Abstract
Maternal Embryonic Leucine zipper Kinase (MELK) is expressed in several developing tissues, in the adult germ line, and in adult neural progenitors. MELK expression is elevated in aggressive undifferentiated tumors correlating with poor patient outcome in human breast cancer. To investigate the role of MELK in mammary tumorigenesis in vivo we used a MELK-GFP reporter mouse, which allows prospective isolation of MELK expressing cells based on GFP fluorescence. We found that in the normal mammary gland, cells expressing high levels of MELK were enriched for proliferating cells, expressing markers of mammary progenitors. The isolation of cells with high levels of MELK in mammary tumors from MMTV-Wnt1/MELK-GFP bitransgenic mice resulted in a significant enrichment of tumorsphere formation in culture and tumor initiation after transplantation into mammary fat pads of syngeneic mice. Furthermore, using lentiviral delivery of MELK-specific shRNA and limiting dilution cell transplantations we demonstrated that MELK function is required for mammary tumorigenesis in vivo. Our findings identify MELK as potential target in breast tumor initiating cells.
Keywords: Mammary tumor, MMTV-Wnt1, tumor-initiating cells, MELK, breast cancer therapy
Introduction
The cancer stem cell model originates from the observed heterogeneity of tumors and the presence of a minority population of tumor cells that are capable of both self-renewal and differentiation (1-5). Targeting cancer stem cells, also called tumor-initiating cells, critically depends on the identification of therapeutic targets. In breast cancer, cells with CD44+CD24low/− phenotype isolated from human tumors are enriched for tumor-forming capacity (6). ALDH-1 is one of the few intracellular, potenti ally functional, markers of human breast tumor-initiating cells that have been reported (7). In mice, cells with the CD24+Thy-1+ phenotype, as well as a subset of luminal progenitors with CD29lowCD24+CD61+ phenotype, from MMTV-Wnt1 tumors are found to be enriched in tumor-initiating cells (8). The Wnt/β-catenin pathway is implicated in mammary tumorigenesis (9). However, the paucity of current molecular targets warrants the search for new candidates that allow developing therapeutic strategies to specifically target and eliminate tumor-initiating cells.
Maternal Embryonic Leucine zipper Kinase (MELK) (10) is a member of the Snf1/AMPK family of kinases, but is unique in that is not regulated by LKB1 kinase (11). In addition to a Ser/Thr kinase domain, MELK contains an unstructured C-terminal ubiquitin associated (UBA) domain that prevents ubiquitin-mediated degradation (12), a leucine zipper motif and a C-terminal kinase-associated (KA1) domain. During development, it is expressed in tissues that contain normal progenitor cells (13) and in adult brain progenitor cells (14, 15). The MELK gene was first cloned from a human myeloid leukemic cell line (16) and subsequently shown to be commonly expressed in poorly differentiated aggressive cancers (17, 18). For example, MELK RNA is dramatically upregulated in glioblastomas and is correlated with malignancy grade in human astrocytomas (19, 20). We have previously demonstrated in vitro that MELK siRNA inhibits the growth of primary glioblastoma cell lines (21). The in vivo function of MELK is currently unknown, however, CDC25B phosphatase, a critical G2/M checkpoint protein, and apoptosis signal-regulating kinase 1 (ASK1) were suggested as potential MELK targets (22, 23).
Elevated expression of MELK was found to be associated with the poor prognosis of breast cancer patients (24) and glioblastoma patients (21). MELK was found to physically interact with and phosphorylate pro-apoptotic Bcl-G. The over expression of wild-type (wt) MELK, but not a kinase-dead mutant, was reported to suppress Bcl-G–induced apoptosis promoting mammary carcinogenesis (25). Based on the growth inhibition of several cancer cell lines in vitro, MELK was proposed to be a promising target for multiple cancer types (26). However, two critical questioned remained unanswered: First, do tumor initiating cells express MELK? Second, is MELK required for mammary tumorigenesis in vivo? Here we used the MMTV-Wnt1 mouse mammary tumor model to demonstrate that cells expressing elevated levels of MELK are enriched for tumor-initiating cells. Critically, we demonstrated that MELK function is required for mammary tumor growth in vivo.
Materials and Methods
Tumorsphere cultures
For culture, the cells were resuspended in Mammary Stem Cell Medium (MSCM) composed of: MEBM (Lonza), 20 ng/ml EGF, 20 ng/ml bFGF, 4 μg/ml Heparin, 1× B27 (Invitrogen) and 2× penicillin/streptomycin. For sphere forming assays, cells in MSCM were mixed (12:1) with growth factor deficient Matrigel (BD Bioscience) and MSCM overlaid each week and images taken after two weeks with a Nikon fluorescent microscope. Unless otherwise stated reagents were from Sigma Aldrich.
Limiting dilution transplantation of Wnt1-induced tumor cells
The transplantation was performed as previously described (27). Briefly, primary tumors were dissociated into single cells. Cells were sorted by FACS into GFPhigh (top 10 %) and GFPlow (bottom 10%) fractions and injected into cleared mammary fat pads of 19 day old FVB/N females at limiting dilutions of 1000, 100 and 10 cells mixed with a 1:1 ratio of MSCM : Matrigel. Tumor development was checked by palpitation three times a week. Mammary glands were removed and imaged with a LEICA fluorescent dissecting scope, after which they were fixed in Carnoys, stained with Carmin Alum and mounted with Permount.
Inhibition of tumorsphere growth by MELK shRNA
Tumors were dissociated into single cells and cultivated for two passages (3 weeks per passage) in a mix of MSCM and Matrigel (1:1) in low attachment dishes. 0.5ml MSCM was added every week to the culture. The resulting tumorspheres were separated from the Matrigel using a Dispase treatment and dissociated into single cells with Accutase. 2×105 cells were treated with 10μl of two different lentiviral constructs (MOI~10) one carrying a MELK shRNA construct the other a control shRNA. Cells were incubated with the virus overnight at 37°C and plated in a mix of MSCM and Matrigel (1:1) in low-attachment dishes the following day.
Statistical analysis
The frequencies of tumor-initiating cells and 95% confidence intervals were calculated using Poisson statistics and maximum likelihood estimate as implemented in ELDA software http://bioinf.wehi.edu.au/software/elda/index.html (28). The ELDA software was specifically designed to handle the extreme situations when all transplanted pads are either negative or positive. The goodness of fit to a single-hit Poisson model was tested using Chi-square as implemented in ELDA software.
Results
MELK is expressed in proliferating luminal cells in normal mammary epithelium
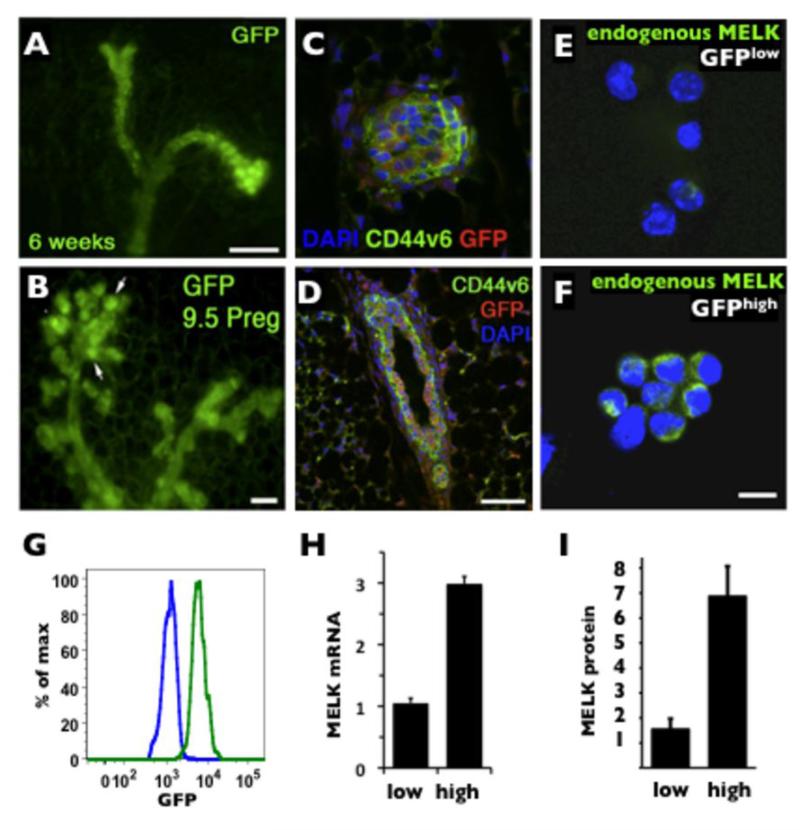
Mammary epithelial tissue consists of the ducts, alveoli, and terminal end buds. The ducts are organized as layers of internal luminal epithelial cells covered by the basally located myoepithelial cells (29, 30). First, we investigated which population of cells expresses MELK in the normal mammary gland. Due to the lack of antibodies that robustly detect MELK, we took advantage of the MELK-GFP promoter-reporter mouse, which we previously used to trace the expression of endogenous MELK in the neural stem/progenitor population (31). We found that MELK is selectively expressed in both the ducts and the terminal end buds of virgin mammary epithelial tissue. In whole mounts, GFP fluorescence is increased in terminal end buds and alveolar expansion during pregnancy (Fig.1A, B). Immunofluorescent detection of GFP expression in sectioned material and co-staining with the epithelial marker CD44v6 (32) indicates enhanced expression in the luminal epithelium (Fig.1C, D). To confirm that the MELK-GFP reporter truly represents the levels of endogenous MELK protein, we sorted GFPlow (10% bottom) and GFPhigh (10% top) cells and immunostained both populations for the endogenous MELK protein (Fig.1E, F). Quantification of the fluorescent signal showed a ~4.7 fold increase in immunoreactivity for the endogenous MELK protein in the GFPhigh cells as compared to GFPlow cells (Fig. 1G), which correlated with a ~ 5 fold increase in GFP fluorescence between GFPhigh (median=5.5×103) and GFPlow (median=1.1×103) populations (Fig. 1I). In addition, the Q-PCR analysis of endogenous MELK mRNA illustrated a ~3 fold increase between GFPhigh and GFPlow populations (Fig. 1H). Taken together, these results confirm that the GFP signal in the mammary tissue of MELK-GFP mice correlates with the expression level of the endogenous MELK mRNA and MELK protein, and that MELK expression is predominantly localized to the luminal epithelium of the mammary gland.
Figure 1. Characterization of MELK expression in normal mammary glands.
A, whole mount mammary fat pad from a 6 weeks-old virgin mouse (Scale bar 100 μm). B, 9.5 days pregnant MELK-GFP female. Note the increased GFP expression in the proliferative alveoli (arrows). Scale bar 50μm. C and D, immunofluorescence of a 6 weeks-old virgin MELK-GFP transgenic mouse mammary gland demonstrating colocalization of GFP expression (red) and CD44v6 (green) in a terminal end bud (C) and predominant GFP expression in epithelial cells as demarcated by CD44v6 in a cross section of a mammary duct (D). Scale bar 30μm. E and F, detection of endogenous MELK protein by immunofluorescence in FACS isolated GFPlow (bottom 10%) and GFPhigh (top 10%) cells. Scale bar 10μm. G. Fluorescent histograms of GFPlow (blue line, median=1.1×103) and GFPhigh (green line median=5.5×103) populations. H. Q-PCR detection of MELK mRNA in GFPlow and GFPhigh populations (normalized to 18s expression). I. Quantification of endogenous MELK protein shown in E, F normalized to DAPI).
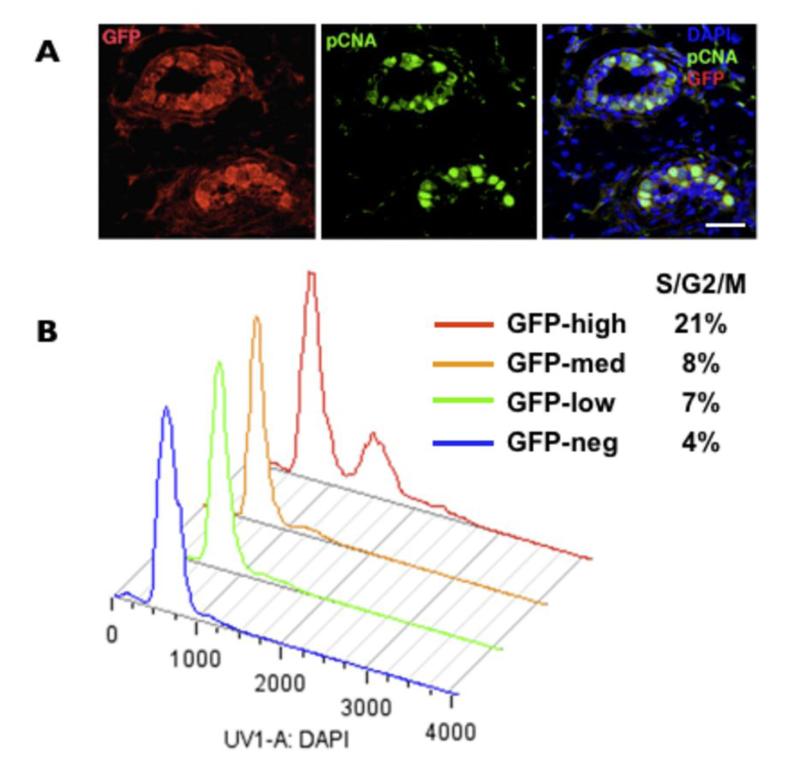
We have previously demonstrated selective expression of MELK in proliferative progenitors in developing and neonatal nervous system (31) and in proliferative cancer cells in culture (33). To determine the cell cycle status of MELK-GFP cells in the normal mammary gland we co-stained GFP-positive cells for proliferating cell nuclear antigen (PCNA). The GFP-positive luminal cells were positive for PCNA, suggesting that MELK is upregulated in proliferating luminal cells (Fig. 2A). We then determined the cell cycle status of various GFP fractions. The high, medium, low, and negative fractions were isolated by FACS and analyzed for DNA content. The GFPhigh cells had the highest proportion of cells (21%) in the S/G2/M phase (Fig. 2B). Together with the PCNA staining these results indicate that MELK expression is associated with proliferating cells and that the GFPhigh fraction is enriched for cycling cells.
Figure 2. Levels of MELK expression correlate with proliferation in normal mammary cells.
A, colocalization of PCNA (green) and GFP (red) in the luminal cells of the ducts in 6 weeks-old virgin mammary. DAPI staining is in blue. Scale bar 40μm. B, the cell cycle analysis of various GFP populations from freshly dissociated 6 weeks-old virgin mammary glands.
Increased MELK expression in mammary cells with markers of progenitor cells
Previous studies indicate that in the normal mammary gland stem cell activity is found in the Lin−, (CD45− CD31− TER119−) CD29highCD24+ compartment, whereas progenitors were found in the Lin−CD29lowCD24+ compartment (34). In addition, the mammary stem cell compartment was positive for keratin 14 (K14), a basal epithelium marker, whereas progenitors were positive for K8, area luminal marker (34). We used MELK-GFP transgenic mice to investigate the expression of MELK with respect to the previously defined stem cell and progenitor cell compartments in the normal mammary epithelium. We found a significantly higher expression of MELK in cells that contain progenitors compared to the population that include stem cells cell (Fig. 3A). These results are consistent with the immunofluorescent detection of MELK expression in situ (Fig. 1C,D).
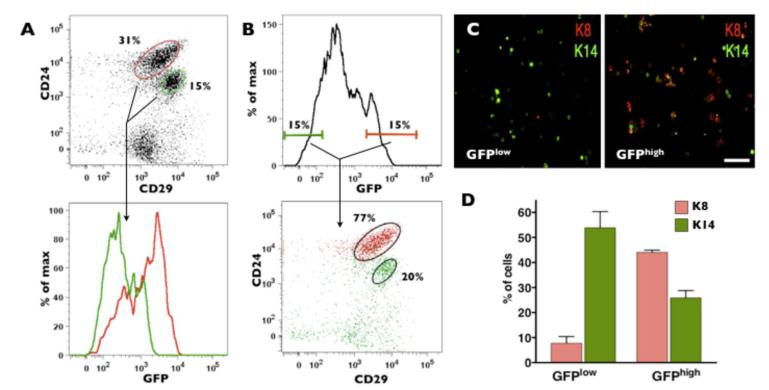
Figure 3. Cells with highest levels of MELK expression include mammary progenitors.
A, flow cytometry analysis of dissociated normal mammary gland revealed up-regulation of GFP signal in CD24+CD29low cells compared to CD24+CD29high cells. B, the GFPhigh cells (top 10-15%) are enriched for cells with progenitor markers. Dot plot and histogram of live Lin− cells (CD45−CD31−TER119−PI−) are shown in the top panels. Cells in the lower panels are color-coded and gated as indicated. C, immunostaining for K8 and K14 demonstrated that GFPhigh cells are enriched for K8 cells, a luminal marker, while GFPlow cells are enriched for K14, a basal marker. Scale bar 50μm. D, quantification of K8 and K14 expression in GFPHigh and GFPLow fractions from MELK-GFP virgin mammary glands. GFPLow and GFPHigh cells express significantly different levels of K8 and K14, p=0.002 and p=0.0164, respectively, n = 3.
Next, we examined the expression of the CD24/29 markers within the GFPhigh (top 10-15%) and GFPlow (bottom 10-15%) cells freshly isolated from normal mammary glands. We found that a majority of GFPhigh cells (77%) express levels of CD24 and CD29 similar to that previously found in a cell population enriched for mammary progenitors (34). GFPlow cells were broadly distributed with a minority (20%) clustered in a population with the levels of CD24 and CD29 typical for mammary stem cells (Fig. 3B). These results suggest that within the normal mammary gland of MELK-GFP mice the top 10-15% of GFP-positive cells are within the population that is enriched for normal mammary progenitors (34).
Next, we isolated the GFPhigh (top 10%) and GFPlow (bottom 10%) cells and immunostained these populations for keratins. The GFPlow fraction predominantly expressed basal associated K14 (~55% K14+ cells vs ~10% K8+ cells) whereas GFPhigh cells were enriched for luminal associated K8 (25% K14+ vs 45% K8+) (Fig.3C, D). The increased proportion of K8 expressing cells in the GFPhigh fraction corresponds with previous reports showing that luminal progenitor enrichment is associated with K8/K18 expression, and intermediate/low levels of K14 (34).
Taken together these results suggest that MELK is upregulated in normal proliferating mammary progenitors and that isolated GFPhigh cells are enriched for such progenitors. The presence of both K8 and K14 positive cells suggests that GFPhigh expressing cells may contain both luminal and basal epithelial proliferating progenitors.
Tumor-initiating cells in MMTV-Wnt1 tumors express high levels MELK
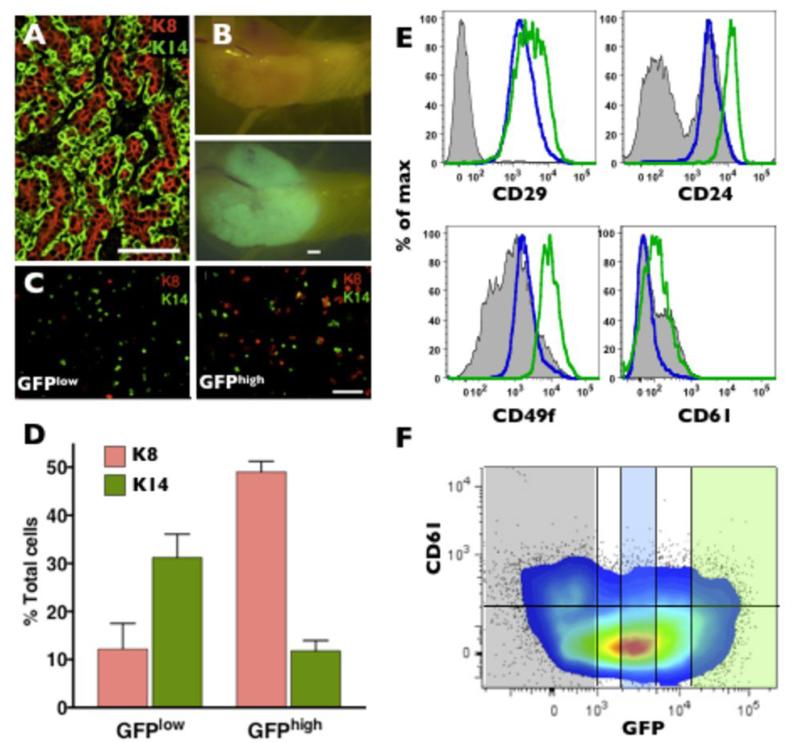
Mammary tumors induced by the Wnt1 gene under the influence of the MMTV enhancer are heterogeneous, containing both luminal and basal epithelial cells (Fig. 4A) (35) and are suggested to originate from progenitor-like cells (36). We crossed the MELK-GFP mice with MMTV-Wnt1 mice and analyzed for MELK expression in these tumors. Whole mounts of mammary fat pads of MMTV-Wnt1/ MELK-GFP bitransgenic mice consistently revealed GFP expression within tumors (Fig. 4B). We isolated GFPlow and GFPhigh cells (top 10% and bottom 10% of the GFP-positive cells) from MMTV-Wnt1/MELK-GFP bitransgenic mice using flow cytometry and determined the expression of K8 and K14 (Fig. 4C). The GFPlow fraction predominantly expressed K14 (30% K14+ and 10% K8+) while GFPhigh cells were significantly enriched (five fold) for K8 (10% K14+ and 50% K8+) (Fig. 4D). These results parallel MELK expression in the normal mammary gland. We also examined the expression of CD29, CD24, CD49f and CD61 surface markers in MMTV-Wnt1/MELK-GFP bitransgenic tumors. We found elevated expression of CD29, CD24, and CD49f in the GFPhigh population (Fig.4E) consistent with the concept that GFPhigh cells contain luminal progenitors in Wnt1 tumors (30, 34, 37). The marker CD61 was recently suggested to identify cancer stem cells in preneoplastic cells in the MMTV-Wnt1 model (38). Indeed, the CD61 expression in GFPhigh cells (median fluorescence 134) was higher than that of GFPlow population (median fluorescence 39). However, a population of CD61+ cells in the GFP-negative fraction was also identified (Fig.4F). Collectively, these results suggest that although MELK expression is not strictly restricted to one type of tumor cell, the GFPhigh fraction in MMTV-Wnt1 tumors is enriched for the cells expressing luminal epithelial markers.
Figure 4. Heterogeneous expression of GFP in MMTV-Wnt1/MELK-GFP bitransgenic tumors.
A, immunostaining for K8 and K14 in a wild-type MMTV-Wnt1 tumor. Scale bar 50μm. B, whole mount bright field and green fluorescent images of a bitransgenic tumor. Scale bar 50μm. C and D, immunostaining (scale bar 50μm) and quantification of K8 and K14 expression in GFPHigh and GFPLow fractions of GFP Wnt1 tumors. GFPLow and GFPHigh cells express significantly different levels of K8 and K14, p=0.003 and p=0.0215, respectively, n=3. E, flow cytometry analysis of stem/progenitor marker expression in GFPnegative(grey area), GFPlow(blue line), and GFPhigh (green line) cells from a MMTV-Wnt1/MELK-GFP bitransgenic tumor. Note GFPhigh cells express elevated CD24, CD49f, CD61 as compared to GFPlow cells. F, flow cytometry analysis (colored dot plot 5% probability with outliers) of CD61 vs GFP expression in MMTV-Wnt1/MELK-GFP bitransgenic tumor. Semi-transparent shaded areas correspond to GFPhigh (green), GFPlow (blue), and GFPnegative (grey) populations. Horizontal line denotes the level of background fluorescence (CD61 negative cells).
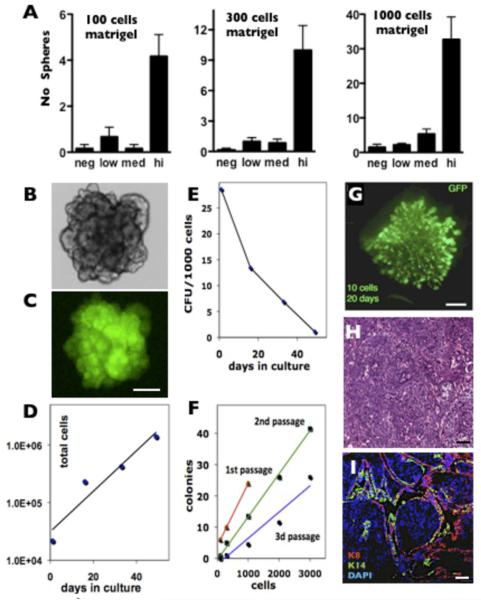
Next, we investigated the proliferative capacity of GFPlow and GFPhigh cells in culture using a tumorsphere assay (39) modified to include Matrigel to decrease anoikis, increase colony formation efficiency, and eliminate cellular aggregation during sphere formation (see Materials and Methods for details). Time-lapse observation of individual cells in Matrigel confirmed the clonal origins of the tumorspheres under these conditions (data not shown). We found that the GFPhigh population contained the vast majority of tumorsphere-forming cells, providing 5-10 fold enrichment compared to other populations at all tested cell densities (Fig. 5A). The spheres can be serially cultivated and maintain GFP expression (Fig. 5B, C). However, despite the linear increase in cumulative total number (Fig. 5D), the sphere forming capacity progressively decreased with passage (Fig. 5E), suggesting that under these conditions the sphere-initiating cells undergo progressive differentiation (Fig. 5F).
Figure 5. The GFPhigh population in MMTV-Wnt1/MELK-GFP bitransgenic tumors is enriched for tumor initiating cells.
A, GFPHigh population is greatly enriched in spheroid forming activity using a sphere forming culture assay under all conditions tested; p<0.05 for all GFPhigh, one-way ANOVA. Proliferating tumorspheres (B, bright field) retain GFP expression (C). Scale bar 100μm. The matrigel cultures of GFPhigh cells have an increased total number of cells (D) but a decreased frequency of sphere forming cells (E). The mammosphere assay is linear for all cell densities and at all passages tested (F). Colony formation efficiency decreased with the first three passages. 1st passage, red, linear regression y = 0.02x + 4□R2 = 1; 2nd passage, green, y = 0.0138x - 0.4401□R2 = 0.9962; 3d passage, blue, y = 0.0084x - 1.9418□R2 = 0.9385). G. Whole-mount GFP fluorescence of a 20 days outgrowth after transplantation of 10 GFPhigh cells derived from WMG49 primary tumor. Scale bar 1mm. H&E (H) and K8, K14 and DAPI (I) staining of a solid tumor developed from transplanted GFPhigh cells clonally derived from WMG300 primary tumor. Scale bar 50μm.
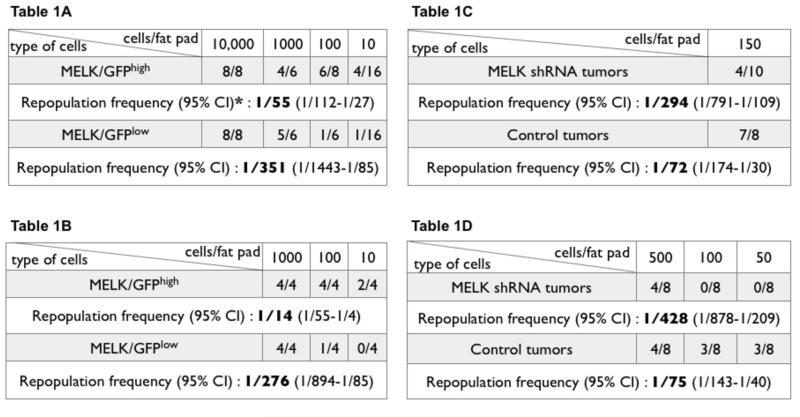
To investigate the in vivo tumorigenicity of primary MMTV-Wnt1/MELK-GFP bitransgenic tumors, we performed limiting-dilution transplantation into cleared mammary fat pads. Based on the in vitro results we chose to compare the GFPhigh and GFPlow populations. In the first tumor analyzed (WMG49), we found that GFPhigh cells are enriched ~6.5-fold in tumorigenic cells compared to GFPlow cells (Table 1A). In this experiment the calculated frequency of cells with tumor-initiating activity for GFPhigh cells was 1/55 cells (95% confidence interval (CI) 1/112-1/27) compared to 1/351 (95% CI, 1/1443-1/85) for GFPlow cells (~6.4 fold enrichment, χ2=7.53, p=0.0061).
Table 1.
Tumor initiating frequencies of GFPhigh/low cells transplanted into the cleared mammary fat pad A, WMG49 tumor: χ2=7.53, p=0.006; * the 1000 cell point was censored from the calculation to satisfy the goodness of fit to a single-hit Poisson model; B, WMG300 tumor: χ2=10, p=0.0013.
|
In a separate experiment, the GFPhigh cells from another primary tumor (WMG300) were isolated, cultured as spheres for 5 passages and then GFPhigh/low fractions isolated and transplanted to determine the frequency of tumor-initiating cells (Table 1B). The calculated frequency of cells with tumor-initiating activity for GFPhigh cells was 1/14 cells (95% confidence interval (CI) 1/55-1/4) compared to 1/276 (95% CI, 1/894-1/85) for GFPlow cells (~20 fold enrichment, χ2=20.3, p=0.0013). When transplanted into a cleared fat pad, as few as 10 GFPhigh cells formed neoplastic outgrowths (WMG49, Fig. 5G, analyzed after 20 days) or solid tumor mass (WMG300, Fig. 5H, I, analyzed after tumors reached 1 cm in diameter). These results demonstrate that mammary tumor cells expressing the highest levels of MELK are enriched for both tumorsphere forming activity in culture and for tumor-initiating activity upon transplantation.
MELK is required for the development of MMTV-Wnt1 tumors in vivo
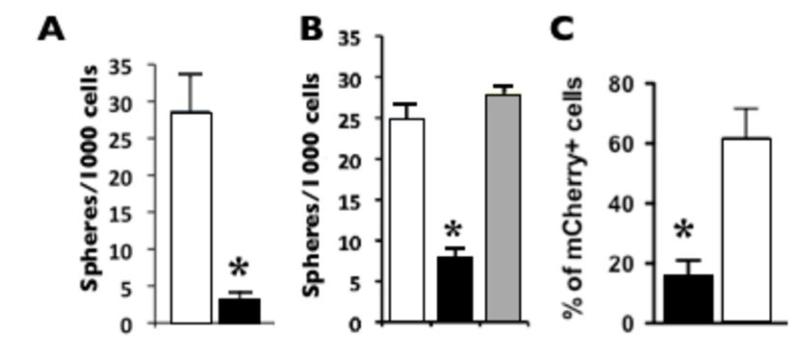
Finally, we investigated whether MELK is required for the initiation and/or propagation of Wnt1-induced mammary tumors in vitro and in vivo. We used lentiviral delivery of MELK shRNA, which was previously documented to specifically down regulate MELK expression in primary cells and several cell lines (21, 31). First, we evaluated the ability of lentivirus coded MELK shRNA to affect the formation of tumorspheres in the 3D Matrigel tumorsphere-forming assay (modified from (39)). MELK shRNA (directed against the 3’UTR of endogenous MELK transcript) but not control shRNA dramatically reduced the number of spheres (~10 fold) formed by the GFPhigh cells from a primary Wnt1 tumor (Fig. 6A). To verify that the reduction in frequency of tumorspheres occurred specifically as a result of MELK downregulation we performed a rescue experiment. We transduced MELK shRNA treated mammary cells with a MELK cDNA expressing lentivirus and observed a complete rescue of tumorsphere formation (Fig. 6B). These results confirm that the inhibitory function of the MELK shRNA on tumorsphere formation shRNA is specific for MELK.
Figure 6. MELK function is required for Wnt1-induced tumorigenesis in vitro and in vivo.
Black bars = MELK shRNA, white bars = control shRNA, grey bar = MELK cDNA rescue. A and B, MELK shRNA reduced the frequency of tumorsphere-initiating cells in Wnt1 tumors. B, co-infection with virus expressing the MELK cDNA rescues tumorsphere proliferation; p < 0.05, n = 3. C, the percentage (%) of mCherry-positive cells in tumors (from D) after transduction with MELK shRNA is reduced compared to control shRNA. Unpaired t-test, p=0.0072.
To investigate if MELK shRNA can inhibit mammary tumorigenesis in vivo we transduced WMG300 MMTV-Wnt1 tumor cells with lentiviruses expressing MELK shRNA or control shRNA followed by transplantation into cleared mammary fat pads. We observed a significant decrease in the number of tumors formed when the tumor cells were transduced with MELK shRNA virus as compared to control shRNA (Table 2A). Statistically, the tumor initiation frequency was decreased ~4 fold from 1/72 to 1/294 (95% CI, 1/791-1/109 to 1/174-1/30, χ2=4.6, p=0.032).
Nevertheless, tumors did arise from shRNA MELK transduced cells, suggesting that some cells had escaped the action of the shRNA MELK lentivirus. To investigate this, we undertook additional experiments using lentiviral vectors co-expressing mCherry in addition to the shRNAs. This permitted the evaluation of the efficiency of viral transduction and fate of the tumors cells through the evaluation of mCherry expression. Similar to the earlier experiments, we observed a significant reduction in the number of tumors arising from MELK shRNA transduced MMTV-Wnt1 cells as compared to the control vector (Table 2B). The tumor initiation frequency was decreased ~6 fold from 1/75 to 1/428 (95% CI, 1/878-1/ 209 to 1/140-1/40, χ2=14.2, p=0.0001). Moreover, the tumors emerging from MELK shRNA transduced cells contained significantly fewer cells with mCherry expression as compared to control lentivirus expressing scrambled siRNA and mCherrry, (unpaired t-test, p=0.0072) (Fig. 6C). Thus the tumors arising from MMTV-Wnt1 cells transduced with the MELK shRNA appear to originate predominately from cells that were either not initially infected or silenced by mCherry/MELK shRNA expression during tumor growth. This indicates a strong selection against MELK shRNA transduced cells during the tumor development in vivo (the MELK shRNA and the control lentiviruses had equal titers and the infection frequency were close to 90% for both). Taken together these data suggest that MELK shRNA reduces tumorigenicity and a selective advantage is afforded to tumor-initiating cells that fail to maintain the expression of the MELK shRNA vector. We conclude that MELK is required for MMTV-Wnt1 tumorigenesis in vivo.
Discussion
We demonstrate that MMTV-Wnt1 mammary tumor cells with elevated MELK expression are enriched for tumor-initiating cells as assayed by orthotopic transplantation. Critically, we demonstrate that MELK is required for growth of tumorspheres in 3D cultures and mammary tumors in vivo. Our data suggests that MMTV-Wnt1 tumor-initiating cells express elevated levels of MELK and provide the first in vivo validation of MELK as a promising therapeutic target for breast cancer.
In normal mammary glands MELK is preferentially expressed in proliferative cells with a luminal location. This was supported by enrichment in cells expressing the luminal marker K8 within the GFPhigh fraction. Nearly 80% of GFPhigh cells are found within the CD24+CD29low population, which was previously identified to contain progenitors, but not multipotent stem cells (34). Luminal progenitors are expanded in pre-neoplastic MMTV-Wnt1 mammary glands (38) and in breast tissue of patients harboring BRCA1 mutations (40). Moreover, luminal progenitors have been suggested to be the origin of subsequent mammary tumors in mouse and human (38, 40, 41). Therefore, our data are consistent with the current hypothesis that breast cancer stem cells may originate from the luminal progenitors rather than stem cells (38, 40, 41).
In fact, we have previously documented MELK expression in progenitor rather than the stem cell compartment in neural tissue where MELK is required in neural progenitors both during development and in adult life (31)(A.V.T unpublished observations). In addition, MELK is expressed in human colon cancer cell lines and in the middle and basal part of normal colonic crypt epithelia, which is consistent with MELK expression in the proliferative progenitor compartment (26). Similarly, MELK expression in the testis is mainly localized to the α6+ c-kit+ progenitor cells directly contacting the basal membrane (13) (A.V.T. unpublished observations). The high proliferation activity of progenitors compared to more quiescent stem cells is consistent with the suggested involvement of MELK with the cell cycle, in particular G2/M phase (22, 42).
Some cancer, targets may be expressed and be functionally important in the normal stem cells of a tissue (e.g. Bmi1 is expressed in leukemia and normal hematopoietic stem cells (43)). Targeting genes that are commonly expressed in the tumor and normal stem cells may eliminate normal stem cells, resulting in tissue collapse. On the other hand, if the tumor target is expressed in progenitor cells, then the aggressive targeting will likely eliminate both tumor cells and the normal progenitors. The advantage of such a target is that the stem cell compartment is preserved, allowing progenitor division and tissue regeneration after therapy withdrawal. In this respect MELK could be uniquely positioned, representing a promising therapeutic target that is preferentially expressed in progenitors.
The expression of MELK in both normal progenitors and tumor initiating cells may reflect the cell cycle regulation of MELK (26, 33). The MMTV-Wnt1 oncogene results in expanded stem/progenitor cell compartment before solid tumors arise, due to secondary mutations like activated Ras (44). However, not all proliferating cells express MELK. For example, non-transformed proliferating cells such as IMR90 and WI-38 human fibroblasts don’t express detectable levels of MELK (26). As expected, IMR90 cells continue to proliferate after transduction with MELK shRNA (A.V.T. unpublished observation).
The frequency of tumorigenic cells in MMTV-Wnt1 tumors has been reported to be from 1/177 (38) to 1 /7,496 cell (8). The variability of cancer stem cell abundance may be due to both technical issues of transplant location, the use of Matrigel, and the requirement for a secondary oncogenic event, such as mutated Ras to convert the MMTV-Wnt1 induced ductal hyperplasia to solid tumors. Thus each tumor is likely to be genetically distinct and therefore have a variable abundance of tumor-initiating cells (44, 45). It is difficult to judge the utility of a given marker to enrich for tumor-initiating cells between different MMTV-Wnt1 tumors because of the expected oncogenic heterogeneity. However, within the cells of an individual tumor the GFPhigh fraction of cells was enriched for tumor-initiating cells 5-10 fold greater than all other fractions (i.e. low and medium) in a 3D Matrigel tumorsphere-forming assay. Previously, using primary pleural effusions from breast cancer patients, the tumorsphere-forming capacity was directly correlated with in vivo tumorigenicity upon transplantation into SCID mice (46). In vivo, the GFPhigh cells were enriched 6-20 fold in tumor-initiating cells compared to GFPlow cells from the same tumor. However, the GFPhigh fraction is heterogeneous as reflected by the presence of both K8 and K14 positive cells in the GFP high fraction. It will be important to determine if the tumor-initiating cells reside in the K8-positive fraction, K14-positive fraction or in cells that express both keratins.
The CD61/beta3 integrin is a suggested marker of mammary luminal progenitor cells and cancer stem cells in MMTV-Wnt1 mammary tumors (38). GFPhigh cells express higher levels of CD61 than GFPlow cells. However, a low-tumorigenic GFPnegative fraction also expresses CD61, suggesting that GFPhigh cells contain only a fraction of CD61+ cells. Our enrichment for tumorigenic cells based on MELK-GFP appears comparable or higher than the use of CD61, or the combination of CD24 and Thy1 (8).
The expression analysis of human breast cancers identified hundreds of gene candidates (18). Here we provide evidence that MELK is a promising target for therapeutic intervention in breast cancer, particularly due to its expression in tumor initiating cells. Given the success of small molecule inhibitors of kinase catalytic activity for other cancer types (e.g., Gleevec, Iressa, Tarceva), the catalytic domain of MELK is an intriguing candidate target for drug development.
Acknowledgements
We are very grateful to Yoav Altman for his expert help with flow cytometry procedures and data analysis, Kutbuddin Doctor for statistical analysis support. This work was supported in part by a pilot grant from NCI Cancer Center Support Grant 5 P30 CA030199, California Breast Cancer Research Program grants 14IB-0082 (R.G.O.) and 12IB-0079 (A.V.T.) and the National Cancer Institute grant R21 CA123353 (A.V.T.) .
References
- 1.Stevens LC. Studies on transplantable testicular teratomas of strain 129 mice. Journal of the National Cancer Institute. 1958;20:1257–75. doi: 10.1093/jnci/20.6.1257. [DOI] [PubMed] [Google Scholar]
- 2.Kleinsmith LJ, Pierce GB., Jr Multipotentiality of Single Embryonal Carcinoma Cells. Cancer research. 1964;24:1544–51. [PubMed] [Google Scholar]
- 3.Lapidot T, Sirard C, Vormoor J, et al. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 1994;367:645–8. doi: 10.1038/367645a0. [DOI] [PubMed] [Google Scholar]
- 4.Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–11. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 5.Visvader JE, Lindeman GJ. Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. Nat Rev Cancer. 2008;8:755–68. doi: 10.1038/nrc2499. [DOI] [PubMed] [Google Scholar]
- 6.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003;100:3983–8. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ginestier C, Hur MH, Charafe-Jauffret E, et al. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell stem cell. 2007;1:555–67. doi: 10.1016/j.stem.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cho RW, Wang X, Diehn M, et al. solation and molecular characterization of cancer stem cells in MMTV-Wnt-1 murine breast tumors. Stem cells (Dayton, Ohio) 2008;26:364–71. doi: 10.1634/stemcells.2007-0440. [DOI] [PubMed] [Google Scholar]
- 9.Liu BY, McDermott SP, Khwaja SS, Alexander CM. The transforming activity of Wnt effectors correlates with their ability to induce the accumulation of mammary progenitor cells. PNAS. 2004;101:4158–63. doi: 10.1073/pnas.0400699101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heyer BS, Warsowe J, Solter D, Knowles BB, Ackerman SL. New member of the Snf1/AMPK kinase family, Melk, is expressed in the mouse egg and preimplantation embryo. Molecular reproduction and development. 1997;47:148–56. doi: 10.1002/(SICI)1098-2795(199706)47:2<148::AID-MRD4>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 11.Lizcano JM, Goransson O, Toth R, et al. LKB1 is a master kinase that activates 13 kinases of the AMPK subfamily, including MARK/PAR-1. Embo J. 2004;23:833–43. doi: 10.1038/sj.emboj.7600110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boudrez A, Beullens M, Waelkens E, Stalmans W, Bollen M. Phosphorylation-dependent interaction between the splicing factors SAP155 and NIPP1. The Journal of biological chemistry. 2002;277:31834–41. doi: 10.1074/jbc.M204427200. [DOI] [PubMed] [Google Scholar]
- 13.Heyer BS, Kochanowski H, Solter D. Expression of Melk, a new protein kinase, during early mouse development. Dev Dyn. 1999;215:344–51. doi: 10.1002/(SICI)1097-0177(199908)215:4<344::AID-AJA6>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 14.Terskikh A, Easterday M, Li L, et al. From Hematopoiesis to Neuropoiesis. Evidence of Ovelapping Genetic Program. Proc Natl Acad Sci U S A. 2001;98:7934–9. doi: 10.1073/pnas.131200898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Geschwind DH, Ou J, Easterday MC, et al. A genetic analysis of neural progenitor differentiation. Neuron. 2001;29:325–39. doi: 10.1016/s0896-6273(01)00209-4. [DOI] [PubMed] [Google Scholar]
- 16.Nagase T, Seki N, Ishikawa K, Tanaka A, Nomura N. Prediction of the coding sequences of unidentified human genes. V. The coding sequences of 40 new genes (KIAA0161-KIAA0200) deduced by analysis of cDNA clones from human cell line KG-1. DNA Res. 1996;3:17–24. doi: 10.1093/dnares/3.1.17. [DOI] [PubMed] [Google Scholar]
- 17.Hemmati HD, Nakano I, Lazareff JA, et al. Cancerous stem cells can arise from pediatric brain tumors. Proc Natl Acad Sci U S A. 2003;100:15178–83. doi: 10.1073/pnas.2036535100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rhodes DR, Yu J, Shanker K, et al. Large-scale meta-analysis of cancer microarray data identifies common transcriptional profiles of neoplastic transformation and progression. Proc Natl Acad Sci U S A. 2004;101:9309–14. doi: 10.1073/pnas.0401994101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu G, Yuan X, Zeng Z, et al. Analysis of gene expression and chemoresistance of CD133+ cancer stem cells in glioblastoma. Mol Cancer. 2006;5:67. doi: 10.1186/1476-4598-5-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marie SK, Okamoto OK, Uno M, et al. Maternal embryonic leucine zipper kinase transcript abundance correlates with malignancy grade in human astrocytomas. International journal of cancer. 2008;122:807–15. doi: 10.1002/ijc.23189. [DOI] [PubMed] [Google Scholar]
- 21.Nakano I, Masterman-Smith M, Saigusa K, et al. Maternal embryonic leucine zipper kinase is a key regulator of the proliferation of malignant brain tumors, including brain tumor stem cells. Journal of neuroscience research. 2008;86:48–60. doi: 10.1002/jnr.21471. [DOI] [PubMed] [Google Scholar]
- 22.Mirey G, Chartrain I, Froment C, et al. CDC25B phosphorylated by pEg3 localizes to the centrosome and the spindle poles at mitosis. Cell cycle (Georgetown, Tex. 2005;4:806–11. doi: 10.4161/cc.4.6.1716. [DOI] [PubMed] [Google Scholar]
- 23.Jung H, Seong HA, Ha H. Murine protein serine/threonine kinase 38 activates apoptosis signal-regulating kinase 1 via Thr 838 phosphorylation. The Journal of biological chemistry. 2008;283:34541–53. doi: 10.1074/jbc.M807219200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pickard MR, Green AR, Ellis IO, et al. Dysregulated expression of Fau and MELK is associated with poor prognosis in breast cancer. Breast Cancer Res. 2009;11:R60. doi: 10.1186/bcr2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin ML, Park JH, Nishidate T, Nakamura Y, Katagiri T. Involvement of maternal embryonic leucine zipper kinase (MELK) in mammary carcinogenesis through interaction with Bcl-G, a pro-apoptotic member of the Bcl-2 family. Breast Cancer Res. 2007;9:R17. doi: 10.1186/bcr1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gray D, Jubb AM, Hogue D, et al. Maternal embryonic leucine zipper kinase/murine protein serine-threonine kinase 38 is a promising therapeutic target for multiple cancers. Cancer research. 2005;65:9751–61. doi: 10.1158/0008-5472.CAN-04-4531. [DOI] [PubMed] [Google Scholar]
- 27.Young LJT. Methods in Mammary Gland Biology and Breast Cancer Research. Plenum Publishers; New York: 2000. The cleared mammary fat pad and the transplantation of mammary gland morphological structures and cells; pp. 67–74. I a A K A P Publishers. 2000. [Google Scholar]
- 28.Hu Y, Smyth GK. ELDA: extreme limiting dilution analysis for comparing depleted and enriched populations in stem cell and other assays. J Immunol Methods. 2009;347:70–8. doi: 10.1016/j.jim.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 29.Hovey RC, Trott JF. Morphogenesis of mammary gland development. Advances in experimental medicine and biology. 2004;554:219–28. doi: 10.1007/978-1-4757-4242-8_19. [DOI] [PubMed] [Google Scholar]
- 30.Stingl J, Raouf A, Emerman JT, Eaves CJ. Epithelial progenitors in the normal human mammary gland. J Mammary Gland Biol Neoplasia. 2005;10:49–59. doi: 10.1007/s10911-005-2540-7. [DOI] [PubMed] [Google Scholar]
- 31.Nakano I, Paucar AA, Bajpai R, et al. Maternal embryonic leucine zipper kinase (MELK) regulates multipotent neural progenitor proliferation. The Journal of cell biology. 2005;170:413–27. doi: 10.1083/jcb.200412115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hebbard L, Steffen A, Zawadzki V, et al. CD44 expression and regulation during mammary gland development and function. Journal of cell science. 2000;113:2619–30. doi: 10.1242/jcs.113.14.2619. Pt 14. [DOI] [PubMed] [Google Scholar]
- 33.Jaksch M, Munera J, Bajpai R, Terskikh A, Oshima RG. Cell cycle-dependent variation of a CD133 epitope in human embryonic stem cell, colon cancer, and melanoma cell lines. Cancer research. 2008;68:7882–6. doi: 10.1158/0008-5472.CAN-08-0723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shackleton M, Vaillant F, Simpson KJ, et al. Generation of a functional mammary gland from a single stem cell. Nature. 2006;439:84–8. doi: 10.1038/nature04372. [DOI] [PubMed] [Google Scholar]
- 35.Tsukamoto AS, Grosschedl R, Guzman RC, Parslow T, Varmus HE. Expression of the int-1 gene in transgenic mice is associated with mammary gland hyperplasia and adenocarcinomas in male and female mice. Cell. 1988;55:619–25. doi: 10.1016/0092-8674(88)90220-6. [DOI] [PubMed] [Google Scholar]
- 36.Li Y, Welm B, Podsypanina K, et al. Evidence that transgenes encoding components of the Wnt signaling pathway preferentially induce mammary cancers from progenitor cells. Proc Natl Acad Sci U S A. 2003;100:15853–8. doi: 10.1073/pnas.2136825100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stingl J, Eirew P, Ricketson I, et al. Purification and unique properties of mammary epithelial stem cells. Nature. 2006;439:993–7. doi: 10.1038/nature04496. [DOI] [PubMed] [Google Scholar]
- 38.Vaillant F, Asselin-Labat ML, Shackleton M, Forrest NC, Lindeman GJ, Visvader JE. The mammary progenitor marker CD61/beta3 integrin identifies cancer stem cells in mouse models of mammary tumorigenesis. Cancer research. 2008;68:7711–7. doi: 10.1158/0008-5472.CAN-08-1949. [DOI] [PubMed] [Google Scholar]
- 39.Dontu G, Abdallah WM, Foley JM, et al. In vitro propagation and transcriptional profiling of human mammary stem/progenitor cells. Genes & development. 2003;17:1253–70. doi: 10.1101/gad.1061803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lim E, Vaillant F, Wu D, et al. Aberrant luminal progenitors as the candidate target population for basal tumor development in BRCA1 mutation carriers. Nat Med. 2009;15:907–13. doi: 10.1038/nm.2000. [DOI] [PubMed] [Google Scholar]
- 41.Bouras T, Pal B, Vaillant F, et al. Notch signaling regulates mammary stem cell function and luminal cell-fate commitment. Cell stem cell. 2008;3:429–41. doi: 10.1016/j.stem.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 42.Karsten SL, Kudo LC, Jackson R, Sabatti C, Kornblum HI, Geschwind DH. Global analysis of gene expression in neural progenitors reveals specific cell-cycle, signaling, and metabolic networks. Developmental biology. 2003;261:165–82. doi: 10.1016/s0012-1606(03)00274-4. [DOI] [PubMed] [Google Scholar]
- 43.Pietersen AM, Evers B, Prasad AA, et al. Bmi1 regulates stem cells and proliferation and differentiation of committed cells in mammary epithelium. Curr Biol. 2008;18:1094–9. doi: 10.1016/j.cub.2008.06.070. [DOI] [PubMed] [Google Scholar]
- 44.Podsypanina K, Li Y, Varmus HE. Evolution of somatic mutations in mammary tumors in transgenic mice is influenced by the inherited genotype. BMC medicine. 2004;2:24. doi: 10.1186/1741-7015-2-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li Y, Hively WP, Varmus HE. Use of MMTV-Wnt-1 transgenic mice for studying the genetic basis of breast cancer. Oncogene. 2000;19:1002–9. doi: 10.1038/sj.onc.1203273. [DOI] [PubMed] [Google Scholar]
- 46.Grimshaw MJ, Cooper L, Papazisis K, et al. Mammosphere culture of metastatic breast cancer cells enriches for tumorigenic breast cancer cells. Breast Cancer Res. 2008;10:R52. doi: 10.1186/bcr2106. [DOI] [PMC free article] [PubMed] [Google Scholar]