Abstract
Neonatal diabetes mellitus (NDM) is a type of diabetes mellitus caused by genetic abnormality which develops in insulin dependent state within 6 mo after birth. HbA1c is widely used in clinical practice for diabetes mellitus as the gold standard glycemic control indicator; however, fetal hemoglobin (HbF) is the main hemoglobin in neonates and so HbA1c cannot be used as a glycemic control indicator in NDM. Glycated albumin (GA), another glycemic control indicator, is not affected by HbF. We reported that GA can be used as a glycemic control indicator in NDM. However, it was later found that because of increased metabolism of albumin, GA shows an apparently lower level in relation to plasma glucose in NDM; measures to solve this problem were needed. In this review, we outlined the most recent findings concerning glycemic control indicators in neonates or NDM.
Keywords: Glycemic control; HbA1c; Glycated albumin; Fructosamine; 1,5-anhydroglucitol; Neonatal diabetes mellitus
Core tip: Neonatal diabetes mellitus (NDM) is a type of diabetes mellitus caused by genetic abnormality which develops in insulin dependent state within 6 mo after birth. Because fetal hemoglobin (HbF) is the main hemoglobin in neonates, HbA1c cannot be used as a glycemic control indicator in NDM. On the other hand, glycated albumin (GA), another glycemic control indicator, is not affected by HbF. We reported that GA can be used as a glycemic control indicator in NDM. In this review, we outlined the most recent findings concerning glycemic control indicators in neonates or NDM.
INTRODUCTION
To prevent chronic diabetic complications, it is necessary to try to achieve normoglycemia as much as possible. Previously, glycemic control used to be evaluated by plasma glucose or urinary glucose. However, these indicators fluctuate continuously due to factors such as dietary intake, and it was difficult to evaluate glycemic control correctly by taking measurements at a particular time. Therefore, hemoglobin A1c (HbA1c), which reflects mean plasma glucose during the past 1 to 2 mo, was introduced as a glycemic control indicator[1], and is now widely used in clinical practice for diabetes mellitus. HbA1c can be used to evaluate glycemic control status; if poor glycemic control is observed, it is possible to make additions, changes, etc. to the treatment of diabetes mellitus[2].
Large-scale researches such as the Diabetes Control and Complications Trial revealed that HbA1c is related to the development and progression of diabetic microangiopathy[3]. That is, the development and progression of diabetic microangiopathy can be prevented by maintaining excellent glycemic control using HbA1c as an indicator. Recently, it also became possible to use HbA1c for the diagnosis of diabetes mellitus[4].
However, the following problems of HbA1c were pointed out: (1) abnormal HbA1c values may be observed because of variantl hemoglobin, hemolytic anemia, etc.; (2) HbA1c does not correctly reflect short-term glycemic control status; and (3) HbA1c does not correctly reflect postprandial plasma glucose/fluctuation of plasma glucose. Accordingly, new glycemic control indicators such as fructosamine, 1,5-anhydroglucitol (1,5-AG), and glycated albumin (GA) were introduced. Although these indicators compensate the disadvantages of HbA1c, they have their own disadvantages. For example, 1,5-AG is affected by the threshold of urinary glucose excretion in the kidney, and fructosamine and GA are affected by albumin metabolism[5].
Because fetal hemoglobin (HbF) is the main hemoglobin in neonates, HbA1c cannot be used as a glycemic control indicator in neonates. Therefore, glycemic control in neonatal diabetes mellitus (NDM) was traditionally performed using blood glucose measured by self-monitoring of blood glucose as an indicator, without using a glycemic control indicator. We demonstrated that GA, which is not affected by HbF, reflects glycemic control in NDM and can be used as a glycemic control indicator in NDM[6]. We also obtained various other findings about GA and HbA1c in neonates/infants or NDM. In this review, we outlined the most recent findings concerning glycemic control indicators in neonates or NDM.
NEONATAL DIABETES MELLITUS
NDM is a type of diabetes mellitus caused by single-gene abnormality which develops acutely in insulin dependent state; NDM accounts for the majority of cases of diabetes mellitus which develops within 6 mo after birth[7]. The frequency of NDM according to this definition is 1 in 89000 births, showing that NDM is a rare disease[8]. So far, more than 20 causative genes of NDM have been discovered; genetic mutations of some kind have been identified in not less than 70% of patients[8,9]. NDM is similar to type 1 diabetes mellitus in terms of the form of development (diabetes mellitus develops acutely); however, type 1 diabetes mellitus very rarely develops within 6 mo after birth, judging from studies on the frequency of human leukocyte antigen risk alleles and the presence of pancreatic autoantibodies[10,11]. Based on the clinical course, NDM is classified into two major categories: transient NDM (TNDM) and permanent NDM (PNDM)[12]. TNDM is a condition in which insulin secretion is restored spontaneously and normoglycemia is achieved without treatment; PNDM is a condition in which remission is not achieved and life-long treatment is required. The frequency of TNDM is about 60%, and that of PNDM is about 40%.
Although patients with TNDM require insulin therapy at the time of onset because of marked hyperglycemia, they can be weaned from insulin therapy at an average of 3 mo after the start of treatment[13-16]. This is called the remission period. However, in about half of patients, diabetes mellitus relapses from childhood to adolescence[14,17]. In 70% of patients with TNDM, the cause is overexpression of an imprinted gene PLAGL1 which is located in the chromosome 6q24 region and is expressed from paternal allele (6q24-TNDM)[14,15,18,19]. In 25% of patients with TNDM, mutations of KCNJ11 and ABCC8 genes which encode the ATP-sensitive potassium channel (KATP channel) essential for glucose-stimulated insulin secretion have been identified (KATP-TNDM)[14,20,21]. 6q24-TNDM has the following characteristics: (1) it often develops within 1 wk after birth; (2) it is often diagnosed asymptomatically on routine blood collection; and (3) it is rarely accompanied by ketoacidosis[15,19]. On the other hand, the time of diagnosis of KATP-TNDM is 1 to 4 mo after birth, which is later than that of 6q24-TNDM[14].
The main causes of PNDM are KATP channel abnormality [KCNJ11 gene (31%); ABCC8 gene (10%)] and insulin gene mutations (12%); the median age at the time of diagnosis is 8 wk after birth and 10 wk after birth, respectively[9]. In contrast to TNDM, PNDM shows symptoms such as dehydration, poor sucking, and poor weight gain at the time of onset and is often accompanied by ketoacidosis[15,19]. A large proportion of other causative genes are expressed by autosomal recessive inheritance and account for about 10% of PNDM. In about 35% of patients with NDM, causative genes have not been identified[7].
Insulin therapy is required at the time of onset of NDM regardless of disease type in order to improve metabolic abnormality and weight increase[22]. It has been reported that because neonates have a small body and then receive a small dose of insulin, excellent glycemic control is achieved by an insulin pump which is capable of fine regulation[23-25]. As a treatment after withdrawal from the acute phase, a switch to high-dose administration of sulfonylurea (SU) drugs is an effective causal therapy for KATP channel abnormality; in not less than 90% of patients, a dramatic improvement of glycemic control is observed immediately without hypoglycemia and is maintained for a long period[26-28]. Therefore, when NDM is diagnosed, it is important to determine by gene analysis whether or not KATP channel abnormality is present. Early diagnosis makes it possible to switch to SU drugs during infancy, resulting in an extremely high quality-of-life[29-32].
1,5-ANHYDROGLUCITOL IN NEONATES
1,5-AG is a polyol with a structure in which hydroxyl at the 1st position of glucose is reduced; 1,5-AG is contained in a wide variety of food, but is hardly metabolized in the body[33]. Therefore, after being absorbed from the intestine, 1,5-AG contained in food is widely distributed in various organs to form an internal pool. The amount of 1,5-AG supplied from daily food intake is smaller than the internal pool, and so there is no change in serum 1,5-AG concentration before and after meal. Excessive intake of 1,5-AG is excreted in urine.
Usually, about 180 g of glucose is excreted daily from glomeruli; about 100% of the excreted glucose is reabsorbed by sodium glucose cotransporter 2 (SGLT2), which is located in proximal renal tubules and are specific to glucose[34], and SGTL1, which is located downstream of SGLT2. After the onset of diabetes mellitus, excretion of glucose will increase; when the increased excretion of glucose exceeds the reabsorption capacity of SGLT2 and SGLT1, reabsorption of glucose via 1,5-AG/mannose/fructose cotransporter (SGLT4), which is located downstream of SGLT2 and SGLT1, will start. Because glucose is usually not present, 99.9% of 1,5-AG is reabsorbed by SGLT4; however, this reabsorption mechanism is common to glucose; therefore, if inflow of glucose into tubules increases, reabsorption of 1,5-AG will be inhibited[35-37]. Therefore, in a hyperglycemic condition, excretion of 1,5-AG into urine will increase and serum 1,5-AG will decrease. Thus, serum 1,5-AG is a glycemic control indicator which reflects the degree of urinary glucose excretion.
Because serum 1,5-AG increases and decreases by excretion of urinary glucose, serum 1,5-AG reflects short-term changes in glycemic control more subtly than HbA1c. When glycemic control has worsened rapidly, serum 1,5-AG will decrease rapidly because the increased excretion of a large amount of glucose will inhibit reabsorption of 1,5-AG via SGLT4. In patients with marked hyperglycemia and a high excretion of urinary glucose, serum 1,5-AG will not increase in a short period even if glycemic control has improved rapidly because the internal pool of 1,5-AG has decreased.
Serum 1,5-AG is also affected by the threshold for urinary glucose excretion, and therefore shows a low level in renal glycosuria in which the threshold decreases. In addition, serum 1,5-AG shows an abnormally low level in conditions such as chronic renal failure in which reabsorption of 1,5-AG decreases[38-40], pregnancy[41], oxyhyperglycemia in which urinary glucose is observed transiently[42], patients receiving long-term hyperalimentation[43], and liver cirrhosis[44,45]. One of the causes of an abnormally high level of 1,5-AG is oral administration of a kind of Chinese medicines such as Ninjin-yoei-to and Kami-kihi-to which contain large amounts of 1,5-AG[46].
It is known that serum 1,5-AG during the neonatal period shows an apparently low level[47]. This is considered to be due to a small intake of 1,5-AG during the neonatal period. We reported that serum 1,5-AG is significantly lower in subjects with a habit of consuming dairy products than in subjects without such a habit[48]. The fact that breast milk or formula which contains galactose is the main source of nutrition during the neonatal period may be related to a low level of serum 1,5-AG in neonates.
FRUCTOSAMINE IN NEONATES
Protein undergoes glycation reaction in accordance with plasma glucose concentration, and ketoamine, an early Maillard reaction product, is produced via aldimine. Because the side chain binding of ketoamine takes a fructose structure, ketoamine is generically named fructosamine. Fructosamine is measured using the property that fructose-lysine (fructosamine), in which glucose is bound to the lysine residues of protein, has reducing ability under alkaline conditions. A large proportion of measurements are made by the chemical method; measurements are made by colorimetric determination by producing reduction color reaction using nitroblue tetrazolium (NBT) as a chromogen. Because 60% to 70% of serum protein is albumin, the main component of fructosamine is glycated albumin, but fructosamine contains glycated lipoprotein and glycated globulin as well. Fructosamine is not affected by anemia or variant hemoglobin. In addition, because the turnover of albumin, which accounts for the most part of serum protein, is faster than that of hemoglobin, it is possible to evaluate short-term glycemic control by measuring fructosamine[49]. A low fructosamine level is observed in hyperthyroidism[50,51] and nephrotic syndrome[52] in which protein (albumin) metabolism is accelerated; a high fructosamine level is observed in hypothyroidism[50,51] in which protein (albumin) metabolism is prolonged.
HbA1c and GA are glycation products of hemoglobin and albumin (single proteins), respectively, whereas fructosamine is the generic name of all glycated proteins and lacks specificity. Because albumin accounts for 60% to 70% of serum protein, fructosamine has similar properties to GA; however, there is a problem that because other glycated proteins are measured as well, a high fructosamine level is observed in myeloma[53]. Because HbA1c and GA are expressed as the ratio of hemoglobin and the ratio of albumin, respectively, they are not affected by dilution of serum; on the other hand, because fructosamine is expressed as reducing ability per 1 mL of serum, it is affected by serum protein concentration, and an apparently low level of fructosamine is observed in dilutional anemia. The level of fructosamine in young children is lower than that in adults[54], which is also partly due to low serum protein concentration. Because fructosamine is measured by colorimetric determination based on reduction color reaction, fructosamine is affected by bilirubin with reducing ability, etc. It is considered that the effects of ascorbic acid and vitamin E are mild; however, if a large amount of ascorbic acid or vitamin E is consumed, measurement of fructosamine may be affected.
GLYCEMIC CONTROL INDICATORS OF CORD BLOOD
The composition of hemoglobin in healthy adults is as follows: adult hemoglobin (HbA): 97%; HbA2: 2.5%; HbF: 0.5%[55]. On the other hand, HbF accounts for 80% to 90%, and HbA accounts for only 10% to 20% immediately after birth. After then, HbF decreases logarithmically and is replaced by HbA; by 6 mo after birth, the largest proportion of Hb is HbA; however, it is not until 1 year after birth when the proportion of HbF decreases to less than 1% (level of HbF in adults)[56,57]. Therefore, it is difficult to use the cation exchange high-performance liquid chromatography (HPLC) method, the immunological (latex immunoturbidimetry; LA) method, and the enzyme method which specifically measure HbA1c as glycemic control indicators in NDM.
We measured glycohemoglobin (GHb) in cord blood by various methods[58]. GHb measured by the HPLC method was less than the detection limit when Arkray’s HA-8180 was used and was as low as 1.8% ± 0.2% when Tosoh’s G8 was used. GHb measured by the LA method was less than the detection limit; HbA1c measured by the enzyme method was 1.1% ± 0.3%. Because these methods for measuring GHb measure HbA1c specifically and do not measure glycated HbF, the result is less than sensitivity or a very low level, and it was confirmed that these methods cannot be used as glycemic control indicators in NDM.
It is considered that measurement of GHb by the affinity method using boronic acid may be used as a glycemic control indicator during the neonatal period as well because it measures all glycated hemoglobins[59,60]. It has been reported that GHb in cord blood is higher in patients whose mother has diabetes mellitus than in patients whose mother does not have diabetes mellitus[61-63]. Our investigation revealed that GHb was 3.9% ± 0.2%, which was slightly lower than the reference value for adults (4.6% to 6.2%)[58]. Plasma glucose in cord blood was normal (94 ± 27 mg/dL); therefore, it is considered that the low GHb levels were due to shortened life span of red blood cells[64].
GA in cord blood was 9.4% ± 1.1%, which was slightly lower than the reference value for adults (11.6% to 16.2%)[58]. We demonstrated that low GA levels are observed in neonates because albumin metabolism in neonates is accelerated[65,66]. Low GA levels in cord blood are considered to be due to accelerated metabolism of albumin.
The level of 1,5-AG in cord blood measured in pregnant women including those with diabetes mellitus was similar to that in maternal blood at the time of delivery[67]. This finding was considered to be due to the fact that 1,5-AG in maternal blood was distributed in the fetus via the placenta.
The above results show that both GHb measured by the affinity method and GA were slightly lower than the reference value for adults, but could be used as glycemic control indicators in NDM. On the other hand, HbA1c measured by the HPLC method, the LA method, or the enzyme method and 1,5-AG cannot be used as glycemic control indicators.
GLYCEMIC CONTROL INDICATORS IN NDM: HBA1C AND GA
The etiologic diagnosis and treatment of NDM have been making rapid progress; however, there have been few studies on glycemic control indicators useful for evaluating the diagnosis of NDM and effects of therapy. Therefore, we hypothesized that GA is a useful glycemic control indicator in NDM[58] and conducted an investigation[6]. We found that GA, as a glycemic control indicator in NDM, has various advantages: (1) GA is not affected by HbF; (2) unlike fructosamine, GA is not affected by serum protein (albumin) because it is expressed as a ratio to albumin; (3) unlike fructosamine, GA is not affected by other proteins and has a high specificity because it reflects glycation products of a single protein (albumin); (4) GA reflects plasma glucose during a shorter period than HbA1c; and (5) HbA1c reflects mean plasma glucose, whereas GA reflects fluctuation of plasma glucose (postprandial hyperglycemia) in addition to mean plasma glucose[68-70]. HbA1c (%) is expressed as HbA1c/total Hb; therefore, if HbF is high, a relatively low HbA1c level will be observed. At the time of onset of NDM (mostly 1 to 2 mo after birth), a large amount of HbF remains in blood; therefore, a lower HbA1c level is observed in relation to plasma glucose level. In addition, it is estimated that during infancy, during which HbA increases, if plasma glucose level is constant, HbA1c will increase. In fact, in an investigation of five patients with NDM (age at the time of diagnosis: 38 ± 20 d), plasma glucose was markedly high [29.7 ± 13.1 mmol/L (535 ± 236 mg/dL)], whereas HbA1c measured by the HPLC method was within the normal range (5.4% ± 2.6%)[6]. As the course of treatment progressed, plasma glucose tended to decrease (Figure 1A), whereas HbA1c tended to increase (Figure 1B). A significant negative correlation was observed between HbA1c and HbF (Figure 2A), whereas no significant correlation was observed between HbA1c and plasma glucose level (Figure 2B). On the other hand, GA at the time of diagnosis was abnormally high (33.3% ± 6.9%)[6]. In contrast to HbA1c, GA decreased as treatment progressed (Figure 1C) and showed a strong positive correlation with plasma glucose level (Figure 2C). Thus, it was found that GA, but not HbA1c, is an appropriate glycemic control indicator in NDM.
Figure 1.
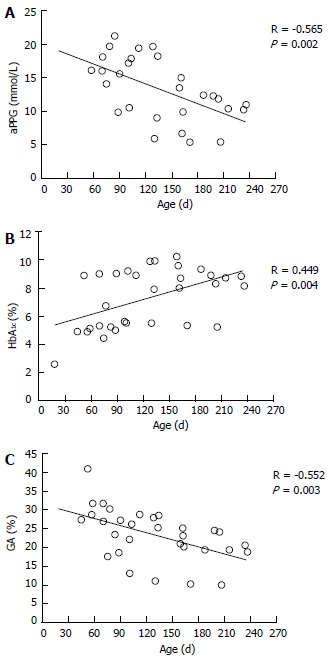
Time course of average preprandial plasma glucose for 1 mo (A), HbA1c (B), and glycated albumin (C) according to treatment in 5 patients with neonatal diabetes mellitus (modified from Ref[6], with permission from Copyright Clearance Center Inc.).
Figure 2.
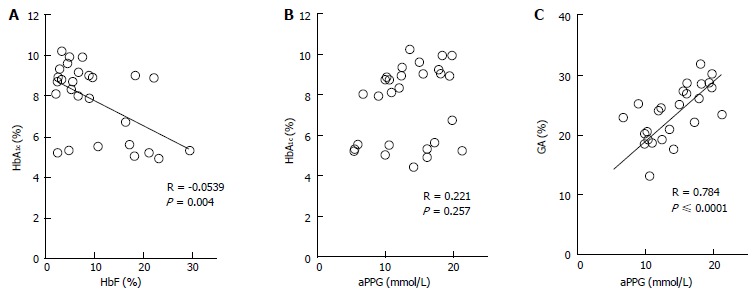
Correlations between HbA1c and HbF (A) and between HbA1c and average preprandial plasma glucose for 1 mo (B) and correlation between glycated albumin and average preprandial plasma glucose in 5 patients with neonatal diabetes mellitus (C) (modified from Ref[6], with permission from Copyright Clearance Center Inc.).
From what age can HbA1c be used as a glycemic control indicator? Alternatively, if the effect of HbF is excluded or if a different principle of measurement is employed, might HbA1c be an appropriate indicator? And when using GA as a glycemic control indicator in NDM, what should be taken into account? In the following chapters, we will discuss these issues in relation to the current status and challenges in infants and NDM.
HBA1C IN NEONATES AND NDM
As mentioned above, when HbA1c is expressed as HbA1c/total Hb, it cannot be used as a glycemic control indicator in NDM. There are two ways to eliminate the effect of HbF. One way is to determine the HbA1c level corrected by HbF (HbF corrected HbA1c) by the formula: HbA1c/(total Hb-HbF), resulting in the correction of an apparently low HbA level. The other way is to determine GHb relative to all hemoglobins including HbF and to use this as a glycemic control indicator. For the latter, it is possible to measure all GHb by the affinity method[71].
We measured HbA1c by the HPLC method and the LA method in 26 healthy infants (0 to 8 mo old), calculated HbA1c values corrected by HbF [Adj-HbA1c (HPLC) and Adj-HbA1c (LA), respectively], measured GHb by the affinity method [GHb (Affinity)], and evaluated correlations between these values and plasma glucose and between these values and GA[72]. As a result, only GHb (Affinity) had a significant correlation with both plasma glucose and GA (Figure 3A). Adj-HbA1c (LA) was correlated only with GA (Figure 3B); Adj-HbA1c (HPLC) was not correlated with either plasma glucose or GA (Figure 3C). These results suggest that GHb (Affinity) may be used as a glycemic control indicator in NDM. In this research, however, GHb (Affinity) within one month was lower than the reference range of HbA1c during 8 to 12 mo (4.8% to 6.0%)[73], and a large proportion of GHb values from 1 to 5 mo were lower than the reference range. The following three factors are thought to contribute together to this finding. The first factor is the effect of a low plasma glucose level during infancy, especially within one month after birth[65,74]. The second factor is the short half-life of red blood cells (about 90 d) during infancy[64]. The third factor is the glycation rate of HbF which is considered to be lower than that of HbA. In this regard, Little et al[75] reported that GHb measured by the affinity method is low when a sample which contains not less than 15% of HbF is used. In the LA method, HbA1c is measured using antibodies which specifically recognize peptides including glycated valine of hemoglobin β-chain N-terminal[76]. Theoretically, when interpreting Adj-HbA1c (LA) levels, it is necessary to consider a low plasma glucose level and shortened half-life of red blood cells of the infant; however, it is considered that Adj-HbA1c (LA) may be used as a glycemic control indicator; in fact, a correlation between Adj-HbA1c (LA) and GA was observed. However, the LA method is too complicated to be used in clinical settings because it is necessary to measure HbF using the HPLC method. In addition, our investigation revealed that Adj-HbA1c (HPLC) is not an appropriate indicator for the evaluation of HbA1c in infants. In the HPLC analysis, HbF and HbA1c migrate to adjacent locations. When a high HbF level is observed, separation of HbF and HbA1c becomes insufficient and so HbA1c cannot be measured correctly, which is considered to be one of the causes of the above-mentioned phenomenon. On the other hand, Little et al[75] and Rohlfing et al[77] reported on HbF-corrected HbA1c as follows: if HbF is not more than 30%, HbA1c measured by the HPLC method using Tosoh’s G7 and G8 can be used as a glycemic control indicator. However, they did not use samples which contained 30% or more of HbF, and they did not state whether or not Hb in the samples used was derived from infants; therefore, these facts may be the reason for the difference from our data obtained from samples of infants.
Figure 3.
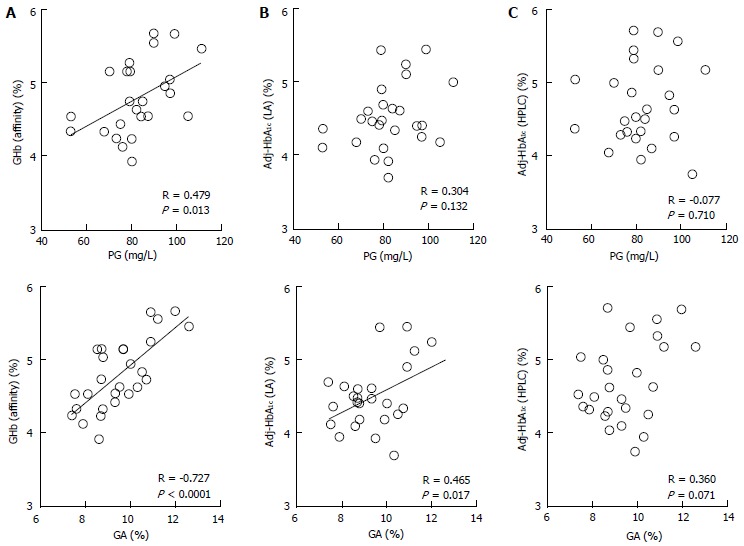
Correlations between glycated hemoglobin measure by various methods and plasma glucose or glycated albumin. Correlations between GHb measured by the affinity method [GHb (affinity)] (A), HbF-adjusted HbA1c measured by the immunological method [Adj-HbA1c (LA)] (B), and HbF-adjusted HbA1c measured by the HPLC method [Adj-HbA1c (HPLC)] (C), and PG or GA in 26 healthy infants were shown (modified from Ref[72], with permission from Copyright Clearance Center Inc.). GA: Glycated albumin; PG: Plasma glucose; GHb: Glycated hemoglobin; HbF: Fetal hemoglobin.
So far, there have been no studies on the age at which HbA1c can be used for patients with NDM, and so research is needed to clarify the relationship between mean plasma glucose and HbA1c and between CGM and HbA1c. Regarding the reference value of HbA1c in healthy infants, there is only a report by Jansen et al[73] who investigated 100 healthy infants of 8 to 12 mo old. In that report, the reference value of HbA1c for infants was 4.8% to 6.0%, which was similar to the reference value of HbA1c for adults (4.6% to 6.2%). From our results, HbA1c levels in most infants of 6 mo of age or older were also within the reference range shown by Jansen et al[73] HbF decreases to less than 5% by 6 mo after birth[56,57]; therefore, it is considered possible to use HbA1c as a glycemic control indicator in patients with NDM of 6 mo of age or older.
GA IN NEONATES AND NDM
GA is a useful glycemic control indicator under conditions in which hemoglobin metabolism is affected. On the other hand, abnormal albumin metabolism affects GA. It has been reported under various conditions that GA shows a low level when albumin metabolism is accelerated and shows a high level when albumin metabolism is suppressed[78].
While GA is a useful glycemic control indicator in patients with NDM, it is necessary to keep in mind the following characteristics of GA during infancy: (1) it shows a lower level in relation to plasma glucose; and (2) it shows a positive correlation with logarithmically transformed age[65,66]. For GA in healthy infants, before the currently widely used enzyme method was developed[79], it had already been reported that GA measured by the HPLC method was lower than the reference value for adults[54]. It is known that protein metabolism is accelerated during infancy[80,81]. In addition, it has been reported that albumin synthesis is accelerated as well[82]. Therefore, acceleration of albumin metabolism may contribute to a low GA level during infancy. We compared the relationship between GA and plasma glucose level in patients with NDM and in patients with juvenile type 1 diabetes mellitus (T1DM), and found that patients with NDM had higher plasma glucose levels but lower GA levels than patients with T1DM (Figure 4); thus, we obtained a result which supports the phenomenon of accelerated metabolism of albumin during infancy[65]. In addition, we investigated in healthy infants the relationship between change in GA according to age and plasma glucose and between change in GA according to age and serum albumin. As a result, a strong positive correlation was observed between GA and logarithmically transformed age in days (Figure 5A), and multivariate analysis revealed that age and serum albumin affect GA levels more significantly than plasma glucose[66]. Because GA is expressed as a percentage relative to serum albumin, it is not affected by serum albumin, which is an advantage of GA over fructosamine[54]. However, an increase in serum albumin associated with aging is observed during infancy (Figure 5B) and there is a positive correlation between GA and serum albumin during this period (Figure 5C)[66]. Accordingly, we determined the reference value of GA in infants according to age in mo from the regression equation of GA and age, and proposed that a comparison between GA level and the reference value[65].
Figure 4.
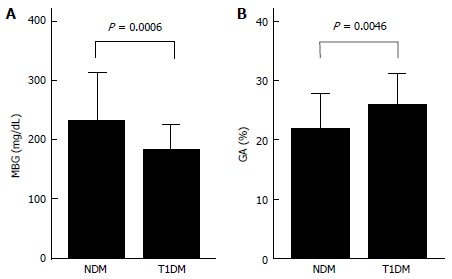
Comparisons of mean blood glucose for 1 mo (A) and GA (B) in 6 patients with neonatal diabetes mellitus and in 18 patients with type 1 diabetes mellitus (modified from Reference[65], with permission from Copyright Clearance Center Inc.).
Figure 5.
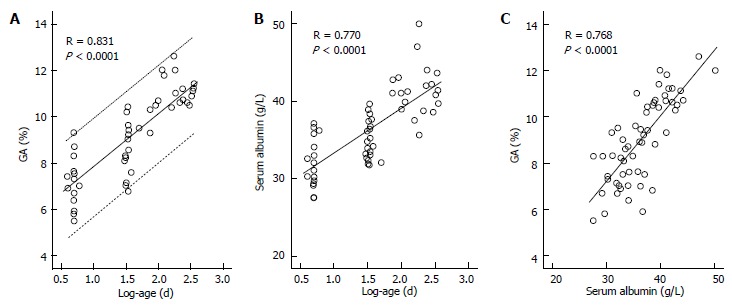
Correlations between glycated albumin and age and between serum albumin and age and correlation between glycated albumin and serum albumin in healthy infants. A: Correlation between glycated albumin (GA) and log-age. The dotted line shows the 95%CI; B: Correlation between serum albumin and log-age; C: Correlation between GA and serum albumin (modified from Ref[66], with permission from Copyright Clearance Center Inc.).
On the other hand, we found that regardless of age, GA can be evaluated based on the reference value for adults without using the reference value for infants by determining age adjusted GA (Aa-GA)[83]. We investigated GA in 376 subjects without diabetes mellitus of a wide range of age (neonates, children, and adults), and found that GA can be expressed as a primary regression equation of logarithmically transformed age (Figure 6). Based on this equation, the following formula for calculating Aa-GA was derived: Aa-GA = GA × 14.0 /[1.77 × log-age (d) + 6.55] or Aa-GA = GA × 14.0 /[1.77 × log-age (yr) + 11.1]. As mentioned above, GA in NDM shows an apparently low level; therefore, if GA in NDM is compared with the reference value for adults, the glycemic control status may be underestimated. By calculating Aa-GA and comparing it with the reference value for adults, it is possible to accurately evaluate the glycemic control status in NDM. The advantages of evaluating Aa-GA by the reference value for adults instead of evaluating GA by the reference value for infants according to age in month are as follows: (1) it is not necessary to consider the reference value according to age in month; and (2) regardless of age, it is possible to make comparisons of longitudinal changes in glycemic control status.
Figure 6.
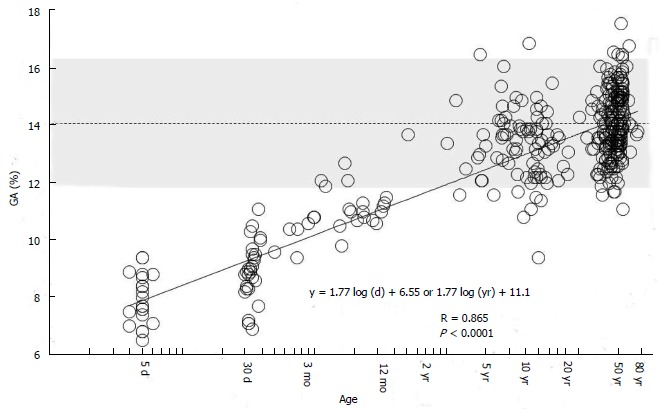
Correlation between glycated albumin and age in days (logarithmic transformation) in 376 healthy subjects (age: 4 d to 78 years). The dotted line indicates the mean reference value for adults (14%), and the shading indicates the range of reference values for adults (11.7% to 16.2%) (modified from Ref[83], with permission from Royal Society of Medicine).
It is known that because the half-life of GA is shorter than that of HbA1c, GA reflects short-term plasma glucose correctly[84,85]. This characteristic also indicates the usefulness of GA as a glycemic control indicator in NDM. Because a large proportion of NDM develops within one month, the duration of the hyperglycemic status is short. This form of development is similar to that of fulminant type 1 diabetes mellitus[86]. In fulminant type 1 diabetes mellitus, pancreatic beta cells are destroyed in a very short period, and ketoacidosis develops shortly after the onset of diabetic symptoms. Therefore, at the time of onset, HbA1c is normal or only slightly high, but GA is already obviously high[87]. We reported that GA at the time of onset of NDM was abnormally high (33.6 ± 6.9%) in all patients[6], and an abnormally high GA level in NDM may be useful for differential diagnosis from transient hyperglycemia. In addition, when evaluating remission of patients with TNDM and when evaluating the effect of SU drugs administered to patients with PNDM, it will be possible to promptly evaluate an improvement of such glycemic control by using GA[5].
CONCLUSION
The usefulness of GA as a glycemic control indicator in NDM was demonstrated. However, it was found that GA is affected by albumin metabolism and shows an apparently low level. Therefore, it is necessary to compare GA with the reference value according to age or to calculate age-adjusted GA (Aa-GA). On the other hand, HbA1c measured by the HPLC method, the LA method, or the enzyme method does not correctly reflect the glycemic control status because it is affected by a high HbF level. GHb measured by the affinity method reflects the glycemic control status in NDM; however, this method is currently hardly used and cannot easily measure GHb routinely. In addition, it is unknown whether the kinetics of glycation reaction of HbF are similar to those of HbA. Taking into account such circumstances, it is desirable to select GA as a glycemic control indicator for patients with NDM and to evaluate the glycemic control status using Aa-GA.
Footnotes
P- Reviewers: Bagdure D, Izawa KP, Tamemoto H S- Editor: Wen LL L- Editor: A E- Editor: Wu HL
References
- 1.Koenig RJ, Peterson CM, Jones RL, Saudek C, Lehrman M, Cerami A. Correlation of glucose regulation and hemoglobin AIc in diabetes mellitus. N Engl J Med. 1976;295:417–420. doi: 10.1056/NEJM197608192950804. [DOI] [PubMed] [Google Scholar]
- 2.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010;33 Suppl 1:S62–S69. doi: 10.2337/dc10-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 4.Gillett MJ. International Expert Committee report on the role of the A1c assay in the diagnosis of diabetes: Diabetes Care 2009; 32(7): 1327-1334. Clin Biochem Rev. 2009;30:197–200. [PMC free article] [PubMed] [Google Scholar]
- 5.Koga M. Glycated albumin and 1,5-anhdroglucitol as alternative markers of glycemia. Adv Clin Chem. 2013:In press. doi: 10.1016/b978-0-12-800263-6.00007-0. [DOI] [PubMed] [Google Scholar]
- 6.Suzuki S, Koga M, Amamiya S, Nakao A, Wada K, Okuhara K, Hayano S, Sarhat AR, Takahashi H, Matsuo K, et al. Glycated albumin but not HbA1c reflects glycaemic control in patients with neonatal diabetes mellitus. Diabetologia. 2011;54:2247–2253. doi: 10.1007/s00125-011-2211-8. [DOI] [PubMed] [Google Scholar]
- 7.Rubio-Cabezas O, Ellard S. Diabetes mellitus in neonates and infants: genetic heterogeneity, clinical approach to diagnosis, and therapeutic options. Horm Res Paediatr. 2013;80:137–146. doi: 10.1159/000354219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grulich-Henn J, Wagner V, Thon A, Schober E, Marg W, Kapellen TM, Haberland H, Raile K, Ellard S, Flanagan SE, et al. Entities and frequency of neonatal diabetes: data from the diabetes documentation and quality management system (DPV) Diabet Med. 2010;27:709–712. doi: 10.1111/j.1464-5491.2010.02965.x. [DOI] [PubMed] [Google Scholar]
- 9.Edghill EL, Flanagan SE, Patch AM, Boustred C, Parrish A, Shields B, Shepherd MH, Hussain K, Kapoor RR, Malecki M, et al. Insulin mutation screening in 1,044 patients with diabetes: mutations in the INS gene are a common cause of neonatal diabetes but a rare cause of diabetes diagnosed in childhood or adulthood. Diabetes. 2008;57:1034–1042. doi: 10.2337/db07-1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Edghill EL, Dix RJ, Flanagan SE, Bingley PJ, Hattersley AT, Ellard S, Gillespie KM. HLA genotyping supports a nonautoimmune etiology in patients diagnosed with diabetes under the age of 6 months. Diabetes. 2006;55:1895–1898. doi: 10.2337/db06-0094. [DOI] [PubMed] [Google Scholar]
- 11.Iafusco D, Stazi MA, Cotichini R, Cotellessa M, Martinucci ME, Mazzella M, Cherubini V, Barbetti F, Martinetti M, Cerutti F, et al. Permanent diabetes mellitus in the first year of life. Diabetologia. 2002;45:798–804. doi: 10.1007/s00125-002-0837-2. [DOI] [PubMed] [Google Scholar]
- 12.Aguilar-Bryan L, Bryan J. Neonatal diabetes mellitus. Endocr Rev. 2008;29:265–291. doi: 10.1210/er.2007-0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Docherty LE, Kabwama S, Lehmann A, Hawke E, Harrison L, Flanagan SE, Ellard S, Hattersley AT, Shield JP, Ennis S, et al. Clinical presentation of 6q24 transient neonatal diabetes mellitus (6q24 TNDM) and genotype-phenotype correlation in an international cohort of patients. Diabetologia. 2013;56:758–762. doi: 10.1007/s00125-013-2832-1. [DOI] [PubMed] [Google Scholar]
- 14.Flanagan SE, Patch AM, Mackay DJ, Edghill EL, Gloyn AL, Robinson D, Shield JP, Temple K, Ellard S, Hattersley AT. Mutations in ATP-sensitive K+ channel genes cause transient neonatal diabetes and permanent diabetes in childhood or adulthood. Diabetes. 2007;56:1930–1937. doi: 10.2337/db07-0043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Suzuki S, Makita Y, Mukai T, Matsuo K, Ueda O, Fujieda K. Molecular basis of neonatal diabetes in Japanese patients. J Clin Endocrinol Metab. 2007;92:3979–3985. doi: 10.1210/jc.2007-0486. [DOI] [PubMed] [Google Scholar]
- 16.Temple IK, Gardner RJ, Mackay DJ, Barber JC, Robinson DO, Shield JP. Transient neonatal diabetes: widening the understanding of the etiopathogenesis of diabetes. Diabetes. 2000;49:1359–1366. doi: 10.2337/diabetes.49.8.1359. [DOI] [PubMed] [Google Scholar]
- 17.von Mühlendahl KE, Herkenhoff H. Long-term course of neonatal diabetes. N Engl J Med. 1995;333:704–708. doi: 10.1056/NEJM199509143331105. [DOI] [PubMed] [Google Scholar]
- 18.Kamiya M, Judson H, Okazaki Y, Kusakabe M, Muramatsu M, Takada S, Takagi N, Arima T, Wake N, Kamimura K, et al. The cell cycle control gene ZAC/PLAGL1 is imprinted--a strong candidate gene for transient neonatal diabetes. Hum Mol Genet. 2000;9:453–460. doi: 10.1093/hmg/9.3.453. [DOI] [PubMed] [Google Scholar]
- 19.Metz C, Cavé H, Bertrand AM, Deffert C, Gueguen-Giroux B, Czernichow P, Polak M. Neonatal diabetes mellitus: chromosomal analysis in transient and permanent cases. J Pediatr. 2002;141:483–489. doi: 10.1067/mpd.2002.127089. [DOI] [PubMed] [Google Scholar]
- 20.Gloyn AL, Reimann F, Girard C, Edghill EL, Proks P, Pearson ER, Temple IK, Mackay DJ, Shield JP, Freedenberg D, et al. Relapsing diabetes can result from moderately activating mutations in KCNJ11. Hum Mol Genet. 2005;14:925–934. doi: 10.1093/hmg/ddi086. [DOI] [PubMed] [Google Scholar]
- 21.Patch AM, Flanagan SE, Boustred C, Hattersley AT, Ellard S. Mutations in the ABCC8 gene encoding the SUR1 subunit of the KATP channel cause transient neonatal diabetes, permanent neonatal diabetes or permanent diabetes diagnosed outside the neonatal period. Diabetes Obes Metab. 2007;9 Suppl 2:28–39. doi: 10.1111/j.1463-1326.2007.00772.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karges B, Meissner T, Icks A, Kapellen T, Holl RW. Management of diabetes mellitus in infants. Nat Rev Endocrinol. 2012;8:201–211. doi: 10.1038/nrendo.2011.204. [DOI] [PubMed] [Google Scholar]
- 23.Beardsall K, Pesterfield CL, Acerini CL. Neonatal diabetes and insulin pump therapy. Arch Dis Child Fetal Neonatal Ed. 2011;96:F223–F224. doi: 10.1136/adc.2010.196709. [DOI] [PubMed] [Google Scholar]
- 24.Olinder AL, Kernell A, Smide B. Treatment with CSII in two infants with neonatal diabetes mellitus. Pediatr Diabetes. 2006;7:284–288. doi: 10.1111/j.1399-5448.2006.00203.x. [DOI] [PubMed] [Google Scholar]
- 25.Tubiana-Rufi N. Insulin pump therapy in neonatal diabetes. Endocr Dev. 2007;12:67–74. doi: 10.1159/000109606. [DOI] [PubMed] [Google Scholar]
- 26.Iafusco D, Bizzarri C, Cadario F, Pesavento R, Tonini G, Tumini S, Cauvin V, Colombo C, Bonfanti R, Barbetti F. No beta cell desensitisation after a median of 68 months on glibenclamide therapy in patients with KCNJ11-associated permanent neonatal diabetes. Diabetologia. 2011;54:2736–2738. doi: 10.1007/s00125-011-2273-7. [DOI] [PubMed] [Google Scholar]
- 27.Pearson ER, Flechtner I, Njølstad PR, Malecki MT, Flanagan SE, Larkin B, Ashcroft FM, Klimes I, Codner E, Iotova V, et al. Switching from insulin to oral sulfonylureas in patients with diabetes due to Kir6.2 mutations. N Engl J Med. 2006;355:467–477. doi: 10.1056/NEJMoa061759. [DOI] [PubMed] [Google Scholar]
- 28.Rafiq M, Flanagan SE, Patch AM, Shields BM, Ellard S, Hattersley AT. Effective treatment with oral sulfonylureas in patients with diabetes due to sulfonylurea receptor 1 (SUR1) mutations. Diabetes Care. 2008;31:204–209. doi: 10.2337/dc07-1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chan YM, Laffel LM. Transition from insulin to glyburide in a 4-month-old girl with neonatal diabetes mellitus caused by a mutation in KCNJ11. Pediatr Diabetes. 2007;8:235–238. doi: 10.1111/j.1399-5448.2007.00231.x. [DOI] [PubMed] [Google Scholar]
- 30.Joshi R, Phatarpekar A. Neonatal diabetes mellitus due to L233F mutation in the KCNJ11 gene. World J Pediatr. 2011;7:371–372. doi: 10.1007/s12519-011-0254-z. [DOI] [PubMed] [Google Scholar]
- 31.Shah RP, Spruyt K, Kragie BC, Greeley SA, Msall ME. Visuomotor performance in KCNJ11-related neonatal diabetes is impaired in children with DEND-associated mutations and may be improved by early treatment with sulfonylureas. Diabetes Care. 2012;35:2086–2088. doi: 10.2337/dc11-2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wambach JA, Marshall BA, Koster JC, White NH, Nichols CG. Successful sulfonylurea treatment of an insulin-naïve neonate with diabetes mellitus due to a KCNJ11 mutation. Pediatr Diabetes. 2010;11:286–288. doi: 10.1111/j.1399-5448.2009.00557.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yamanouchi T, Tachibana Y, Akanuma H, Minoda S, Shinohara T, Moromizato H, Miyashita H, Akaoka I. Origin and disposal of 1,5-anhydroglucitol, a major polyol in the human body. Am J Physiol. 1992;263:E268–E273. doi: 10.1152/ajpendo.1992.263.2.E268. [DOI] [PubMed] [Google Scholar]
- 34.Wright EM. Renal Na(+)-glucose cotransporters. Am J Physiol Renal Physiol. 2001;280:F10–F18. doi: 10.1152/ajprenal.2001.280.1.F10. [DOI] [PubMed] [Google Scholar]
- 35.Yamanouchi T, Ogata N, Tagaya T, Kawasaki T, Sekino N, Funato H, Akaoka L, Miyashita H. Clinical usefulness of serum 1,5-anhydroglucitol in monitoring glycaemic control. Lancet. 1996;347:1514–1518. doi: 10.1016/s0140-6736(96)90672-8. [DOI] [PubMed] [Google Scholar]
- 36.Yamanouchi T, Shinohara T, Ogata N, Tachibana Y, Akaoka I, Miyashita H. Common reabsorption system of 1,5-anhydro-D-glucitol, fructose, and mannose in rat renal tubule. Biochim Biophys Acta. 1996;1291:89–95. doi: 10.1016/0304-4165(96)00050-5. [DOI] [PubMed] [Google Scholar]
- 37.Tazawa S, Yamato T, Fujikura H, Hiratochi M, Itoh F, Tomae M, Takemura Y, Maruyama H, Sugiyama T, Wakamatsu A, et al. SLC5A9/SGLT4, a new Na+-dependent glucose transporter, is an essential transporter for mannose, 1,5-anhydro-D-glucitol, and fructose. Life Sci. 2005;76:1039–1050. doi: 10.1016/j.lfs.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 38.Shimizu H, Shouzu A, Nishikawa M, Omoto S, Hayakawa T, Miyake Y, Yonemoto T, Inada M. Serum concentration and renal handling of 1,5-anhydro-D-glucitol in patients with chronic renal failure. Ann Clin Biochem. 1999;36(Pt 6):749–754. doi: 10.1177/000456329903600608. [DOI] [PubMed] [Google Scholar]
- 39.Emoto M, Tabata T, Inoue T, Nishizawa Y, Morii H. Plasma 1,5-anhydroglucitol concentration in patients with end-stage renal disease with and without diabetes mellitus. Nephron. 1992;61:181–186. doi: 10.1159/000186868. [DOI] [PubMed] [Google Scholar]
- 40.Kim WJ, Park CY, Lee KB, Park SE, Rhee EJ, Lee WY, Oh KW, Park SW. Serum 1,5-anhydroglucitol concentrations are a reliable index of glycemic control in type 2 diabetes with mild or moderate renal dysfunction. Diabetes Care. 2012;35:281–286. doi: 10.2337/dc11-1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tetsuo M, Hamada T, Yoshimatsu K, Ishimatsu J, Matsunaga T. Serum levels of 1,5-anhydro-D-glucitol during the normal and diabetic pregnancy and puerperium. Acta Obstet Gynecol Scand. 1990;69:479–485. doi: 10.3109/00016349009013322. [DOI] [PubMed] [Google Scholar]
- 42.Murai J, Koga M, Saito H, Mukai M, Kasayama S. Serum 1,5-anhydroglucitol is low in gastrectomized men. Acta Diabetol. 2014;51:337–338. doi: 10.1007/s00592-011-0354-1. [DOI] [PubMed] [Google Scholar]
- 43.Yamanouchi T, Minoda S, Ogata N, Tachibana Y, Sekino N, Miyashita H, Akaoka I. Prolonged hyperalimentation as a possible cause of renal tubular dysfunction: evaluation of 1,5-anhydro-D-glucitol resorption and N-acetylglucosaminidase excretion in humans. Clin Sci (Lond) 1995;88:203–210. doi: 10.1042/cs0880203. [DOI] [PubMed] [Google Scholar]
- 44.Yamagishi S, Ohta M. Serum 1,5-anhydro-D-glucitol levels in liver cirrhosis. Acta Diabetol. 1998;35:65–66. doi: 10.1007/s005920050104. [DOI] [PubMed] [Google Scholar]
- 45.Koga M, Murai J, Saito H, Mukai M, Toya D, Tanaka N, Kanehara H, Bando Y, Kasayama S. 1,5-Anhydroglucitol levels are low irrespective of plasma glucose levels in patients with chronic liver disease. Ann Clin Biochem. 2011;48:121–125. doi: 10.1258/acb.2010.010053. [DOI] [PubMed] [Google Scholar]
- 46.Kawasaki T, Yamanouchi T, Kashiwabara A, Inoue T, Yoshimura T, Fujimori S, Tanabe T, Aiso Y. The influence of traditional Chinese herbal drugs on serum 1, 5-anhydroglucitol levels. Diabetes Res Clin Pract. 2000;50:97–101. doi: 10.1016/s0168-8227(00)00167-4. [DOI] [PubMed] [Google Scholar]
- 47.Yoshioka S. New metabolic parameter for diabetes-1-deoxyglucose (1,5-anhdroglucitol) Shonika. 1983;24:405–410 (In Japanese). [Google Scholar]
- 48.Koga M, Murai J, Saito H, Mukai M, Kasayama S. Habitual intake of dairy products influences serum 1,5-anhydroglucitol levels independently of plasma glucose. Diabetes Res Clin Pract. 2010;90:122–125. doi: 10.1016/j.diabres.2010.06.023. [DOI] [PubMed] [Google Scholar]
- 49.Armbruster DA. Fructosamine: structure, analysis, and clinical usefulness. Clin Chem. 1987;33:2153–2163. [PubMed] [Google Scholar]
- 50.Ford HC, Lim WC, Crooke MJ. Hemoglobin A1 and serum fructosamine levels in hyperthyroidism. Clin Chim Acta. 1987;166:317–321. doi: 10.1016/0009-8981(87)90435-9. [DOI] [PubMed] [Google Scholar]
- 51.Sako Y, Umeda F, Hashimoto T, Haji M, Nawata H. Serum fructosamine in assessment of diabetic control and relation to thyroid function. Horm Metab Res. 1989;21:669–672. doi: 10.1055/s-2007-1009316. [DOI] [PubMed] [Google Scholar]
- 52.Constanti C, Simo JM, Joven J, Camps J. Serum fructosamine concentration in patients with nephrotic syndrome and with cirrhosis of the liver: the influence of hypoalbuminaemia and hypergammaglobulinaemia. Ann Clin Biochem. 1992;29(Pt 4):437–442. doi: 10.1177/000456329202900412. [DOI] [PubMed] [Google Scholar]
- 53.Montagna MP, Laghi F, Cremona G, Zuppi C, Barbaresi G, Castellana ML. Influence of serum proteins on fructosamine concentration in multiple myeloma. Clin Chim Acta. 1991;204:123–130. doi: 10.1016/0009-8981(91)90223-y. [DOI] [PubMed] [Google Scholar]
- 54.Abe F, Yano M, Minami Y, Ueda T, Chikakiyo H, Miyamoto N, Shirakawa N, Shima K. Alterations in fructosamine and glycated albumin levels during childhood. Ann Clin Biochem. 1989;26(Pt 4):328–331. doi: 10.1177/000456328902600405. [DOI] [PubMed] [Google Scholar]
- 55.Sacks DB. Carbohydrates. In: Burtis CA, Ashwood ER, eds , editors. Tietz textbook of clinical chemistry. 3rd ed. Philadelphia: WB Saunders; 1999. pp. 790–796. [Google Scholar]
- 56.Bard H. The postnatal decline of hemoglobin F synthesis in normal full-term infants. J Clin Invest. 1975;55:395–398. doi: 10.1172/JCI107943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ohls RK, Christensen RD. Developmental of the hematopietic system. In: Kliegman RM, Beheman RE, Jenson HB, Santon BF, eds , et al., editors. Nelson’s Textbook of Pediatrics. 18th ed. Philadelphia: Saunders Elsevier; 2008. pp. 1997–2003. [Google Scholar]
- 58.Koga M, Murai J, Saito H, Yamada Y, Mori T, Suno S, Takeuchi K, Suzuki S, Fujieda K, Kasayama S. Measurement of glycated hemoglobin and glycated albumin in umbilical cord: evaluation of the glycemic control indicators in neonates. J Perinatol. 2011;31:430–433. doi: 10.1038/jp.2010.144. [DOI] [PubMed] [Google Scholar]
- 59.Moiz B, Hashmi MR, Sadaf S. Performance evaluation of ion exchange and affinity chromatography for HbA1c estimation in diabetic patients with HbD: a study of 129 samples. Clin Biochem. 2008;41:1204–1210. doi: 10.1016/j.clinbiochem.2008.06.015. [DOI] [PubMed] [Google Scholar]
- 60.Weykamp CW, Penders TJ, Muskiet FA, van der Slik W. Influence of hemoglobin variants and derivatives on glycohemoglobin determinations, as investigated by 102 laboratories using 16 methods. Clin Chem. 1993;39:1717–1723. [PubMed] [Google Scholar]
- 61.Hall PM, Cawdell GM, Cook JG, Gould BJ. Measurement of glycosylated haemoglobins and glycosylated plasma proteins in maternal and cord blood using an affinity chromatography method. Diabetologia. 1983;25:477–481. doi: 10.1007/BF00284454. [DOI] [PubMed] [Google Scholar]
- 62.Worth R, Ashworth L, Home PD, Gerrard J, Lind T, Anderson J, Alberti KG. Glycosylated haemoglobin in cord blood following normal and diabetic pregnancies. Diabetologia. 1983;25:482–485. doi: 10.1007/BF00284455. [DOI] [PubMed] [Google Scholar]
- 63.John WG, Webb AM, Jones AE. Glycosylated haemoglobin and glycosylated albumin in non-diabetic and diabetic mothers, and their babies. Diabet Med. 1985;2:103–104. doi: 10.1111/j.1464-5491.1985.tb00609.x. [DOI] [PubMed] [Google Scholar]
- 64.Hann IM. The normal blood picture in neonates. In: Hann IM, Gibson BES, Letsky EA, eds , et al., editors. Fetal and neonatal haematology. London: W.B. Saunders; 1991. pp. 29–50. [Google Scholar]
- 65.Suzuki S, Koga M, Takahashi H, Matsuo K, Tanahashi Y, Azuma H. Glycated albumin in patients with neonatal diabetes mellitus is apparently low in relation to glycemia compared with that in patients with type 1 diabetes mellitus. Horm Res Paediatr. 2012;77:273–276. doi: 10.1159/000337914. [DOI] [PubMed] [Google Scholar]
- 66.Suzuki S, Koga M, Niizeki N, Furuya A, Takahashi H, Matsuo K, Tanahashi Y, Kawata Y, Asai H, Tsuchida E, et al. Glycated albumin is lower in infants than in adults and correlated with both age and serum albumin. Pediatr Diabetes. 2013;14:25–30. doi: 10.1111/j.1399-5448.2012.00895.x. [DOI] [PubMed] [Google Scholar]
- 67.Sanaka M, Minei S, Shimizu M, Tetsuou T, Yanagisawa K, Omori Y, Saitoh S, Yoshioka S. Serum 1, 5-Anhydroglucitol (1, 5-AG) in pregnant diabetics at delivery and in cord serum. J Japan Diabetes Society. 1994;37:895–900 (In Japanese). [Google Scholar]
- 68.Yoshiuchi K, Matsuhisa M, Katakami N, Nakatani Y, Sakamoto K, Matsuoka T, Umayahara Y, Kosugi K, Kaneto H, Yamasaki Y, et al. Glycated albumin is a better indicator for glucose excursion than glycated hemoglobin in type 1 and type 2 diabetes. Endocr J. 2008;55:503–507. doi: 10.1507/endocrj.k07e-089. [DOI] [PubMed] [Google Scholar]
- 69.Saisho Y, Tanaka K, Abe T, Shimada A, Kawai T, Itoh H. Glycated albumin to glycated hemoglobin ratio reflects postprandial glucose excursion and relates to beta cell function in both type 1 and type 2 diabetes. Diabetol Int. 2011;2:146–153. [Google Scholar]
- 70.Ogawa A, Hayashi A, Kishihara E, Yoshino S, Takeuchi A, Shichiri M. New indices for predicting glycaemic variability. PLoS One. 2012;7:e46517. doi: 10.1371/journal.pone.0046517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schnedl WJ, Lahousen T, Lang T, Lipp RW, Yonehara S, Fukunaga S, Imai T, Little RR. Determination of glycated hemoglobin in clinically silent hemoglobin variants. Diabetes Metab Res Rev. 2004;20:460–465. doi: 10.1002/dmrr.483. [DOI] [PubMed] [Google Scholar]
- 72.Suzuki S, Koga M, Niizeki N, Furuya A, Matsuo K, Tanahashi Y, Tsuchida E, Nohara F, Okamoto T, Nagaya K, et al. Evaluation of glycated hemoglobin and fetal hemoglobin-adjusted HbA1c measurements in infants. Pediatr Diabetes. 2013;14:267–272. doi: 10.1111/pedi.12013. [DOI] [PubMed] [Google Scholar]
- 73.Jansen H, Huiting HG, Scholtens S, Sauer PJ, Stolk RP. HbA1c in nondiabetic Dutch infants aged 8-12 months: the GECKO-Drenthe birth cohort study. Diabetes Care. 2011;34:403–405. doi: 10.2337/dc10-1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hawdon JM, Ward Platt MP, Aynsley-Green A. Patterns of metabolic adaptation for preterm and term infants in the first neonatal week. Arch Dis Child. 1992;67:357–365. doi: 10.1136/adc.67.4_spec_no.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Little RR, Rohlfing CL, Hanson SE, Schmidt RL, Lin CN, Madsen RW, Roberts WL. The effect of increased fetal hemoglobin on 7 common Hb A1c assay methods. Clin Chem. 2012;58:945–947. doi: 10.1373/clinchem.2012.181933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Guthrie R, Hellman R, Kilo C, Hiar CE, Crowley LE, Childs B, Fisher R, Pinson MB, Suttner A, Vittori C. A multisite physician’s office laboratory evaluation of an immunological method for the measurement of HbA1c. Diabetes Care. 1992;15:1494–1498. doi: 10.2337/diacare.15.11.1494. [DOI] [PubMed] [Google Scholar]
- 77.Rohlfing CL, Connolly SM, England JD, Hanson SE, Moellering CM, Bachelder JR, Little RR. The effect of elevated fetal hemoglobin on hemoglobin A1c results: five common hemoglobin A1c methods compared with the IFCC reference method. Am J Clin Pathol. 2008;129:811–814. doi: 10.1309/YFVTUD0GHJF7D16H. [DOI] [PubMed] [Google Scholar]
- 78.Koga M, Kasayama S. Clinical impact of glycated albumin as another glycemic control marker. Endocr J. 2010;57:751–762. doi: 10.1507/endocrj.k10e-138. [DOI] [PubMed] [Google Scholar]
- 79.Kouzuma T, Usami T, Yamakoshi M, Takahashi M, Imamura S. An enzymatic method for the measurement of glycated albumin in biological samples. Clin Chim Acta. 2002;324:61–71. doi: 10.1016/s0009-8981(02)00207-3. [DOI] [PubMed] [Google Scholar]
- 80.Duffy B, Gunn T, Collinge J, Pencharz P. The effect of varying protein quality and energy intake on the nitrogen metabolism of parenterally fed very low birthweight (less than 1600 g) infants. Pediatr Res. 1981;15:1040–1044. doi: 10.1203/00006450-198107000-00013. [DOI] [PubMed] [Google Scholar]
- 81.Micheli JL, Schutz Y, Jequier E. Protein metabolism in the newborn. In: Richard AP, Fox WW. eds. Fetal and neonatal physiology, 2nd ed. Philadelphia; W.B. Saunders Company; 1998. pp. 642–653. [Google Scholar]
- 82.Bunt JE, Rietveld T, Schierbeek H, Wattimena JL, Zimmermann LJ, van Goudoever JB. Albumin synthesis in preterm infants on the first day of life studied with [1-13C]leucine. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1157–G1161. doi: 10.1152/ajpgi.00300.2006. [DOI] [PubMed] [Google Scholar]
- 83.Suzuki S, Koga M, Niizeki N, Furuya A, Matsuo K, Tanahashi Y, Azuma H. Age-adjusted glycated albumin is a useful indicator for glycemic control in patients with neonatal diabetes mellitus. Ann Clin Biochem. 2013:published online. doi: 10.1177/0004563213512617. [DOI] [PubMed] [Google Scholar]
- 84.Tahara Y, Shima K. Kinetics of HbA1c, glycated albumin, and fructosamine and analysis of their weight functions against preceding plasma glucose level. Diabetes Care. 1995;18:440–447. doi: 10.2337/diacare.18.4.440. [DOI] [PubMed] [Google Scholar]
- 85.Takahashi S, Uchino H, Shimizu T, Kanazawa A, Tamura Y, Sakai K, Watada H, Hirose T, Kawamori R, Tanaka Y. Comparison of glycated albumin (GA) and glycated hemoglobin (HbA1c) in type 2 diabetic patients: usefulness of GA for evaluation of short-term changes in glycemic control. Endocr J. 2007;54:139–144. doi: 10.1507/endocrj.k06-103. [DOI] [PubMed] [Google Scholar]
- 86.Imagawa A, Hanafusa T, Miyagawa J, Matsuzawa Y. A novel subtype of type 1 diabetes mellitus characterized by a rapid onset and an absence of diabetes-related antibodies. Osaka IDDM Study Group. N Engl J Med. 2000;342:301–307. doi: 10.1056/NEJM200002033420501. [DOI] [PubMed] [Google Scholar]
- 87.Koga M, Murai J, Saito H, Kasayama S, Imagawa A, Hanafusa T, Kobayashi T. Serum glycated albumin to haemoglobin A(1C) ratio can distinguish fulminant type 1 diabetes mellitus from type 2 diabetes mellitus. Ann Clin Biochem. 2010;47:313–317. doi: 10.1258/acb.2010.009234. [DOI] [PubMed] [Google Scholar]


