Abstract
The P2X7 receptor is one of the members of the family of purinoceptors which are ligand-gated membrane ion channels activated by extracellular adenosine 5’-triphosphate. A unique feature of the P2X7 receptor is that its activation can result in the formation of large plasma membrane pores that allow not only the flux of ions but also of hydrophilic molecules of up to 900 Da. Recent studies indicate that P2X7-mediated signaling can trigger apoptotic cell death after ischemia and during the course of certain neurodegenerative disorders. Expression of the P2X7 receptor has been demonstrated in most types of cells in the retina. This purinoceptor mediates the contraction of pericytes and regulates the spatial and temporal dynamics of the vasomotor response through cell-to-cell electrotonic transmission within the microvascular networks. Of potential clinical significance, investigators have found that diabetes markedly boosts the vulnerability of retinal microvessels to the lethal effect of P2X7 receptor activation. This purinergic vasotoxicity may result in reduced retinal blood flow and disrupted vascular function in the diabetic retina. With recent reports indicating an association between P2X7 receptor activation and inflammatory cytokine expression in the retina, this receptor may also exacerbate the development of diabetic retinopathy by a mechanism involving inflammation.
Keywords: P2X7 receptor, Diabetic retinopathy, Vasotoxicity, Retinal microvessels, Interleukin-1β, Tumor necrosis factor-α
Core tip: This review summarizes the studies regarding the putative role of the P2X7 receptor in triggering purinergic vasotoxicity in the retina and thereby contributing to the progression of diabetic retinopathy.
INTRODUCTION
One of the most important characteristics of diabetic retinopathy (DR) is the death of microvascular pericytes and endothelial cells[1]. The loss of pericytes, contractile cells located on the abluminal wall of capillaries[2], appears to play a critical role in the development of microaneurysms and neovascular tufts[3]. Damage in the endothelial cells can result in a breakdown of the blood–retinal barrier and macular edema[4].
Currently, the mechanisms by which diabetes induces apoptosis in the retinal microvasculature remain uncertain, although oxidative stress, formation of advanced glycation end products, upregulation of protein kinase C, increased polyol pathway flux and focal leukostasis may be taken as important factors[5]. In fact, multiple lethal pathways may be activated during chronic hyperglycemia[6].
Extracellular adenosine 5’-triphosphate (ATP) is an excitatory transmitter both in the peripheral and central nervous systems. P2X receptors are a family of ligand-gated membrane ion channels activated by extracellular ATP. P2X receptors consist of seven isoforms designated P2X1 to P2X7[7,8]. They are widely distributed in most types of cells in nearly every origin. They are involved in many actions, such as synaptic transmission in the peripheral and central nervous systems, contraction of smooth muscle, platelet aggregation, macrophage activation, cell death and immunomodulation[9,10].
In contrast to other ligand-gated channels in the purinoceptor family, the P2X7 receptor possesses unique features that are likely to be of both physiological and pathophysiological significance. Most importantly, not only does the initial activation of these receptors result in the opening of a non-selective plasma membrane channel, but with sustained activation there is in many types of cells the formation of trans-membrane pores that are permeable to hydrophilic molecules of up to 900 Da[11,12]. Indicative of P2X7 receptors having a role in cell pathology, this receptor has been found to be highly up-regulated in neurons and glial cells located in the ischemic cerebral cortex[13]. P2X7-mediated signaling is also implicated in neurodegenerative diseases, such as Parkinson’s disease, Alzheimer’s disease and multiple sclerosis[14].
P2X7 RECEPTOR IN THE RETINA
Expression of the P2X7 receptor has been demonstrated in most types of cells in the retina; these include neurons such as the ganglion cells[15,16], as well as glia[17,18] and vascular cells[19]. The P2X7 receptor was found to mediate the contraction of pericytes through an increase in intracellular calcium levels[19]. Interestingly, the spatial and temporal dynamics of this vasomotor response are established by the ability of P2X7 activation to potently inhibit cell-to-cell electrotonic transmission within the retinal microvascular network[19].
In the adult rat retina, immunolabeling for the P2X7 receptor is detected in a number of cells in the inner nuclear layer and ganglion cell layer, suggesting amacrine cells and ganglion cells[15]. This receptor was also found in processes presynaptic to rod bipolar cells, as well as other conventional synapses, suggesting that purines play a role in neurotransmission within the retina and may modulate both photoreceptor and rod bipolar cell responses[20].
In addition to the putative physiological roles of P2X7 receptors, it is reported that stimulation of these receptors can kill retinal ganglion cells in vitro and in vivo by a mechanism that appears to be dependent on a rise in intracellular Ca2+[21,22]. One of those reports also suggested that the balance between extracellular ATP and its protective metabolite adenosine can influence ganglion cell survival in the living eye[22]. Another study suggested that an early up-regulation of neuronal P2X7 receptors may cause injury of retinal neurons and thereby contribute to the retinal damage[23]. Furthermore, data from our laboratory indicate that the activation of P2X7 receptors is involved in hypoxia-induced death of retinal neurons[24]. Other researchers have indicated mechanical strain triggers ATP release directly from retinal ganglion cells and that this released ATP autostimulates P2X7 receptors. Since extracellular ATP levels in the retina increase with elevated intraocular pressure and stimulation of P2X7 receptors on retinal ganglion cells can be lethal, this autocrine response may exert a deleterious effect on retinal ganglion cells in glaucomatous eyes[25].
P2X7 RECEPTOR AND DIABETIC RETINOPATHY
A study showed that human primary fibroblasts in a medium with a high glucose concentration underwent substantial ATP-mediated morphological changes and increased apoptosis. P2X7 was identified as the main purinergic receptor involved in these responses[26]. It has also been reported that fibroblasts from type 2 diabetes patients are characterized by a hyperactive purinergic loop based either on a higher level of ATP release or on increased P2X7 reactivity[27]. Another study revealed that changes in Müller cell membrane conductance in proliferative diabetic retinopathy (PDR), i.e., the down-regulation of active Kir channels and the membrane depolarization, likely disturb voltage-dependent Müller cell functions, such as regulation of local ion concentrations and uptake of neurotransmitters[28]. The enhanced entry of calcium ions from the extracellular space and the subsequent stimulation of calcium-activated potassium channels may trigger Müller cell proliferation in PDR. Others reported that prolonged stimulation of the P2X7 receptor elicited permeabilization exclusively in microglial cells but not in neurons of the inner retina[29].
Our experiments, using pericyte-containing retinal microvessels, have shown a diabetes-induced increase in the vulnerability of retinal microvessels to the lethal effect of P2X7 receptor activation[30]. In other words, the agonist concentration needed to open large membrane pores and trigger apoptosis decreased markedly soon after the onset of streptozotocin-induced hyperglycemia in rats (Figure 1). It was also found that extracellular nicotinamide adenosine dinucleotide (NAD+) caused cell death in the retinal microvasculature by a mechanism involving the activation of the P2X7 purinoceptor and the formation of transmembrane pores. Soon after the onset of diabetes, the sensitivity of retinal microvessels to the vasotoxic effect of extracellular NAD+ increased by approximately 100-fold[31]. In our in vivo study using the laser speckle circulation analyzer and electroretinography, soon after the onset of alloxan-induced diabetes, retinal blood velocity and function become more vulnerable to reduction initiated through the P2X7 receptor (Figure 2)[32]. Additional investigations indicate that, under physiological conditions, the formation of P2X7 pores is tightly regulated via a nitric oxide- and P2Y4-dependent pathway that limits the rise in pericyte calcium during the activation of these purinoceptors[33]. However, if this regulatory mechanism becomes dysfunctional, as appears to occur in the diabetic retina (Figure 3)[33], then purinergic vasotoxicity may contribute to the microvascular cell death that is a hallmark of DR.
Figure 1.
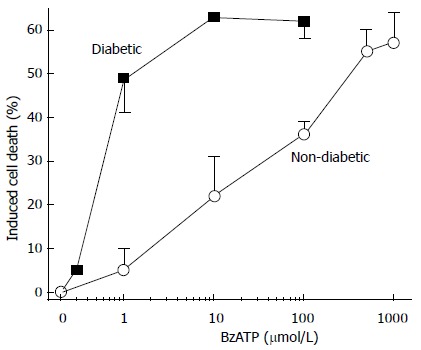
Cell death induced in non-diabetic and diabetic retinal microvessels by the P2X7 agonist, benzoylbenzoyl adenosine triphosphate. From Sugiyama et al[30] with permission from Investigative Ophthalmology and Visual Sciences. BzATP: Benzoylbenzoyl adenosine triphosphate.
Figure 2.
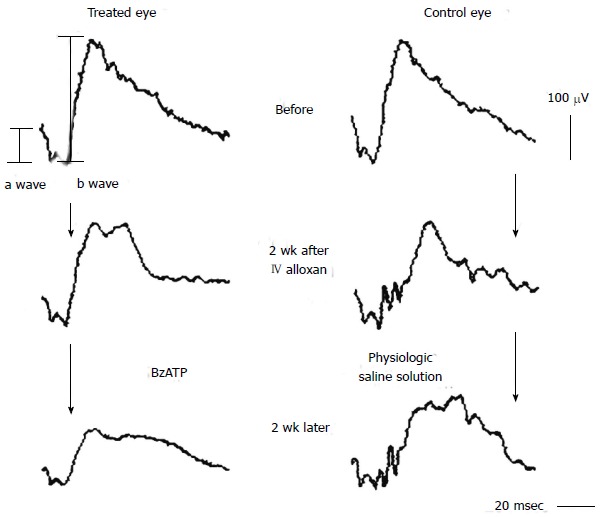
Typical changes of electroretinography after intravitreal injection (IV) of benzoylbenzoyl adenosine triphosphate (50 nmol) or physiological saline solution in an alloxan-induced diabetic rabbit. The amplitudes of a and b waves and oscillatory potentials were reduced in the BzATP-treated eye. From Sugiyama et al[32] with permission from Archives of Ophthalmology. BzATP: Benzoylbenzoyl adenosine triphosphate.
Figure 3.
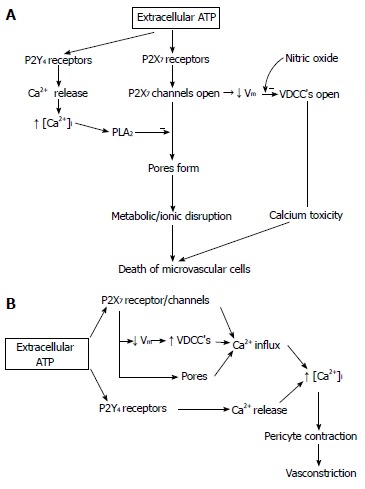
Models of the physiological and pathobiological effects of adenosine 5’-triphosphate in the retinal microvasculature. A: Putative mechanisms regulating purinergic vasotoxicity; B: Putative mechanisms by which extracellular adenosine 5’-triphosphate (ATP) causes pericyte Ca2+ levels to rise and thereby the contraction of these mural cells and the constriction of adjacent lumens. From Sugiyama et al[33].
Of additional interest, recent studies of DR in experimental models suggest the P2X7 receptors may have a role in mediating cytokine-induced vascular inflammatory reactions that can degrade the integrity of the blood-retinal barrier and thereby contribute to retinal vascular occlusion and ischemia[34]. More specifically, there are a number of reports linking P2X7 receptor activation in the retina with the expression of inflammatory cytokines[35]. For example, P2X7 agonists enhance the release of interleukin (IL)-1β and tumor necrosis factor (TNF)-α from hypoxia-activated retinal microglia[17]. In addition, our recent data suggest that the up-regulation of TNF-α, IL-1β and IL-6 may be involved in the retinal ganglion cell death that can occur with P2X7 receptors activated after an elevation in the intraocular pressure[36]. Although it is clear that more investigation is needed, these new findings further suggest that this purinoceptor may have a role in the progression of DR.
In conclusion, a variety of recent experimental studies are providing evidence that the P2X7 purinoceptor is a potential therapeutic target of a pharmacological strategy designed to diminish or prevent cell death in the diabetic retina.
ACKNOWLEDGMENTS
I would like to express my appreciation to Professor Donald G. Puro for leading me to this research topic during my stay in 2001 as a research fellow in his laboratory at the University of Michigan as well as for kindly editing this manuscript, and also to Professor Tsunehiko Ikeda for giving me the opportunity to study abroad and then to continue investigating this topic in Department of Ophthalmology at Osaka Medical College.
Footnotes
P- Reviewers: Gurunathan S, Koleva-Georgieva DN, Le YZ S- Editor: Ma YJ L- Editor: Roemmele A E- Editor: Wu HL
References
- 1.Mizutani M, Kern TS, Lorenzi M. Accelerated death of retinal microvascular cells in human and experimental diabetic retinopathy. J Clin Invest. 1996;97:2883–2890. doi: 10.1172/JCI118746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shepro D, Morel NM. Pericyte physiology. FASEB J. 1993;7:1031–1038. doi: 10.1096/fasebj.7.11.8370472. [DOI] [PubMed] [Google Scholar]
- 3.Enge M, Bjarnegård M, Gerhardt H, Gustafsson E, Kalén M, Asker N, Hammes HP, Shani M, Fässler R, Betsholtz C. Endothelium-specific platelet-derived growth factor-B ablation mimics diabetic retinopathy. EMBO J. 2002;21:4307–4316. doi: 10.1093/emboj/cdf418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Vriese AS, Verbeuren TJ, Van de Voorde J, Lameire NH, Vanhoutte PM. Endothelial dysfunction in diabetes. Br J Pharmacol. 2000;130:963–974. doi: 10.1038/sj.bjp.0703393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Archer DB. Bowman Lecture 1998. Diabetic retinopathy: some cellular, molecular and therapeutic considerations. Eye (Lond) 1999;13(Pt 4):497–523. doi: 10.1038/eye.1999.130. [DOI] [PubMed] [Google Scholar]
- 6.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813–820. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 7.North RA. Molecular physiology of P2X receptors. Physiol Rev. 2002;82:1013–1067. doi: 10.1152/physrev.00015.2002. [DOI] [PubMed] [Google Scholar]
- 8.Kaczmarek-Hájek K, Lörinczi E, Hausmann R, Nicke A. Molecular and functional properties of P2X receptors--recent progress and persisting challenges. Purinergic Signal. 2012;8:375–417. doi: 10.1007/s11302-012-9314-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burnstock G, Fredholm BB, North RA, Verkhratsky A. The birth and postnatal development of purinergic signalling. Acta Physiol (Oxf) 2010;199:93–147. doi: 10.1111/j.1748-1716.2010.02114.x. [DOI] [PubMed] [Google Scholar]
- 10.Burnstock G, Kennedy C. P2X receptors in health and disease. Adv Pharmacol. 2011;61:333–372. doi: 10.1016/B978-0-12-385526-8.00011-4. [DOI] [PubMed] [Google Scholar]
- 11.Valera S, Hussy N, Evans RJ, Adami N, North RA, Surprenant A, Buell G. A new class of ligand-gated ion channel defined by P2X receptor for extracellular ATP. Nature. 1994;371:516–519. doi: 10.1038/371516a0. [DOI] [PubMed] [Google Scholar]
- 12.Falzoni S, Munerati M, Ferrari D, Spisani S, Moretti S, Di Virgilio F. The purinergic P2Z receptor of human macrophage cells. Characterization and possible physiological role. J Clin Invest. 1995;95:1207–1216. doi: 10.1172/JCI117770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Franke H, Günther A, Grosche J, Schmidt R, Rossner S, Reinhardt R, Faber-Zuschratter H, Schneider D, Illes P. P2X7 receptor expression after ischemia in the cerebral cortex of rats. J Neuropathol Exp Neurol. 2004;63:686–699. doi: 10.1093/jnen/63.7.686. [DOI] [PubMed] [Google Scholar]
- 14.Romagnoli R, Baraldi PG, Cruz-Lopez O, Lopez-Cara C, Preti D, Borea PA, Gessi S. The P2X7 receptor as a therapeutic target. Expert Opin Ther Targets. 2008;12:647–661. doi: 10.1517/14728222.12.5.647. [DOI] [PubMed] [Google Scholar]
- 15.Brändle U, Kohler K, Wheeler-Schilling TH. Expression of the P2X7-receptor subunit in neurons of the rat retina. Brain Res Mol Brain Res. 1998;62:106–109. doi: 10.1016/s0169-328x(98)00254-x. [DOI] [PubMed] [Google Scholar]
- 16.Ishii K, Kaneda M, Li H, Rockland KS, Hashikawa T. Neuron-specific distribution of P2X7 purinergic receptors in the monkey retina. J Comp Neurol. 2003;459:267–277. doi: 10.1002/cne.10608. [DOI] [PubMed] [Google Scholar]
- 17.Morigiwa K, Quan M, Murakami M, Yamashita M, Fukuda Y. P2 Purinoceptor expression and functional changes of hypoxia-activated cultured rat retinal microglia. Neurosci Lett. 2000;282:153–156. doi: 10.1016/s0304-3940(00)00887-9. [DOI] [PubMed] [Google Scholar]
- 18.Pannicke T, Fischer W, Biedermann B, Schädlich H, Grosche J, Faude F, Wiedemann P, Allgaier C, Illes P, Burnstock G, et al. P2X7 receptors in Müller glial cells from the human retina. J Neurosci. 2000;20:5965–5972. doi: 10.1523/JNEUROSCI.20-16-05965.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kawamura H, Sugiyama T, Wu DM, Kobayashi M, Yamanishi S, Katsumura K, Puro DG. ATP: a vasoactive signal in the pericyte-containing microvasculature of the rat retina. J Physiol. 2003;551:787–799. doi: 10.1113/jphysiol.2003.047977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Puthussery T, Fletcher EL. Synaptic localization of P2X7 receptors in the rat retina. J Comp Neurol. 2004;472:13–23. doi: 10.1002/cne.20045. [DOI] [PubMed] [Google Scholar]
- 21.Zhang X, Zhang M, Laties AM, Mitchell CH. Stimulation of P2X7 receptors elevates Ca2+ and kills retinal ganglion cells. Invest Ophthalmol Vis Sci. 2005;46:2183–2191. doi: 10.1167/iovs.05-0052. [DOI] [PubMed] [Google Scholar]
- 22.Hu H, Lu W, Zhang M, Zhang X, Argall AJ, Patel S, Lee GE, Kim YC, Jacobson KA, Laties AM, et al. Stimulation of the P2X7 receptor kills rat retinal ganglion cells in vivo. Exp Eye Res. 2010;91:425–432. doi: 10.1016/j.exer.2010.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Franke H, Klimke K, Brinckmann U, Grosche J, Francke M, Sperlagh B, Reichenbach A, Liebert UG, Illes P. P2X7 receptor-mRNA and -protein in the mouse retina; changes during retinal degeneration in BALBCrds mice. Neurochem Int. 2005;47:235–242. doi: 10.1016/j.neuint.2005.04.022. [DOI] [PubMed] [Google Scholar]
- 24.Sugiyama T, Oku H, Shibata M, Fukuhara M, Yoshida H, Ikeda T. Involvement of P2X7 receptors in the hypoxia-induced death of rat retinal neurons. Invest Ophthalmol Vis Sci. 2010;51:3236–3243. doi: 10.1167/iovs.09-4192. [DOI] [PubMed] [Google Scholar]
- 25.Xia J, Lim JC, Lu W, Beckel JM, Macarak EJ, Laties AM, Mitchell CH. Neurons respond directly to mechanical deformation with pannexin-mediated ATP release and autostimulation of P2X7 receptors. J Physiol. 2012;590:2285–2304. doi: 10.1113/jphysiol.2012.227983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Solini A, Chiozzi P, Falzoni S, Morelli A, Fellin R, Di Virgilio F. High glucose modulates P2X7 receptor-mediated function in human primary fibroblasts. Diabetologia. 2000;43:1248–1256. doi: 10.1007/s001250051520. [DOI] [PubMed] [Google Scholar]
- 27.Solini A, Chiozzi P, Morelli A, Adinolfi E, Rizzo R, Baricordi OR, Di Virgilio F. Enhanced P2X7 activity in human fibroblasts from diabetic patients: a possible pathogenetic mechanism for vascular damage in diabetes. Arterioscler Thromb Vasc Biol. 2004;24:1240–1245. doi: 10.1161/01.ATV.0000133193.11078.c0. [DOI] [PubMed] [Google Scholar]
- 28.Bringmann A, Pannicke T, Uhlmann S, Kohen L, Wiedemann P, Reichenbach A. Membrane conductance of Müller glial cells in proliferative diabetic retinopathy. Can J Ophthalmol. 2002;37:221–227. doi: 10.1016/s0008-4182(02)80113-2. [DOI] [PubMed] [Google Scholar]
- 29.Innocenti B, Pfeiffer S, Zrenner E, Kohler K, Guenther E. ATP-induced non-neuronal cell permeabilization in the rat inner retina. J Neurosci. 2004;24:8577–8583. doi: 10.1523/JNEUROSCI.2812-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sugiyama T, Kobayashi M, Kawamura H, Li Q, Puro DG. Enhancement of P2X7-induced pore formation and apoptosis: an early effect of diabetes on the retinal microvasculature. Invest Ophthalmol Vis Sci. 2004;45:1026–1032. doi: 10.1167/iovs.03-1062. [DOI] [PubMed] [Google Scholar]
- 31.Liao SD, Puro DG. NAD+-induced vasotoxicity in the pericyte-containing microvasculature of the rat retina: effect of diabetes. Invest Ophthalmol Vis Sci. 2006;47:5032–5038. doi: 10.1167/iovs.06-0422. [DOI] [PubMed] [Google Scholar]
- 32.Sugiyama T, Oku H, Komori A, Ikeda T. Effect of P2X7 receptor activation on the retinal blood velocity of diabetic rabbits. Arch Ophthalmol. 2006;124:1143–1149. doi: 10.1001/archopht.124.8.1143. [DOI] [PubMed] [Google Scholar]
- 33.Sugiyama T, Kawamura H, Yamanishi S, Kobayashi M, Katsumura K, Puro DG. Regulation of P2X7-induced pore formation and cell death in pericyte-containing retinal microvessels. Am J Physiol Cell Physiol. 2005;288:C568–C576. doi: 10.1152/ajpcell.00380.2004. [DOI] [PubMed] [Google Scholar]
- 34.Liou GI. Diabetic retinopathy: Role of inflammation and potential therapies for anti-inflammation. World J Diabetes. 2010;1:12–18. doi: 10.4239/wjd.v1.i1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weisman GA, Camden JM, Peterson TS, Ajit D, Woods LT, Erb L. P2 receptors for extracellular nucleotides in the central nervous system: role of P2X7and P2Y2 receptor interactions in neuroinflammation. Mol Neurobiol. 2012;46:96–113. doi: 10.1007/s12035-012-8263-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sugiyama T, Lee SY, Horie T, Oku H, Takai S, Tanioka H, Kuriki Y, Kojima S, Ikeda T. P2X7 receptor activation may be involved in neuronal loss in the retinal ganglion cell layer after acute elevation of intraocular pressure in rats. Mol Vis. 2013;19:2080–2091. [PMC free article] [PubMed] [Google Scholar]


