Abstract
Background
Hemorrhagic shock results in cellular damage and cell death. A primary mechanism is cellular apoptosis from mitochondrial damage. This study demonstrated that administration of crocetin to experimental animals during resuscitation from shock significantly improved postshock survival and reduced apoptosis. Crocetin is a component of saffron and has long been used in traditional medicine in Asia.
Methods
Male Sprague-Dawley rats (350 ± 30g) were randomly assigned to 1 of 4 groups of 8 animals. Hemorrhagic shock was induced by withdrawing blood until the mean arterial pressure was 35–40 mm Hg, and blood pressure was maintained at that level for 60 minutes with further withdrawals as needed. Resuscitation was carried out by administration of 21 mL/kg lactated Ringer’s solution and return of shed blood, with or without concurrent administration of crocetin (2 mg/kg). Control animals were sham-treated with surgical preparation, without shock or resuscitation, and with and without crocetin. Rats were sacrificed 24 hours after completion of resuscitation. The extent of activation of hepatic apoptosis was established by measuring levels of hepatic cytosolic cytochrome c, caspase-3, and bcl-2. A separate group of 53 animals treated identically was used to assess survival.
Results
Crocetin administration during resuscitation resulted in less extensive activation of hepatic apoptosis and significantly increased survival relative to controls.
Conclusions
Crocetin administration to experimental animals during resuscitation post hemorrhage increased survival, at least in part by protecting the liver from activation of apoptotic cell death. This agent continues to show promise as a potential treatment strategy for hemorrhagic shock.
Keywords: inflammatory mediators, cytokines, resuscitation, cytochrome c, caspase-3, Bcl-2 protein, saffron, oxygen diffusion
Hemorrhagic shock, a major complication of trauma, is a relatively common and potentially critical medical problem for which optimal therapeutic interventions have yet to be identified. A primary cause of hemorrhage-associated problems is the development of extensive postresuscitation tissue injury mediated by inflammatory mediators, leading to cellular apoptosis. We and others earlier reported that in animals subjected to controlled hemorrhagic shock followed by resuscitation, extensive hepatic apoptosis can be readily detected within 24 hours. It is highly likely that this cellular apoptosis is at least one of the major mechanisms by which significant organ damage results during and following reperfusion leading to tissue injury.1,2
Crocetin has long been used in traditional Chinese medicine as an agent for the treatment for many human diseases, including gout, cancer, depression, constipation, and infections, among others. Structurally, crocetin has been shown to consist of a linear 16-carbon chain, of which 8 of the linkages are double bonds. There are also 4 attached internal methyl groups, and a carboxylic acid (—COOH) group is positioned at each end of the molecule. The molecular weight of this compound is 328 Da (Figure 1).
Figure 1.
Structure of the crocetin molecule.
Present in many foods, crocetin is a major constituent of saffron and is responsible for the characteristic yellow color of that spice. Besides the crocus plant (Crocus sativus L), crocetin is found in a number of other relatively common plants and is commercially extracted from gardenia root (Gardenia jasminoides Ellis). It is commonly sold in the United States as an herbal supplement.
In terms of crocetin’s fundamental mode of action in host tissues that might explain some of its therapeutic properties, crocetin was initially reported by Gainer and others to cause increases in oxygen diffusion in vitro and to improve tissue oxygenation post resuscitation in vivo.3–7 However, the effects of this agent on actual survival were not evaluated in those studies. In our own earlier published studies, we showed that administration of crocetin to adult male rats during resuscitation following experimentally induced hemorrhagic shock improved the recovery of cellular adenosine triphosphate (ATP) in liver and resulted in significantly reduced expression of inflammation-related genes in a rat model of hemorrhagic shock and resuscitation.8–10
To better understand the underlying mechanisms by which crocetin can potentially modulate the host response to hemorrhagic shock, we evaluated the effect of crocetin administration on the level of postresuscitation hepatic apoptosis in a sublethal experimental model of hemorrhagic shock followed by resuscitation. In the studies reported here, we evaluated the extent of hepatic cellular apoptosis by quantitating levels of hepatic cytochrome c, caspase 3, and bcl-2 during the early period post shock (24 hours). In a separate group of rats, we monitored survival over 72 hours following treatment with crocetin at the time of resuscitation relative to control rats treated with resuscitation alone. Our hypothesis in this study was that crocetin administration would significantly reduce the levels of hepatic cellular apoptosis, which would correlate with an increased number of animals surviving relative to untreated controls.
Methods
Experimental Animal Hemorrhagic Shock Model
Sprague-Dawley male rats (n = 32, 350 ± 30 g; purchased from Charles River, Wilmington, MA) were randomly assigned to 4 groups, each group containing 8 animals:
Sham control group—anesthesia and surgical preparation, no hemorrhagic shock, no crocetin intervention
Sham crocetin group—anesthesia and surgical preparation, no hemorrhagic shock, crocetin administered
Control group—hemorrhagic shock, no crocetin intervention, resuscitated with lactated Ringer’s solution (LR) alone
Crocetin group—hemorrhagic shock, resuscitated with LR and administered crocetin during resuscitation
All rats were anesthetized with 3% isoflurane (Baxter Healthcare Corporation, Deerfield, IL) with an anesthesia vaporizer (SurgiVet, Waukesha, WI) with 100% oxygen while breathing spontaneously. Isoflurane was adjusted from 3% to 1% after induction and then maintained at 1%–1.5% until the conclusion of the resuscitation.
After anesthetization, animals were covered with a sterile drape. The temperature under the drape, controlled by a lamp over the table, was maintained at approximately 28°C during the shock period and 36°C during the resuscitation period. After sterile skin preparation, a cervical incision was made. The left carotid artery and jugular vein were cannulated using PE50 polyethylene tubing (Becton Dickinson, Sparks, MD). All animals were anticoagulated with heparin (300 units/kg) through the venous cannula after the procedure was completed. A digital blood pressure analyzer (Micro-Med, Louisville, KY) was used to monitor pressure continuously. Hemorrhagic shock was initiated by withdrawing blood through the carotid arterial cannula over a 5-minute period, until the mean arterial pressure (MAP) stabilized at 35–40 mm Hg, MAP was maintained at that level with further withdrawals of blood, as required, for 60 minutes. MAP was monitored continuously and recorded every 5 minutes.
In all animals subjected to hemorrhagic shock, resuscitation was carried out by administration of LR, 21 mL/kg, followed by return of the shed blood to the animals previously subjected to hemorrhagic shock. Resuscitation fluid and blood were given over a total of 30 minutes. In the crocetin treatment group, crocetin (ICN Biomedicals, Aurora, OH), dissolved in normal saline (0.9%), was administered as a bolus of 2 mg/kg (1 mL/kg of solution, about 0.35 mL) at the time of resuscitation. The crocetin dosage used was determined on the basis of dose–response studies carried out in conjunction with previously reported studies.8–10 As noted below, a larger dose was used following the longer period of shock used in the chronic study.
Control shock animals received an equivalent bolus of normal saline (1 mL/kg). For the sham control and sham crocetin groups, either normal saline or crocetin as above was given, without administration of LR.
All animals were allowed to recover from anesthesia. At 24 hours following resuscitation, a midline laparotomy incision was made, and liver biopsies were taken. Samples were frozen immediately in liquid nitrogen and then stored in a refrigerator at −80°C for further studies. After sampling, animals were sacrificed by exsanguination.
Measurements of Apoptosis
Frozen tissue samples were thawed and gently homogenized, and particulate material was removed by centrifugation. The resulting liver cytosolic extracts were analyzed for markers indicative of relative levels of hepatic apoptosis and levels of cytochrome c, caspase-3, and bcl-2 quantified using enzyme-linked immunosorbent assays (ELISAs) or enzyme activity assays. All assays were compared to purified standards provided by the manufacturers run in parallel. Relative extent of apoptosis was measured using the Cell Death Detection ELISA-PLUS kit (Roche Applied Science, Indianapolis, IN). Cytochrome c was analyzed using a commercially available assay kit (MBL International, Woburn, MA). Caspase-3 was assayed using the Caspase-3 Protease Assay kit (BioSource International, Camarillo, CA). Bcl-2 was analyzed with a bcl-2 ELISA kit (Oncogene Research Products, San Diego, CA).
Animal Survival Studies
For survival studies, male Sprague-Dawley rats (350 ± 30 g, n = 53) were subjected to hemorrhagic shock using a protocol almost identical to that described above but with the difference that a mean arterial pressure of 25–30 mm Hg was maintained for 60 minutes. Two groups of rats were studied, 1 resuscitated with LR (21 mL/kg) alone and the other with LR (21 mL/kg) plus crocetin (4 mg/kg), with both groups receiving return of shed blood. Following recovery from the surgery, all animals were monitored until death or for 72 hours. The larger dose of crocetin was used to compensate for the longer period of shock, in an effort to maximize any potential contribution of the intervention on survival.
Statistical Analysis
Sample size for rats used in both the acute and chronic experiments was calculated using power analysis (PASS, NCSS, Kaysville, UT). For the acute survival experiments, a sample size of 8 was determined to provide a 98% chance of detecting a 2:1 variation in the levels of apoptosis and cytochrome c detected in hepatic extracts of the experimental group vs controls. For the longer term survival experiments, a sample size of 26 was estimated to provide an 87% chance of detecting a 2:1 difference in overall survival between the treated and control animals assuming an approximate control mortality of 80%.
Data analysis was carried out with Microsoft Excel (Microsoft, Redmond, OR) and with NCSS (NCSS, Kaysville, UT). Data results and graphs were expressed as mean ± standard error of the mean. Data were further analyzed using analysis of variance, Student’s t test, and Kruskal-Wallis multiple-comparison z-value test (NCSS, Kaysville, UT). One-tailed analysis of variance was used to compare survival rates (Microsoft Excel). Survival data were further analyzed with Fisher’s exact test. The significance level was .05 for all analyses.
Institutional Approval
All animal care and experimental procedures were carried out strictly in accordance with the Guidelines of the Laboratory Animal Center of the University of Missouri–Kansas City, which is a facility accredited by the American Association for the Accreditation of Laboratory Animal Care. Before and after the surgical procedures, rats were maintained in the Animal Care Facility of the University of Missouri–Kansas City and were maintained on a 12:12-hour light–dark cycle with free access to food and water. The specific experimental protocols under which this work was carried out were approved by the Institutional Animal Care and Use Committee of the University of Missouri–Kansas City.
Results
Crocetin Administration Reduced Levels of Hepatic Apoptosis
As summarized in the Methods section, rats were subjected to 1 hour of sublethal hemorrhagic shock, after which they were resuscitated with LR and shed blood either with or without crocetin at 2 mg/kg. Sham animals were not subjected to shock but received a bolus administration of either saline or crocetin. Hepatic biopsy tissues taken at 24 hours post resuscitation were then assessed for levels of tissue apoptosis. The results from these studies, summarized in Figure 2, indicate that the extent of hepatic apoptosis was significantly increased at 24 hours in the control group whereas hepatic tissue obtained from the crocetin-treated rats showed no significant increase above baseline. We conclude from this experiment that crocetin provides significant protection of experimental animals against hepatic apoptosis induced by hemorrhagic shock.
Figure 2.
Acute studies. Liver cell apoptosis in sham and shock, comparing control and crocetin groups, measured at 24 hours following 60 minutes of hemorrhagic shock and 30 minutes of resuscitation or an equivalent length of time for the sham-operated animals. *P < .05 (control vs crocetin).
It is generally recognized that an early event in development of apoptosis is the release of cytochrome c from mitochondria. Therefore, it was postulated that levels of free cytosolic cytochrome c should be significantly decreased in hepatic tissues of rats treated with crocetin compared with rats treated with LR solution alone. We measured cytosolic extracts of tissue biopsy samples obtained from the rats at 24 hours for levels of cytosolic cytochrome c. The results of these studies, shown in Figure 3, indicate that although cytosolic cytochrome c was clearly present in both groups 24 hours post resuscitation, the levels were significantly reduced in the crocetin-treated rats compared with the control rats.
Figure 3.
Acute studies. Liver cytosolic cytochrome c levels in sham and shock, comparing control and crocetin groups, measured at 24 hours following 60 minutes of hemorrhagic shock and 30 minutes of resuscitation or an equivalent length of time for the sham-operated animals. *P < .05 (control vs crocetin).
It is also well established that early events of cellular apoptosis are accompanied by increases in levels of caspase 3, an important intermediary in the pathway. Furthermore, there is a reduction in levels of bcl-2, a protein known to inhibit development of apoptosis. In support of our results with cytosolic cytochrome c, significant hepatic caspase-3 activation was observed at 24 hours in the control rats, but levels were markedly reduced in the crocetin-treated animals (Figure 4). Correspondingly, hepatic bcl-2 protein levels were diminished at 24 hours post resuscitation in both of the shock groups, but bcl-2 levels in the crocetin-treated rats were significantly higher than in the control rats (Figure 5). We conclude from these findings that crocetin administration at the time of resuscitation significantly reduces the activation of the apoptosis pathway in hepatic tissue relative to controls.
Figure 4.
Acute studies. Caspase-3 activity in sham and shock, comparing control and crocetin groups, measured at 24 hours following 60 minutes of hemorrhagic shock and 30 minutes of resuscitation or an equivalent length of time for the sham-operated animals. *P < .05 (control vs crocetin).
Figure 5.
Acute studies. Bcl-2 levels in sham and shock, comparing control and crocetin groups, measured at 24 hours following 60 minutes of hemorrhagic shock and 30 minutes of resuscitation or an equivalent length of time for the sham-operated animals. * P < .05 (control vs crocetin).
Crocetin Improved Survival Following Hemorrhagic Shock
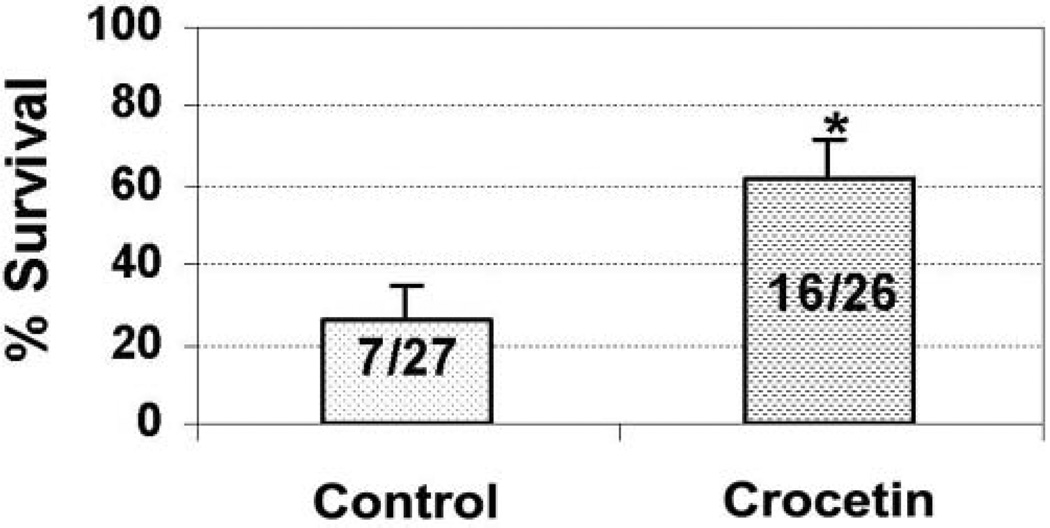
In a separate series of studies to assess survival following hemorrhagic shock, rats were subjected to shock at MAP of 25–30 mm Hg for 60 minutes prior to resuscitation. The MAP in the control group (n = 27) and the crocetin group (n = 26) was reduced to the same level during shock. MAP in both groups recovered to baseline within 10 minutes of beginning fluid resuscitation and actually exceeded baseline values during resuscitation (Figure 6). Animals were then closely followed for 3 days and monitored for survival. As seen in Figure 7, administration of crocetin during resuscitation, compared with resuscitation with LR only, significantly reduced mortality following lethal hemorrhagic shock.
Figure 6.
Chronic studies. Time course of mean arterial pressure (MAP) during 60 minutes of hemorrhagic shock followed by 30 minutes of resuscitation.
Figure 7.
Chronic studies. Survival rates at 72 hours after 60 minutes of hemorrhagic shock and 30 minutes of resuscitation. *P < .05 (control vs crocetin).
Discussion
Our studies provide strong evidence that inclusion of crocetin in resuscitation fluids administered to experimental animals following a period of hypovolemic shock significantly reduces mortality. One possible mechanism for this reduction is that crocetin administration may restrict the extent of cellular apoptosis that normally accompanies fluid resuscitation following hemorrhagic shock. Evidence of reduced hepatic apoptosis is provided by reductions in levels of postshock cytosolic cytochrome c, reduced levels of activated caspase 3, and increases in the levels of protective bcl-2 protein. These results collectively support the concept that administration of crocetin—or crocetin-like molecules—might be a viable treatment option for injured patients and others presenting with shock due to hemorrhage. Mitochondrial damage, either from ischemia or from reperfusion, leads to the release of cytochrome c from the mitochondria into the cytoplasm, thereby initiating activation of the apoptotic pathway. Given that crocetin administration both protects animals from mortality following resuscitation from shock and reduces markers associated with cellular apoptosis, it is highly likely that apoptosis is a major mechanism responsible for cellular and organ damage following shock.1,2 Specifically, we observed a marked increase in free cytochrome c detectable in the cytoplasmic fraction of hepatic tissues compared with baseline at 24 hours. With crocetin administration, however, cytosolic cytochrome c levels were inhibited significantly compared with control animals resuscitated with LR alone. Caspases are well established to be cysteine proteases that serve as key effectors in apoptotic cell death. Caspases are usually present in the cellular cytoplasm as inactive zymogens that become activated in response to a variety of specific death stimuli. The appearance of active caspase-3 is generally accepted as strong evidence that the apoptotic pathway has been activated and that a cell will proceed to apoptotic cell death.11 Our data also provide strong evidence of a marked increase in hepatic caspase-3 at 24 hours, compared with baseline, following a period of shock. Once again, crocetin administration significantly inhibited caspase- 3 levels compared with controls.
The bcl-2 protein, a constituent of the mitochondrial membrane, is thought to serve a protective function for the mitochondrion against ischemic damage. Results of previous research have helped to establish that levels of bcl-2 protein are severely depressed in experimental animals following shock and resuscitation.12–15 As might be anticipated, given the results with cytochrome c and caspase 3, levels of bcl-2 were markedly increased at 24 hours in the crocetin-treated animals relative to controls. Thus, treatment with crocetin at the time of resuscitation appears to contribute significantly to recovery of hepatic bcl-2 levels.
Following ischemia and resuscitation, both apoptosis and necrosis can be seen. They are more closely related than previously thought and they share certain common pathways. Indeed, the term “necrapoptosis” has been coined to reflect the overlap between them.16 Paxian et al17 observed, as we have,8,9 that hemorrhagic shock markedly depresses hepatic ATP. They made the additional observation that resuscitation after a brief period of shock compared with a longer period of shock was associated with more rapid recovery of ATP, better return of hepatic function, and, significantly, more apoptosis compared with necrosis. Shorter periods of shock (1 and 2 hours) were associated predominantly with postresuscitation apoptosis, whereas the longest period of shock (3 hours) was associated predominantly with necrosis. Necrosis appeared to be associated with failure of ATP levels to recover after resuscitation. Although the models are somewhat different, the present studies, both acute and chronic, used relatively shorter periods of shock (30 and 90 minutes, respectively), within the range that produced apoptosis in the studies of Paxian et al. To the extent that crocetin administration is associated with more rapid recovery of ATP, it might be expected to diminish necrosis as well. However, this study was intended to examine the apoptotic pathway and provided no evidence on whether necrosis was affected.
This study was limited to hepatic tissue. We have confirmed that intestinal mucosa exhibit similar changes to hepatic tissue in terms of ATP and adenosine metabolites. However, these experiments were not repeated in intestinal mucosa or in other tissues. It would be unwise to generalize these findings without further experimental verification.
Our findings indicate that administration of crocetin during resuscitation reduces the extent of liver apoptosis and significantly increases survival compared with resuscitation using LR alone. These results support the validity of our hypothesis that crocetin reduces cell apoptosis and increases survival. It is likely that crocetin modifies the cellular death pathway of apoptosis and protects from cellular reperfusion injuries. Of note, this may not be the only mechanism of action for this agent.
Results from our previous experiments have provided convincing evidence that crocetin administration at the time of resuscitation from hemorrhagic shock in rats accelerates the return of cellular energy stores to normal. The mechanism of action probably involves, at least in part, prevention of mitochondrial damage.8,9 Because crocetin is highly hydrophobic, this could occur through crocetin-mediated stabilization of the mitochondrial membrane. It is also possible, as others have hypothesized, that crocetin increases oxygen transport in the plasma and may thereby enhance oxygen supply at the cellular level.3–7 Other investigators have found that crocetin can function as an anti-inflammatory agent or as an antioxidant18–21 and can protect against hepatotoxic agents.20,22 Crocin, a closely related molecule, has been shown to protect against cell death in retinal photoreceptor cells.23
The precise mechanism or mechanisms by which crocetin exerts its protective effects remain to be fully elucidated. Given that other lipids, such as leukotriene B4, can serve as relatively potent activators of the peroxisome proliferator–activated receptor-α, which has well-established functions in inhibiting inflammatory responses, it is attractive to consider a similar mode of action for crocetin. Alternatively, others have published results that strongly implicate a role for inducible nitric oxide synthase in the hemorrhage-induced increase in caspase-3 activity.24–26 We noted previously that crocetin administration following shock inhibits messenger RNA for inducible nitric oxide synthase.10 Our continuing research efforts are directed toward further understanding these mechanisms, with the ultimate goal of developing effective strategies to treat hemorrhagic shock.
Conclusion
Crocetin has potential for treatment of hemorrhagic shock. The development of new pharmacologic strategies to minimize late tissue and organ damage can be expected to greatly improve the clinical treatment of hemorrhagic shock. Subsequent investigations will be designed to determine the specific underlying mechanisms and whether action is primary or secondary to increased oxygen transport.
Acknowledgments
Financial disclosure: This work was supported by grants or contracts from the Office of Naval Research (N00014-01-1-0151), the American Heart Association (00000981), the U.S. Department of Defense (Army Medical Research and Materials Command, USAMRIC, W81XSH-06-1-530), the National Institutes of Health (GM-50870), and by the Coffey Foundation and the Sosland Foundation, both of Kansas City, Missouri.
References
- 1.Genesca M, Sola A, Miquel R, et al. Role of changes in tissue nucleotides on the development of apoptosis during ischemia/reperfusion in rat small bowel. Am J Pathol. 2002;161:1839–1847. doi: 10.1016/S0002-9440(10)64460-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chopp M, Chan PH, Hsu CY, Cheung ME, Jacobs TP. DNA damage and repair in central nervous system injury: National Institute of Neurological Disorders and Stroke Workshop Summary. Stroke. 1996;27:363–369. doi: 10.1161/01.str.27.3.363. [DOI] [PubMed] [Google Scholar]
- 3.Gainer JL, Rudolph DB, Caraway DL. The effect of crocetin on hemorrhagic shock in rats. Circ Shock. 1993;41:1–7. [PubMed] [Google Scholar]
- 4.Roy JW, Graham MC, Griffin AM, Gainer JL. A novel fluid resuscitation therapy for hemorrhagic shock [editorial] Shock. 1998;10:213–217. doi: 10.1097/00024382-199809000-00010. [DOI] [PubMed] [Google Scholar]
- 5.Singer M, Stidwill RP, Nathan A, Gainer JL. Intravenous crocetinate prolongs survival in a rat model of lethal hypoxemia. Crit Care Med. 2000;28:1968–1972. doi: 10.1097/00003246-200006000-00047. [DOI] [PubMed] [Google Scholar]
- 6.Giassi LJ, Gilchrist MJ, Graham BA, Gainer JL. Trans-sodium crocetinate restores blood pressure, heart rate, and plasma lactate after hemorrhagic shock. J Trauma. 2001;51:932–938. doi: 10.1097/00005373-200111000-00018. [DOI] [PubMed] [Google Scholar]
- 7.Giassi LJ, Poynter AK, Gainer JL. Trans sodium crocetinate for hemorrhagic shock: effect of time delay in initiating therapy. Shock. 2002;18:585–588. doi: 10.1097/00024382-200212000-00017. [DOI] [PubMed] [Google Scholar]
- 8.Van Way CW, III, Dhar A, Morrison DC, Longorio MA, Maxfield DM. Cellular energetics in hemorrhagic shock restoring adenosine triphosphate to the cells. J Trauma. 2003;54:S169–S176. doi: 10.1097/01.TA.0000047226.36678.EE. [DOI] [PubMed] [Google Scholar]
- 9.Van Way CW, III, Dhar A, Morrison D. Hemorrhagic shock: a new look at an old problem. Mo Med. 2003;100:518–523. [PubMed] [Google Scholar]
- 10.Yang R, Tan X, Thomas AM, et al. Crocetin inhibits mRNA expression for tumor necrosis factor-alpha, interleukin-1beta, and inducible nitric oxide synthase in hemorrhagic shock. JPEN J Parenter Enteral Nutr. 2006;30:297–301. doi: 10.1177/0148607106030004297. [DOI] [PubMed] [Google Scholar]
- 11.Boatright KM, Salvesen GS. Mechanisms of caspase activation. Curr Opin Cell Biol. 2003;15:725–731. doi: 10.1016/j.ceb.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 12.Inoue I, Nagase H, Kishi K, Higuti T. ATP-sensitive K+ channel in the mitochondrial inner membrane. Nature. 1991;352:244–247. doi: 10.1038/352244a0. [DOI] [PubMed] [Google Scholar]
- 13.Dhar A, Kujath S, Van Way CW., III Glutamine administration during total parenteral nutrition protects liver adenosine nucleotides during and after subsequent hemorrhagic shock. JPEN J Parenter Enteral Nutr. 2003;27:246–251. doi: 10.1177/0148607103027004246. [DOI] [PubMed] [Google Scholar]
- 14.Darlington DN, Gann DS. Adenosine stimulates Na/K ATPase and prolongs survival in hemorrhagic shock. J Trauma. 2005;58:1–6. doi: 10.1097/01.ta.0000151185.63058.e3. [DOI] [PubMed] [Google Scholar]
- 15.Zager RA. Adenine nucleotide changes in kidney, liver, and small intestine during different forms of ischemic injury. Circ Res. 1991;68:185–196. doi: 10.1161/01.res.68.1.185. [DOI] [PubMed] [Google Scholar]
- 16.Lemasters JJ. Necrapoptosis and the mitochondrial permeability transition: shared pathways to necrosis and apoptosis. Am J Physiol. 1999;276:G1–G6. doi: 10.1152/ajpgi.1999.276.1.G1. [DOI] [PubMed] [Google Scholar]
- 17.Paxian M, Bauer I, Rensing H, et al. Recovery of hepatocellular ATP and “pericentral apoptosis” after hemorrhage and resuscitation. FASEB J. 2003;17:993–1002. doi: 10.1096/fj.02-0624com. [DOI] [PubMed] [Google Scholar]
- 18.Hosseinzadeh H, Younesi HM. Antinociceptive and anti-inflammatory effects of Crocus sativus L. stigma and petal extracts in mice. [Accessed December 19, 2006];2002 May 15; doi: 10.1186/1471-2210-2-7. http://www.biomedcentral.com/1471-2210/2/7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang CJ, Lee MJ, Chang MC, Lin JK. Inhibition of tumor promotion in benzo[a]pyrene-initiated CD-1 mouse skin by crocetin. Carcinogenesis. 1995;16:187–191. doi: 10.1093/carcin/16.2.187. [DOI] [PubMed] [Google Scholar]
- 20.Wang CJ, Hsu JD, Lin JK. Suppression of aflatoxin B1-induced hepatotoxic lesions by crocetin (a natural carotenoid) Carcinogenesis. 1991;12:1807–1810. doi: 10.1093/carcin/12.10.1807. [DOI] [PubMed] [Google Scholar]
- 21.Hsu JD, Chou FP, Lee MJ, et al. Suppression of the TPA-induced expression of nuclear proto-oncogenes in mouse epidermis by crocetin via antioxidant activity. Anticancer Res. 1999;19:4221–4227. [PubMed] [Google Scholar]
- 22.Tseng TH, Chu CY, Huang JM, Shiow SJ, Wang CJ. Crocetin protects against oxidative damage in rat primary hepatocytes. Cancer Lett. 1995;97:61–67. doi: 10.1016/0304-3835(95)03964-x. [DOI] [PubMed] [Google Scholar]
- 23.Laabich A, Vissvesvaran GP, Lieu KL, et al. Protective effect of crocin against blue light- and white light-mediated photoreceptor cell death in bovine and primate retinal primary cell culture. Invest Ophthalmol Vis Sci. 2006;47:3156–3163. doi: 10.1167/iovs.05-1621. [DOI] [PubMed] [Google Scholar]
- 24.Kiang JG, Bowman PD, Lu X, et al. Geldanamycin inhibits hemorrhage-induced increases in caspase-3 activity: role of inducible nitric oxide synthase. J Appl Physiol. 2007;103:1045–1055. doi: 10.1152/japplphysiol.00100.2007. [DOI] [PubMed] [Google Scholar]
- 25.Kiang JG, Peckham RM, Duke LE, Shimizu T, Chaudry IH, Tsokos GC. Androsetenediol inhibits the trauma-hemorrhage-induced increase in caspase-3 by downregulating the inducible nitric oxide synthase pathway. J Appl Physiol. 2007;102:933–941. doi: 10.1152/japplphysiol.00919.2006. [DOI] [PubMed] [Google Scholar]
- 26.Kan WH, Hsu JT, Schwacha MG, et al. Selective inhibition of iNOS attenuates trauma-hemorrhage/resuscitation-induced hepatic injury. J Appl Physiol. 2008;105:1076–1082. doi: 10.1152/japplphysiol.90495.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]