Abstract
Francisella tularensis is a coccobacillus that causes tularemia. F. tularensis lipopolysaccharide (LPS) has nominal biological activity. Currently there is controversy regarding the structure of the lipid A obtained from F. tularensis LVS. Therefore, to resolve this controversy, the purification and structural identification of this LPS was crucial. To achieve this, LPS from F. tularensis was acid hydrolyzed to obtain crude lipid A, which was methylated and purified by HPLC. HPLC peak fractions were analyzed by mass spectrometry. The structure of the major lipid A of F. tularensis LVS comprised the glucosamine disaccharide backbone substituted with four fatty acyl groups, and a phosphate (1-position) with molecular masses of 1505. The major lipid A component had 18:0[3-O (16)] in the distal subunit and two 18:0 (3-OH) fatty acyl chains at the 2- or the 3- positions of the reducing subunit. Other lipid A variants in fatty acyl groups, include a phosphate or a phosphoryl galactosamine at the 1-position, and a hexose attached to the lipid A at the 4’ or 6’ position and these have not been described before for the F. tularensis LVS. This analysis revealed that the lipid A from F. tularensis LVS is far more complex than originally believed.
Introduction
Lipopolysaccharide (LPS) present in the outer membrane of Gram-negative bacteria can trigger macrophages to produce inflammatory cytokines such as tumor necrosis factor (TNF-α) and interleukin-1 (IL-1β) and other mediators such as nitric oxide which collectively, when produced excessively, may lead to inflammation and development of shock. LPS usually consists of the O-antigen, the core and the lipid A (lipophilic region) (reviewed in 1). Qureshi et al. initially determined the complete structure of the enterobacterial lipid A, which consists of a (β 1→6) linked glucosamine disaccharide backbone to which 6 fatty acyl groups are attached in a precise manner at the 2, 3 and 2’ and 3’ positions. The phosphates are attached at the 1 and 4’ positions of the glucosamine disaccharide. 1–6 It is the hexaacylated lipid A with two phosphates that is responsible for stimulating the signaling cascade leading to inflammation, sepsis and septic shock.1–6 Francisella tularensis is an intracellular Gram-negative bacterium that causes tularemia in humans. 7, 8 Our studies have been focused on the subspecies LVS that causes a similar disease in mice, but is attenuated in humans. The highly purified F. tularensis LVS LPS was found to be minimally stimulatory in primary murine macrophages and in HEK293T cells transiently transfected with Toll-like receptor TLR4/MD-2/CD14, whereas live F. tularensis LVS bacteria are highly stimulatory for macrophages and TLR2-expressing HEK293T cells.
The first structural analysis of F. tularensis LVS lipid A was carried out by Vinogradov et al. 9 They showed a partial MALDI mass spectrum of just the molecular ions and suggested that the major lipid A structure comprised the diglucosamine backbone substituted with four fatty acyl groups. The major lipid A component had 18:0[3-O (14)] in the distal subunit and two 16:0 (3-OH) and 18:0 (3-OH) fatty acyl chains at either the 2- or the 3-positions of the reducing subunit. However, they did not identify phosphate or sugars at the reducing end. The positions of the fatty acyl groups were determined by NMR analysis and not by mass spectrometry. Phillips et al have reported only one lipid A for the F. tularensis LVS that lacked a galactosamine or a hexose. 10 In contrast, Phillips et al. showed that F. tularensis subsp. holarctica strain 1547-57 lipid A had a similar structure, except that it contained a phosphate-linked galactosamine at the 1-position. The major lipid A structure comprised the glucosamine disaccharide backbone substituted with four fatty acyl groups. The major lipid A component had 18:0[3-O (16)] in the distal subunit and two 18:0 (3-OH) fatty acyl chains at either the 2- or the 3- positions of the reducing subunit. 10 However, the lipid A samples were not purified and several different species were identified by MALDI-TOF, whose complete structure could not be determined with respect to the location of fatty acyl groups unless they resorted to multistage mass spectrometry. F. novicida lipid A has been also characterized by Wang et al. and Gunn and Ernst and they also showed the presence of galactosamine attached to the phosphate at the 1-position and another hexosamine attached to the 4’ position, 11–13 whereas the F. tularensis LVS lacks the phosphate and galactosamine.11 The structure of the lipid A from F. tularensis LVS has not been completely analyzed. Therefore, there is controversy regarding the structure of the F. tularensis LVS lipid A. 11
Our objective in the present study is to determine whether the F. tularensis LVS lipid A structure is really different from F. tularensis and F. novicida. Therefore, we performed the first HPLC purification of the methylated (at the phosphate) lipid A and structural determination of the highly purified lipid A obtained from the LPS of F. tularensis LVS. We also purified the unmethylated free lipid A (not bound to the LPS, normally present in Francisella novicida) by thin-layer chromatography (TLC). Our data revealed that the methylated lipid A is amenable to purification by reversed-phase high performance liquid chromatography (RP-HPLC) and its structures can be fully defined with respect to phosphates and fatty acyl groups using mass spectrometry. The TLC purified free lipid A was also analyzed by mass spectrometry. We found that the F. tularensis LVS lipid A contains the phosphate and the phosphate-linked galactosamine to some extent, but lacks the hexosamine attached to the 4’-position in the aqueous layer. The phenol layer however, contains all three substituents. These latter substituents have not been described previously for the F. tularensis LVS lipid A.
Experimental procedures
Materials
HPLC grade solvents such as acetonitrile, chloroform, methanol and isopropanol were purchased from Burdick & Jackson (Muskegon, MI). Silica gel H thin layer plates were purchased from Analabs Inc. (North Haven, CT) Dowex 50W-X8, Chelex 100, and Cellex-D were obtained from Bio-Rad as described previously. 14
Growth of bacteria and preparation of LPS
F. tularensis LVS cells were cultivated by List Biological Laboratories as described previously. 7 Briefly, LPS was extracted by using the modified Westphal/Jann protocol. The aqueous phase fraction obtained after the water/phenol extraction was treated with RNase, DNase and proteinase K. The LPS was then centrifuged at 3076 X g to remove solids and then supernatant was dialyzed against 0.85% NaCl and distilled water using a 100 kDa AGT hollow fiber cartridge. Lyophilized LPS was reconstituted in water and re-extracted using a modified deoxycholate-phenol extraction and again treated with RNASE, DNase and proteinase K.7 Finally, LPS was extracted with chloroform/methanol (2:1, v/v) to remove the remaining free lipid A and phospholipids present in the LPS as reported earlier. 8 This highly purified LPS was dialyzed against water and suspended in pyrogen-free saline. The phenol phase fraction was saved and processed similarly, except the chloroform/methanol extraction step was omitted.
Preparation of lipid A
Aqueous Phase Lipid A. F. tularensis LVS LPS aqueous phase (List Biologicals, 78 mg, Fig. 1A, lane 1) was suspended in 20 mM sodium acetate (pH 2.5, 20 mL) and heated to 100°C for 90 min as described by Qureshi et al. This is a milder acid treatment used for the preparation of diphosphoryl lipid A from LPS of Rhodobacter sphaeroides. 15 The resulting crude LVS lipid A was then centrifuged at 9,000×g for 20 min, and the pellet was suspended in a two phase chloroform/methanol/water solvent (10:5:6) as described previously. This mixture was centrifuged again and the lower organic layer was recovered and evaporated to dryness. This fraction was analyzed by analytical TLC. The lipid A (13 mg, Fig. 1A, lane 2) was converted into the free acid form and methylated with diazomethane. 16 RP-HPLC was performed with two Shimadzu LC-1080 solvent delivery systems, a Shimadzu solvent programmer, and a variable wavelength detector (Model 10AV, Shimadzu). A Nova-Pak cartridge (8 mm×10 cm, C18-bonded, 5 µm, Waters Associates, Milford MA) was used at a flow rate of 2 mL/min. A linear gradient of 10–80% of isopropanol in acetonitrile was used for 1 h as described previously. 16 Fractions from five runs were collected, evaporated to dryness and analyzed by mass spectrometry.
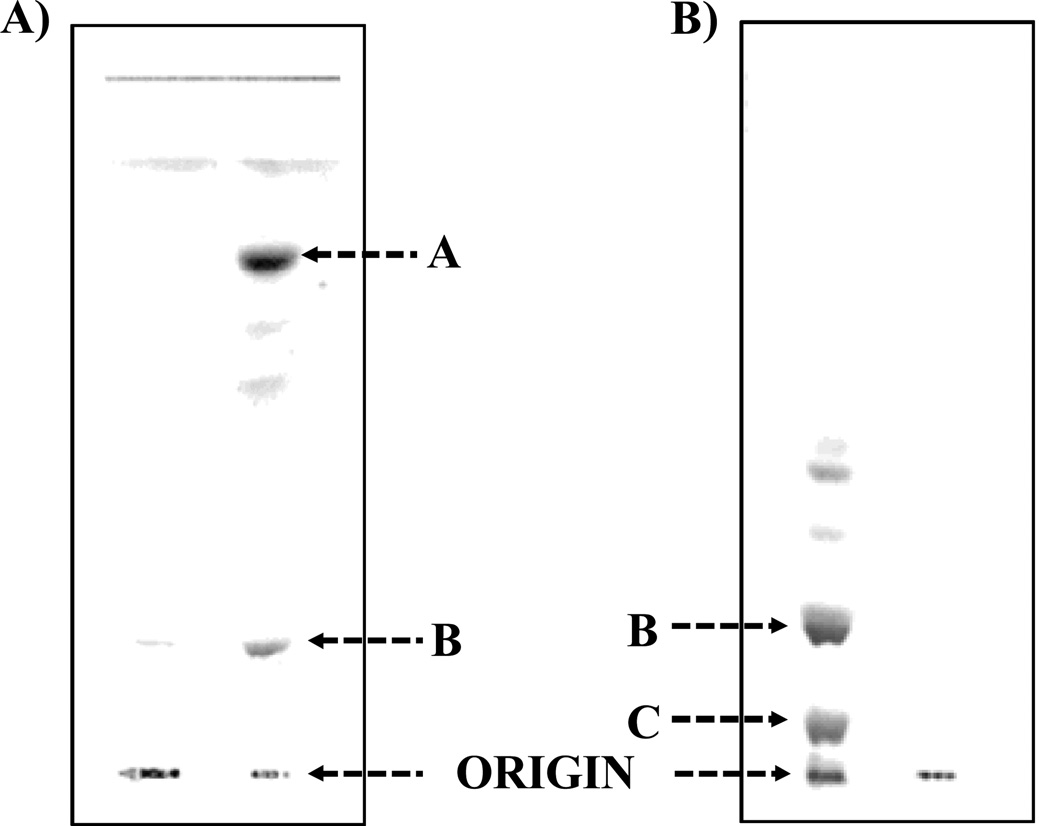
Figure 1.
Thin layer chromatography (TLC) was performed on silica gel GHL plates with a solvent system composed of chloroform/methanol/water/ammonia (50:25:4:2, v/v/v/v). 1A illustrates the TLC analysis of the aqueous phase purified LPS of F. tularensis (List Biologicals, 20 µg, Lane 1, origin) and lipid A bound to LPS of F. tularensis (Lane 2). Band A (Lane 2) contains the unphosphorylated lipid A species and band B (Lane 2) represents the phosphoryl galactosamine lipid A species. 1B illustrates the TLC analysis of the F. tularensis LVS (CDC) LPS (Inzana group) prepared before and after the chloroform/methanol (2:1,v/v) extraction. The free lipid A fractionated into this extract and the LPS was purified away from it. Lane 1 is the free lipid A species and LPS (origin) and 2 is the purified LPS. Band B is composed of the phosphoryl galactosamine lipid A species and was present predominantly in the aqueous layer. In contrast, band C contains the phosphorylated lipid A species with both a galactosamine and hexose residue and was only present in the phenol layer.
Phenol Phase Lipid A. F. tularensis LVS LPS phenol phase (List Biologicals) was also analyzed by mass spectrometry without prior chloroform/methanol (2:1, v/v) extraction. This fraction was analyzed by analytical TLC and it contained mainly the LPS at the origin and band C (Fig. 1B, lane 1), TLC data not presented.
Preparation of F. tularensis LVS (CDC) LPS
F. tularensis LVS (CDC) LPS was also prepared by the Inzana group, according to the method of Vinogradov et al. (9) but instead of separating the two water/phenol layers, the two layers were extensively dialyzed with water to remove the phenol as described previously. 17 This crude LPS was extracted with chloroform/methanol (2:1,v/v) to obtain the free lipid A that is shown to be normally present in the F. novicida LPS (12) but not in the F. tularensis LVS LPS preparations. LPS (249 mg) was extracted twice with chloroform/methanol (2:1,v/v; 100 ml). The extracts were evaporated to dryness using a rotary evaporator. The free lipid A (30 mg, Fig. 1B, lane 1) was purified using the silica gel GHL plates developed with chloroform/methanol/ water/ammonia (50:25:4:2,v/v/v/v). The major band (band B, Fig. 1B) was identified using iodine vapors and scraped and eluted with chloroform/methanol (2:1,v/v) containing 4% water (4). The purified fractions were analyzed by mass spectrometry.
Analytical Procedures
Samples for the glucosamine assay were hydrolyzed in 3 N HCl at 95°C for 4 h as described by Enghofer and Kress (18). Fatty acid Analysis. Fatty acid analysis was carried out as described previously.19 TLC Analyses. Analytical TLC was performed on lipid A samples using silica gel GHL plates (250 µm) and a solvent system of chloroform/methanol/water/ concentrated ammonium hydroxide (50:25:4:2,v/v/v/v) as described by Qureshi et al. 2 Standard lipid A was also prepared from E. coli D31m4 ReLPS for calibration purposes. 3
Mass Spectrometry of Lipid A Species
The mass spectra of the lipid A species were acquired in both the negative ion and positive ion linear mode using the matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) mass spectrometer (AXIMA-CFR; Kratos, Manchester, England), equipped with a 337-nm nitrogen laser, a 20-kV extraction voltage, and time-delayed extraction. Prior to MALDI-TOF analysis, the samples were dissolved in chloroform-methanol solution (4:1,v/v) and deposited in a 1:1 ratio with the saturated matrix solution of 6-aza-2-thiothymine in 50% acetonitrile and 10% tribasic ammonium citrate (9:1,v/v) on the stainless steel MALDI target plate. The samples were dried at 25°C prior to mass analysis. Each spectrum represented the average of 100 laser shots and E. coli Lipid A (Sigma, St. Louis, MO) was used as the external calibrate.
Results
Characterization of the lipid A obtained from the LPS of F. tularensis LVS
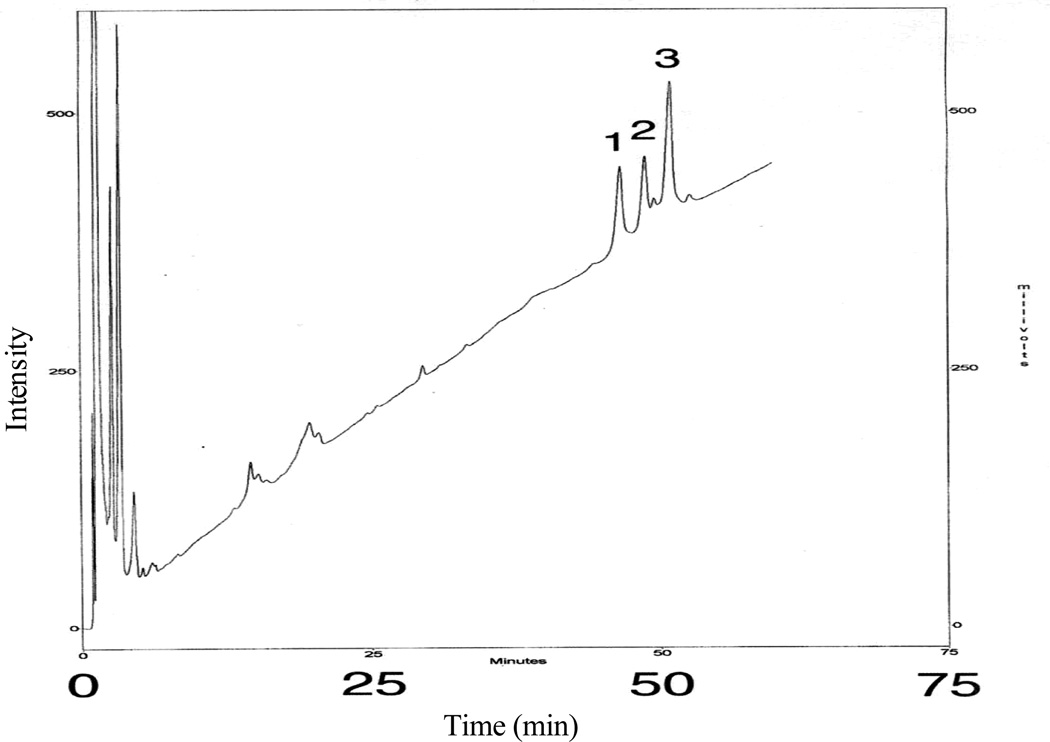
The F. tularensis LVS lipid A from the aqueous phase layer was analyzed by silica gel GHL TLC as shown in Fig. 1A. Lanes 1 and 2 show the migration of the purified F. tularensis LPS (remained at the origin) and lipid A, respectively. Band A (lane 2) corresponds to the tetraacyl lipid A without any phosphates and the slower moving bands represent lipid A species with differing types of fatty acyl chains present. The more polar band B, close to the origin, corresponds to the tetraacyl monophosphoryl lipid A with a galactosamine. After methylation, the lipid A species from band B migrated close to the solvent front, thus suggesting the presence of a phosphoryl group in the lipid A (data not presented). The lipid A was methylated with diazomethane, as described previously (3), to confirm the presence of a phosphate group, to obtain optimal fractionation on a reversed-phase column, and to enhance fragmentation for MALDI-TOF MS analysis. The resulting methylated lipid A was then fractionated by RP-HPLC with a C18-bonded silica cartridge and the representative profile of the RP-HPLC a fractionation is shown in Fig. 2. RP-HPLC analysis of the methylated lipid A showed the presence of three major fractions (HPLC Fractions 1–3) and a few minor components that varied in the number and types of fatty acyl chains, as shown in Tables 1–3.
Figure 2.
Reversed-phase HPLC of the methylated lipid A from F. tularensis LVS and E. coli D31m4. A Nova-Pak, high resolution 5µ silica cartridge was used. The mobile phase was a linear gradient of 10–80% of isopropanol in acetonitrile, at a flow rate of 2 ml/min over 60 min. The sensitivity of the UV detector at 210 nm was set at 0.16 absorbance units full scale for 1.5 mg of lipid A. The chromatogram was not baseline corrected and HPLC Fractions 1–3 were collected for mass spectrometry and NMR spectroscopy.
Table1.
Proposed fatty acyl distribution of methyl-lipid A species of HPLC fractions 1–3.
| Fatty Acyl Groups | ||||||||
|---|---|---|---|---|---|---|---|---|
| Lipid A Species |
a[M-H]−1 | a[M(CH2)-H]−1 | DG Distal | DG Reducing | ||||
| FA 3' | FA2' | FA3 | FA2 | P** | GalN** | |||
| Species I | 1477 | 1491 | - | 18:0[3-OH(14:0)] | 18:0(3-OH) | 18:0(3-OH) | 1 | - |
| Species Ia | 1638 | 1653 | - | 18:0[3-OH(14:0)] | 18:0(3-OH) | 18:0(3-OH) | 1 | 1 |
| Species II | 1505 | 1519 | - | 18:0[3-OH(16:0)] | 18:0(3-OH) | 18:0(3-OH) | 1 | - |
| Species IIa | 1666 | 1681 | - | 18:0[3-OH(16:0)] | 18:0(3-OH) | 18:0(3-OH) | 1 | 1 |
| Species III | 1533 | 1547 | - | 18:0[3-OH(18:0)] | 18:0(3-OH) | 18:0(3-OH) | 1 | - |
| Species IIIa | 1694 | 1709 | - | 18:0[3-OH(18:0)] | 18:0(3-OH) | 18:0(3-OH) | 1 | 1 |
Table 3.
Proposed fatty acyl distribution for lipid A species of LPS and free lipid A present in the phenol phase; or in the chloroform/methanol washes of aqueous plus phenol phase LPS (Inzana Prep).
| Fatty Acyl Groups | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Lipid A Species | a[M+H]+1 | a[M+2Na+H]+1 | DG Distal | DG Reducing | |||||
| FA 3' | FA2' | FA3 | FA2 | P** | GalN** | Hexose | |||
| Species I | 1477 | 1523 | - | 18:0[3-OH(14:0)] | 18:0(3-OH) | 18:0(3-OH) | 1 | - | |
| Species Ia | 1799 | 1845 | - | 18:0[3-OH(14:0)] | 18:0(3-OH) | 18:0(3-OH) | 1 | 1 | 1 |
| Species Ib | 1845 | 2332*** | 18:0(3-OH) | 18:0[3-OH(14:0)] | 18:0(3-OH) | 18:0(3-OH) | 1 | 1 | 1*** |
| Species II | 1505 | 1551 | - | 18:0[3-OH(16:0)] | 18:0(3-OH) | 18:0(3-OH) | 1 | - | |
| Species IIa | 1666 | 1712 | - | 18:0[3-OH(16:0)] | 18:0(3-OH) | 18:0(3-OH) | 1 | 1 | |
| Species IIb | 1666 | 1689b | - | 18:0[3-OH(16:0)] | 18:0(3-OH) | 18:0(3-OH) | 1 | 1 | |
| Species IIc | 2314 | 2360*** | 18:0(3-OH) | 18:0[3-OH(16:0)] | 18:0(3-OH) | 18:0(3-OH) | 1 | 1 | 1*** |
Mass spectrometry of the HPLC-purified methylated lipid A obtained from F. tularensis LVS LPS
HPLC Fractions 1–3 were analyzed via MALDI-TOF MS in the negative ion mode. The mass spectrum of the lipid A HPLC Fractions 1–3 in Fig. 3A-C contained an abundant molecular ion [M-H]−1 at m/z 1519 and 1533, which is consistent with the mono- and di-methylated tetraacyl phosphorylated lipid A Species II (Table 1) with 18:0[3-O(16:0)] in 2’-position on the distal subunit. The molecular ion observed at m/z 1491 (1519–28) in Fraction 1 (Fig. 3A) contains 18:0[3-O(14:0)] at the 2’-position on the distal subunit and the phosphate is mono-methylated, creating a mass shift of 14 Da (Species I, Table 1). The peak observed at m/z 1653 (1491+162) in Fraction 1 represents Species Ia with the addition of the phosphate-galactosamine at the 1-position (Table 1, Fig. 3A). This peak has not been previously observed for lipid A from F. tularensis LVS. The ion observed in Fraction 2 at m/z 1681 (1519+162) represents the mono-methylated form of Species IIa with the addition of phosphate-galactosamine at the 1’-position (Table 1, Fig. 3B). Similar to the m/z 1653 ion, this lipid A species has not been previously reported for the LVS strain. The ion observed in Fraction 3 at m/z 1709 (1547+162) represents the mono-methylated form of Species IIIa with the addition of phosphate-galactosamine (Table 1, Fig. 3C). No unphosphorylated lipid A species were identified by negative ion MALDI-TOF mass spectrometry.
Figure 3.
Negative-ion MALDI-TOF spectra of HPLC Fractions 1–3 (A-C).
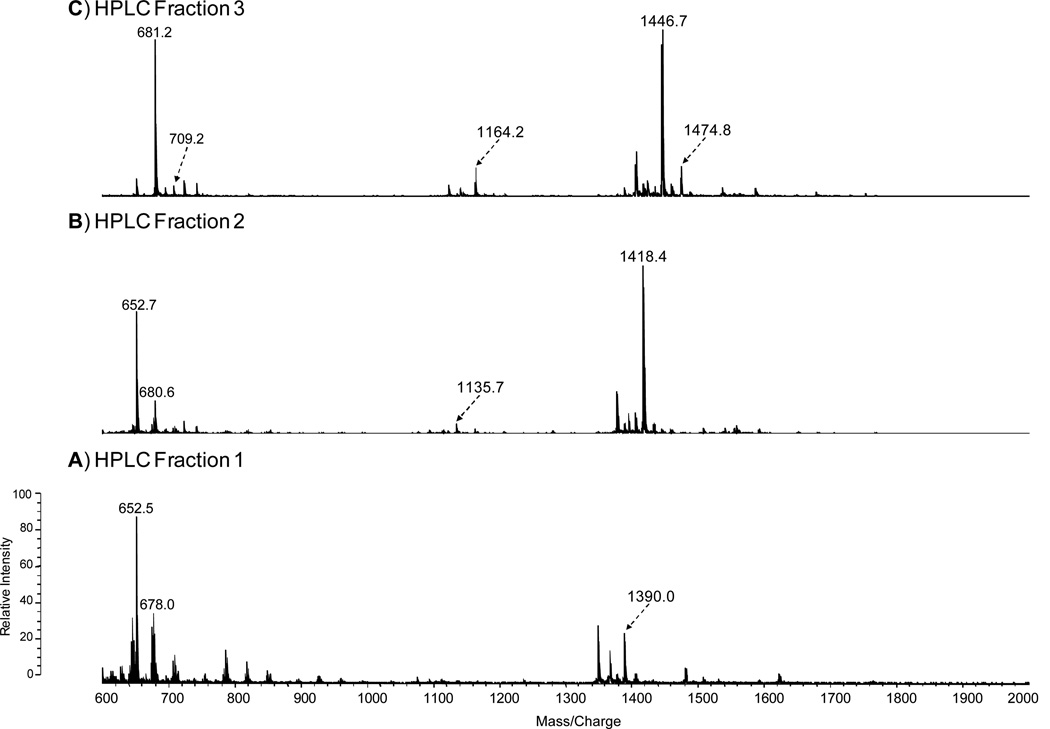
To investigate the positions and types of fatty acyl groups attached to the lipid A species, we analyzed the HPLC Fractions 1–3 by positive ion MALDI-TOF MS (Fig. 4A-C, Table 2). The mass spectrum of the Fraction 1 in Fig. 4A contained an abundant molecular ion [MH+Na]+1 at m/z 1390, which is consistent with the mono-methylated lipid A containing 18:0[3-O(14:0)] at the 2’-position on the distal subunit and 16:0[3-OH] at the 2-position at the reducing subunit (Species IV, Table 2). To further confirm the position of the fatty acyl chains, the oxonium ion of the non-methylated Species IV, with a molecular mass of 1369, was observed at m/z 653 (Table 2). This ion resulted from the cleavage of the distal glucosamine from the reducing glucosamine at the oxygen bond. The most abundant ion in the Fraction 2 mass spectrum at m/z 1418 is consistent with the mono-methylated lipid A containing 18:0[3-O(14:0)] at the 2’-position in the distal end and 18:0[3-OH] at the 2-position at the reducing end (Species I, Table 2). The oxonium ion observed at m/z 653 again suggests the presence of the 18:0[3-O(14:0)] in the distal subunit of the glucosamine disaccharide. The most abundant ions observed in the Fraction 3 mass spectrum, m/z 1446 and 1474, are consistent with the mono-methylated form of the lipid A species with molecular mass of 1425 and 1453, respectively (Fig. 4C). The oxonium ions observed at m/z 681 and 709 suggest the presence of a 18:0[3-O (16:0)] on the distal subunit of lipid A Species II and 18:0[3-O (18:0)] on the distal subunit of lipid A Species III, respectively (Fig. 4C, Table 2). The species containing the phosphates are not detectable in the positive ion mode due to the presence of the net negative charge. Overall, the mass spectral data are consistent with the proposed structures of the lipid A HPLC Fractions 1–3 as summarized in Tables 1–2.
Figure 4.
Positive-ion MALDI-TOF spectra of HPLC Fractions 1–3 (A-C).
Table 2.
Proposed fatty acyl distribution of methyl-lipid A species of HPLC fractions 1–3.
| Fatty Acyl Groups | |||||||
|---|---|---|---|---|---|---|---|
| Lipid A Species |
a[M+H]+1 | a[M(CH2)+H]+1 | Oxonium ion |
DG Distal | DG Reducing | ||
| FA 3' | FA2' | FA3 | FA2 | ||||
| Species I | 1397 | 1418 | 653 | - | 18:0[3-OH(14:0)] | 18:0(3-OH) | 18:0(3-OH) |
| Species II | 1425 | 1446 | 681 | - | 18:0[3-OH(16:0)] | 18:0(3-OH) | 18:0(3-OH) |
| Species III | 1453 | 1474 | 709 | - | 18:0[3-OH(18:0)] | 18:0(3-OH) | 18:0(3-OH) |
| Species IV | 1369 | 1390 | 653 | - | 18:0[3-OH(14:0)] | 18:0(3-OH) | 16:0(3-OH) |
Mass spectrometry of the TLC-purified non-methylated lipid A obtained from F. tularensis LVS (CDC) LPS
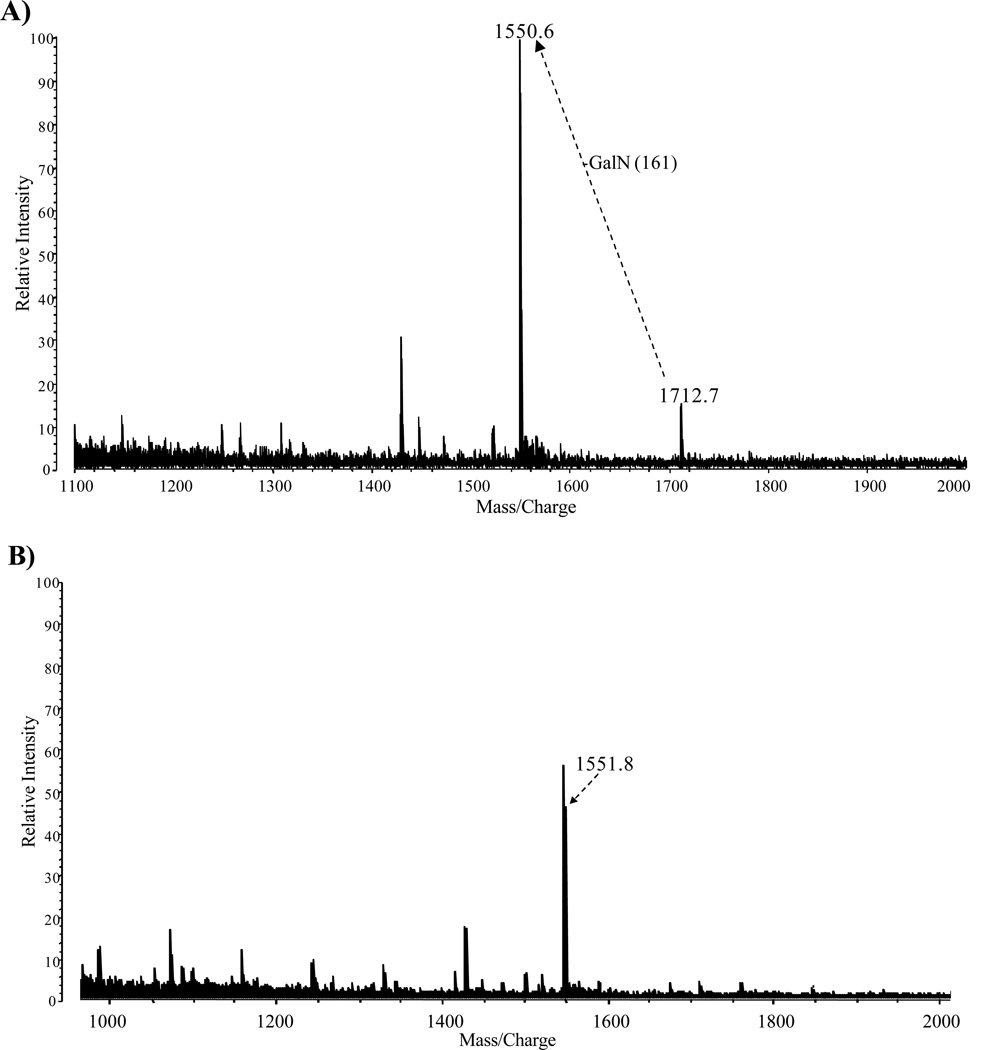
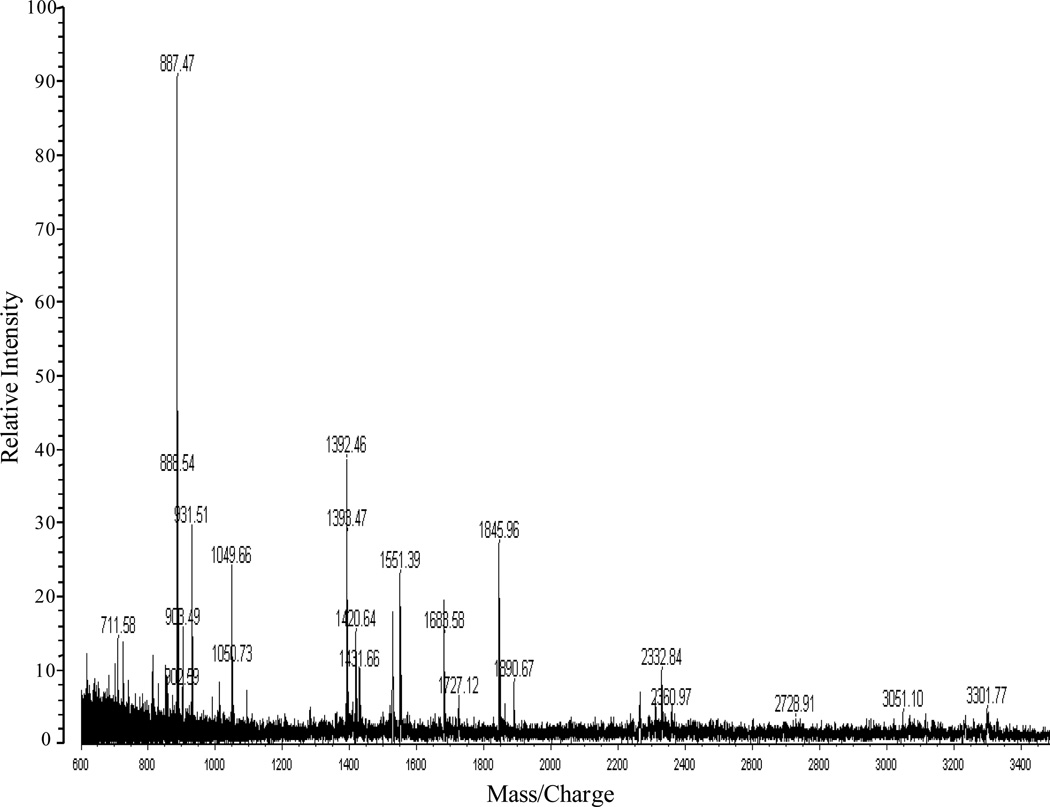
The major fractions of the free lipid A that were extracted from the crude LPS with chloroform/methanol (2:1,v/v) and purified by TLC without acid hydrolysis or methylation to minimize any loss of fatty acyl groups. The positive ion MALDI-TOF MS analysis of the TLC band B (Fig. 1B, lane 2) and the TLC band above band B (band not observed in Fig. 1B) is illustrated in Fig. 5A and 5B, respectively. The molecular ion observed in Fig. 5A at m/z 1712 ([MH+2Na]+1) is representative of the tetraacyl lipid A with the addition of galactosamine attached to the phosphate (Species IIa, Table 3). The peak observed at m/z 1551 was due to a loss of galactosamine (Species II, Table 3). MALDI-TOF MS analysis of the TLC band above band B (Fig. 1B, lane 2) showed a major peak at m/z 1551 which contained the tetraacyl phosphorylated lipid A observed in Fig. 5A with the absence of galactosamine (Fig. 5B). These data revealed the presence of a phosphate and galactosamine in the F. tularensis LVS lipid A structure for the first time. In conclusion, the LVS lipid A is similar to that of other Francisella lipid A species. The LVS lipid A definitely contains a phosphate (which could be methylated) and a galactosamine but the substitution with these components may be partial as compared to that observed in the lipid A species of other Francisella tularensis subspecies or in F. novicida. Moreover, we did not observe hexose in the purified lipid A fractions obtained from the aqueous phase layer. However, in the MS analysis of the phenol phase LPS (List Biologicals) a peak was observed at m/z 1846 ([MH+2Na]+1), which represents Species Ia of Table 3 (Fig. 6). Lipid A Species Ia contains a hexose at the 4’ or 6’-position, which is consistent with previous MS analysis of lipid A from F. novicida (12). Another ion observed at m/z 2333 corresponds to Species Ib (Table 3) and is composed of a pentacyl LPS containing Kdo-O and an 18:[3-OH], which has not been previously described for the LVS strain (Fig. 6). Ions observed at m/z 1551 (Species II) and m/z 1523 (Species I) result from the addition of two sodium atoms during MS analysis to m/z 1505 and m/z 1477, respectively (Table 3, Fig. 6). The oxonium ion peak at m/z 681 was not observed; however, a peak at m/z 887 (681+161+2Na) was observed in the phenol phase layer, which is consistent with a hexose attached to the lipid A species. This would then suggest that during the initial aqueous/phenol extraction of the crude LPS, the purified LPS and free lipid A are present in both phases. Therefore, the absence of the various lipid A species in previous analysis could possibly be due to the fact that various investigators used different phases for analysis.
Figure 5.
Positive-ion MALDI-TOF spectra of free lipid A extracted from crude LPS of F. tularensis from the TLC band B (Fig. 1B, Lane 1), which was fractionated into two fractions 1 (A) and 2 (B).
Figure 6.
Positive-ion MALDI-TOF spectra of LPS obtained from the phenol phase (List Biologicals) before the chloroform/methanol wash.
Discussion
The lipid A of F. tularensis LVS has not been fully characterized due to difficulties associated with purifying the free lipid A species. Such issues include solubility and the absence of the 1’-phosphate, which is removed during the hydrolysis of the free lipid A from LPS. In the present manuscript, we present structural determination of F. tularensis lipid A that is different from the lipid A structures biosynthesized by Enterobacteriaceae, Rhodobacter, Chlamydia, Brucella, Aquiflex, Neisseria, and Caulobacter with respect to the number of phosphates, fatty acyl chain length, and the presence of a phosphate-galactosamine at the 1’-position.2, 15, 20–26 In collaboration with List Biologicals, our initial objective was to study the lipid A bound to LPS; therefore, we utilized a chloroform/methanol (2:1,v/v) extraction to purify LPS from the aqueous phase layer. This purification was necessary to remove free lipid A (not bound to LPS) because large amounts of free lipid A is present in crude LPS mixtures, as reported previously for F. novicida lipid A. 12 A portion of the lipid A contained phosphate, therefore the mixture was methylated following hydrolysis of the purified LPS to enhance resolution and recovery from the RP-HPLC column. Fatty acid analysis after acid hydrolysis coupled with MS analysis of the fatty acids revealed the presence of predominantly 18:0[3-OH], 14:0, 16:0 and 18:0 fatty acids. The backbone also contained two glucosamine residues that are normally present in the lipid A of Enterbacteriaceae, Neisseria gonorrhoeae and R. sphaeoides.2, 4, 20, 15 The MS analysis of the highly purified HPLC fractions (Fraction 1–3) revealed that we were able to purify and analyze the F. tularensis lipid A, despite problems associated with purification of the lipid A species.
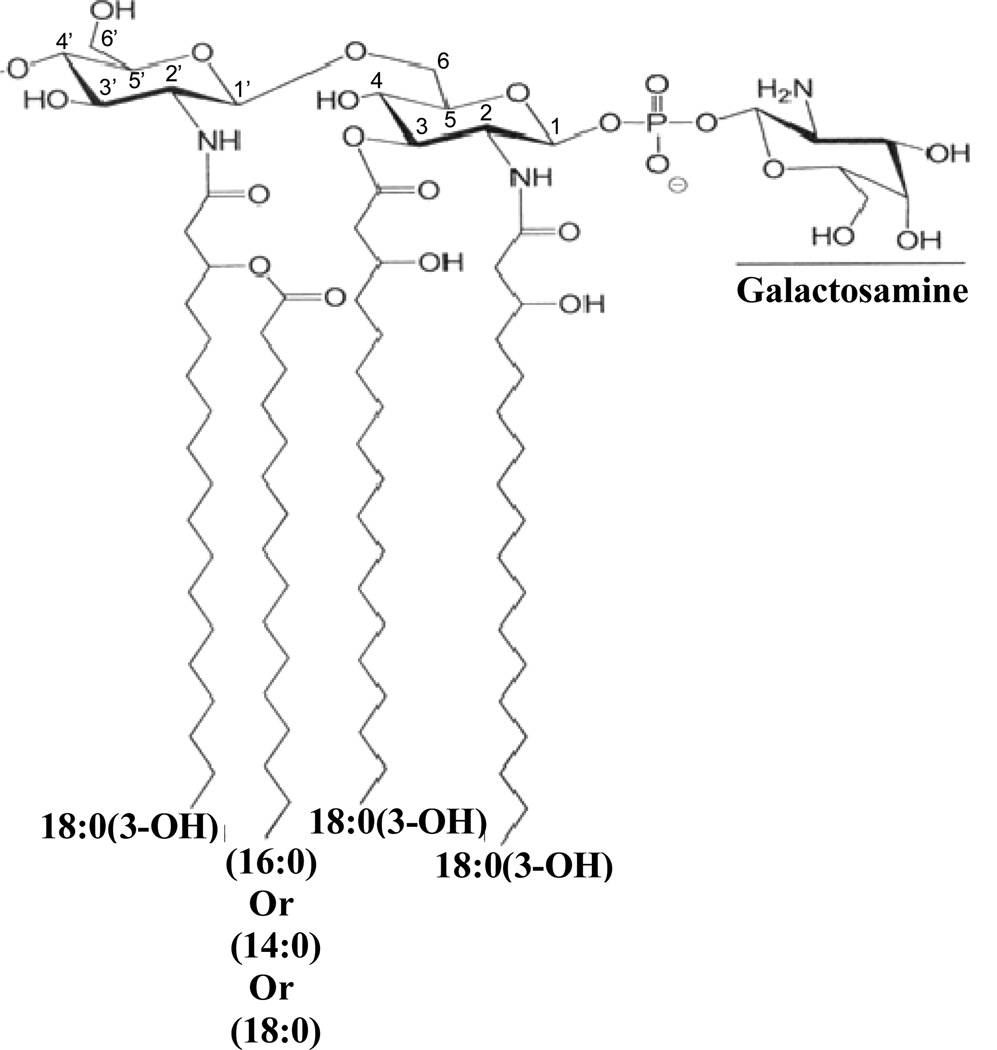
From the current analysis, we can completely describe the structure of the F. tularensis LVS lipid A, as shown in Fig. 7. Collectively, the major lipid A of F. tularensis has a diglucosamine backbone containing four fatty acids with a molecular mass of 1425. The major component contains 18:0[3-O(16:0)] at the 2’-position on the distal subunit and two 18:0(3-OH) at the 2- and the 3-positions on the reducing subunit. The 16:0 in the acyloxyacyl linkage can be substituted by either 14:0 or 18:0. In addition, other tetraacyl lipid A with either a phosphate or phosphate and galactosamine attached at the 1-position were also observed, as shown in Fig. 3 and Tables 2 and 3. The phosphate linked galactosamine has not been previously observed in the lipid A of F. tularensis LVS. However, we did not observe high concentrations of the lipid A species containing a hexose attached at the 4’-position in these fractions.
Figure 7.
Proposed structure of the lipid A derived from the LPS of F. tularensis. The locations of the fatty acyl groups attached to the backbone are shown in Tables 1 and 2. Some of the lipid A species lacked the phosphoryl galactosamine moiety and heterogeneity was observed in the fatty acyl groups regarding the chain lengths. The presence of a hexose was observed only from the phenol phase LPS extract and this hexose was present at either the 4’ or 6’ positions. An additional 18:0(3-OH) at the 3’-position was also observed.
Moreover, use of the mild acid hydrolysis of the LPS to remove bound lipid A also removes some of the phosphate and galactosamine, which increased the difficulty in the structural determination of the lipid A species. Therefore, to circumvent this problem, we used the free lipid A that is present in large concentrations in the crude LPS preparations, 12 unless the free lipid A is removed in the chloroform/methanol wash. However, MS analysis of the crude LPS, with only one major peak obtained at m/z 1505 did not show any interpretable results. However, we obtained interpretable mass spectral data with our TLC fractions. These fractions also showed unequivocally the presence of both galactosamine and phosphate in the free lipid A of F. tularensis LVS (Fig. 5 and 7). Our results are consistent with those obtained by Vinogradov et al., who initially described the F. tularensis LVS lipid A structure. The mass spectra in the earlier studies were not well-defined with respect to cleavage of lipid A, and therefore, the positions of fatty acyl groups could not be clearly defined. The lipid A structure determined from previous studies did not appear to contain any phosphates or galactosamine. Much of the structure was deduced from the nuclear magnetic resonance studies. It is not clear whether previous investigators studied the structure of the F. tularensis LPS obtained from the aqueous or the phenol layer, however, none of them purified their LPS with chloroform/methanol prior to their studies, therefore, it was a mixture of several components.
Analyses of our data reveal that the purified fractions were amenable to MALDI-TOF MS and the lipid A obtained from the LPS of F. tularensis LVS contains either no phosphate, one phosphate at the 1-position of the glucosamine disaccharide or a 1-phospho-galactosamine. The free lipid A from this organism contained both the 1-phosphate and the 1-phospho-galactosamine. The LPS and the free lipid A obtained from the phenol phase contained the lipid A with one phosphate at the 1-position, a 1-phospho-galactosamine, a hexose attached to either the 4’ or 6’-position and in some cases a Kdo-O and extra 18:0(3-OH) was also observed. There were a few variants as far as fatty acyl groups, for instance a 16:0(3-OH) instead of 18:0(3-OH) but those were present in small amounts.
In summary, all types of lipid A both bound to the LPS and free lipid A were observed in the F. tularensis LVS preparations, depending on whether the aqueous or the phenol phase preparations were analyzed by mass spectrometry. Most of these purified LPS and lipid A are biologically inactive. These results are consistent with the diminished activity of intact F. tularensis LPS and are a likely consequence of the unusual lipid A structure, a combined effect of the lack of the 4’-phosphate, the presence of the galactosamine, fewer fatty acyl groups or the long chain fatty acids present in the lipid A.
Acknowledgments
This research was supported by NIH grants GM50870 and U54-AI-15571168 (NQ), NIH NIAID Mid-Atlantic Regional Center of Excellence grant U54 AI-15571168 (SNV, TJI) and grants to RJC. We thank Robert Ernst for helpful suggestions with the manuscript.
Abbreviations
- LPS
lipopolysaccharide
- TNF
tumor necrosis factor
- IL-1
interleukin-1
- MS
mass spectrometry
- MALDI-TOF
matrix assisted laser desorption/ionization time-of-flight
- HPLC
high performance liquid chromatography
- TLC
thin-layer chromatography
References
- 1.Rietschel ET, Westphal O. Endotoxin Historical Perspectives. In: Brade H, Opal SM, Vogel SN, Morrison DC, editors. Endotoxin in Health and Disease. New York: Marcel Dekker, Inc.; 1999. pp. 1–30. [Google Scholar]
- 2.Qureshi N, Takayama K, Heller D, Fenselau C. Position of ester groups in the lipid A backbone of lipopolysaccharides obtained from Salmonella typhimurium. J. Biol. Chem. 1983;258:12947–12951. [PubMed] [Google Scholar]
- 3.Qureshi N, Takayama K, Mascagni P, Honovich J, Wong R, Cotter RJ. Complete structural determination of lipopolysaccharide obtained from deep rough mutant of Escherichia coli. Purification by high performance liquid chromatography and direct analysis by plasma desorption mass spectrometry. J. Biol. Chem. 1988;263:11971–11976. [PubMed] [Google Scholar]
- 4.Qureshi N, Takayama K, Ribi E. Purification and structural determination of nontoxic lipid A obtained from the lipopolysaccharide of Salmonella typhimurium. J. Biol. Chem. 1982;257:11808–11815. [PubMed] [Google Scholar]
- 5.Takayama K, Qureshi N, Mascagni P. Complete structure of lipid A obtained from the lipopolysaccharides of the heptoseless mutant of Salmonella typhimurium. J Biol Chem. 1983;258:12801–12803. [PubMed] [Google Scholar]
- 6.Agrawal PK, Bush CA, Qureshi N, Takayama K. Structural analysis of lipid A and Re-lipopolysaccharides by NMR spectroscopic methods. In: Bush CA, editor. Advances in Biophysical Chemistry. Vol 4. 1994. pp. 179–236. [Google Scholar]
- 7.Cole LE, Elkins KL, Michalek SM, Qureshi N, Eaton LJ, Rallabhandi P, Cuesta N, Vogel SN. Immunologic consequences of Francisella tularensis live vaccine strain infection: role of the innate immune response in infection and immunity. J. Immunol. 2006;176:6888–6899. doi: 10.4049/jimmunol.176.11.6888. [DOI] [PubMed] [Google Scholar]
- 8.Cole LE, Yang Y, Elkins KL, Fernandez ET, Qureshi N, Shlomchik MJ, Herzenberg LA, Herzenberg LA, Vogel SN. Antigen-specific B-1a antibodies induced by Francisella tularensis LPS provide long-term protection against F. tularensis LVS challenge. PNAS. 2009;106:4343–4348. doi: 10.1073/pnas.0813411106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vinogradov E, Perry MB, Conlan JW. Structural analysis of Francisella tularensis lipopolysaccharide. Eur J Biochem. 2002;269:6112–6118. doi: 10.1046/j.1432-1033.2002.03321.x. [DOI] [PubMed] [Google Scholar]
- 10.Phillips NJ, Schilling B, McLendon MK, Apicella MA, Gibson BW. Novel modification of lipid A of Francisella tularensis. Infect Immun. 2004;72:5340–5348. doi: 10.1128/IAI.72.9.5340-5348.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gunn JS, Ernst RK. The structure and function of Francisella lipopolysaccharide. Ann N.Y. Acad Sci. 2007;1105:202–218. doi: 10.1196/annals.1409.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang X, Ribeiro AA, Guan Z, McGrath SC, Cotter RJ, Raetz CR. Structure and biosynthesis of free lipid A molecules that replace lipopolysaccharide in Francisella tularensis subsp. novicida. Biochemistry. 2006;45:14427–40. doi: 10.1021/bi061767s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shaffer SA, Harvey MD, Goodlett DR, Ernst RK. Structural heterogeneity and environmentally regulated remodeling of Francisella tularensis subspecies novicida lipid A characterized by tandem mass spectrometry. J. Am. Soc. Mass. Spectrom. 2007;18:1080–1092. doi: 10.1016/j.jasms.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smit J, Kaltashov IA, Cotter RJ, Vinogradov E, Perry MB, Haider H, Qureshi N. Structure of a novel lipid A obtained from the lipopolysaccharide of Caulobacter crescentus. Innate Immunity. 2008;14:25–36. doi: 10.1177/1753425907087588. [DOI] [PubMed] [Google Scholar]
- 15.Qureshi N, Takayama K, Meyer KC, Kirkland TN, Bush CA, Chen L, Wang R, Cotter RJ. Chemical reduction of 3-oxo and unsaturated groups in fatty acids of diphosphoryl lipid A from the lipopolysaccharide of Rhodopseudomonas sphaeroides. Comparison of biological properties before and after reduction. J. Biol. Chem. 1991;266:6532–6538. [PubMed] [Google Scholar]
- 16.Qureshi N, Mascagni P, Ribi E, Takayama K. Monophosphoryl lipid A obtained from lipopolysaccharides of Salmonella minnesota R595. Purification of the dimethyl derivative by high performance liquid chromatography and complete structural determination. J. Biol. Chem. 1985;260:5271–5278. [PubMed] [Google Scholar]
- 17.Li J, Ryder C, Mandal M, Ahmed F, Azadi P, Snyder DS, Pechous RD, Zahrt T, Inzana TJ. Attenuation and protective efficacy of an O-antigen-deficient mutant of Francisella tularensis LVS. Microbiology. 2007;153:3141–3153. doi: 10.1099/mic.0.2007/006460-0. [DOI] [PubMed] [Google Scholar]
- 18.Enghofer E, Kress H. An evaluation of the Morgan--Elson assay for 2-amino-2-deoxy sugars. Carbohydr. Res. 1979;76:233–238. doi: 10.1016/0008-6215(79)80022-1. [DOI] [PubMed] [Google Scholar]
- 19.Qureshi N, Takayama K, Seydel U, Wang R, Cotter RJ, Agraval PK, Bush CA, Kurtz R, Berman DT. Isolation and purification of lipopolysaccharide from Brucella abortus and characterization of the lipid A moiety. J. Endotoxin. Res. 1994;1:137–148. [Google Scholar]
- 20.Takayama K, Qureshi N, Hyver K, Honovich J, Cotter RJ, Mascagni P, Schneider H. Characterization of a structural series of lipid A obtained from the lipopolysaccharides of Neisseria gonorrhoeae. Combined laser desorption and fast atom bombardment mass spectral analysis of high performance liquid chromatography-purified dimethyl derivatives. J. Biol. Chem. 1986;261:10624–10631. [PubMed] [Google Scholar]
- 21.Qureshi N, Kaltashov I, Walker K, Doroshenko V, Cotter RJ, Takayama K, Sievert TR, Rice PA, Lin J-S L, Golenbock DT. J. Biol. Chem. 1997;14:25–36. doi: 10.1074/jbc.272.16.10594. [DOI] [PubMed] [Google Scholar]
- 22.Plötz BM, Lindner B, Stetter KO, Holst O. Characterization of a novel lipid A containing D-galacturonic acid that replaces phosphate residues. The structure of the lipid a of the lipopolysaccharide from the hyperthermophilic bacterium Aquifex pyrophilus. J. Biol. Chem. 2000;275:11222–11228. doi: 10.1074/jbc.275.15.11222. [DOI] [PubMed] [Google Scholar]
- 23.Kaltashov IA, Doroshenko V, Cotter RJ, Takayama K, Qureshi N. Confirmation of the structure of lipid A derived from the lipopolysaccharide of Rhodobacter sphaeroides by a combination of MALDI, LSIMS, and tandem mass spectrometry. Anal. Chem. 1997;69:2317–2322. doi: 10.1021/ac9612943. [DOI] [PubMed] [Google Scholar]
- 24.Roppel J, Mayer H, Weckesser J. Identification of a 2,3-diamino-2,3-dideoxyhexose in the lipid A component of lipopolysaccharides of Rhodopuedomonas viridis and Rhodopseudomonas palustris. Carbohydr Res. 1975;40:31–40. doi: 10.1016/s0008-6215(00)82666-x. [DOI] [PubMed] [Google Scholar]
- 25.Que NLR, Ribeiro AA, Raetz CR. Two-dimensional NMR spectroscopy and structures of six lipid A species from Rhizobium etli CE3. Detection of an acyloxyacyl residue in each component and origin of the aminogluconate moiety. J. Biol. Chem. 2000;275:28017–28027. doi: 10.1074/jbc.M004009200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bhat UR, Forsberg LS, Carlson RW. Structure of lipid A component of Rhizobium leguminosarum bv phaseoli lipopolysaccharide. Unique nonphosphorylated lipid A containing 2-amino-2-deoxygluconate, galacturonate, and glucosamine. J. Biol. Chem. 1994;269:14402–14410. [PubMed] [Google Scholar]