Abstract
Some individual fish like to be close together in ‘schools’, while other individuals like to be alone. A pair of recent papers dissects the genetic basis of schooling behavior, showing that genetic changes in sensory systems are involved when this social behavior is lost during evolution.
Did you ever stand in a Cavern’s Mouth—
Widths out of the Sun—
And look—and shudder, and block your breath—
And deem to be alone
Emily Dickinson
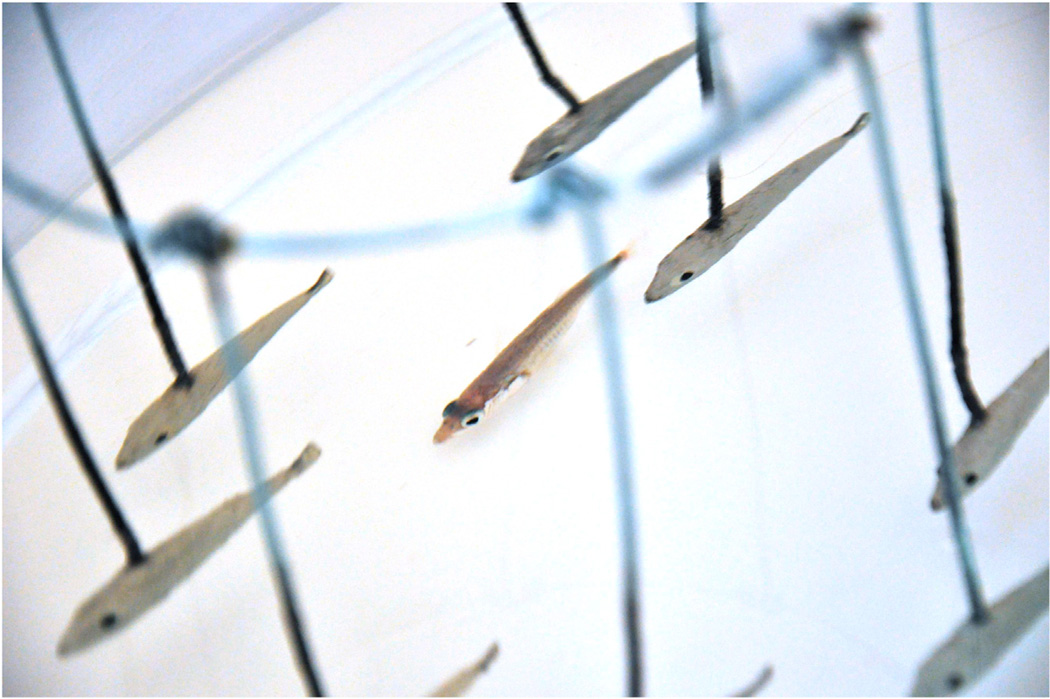
For the small cave-dwelling fish Astyanax mexicanus, the world must indeed appear to be a dark and lonely place. They can’t see — not only because it’s dark, but because they don’t have eyes. It turns out that compared to their sighted relatives of the same species that live in surface waters, A. mexicanus in caves are indeed often alone. While their surface-dwelling relatives swim together in tight aggregations known as schools, cave-dwellers lead a more solitary existence. In many species, individuals aggregate in order to guard themselves against predators or to find food. Within-species variability in schooling behavior has been documented in other fishes. Two new papers [1,2] in this issue of Current Biology tackle the genetic basis for schooling behavior. In the case of A mexicanus, Kowalko et al. [2] show that vision is required for this social behavior, but the loss of schooling behavior in cave-dwellers evolved independently of the loss of vision. In another paper, Greenwood et al. [1] show that this social behavior can be broken into different components that map to different regions of the genome in sticklebacks (Figure 1).
Figure 1.
Schooling in sticklebacks. A threespine stickleback (center) ‘schooling’ with a model school. Photo by Anna Greenwood.
Both cavefish and sticklebacks have proven to be profitable systems for identifying the genetic basis of how traits are lost during evolution. Crosses between surface- and cave-dwelling fish followed by genetic mapping have narrowed-down genomic regions harboring genes related to the loss of eyes [3] and pigmentation [4]. Similarly, genetic mapping based on crosses between marine and freshwater sticklebacks has revealed genomic regions (and even genes) related to the loss of skeletal traits [5,6]. But tackling the genetic basis of differences in social behavior is more challenging. Behavioral traits are notoriously complex, and social behaviors are particularly fraught with environmental influences, including, in particular, the behavior of other individuals [7]. That being said, while schooling behavior might at first glance appear to be hopelessly complex given that it requires coordination among school-mates, relatively simple decision rules can generate complex coordinated group movement [8]; therefore, it might be genetically tractable after all. Indeed the two new papers [1,2] show that it is possible to track down regions of the genome that influence schooling behavior. One of the key insights from the stickleback study [3] is that by carefully measuring different elements of schooling (attending to the behavior of others versus position while schooling, for example), the authors could dissect this complex behavior into more manageable components, which then could be mapped to different regions of the genome.
The lab must have been like a science fair while the series of experiments by Kowalko et al. [2] were ongoing. Not only did they build an artificial school of fish that ‘swam’ around a tank, they ran experiments in the dark, put fish on antidepressants and gave their subjects the cavefish equivalent of choices at the optometrist’s office: which do you prefer — light or dark? The authors complemented these studies with surgery (ablating the lateral line, removing lenses from embryos), genomics and QTL mapping. Altogether this paper comprises a series of diverse and often clever experiments to answer some hard questions.
First, the authors establish that cave-dwelling fish really don’t school, and show that there is a heritable basis to this trait. Why did they lose schooling behavior? The authors mention a few possible reasons — perhaps because there are few predators in caves, and therefore no benefit to hiding behind the group. But their real interest is in how schooling was lost, i.e. the underlying mechanism. One possibility is that the loss of schooling is related to the enhancement of the lateral line in cave-dwellers. The lateral line system is a peripheral mechanoreceptive sensory system that is more developed in cave-dwellers because it improves their ability to find food in darkness [9]. However, the cave-dwellers’ hyperactive lateral line system might interfere with schooling — providing a repulsive rather than attractive force. Kowalko et al. [2] rejected this hypothesis after experimentally ablating the lateral line, and observing no effect on schooling behavior.
Another possibility is that cave-dwellers don’t school because they have higher levels of monoamines such as serotonin and dopamine in the brain compared to surface-dwellers, probably because these systems have been co-opted by cave-dwellers to focus on finding food rather than fighting with others [10]. The authors find some support for this idea — fish on the antidepressant R-deprenyl schooled less.
But the crux of the Kowalko et al. [2] paper is about the possibility that cave-dwellers don’t school because they can’t see each other. It turns out that surface-dwellers like to be in the dark, but when they’re in the dark, they don’t school. Moreover, when the eye lenses of surface-dwelling fish are removed, they don’t school — strong evidence that vision is required for schooling. These observations suggest that when A. mexicanus first entered a pitch-dark cave (probably because they like to be in the dark), they could not see each other, and as vision is necessary for schooling, they didn’t school. Other studies have shown that there is a genetic basis to the loss of vision in cave-dwellers [3]. Therefore, at first the authors thought vision and schooling might have been lost together over evolutionary time if the same genes influence vision and schooling.
Kowalko et al. [2] address this possibility using the time-honored method of a simple genetic cross between cave and surface fish. When the F1 hybrids are crossed, a wide range of schooling behaviors among the F2 hybrid offspring between surface- and cave-dwellers is observed, but schooling segregates independent of vision. For example, there are sighted F2 hybrids who don’t school, and there are F2 hybrids that strongly prefer the dark and don’t school. Therefore, vision is necessary but not sufficient for schooling behavior, and vision, schooling and preference for dark can be uncoupled at the genetic level.
The authors then harnessed the power of genomics to determine whether the same or different regions of the genome influence schooling, preference for dark and vision. Like most interesting questions in science, the answer is a mix of all of the above. By performing QTL analysis on the subset of the F2s that were light-perceiving, the authors could remove the effects of vision on schooling. Altogether they found QTL that influence vision only, QTL that influence schooling only, and some QTL that influence both preference for dark and schooling. In other words, there are both vision-dependent, and vision-independent loci influencing schooling. This suggests that the loss of schooling in cave-dwellers evolved by multiple genetic changes, only some of which are vision-dependent.
The paper by Greenwood et al. [1] offers a different evolutionary scenario for the loss of schooling in threespine sticklebacks. Like A. mexicanus, there are populations of sticklebacks that differ in schooling behavior, and the populations are inter-fertile. Unlike A. mexicanus, though, in the stickleback case the populations differ in how tightly they school — sticklebacks from the ocean school tightly together, while sticklebacks in lakes still form schools, but they are looser. Moreover, while the lateral line has a small effect on schooling in A. mexicanus, Greenwood et al. [1] found a genetic link between schooling and the lateral line in sticklebacks.
Together, these studies show that schooling behavior in both species was lost through modifications to sensory systems, but that convergent loss of schooling occurred via different sensory mechanisms (vision versus lateral line [11]). It will be fascinating to learn whether this generalization also applies within species. That is, was schooling lost via the same genetic changes in different populations of cavefish, for example? And did the genetic changes originate once (selection on standing genetic variation) or multiple times? This question is tractable in both the cavefish and stickleback systems as there are multiple populations of both species that have independently lost schooling behavior. Another outstanding question is whether the loss of schooling behavior is actually an adaptive response to relaxed predation pressure in caves or lakes, or if it reflects neutral evolution and genetic drift. Perhaps genes influencing schooling accumulate mutations, which eventually result in loss of function and disappearance of the trait. Finally, there will be great interest in knowing the identity of the mutations that can turn a socialite fish into a loner.
References
- 1.Greenwood AK, Wark AR, Yoshida K, Peichel CL. Genetic and neural modularity underlie the evolution of schooling behavior in threespine sticklebacks. Curr. Biol. 2013;23:1884–1888. doi: 10.1016/j.cub.2013.07.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kowalko JE, Rohner N, Rompani SB, Peterson BK, Linden TA, Yoshizawa M, Kay EH, Weber J, Hoekstra HE, Jeffery WR, et al. Loss of schooling behavior in cavefish through sight-dependent and sight-independent mechanisms. Curr. Biol. 2013;23:1874–1883. doi: 10.1016/j.cub.2013.07.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O’Quin KE, Yoshizawa M, Doshi P, Jeffery WR. Quantitative genetic analysis of retinal degeneration in the blind cavefish Astyanax mexicanus. PloS One. 2013;8:e57281. doi: 10.1371/journal.pone.0057281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Protas ME, Hersey C, Kochanek D, Zhou Y, Wilkens H, Jeffery WR, Zon LI, Borowsky R, Tabin CJ. Genetic analysis of cavefish reveals molecular convergence in the evolution of albinism. Nat. Genet. 2006;38:107–111. doi: 10.1038/ng1700. [DOI] [PubMed] [Google Scholar]
- 5.Colosimo PF, Hosemann KE, Balabhadra S, Guadalupe Villarreal J, Dickson M, Grimwood J, Schmutz J, Myers RM, Schluter D, Kingsley DM. Widespread parallel evolution in sticklebacks by repeated fixation of ectodysplasin alleles. Science. 2005;307:1928–1933. doi: 10.1126/science.1107239. [DOI] [PubMed] [Google Scholar]
- 6.Shapiro MD, Marks ME, Peichel CL, Blackman BK, Nereng KS, Jonsson B, Schluter D, Kingsley DM. Genetic and developmental basis of evolutionary pelvic reduction in threespine sticklebacks. Nature. 2004;428:717–723. doi: 10.1038/nature02415. [DOI] [PubMed] [Google Scholar]
- 7.Robinson GE, Fernald RD, Clayton DF. Genes and social behavior. Science. 2008;322:896–900. doi: 10.1126/science.1159277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sumpter DJT, Krause J, James R, Couzin ID, Ward AJW. Consensus decision making by fish. Curr. Biol. 2008;18:1773–1777. doi: 10.1016/j.cub.2008.09.064. [DOI] [PubMed] [Google Scholar]
- 9.Yoshizawa M, Goricki S, Soares D, Jeffery WR. Evolution of a behavioral shift mediated by superficial neuromasts helps cavefish find food in darkness. Curr. Biol. 2010;20:1631–1636. doi: 10.1016/j.cub.2010.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elipot Y, Hinaux H, Callebert J, Retaux S. Evolutionary shift from fighting to foraging in blind cavefish through changes in the serotonin network. Curr. Biol. 2013;23:1–10. doi: 10.1016/j.cub.2012.10.044. [DOI] [PubMed] [Google Scholar]
- 11.Arendt J, Reznick D. Convergence and parallelism reconsidered: what have we learned about the genetics of adaptation? Trends Ecol. Evol. 2008;23:26–32. doi: 10.1016/j.tree.2007.09.011. [DOI] [PubMed] [Google Scholar]