Abstract
AIM: To investigate the presence and potency of natural enzyme inhibitors with hypoglycemic potentials amongst Eucalyptus Spp. by in vitro assays.
METHODS: The leaf extracts of the three different Eucalyptus species [E. globulus (EG), E. citriodora (EC), E. camaldulensis (ECA)] were subjected to in vitro assay procedures to explore the prevalence of natural enzyme inhibitors (NEIs) after preliminary qualitative and quantitative phytochemical evaluations, to study their inhibitory actions against the enzymes like α-amylase, α-glucosidase, aldose reductase, angiotensin converting enzyme and dipeptidyl peptidase 4 playing pathogenic roles in type 2 diabetes. The antioxidant potential and total antioxidant capacity of the species were also evaluated.
RESULTS: Major bioactive compounds like polyphenols (341.75 ± 3.63 to 496.85 ± 3.98) and flavonoids (4.89 ± 0.01 to 7.15 ± 0.02) were found in appreciable quantity in three species. Based on the IC50 values of the extracts under investigation, in all assays the effectivity was in the order of EG > ECA > EC. The results of the ferric reducing antioxidant power assay showed that the reducing ability of the species was also in the order of EG > ECA > EC. A strong correlation (R2 = 0.81-0.99) was found between the phenolic contents and the inhibitory potentials of the extracts against the targeted enzymes.
CONCLUSION: These results show immense hypoglycemic potentiality of the Eucalyptus Spp. and a remarkable source of NEIs for a future phytotherapeutic approach in Type 2 diabetes.
Keywords: Natural enzyme inhibitors, Hypoglycemic, Eucalyptus, In vitro assays, Pathogenic, Polyphenols, Flavonoids
Core tip: Enzymes play an essential role in mediating important biochemical processes of life but hyper or hypo activity of such enzymes leads to malfunctions of the processes. Etiopathogenesis of diseases at molecular level has shown that enzyme inhibitors can serve as effective therapeutic bullets for several diseases. The plant kingdom is a giant hub of phytomolecules with variant pharmacology, largely unexplored. Volatile and non-volatile fractions of Eucalyptus include bioactive compounds like terpenes, triterpenoids, flavonoids, polyphenols, etc. The exploration of enzyme inhibitors amongst Eucalyptus species by in vitro assays will help in bioactivity guided isolations of such inhibitors to be targeted as natural hypoglycemics.
INTRODUCTION
Diabetes mellitus (DM) is fast becoming the epidemic of the 21st century, becoming one of the major killers of the health of mankind after Acquired Immuno Deficiency Syndrome, cancer and cerebrovascular diseases[1]. The statistics of the global diabetic population is expected to show a steady growth to 366 million by 2030. The international diabetes federation has estimated the number of diabetics in India to be 40.9 million, which is expected to grow to 60.9 million by 2025[1,2]. Diabetes is a common metabolic disorder with abnormal elevations in the blood glucose lipid profile, leading to major complications like diabetic neuropathy, nephropathy leading to end stage renal disease, retinopathy leading to blindness and diabetic foot ulcers necessitating limb amputations[1,2]. But despite tremendous strides in modern medicines, the availability of insulin therapy and synthetic hypoglycemics, their failure to restore normoglycemia without adverse effects calls for phytotherapy and alternative medicine[3,4]. Enzymes play a vital role in mediating essential biochemical life processes like metabolism, cell cycling, signal transduction, etc. However, hyper or hypo activity of such enzymes leads to malfunctions of the respective biochemical processes which in many cases are the underlying causes of diseases like diabetes, Alzheimer’s disease, myasthenia gravis and Parkinson’s disease, as depicted by their etiopathogenesis at the molecular level. It is anticipated that enzyme inhibitors serve as important therapeutic targets for these diseases[5]. It has been found that enzymes like α-amylase, α-glucosidase, dipeptidyl peptidase 4 (DPP4), aldose reductase (AR), angiotensin converting enzyme (ACE) and peroxisome proliferator activated receptor-γ (PPAR-γ) contribute significantly to the pathogenesis of type 2DM. Reactive oxygen species (ROS) also play a pathogenic role in type 2DM.
Phytomolecules, as natural enzyme inhibitors (NEIs), can serve as successful therapeutic bullets in the control of this chronic disease[2,5-8]. The World Health Organization has recommended phytotherapy for diabetes due to safety, effectivity, availability and affordability. The NAPRALERT database (NAtural PRoducts ALERT) and the ethnobotanical literature have reported more than 800 anti-diabetic plant species[4,7-9].
Eucalyptus, also known as “gum tree”, is taxonomically from the family Myrtaceae, indigenous to Tasmania, Australia and cultivated mostly in subtropical and warm temperate regions of the world. The bark and leaves of Eucalyptus Spp. have been used in folk medicine for the treatment of ailments such as colds, fever, toothache, diarrhea and snake bites. Uses of Eucalyptus leaf hot decoctions as “herbal tea” have been recorded in Aboriginal, European and British Pharmacopeias for the traditional remedy of type DM[10-21]. A rich literature exists, reporting over 500 Eucalyptus species with different pharmacological actions[11-22]. Hypoglycemic potentials of Eucalyptuses are documented, but the mechanistic actions need to be explored further [11-21].
Inhibiting the actions of carbohydrate hydrolyzing enzymes like α-amylase and α-glucosidase helps to reduce post-prandial (PP) hyperglycemia. Inhibition of other enzymes like AR, DPP-4, ACE and PPAR-γ also presents an effective strategy to combat type 2 DM naturally[5,6,8,11]. Extensive literature surveys and our previous works have reported that Eucalyptus shows the presence of terpenoids, triterpenoids, flavonoids, polyphenols and tannins in its various volatile and non-volatile fractions[8,21,22]. Major phytomolecules isolated from the Eucalyptus Spp and their inhibitory activity against the enzymes are depicted in Table 1.
Table 1.
List of phytochemicals of Eucalyptus Spp. inhibiting the enzymes
| Phytochemicals | Enzymes inhibited ↓ |
| Flavonoids, like quercetin, kaempferol, myricetin; Phenolics-tannins, ellagic acid, and gallic acid; terpinoids-ursolic acid, oleanolic acid, p-cymene, and 1,8-cineole, 1-(S)-α-pinene | α-amylase |
| Polyphenols, proanthocyanidins, anthocyanins | α-glucosidase |
| Flavonoids, flavonols, terpenoids, mono-terpenes | Aldose reductase |
| Flavonoids, flavonols, terpenoids, mono-terpenes | Angiotensin converting enzyme |
| Terpenoids | Peroxisome proliferator activated receptor |
| Triterpenoids, phenolic compounds | Di-peptidyl peptidase 4 |
AR, a member of the aldo-keto reductase super family, is the first and rate-limiting enzyme in the polyol pathway and reduces glucose to sorbitol, utilizing reduced form of nicotinamide adenine dinucleotide phosphate (NADPH) as a cofactor. In type 2DM, due to increased availability of glucose in sensitive tissues like lens, nerves and retina, there is an increased formation of sorbitol through the polyol pathway. Intracellular accumulation of sorbitol leads to cataract, retinopathy and neuropathy. AR-inhibitors prevent the conversion of glucose to sorbitol and are capable of controlling diabetic complications[8,23-32]. Limited literature data and molecular docking analysis have shown that natural biomolecules with potent aldose reductase inhibitory actions include flavonoids like quercetin, quercitrin, myricitrin, coumarins, monoterpenes, stilbenes, etc. Flavonoids with binding energy (BE) ranging between -9.33 to -7.23 kcal/mol exhibited AR inhibitory properties, as evidenced by in-silico docking studies[8,32,33]. Five bioactive compounds, namely macrocarpals A-E detected in the ethanol extracts of the leaves of E. globulus, showed antibacterial actions, HIV-RTase (HIV-reverse transcriptase) inhibitory activity and also inhibited AR[8,32,33].
Attenuation in ROS level may be due to increased production or diminished depletion of enzymes, like catalase, glutathione peroxidase and superoxide dismutase. Natural antioxidants which scavenge free radicals can provide a synergistic action to the overall antidiabetic potential of a plant[12,13]. Osawa and Namiki (1981, 1985) reported the presence of a powerful antioxidant, 4-hydroxytritriacontane-16,18 dione, in the leaf wax of different Eucalyptus species[24,25].
The enzyme ACE is associated with hypertension, a long term complication of diabetes. ACE activates histidyl leucine dipeptide called angiotensin-І into a potent vasoconstrictor called angiotensin-II. Angiotensin-ІІ influences aldosterone release which increases blood pressure by promoting sodium retention in distal tubules. Biomolecules like flavonoids, flavonols, anthocyanins and triterpenes are potent ACE inhibitors[8,34,35]. Molecular docking studies also recommend the use of herbal ACE inhibitors in the management of type 2 DM[8,34,35].
PPAR-γ is a key receptor in lipid and glucose homeostasis because of its ability to reduce the plasma free fatty acids and phytomolecules can exert their insulin sensitizing actions with their high affinity for the receptor PPAR-γ. Terpenoids act as PPAR modulators regulating carbohydrate and lipid metabolism. Several terpenoids have been isolated from the Eucalyptus species and PPAR antagonism is amongst the suggested modes of hypoglycemic action of Eucalyptus[8,36].
Glucagon-like peptide-1 (GLP-1) is a remarkable antidiabetic gut hormone with combinatorial actions of stimulating insulin secretion, inhibiting glucagon secretion, increasing beta cell mass, reducing the rate of gastric emptying and inducing satiety. DPP4 rapidly deactivates GLP-1. Phytomolecules, mostly triterpenoids, steroids and phenolic constituents with DPP4 inhibitory activity, help to increase the levels of endogenous active GLP-1 and act as an important therapeutic compound against type 2 DM, the fact being further supported by molecular docking studies[8,37].
The present report documents our studies aiming to explore the major phytochemicals amongst three Eucalyptus species, E. globulus (EG, blue gum or Tasmanian blue gum), E. citriodora (EC, lemon scented gum) and E. camaldulensis (ECA, river red gum or Murray red gum), along with the existence of NEIs of enzymes like α-amylase, α-glucosidase, AR, DPP4, ACE and antioxidant enzymes by in vitro assays, with the perspective to evaluate the potentiality of these three species to combat type 2 DM and its complications. Furthermore, such research will help in bioactivity guided isolation of potent NEIs.
MATERIALS AND METHODS
Plant materials
Fresh leaves of EG, EC and ECA were collected from natural and man-made forest areas of IIT Kharaghpur and adjoining areas, like Balarampur, Gopali and Arabari forest areas, and authenticated by Dr Shanta AK, Biotechnologist, Nirmala College of Pharmacy, Guntur, India.
Reagents and chemicals
Yeast α-glucosidase, bovine serum albumin, sodium azide, para-nitrophenyl α-D-glucopyranoside solution (pNPG ), ACE (from rabbit lung, 3.5 units/mg of protein), starch azure, porcine pancreatic amylase, Tris-HCl buffer, hippuryl-L-histidyl-L-leucine (HHL) and 1,1-diphenyl-2-picrylhydrazyl (DPPH) were obtained from Sigma Chemicals, United States. Other chemicals like diagnostic reagents, surfactants, polyphosphate, dextran sulphate, etc., were purchased from Merck Co, India. Acarbose was a kind gift sample from Zota Pharmaceuticals Pvt. Ltd, Chennai, India.
Preparation of eucalyptus leaf extracts
A uniform methodology was followed for preparing the leaf extracts of the three different species of Eucalyptus. Typically, the leaves were washed first with tap water and then with distilled water to remove all dust, subjected to shade drying at 25 ± 3 °C temperature, and then finely powdered in an electrical grinder (Bajaj GX 11, India). The leaf powder was stored at room temperature in an airtight container until use and labeled separately as EG, EC and ECA. Extraction was carried out as described by Sugimoto et al[20,21] with few modifications. Briefly, 500 g of leaf powder of each species was extracted separately with ethanol-water (1:2 v/v) under reflux for 2 h, filtered through a Whatman filter paper no. 1, concentrated using a rotary evaporator (Buchi, Flawil, Switzerland) and dried in a vacuum oven. The percentage yield of extracts was calculated with regard to the initial weight of dry powders and final weight of the extracts. These extracts were then subjected to preliminary and quantitative phytochemical evaluations and in vitro assay procedures.
Phytochemical investigations of the eucalyptus leaf extract
Phytochemical analysis of the major bioactive compounds of interest of the three different extracts (EG, EC and ECA) was performed using the methods of Harbone (1984), Trease and Evans (1989) and other literature methods[22,38]. The three extracts were analyzed for glycosides (Keller Killiani and Borntrager’s tests), alkaloids (Mayer’s, Dragendorff’s reagents), saponins (Foam test), triterpenes (Salkowski and Libermann Burchard tests) and 1,8-cineole (Marquis reagent, Gallic acid reagent, conc. H2SO4 and phloroglucinol).
The total polyphenol content of the extracts was determined by ultra violet (UV) spectrophotometry (Perkin Elmer Lambda 25 UV-vis) at 760 nm using Folin-Ciocalteu reagent by the method of Othman et al[39] and Modnicki et al[40] (2009)[41,42]. The concentrations of the total polyphenols were determined in Gallic equivalents (GAE) per gram of the extract. The polyphenol content was calculated by the formula: X = (5.6450 × A)/m. Where X is total phenolic compounds (%), A is absorbance of investigated extract and m is mass (g) of the investigated sample.
The total flavonoid content of the extracts was determined by the method of Djeridane et al[43], 2006, which is based on the formation of a complex of flavonoid-aluminium, and the concentration of the flavonoids was expressed in terms of QE per gram extract.
The flavonol content of the extracts was determined according to Abdel-Hameed, 2009, which is based on the formation of a complex between the extract with AlCl3 and sodium acetate and the total flavonol content was expressed in terms of quercetin equivalent (QE) per gram extract[42].
Tannins were measured according to the protocol of Hagerman and Butler, 1978, which is based on the obtention of a colored complex Fe2+-phenol whose absorbance was measured spectrophotometrically at 510 nm. The tannin content was obtained in mg of tannic acid equivalent (TAE) per gram extract[44].
The three extracts were subjected to color reactions with Marquis Reagent, gallic acid reagent, concentrated H2SO4 and phloroglucinol reagent. Standard 1,8-cineole gives orange color with Marquis reagent, yellow color with gallic acid, dark yellow color with concentrated H2SO4, and no coloration with phloroglucinol reagent[22,38].
Gas chromatographic analysis of 1,8-cineole
Fresh leaves of the three Eucalyptus spp. (EG, EC and ECA) were air dried and 100 g leaves of each variety were subjected to hydrodistillation for 3-4 h to extract the essential oil, employing a Clevenger type apparatus[45]. Extracted oils were decanted from the water layer and dried over anhydrous sodium sulfate. The extracted oils of the three species were subjected to gas chromatographic (GC) analysis (perkin elmer clarus 500, with Flame Ionization Detector) as described by Quereshi et al[45]. The operating conditions were: nitrogen as carrier gas, injector and detector temperature of -250 °C; column of 30 m (length) × 0.25 mm (inner diameter) and film thickness of 0.25 μm. The temperature was gradually increased at a rate of 15 °C/min to 240 °C for 4 min.
In vitro assay procedures
After phytochemical investigations of the leaf extracts of EG, EC and ECA, in vitro-inhibitory assays of α-amylase, α-glucosidase, aldose reductase, ACE, DPP4, antioxidant assays like DPPH free radical scavenging activity, scavenging of hydrogen peroxide and total antioxidant activity in the ferric reducing antioxidant power (FRAP) assay were carried out following standard methods[46-57].
The enzymes mentioned above contribute to the pathogenesis of type 2 DM in one way or another. Inhibition of such enzymes helps to combat type 2 DM naturally. There are ample research works highlighting the hypoglycemic potentials of Eucalyptus. We explored such NEIs by the above mentioned in vitro assays and once again evaluated the hypoglycemic potentiality of Eucalyptus.
α-Amylase inhibitory assay: The study was carried out following standard literature methodologies with slight modifications[12,46,47]. Briefly, 2 mg of starch azure was suspended in a tube containing 0.2 mL of 0.5 mol tris-phosphate buffer (pH = 6.9) containing 0.01 mol calcium chloride as the substrate. After boiling the tube for 5 min, it was preincubated for 5 min at 37 °C. Different concentrations (10, 20, 40, 60, 80 and 100 μg/mL) of the extracts of EC, EG and ECA were prepared by dissolving in 1 mL of 0.1% dimethyl sulfoxide. Then 0.2 mL of the extract of particular concentrations was put in the tube containing the substrate solution. Next, 0.1 mL of porcine pancreatic amylase in tris-HCL buffer (2 units/mL) was added to the tube containing extracts and substrate, at 37 °C. After 10 min, the reaction was stopped by adding 0.5 mL of 50% acetic acid in each tube and the reaction mixture was centrifuged (Eppendorf-5804R) at 3000 g for 5 min at 4 °C. The absorbance of the resulting supernatant was measured at 595 nm. Acarbose (Acar) in the concentration range of 1.25, 2.5, 5 and 10 μg/mL in distilled water was used to create the calibration curve. The assay was performed in triplicate. The concentration of the Eucalyptus extracts of three species (EG, EC and ECA) required to inhibit 50% of α amylase activity under the assay conditions is referred to as IC50 values. Absorbance was calculated using the formula: a amylase activity = [(Ac+) - (Ac-) - (As-Ab)]/[(Ac+) - (Ac-)] × 100.
α-glucosidase inhibitory assay: The assay procedure was developed as described by Basak et al[12] and Subramanian et al[47], with slight modifications. An aqueous ethanol extract of the three species (EG, EC and ECA) was used for the study. The yeast α-glucosidase enzyme solution was prepared by dissolving at a concentration of 0.1 U/mL in 100 mmol phosphate buffer, pH = 7.0, containing bovine serum albumin and sodium azide which was used as enzyme source. This enzyme solution was added to the aqueous-ethanolic extracts of EG, EC and ECA in increasing concentrations (1, 1.5, 2, 2.5, 3, 3.5 μL/mL). The reaction was initiated by adding 0.20 mL of para-nitrophenyl α-D-glucopyranoside solution (pNPG); 2 mmol pNPG in 50 mmol sodium phosphate buffer pH = 6.9) which acted as the substrate. The reaction was terminated by adding 1 mL 0.1 mol/L Na2HPO4. The test tubes were cooled under tap water and α-glucosidase inhibitory activity was determined at 405 nm by measuring the quantity of P-nitrophenol released from pNPG. The assay was performed in triplicate for each extract and the data presented as mean ± SD. The concentration of the Eucalyptus extracts (EG, EC and ECA) required inhibiting 50% of α-glucosidase activity under experimental conditions is defined as the IC50 value. Acarbose (Acar) was dissolved in distilled water to prepare a series of dilutions (1.25, 2.5, 5, 10 mg/mL) and was used as the positive control. The percentage inhibition was calculated according to the formula: %inhibition = (Abs400 control - Abs400 extarct)/Abs400 control.
IC50 values were determined from the plots of percentage inhibition vs log inhibitor concentration and were calculated by nonlinear regression analysis from the mean inhibitory values.
Aldose reductase inhibitory assay: The assay was carried out following reported literature methods and the experimental protocol approved by the Institutional Ethical Committee[48-50]. Two to three mo old healthy adult Wistar albino rats weighing about 150-200 g were acclimatized to laboratory conditions (12 h light and 12 h dark cycle, 25 ± 5 °C, 30%-60% relative humidity) with free access to pelleted food and water ad libitum. Immediately after sacrifice, lenses were removed from the eyes, washed with saline and the fresh weights of a lens were measured. Next, a 10% homogenate was prepared from the rat lens in 0.1 mol/L phosphate-buffered saline (PBS) at pH 7.4, centrifuged at 5000 g for 10 min in the cold and the supernatant collected. The protein content of the supernatant was determined by literature methods[48-50].
For the determination of the aldose reductase (AR) inhibitory activity, 0.7 mL of phosphate buffer (0.067 mol), 0.1 mL of NADPH (25 × 10-5 mol), 0.1 mL of DL-glyceraldehyde (substrate, 5 × 10-4 mol) and 0.1 mL of lens supernatant were mixed in the sample cuvette. Absorbance was taken against a reference cuvette containing all other components except the substrate, DL-glyceraldehyde. The final pH of the reaction mixture was adjusted to pH = 6.2. On adding substrate to the solution mixture, the enzymatic reaction starts and absorbance (OD) was recorded at 340 nm for 3 min at 30 s intervals. AR activity was calculated and expressed as OD/min per milligram protein.
For the determination of the AR inhibitory activity of the Eucalyptus extracts, a stock solution was prepared by dissolving the Eucalyptus extracts (EG, EC and ECA) in PBS and different concentrations prepared from stock solutions were added to both the reference and standard cuvettes. The reaction was initiated by the addition of 0.1 mL DL-glyceraldehyde and the reaction rate measured as mentioned above. Percentage inhibitions of AR activity of the extracts were calculated with reference to normal rat lens to have 100% activity. The concentrations of the extracts required to inhibit 50% of AR activity under assay conditions is defined as the IC50 values which were calculated for each sample by plotting a graph between log dose concentrations vs percentage inhibition. Quercetin, a known AR inhibitor, was used as the positive control.
ACE inhibitory assay: The assay method was based on the liberation of hippuric acid from hippuryl-L-histidyl-L-leucine (HHL) catalyzed by the ACE. The assay procedure was carried as described [51,52] and other methods with slight modifications. Briefly, 50 μL of sample solutions (extracts of EC, EG and ECA) in the concentration range of 0.1-2.5 mg/mL were preincubated with 50 μL of ACE (25 mU/mL) at 37 °C for 10 min. Next, 150 μL of substrate solution (8.3 mmol HHL in 50mmol sodium borate buffer containing 0.5 mol NaCl at pH 8.3) was added and incubated for 30 min at 37 °C. The reaction was terminated by addition of 250 μL 1.0 mol HCl. To the resulting solution, 0.5 mL of ethyl acetate was added and centrifuged (Eppendorf-5804R) for 15 min. Then, 0.2 mL of the upper layer was transferred to a test tube, evaporated under room temperature in vacuum and the liberated hippuric acid was dissolved in 1 mL distilled water and the absorbance was measured at 228 nm. Experiments were performed in triplicates. Captopril was used as standard (3.5 μg/mL) in the assay. The percentage of inhibition (ACEI) was calculated using the formula: %inhibition = (A-B)/(A - C) × 100. Where A is the OD at 228 nm with ACE but without inhibitor, B is the OD in presence of both ACE and inhibitor, C is the OD without ACE and inhibitor.
DPP4 inhibitory assay: The assay was carried out following reported literature methods using GPN-Tos (Gly-Pro p-nitroanilide toluenesulfonate salt) as the substrate[53-55]. Briefly, 0.5 mL of the assay mixture contained 40 mmol K-Na-phosphate buffer, pH 7.5, an enzyme sample. The reaction was initiated by adding a substrate to a concentration of 0.24 mmol and stopped by adding 0.2 mol acetic buffer at pH 5.5. The differential absorption at 390 nm was recorded against an identical mixture without the enzyme and the amount of p-nitroaniline depleted was evaluated from its extinction coefficient at the wavelength of 9.9 mmol/L/cm-1.
Evaluation of antioxidant activity
Dpph free radical scavenging activity: The antioxidant activity of the Eucalyptus extracts (EC, EG and ECA) was determined on the basis of the scavenging effect on the stable DPPH free radical activity[12,39,51,56]. A stock solution of DPPH in methanol (33 mg in 1 L) was freshly prepared and kept in the dark at 4 °C; after checking its initial absorbance, 5 mL of this stock solution was added to 1 mL of the solution of the extracts prepared in concentrations of 50-500 μg/mL. Next, 2.8 mL of 95% methanol was added and the mixture was shaken vigorously and after 30 min the absorption was measured at 517 nm. Ascorbic acid was used as the standard. The radical scavenging capacities of the test samples were expressed as percentage inhibition and calculated according to the equation: % inhibition of DPPH activity = (Absorbance control - Absorbance)/(Absorbance control) × 100.
Plotting was done of percentage inhibition vs concentration, and the concentration of sample required for 50% inhibition is regarded as IC50 value for each of the test samples.
Total antioxidant activity (FRAP assay): Total antioxidant activity was determined by the FRAP assay as described by Pracheta et al[56] and Shahwar et al[57]. It is a direct test of antioxidant capacity. The assay of reducing activity is based on the reduction of ferric to ferrous form in the presence of antioxidants in the tested samples (extracts of Eucalyptus species). The stock solutions included 10 mmol/L 2,4,6-tripyridyl-s-triazine (TPTZ) in 40 mmol HCl and 20 mmol FeCl3, and 300 mmol acetate buffer (pH 3.6). The working solutions were freshly prepared by mixing 25 mL acetate buffer, 2.5 mL TPTZ and 2.5 mL of FeCl3. The temperature of the solution was raised to 37 °C prior to use. Eucalyptus extracts (200 μL) were allowed to react with FRAP solution (2900-3000 μL) for 30 min in the dark. Absorbance of the colored product formed (ferrous tripyridyl triazine complex) was recorded at 595 nm. Results were expressed in μM equivalent to FeSO4 by extrapolation from the calibration curve.
Statistical analysis
The experimental results were expressed as mean ± SD of three replicates. The data were subjected to one way analysis of variance (ANOVA) using commercially available software (Prism version 5.0; Graph Pad Software, San Diego, CA, United States). Results were analyzed by Student’s t test (paired or unpaired, as appropriate) or Tukey’s multiple comparison test. Statistical analysis was performed by using GraphPad Prism where P < 0.05 was considered statistically significant.
RESULTS
The yield of the Eucalyptus leaf extracts (extractions carried out in triplicates) were 49% ± 3.3% for EG, 46.5% ± 4.2% for EC, and 45.8 ± 3.9% for ECA. The details of phytochemicals amongst Eucalyptus Spp. and the enzymes inhibited by them are presented in Tables 1 and 2 and Figure 1. The color test results for the presence of 1,8-cineole in the extracts of EG, EC and ECA are presented in Table 3. GC analysis of the oils extracted from three species (EG, EC and ECA) showed the highest 1,8-cineole content in EG (about 50%). ECA also showed the presence of 1,8-cineole in addition to several other peaks indicating the presence of other compounds. In EC citronellal was found to be the major component.
Table 2.
Total polyphenol, flavonoid, flavonol and tannin contents of E. globulus, E. citriodora and E. camaldulensis
| Extract1 | Polyphenol (mg/g extract2) | Tannins (mg/g extract2) | Flavonoid (mg/g extract2) | Flavonol (mg/g extract2) |
| EG | 496.85 ± 3.98 | 329.06 ± 6.25 | 7.15 ± 0.02 | 4.98 ± 0.01 |
| EC | 341.75 ± 3.63 | 199.75 ± 5.49 | 4.89 ± 0.01 | 3.87 ± 0.05 |
| ECA | 429.91 ± 4.03 | 253.15 ± 4.96 | 5.01 ± 0.02 | 4.09 ± 0.01 |
Content expressed per gram of relevant extracts (EG, EC and ECA);
Values are expressed as mean ± SD from triplicate determination. EG: E. globulus; EC: E. citriodora; ECA: E. camaldulensis.
Figure 1.
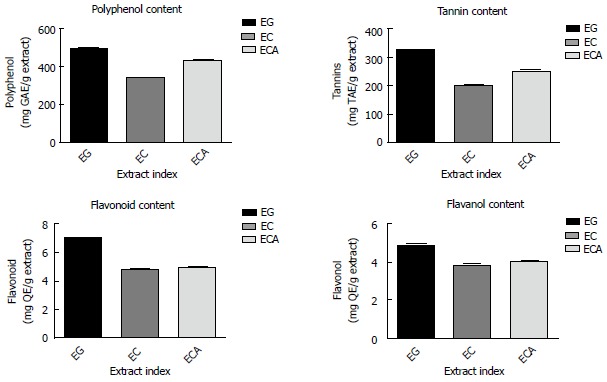
Graphical presentations of the presence of phytochemicals in Eucalyptus extracts. Data are presented as the mean ± SD of each triplicate test. EG: E. globulus; EC: E. citriodora; ECA: E. camaldulensis.
Table 3.
Color test results for the presence of 1,8-cineole in E. globulus, E. citriodora and E. camaldulensis extracts
| Extracts | Marquis test | Gallic acid test | Concentrated H2SO4 | Phloroglucinol |
| EG | Orange | Yellow | Dark yellow | No color |
| EC | Orange | Dark Yellow | Dark yellow | No color |
| ECA | Orange | Yellow | Bright orange-yellow | Pink |
EG: E. globulus; EC: E. citriodora; ECA: E camaldulensis.
All three extracts (EG, EC and ECA) showed promising inhibitory potentials for enzymes, including α-amylase, α-glucosidase, AR, ACE and DPP4. The antioxidative potential of the extracts were determined by DPPH radical scavenging and the total antioxidative capacity by the FRAP assay. The results of all such inhibitory assays are presented in Figure 2 and the summary of the IC50 values of tested samples in Table 4.
Figure 2.
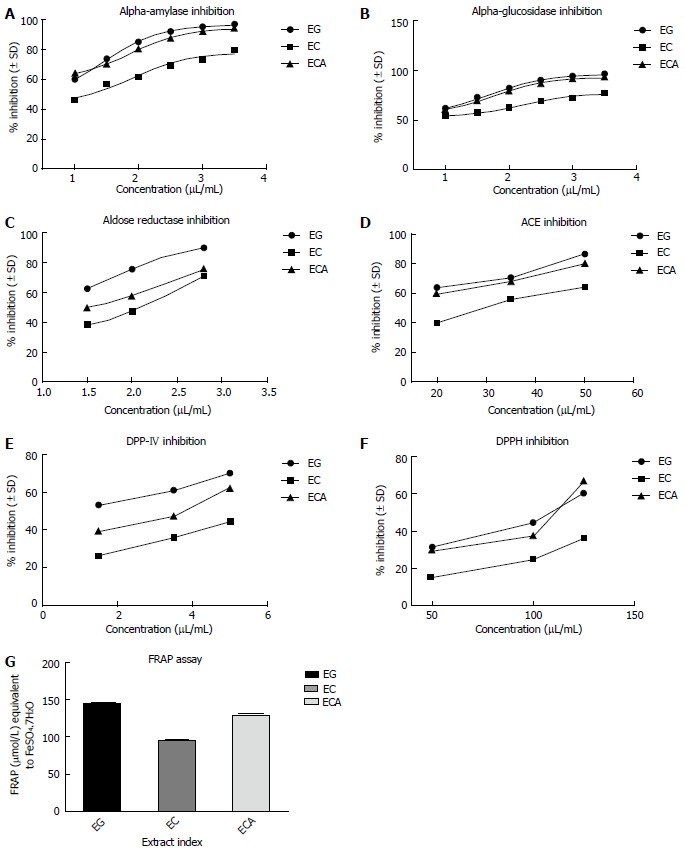
Eucalyptus extracts. A: Alpha-amylase; B: Alpha-glucosidase; C: Aldose-reductase; D: Angiotensin converting enzyme; E: Dipeptidyl peptidase 4; F: 1,1-Diphenyl-2-picrylhydrazyl; G: FRAP assay. Data are presented as the mean ± SD of each triplicate test. EG: E. globulus; EC: E. citriodora; ECA: E. camaldulensis; FRAP: Ferric reducing antioxidant power.
Table 4.
IC50 inhibitory values of Eucalyptus extracts E. globulus, E. citriodora and E. camaldulensis in different assays
| Assays | EG | EC | ECA |
| α-amylase | 3.01 ± 0.01 | 4.13 ± 0.09 | 3.65 ± 1.04 |
| α-glucosidase | 2.08 ± 0.01 | 2.68 ± 0.11 | 2.11 ± 0.19 |
| Aldose reductase | 2.06 ± 0.03 | 6.72 ± 0.65 | 2.56 ± 0.84 |
| Angiotensin converting enzyme | 4.31 ± 0.09 | 30.83 ± 0.45 | 6.85 ± 0.98 |
| Dipeptidyl peptidase | 3.098 ± 0.09 | 6.138 ± 0.68 | 3.99 ± 0.91 |
| 1,1-diphenyl-2- picrylhydrazyl (DPPH) | 12.32 ± 0.91 | 68.42 ± 0.05 | 14.44 ± 1.91 |
EG: E. globulus; EC: E. citriodora; ECA: E. camaldulensis.
The correlation coefficient (R2) between polyphenol and flavonoid content and IC50 inhibitory values of the enzymes ranged between 0.81-0.99 and 0.57-0.99 respectively.
The polyphenol content of three Eucalyptus Spp. (EG, EC and ECA) was compared with the IC50 values of different inhibitory assays using Tukey’s multiple comparison test (one-way ANOVA), considering P < 0.05 as significant. All P values were found to be < 0.05. The results suggested that the inhibitory potentials of the extracts are largely dependent upon the polyphenol content in Eucalyptus Spp.
DISCUSSION
Qualitative and quantitative phytochemical investigations of the Eucalyptus leaf extracts EG, ECA and EC showed appreciable levels of bioactive components like polyphenols and flavonoids. From the IC50 values of Eucalyptus extracts in different assays (Table 4), it appears that all three extracts showed significant inhibitory potentials against the six enzymes assayed, in the order EG > ECA > EC. Based on the results of FRAP assay, the reducing ability of EG was highest and that of EC lowest (Figure 2). 1,8-cineole is the major constituent of the volatile fractions in EG and ECA, whereas in EC the major constituent is citronellal with citronellol and spathulenol. According to the literature, compounds with highest reducing ability have delocalized chemical bonds[56-60]. Prior research suggested a strong positive correlation (R2 = 0.99) between phenolic content and antioxidative potential[12,18,58,59]. Polyphenols received wide attention because of their antioxidant properties which refers to their ability to prevent damage from ROS through radical scavenging or prevent the generation of these species by iron chelation[61]. Polyphenols also bind and inhibit the enzymes α-amylase and α-glucosidase[61]. Polyphenols have also been shown to facilitate insulin response and attenuate secretion of glucose dependent insulinotropic polypeptide and glucagon like GLP-1. Other suggested mechanisms for the hypoglycemic actions of polyphenols were down regulation of the expression of liver glucokinase, upregulation of phosphoenolpyruvate carboxykinase (PEPCK), induction of the AMP-activated protein kinase (AMPK) pathway, enhancing peripheral glucose utilization by stimulating glucose transporter subtype 4 (GLUT-4), etc.[62]. In this context, it is to be mentioned that green tea extract (GTE) contains polyphenols like catechin, epicatechin, etc. Epigallocatechin gallate (EGCG), an abundant form of catechin, is the major attributable factor for the beneficial effects of green tea. EGCG inhibits adipocyte proliferation, increases fat oxidation and enhances the expression of GLUT-4, as shown in animal studies[63,64].
Literature surveys have shown that flavonoids and its subfamilies significantly inhibit the ACE enzyme by generating chelate complexes within the active center of ACE[65]. Flavonoids were found to attenuate hepatic gluconeogenesis by decreasing the activity of glucose-6-phosphate and PEPCK, subsequently improving glycemic control[65]. Our research data are in accordance with this phenomenon. A strong correlation was found between polyphenol (R2 = 0.81-0.99) and flavonoid contents (R2 = 0.57-0.99) with the antioxidative and enzyme inhibitory potentials of the extracts.
NEIs can serve as an important therapeutic tool against type 2 DM. The current research aims to provide the state-of-the-art search of NEIs amongst Eucalyptus Spp. by in vitro assays which can be further utilized for bioactivity-guided isolations of such enzyme inhibitors. Our research results show the hypoglycemic potential of the Eucalyptus Spp. (extracts) for future exploitations in phytotherapy of type 2 DM. However, further extensive pharmacology and toxicological studies in animal and human models are warranted.
ACKNOWLEDGMENTS
The authors deeply acknowledge the research facilities provided by Central Research Facilities, IIT Kharaghpur and the laboratory facilities of Nirmala College of Pharmacy, Guntur. Our special thanks go to Dr. Shanta AK for identification of the plant specimens.
COMMENTS
Background
The current research aims to explore the presence of biomolecules by in vitro assays amongst three eucalyptus species acting as natural enzyme inhibitors for enzymes with significant pathogenic roles in type 2 diabetes.
Research frontiers
Enzymes like α-amylase, α-glucosidase, aldose reductase, angiotensin converting enzyme and dipeptidyl peptidase 4 play important pathogenic roles in type 2 diabetes. Phytomolecules acting as inhibitors of such enzymes can act as effective therapeutic targets in type 2 diabetes. Volatile and non-volatile fractions of Eucalyptus Spp. include biomolecules like terpenes, triterpenoids, flavonoids, polyphenols, etc. The exploration of enzyme inhibitors amongst Eucalyptus Spp. by in vitro assays will help in bioactivity guided isolation of such inhibitors to be targeted as natural hypoglycemics.
Innovations and breakthroughs
Enzymes play a vital role in mediating essential biochemical life processes. However, hyper or hypo activity of such enzymes leads to malfunctions of the respective biochemical processes, which in many cases are the underlying causes of diseases like diabetes. The current research aims to provide the state-of-the-art search of natural enzyme inhibitors amongst Eucalyptus Spp. by in vitro assays which can be further utilized for bioactivity-guided isolations of such enzyme inhibitors. Those research findings have shown that the Eucalyptus Spp. under study have immense hypoglycemic potentials with high IC50 values against the targeted enzymes. Moreover, the inhibitory potentials of the species are also well correlated with the polyphenol-flavonoid contents of the species.
Applications
The Eucalyptus Spp. (extracts) under study showed significant hypoglycemic potentialities for future exploitations in phytotherapy of type 2 DM.
Terminology
Natural Enzyme Inhibitors: Malfunctions of certain enzymes are the root causes of many diseases. Effective enzyme inhibitors have great clinical significance and a substantial role in the drug delivery process. Such enzyme inhibitors of natural origin are more acceptable due to safety and lower incidences of side effects on short and long term treatment modalities.
Peer review
Dey et al investigated the potential hypoglycemic actions of Eucalyptus extracts in vitro. The extracts were found to significantly inhibit a number of enzymes related to T2DM, such as amylase, glucosidase, dipeptidyl peptidase 4, etc. The rationale of this study and methodology were adequately described. The selection of enzymes and antioxidant activity is based on the hypothesis that these activities are involved in the pathogenesis of type 2 diabetes. The three extracts show broad enzyme inhibitory activity and antioxidant activity, which differs in magnitude between the three extracts. The authors conclude that the extracts might serve as starting material for new therapeutic modalities for type 2 diabetes and that their data fit with the idea that leaves from trees could provide a base material for drug discovery and development programs.
Footnotes
P- Reviewers: Charoenphandhu N, Elisaf MS, Schuurman HJ S- Editor: Qi Y L- Editor: Roemmele A E- Editor: Wu HL
References
- 1.Mitra A, Dewanjee D, Dey B. Mechanistic studies of lifestyle interventions in type 2 diabetes. World J Diabetes. 2012;3:201–207. doi: 10.4239/wjd.v3.i12.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.DeFronzo RA. Pathogenesis of type 2 diabetes mellitus. Med Clin North Am. 2004;88:787–835, ix. doi: 10.1016/j.mcna.2004.04.013. [DOI] [PubMed] [Google Scholar]
- 3.Bailey CJ, Day C. Traditional plant medicines as treatments for diabetes. Diabetes Care. 1989;12:553–564. doi: 10.2337/diacare.12.8.553. [DOI] [PubMed] [Google Scholar]
- 4.Swanston-Flatt SK, Day C, Bailey CJ, Flatt PR. Traditional plant treatments for diabetes. Studies in normal and streptozotocin diabetic mice. Diabetologia. 1990;33:462–464. doi: 10.1007/BF00405106. [DOI] [PubMed] [Google Scholar]
- 5.Ata A, Naz S, Elias EM. Naturally occurring enzyme inhibitors and their pharmaceutical applications. Pure Appl Chem. 2011;83:1741–1749. [Google Scholar]
- 6.Kumar S, Kumar V, Rana M, Kumar D. Enzyme inhibitors from plants: An alternate approach to treat diabetes. Pharmacog Communi. 2012;2:18–33. [Google Scholar]
- 7.Gallagher AM, Flatt PR, Duffy G, Abdel-Wahab YH. The effects of traditional anti-diabetic plants on in vitro glucose diffusion. Nutr Res. 2003;23:413–424. [Google Scholar]
- 8.Dey B, Mitra A. Chemo-profiling of eucalyptus and study of its hypoglycemic potential. World J Diabetes. 2013;4:170–176. doi: 10.4239/wjd.v4.i5.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pérez RM, Ocegueda A, Muñoz JL, Avila JG, Morrow WW. A study of the hypoglycemic effect of some Mexican plants. J Ethnopharmacol. 1984;12:253–262. doi: 10.1016/0378-8741(84)90054-0. [DOI] [PubMed] [Google Scholar]
- 10.Bedgood DR, Bishop AG, Prenzler PD, Robards K. Analytical approaches to the determination of simple biophenols in forest trees such as Acer (maple), Betula (birch), Coniferous, Eucalyptus, Juniperus(cedar), Picea(spruce), Quercus (oak) Analyst. 2005;130:809–823. doi: 10.1039/b501788b. [DOI] [PubMed] [Google Scholar]
- 11.Gray AM, Flatt PR. Antihyperglycemic actions of Eucalyptus globulus (Eucalyptus) are associated with pancreatic and extra-pancreatic effects in mice. J Nutr. 1998;128:2319–2323. doi: 10.1093/jn/128.12.2319. [DOI] [PubMed] [Google Scholar]
- 12.Basak SS, Candan F. Chemical composition and in vitro antioxidant and antidiabetic activities of Eucalyptus camaldulensis Dehnh. Essential oil. J Iran Chem Soc. 2010;7:216–226. [Google Scholar]
- 13.Nakhaee A, Bokaeian M, Saravani M, Farhangi A, Akbarzadeh A. Attenuation of oxidative stress in streptozotocin-induced diabetic rats by Eucalyptus globulus. Indian J Clin Biochem. 2009;24:419–425. doi: 10.1007/s12291-009-0075-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Al-Khazraji SM. The effect of aqueous extract of the leaves of Eucalyptus globulus on clinical laboratory parameters in alloxan induced diabetic rats. Available from: http:// www.iasj.net/iasj?func=fulltext&aId=35232.
- 15.Patra A, Jha S. Antidiabetic effect of the aqueous extract of E. citriodora in alloxan induced diabetic rats; Pharmacog Mag. 2009;5:51–54. [Google Scholar]
- 16.Villaseñor IM, Lamadrid MR. Comparative anti-hyperglycemic potentials of medicinal plants. J Ethnopharmacol. 2006;104:129–131. doi: 10.1016/j.jep.2005.08.067. [DOI] [PubMed] [Google Scholar]
- 17.Shahraki A, Shahraki M. The comparison of eucalyptus aqueous extract and insulin on blood sugar and liver enzymes in diabetic male rats. Zahedan J Res Med Sci. 2013;15:25–28. [Google Scholar]
- 18.Gireesh G, Thomas SK, Joseph B, Paulose CS. Antihyperglycemic and insulin secretory activity of Costus pictus leaf extract in streptozotocin induced diabetic rats and in in vitro pancreatic islet culture. J Ethnopharmacol. 2009;123:470–474. doi: 10.1016/j.jep.2009.03.026. [DOI] [PubMed] [Google Scholar]
- 19.Ahlem S, Khaled H, Wafa M, Sofiane B, Mohamed D, Jean-Claude M, Abdelfattah el F. Oral administration of Eucalyptus globulus extract reduces the alloxan-induced oxidative stress in rats. Chem Biol Interact. 2009;181:71–76. doi: 10.1016/j.cbi.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 20.Sugimoto K, Suzuki J, Nakagawa K, Hayashi S, Enomoto T, Fujita T, Yamaji R, Inui H, Nakano Y. Eucalyptus leaf extract inhibits intestinal fructose absorption, and suppresses adiposity due to dietary sucrose in rats. Brit j Nutr. 2005;93:957–963. doi: 10.1079/bjn20051436. [DOI] [PubMed] [Google Scholar]
- 21.Sugimoto K, Hosotani T, Kawasaki T, Nakagawa K, Hayashi S, Nakano Y, Hiroshi I, Yamanouchi T. Eucalyptus Leaf Extract Suppresses the Postprandial Elevation of Portal, Cardiac and Peripheral Fructose Concentrations after Sucrose Ingestion in Rats. J Clin Biochem Nutr. 2010;46:205–211. doi: 10.3164/jcbn.09-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boulekbache ML, Slimani S, Madani K. Antioxidant effects and phytochemical analysis of crude and chromatographic fractions obtained from Eucalyptus globulus bark. Afri J Biotechnol. 2012;11:10048–10055. [Google Scholar]
- 23.Adefegha SA, Oboh G. In vitro inhibition of polyphenol rich extracts from Syzygium aromaticum (L.) Merr. and Perry (Clove) buds against carbohydrate hydrolyzing enzymes linked to type 2 diabetes and Fe2 -induced lipid peroxidation in rat pancreas. Asian Pac J Trop Biomed. 2012;2:774–781. doi: 10.1016/S2221-1691(12)60228-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Osawa T, Namiki M. Natural antioxidants isolated from Eucalyptus leaf waxes. J Agric Food Chem. 1985;33:777–779. [Google Scholar]
- 25.Osawa K, Yasuda H, Morita H, Takeya K, Itokawa H. Eucalyptone from Eucalyptus globulus. Phytochemistry. 1995;40:183–184. doi: 10.1016/0031-9422(95)00233-w. [DOI] [PubMed] [Google Scholar]
- 26.Singh AK, Khare M, Kumar S. Non-volatile constituents of eucalyptus: a review on chemistry and biological activities. J Med Arom Plant Sci. 1999;21:375–407. [Google Scholar]
- 27.Yamakoshi Y, Murata M, Shimizu A, Homma S. Isolation and characterization of macrocarpals B-G, antibacterial compounds from Eucalyptus macrocarpa. Biosci Biotech Biochem. 1992;56:1570–1576. doi: 10.1271/bbb.56.1570. [DOI] [PubMed] [Google Scholar]
- 28.De Sales PM, De Souza PM, Simeoni LA, De Oliveira Magalhaes P, Silveira D. α-amylase Inhibitors: A review of raw materials and isolated compounds from plant source. J Pharm Pharmaceut Sci. 2012;15:141–183. doi: 10.18433/j35s3k. [DOI] [PubMed] [Google Scholar]
- 29.Lamba HS, Bhargava CS, Thakur M, Bhargava S. α-glucosidase and Aldose reductase inhibitory activity in vitro and anti-diabetic activity in vivo of Tribulus terrestris L. (Dunal) Int J Pharm pharm Sci. 2011;3:270–272. [Google Scholar]
- 30.Bachhawat A, Shihabudeen MS, Thirumurugan K. Screening of fifteen Indian Ayurvedic plants for alpha-glucosidase inhibitory activity and enzyme kinetics. Int J Pharm Pharm Sci. 2011;3:267–274. [Google Scholar]
- 31.Guzman A, Guerrero RO. Inhibition of aldose reductase by herbs extracts and natural substances and their role in prevention of cataracts. Rev Cubana Plant Med. 2005;10:1–7. [Google Scholar]
- 32.Vyshali P, Thara Saraswati KJ, Sanakal R, Kaliwal BB. Inhibition of Aldose activity by essential phytochemicals of Cymbopogon Citratus (DC.) Stapf. Int J Biometr Bioinfor. 2011;5:257–267. [Google Scholar]
- 33.Madeswaran A, Muthuswamy UM, Kuppusamy AK, Sivasanmugam T, Varadharajan SD, Puliyath J. In-silico docking studies of aldose reductase inhibitory activity of commercially available flavonoids. Bangladesh J Pharmacol. 2012;7:266–271. [Google Scholar]
- 34.Balasuriya BWN, Rupasinghe HPV. Plant flavonoids as angiotensin converting enzyme inhibitors in regulation of hypertension. Func Foods Heal Diseas. 2011;5:172–188. [Google Scholar]
- 35.Priya V, Jananie RK, Vijayalakshmi K. Molecular docking analysis of compounds present in Trigonella foenum graceum with angiotension converting enzyme in-silico analysis. J Chem Pharm Res. 2011;3:129–139. [Google Scholar]
- 36.Goto T, Takahashi N, Hirai S, Kawada T. Various terpenoids derived from herbal and dietary plants function as PPAR modulators and regulate carbohydrate and lipid metabolism. PPAR res. 2010;20:1–9. doi: 10.1155/2010/483958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Geng Y, Lu ZM, Huang W, Xu HY, Shi JS, Xu ZH. Bioassay guided isolation of DPP-4 inhibitory fractions from extracts of submerged cultured of Inonotus obliquus. Molecules. 2013;18:1150–1161. doi: 10.3390/molecules18011150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Achimugu MD, Chiletugo FO, Ojogbane E. Phytochemical, antibacterial, and toxicity studies of the aqueous extract of Eucalyptus camaldulensis Dehnh. Asian J Plant Sci Res. 2011;1:1–10. [Google Scholar]
- 39.Othman A, Ismail A, Abdul Ghani N, Adenan I. Antioxidant capacity and phenolic content of cocoa beans. Food Chem. 2007;100:1523–1530. [Google Scholar]
- 40.Modnicki D, Balcerek M. Estimation of total phenol contents in Ocimum basilicum L., Origanum vulgare L., Thymus vulgaris L., commercial samples. Herba Pol. 2009;55:35–42. [Google Scholar]
- 41.Ozkok A, Darcy B, Sorkun K. Total phenolic acid and total flavonoid content of Turkish pine honeydew honey. J Apipdt ApiMedsci. 2010;2:65–71. [Google Scholar]
- 42.Abdel-Hameed El-Sayed S. Total phenolic contents and free radical scavenging activity of certain Egyptian Ficus species leaf samples. Food Chem. 2009;114:1271–1277. [Google Scholar]
- 43.Djeridane K, Yousfi M, Nadjemi D, Boutassouna D, Stocker P, Vidal N. Antioxidant activity of some Algerian medicinal plants extracts containing phenolic compounds. Food Chem. 2006;97:654–660. [Google Scholar]
- 44.Hagerman AE, Butler LG. Protein precipitation method for the quantitative determination of tannin. J Agric Food Chem. 1978;26:809–812. [Google Scholar]
- 45.Quereshi S, Upadhyay A, Singh R, Khan NA, Mani A et al. GC analysis of essential oils, TLC profiling of pigments and DNA extraction from Eucalyptus species. Curr Bot. 2011;2:23–26. [Google Scholar]
- 46.Hansawasdi C, Kawabata J, Kasai T. Alpha-amylase inhibitors from roselle (Hibiscus sabdariffa Linn.) tea. Biosci Biotechnol Biochem. 2000;64:1041–1043. doi: 10.1271/bbb.64.1041. [DOI] [PubMed] [Google Scholar]
- 47.Subramanian R, Azmawi ZM, Sadikun A. In vitro α-glucosidase and α-amylase enzyme inhibitory effects of Andrographis Paniculata extract and andrographolide. Acta Biochim Pol. 2008;2:391–398. [PubMed] [Google Scholar]
- 48.Patil DK, Kumar R, Kumar M, Sairam K, Hemalatha S. Evaluation of in vitro aldose reductase inhibitory potential of different fraction of Hybanthus enneaspermus Linn. F Muell. Asian Pac J Trop Biomed. 2012;2:134–139. doi: 10.1016/S2221-1691(11)60207-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Halder N, Joshi S, Gupta SK. Lens aldose reductase inhibiting potential of some indigenous plants. J Ethnopharmacol. 2003;86:113–116. doi: 10.1016/s0378-8741(03)00052-7. [DOI] [PubMed] [Google Scholar]
- 50.Patel DK, Kumar R, Sairam K, Hemalatha S. Aldose reductase inhibitory activity of alcoholic extract of Pedalium murex Linn fruit. Asian Pac J Trop Biomed. 2012:S265–S269. [Google Scholar]
- 51.Chaudhary SK, Mukherjee PK, Maiti N, De AK, Bhadra S, Saha BP. Evaluation of Angiotensin converting enzyme inhibition and antioxidant activity of Piper Longum L. Ind J Trad Know. 2013;12:478–482. [Google Scholar]
- 52.McCue P, Kwon YI, Shetty K. Anti-diabetic and anti-hypertensive potential of sprouted and solid-state bioprocessed soybean. Asia Pac J Clin Nutr. 2005;14:145–152. [PubMed] [Google Scholar]
- 53.Mardanyan S, Sharoyan S, Antonyan A, Zarkanyan N. Dipeptidyl peptidase IV and adenosine deaminase inhibition by Armenian plants and antidiabetic drugs. Int J Diabetes Metab. 2011;19:69–74. [Google Scholar]
- 54.Sharoyan S, Antonyan A, Mardanyan S, Lupidi G, Cristalli G. Influence of dipeptidyl peptidase IV on enzymatic properties of adenosine deaminase. Acta Biochim Pol. 2006;53:539–546. [PubMed] [Google Scholar]
- 55.Mentlein R, Struckhoff G. Purification of two dipeptidyl aminopeptidase II from rat brain and their action on proline containing neuropeptides. J Neurochem. 1989;52:1284–1293. doi: 10.1111/j.1471-4159.1989.tb01877.x. [DOI] [PubMed] [Google Scholar]
- 56.Pracheta , Sharma V, Paliwal R, Sharma S. In vitro free radical scavenging and antioxidant potential of ethanolic extract of Euphorbia Nerifolia Linn. Int J Pharm Pharm Sci. 2011;3:238–242. [Google Scholar]
- 57.Shahwar D, Raza MA, Bukhari S, Bukhari G. Ferric reducing antioxidant power of essential oils extracted from Eucalyptus and Curcuma species. Asian Pac J Trop Biomed. 2012:S1633–S1636. [Google Scholar]
- 58.Munin A, Levy FE. Encapsulation of natural phenolic compounds: A review. Pharmaceutics. 2011;3:793–829. doi: 10.3390/pharmaceutics3040793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.De Vincenzi M, Silano M, De Vincenzi A, Maialetti F, Scazzocchio B. Constituents of aromatic plants: eucalyptol. Fitoterapia. 2002;73:269–275. doi: 10.1016/s0367-326x(02)00062-x. [DOI] [PubMed] [Google Scholar]
- 60.Cristina L, Maria T, Ilona V, Eva S, Clara S. Anti-oxidant properties of volatile oil determined by the Ferric reducing ability. Z Naturforsch. 2004;59:354–358. doi: 10.1515/znc-2004-5-611. [DOI] [PubMed] [Google Scholar]
- 61.Perron NR, Brumaghim JL. A review of the antioxidant mechanisms of polyphenol compounds related to iron binding. Cell Biochem Biophys. 2009;53:75–100. doi: 10.1007/s12013-009-9043-x. [DOI] [PubMed] [Google Scholar]
- 62.Bahadoran Z, Mirmiran P, Azizi F. Dietary polyphenols as potential nutraceuticals in management of diabetes: a review. J Dia Metabol Disor. 2013;12:43. doi: 10.1186/2251-6581-12-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tsuneki H, Ishizuka M, Terasawa M, Wu JB, Sasaoka T, Kimura I. Effect of green tea on blood glucose levels and serum proteomic patterns in diabetic (db/db) mice and on glucose metabolism in healthy humans. BMC Pharmacol. 2004;4:18. doi: 10.1186/1471-2210-4-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kim HM, Kim J. The effects of green tea on obesity and type 2 diabetes. Diabetes Metab J. 2013;37:173–175. doi: 10.4093/dmj.2013.37.3.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Guerero L, Castillo J, Quinones M, Garcia-Vallve S, Arola L, Pujadas G, Muguerza B. Inhibition of angiotensin converting enzyme activity by flavonoids: Structure activity relationship studies. PloS ONE. 2012;7:e49493. doi: 10.1371/journal.pone.0049493. [DOI] [PMC free article] [PubMed] [Google Scholar]


