carey et al., 2007; Damera et al., 2009; Panettieri, 2004; Prakash, 2013; Tam et al., 2011; Townsend et al., 2012). Accordingly, understanding how BDNF expression and signaling occurs in the nervous system may provide insight into airway diseases as well: a theme highlighted in this review, and certainly a topic for future research.
2.1. Production of BDNF
The BDNF gene has at least 9 distinct promoters that allow for multiple mRNA transcripts with each containing the entire open reading frame for the BDNF protein (Aid et al., 2007; Boulle et al., 2012; Zheng et al., 2012). Via alternative promoters, splicing and polyA sites at least 22 transcripts can be generated, each for the same initial BDNF protein product. This level of complexity at the transcriptional level allows for multiple layers of regulation for BDNF generation and may be important in intracellular localization of BDNF mRNA or initial proteins. Furthermore, given multiple promoters, BDNF transcription can be regulated by several upstream intracellular cascades relevant to sex steroid, glucocorticoid or other factors. Agonist or electrical stimulation leading to increased [Ca2+]i induces BDNF transcription highlighting activity-dependent BDNF regulation (Zheng et al., 2012). There are at least 3 Ca2+-responsive elements in the regulatory region of one of the more important BDNF exons. Activation of these elements involves regulatory elements such as cAMP response element binding protein (CREB) and protein kinase A (PKA), calmodulin-dependent protein kinases I, II and IV, NFκB and NFAT (Figure 1). The relevance of many of these elements lies in the fact that they are themselves activated by elevated [Ca2+]i in cells (e.g. PKA, CREB, ERK 1/2, and importantly, these elements are known to regulate several processes in the airway, particularly in the context of inflammation. However, unlike in neurons, there is currently limited data on how BDNF transcription occurs in the airway.
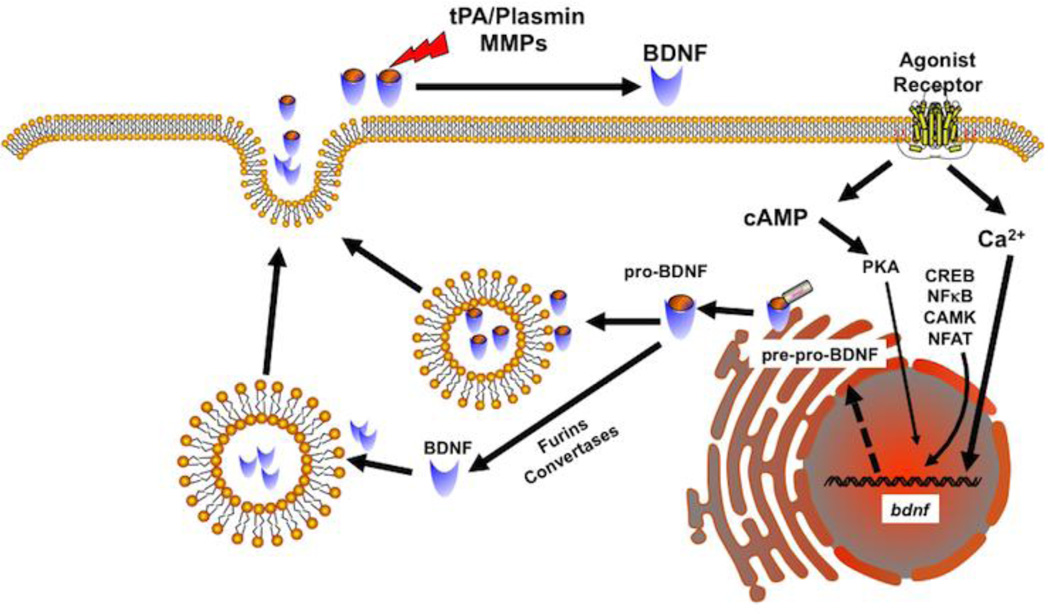
Figure 1. Production of brain-derived neurotrophic factor (BDNF).
Stimulation by agonists or other molecules leading to elevated Ca2+ is a common mechanism for inducing BDNF in cells. Other processes that enhance cAMP, or activate signaling intermediates such as CREB, NFkB, CaM kinases or NFAT may also activate the BDNF gene. BDNF is first produced as a pre-pro-peptide that is then cleaved in the Golgi to produce pro-BDNF that is then packaged into vesicles. Pro-BDNF can also be cleaved intracellularly by furins and vesicular convertases to produce mature BDNF that is also packaged. Vesicular release of pro-BDNF or mature BDNF can then act on neurotrophin receptors to produce effects. Extracellularly, pro-BDNF is cleaved by factors such as matrix metalloproteinases or tissue plasminogens.
In addition to transcriptional regulation, there is now evidence in non-airway cell types (particularly neurons) that epigenetic mechanisms such as chromatin remodeling (histone acetylation/methylation) and DNA methylation also regulate BDNF levels (Boulle et al., 2012; Zheng et al., 2012). Such regulation appears to be highly complex, coordinated, and context-dependent, with further complication introduced by different BDNF exons being influenced differentially. Given that the role of epigenetics in the airway is still being investigated (Clifford et al., 2013; Yang and Schwartz, 2012), examining regulation of BDNF by these mechanisms is likely premature.
BDNF is initially synthesized in the endoplasmic reticulum as a precursor protein (pre-pro-BDNF; ~27 kDa) (Butte et al., 1998; Lessmann and Brigadski, 2009; Lessmann et al., 2003; McDonald and Chao, 1995; Robinson et al., 1995) (Figure 1). The signal peptide is then cleaved to produce pro-BDNF which is then transported to the Golgi where it is sorted into either constitutive or regulated secretory vesicles. Vesicular pro-BDNF can be converted intracellularly into mature BDNF by endoproteases such as furin or within secretory granules by pro-protein convertases (Mowla et al., 2001) allowing for one more level of regulation of BDNF expression within cells. In this regard, vesicular secretion can involve both pro-BDNF and mature BDNF, with the amount of secreted mature BDNF depending on the type and activity of the convertases. Pro-BDNF is then extracellularly cleaved by factors such as plasmin (through tPA conversion of plasminogen) and importantly matrix metalloproteinases (MMPs) into the mature form. Since mature BDNF is highly homologous across species, examination of its biology is facilitated using animal models, particularly transgenics that lack or overexpress specific aspects of BDNF signaling, and exogenous recombinant BDNF.
2.2. BDNF Signaling
Although pro-BDNF was initially thought to be biologically inactive, there is now evidence that both pro- and mature BDNF can bind to and signal through two distinct, principal receptor types: the low-affinity “pan-neurotrophin” receptor p75NTR, and the high-affinity tropomyosin related kinase (Trk) receptor (~140KDa), with BDNF (and NT4) binding to the TrkB isoform (Barker, 2004; Blochl and Blochl, 2007; Chen et al., 2009). Nerve growth factor (NGF) binds specifically to TrkA, while NT3 binds to TrkC (Lu et al., 2005; Reichardt, 2006; Teng and Hempstead, 2004) (Figure 2). In addition, there is some heterologous binding, with NT3 and NT4 also activating TrkA, and NT3 activating TrkB. The present review obviously focuses on TrkB and p75NTR, however, it is important to recognize that the other receptor systems may also be relevant in the airways (see below).
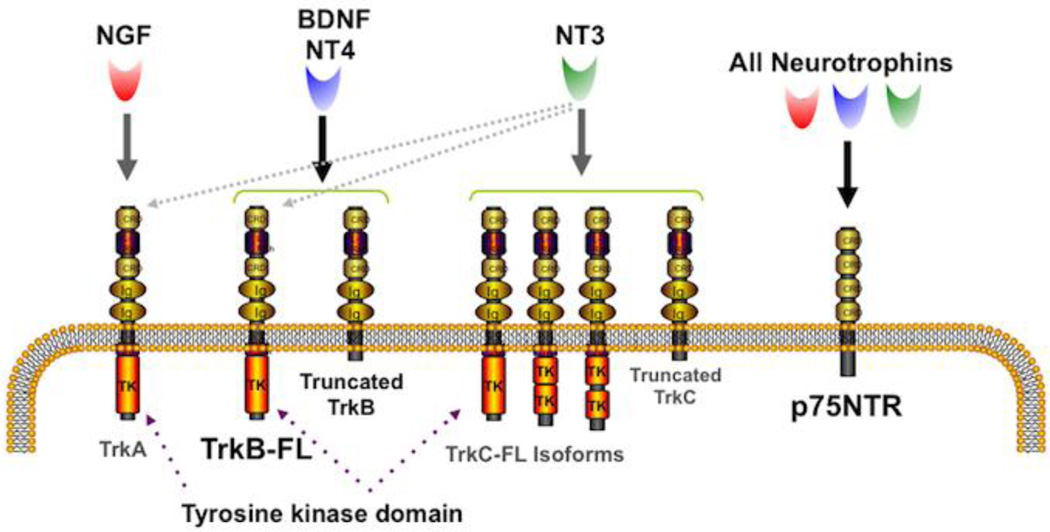
Figure 2. Neurotrophins and their receptors.
The neurotrophin family consists of four polypeptide proteins that activate two classes of receptors. All of the neurotrophins (NGF, BDNF, NT4 and NT3) bind to the low-affinity p75NTR receptor that lacks a tyrosine kinase domain but can use adaptor proteins. P75NTR is a member of the tumor necrosis factor receptor family. The high-affinity receptors are the tropomyosin related kinases (Trks) that in their full-length (FL) form have an intracellular tyrosine kinase domain via which they function. In addition, ‘truncated” Trks also exist that do not have the tyrosine kinase domain but can act via other mechanisms. NGF preferentially binds to TrkA, while TrkB is the preferred receptor for BDNF and NT4, and TrkC preferentially binds to NT3. In addition, NT3 can bind to the other Trks with lower affinity.
P75NTR is a member of the TNF receptor superfamily and lacks intrinsic enzymatic activity, but can recruit signaling adaptors and modulate Trk signaling as well as a number of intracellular processes (Blochl and Blochl, 2007; Chen et al., 2009; Ibanez and Simi, 2012; Lu et al., 2005; Skaper, 2008; Underwood and Coulson, 2008; Yamashita et al., 2005). p75NTR can modulate a number of intracellular pathways including PI3/Akt, NFκB, MAPKs, JNK, RhoA, PKA, and HIF, all of which are familiar in the context of airway contractility, proliferation, growth, and responsiveness to factors such as inflammation (Chiba and Misawa, 2004; Damera et al., 2009; Gosens et al., 2006; Halayko and Amrani, 2003; Halayko and Solway, 2001; Hirota et al., 2005; James, 2005; Panettieri, 2004). Mature BDNF binding to p75NTR, depending on the context, can enhance TrkB receptor signaling through MAPK or Akt, and promote cell survival through NFκB. Alternatively, p75NTR can inhibit Trk effects through JNK and RhoA. Pro-BDNF can bind to sortilin and forms a ternary complex with p75NTR, which then activates cell death pathways (Figure 3). Accordingly p75NTR-induced effects can be diverse and context-specific, and seemingly contradictory: cell survival vs. cell death, proliferation vs. inhibition of cellular outgrowth etc. making for a diverse range of BDNF effects just through the low-affinity receptor.
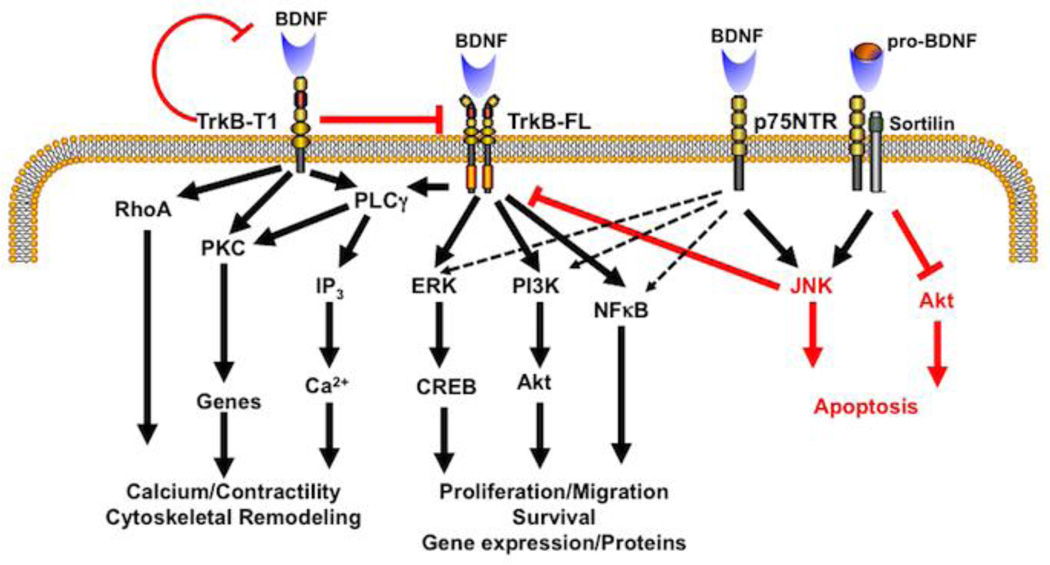
Figure 3. BDNF signaling.
As discussed in the main text, BDNF signaling via TrkB vs. p75NTR is complex but elegant, allowing for a diversity of signaling mechanisms and cellular effects that are cell type- and context-dependent. Activation of the full-length TrkB by mature BDNF classically results in activation of PLCγ with downstream effects on [Ca2+]i via the IP3 pathway that can enhance contractility, the ERK pathway and the PI3K/Akt pathway that can both promote cell proliferation and survival. In addition TrkB can work through the NFκB pathway. In contrast to TrkB-FL, the truncated TrkB-T1 can be both inhibitory (red; by dimerizing with TrkB-FL or chelating BDNF) and activating via RhoA and PKC, leading to cytoskeletal remodeling, gene regulation and other effects. The low-affinity p75NTR, when activated by mature BDNF is typically activating to pathways that TrkB-FL works through, but importantly can be inhibitory via the JNK or Akt pathways. This is particularly the case when p75NTR is activated by pro-BDNF and forms a ternary complex with the adaptor protein sortilin.
The TrkB gene is on chromosome 9q22 and contains 24 exons (Barbacid, 1994; Ohira and Hayashi, 2009; Thiele et al., 2009). Four major protein isoforms are formed, but alternatively splicing can lead to 36 different isoforms being formed. Although BDNF is unlikely to function via all of these isoforms, the full-length version of the transmembrane TrkB receptor (TrkB-FL) is particularly important. TrkB-FL contains an intracellular tyrosine kinase domain that is essential for BDNF signaling (Figure 3). TrkB-FL undergoes autophosphorylation to induce conformational changes, and substrate binding sites for Shc, GRB2, ATP, and PLCγ are exposed. Trk signaling in general occurs through three tyrosine kinase mediated pathways (Barbacid, 1995; Chao and Hempstead, 1995; Ohira and Hayashi, 2009; Pitts et al., 2006; Thiele et al., 2009): the PLCγ1/PKC pathway relevant to IP3 and thus [Ca2+]i signaling, the MAPK-ERK pathway and the PI3/Akt pathway, both of which can modulate cell survival and differentiation. In addition to TrkB-FL, the most important truncated isoform lacking the tyrosine kinase domain (among the different alternatively spliced constructs) is TrkB-T1 (Cheng et al., 2007; Fenner, 2012; Li et al., 1998; Rose et al., 2003). Truncated isoforms are thought to have dominant negative effects, or in sequestering BDNF to prevent signaling, or activating alternative pathways. For example, TrkB-T1 binds to and internalizes BDNF but does not undergo autophosphorylation (unlike the full-length receptor). TrkB-T1 also dimerizes with TrkB-FL to indirectly inhibit BDNF functionality (Fenner, 2012). In addition, TrkB-T1 can regulate cytoskeletal remodeling via a TrkB-T1 homodimer that functions independently of BDNF. Non-neuronal cells use plasma membrane TrkB-T1 expression to regulate extracellular BDNF levels by binding to the ligand, internalization and sequestration of the BDNF into transport vesicles that can then by exocytosed when necessary. TrkB-T1 can also mediate BDNF-induced proliferation, but the underlying mechanisms are not known. Overall, the high-affinity receptors for BDNF can have complex effects on cells as well as BDNF regulation itself.
The above discussion highlights the fact that the actual biological efficacy of BDNF is dependent not only on ligand concentrations, but also the balance between full-length vs. truncated TrkB receptor expression, along with a modulating role for p75NTR. In this regard, data from knockout mice on neuronal survival suggest that Trk receptors are key to biological activity of NTs (Conover and Yancopoulos, 1997), and can influence survival (e.g., transgenic animals lacking TrkB show early mortality). As discussed above, p75NTR also appears to be involved in neuronal survival, but it can conversely influence cell death as well. Nonetheless, differences in expression of TrkB isoforms vs. p75NTR and biological availability of BDNF all allow for considerable heterogeneity in BDNF signaling across different cell types.
Following receptor activation, a number of downstream signaling cascades can be activated by BDNF, with cell-specific transcription factors that contribute to the many effects of BDNF such as differentiation, survival, apoptosis, and growth: events that are genomic in nature. Here, some of the identified transcription factors such as NFκB, AP-1 and CREB (Figure 3) are highly relevant to airway inflammation (Damera et al., 2009; Gosens et al., 2006; Halayko and Amrani, 2003; Halayko and Solway, 2001). In addition, there is increasing recognition that BDNF can have relatively rapid, likely “non-genomic” effects on much shorter timeframes (seconds to minutes), and most likely involve high-affinity TrkB receptors (Blum and Konnerth, 2005; Carvalho et al., 2008; Kovalchuk et al., 2004; Rose et al., 2004). Much of this data comes from neurons where BDNF, which is released in a Ca2+-dependent fashion akin to neurotransmitters, can modulate the efficacy of synaptic transmission (Blum and Konnerth, 2005). In neurons, BDNF can also non-genomically modulate plasma membrane receptors, Ca2+ influx channels and voltage-gated Na+ channels (Blum and Konnerth, 2005; Carvalho et al., 2008; Kovalchuk et al., 2004; Rose et al., 2004). While the repertoire of receptors, ion channels and signaling cascades differs between neurons and non-neuronal cells of the airways, the relevance of these genomic and non-genomic BDNF effects in neurons lies in the potential that any common mechanisms and cascades may be targets for BDNF effects in the airways.
3. BDNF in the Lung
As nutritive factors, the target organs of the central and peripheral nervous systems such as skin, skeletal muscles, epithelia and smooth muscles of different types can be expected to express NTs (Nockher and Renz, 2005; Sariola, 2001), thus modulating their own innervation. Here, the concept of local production of NTs such as BDNF vs. their “targets” is important in that it is entirely possible for a cell type or tissue to only be the recipient of BDNF which is actually derived from a remote source (e.g. neurons or even circulating BDNF) vs. autocrine/paracrine effects of locally produced BDNF. In this regard, it is now clear that both a range of Trk receptors are widely distributed in non-neuronal tissues (Hoyle, 2003; Lommatzsch et al., 2003; Nassenstein et al., 2004; Prakash et al., 2010), and accordingly local release and autocrine/paracrine functions of NTs have to be considered. Relevant to this discussion, all of the NTs (particularly BDNF) and their receptors have now been shown to be expressed, albeit to different extents, in different lung compartments: nerves, immune cells, bronchial and alveolar epithelium, smooth muscle, fibroblasts and vascular endothelium (Prakash et al., 2010; Ricci et al., 2004; Ricci et al., 2007). What is less known is what roles these NTs play in lung structure or function. The relevance of BDNF effects in the airways lies in recent recognition that circulating BDNF levels as well as local receptor expression are both increased in asthma and that there is clinical evidence for increased BDNF levels in both sputum and bronchoalveolar lavages of patients with chronic inflammatory airway diseases following smoking or cigarette smoke exposure, viral infections, allergens and other insults (Andiappan et al., 2011; Dagnell et al., 2010; Ricci et al., 2005; Rochlitzer et al., 2006; Tortorolo et al., 2005). While these studies obviously associate airway disease with elevated BDNF, the question is regarding the source of BDNF and the functional relevance of elevated BDNF. Here, emerging data from several groups including our own suggest important roles for BDNF expression and signaling in airway smooth muscle, innervation and epithelium are potentially important.
3.1. BDNF in Airway Smooth Muscle
There is now increasing evidence that airway smooth muscle (ASM) is a significant source of NTs including BDNF. For example, immunocytochemical staining of human lung sections show constitutive expression of NGF, BDNF and NT3 by ASM (Ricci et al., 2004). Substantial expression of BDNF has also been noted in isolated human ASM cells from non-asthmatic humans by both immunocytochemistry (Prakash et al., 2006) as well as Western analysis (Prakash et al., 2009). The importance of this complementary evidence lies in establishing that while “traditional” sources such as airway nerves (discussed below) can serve as sources and targets for BDNF, the sheer mass of ASM (or other cell types) within the airways would be a much larger source of BDNF for local signaling in the airways. Furthermore, we have previously demonstrated TrkB expression in human ASM cells and tissues (Abcejo et al., 2012; Meuchel et al., 2011; Prakash et al., 2006; Prakash et al., 2009; Vohra et al., 2013) and have also found p75NTR expression (Prakash et al., 2006).
In addition to constitutive expression, emerging data suggest that factors relevant to airway diseases can enhance ASM BDNF and receptor expression (Braun et al., 1999; Kemi et al., 2006; Meuchel et al., 2011; Prakash et al., 2009; Sathish et al., 2013; Vohra et al., 2013), thus raising the possibility that BDNF is a “player” in mediating or modulating the effects of these factors. For example, Kemi et al. (Kemi et al., 2006) reported that IL-1β produces sustained increases in BDNF (but only transient increase in NGF) in human ASM cells, an effect mediated through COX. In contrast IFNγ decreases BDNF expression while IL-4 had no effect. Interestingly, neurokinins such as Substance P (that can be released by airway nerves) can also enhance BDNF/TrkB expression, as we reported in rat ASM (Meuchel et al., 2011). Oxidant stress, e.g. induced by cigarette smoke increases BDNF and TrkB-FL (with relative decrease in TrkB-T1) in human ASM (Sathish et al., 2013). Indirect evidence of TNFα effects can further be derived from the blunting of siRNAs against BDNF or TrkB-FL on cell proliferation (Aravamudan et al., 2012) or [Ca2+]i (Prakash et al., 2009).
Overall, the limited but exciting data point to baseline, endogenous production of BDNF. The question then is how is BDNF production or secretion regulated in ASM. As discussed above, in neurons elevation in [Ca2+]i (e.g. following electrical stimulation) leads to vesicular release (Sadakata and Furuichi, 2009). While it is unlikely that ASM requires rapid secretion of BDNF as in neurons, vesicular secretion pathways do exist in airway cells (Proskocil et al., 2004). Here, [Ca2+]i is known to modulate secretion in general (with a well-recognized role for cAMP (Furman et al., 2010; Hofer, 2012; Schmidt et al., 2013; Seino et al., 2009)), and elevated [Ca2+]i does increase BDNF secretion in neurons and vasculature (Lessmann and Brigadski, 2009; Nakahashi et al., 2000). Thus mechanisms that regulate [Ca2+]i in ASM may also play a role in modulating BDNF secretion. The mechanisms involved in [Ca2+]i regulation in ASM are manifold and have been reviewed elsewhere (Amrani and Panettieri, 2002; Gerthoffer, 1991; Hall, 2000; Janssen, 1998). In non-ASM cells, canonical transient receptor potential (TRPC) channels are involved in fluid secretion and exocytosis of other intracellular proteins (Bollimuntha et al., 2011). ASM cells express a variety of TRPC channels, with TRPC3 being particularly important for modulating Ca2+ entry and in airway responses to inflammation (Amrani, 2007; Sweeney et al., 2002; Vohra et al., 2013; White et al., 2006). In non-neuronal cells, TRPC3 regulates secretion (Kim et al., 2011; Lavender et al., 2008). In a recent study, using DNA constructs to produce GFP-tagged BDNF, and real-time fluorescence imaging, we showed that vesicular BDNF release occurs in human ASM cells, and is regulated by TRPC3 (Vohra et al., 2013). Furthermore, inflammation induced by TNFα or mixed allergens relevant to asthma enhances TRPC3 expression and its effects on BDNF release. [Ca2+]i does play a part, as suggested by reduced BDNF secretion when [Ca2+]i levels are chelated with a substance like BAPTA, vs. increased BDNF secretion when a bronchoconstrictor agonist such as ACh is applied. The latter effect is particularly interesting since it is possible that activated ASM, e.g. during bronchoconstriction, can enhance BDNF secretion in the airway. The next question then is what effects BDNF has on ASM or other cell types.
In previous studies, we reported that exogenous BDNF acutely (within 30 min) enhances [Ca2+]i and force responses of human ASM to agonists such as ACh (Prakash et al., 2006). Such effects are further enhanced in the presence of inflammatory cytokines such as TNFα (Prakash et al., 2009). These data indicate that regardless of the source of BDNF, this NT can directly enhance ASM contractility via non-genomic mechanisms. BDNF exerts its effects on ASM [Ca2+]i by enhancing Ca2+ influx across the plasma membrane (Prakash et al., 2006), particularly via store-operated Ca2+ entry (SOCE) that involves TRPC3 and the regulatory proteins STIM1 and Orai1 (Abcejo et al., 2012). BDNF can also enhance intracellular Ca2+ release via the IP3 pathway (important in ASM), consistent with TrkB activation of PLCγ. Here, it is important to note that the non-genomic effects of BDNF appear to be mediated almost exclusively by TrkB, and not p75NTR (Prakash et al., 2009).
From a genomic standpoint, BDNF can enhance expression and function of [Ca2+]i regulatory proteins (Abcejo et al., 2012), leading to increased Ca2+ responses and contractility in response to bronchoconstrictor stimuli. In this regard, our recently demonstrated involvement of TRPC3 in BDNF secretion is relevant, since it is possible that enhanced secretion of BDNF could then act in an autocrine fashion to promote BDNF expression. Furthermore, in other cell types such as HEK293 and dorsal root ganglion, the neurotrophin NGF can enhance trafficking of TRPV1 channels to the plasma membrane (Stein et al., 2006). Whether BDNF can promote trafficking of TRPC channels is not known, but could present a novel positive feedback mechanism for BDNF to maintain its release and signaling. The relevance of such novel BDNF effects further lies in the potential for BDNF enhancement of plasma membrane mechanisms such as caveolae (invaginations that harbor receptors, channels and signaling proteins) (Suzuki et al., 2007; Suzuki et al., 2004), thus promoting integration and amplification of signaling at the cell surface.
Separately, BDNF can also increase ASM proliferation (Aravamudan et al., 2012). Indeed, chelation of extracellular BDNF in unstimulated human ASM cells using the chimeric TrkB-Fc protein blunts baseline proliferation (Aravamudan et al., 2012). Importantly, TNFα or IL-13 enhancement of cell proliferation is substantially blunted by TrkB-Fc, TrkB siRNA or BDNF siRNA highlighting the importance of ASM-derived BDNF and its autocrine effects on ASM proliferation. Here, it appears that BDNF effects are largely via TrkB (p75NTR siRNA had only small effect). In terms of signaling, BDNF activates p42/p44 MAP kinase and NFκB to enhance ASM proliferation (Aravamudan et al., 2012).
In vascular smooth muscle, NGF (which activates TrkA) induces migration but not proliferation (Kraemer et al., 1999), while p75NTR activation induces apoptosis (Wang et al., 2000). Whether BDNF enhance ASM migration has been examined only to a limited extent. The importance of ASM migration lies in airway remodeling that occurs in diseases such as asthma and perhaps COPD, especially in the setting of altered extracellular matrix (ECM) production and composition (Bai, 2010; Halayko and Amrani, 2003; Holgate, 2002; Joubert and Hamid, 2005; Lagente and Boichot, 2009). Here, the link to BDNF is particularly interesting and relevant since MMPs, particularly the gelatinases MMP-2 and MMP-9 extracellularly cleave pro-BDNF (Ethell and Ethell, 2007; Lee et al., 2001; Soleman et al., 2013), while these MMPs are also key to breakdown and recomposition of ECM proteins such as collagens and fibronectin. Accordingly, BDNF effects on MMPs in the airway could be particularly important in the context of remodeling. There is currently only one study that has examined this issue in human ASM cells, and reported that BDNF increases MMP-9 but not MMP-2 secretion (Dagnell et al., 2007). Furthermore, the study also reported that BDNF enhances ASM cell migration. In ongoing studies, we have found that physiologically-relevant concentrations of BDNF enhance MMP-9 mRNA, protein and extracellular levels (Thompson, Prakash, Britt, unpublished observations). Furthermore, the inflammatory cytokines TNFα, and to a lesser extent IL-13 enhance MMP-9 expression and activity via BDNF (chelation of extracellular BDNF blunts cytokine effects).
Overall, these emerging data show that BDNF has both non-genomic and genomic effects on ASM Ca2+, cell proliferation, migration and ECM formation and deposition, aspects very relevant to normal airway structure and function, and airway hyperresponsiveness and remodeling in diseases such as asthma. What is not known is whether these longer-term effects of BDNF occur through TrkB only or via p75NTR. For example, our previous proliferation data suggest TrkB-FL (Aravamudan et al., 2012), but the effects on cell migration could involve cytoskeletal remodeling (as occurs in neurons) which could occur through homodimerization of TrkB-T1, a potentially novel regulatory pathway. Here, the lack of information on TrkB-T1 is unfortunate, given that in spite of TrkB-FL expression in ASM (or other cell types in the airway), the eventual functional efficacy of BDNF will be dictated by the relative levels of TrkB-FL v. TrkB-T1, especially if the latter serves a negative dominant role. In addition to TrkB, whether nuanced effects of p75NTR via signaling pathways such as NFκB or RhoA are also involved in ASM remains to be determined.
3.2. BDNF and Airway Innervation
Neural control of the airway is extensive and exquisite (see (Canning, 2006; Kc and Martin, 2010; Racke and Matthiesen, 2004; Undem and Nassenstein, 2009; Undem and Potenzieri, 2012; Verhein et al., 2009) for review). CNS control involves integrated networks, particularly medullary vagal preganglionic neurons which send signals to intrinsic tracheobronchial ganglia in close proximity to effector systems of the postganglionic axons that are diffusely distributed within the airways. In addition to cholinergic pathways, intrinsic airway neurons release a number of neuromodulators including vasoactive intestinal peptide and nitric oxide (the so called non-adrenergic, non-cholinergic system) that promote bronchodilation (although an excitatory component can also exist). Vagal afferents of sensory ganglia are present throughout the bronchopulmonary tree and sense chemical, mechanical, or thermal stimuli. There is considerable heterogeneity here, in that some receptors contain the pro-inflammatory bronchoconstricting neuropeptides substance P and neurokinin A (Groneberg et al., 2006), while other mechanosensitive reflex systems control breathing and parasympathetic outflow to regulate airway tone. The relevance of complex neural pathways lies in the fact that increased airway hyperresponsiveness in diseases such as asthma or bronchitis can be modulated by increased cholinergic outflow from the parasympathetic nervous system (neurogenic asthma) (Butler and Heaney, 2007; Nassini et al., 2010; Nockher and Renz, 2006; Pisi et al., 2009) and that commonly used drugs such as ipratropium bromide target cholinergic innervation. Accordingly, by virtue of being a growth factor for neurons, BDNF could potentially affect several neural mechanisms relevant to airway tone and thus influence airway diseases.
Initial immunohistochemical studies of human lung showed obvious presence of NGF and BDNF in neurons and to a lesser extent satellite cells of parasympathetic ganglia (Ricci et al., 2004). While one would expect such ganglia to respond to BDNF, immunoreactivity for Trks was not observed, but p75NTR was present. On the other hand, in lung sections, nerve fibers that may form the sympathetic component showed moderate immunoreactivity for both TrkB and p75NTR. The functional role of such NT receptors in airway innervation has been examined to some extent, but mostly for NGF (reviewed in (Freund-Michel and Frossard, 2008; Frossard et al., 2004)) which may or may not be representative of BDNF effects. In mouse airway, NGF increases Substance P content of sensory nerves (Freund-Michel and Frossard, 2008). Inflammation due to allergens or respiratory syncytial virus can induce de novo neuropeptide production in vagal afferents that are important for cough and reflex airway responses. In mice overexpressing airway NGF, increased sensory innervation has been reported (Hoyle et al., 1998) suggesting neural outgrowth. These neurons express p75NTR and may therefore respond to BDNF (although this remains to be shown).
The relevance of expanded airway innervation or increased neurogenic stimulation by NTs lies in increased sensitivity of the airway to a variety of stimuli in diseases such as asthma or with environmental exposures (allergens, cigarette smoke, pollutants). Here, NTs may actually mimic inflammation or other such stimuli. For example, in mice, exogenous NGF enhances airway hyperresponsiveness even in the absence of allergen sensitization and challenge) (Freund-Michel and Frossard, 2008), although this effect does not appear to occur in guinea pigs treated with NGF (Pan et al., 2010). On the other hand, in guinea pigs, BDNF can mimic allergen sensitization by enhancing expression of TRPV1 channels in vagal tracheal cough-causing mechanosensitive neurons of the nodose ganglia, thus enhancing cough reflexes (Lieu et al., 2012). Sensory neurons of mice overexpressing NGF show enhanced responses to capsaicin and hyperinnervation of SP-containing nerves (Hoyle et al., 1998). In addition to NGF, lesser numbers of studies have shown that BDNF may also play a role in airway innervation. For example, mice treated with functional antibodies targeting BDNF show reduced airway responsiveness to capsaicin (Braun et al., 2004). In ferrets, in situ hybridization, immunohistochemistry and ELISA of BDNF levels showed that parasympathetic premotor neurons innervating the extrathoracic trachea (airway-related vagal preganglionic neurons) produce BDNF and express TrkB receptors, suggesting they are both source and target for BDNF (Zaidi et al., 2005). Here, BDNF may increase cholinergic outflow from these neurons to postganglionic parasympathetic nerves, indirectly leading to enhanced bronchoconstriction and bronchopulmonary reflexes.
Overall, these data, although exclusively in animals, underline a central role for NTs such as NGF and BDNF in neural control of the airways. What has been examined to a lesser extent are the mechanisms by which the function of airway innervation can be modulated. As a growth factor, BDNF (potentially derived from local tissues or nerves themselves) could enhance survival or expansion of receptive fields of sensory nerve endings. Certainly, increased BDNF expression and secretion by ASM in the inflamed airway may act on postganglionic parasympathetic airway nerves. Furthermore, given that neuronal activity itself can enhance BDNF expression and release, a positive feedback cycle may be set up in the setting of disease which would exaggerate nerve expansion. Separately, BDNF can enhance expression of other receptors or enhance synaptic transmission, again enhancing bronchoconstriction. For example, upregulated neurokinin receptor expression can occur as demonstrated with NGF (Piedimonte, 2003) as well as with BDNF (Meuchel et al., 2011). However, beyond this rudimentary understanding, there is much not known about how BDNF or other NTs can influence airway innervation and neuronal function, especially given that there is currently no clear cut information on the distribution of receptors (TrkB, p75NTR) in airway nerve endings.
3.3. BDNF in Bronchial Epithelium
The airway epithelium serves as a barrier to allergens and infectious agents, but also as a modulator of airway responses to insults, and a source for bronchoconstrictive and bronchodilatory factors (Crystal et al., 2008; Dekkers et al., 2009; Gras et al., 2013; Hirota and Martin, 2013; Holgate, 2013). Accordingly BDNF expression or function in this important cell type is relevant to basic airway function and its modulation in disease. However, there are very few studies on NTs in airway epithelium in general, and are mostly focused on NGF (Hahn et al., 2006; Othumpangat et al., 2009; Ricci et al., 2004). Immunocytochemistry shows that airway epithelium constitutively expresses NGF, BDNF and NT3. Increased expression of epithelial BDNF has been reported in mouse models of allergic airway hyperresponsiveness (Hahn et al., 2006; Lommatzsch et al., 2003). While these data show epithelial production of BDNF, its function in epithelium is less clear. In situ hybridization of mouse lung epithelium as well as immunohistochemistry of surgical human lung samples do not show receptor expression (Ricci et al., 2004), raising the question of whether airway epithelia are only a source, but not a target (unlike ASM). However, in a recent study using isolated human bronchial epithelial cells, we detected TrkB protein expression by Western blot (Meuchel et al., 2010). Separately, the NGF receptor TrkA is expressed by human bronchial epithelial cells (Hahn et al., 2006), and infection induced epithelial cell death is worsened in the absence of NGF (Othumpangat et al., 2009). Given some cross-activation of TrkA by BDNF, its function on epithelium cannot be entirely ruled out.
Assuming that TrkB is present in epithelium, as with ASM, BDNF may also have rapid (i.e. non-genomic) effects on airway epithelium. For example in isolated human bronchial epithelial cells, we found that BDNF, acting via TrkB, can elevate [Ca2+]I, phosphorylate eNOS and induce nitric oxide production within minutes (Meuchel et al., 2010). Furthermore, in ongoing studies using human bronchial epithelial cells, we have found that BDNF enhances expression of arginase 1 and 2 via a TrkB and NFκB mediated mechanism (Thompson, Prakash, unpublished observations). The relevance of this novel data lies in the fact that arginase can limit availability of L-arginine for production of NO, and furthermore enhance production of proline, a precursor to collagen formation. Thus, these preliminary and limited data at least suggest that BDNF (regardless of source) can potentially influence epithelial structure and function. Certainly much more study is needed to determine the contribution of BDNF signaling and of its receptors in normal epithelium and role in airway diseases.
4. BDNF in the Developing Lung
4.1 Normal Lung Development
NTs such as BDNF are critical for embryonic development of the central and peripheral nervous systems (Reichardt, 2006). Accordingly, detailed studies on BDNF in development of any organ including lung are severely limited by early mortality of transgenic animals lacking BDNF receptors, especially TrkB. In the developing mouse, a dynamic pattern of increasing vs. decreasing levels of NGF vs. BDNF has been observed in whole lung lysates (Lommatzsch et al., 2005). However, the relevance of these patterns are not clear, given that different cell type sin the lung likely express various NTs or their receptors to different levels, further leading to cell-dependent effects‥ Nonetheless, in ongoing studies in whole embryonic lung, we found that BDNF mRNA levels rise at ~E12 and TrkB mRNA at ~E13, a period of initiation of lung development, and are maintained until birth (Hartman, Prakash, unpublished observations). A single published study examined TrkB during lung development in the mouse, comparing wild-type animals to those carrying a trkB gene mutation leading to a non-functional protein (resulting in postnatal survival of a few weeks) (Garcia-Suarez et al., 2009). They reported higher TrkB mRNA in early postnatal development, lasting until 15 days postnatal, with progressive decline after that age.
The function(s) of BDNF/TrkB in the developing lung are not entirely clear. The single study using transgenic TrkB mice (Garcia-Suarez et al., 2009) reported that neuroepithelial bodies and lung innervation have high TrkB expression. Interestingly, transgenic animals showed thinner bronchial epithelium, larger airway luminal diameter and larger alveolar spaces compared to wildtype animals. These data suggest that BDNF/TrkB is important for cell proliferation and/or migration and thus proper formation of functioning airways with adequate amounts of epithelium and ASM. On the other hand, another study found that that absence of BDNF does not significantly affect lung development (Lommatzsch et al., 1999). Separately, given its role as a neurotrophic factor, BDNF effects on airway innervation need to be considered. During embryogenesis, axon outgrowth into the distal lung is tied to formation of ASM, with progressive growth and expansion of ASM layers leading to elongation and spreading of adjacent neural branches (Sparrow et al., 1999; Tollet et al., 2001). These data would suggest an ASM-derived neurotrophic factor. Again, a single study in mice reported BDNF is expressed by embryonic ASM and that the BDNF knockout embryos display reduced axonal branching in targeting the ASM (Radzikinas et al., 2011). Here, BDNF expression in the embryonic lung appears to be post-transcriptionally coordinated with ASM development (via a sonic hedgehog pathway where a microRNA 206 inhibits BDNF expression until the appropriate time). What is less clear is the source(s) of BDNF in the developing airway. Certainly, consistent with its presence in adult ASM, fetal or neonatal ASM could be a source, as could bronchial epithelium. However, there is currently no published data on this topic, but represents an important topic for future research.
Overall, these limited data suggest that BDNF is involved in lung and airway development, potentially by affecting neuronal regulation of growth and proliferation of lung elements. However, the actual physiology is likely more complex and remains to be unexplored. Here, a potentially elegant technique to examine the role of BDNF/TrkB signaling in lung development may be the TrkB “knock-in” mouse model developed by Ginty and colleagues (Chen et al., 2005) where small molecule inhibitors of mutated TrkB tyrosine kinase domains allow for normal receptor expression and function until the kinase activity is transiently and reversibly inhibited by a small peptide inhibitor without affecting expression. The appeal of this model lies in the fact that TrkB expression and function are normal throughout critical periods in lung development and in the postnatal period, and can be interrupted at specific time points to examine the role of BDNF/TrkB signaling in the context of ASM growth in the fetal/perinatal period vs. that which occurs during postnatal lung growth, thus setting the stage to examine the importance of BDNF in different aspects of perinatal airway disease.
4.2. Neonatal Airway Disease
Prematurity and associated neonatal lung injury, characterized by bronchopulmonary dysplasia (BPD) can result in high incidence of childhood asthma (Hack et al., 2005). Neonatal hyperoxic exposure, e.g. as occurs in the intensive care unit, can be particularly contributory. The role of hyperoxia has been examined in animal models such as rat pups, and is associated with increased cholinergically-mediated airway contractile responses in vitro and in vivo (reviewed in (Yao et al., 2006)). However, the mechanisms by which cholinergic effects are upregulated are not clear. We have evaluated hyperoxia effects on lung expression of BDNF and TrkB (Yao et al., 2005; Yao et al., 2006) and found that hyperoxia (95% O2) starting at birth (thus mimicking the ICU experience) increases BDNF and TrkB mRNA and protein in the airways, particularly in the peribronchial smooth muscle. Here, BDNF may be the NT of interest in that hyperoxia does not appear to increase NGF (as opposed to elevated NGF when neonates are exposed to infection (Tortorolo et al., 2005)). These findings raise the possibility that upregulation of BDNF and its autocrine signaling may result in enhanced airway reactivity in neonates, thus contributing to neonatal/pediatric asthma, and other inflammatory airway disease in infants and children (Yao et al., 2006). This scenario is also consistent with the finding that BDNF is expressed in airway preganglionic neurons (Zaidi et al., 2005), and additionally that hyperoxia substantially increases ACh content in the lung: an effect inhibited by tyrosine kinase inhibitors. These findings, albeit in animals, suggest that BDNF/TrkB signaling increases neuronal ACh production and resultant cholinergic outflow to the airways under conditions of hyperoxia (Sopi et al., 2008). Accordingly, hyperoxia increases contraction, and tyrosine kinase inhibition reduces it (Sopi et al., 2008).
Overall, these limited but interesting data show that factors such as hyperoxia increase BDNF/TrkB signaling in the airways in early life. However, the mechanisms underlying enhanced BDNF, or downstream pathways activated by BDNF in the context of airway disease are not known. As discussed above, in adult ASM, BDNF can enhance [Ca2+]I, cell proliferation, migration and airway remodeling in general. These processes are equally important in the developing airway, perhaps more so given that a stiffer, thicker airway can lead to severe respiratory decompensation in children. Here, it is possible that many of the signaling cascades such as MAPK, NFκB etc. are activated by BDNF in developing ASM, but remain to be demonstrated. The significance of such studies lies in the potential for pharmacological modulation of BDNF/TrkB signaling to counter airway hyperreactivity and remodeling.
5. BDNF and Asthma
Asthma involves inflammation-driven changes in airway structure and function, represented by hyperresponsiveness to bronchoconstrictors, impaired bronchodilation and a largely irreversible remodeling of the airway leading to obstruction. With evidence for BDNF expression and signaling in airway innervation, epithelium and smooth muscle there is obviously significant interest in the potential role for targeting NTs such as BDNF in asthma (Abram et al., 2009; Bennedich Kahn et al., 2008; Dagnell et al., 2007; Freund-Michel and Frossard, 2008; Frossard et al., 2004; Nassenstein et al., 2006; Nockher and Renz, 2006; Prakash et al., 2009; Renz et al., 2004; Rochlitzer et al., 2006). In this regard, there is some evidence for an association between BDNF and asthma, particularly in children as well as increased asthma association with a variant of the BDNF gene (Andiappan et al., 2011; Koskela et al., 2010; Szczepankiewicz et al., 2007; Szczepankiewicz et al., 2010; Szczepankiewicz et al., 2009; Yinli et al., 2013; Zeilinger et al., 2009). Furthermore, in humans as well as in mouse models of asthma, allergen challenge increases NGF and BDNF levels in bronchoalveolar lavage fluid (Braun et al., 1999; Virchow et al., 1998). Certainly such increases may be a result of enhanced BDNF secretion by resident airway cells, as well as by immune cells that also happen to expression BDNF and its receptors (reviewed in (Prakash et al., 2010)). Increased BDNF may then promote airway irritability via the nerves, modulate epithelium-derived bronchodilator responses, or increase ASM [Ca2+]i and contractility, especially in the presence of inflammatory cytokines (Prakash et al., 2009). Finally, BDNF may modulate airway remodeling. via proliferation, migration and secretion of inflammatory mediators and modulators such as MMPs (Bai, 2010; Dekkers et al., 2009; Lagente and Boichot, 2009). Indeed, BDNF does increase MMP9 (but not MMP2), and enhances smooth muscle migration (Dagnell et al., 2007). We have shown increased human ASM cell proliferation by BDNF in the presence of cytokines such as TNFα and IL-13 that are relevant to asthma pathophysiology (Aravamudan et al., 2012). Whether BDNF expression is intrinsically higher in ASM of asthmatics, or altered to a different level in the presence of inflammation is not known. Similarly, the effect of BDNF in asthmatic ASM may be enhanced or may differ in the contribution of downstream signaling pathways. There is currently no information on these important issues. Nonetheless, based on the diverse data to date, it is very likely that BDNF plays a role in asthma pathophysiology. Given that BDNF can be targeted via TrkB-Fc, it may therefore represent a potential therapeutic avenue in asthma.
6. BDNF and Environmental Exposures
Air pollutants are risk factors in development of allergic diseases and for respiratory infections. For example, active smoking as well as secondhand smoke can potentiate effects of other allergens. The role of BDNF expression and/or signaling in this context has been examined to a limited extent. In patients with atopic keratoconjuctivitis (where BDNF levels are elevated in tears), smoking elevates BDNF levels (Kimata, 2004). In rats, nicotine exposure increases expression of p75NTR (but not Trk receptors) (Urrego et al., 2009). In a recent study using human ASM, we found that exposure to cigarette smoke substantially increases both TrkB and p75NTR expression, and that BDNF potentiates cigarette smoke enhancement of ASM [Ca2+]i responses and thus contractility (Sathish et al., 2013). While the mechanisms by which cigarette smoke enhances TrkB or p75NTR expression are not known, factors such as nicotine and oxidant stress are possibilities that remains to be explored. Here, it is interesting to note that nicotine by itself does not increase the NGF receptor TrkA in rat lung (Urrego et al., 2009). More complex mechanisms may involve nicotine-induced increase in BDNF (as occurs in neuroblastoma cells (Serres and Carney, 2006)) with autocrine effects on enhancing TrkB. While there is much more information needed in this area, these limited data point to participation of BDNF in enhanced airway inflammation and reactivity following environmental exposures. In response, BDNF may potential neuronal or immune effects in the airway as well as directly influence airway remodeling, increase airway irritability or hyperresponsiveness. Again, as with asthma, targeting of BDNF may be an attractive therapeutic avenue.
7. BDNF and Fibrosis
One aspect of airway remodeling in diseases such as asthma or COPD is increased fibrosis. Here, altered MMPs and ECM components are obviously contributory, and BDNF may play a role in this regard. A single study reported that fibroblast-rich areas in lungs of patients with pulmonary fibrosis (Ricci et al., 2007) stained for BDNF and TrkB. In fibroblasts from these patients, BDNF increased cell proliferation, compared to control patients. A more recent study (Avcuoglu et al., 2011) reported higher expression of TrkB in patients with idiopathic pulmonary fibrosis and in mice with bleomycin-induced fibrosis. Importantly, the pro-fibrotic cytokine TGFβ1 was found to enhance TrkB expression. Furthermore, the enhanced TrkB was found to have functional effects in enhancing proliferation that would only further promote the cycle of fibrosis. Separately, in pulmonary sarcoidosis, epithelioid cells and multinucleated giant cells within granulomas have been found to show immunoreactivity for BDNF and TrkB (Dagnell et al., 2010). Overall these limited but interesting data suggest that BDNF expression or signaling is altered in lung fibrosis, although the actual mechanisms and end effects of BDNF in the fibrotic lung remain to be determined. Nonetheless, the potential for targeting BDNF in fibroblasts or other associated cell types offers a novel target for this disease.
8. BDNF and TrkB as Therapeutic Targets
From the perspective of understanding normal airway structure/function as well as exploring novel therapeutic options for airway diseases, BDNF and its receptors represent attractive targets. However, the very pleiotropic effects of BDNF on different airway components which would be attractive in terms of therapy would also make it a challenge to specifically target particular cell types, especially if they are not as easy to access. A prime example is targeting ASM via inhalational routes without disrupting the epithelium. Simple nebulization or intratracheal administration of BDNF has been performed in animal studies (Braun et al., 2004; Lommatzsch et al., 2003), but it is not clear that the extent of penetration into the lung, local vs. systemic levels, or degradation vs. elimination has actually been studied. Here, approaches such as nanoparticle carriers and cell-specific markers to facilitate preferential uptake by cells of interest, viral vectors, or cellular scaffolds may be appealing as we move forward. Small molecule activators (or inhibitors) of Trks vs. p75NTR are potential options as well (Longo and Massa, 2008; 2013; Skaper, 2008; Thiele et al., 2009; Webster and Pirrung, 2008). Molecules such as TrkB-Fc that bind extracellular BDNF and prevent its action could be helpful, but again access to specific cell types may be limiting. However, even these novel approaches are limited by the complexity of NT receptors and signaling. For example, depending on the relative expression of TrkB-FL vs. TrkB-T1 vs. p75NTR, small molecules that act on TrkB may have differential effects on cell survival vs. cell death. On the other hand, small, synthetic peptides have been generated that can selectively activate specific aspects of TrkB or p75NTR and thus mimic BNDF (Longo and Massa, 2013). However, due to limitations of peptides for drug development including stability, bioavailability and barrier penetration, small peptides may not be ideal options. Ligands for TrkB have also been developed (Longo and Massa, 2013), in particular the specific agonists 7,8-dihydroxyflavone (Jang et al., 2010b) or deoxygedunin (Jang et al., 2010a)
While these exciting developments in approaches to modulating BDNF/TrkB are very interesting, their applicability to airway diseases is less clear for the simple reason that in the airways, BDNF appears to be pro-inflammatory, pro-contractility and pro-remodeling, and therefore inhibition of BDNF expression and signaling is needed. However, much of the focus on BDNF-based therapies are geared towards enhancing BDNF/TrkB effects in the context of neurological diseases where loss of trophic influences is the problem. Accordingly, there is much need for identifying novel approaches to inhibiting BDNF/TrkB in the context of airway diseases.
9. Summary and Conclusions
While signaling via BDNF in different airway components has now been recognized (Figure 4), a complete, integrated physiological model for BDNF function in the airway needs to be developed. Here, a number of questions can be put forth: A) What are the normal, major sources of BDNF in the airway? Here ASM appears to be particularly important; B) What are the primary target cell types for BDNF? As discussed in this review, it appears that epithelium, ASM, fibroblasts and nerves are all potential targets. What is less clear is whether BDNF acts on each of these cells under normal circumstances, i.e. If BDNF is widely expressed in the airway, from an evolutionary standpoint, what is its intended function: modulation of neurogenic airway tone vs. airway structure (remodeling) vs. immune function? C) If BDNF is important in neuronal development, is there a role in lung development as well? D) Under disease conditions, is altered BDNF expression and function secondary or due to initiating insults such as inflammation or infection, or is BDNF itself a trigger of disease? Ongoing research integrating in vitro and in vivo approaches should help address many of these issues. Given substantial homology of BDNF, TrkB and p75NTR across species, corroboration of animal experiments with human samples, especially patients with specific, well-defined airway diseases will be particularly helpful.
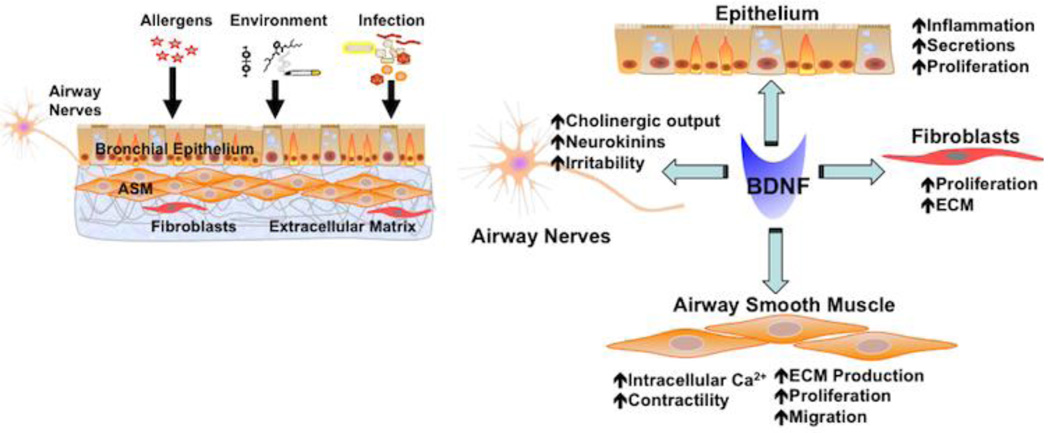
Figure 4. BDNF and the airway.
There is increasing evidence that BDNF can be produced by cells of the airway such as epithelium, airway smooth muscle (ASM), airway nerves, and even fibroblasts. Furthermore, each of these cell types can be a target of BDNF. In the context of airway diseases, BDNF can enhance airway irritability and responsiveness to agonist via the nerves or ASM itself (where [Ca2+]i is increased) or enhance airway remodeling in diseases such as asthma or COPD by increasing cell proliferation, migration, fibrosis and formation of extracellular matrix.
Table 1.
BDNF in the Airways
| Cell/Tissue Component |
Receptors | Reported Effects | Reported Role in Diseases |
|---|---|---|---|
| Bronchial Epithelium | TrkB p75NTR |
Essential for airway and lung development Cell survival (decreased apoptosis) NO release Airway remodeling |
↑ BDNF in asthma ↑ BDNF and TrkB, ↓ p75NTR with infection ↑ Airway thickening in asthma or inflammation ↑ TrkB, p75NTR with cigarette smoke |
| Airway Smooth Muscle | TrkB p75NTR |
Airway development Enhanced airway contractility with exogenous and endogenous BDNF Increased cell proliferation Airway remodeling |
↑ BDNF, TrkB with hyperoxia in fetal or neonatal ASM ↑ Endogenous BDNF production and secretion with airway inflammation ↑ TrkB, p75NTR with allergic airway inflammation ↑ ASM contractility in inflammation Mediates and enhances ASM cell proliferation in the presence of cytokines ↑ Extracellular matrix deposition ↑ TrkB, p75NTR with cigarette smoke ↑ ASM contractility with smoke exposure |
| Nerves | TrkB p75NTR |
↑ SP and ACh content of sensory nerves, vagal afferents ↑ NK1 receptor expression ↑ neurotransmitter release |
↑ Cholinergic outflow ↑ BDNF in asthma ↑ Neurally-mediated airway hyperresponsiveness ↑ neuronal plasticity |
| Fibroblasts | TrkB p75NTR |
Proliferation Migration Myofibroblast differentiation |
↑ BDNF and TrkB in inflammation ↑ Proliferation |
| Blood | ↑ BDNF in asthma ↑ p75NTR with nicotine exposure Association between BDNF gene variants and asthma |
||
| BAL | ↑ BDNF in asthma |
Information gleaned from multiple studies in human or animals, all included in bibliography.
Acknowledgments
Funding Acknowledgement: Supported by NIH grants HL088029 (Prakash) and HL056470 (Prakash, Martin).
Abbreviations
- BDNF
Brain-derived neurotrophic factor
- NGF
Nerve growth factor
- NT-3
Neurotrophin 3
- TrkB
Tropomyosin related kinase B
- P75NTR
Pan-neurotrophin receptor
- NT
Neurotrophin
- COPD
Chronic obstructive pulmonary disease
- CREB
cAMP response element binding protein
- PKA
Protein kinase A
- NFkB
Nuclear factor kappa B
- ERK
Extracellular signal regulated kinase
- PLC
Phospholipase C
- ECM
Extracellular matrix
- MMP
Matrix metalloproteinase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial and Competing Interests Disclosure: The authors have nothing to declare in terms of financial or other conflicts of interest relevant to the subject matter of materials discussed in this review. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
REFERENCES
- Abcejo AJ, Sathish V, Smelter DF, Aravamudan B, Thompson MA, Hartman WR, Pabelick CM, Prakash YS. Brain-derived neurotrophic factor enhances calcium regulatory mechanisms in human airway smooth muscle. PloS One. 2012;7:e44343. doi: 10.1371/journal.pone.0044343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abram M, Wegmann M, Fokuhl V, Sonar S, Luger EO, Kerzel S, Radbruch A, Renz H, Zemlin M. Nerve growth factor and neurotrophin-3 mediate survival of pulmonary plasma cells during the allergic airway inflammation. J Immunol. 2009;182:4705–4712. doi: 10.4049/jimmunol.0802814. [DOI] [PubMed] [Google Scholar]
- Aid T, Kazantseva A, Piirsoo M, Palm K, Timmusk T. Mouse and rat BDNF gene structure and expression revisited. J Neurosci Res. 2007;85:525–535. doi: 10.1002/jnr.21139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen SJ, Watson JJ, Shoemark DK, Barua NU, Patel NK. GDNF, NGF and BDNF as therapeutic options for neurodegeneration. Pharmacol Ther. 2013;138:155–175. doi: 10.1016/j.pharmthera.2013.01.004. [DOI] [PubMed] [Google Scholar]
- Amrani Y. TNF-alpha and calcium signaling in airway smooth muscle cells: a never-ending story with promising therapeutic relevance. Am J Respir Cell Mol Biol. 2007;36:387–388. doi: 10.1165/ajrcmb.36.3.387. [DOI] [PubMed] [Google Scholar]
- Amrani Y, Panettieri RA., Jr Modulation of calcium homeostasis as a mechanism for altering smooth muscle responsiveness in asthma. Curr Opin Allergy Clin Immunol. 2002;2:39–45. doi: 10.1097/00130832-200202000-00007. [DOI] [PubMed] [Google Scholar]
- Andiappan AK, Parate PN, Anantharaman R, Suri BK, Wang de Y, Chew FT. Genetic variation in BDNF is associated with allergic asthma and allergic rhinitis in an ethnic Chinese population in Singapore. Cytokine. 2011;56:218–223. doi: 10.1016/j.cyto.2011.05.008. [DOI] [PubMed] [Google Scholar]
- Angelucci F, Mathe AA, Aloe L. Neurotrophic factors and CNS disorders: findings in rodent models of depression and schizophrenia. Prog Brain Res. 2004;146:151–165. doi: 10.1016/s0079-6123(03)46011-1. [DOI] [PubMed] [Google Scholar]
- Aravamudan B, Thompson M, Pabelick C, Prakash YS. Brain-derived neurotrophic factor induces proliferation of human airway smooth muscle cells. J Cell Mol Med. 2012;16:812–823. doi: 10.1111/j.1582-4934.2011.01356.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avcuoglu S, Wygrecka M, Marsh LM, Gunther A, Seeger W, Weissmann N, Fink L, Morty RE, Kwapiszewska G. Neurotrophic tyrosine kinase receptor B/neurotrophin 4 signaling axis is perturbed in clinical and experimental pulmonary fibrosis. Am J Respir Cell Mol Biol. 2011;45:768–780. doi: 10.1165/rcmb.2010-0195OC. [DOI] [PubMed] [Google Scholar]
- Aven L, Ai X. Mechanisms of respiratory innervation during embryonic development. Organogenesis. 2013;9:194–198. doi: 10.4161/org.24842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awad BI, Carmody MA, Steinmetz MP. Potential Role of Growth Factors in the Management of Spinal Cord Injury. World neurosurgery. 2013 doi: 10.1016/j.wneu.2013.01.042. S1878-8750(13)00103-4. [DOI] [PubMed] [Google Scholar]
- Babayan AH, Kramar EA. Rapid Effects of Oestrogen on Synaptic Plasticity: Interactions with Actin and its Signaling Proteins. J Neuroendocrinol. 2013 doi: 10.1111/jne.12108. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai TR. Evidence for airway remodeling in chronic asthma. Curr Opin Allergy Clin Immunol. 2010;10:82–86. doi: 10.1097/ACI.0b013e32833363b2. [DOI] [PubMed] [Google Scholar]
- Barbacid M. The Trk family of neurotrophin receptors. J Neurobiol. 1994;25:1386–1403. doi: 10.1002/neu.480251107. [DOI] [PubMed] [Google Scholar]
- Barbacid M. Structural and functional properties of the TRK family of neurotrophin receptors. Ann N Y Acad Sci. 1995;766:442–458. doi: 10.1111/j.1749-6632.1995.tb26693.x. [DOI] [PubMed] [Google Scholar]
- Barker PA. p75NTR is positively promiscuous: novel partners and new insights. Neuron. 2004;42:529–533. doi: 10.1016/j.neuron.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Bennedich Kahn L, Gustafsson LE, Olgart Hoglund C. Brain-derived neurotrophic factor enhances histamine-induced airway responses and changes levels of exhaled nitric oxide in guinea pigs in vivo. Eur J Pharmacol. 2008;595:78–83. doi: 10.1016/j.ejphar.2008.07.041. [DOI] [PubMed] [Google Scholar]
- Blesch A, Tuszynski MH. Spontaneous and neurotrophin-induced axonal plasticity after spinal cord injury. Prog Brain Res. 2002;137:415–423. doi: 10.1016/s0079-6123(02)37033-x. [DOI] [PubMed] [Google Scholar]
- Blochl A, Blochl R. A cell-biological model of p75NTR signaling. J Neurochem. 2007;102:289–305. doi: 10.1111/j.1471-4159.2007.04496.x. [DOI] [PubMed] [Google Scholar]
- Blum R, Konnerth A. Neurotrophin-mediated rapid signaling in the central nervous system: mechanisms and functions. Physiology (Bethesda) 2005;20:70–78. doi: 10.1152/physiol.00042.2004. [DOI] [PubMed] [Google Scholar]
- Bollimuntha S, Selvaraj S, Singh BB. Emerging roles of canonical TRP channels in neuronal function. Adv Exp Med Biol. 2011;704:573–593. doi: 10.1007/978-94-007-0265-3_31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulle F, van den Hove DL, Jakob SB, Rutten BP, Hamon M, van Os J, Lesch KP, Lanfumey L, Steinbusch HW, Kenis G. Epigenetic regulation of the BDNF gene: implications for psychiatric disorders. Mol Psychiatr. 2012;17:584–596. doi: 10.1038/mp.2011.107. [DOI] [PubMed] [Google Scholar]
- Braun A, Lommatzsch M, Lewin GR, Virchow JC, Renz H. Neurotrophins: a link between airway inflammation and airway smooth muscle contractility in asthma? Int Arch Allergy Immunol. 1999;118:163–165. doi: 10.1159/000024056. [DOI] [PubMed] [Google Scholar]
- Braun A, Lommatzsch M, Neuhaus-Steinmetz U, Quarcoo D, Glaab T, McGregor GP, Fischer A, Renz H. Brain-derived neurotrophic factor (BDNF) contributes to neuronal dysfunction in a model of allergic airway inflammation. Br J Pharmacol. 2004;141:431–440. doi: 10.1038/sj.bjp.0705638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun A, Lommatzsch M, Renz H. The role of neurotrophins in allergic bronchial asthma. Clin Exp Allergy. 2000;30:178–186. doi: 10.1046/j.1365-2222.2000.00741.x. [DOI] [PubMed] [Google Scholar]
- Butler CA, Heaney LG. Neurogenic inflammation and asthma. Inflammation Allergy Drug Targets. 2007;6:127–132. doi: 10.2174/187152807780832238. [DOI] [PubMed] [Google Scholar]
- Butte MJ, Hwang PK, Mobley WC, Fletterick RJ. Crystal structure of neurotrophin-3 homodimer shows distinct regions are used to bind its receptors. Biochemistry. 1998;37:16846–16852. doi: 10.1021/bi981254o. [DOI] [PubMed] [Google Scholar]
- Canning BJ. Reflex regulation of airway smooth muscle tone. J Appl Physiol. 2006;101:971–985. doi: 10.1152/japplphysiol.00313.2006. [DOI] [PubMed] [Google Scholar]
- Carey MA, Card JW, Voltz JW, Germolec DR, Korach KS, Zeldin DC. The impact of sex and sex hormones on lung physiology and disease: lessons from animal studies. Am J Physiol Lung Cell Mol Physiol. 2007;293:L272–L278. doi: 10.1152/ajplung.00174.2007. [DOI] [PubMed] [Google Scholar]
- Carvalho AL, Caldeira MV, Santos SD, Duarte CB. Role of the brain-derived neurotrophic factor at glutamatergic synapses. Br J Pharmacol. 2008;153(Suppl 1):S310–S324. doi: 10.1038/sj.bjp.0707509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao MV, Hempstead BL. p75 and Trk: a two-receptor system. Trends Neurosci. 1995;18:321–326. [PubMed] [Google Scholar]
- Chao MV, Rajagopal R, Lee FS. Neurotrophin signalling in health and disease. Clin Sci (Lond) 2006;110:167–173. doi: 10.1042/CS20050163. [DOI] [PubMed] [Google Scholar]
- Chen X, Ye H, Kuruvilla R, Ramanan N, Scangos KW, Zhang C, Johnson NM, England PM, Shokat KM, Ginty DD. A chemical-genetic approach to studying neurotrophin signaling. Neuron. 2005;46:13–21. doi: 10.1016/j.neuron.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Chen Y, Zeng J, Cen L, Chen Y, Wang X, Yao G, Wang W, Qi W, Kong K. Multiple roles of the p75 neurotrophin receptor in the nervous system. J Intl Med Res. 2009;37:281–288. doi: 10.1177/147323000903700201. [DOI] [PubMed] [Google Scholar]
- Cheng A, Coksaygan T, Tang H, Khatri R, Balice-Gordon RJ, Rao MS, Mattson MP. Truncated tyrosine kinase B brain-derived neurotrophic factor receptor directs cortical neural stem cells to a glial cell fate by a novel signaling mechanism. J Neurochem. 2007;100:1515–1530. doi: 10.1111/j.1471-4159.2006.04337.x. [DOI] [PubMed] [Google Scholar]
- Chiba Y, Misawa M. The role of RhoA-mediated Ca2+ sensitization of bronchial smooth muscle contraction in airway hyperresponsiveness. J Smooth Muscle Res. 2004;40:155–167. doi: 10.1540/jsmr.40.155. [DOI] [PubMed] [Google Scholar]
- Clifford RL, Singer CA, John AE. Epigenetics and miRNA emerge as key regulators of smooth muscle cell phenotype and function. Pulm Pharmacol Ther. 2013;26:75–85. doi: 10.1016/j.pupt.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conover JC, Yancopoulos GD. Neurotrophin regulation of the developing nervous system: analyses of knockout mice. Rev Neurosci. 1997;8:13–27. doi: 10.1515/revneuro.1997.8.1.13. [DOI] [PubMed] [Google Scholar]
- Crystal RG, Randell SH, Engelhardt JF, Voynow J, Sunday ME. Airway epithelial cells: current concepts and challenges. Proc Am Thoracic Soc. 2008;5:772–777. doi: 10.1513/pats.200805-041HR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagnell C, Grunewald J, Kramar M, Haugom-Olsen H, Elmberger GP, Eklund A, Olgart Hoglund C. Neurotrophins and neurotrophin receptors in pulmonary sarcoidosis - granulomas as a source of expression. Respir Res. 2010;11:156. doi: 10.1186/1465-9921-11-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagnell C, Kemi C, Klominek J, Eriksson P, Skold CM, Eklund A, Grunewald J, Olgart Hoglund C. Effects of neurotrophins on human bronchial smooth muscle cell migration and matrix metalloproteinase-9 secretion. Transl Res. 2007;150:303–310. doi: 10.1016/j.trsl.2007.05.001. [DOI] [PubMed] [Google Scholar]
- Dale-Nagle EA, Hoffman MS, MacFarlane PM, Satriotomo I, Lovett-Barr MR, Vinit S, Mitchell GS. Spinal plasticity following intermittent hypoxia: implications for spinal injury. Ann N Y Acad Sci. 2010;1198:252–259. doi: 10.1111/j.1749-6632.2010.05499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damera G, Tliba O, Panettieri RA., Jr Airway smooth muscle as an immunomodulatory cell. Pulm Pharmacol Ther. 2009;22:353–359. doi: 10.1016/j.pupt.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekkers BG, Maarsingh H, Meurs H, Gosens R. Airway structural components drive airway smooth muscle remodeling in asthma. Proc Am Thoracic Soc. 2009;6:683–692. doi: 10.1513/pats.200907-056DP. [DOI] [PubMed] [Google Scholar]
- Dooley D, Vidal P, Hendrix S. Immunopharmacological intervention for successful neural stem cell therapy: New perspectives in CNS neurogenesis and repair. Pharmacol Ther. 2013 doi: 10.1016/j.pharmthera.2013.08.001. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Dwivedi Y. Brain-derived neurotrophic factor: role in depression and suicide. Neuropsychiatr Dis Treatment. 2009;5:433–449. doi: 10.2147/ndt.s5700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ethell IM, Ethell DW. Matrix metalloproteinases in brain development and remodeling: synaptic functions and targets. J Neurosci Res. 2007;85:2813–2823. doi: 10.1002/jnr.21273. [DOI] [PubMed] [Google Scholar]
- Fenner BM. Truncated TrkB: beyond a dominant negative receptor. Cytokine Growth Factor Rev. 2012;23:15–24. doi: 10.1016/j.cytogfr.2012.01.002. [DOI] [PubMed] [Google Scholar]
- Freund-Michel V, Frossard N. The nerve growth factor and its receptors in airway inflammatory diseases. Pharmacol Ther. 2008;117:52–76. doi: 10.1016/j.pharmthera.2007.07.003. [DOI] [PubMed] [Google Scholar]
- Frossard N, Freund V, Advenier C. Nerve growth factor and its receptors in asthma and inflammation. Eur J Pharmacol. 2004;500:453–465. doi: 10.1016/j.ejphar.2004.07.044. [DOI] [PubMed] [Google Scholar]
- Furman B, Ong WK, Pyne NJ. Cyclic AMP signaling in pancreatic islets. Adv Exp Med Biol. 2010;654:281–304. doi: 10.1007/978-90-481-3271-3_13. [DOI] [PubMed] [Google Scholar]
- Garcia-Suarez O, Perez-Pinera P, Laura R, Germana A, Esteban I, Cabo R, Silos-Santiago I, Cobo JL, Vega JA. TrkB is necessary for the normal development of the lung. Respir Physiol Neurobiol. 2009;167:281–291. doi: 10.1016/j.resp.2009.06.001. [DOI] [PubMed] [Google Scholar]
- Gerthoffer WT. Regulation of the contractile element of airway smooth muscle. Am J Physiol. 1991;261:L15–L28. doi: 10.1152/ajplung.1991.261.2.L15. [DOI] [PubMed] [Google Scholar]
- Gosens R, Zaagsma J, Meurs H, Halayko AJ. Muscarinic receptor signaling in the pathophysiology of asthma and COPD. Respir Res. 2006;7:73. doi: 10.1186/1465-9921-7-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gras D, Chanez P, Vachier I, Petit A, Bourdin A. Bronchial epithelium as a target for innovative treatments in asthma. Pharmacol Ther. 2013;140:290–305. doi: 10.1016/j.pharmthera.2013.07.008. [DOI] [PubMed] [Google Scholar]
- Groneberg DA, Harrison S, Dinh QT, Geppetti P, Fischer A. Tachykinins in the respiratory tract. Curr Drug Targets. 2006;7:1005–1010. doi: 10.2174/138945006778019318. [DOI] [PubMed] [Google Scholar]
- Hack M, Taylor HG, Drotar D, Schluchter M, Cartar L, Andreias L, Wilson-Costello D, Klein N. Chronic conditions, functional limitations, and special health care needs of school-aged children born with extremely low-birth-weight in the 1990s. Jama. 2005;294:318–325. doi: 10.1001/jama.294.3.318. [DOI] [PubMed] [Google Scholar]
- Hahn C, Islamian AP, Renz H, Nockher WA. Airway epithelial cells produce neurotrophins and promote the survival of eosinophils during allergic airway inflammation. J Allergy Clin Immunol. 2006;117:787–794. doi: 10.1016/j.jaci.2005.12.1339. [DOI] [PubMed] [Google Scholar]
- Halayko AJ, Amrani Y. Mechanisms of inflammation-mediated airway smooth muscle plasticity and airways remodeling in asthma. Respir Physiol Neurobiol. 2003;137:209–222. doi: 10.1016/s1569-9048(03)00148-4. [DOI] [PubMed] [Google Scholar]
- Halayko AJ, Solway J. Molecular mechanisms of phenotypic plasticity in smooth muscle cells. J Appl Physiol. 2001;90:358–368. doi: 10.1152/jappl.2001.90.1.358. [DOI] [PubMed] [Google Scholar]
- Hall IP. Second messengers, ion channels and pharmacology of airway smooth muscle. Eur Respir J. 2000;15:1120–1127. doi: 10.1034/j.1399-3003.2000.01523.x. [DOI] [PubMed] [Google Scholar]
- He YY, Zhang XY, Yung WH, Zhu JN, Wang JJ. Role of BDNF in central motor structures and motor diseases. Mol Neurobiol. 2013;48:783–793. doi: 10.1007/s12035-013-8466-y. [DOI] [PubMed] [Google Scholar]
- Hennigan A, O'Callaghan RM, Kelly AM. Neurotrophins and their receptors: roles in plasticity, neurodegeneration and neuroprotection. Biochem Soc Trans. 2007;35:424–427. doi: 10.1042/BST0350424. [DOI] [PubMed] [Google Scholar]
- Hirota N, Martin JG. Mechanisms of airway remodeling. Chest. 2013;144:1026–1032. doi: 10.1378/chest.12-3073. [DOI] [PubMed] [Google Scholar]
- Hirota S, Helli PB, Catalli A, Chew A, Janssen LJ. Airway smooth muscle excitation-contraction coupling and airway hyperresponsiveness. Can J Physiol Pharmacol. 2005;83:725–732. doi: 10.1139/y05-070. [DOI] [PubMed] [Google Scholar]
- Hofer AM. Interactions between calcium and cAMP signaling. Curr Med Chem. 2012;19:5768–5773. doi: 10.2174/092986712804143286. [DOI] [PubMed] [Google Scholar]
- Holgate ST. Airway inflammation and remodeling in asthma: current concepts. Mol Biotechnol. 2002;22:179–189. doi: 10.1385/MB:22:2:179. [DOI] [PubMed] [Google Scholar]
- Holgate ST. Mechanisms of Asthma and Implications for Its Prevention and Treatment: A Personal Journey. Allergy Asthma Immunol Res. 2013;5:343–347. doi: 10.4168/aair.2013.5.6.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyle GW. Neurotrophins and lung disease. Cytokine Growth Factor Rev. 2003;14:551–558. doi: 10.1016/s1359-6101(03)00061-3. [DOI] [PubMed] [Google Scholar]
- Hoyle GW, Graham RM, Finkelstein JB, Nguyen KP, Gozal D, Friedman M. Hyperinnervation of the airways in transgenic mice overexpressing nerve growth factor. Am J Respir Cell Mol Biol. 1998;18:149–157. doi: 10.1165/ajrcmb.18.2.2803m. [DOI] [PubMed] [Google Scholar]
- Ibanez CF, Simi A. p75 neurotrophin receptor signaling in nervous system injury and degeneration: paradox and opportunity. Trends Neurosci. 2012;35:431–440. doi: 10.1016/j.tins.2012.03.007. [DOI] [PubMed] [Google Scholar]
- Jacoby DB. Pathophysiology of airway viral infections. Pulm Pharmacol Ther. 2004;17:333–336. doi: 10.1016/j.pupt.2004.09.014. [DOI] [PubMed] [Google Scholar]
- James A. Airway remodeling in asthma. Curr Opin Pulm Med. 2005;11:1–6. doi: 10.1097/01.mcp.0000146779.26339.d8. [DOI] [PubMed] [Google Scholar]
- Jang SW, Liu X, Chan CB, France SA, Sayeed I, Tang W, Lin X, Xiao G, Andero R, Chang Q, Ressler KJ, Ye K. Deoxygedunin, a natural product with potent neurotrophic activity in mice. PloS one. 2010a;5:e11528. doi: 10.1371/journal.pone.0011528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang SW, Liu X, Yepes M, Shepherd KR, Miller GW, Liu Y, Wilson WD, Xiao G, Blanchi B, Sun YE, Ye K. A selective TrkB agonist with potent neurotrophic activities by 7,8-dihydroxyflavone. Proc Natl Acad Sci U S A. 2010b;107:2687–2692. doi: 10.1073/pnas.0913572107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen LJ. Calcium handling in airway smooth muscle: mechanisms and therapeutic implications. Can Respir J. 1998;5:491–498. doi: 10.1155/1998/678027. [DOI] [PubMed] [Google Scholar]
- Joubert P, Hamid Q. Role of airway smooth muscle in airway remodeling. J Allergy Clin Immunol. 2005;116:713–716. doi: 10.1016/j.jaci.2005.05.042. [DOI] [PubMed] [Google Scholar]
- Kalb R. The protean actions of neurotrophins and their receptors on the life and death of neurons. Trends Neurosci. 2005;28:5–11. doi: 10.1016/j.tins.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Kapfhammer JP. Cellular and molecular control of dendritic growth and development of cerebellar Purkinje cells. Prog Histochem Cytochem. 2004;39:131–182. doi: 10.1016/j.proghi.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Kc P, Martin RJ. Role of central neurotransmission and chemoreception on airway control. Respir Physiol Neurobiol. 2010;173:213–222. doi: 10.1016/j.resp.2010.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemi C, Grunewald J, Eklund A, Hoglund CO. Differential regulation of neurotrophin expression in human bronchial smooth muscle cells. Respir Res. 2006;7:18. doi: 10.1186/1465-9921-7-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MS, Lee KP, Yang D, Shin DM, Abramowitz J, Kiyonaka S, Birnbaumer L, Mori Y, Muallem S. Genetic and pharmacologic inhibition of the Ca2+ influx channel TRPC3 protects secretory epithelia from Ca2+-dependent toxicity. Gastroenterology. 2011;140:2107–2115. doi: 10.1053/j.gastro.2011.02.052. 2115 e2101–2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimata H. Passive smoking elevates neurotrophin levels in tears. Hum Exp Toxicol. 2004;23:215–217. doi: 10.1191/0960327104ht445oa. [DOI] [PubMed] [Google Scholar]
- Koskela HO, Purokivi MK, Romppanen J. Neurotrophins in chronic cough: association with asthma but not with cough severity. Clin Respir J. 2010;4:45–50. doi: 10.1111/j.1752-699X.2009.00143.x. [DOI] [PubMed] [Google Scholar]
- Kovalchuk Y, Holthoff K, Konnerth A. Neurotrophin action on a rapid timescale. Curr Opin Neurobiol. 2004;14:558–563. doi: 10.1016/j.conb.2004.08.014. [DOI] [PubMed] [Google Scholar]
- Kraemer R, Nguyen H, March KL, Hempstead B. NGF activates similar intracellular signaling pathways in vascular smooth muscle cells as PDGF-BB but elicits different biological responses. Arterioscler Thromb Vasc Biol. 1999;19:1041–1050. doi: 10.1161/01.atv.19.4.1041. [DOI] [PubMed] [Google Scholar]
- Lagente V, Boichot E. Role of matrix metalloproteinases in the inflammatory process of respiratory diseases. J Mol Cell Cardiol. 2009;48:440–444. doi: 10.1016/j.yjmcc.2009.09.017. [DOI] [PubMed] [Google Scholar]
- Lavender V, Chong S, Ralphs K, Wolstenholme AJ, Reaves BJ. Increasing the expression of calcium-permeable TRPC3 and TRPC7 channels enhances constitutive secretion. Biochem J. 2008;413:437–446. doi: 10.1042/BJ20071488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leal G, Comprido D, Duarte CB. BDNF-induced local protein synthesis and synaptic plasticity. Neuropharmacology. 2013;76:639–656. doi: 10.1016/j.neuropharm.2013.04.005. [DOI] [PubMed] [Google Scholar]
- Lee R, Kermani P, Teng KK, Hempstead BL. Regulation of cell survival by secreted proneurotrophins. Science. 2001;294:1945–1948. doi: 10.1126/science.1065057. [DOI] [PubMed] [Google Scholar]
- Lessmann V, Brigadski T. Mechanisms, locations, and kinetics of synaptic BDNF secretion: an update. Neurosci Res. 2009;65:11–22. doi: 10.1016/j.neures.2009.06.004. [DOI] [PubMed] [Google Scholar]
- Lessmann V, Gottmann K, Malcangio M. Neurotrophin secretion: current facts and future prospects. Prog Neurobiol. 2003;69:341–374. doi: 10.1016/s0301-0082(03)00019-4. [DOI] [PubMed] [Google Scholar]
- Levi-Montalcini R. The saga of the nerve growth factor. Neuroreport. 1998;9:R71–R83. [PubMed] [Google Scholar]
- Li YX, Xu Y, Ju D, Lester HA, Davidson N, Schuman EM. Expression of a dominant negative TrkB receptor, T1, reveals a requirement for presynaptic signaling in BDNF-induced synaptic potentiation in cultured hippocampal neurons. Proc Natl Acad Sci U S A. 1998;95:10884–10889. doi: 10.1073/pnas.95.18.10884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieu TM, Myers AC, Meeker S, Undem BJ. TRPV1 induction in airway vagal low-threshold mechanosensory neurons by allergen challenge and neurotrophic factors. Am J Physiol Lung Cell Mol Physiol. 2012;302:L941–L948. doi: 10.1152/ajplung.00366.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lommatzsch M, Braun A, Mannsfeldt A, Botchkarev VA, Botchkareva NV, Paus R, Fischer A, Lewin GR, Renz H. Abundant production of brain-derived neurotrophic factor by adult visceral epithelia. Implications for paracrine and target-derived Neurotrophic functions. Am J Pathol. 1999;155:1183–1193. doi: 10.1016/S0002-9440(10)65221-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lommatzsch M, Braun A, Renz H. Neurotrophins in allergic airway dysfunction: what the mouse model is teaching us. Ann N Y Acad Sci. 2003;992:241–249. doi: 10.1111/j.1749-6632.2003.tb03154.x. [DOI] [PubMed] [Google Scholar]
- Lommatzsch M, Quarcoo D, Schulte-Herbruggen O, Weber H, Virchow JC, Renz H, Braun A. Neurotrophins in murine viscera: a dynamic pattern from birth to adulthood. Int J Dev Neurosci. 2005;23:495–500. doi: 10.1016/j.ijdevneu.2005.05.009. [DOI] [PubMed] [Google Scholar]
- Longo FM, Massa SM. Small molecule modulation of p75 neurotrophin receptor functions. CNS & neurological disorders drug targets. 2008;7:63–70. doi: 10.2174/187152708783885093. [DOI] [PubMed] [Google Scholar]
- Longo FM, Massa SM. Small-molecule modulation of neurotrophin receptors: a strategy for the treatment of neurological disease. Nat Rev Drug Discovery. 2013;12:507–525. doi: 10.1038/nrd4024. [DOI] [PubMed] [Google Scholar]
- Lu B, Pang PT, Woo NH. The yin and yang of neurotrophin action. Nat Rev Neurosci. 2005;6:603–614. doi: 10.1038/nrn1726. [DOI] [PubMed] [Google Scholar]
- Lykissas MG, Batistatou AK, Charalabopoulos KA, Beris AE. The role of neurotrophins in axonal growth, guidance, and regeneration. Curr Neurovasc Res. 2007;4:143–151. doi: 10.2174/156720207780637216. [DOI] [PubMed] [Google Scholar]
- McDonald NQ, Chao MV. Structural determinants of neurotrophin action. J Biol Chem. 1995;270:19669–19672. doi: 10.1074/jbc.270.34.19669. [DOI] [PubMed] [Google Scholar]
- Meuchel L, Townsend E, Thompson M, Pabelick C, Prakash Y. International Conference of the American Thoracic Society. New Orleans, LA: 2010. Effect of neurotrophins on NO generation in airway epithelial cells. [Google Scholar]
- Meuchel LW, Stewart A, Smelter DF, Abcejo AJ, Thompson MA, Zaidi SI, Martin RJ, Prakash YS. Neurokinin-neurotrophin interactions in airway smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2011;301:L91–L98. doi: 10.1152/ajplung.00320.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda RC, Sohrabji F, Toran-Allerand D. Interactions of estrogen with the neurotrophins and their receptors during neural development. Horm Behav. 1994;28:367–375. doi: 10.1006/hbeh.1994.1033. [DOI] [PubMed] [Google Scholar]
- Mowla SJ, Farhadi HF, Pareek S, Atwal JK, Morris SJ, Seidah NG, Murphy RA. Biosynthesis and post-translational processing of the precursor to brain-derived neurotrophic factor. J Biol Chem. 2001;276:12660–12666. doi: 10.1074/jbc.M008104200. [DOI] [PubMed] [Google Scholar]
- Murray M, Kim D, Liu Y, Tobias C, Tessler A, Fischer I. Transplantation of genetically modified cells contributes to repair and recovery from spinal injury. Brain Res Brain Res Rev. 2002;40:292–300. doi: 10.1016/s0165-0173(02)00211-4. [DOI] [PubMed] [Google Scholar]
- Nagatsu T, Mogi M, Ichinose H, Togari A. Changes in cytokines and neurotrophins in Parkinson's disease. J Neural Transm Suppl. 2000:277–290. doi: 10.1007/978-3-7091-6301-6_19. [DOI] [PubMed] [Google Scholar]
- Nagatsu T, Sawada M. Inflammatory process in Parkinson's disease: role for cytokines. Curr Pharm Des. 2005;11:999–1016. doi: 10.2174/1381612053381620. [DOI] [PubMed] [Google Scholar]
- Nakahashi T, Fujimura H, Altar CA, Li J, Kambayashi J, Tandon NN, Sun B. Vascular endothelial cells synthesize and secrete brain-derived neurotrophic factor. FEBS Lett. 2000;470:113–117. doi: 10.1016/s0014-5793(00)01302-8. [DOI] [PubMed] [Google Scholar]
- Nassenstein C, Dawbarn D, Pollock K, Allen SJ, Erpenbeck VJ, Spies E, Krug N, Braun A. Pulmonary distribution, regulation, and functional role of Trk receptors in a murine model of asthma. J Allergy Clin Immunol. 2006;118:597–605. doi: 10.1016/j.jaci.2006.04.052. [DOI] [PubMed] [Google Scholar]
- Nassenstein C, Kerzel S, Braun A. Neurotrophins and neurotrophin receptors in allergic asthma. Prog Brain Res. 2004;146:347–367. doi: 10.1016/S0079-6123(03)46022-6. [DOI] [PubMed] [Google Scholar]
- Nassini R, Materazzi S, De Siena G, De Cesaris F, Geppetti P. Transient receptor potential channels as novel drug targets in respiratory diseases. Curr Opin Investig Drugs. 2010;11:535–542. [PubMed] [Google Scholar]
- Nockher WA, Renz H. Neurotrophins in clinical diagnostics: pathophysiology and laboratory investigation. Clin Chim Acta. 2005;352:49–74. doi: 10.1016/j.cccn.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Nockher WA, Renz H. Neurotrophins and asthma: novel insight into neuroimmune interaction. J Allergy Clin Immunol. 2006;117:67–71. doi: 10.1016/j.jaci.2005.08.029. [DOI] [PubMed] [Google Scholar]
- Nowacka M, Obuchowicz E. BDNF and VEGF in the pathogenesis of stress-induced affective diseases: an insight from experimental studies. Pharmacol Rep. 2013;65:535–546. doi: 10.1016/s1734-1140(13)71031-4. [DOI] [PubMed] [Google Scholar]
- Numakawa T, Adachi N, Richards M, Chiba S, Kunugi H. Brain-derived neurotrophic factor and glucocorticoids: reciprocal influence on the central nervous system. Neuroscience. 2013;239:157–172. doi: 10.1016/j.neuroscience.2012.09.073. [DOI] [PubMed] [Google Scholar]
- Ohira K, Hayashi M. A new aspect of the TrkB signaling pathway in neural plasticity. Curr Neuropharmacol. 2009;7:276–285. doi: 10.2174/157015909790031210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Othumpangat S, Gibson LF, Samsell L, Piedimonte G. NGF is an essential survival factor for bronchial epithelial cells during respiratory syncytial virus infection. PloS One. 2009;4:e6444. doi: 10.1371/journal.pone.0006444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan J, Rhode HK, Undem BJ, Myers AC. Neurotransmitters in airway parasympathetic neurons altered by neurotrophin-3 and repeated allergen challenge. Am J Respir Cell Mol Biol. 2010;43:452–457. doi: 10.1165/rcmb.2009-0130OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panettieri RA., Jr Effects of corticosteroids on structural cells in asthma and chronic obstructive pulmonary disease. Proc Am Thoracic Soc. 2004;1:231–234. doi: 10.1513/pats.200402-021MS. [DOI] [PubMed] [Google Scholar]
- Park H, Poo MM. Neurotrophin regulation of neural circuit development and function. Nat Rev Neurosci. 2013;14:7–23. doi: 10.1038/nrn3379. [DOI] [PubMed] [Google Scholar]
- Pezet S, Malcangio M. Brain-derived neurotrophic factor as a drug target for CNS disorders. Expert Opin Ther Targets. 2004;8:391–399. doi: 10.1517/14728222.8.5.391. [DOI] [PubMed] [Google Scholar]
- Piedimonte G. Contribution of neuroimmune mechanisms to airway inflammation and remodeling during and after respiratory syncytial virus infection. Pediatr Infect Dis J. 2003;22:S66–S74. doi: 10.1097/01.inf.0000053888.67311.1d. discussion S74-65. [DOI] [PubMed] [Google Scholar]
- Pisi G, Olivieri D, Chetta A. The airway neurogenic inflammation: clinical and pharmacological implications. Inflammation Allerg Drug Targets. 2009;8:176–181. doi: 10.2174/187152809788681047. [DOI] [PubMed] [Google Scholar]
- Pitts EV, Potluri S, Hess DM, Balice-Gordon RJ. Neurotrophin and Trk-mediated signaling in the neuromuscular system. Int Anesthesiol Clin. 2006;44:21–76. doi: 10.1097/00004311-200604420-00004. [DOI] [PubMed] [Google Scholar]
- Pluchino N, Russo M, Santoro AN, Litta P, Cela V, Genazzani AR. Steroid hormones and BDNF. Neuroscience. 2013;239:271–279. doi: 10.1016/j.neuroscience.2013.01.025. [DOI] [PubMed] [Google Scholar]
- Prakash Y, Thompson MA, Meuchel L, Pabelick CM, Mantilla CB, Zaidi S, Martin RJ. Neurotrophins in lung health and disease. Expert Rev Respir Med. 2010;4:395–411. doi: 10.1586/ers.10.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakash YS. Airway Smooth Muscle in Airway Reactivity and Remodeling: What Have We Learned? Am J Physiol Lung Cell Mol Physiol. 2013 doi: 10.1152/ajplung.00259.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakash YS, Iyanoye A, Ay B, Mantilla CB, Pabelick CM. Neurotrophin effects on intracellular Ca2+ and force in airway smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2006;291:L447–L456. doi: 10.1152/ajplung.00501.2005. [DOI] [PubMed] [Google Scholar]
- Prakash YS, Thompson MA, Pabelick CM. Brain-derived neurotrophic factor in TNF-alpha modulation of Ca2+ in human airway smooth muscle. Am J Respir Cell Mol Biol. 2009;41:603–611. doi: 10.1165/rcmb.2008-0151OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proskocil BJ, Sekhon HS, Jia Y, Savchenko V, Blakely RD, Lindstrom J, Spindel ER. Acetylcholine is an autocrine or paracrine hormone synthesized and secreted by airway bronchial epithelial cells. Endocrinology. 2004;145:2498–2506. doi: 10.1210/en.2003-1728. [DOI] [PubMed] [Google Scholar]
- Racke K, Matthiesen S. The airway cholinergic system: physiology and pharmacology. Pulm Pharmacol Ther. 2004;17:181–198. doi: 10.1016/j.pupt.2004.03.001. [DOI] [PubMed] [Google Scholar]
- Radzikinas K, Aven L, Jiang Z, Tran T, Paez-Cortez J, Boppidi K, Lu J, Fine A, Ai X. A Shh/miR-206/BDNF cascade coordinates innervation and formation of airway smooth muscle. J Neurosci. 2011;31:15407–15415. doi: 10.1523/JNEUROSCI.2745-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramer MS. Endogenous neurotrophins and plasticity following spinal deafferentation. Exp Neurol. 2012;235:70–77. doi: 10.1016/j.expneurol.2010.12.021. [DOI] [PubMed] [Google Scholar]
- Reichardt LF. Neurotrophin-regulated signalling pathways. Philos Trans R Soc Lond B Biol Sci. 2006;361:1545–1564. doi: 10.1098/rstb.2006.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renz H, Kerzel S, Nockher WA. The role of neurotrophins in bronchial asthma: contribution of the pan-neurotrophin receptor p75. Prog Brain Res. 2004;146:325–333. doi: 10.1016/s0079-6123(03)46020-2. [DOI] [PubMed] [Google Scholar]
- Ricci A, Felici L, Mariotta S, Mannino F, Schmid G, Terzano C, Cardillo G, Amenta F, Bronzetti E. Neurotrophin and neurotrophin receptor protein expression in the human lung. Am J Respir Cell Mol Biol. 2004;30:12–19. doi: 10.1165/rcmb.2002-0110OC. [DOI] [PubMed] [Google Scholar]
- Ricci A, Graziano P, Bronzetti E, Saltini C, Sciacchitano S, Cherubini E, Renzoni E, Du Bois RM, Grutters JC, Mariotta S. Increased pulmonary neurotrophin protein expression in idiopathic interstitial pneumonias. Sarcoidosis Vasc Diffuse Lung Dis. 2007;24:13–23. [PubMed] [Google Scholar]
- Ricci A, Mariotta S, Saltini C, Falasca C, Giovagnoli MR, Mannino F, Graziano P, Sciacchitano S, Amenta F. Neurotrophin system activation in bronchoalveolar lavage fluid immune cells in pulmonary sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 2005;22:186–194. [PubMed] [Google Scholar]
- Robinson RC, Radziejewski C, Stuart DI, Jones EY. Structure of the brain-derived neurotrophic factor/neurotrophin 3 heterodimer. Biochemistry. 1995;34:4139–4146. doi: 10.1021/bi00013a001. [DOI] [PubMed] [Google Scholar]
- Rochlitzer S, Nassenstein C, Braun A. The contribution of neurotrophins to the pathogenesis of allergic asthma. Biochem Soc Trans. 2006;34:594–599. doi: 10.1042/BST0340594. [DOI] [PubMed] [Google Scholar]
- Rose CR, Blum R, Kafitz KW, Kovalchuk Y, Konnerth A. From modulator to mediator: rapid effects of BDNF on ion channels. Bioessays. 2004;26:1185–1194. doi: 10.1002/bies.20118. [DOI] [PubMed] [Google Scholar]
- Rose CR, Blum R, Pichler B, Lepier A, Kafitz KW, Konnerth A. Truncated TrkB-T1 mediates neurotrophin-evoked calcium signalling in glia cells. Nature. 2003;426:74–78. doi: 10.1038/nature01983. [DOI] [PubMed] [Google Scholar]
- Sadakata T, Furuichi T. Developmentally regulated Ca2+-dependent activator protein for secretion 2 (CAPS2) is involved in BDNF secretion and is associated with autism susceptibility. Cerebellum. 2009;8:312–322. doi: 10.1007/s12311-009-0097-5. [DOI] [PubMed] [Google Scholar]
- Saragovi HU, Hamel E, Di Polo A. A neurotrophic rationale for the therapy of neurodegenerative disorders. Curr Alzheimer Res. 2009;6:419–423. doi: 10.2174/156720509789207912. [DOI] [PubMed] [Google Scholar]
- Sariola H. The neurotrophic factors in non-neuronal tissues. Cell Mol Life Sci. 2001;58:1061–1066. doi: 10.1007/PL00000921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sathish V, Vanoosten SK, Miller BS, Aravamudan B, Thompson MA, Pabelick CM, Vassallo R, Prakash YS. Brain-derived neurotrophic factor in cigarette smoke-induced airway hyperreactivity. Am J Respir Cell Mol Biol. 2013;48:431–438. doi: 10.1165/rcmb.2012-0129OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaaf MJ, De Kloet ER, Vreugdenhil E. Corticosterone effects on BDNF expression in the hippocampus. Implications for memory formation. Stress. 2000;3:201–208. doi: 10.3109/10253890009001124. [DOI] [PubMed] [Google Scholar]
- Schmidt M, Dekker FJ, Maarsingh H. Exchange protein directly activated by cAMP (epac): a multidomain cAMP mediator in the regulation of diverse biological functions. Pharmacol Rev. 2013;65:670–709. doi: 10.1124/pr.110.003707. [DOI] [PubMed] [Google Scholar]
- Schulte-Herbruggen O, Jockers-Scherubl MC, Hellweg R. Neurotrophins: from pathophysiology to treatment in Alzheimer's disease. Curr Alzheimer Res. 2008;5:38–44. doi: 10.2174/156720508783884620. [DOI] [PubMed] [Google Scholar]
- Seino S, Takahashi H, Fujimoto W, Shibasaki T. Roles of cAMP signalling in insulin granule exocytosis. Diabetes Obesity Metabol. 2009;11(Suppl 4):180–188. doi: 10.1111/j.1463-1326.2009.01108.x. [DOI] [PubMed] [Google Scholar]
- Serres F, Carney SL. Nicotine regulates SH-SY5Y neuroblastoma cell proliferation through the release of brain-derived neurotrophic factor. Brain Res. 2006;1101:36–42. doi: 10.1016/j.brainres.2006.05.023. [DOI] [PubMed] [Google Scholar]
- Shoval G, Weizman A. The possible role of neurotrophins in the pathogenesis and therapy of schizophrenia. Eur Neuropsychopharmacol. 2005;15:319–329. doi: 10.1016/j.euroneuro.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Sieck GC, Mantilla CB. Role of neurotrophins in recovery of phrenic motor function following spinal cord injury. Respir Physiol Neurobiol. 2009;169:218–225. doi: 10.1016/j.resp.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpkins JW, Green PS, Gridley KE, Singh M, de Fiebre NC, Rajakumar G. Role of estrogen replacement therapy in memory enhancement and the prevention of neuronal loss associated with Alzheimer's disease. Am J Med. 1997;103:19S–25S. doi: 10.1016/s0002-9343(97)00260-x. [DOI] [PubMed] [Google Scholar]
- Skaper SD. The biology of neurotrophins, signalling pathways, and functional peptide mimetics of neurotrophins and their receptors. CNS Neurol Disorders Drug Targets. 2008;7:46–62. doi: 10.2174/187152708783885174. [DOI] [PubMed] [Google Scholar]
- Skaper SD, Giusti P, Facci L. Microglia and mast cells: two tracks on the road to neuroinflammation. FASEB J. 2012;26:3103–3117. doi: 10.1096/fj.11-197194. [DOI] [PubMed] [Google Scholar]
- Soleman S, Filippov MA, Dityatev A, Fawcett JW. Targeting the neural extracellular matrix in neurological disorders. Neuroscience. 2013;253:194–213. doi: 10.1016/j.neuroscience.2013.08.050. [DOI] [PubMed] [Google Scholar]
- Sopi RB, Martin RJ, Haxhiu MA, Dreshaj IA, Yao Q, Jafri A, Zaidi SI. Role of brain-derived neurotrophic factor in hyperoxia-induced enhancement of contractility and impairment of relaxation in lung parenchyma. Am J Physiol Lung Cell Mol Physiol. 2008;295:L348–L355. doi: 10.1152/ajplung.00067.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparrow MP, Weichselbaum M, McCray PB. Development of the innervation and airway smooth muscle in human fetal lung. Am J Respir Cell Mol Biol. 1999;20:550–560. doi: 10.1165/ajrcmb.20.4.3385. [DOI] [PubMed] [Google Scholar]
- Stein AT, Ufret-Vincenty CA, Hua L, Santana LF, Gordon SE. Phosphoinositide 3-kinase binds to TRPV1 and mediates NGF-stimulated TRPV1 trafficking to the plasma membrane. J Gen Physiol. 2006;128:509–522. doi: 10.1085/jgp.200609576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suliman S, Hemmings SM, Seedat S. Brain-Derived Neurotrophic Factor (BDNF) protein levels in anxiety disorders: systematic review and meta-regression analysis. Front Integr Neurosci. 2013;7:55. doi: 10.3389/fnint.2013.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki S, Kiyosue K, Hazama S, Ogura A, Kashihara M, Hara T, Koshimizu H, Kojima M. Brain-derived neurotrophic factor regulates cholesterol metabolism for synapse development. J Neurosci. 2007;27:6417–6427. doi: 10.1523/JNEUROSCI.0690-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki S, Numakawa T, Shimazu K, Koshimizu H, Hara T, Hatanaka H, Mei L, Lu B, Kojima M. BDNF-induced recruitment of TrkB receptor into neuronal lipid rafts: roles in synaptic modulation. J Cell Biol. 2004;167:1205–1215. doi: 10.1083/jcb.200404106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney M, McDaniel SS, Platoshyn O, Zhang S, Yu Y, Lapp BR, Zhao Y, Thistlethwaite PA, Yuan JX. Role of capacitative Ca2+ entry in bronchial contraction and remodeling. J Appl Physiol. 2002;92:1594–1602. doi: 10.1152/japplphysiol.00722.2001. [DOI] [PubMed] [Google Scholar]
- Szczepankiewicz A, Breborowicz A, Skibinska M, Wilkosc M, Tomaszewska M, Hauser J. Association analysis of brain-derived neurotrophic factor gene polymorphisms in asthmatic children. Pediatr Allergy Immunol. 2007;18:293–297. doi: 10.1111/j.1399-3038.2007.00525.x. [DOI] [PubMed] [Google Scholar]
- Szczepankiewicz A, Breborowicz A, Sobkowiak P, Popiel A. Association of BDNF gene polymorphism with asthma in polish children. World Allergy Org J. 2010;3:235–238. doi: 10.1097/WOX.0b013e3181eedb68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szczepankiewicz A, Rose-Zerilli MJ, Barton SJ, Holgate ST, Holloway JW. Association analysis of brain-derived neurotrophic factor gene polymorphisms in asthmatic families. Int Arch Allergy Immunol. 2009;149:343–349. doi: 10.1159/000205580. [DOI] [PubMed] [Google Scholar]
- Tabakman R, Lecht S, Sephanova S, Arien-Zakay H, Lazarovici P. Interactions between the cells of the immune and nervous system: neurotrophins as neuroprotection mediators in CNS injury. Prog Brain Res. 2004;146:387–401. doi: 10.1016/s0079-6123(03)46024-x. [DOI] [PubMed] [Google Scholar]
- Tam A, Morrish D, Wadsworth S, Dorscheid D, Man SF, Sin DD. The role of female hormones on lung function in chronic lung diseases. BMC women's health. 2011;11:24. doi: 10.1186/1472-6874-11-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor-Clark T, Undem BJ. Transduction mechanisms in airway sensory nerves. J Appl Physiol. 2006;101:950–959. doi: 10.1152/japplphysiol.00222.2006. [DOI] [PubMed] [Google Scholar]
- Teng KK, Hempstead BL. Neurotrophins and their receptors: signaling trios in complex biological systems. Cell Mol Life Sci. 2004;61:35–48. doi: 10.1007/s00018-003-3099-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiele CJ, Li Z, McKee AE. On Trk--the TrkB signal transduction pathway is an increasingly important target in cancer biology. Clin Cancer Res. 2009;15:5962–5967. doi: 10.1158/1078-0432.CCR-08-0651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tollet J, Everett AW, Sparrow MP. Spatial and temporal distribution of nerves, ganglia, and smooth muscle during the early pseudoglandular stage of fetal mouse lung development. Dev Dyn. 2001;221:48–60. doi: 10.1002/dvdy.1124. [DOI] [PubMed] [Google Scholar]
- Tortorolo L, Langer A, Polidori G, Vento G, Stampachiacchere B, Aloe L, Piedimonte G. Neurotrophin overexpression in lower airways of infants with respiratory syncytial virus infection. Am J Respir Crit Care Med. 2005;172:233–237. doi: 10.1164/rccm.200412-1693OC. [DOI] [PubMed] [Google Scholar]
- Townsend EA, Miller VM, Prakash YS. Sex differences and sex steroids in lung health and disease. Endocrine reviews. 2012;33:1–47. doi: 10.1210/er.2010-0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Undem BJ, Nassenstein C. Airway nerves and dyspnea associated with inflammatory airway disease. Respir Physiol Neurobiol. 2009;167:36–44. doi: 10.1016/j.resp.2008.11.012. [DOI] [PubMed] [Google Scholar]
- Undem BJ, Potenzieri C. Autonomic neural control of intrathoracic airways. Comprehensive Physiol. 2012;2:1241–1267. doi: 10.1002/cphy.c110032. [DOI] [PubMed] [Google Scholar]
- Underwood CK, Coulson EJ. The p75 neurotrophin receptor. Intl J Biochem Cell Biol. 2008;40:1664–1668. doi: 10.1016/j.biocel.2007.06.010. [DOI] [PubMed] [Google Scholar]
- Urrego F, Scuri M, Auais A, Mohtasham L, Piedimonte G. Combined effects of chronic nicotine and acute virus exposure on neurotrophin expression in rat lung. Pediatr Pulmonol. 2009;44:1075–1084. doi: 10.1002/ppul.21099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhein KC, Fryer AD, Jacoby DB. Neural control of airway inflammation. Curr Allergy Asthma Rep. 2009;9:484–490. doi: 10.1007/s11882-009-0071-9. [DOI] [PubMed] [Google Scholar]
- Virchow JC, Julius P, Lommatzsch M, Luttmann W, Renz H, Braun A. Neurotrophins are increased in bronchoalveolar lavage fluid after segmental allergen provocation. Am J Respir Crit Care Med. 1998;158:2002–2005. doi: 10.1164/ajrccm.158.6.9803023. [DOI] [PubMed] [Google Scholar]
- Vohra PK, Thompson MA, Sathish V, Kiel A, Jerde C, Pabelick CM, Singh BB, Prakash YS. TRPC3 regulates release of brain-derived neurotrophic factor from human airway smooth muscle. Biochim Biophys Acta. 2013;1833:2953–2960. doi: 10.1016/j.bbamcr.2013.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Bray P, McCaffrey T, March K, Hempstead BL, Kraemer R. p75(NTR) mediates neurotrophin-induced apoptosis of vascular smooth muscle cells. Am J Pathol. 2000;157:1247–1258. doi: 10.1016/S0002-9440(10)64640-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster NJ, Pirrung MC. Small molecule activators of the Trk receptors for neuroprotection. BMC neuroscience. 2008;9(Suppl 2):S1. doi: 10.1186/1471-2202-9-S2-S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weishaupt N, Blesch A, Fouad K. BDNF: the career of a multifaceted neurotrophin in spinal cord injury. Exp Neurol. 2012;238:254–264. doi: 10.1016/j.expneurol.2012.09.001. [DOI] [PubMed] [Google Scholar]
- White TA, Xue A, Chini EN, Thompson M, Sieck GC, Wylam ME. Role of TRPC3 in Tumor Necrosis Factor-alpha Enhanced Calcium Influx in Human Airway Myocytes. Am J Respir Cell Mol Biol. 2006 doi: 10.1165/rcmb.2006-0003OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita T, Fujitani M, Hata K, Mimura F, Yamagishi S. Diverse functions of the p75 neurotrophin receptor. Anat Sci Int. 2005;80:37–41. doi: 10.1111/j.1447-073x.2005.00095.x. [DOI] [PubMed] [Google Scholar]
- Yang IV, Schwartz DA. Epigenetic mechanisms and the development of asthma. J Allergy Clin Immunol. 2012;130:1243–1255. doi: 10.1016/j.jaci.2012.07.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Q, Haxhiu MA, Zaidi SI, Liu S, Jafri A, Martin RJ. Hyperoxia enhances brain-derived neurotrophic factor and tyrosine kinase B receptor expression in peribronchial smooth muscle of neonatal rats. Am J Physiol Lung Cell Mol Physiol. 2005;289:L307–L314. doi: 10.1152/ajplung.00030.2005. [DOI] [PubMed] [Google Scholar]
- Yao Q, Zaidi SI, Haxhiu MA, Martin RJ. Neonatal lung and airway injury: a role for neurotrophins. Seminars in perinatology. 2006;30:156–162. doi: 10.1053/j.semperi.2006.04.008. [DOI] [PubMed] [Google Scholar]
- Yinli C, Jie H, Li Z, Jun G, Peiling L, Weihong Y. Association between brain-derived neurothropic factor variants and asthma in Chinese Han children. Acta Paediatr. 2013;102:e247–e250. doi: 10.1111/apa.12224. [DOI] [PubMed] [Google Scholar]
- Zagrebelsky M, Korte M. Form follows function: BDNF and its involvement in sculpting the function and structure of synapses. Neuropharmacology. 2013 doi: 10.1016/j.neuropharm.2013.05.029. [DOI] [PubMed] [Google Scholar]
- Zaidi SI, Jafri A, Doggett T, Haxhiu MA. Airway-related vagal preganglionic neurons express brain-derived neurotrophic factor and TrkB receptors: implications for neuronal plasticity. Brain Res. 2005;1044:133–143. doi: 10.1016/j.brainres.2005.02.037. [DOI] [PubMed] [Google Scholar]
- Zeilinger S, Pinto LA, Nockher WA, Depner M, Klopp N, Illig T, von Mutius E, Renz H, Kabesch M. The effect of BDNF gene variants on asthma in German children. Allergy. 2009;64:1790–1794. doi: 10.1111/j.1398-9995.2009.02131.x. [DOI] [PubMed] [Google Scholar]
- Zheng F, Zhou X, Moon C, Wang H. Regulation of brain-derived neurotrophic factor expression in neurons. Intl J Physiol Pathophysiol Pharmacol. 2012;4:188–200. [PMC free article] [PubMed] [Google Scholar]