Abstract
AIM: To examine the contribution of toll-like receptors (TLRs) expression and activation to the prolonged inflammation often seen in human diabetic wounds.
METHODS: Debridement wound tissue was collected from diabetic patients with informed consent. Total RNA and protein were isolated and subjected to real-time polymerase chain reaction and Western blot analyses.
RESULTS: TLR1, 2, 4, and 6 mRNA expressions were increased significantly in wounds of diabetic patients compared with non-diabetic wounds (P < 0.05). MyD88 protein expression was significantly increased in diabetic wounds compared to non-diabetic wounds. Interleukin-1beta, tumor necrosis factor-alpha concentration nuclear factor-kappa B activation, and thiobarbituric acid reactive substances were increased in diabetic wounds compared to non-diabetic wounds (P < 0.01).
CONCLUSION: Collectively, our novel findings show that increased TLR expression, signaling, and activation may contribute to the hyper inflammation in the human diabetic wounds.
Keywords: Interleukin-1β, Inflammation, Toll-like receptors 2, Toll-like receptors 4, Tumor necrosis factor-α, Type 2-diabetes mellitus, Wound healing
Core tip: Increased TLR2/4-MyD88-nuclear factor-kappa B expression and signaling with attendant oxidative stress may contribute to the hyperinflammation frequently seen in human diabetic wounds.
INTRODUCTION
Diabetes mellitus (DM) is a constellation of metabolic aberrations that collectively manifest as debilitating pathological complications affecting the quality of life in DM patients. Around 348 million people worldwide and 36 million people in United States have DM and 40%-60% of these patients develop foot wounds accounting to more than 20% of all hospitalizations equating to one amputation every 30 s[1-3]. Emerging experimental data and human studies suggest that systemic inflammation orchestrated by innate immune receptors plays a role in the pathogenesis of DM complications[4]. Toll-like receptors (TLRs) are pivotal innate immune receptors that induce inflammatory responses[5] and their expression and activation is increased in a plethora of inflammatory disorders including DM and its complications[6-9]. Recent data from our group and others have provided evidence that TLR expression, activation, and signaling are significantly increased in monocytes of DM patients, non-obese diabetic (NOD) mice, and db/db mice (see review, 4). In addition, we showed that genetic ablation of TLR2/4 in diabetic mice attenuates inflammation as indicated by decreased circulating cytokine/chemokine levels and improved wound healing[8-10]. However, it is not known if TLR expression and activation contributes to the uncontrolled inflammation seen in wounds of DM patients. Thus, in the present study, we examined TLR expression, signaling, and inflammation in human DM wounds.
MATERIALS AND METHODS
Patients
The study population consisted of type 2 DM patients presenting for care of a diabetic ulcer located anywhere on the foot and non-DM patients (controls) with a leg ulcer, aged between 45-65 years. We collected wound tissues from diabetic (n = 8) and non-diabetic subjects (n = 4) during initial debridement as part of standard of care, with informed patient consent at the Sacramento VA clinics. Patient evaluations consisted of a medical history, physical examination, and wound site measurements (including location, size, presence of periulcerative tissue, and clinical infection) were recorded. Serum glucose and HbA1c levels were extracted from patient charts that were done within the last 60 d. All the human study protocols were approved by the Institutional Review Board at University of California at Davis and VA of Northern California, MatherField CA.
Collection of debridement wound tissue
Study inclusion criteria were as follows: age 18 or older; ulcer size > 2 cm2 and < 25 cm2; ulcer duration of ≥ 4 wk; no clinical signs of infection; glycosylated haemoglobin (HbA1c) < 12%; and adequate circulation to the affected extremity Patients were excluded if any of the following preexisting conditions: presence of charcot foot, index ulcer probing to bone; currently receiving radiation or chemotherapy; known or suspected malignancy of current ulcer; diagnosis of autoimmune connective tissue disease; received a biomedical or topical growth factor for their wound within the previous 30 d; taking medications considered to be immune system modulators, antibiotics, with C-reactive protein levels (> 10 mg/dL), and CBC (white blood cells < 4 to > 11 K/mm3) indicative of infection. Debridement tissue was collected using sharp debridement technique[11] and immediately snap frozen in liquid nitrogen for mRNA and protein analyses.
Real time-polymerase chain reaction
Total RNA was isolated from all the snap frozen wound tissues and mRNA expression was determined by REal time-polymerase chain reaction (RT-PCR) using commercial sequence-specific primers and probes purchased from SA Biosciences, Gaithersburg, MD, United States). The first strand of cDNA was synthesized using total RNA (1 μg per reaction). cDNA (50 ng) was amplified using primer probe sets for TLR1, TLR2, TLR4, TLR6, Myeloid differentiation factor-88 (MyD88), Interleukin receptor activated kinase-1 (IRAK-1), myeloid differentiation protein-2 (MD2), nuclear factor-kappa B (NF-κB), tumor necrosis factor-alpha (TNF-α) and 18s (SA Biosciences) following the manufacturer’s cycling parameters. Data were calculated using the 2-ΔΔCt method and are presented as ratio of transcripts for TLR gene normalized to 18s as described previously[8,9].
Western blot and ELISA
For Western blot assays, wound tissues were homogenized in tissue lysis buffer and total protein was determined using bicinchoninic acid protein quantitation method[8-10]. Equal amounts of protein (25 μg) were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis, transferred to polyvinylidene fluoride membranes, and were probed with MyD88 (Imgenix, United States) and β-actin (Santa Cruz, United States) antibodies as reported earlier[8,9]. Densitometric ratios of the bands were calculated as reported earlier[8,9] and expressed as MyD88/β-actin ratio. Interleukin-1beta (IL-1β) and TNF-α levels were measured in the wound tissue lysates using ELISA (R and D systems) assay as reported earlier[8-10]. Intra- and interassay coefficient of variation (CV) of ELISA assays were determined to be < 10%[8-10]. Nuclear extracts were used to perform NF-κB transcription factors activation assays (Active Motif, Carlsbad, CA, United States) to verify activation of NF-κB in the diabetic wounds, indicative of increased inflammation. Assays were performed in accordance to the manufacturer’s protocols. Intra- and inter-assay CV for transcription factor assays was < 8%[8-10].
Thiobarbituric acid reactive substances
We measured oxidative stress through lipid peroxidations [Thiobarbituric acid reactive substances (TBARs)] in wound tissues to reflect the pathogenic mechanisms in impaired wound healing in DM wounds compared with control wounds. TBARs are a surrogate marker of oxidative stress and malondialdehyde equivalents were determined by reading the absorbance at 532 nm using 1,1,3,3-tetramethoxypropane as an external standard[8,12]. Results were expressed as malondialdehyde equivalents (nmol/mg protein) as reported previously[8,12].
Statistical analyses
Data are presented as mean ± SD. We used two-tailed t tests with appropriate post hoc analyses. P < 0.05 was considered statistically significant. All statistical analyses were performed using GraphPad Prism software[8-10].
RESULTS
All the patients had DM for > 5 years (mean glucose of 132 ± 10 mg/dL and HbA1c of 7.5% ± 0.8%) and are on routine standard care for a chronic diabetic foot ulcer of at least 4-wk duration and showed no signs of clinical infection. We first examined mRNA levels of TLRs and associated inflammatory signaling mediators in DM and control wound tissue to test the hypothesis that increased TLR expression and activation accentuate inflammation in diabetic wounds, using RT-PCR. TLR1, TLR2, TLR4, TLR6, MyD88, IRAK-1, NF-κB, IL-1β, and TNF-α mRNA expression were significantly increased compared to non-diabetic wounds (P < 0.05) (Table 1) implicating a role for TLR-MyD88-NF-κB signaling on hyperinflammatory phenotype often seen in DM wounds[7-9]. The mRNA data was validated using Western blot and enzyme-linked immunosorbent assay (ELISA) assays[7-9]. MyD88 is an immediate and common downstream adaptor molecule recruited by activated TLRs through their TIR domain. MyD88, in turn, recruits IRAK-1, leading to the activation of NF-κB transcription factor, and attendant inflammatory cytokine gene expression[5]. Thus, we chose MyD88 for further validation. As shown in Figure 1, MyD88 protein expression was significantly higher in DM wounds compared to the non-diabetic wounds (P < 0.05 vs non-diabetic wounds). Figure 2 depicts significantly increased NF-κB activation in the nuclear extracts of diabetic wounds compared to non-diabetic wounds (P < 0.001). Next, local IL-1β and TNF-α levels known to be expressed as a result of TLR-MyD88-NF-κB activation, were determined using ELISA assay. Figure 3 shows significantly increased IL-1β and TNF-α levels in DM wounds compared to non-diabetic wounds (P < 0.05) supporting our hypothesis that TLR signaling and activation contribute to the prolonged inflammation seen in DM wounds[7-9]. Because oxidative stress and inflammation are linked by TLRs[13] as a surrogate index of oxidative stress, we measured TBARS formation during an acid-heating reaction in wound tissues as described earlier[8,12]. Figure 4 depicts significantly higher TBAR levels in diabetic wounds compared to non-diabetic wounds (P < 0.01). Thus our data for the first time attests to the concept that persistent activation of TLR-MyD88-NF-κB signaling pathway and increased oxidative stress contribute to the hyperinflammation frequently seen in human DM wounds.
Table 1.
Toll-like receptor pathway genes expressed in debridement wound tissue
| Gene | Non-diabetic wounds mRNA/18s ratio | Diabetic wounds mRNA/18s ratioa |
| TLR1 | 0.6 ± 0.1 | 1.9 ± 0.4 |
| TLR2 | 1.2 ± 0.3 | 3.6 ± 0.5 |
| TLR4 | 1.3 ± 0.2 | 3.8 ± 0.2 |
| TLR6 | 0.2 ± 0.1 | 2 ± 0.5 |
| MyD88 | 1.4 ± 0.2 | 3.1 ± 0.4 |
| IRAK-1 | 1.1 ± 0.1 | 2.8 ± 0.6 |
| MD2 | 0.2 ± 0.04 | 1.6 ± 0.3 |
| NF-κB | 0.8 ± 0.05 | 2.3 ± 0.2 |
| TNF-α | 1 ± 0.4 | 2.6 ± 0.6 |
Human diabetic wounds (n = 8) show significantly higher mRNA/18s ratio compared to non-diabetic wounds (n = 4) (aP < 0.05 vs non-diabetic wounds). TLR: Toll-like receptor.
Figure 1.
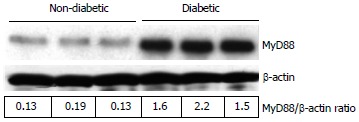
Representative Western blot showing the MyD88 protein expression in non-diabetic and diabetic wound tissues. Wound tissues were collected, lysed and 25 μg protein was blotted for MyD88 and β-actin. Densitometric ratios (MyD88/β-actin) are indicated below. Each lane presents protein from an individual patient wound debridement tissue (n = 3/group).
Figure 2.
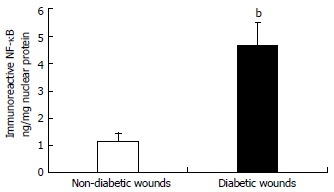
The DNA-binding activity of nuclear nuclear factor-kappa B p65 in wound tissues was determined using ELISA technique. Values are normalized to mg nuclear protein and expressed as mean ± SD. bP < 0.001 vs non-diabetic.
Figure 3.
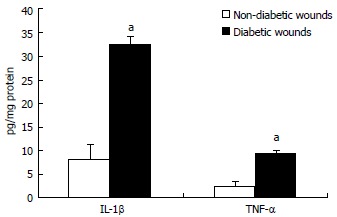
Interleukin-1β and tumor necrosis factor-α concentration in wound tissues were determined by ELISA assay. Values are normalized to mg protein and expressed as mean ± SD. aP < 0.05 vs non-diabetic. IL-1β: Interleukin-1beta; TNF-α: Tumor necrosis factor-alpha.
Figure 4.
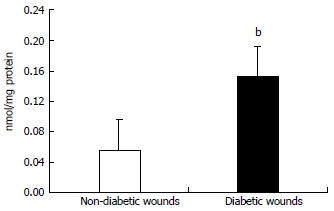
Lipid peroxidation in wound tissue lysates were determined using thiobarbituric acid reactive substances assay as described in Materials and methods. Values are normalized to mg protein and expressed as mean ± SD. bP < 0.01 vs non-diabetic.
DISCUSSION
The interactions among increased glucose levels elevated free fatty acids and resultant proinflammatory cytokines in DM have clear implications for the immune system[14,15]. A diabetic foot ulcer is primarily comprised of keratinocytes, dermal cells, and leukocytes with a coexisting paucity for angiogenesis[16]. All the evidence point towards uncontrolled inflammation and frequent bacterial colonization at the site of injury as the main causes for foot ulcers not healing in a timely manner or not heal at all[7,16]. In addition, chronic diabetic ulcers may also persist due to disrupted formation of granulation tissues and deep tissue necrosis[7,16,17]. Along with cell specific abnormalities, inflammatory cytokine expression such as IL-1β and TNF-α are elevated and sustained by hyperglycemia implying the role of innate immunity[14,18]. TLRs in the wound bed environment play an important role in mediating innate immune functions and inflammation whereby potential healing may be impaired[6,8,9].
Studies in animal models as well as humans have suggested that inflammation is a major contributing factor to DM pathology primarily orchestrated by the innate immune receptors[8-10]. Mohammad et al[19] reported increased TLR2 and TLR4 expression in bone marrow derived macrophages of non-obese diabetic (NOD) mice, correlating with increased NF-κB activation and increased pro-inflammatory cytokines. Kim et al[20] using TLR2-/-, TLR4-/- knockouts, and NOD mice have demonstrated that TLR2 senses beta cell death and contributes to the instigation of autoimmune diabetes. Recently, we showed increased TLR2 and TLR4 expression, intracellular signaling, and TLR2/4 mediated inflammation in monocytes with significant correlation to HbA1c levels in DM patients[21,22]. Creely et al[23] showed increased TLR2 expression in the adipose tissue of type 2 diabetes (T2DM) patients with strong correlates to plasma endotoxin levels. Also, Song et al[24] reported increased TLR4 mRNA expression in differentiating adipose tissue of db/db mice. Furthermore, Davis et al[25] have shown that the TLR4-deficient 10ScN mouse strain fed with diet rich in saturated fat is protected from systemic inflammation. Taken together, these observations suggest a potential role for TLR2 and TLR4 in the pathology of DM. Furthermore, recent findings have shown increased TLR2/4 expression, signaling, ligands, and functional activation in DM subjects with and without complications[20,26]. All the above studies suggest that TLR activation and signaling contribute to the prolonged inflammatory condition seen in DM and may lead to complications in line with our current data.
Functional activation of TLRs includes dimerization and this results in cytokine production. TLR2 requires heterodimerization with TLR1 or TLR6 for activity[27]. We have previously shown that hyperglycemia induces TLR2/TLR6 heterodimerization resulting in cytokine secretion in human monocytes[14] consistent with the increased mRNA expression as seen in this study. However, it is to be noted that characterization of dimerization events in vivo is technically challenging. Besides, we also observed changes in TLR1 mRNA expression and it is not known if either TLR1 or TLR6 by themselves are inflammatory and if TLR2/1 heterodimerization play a role in the peristent inflammation. TLR2 primarily activates MyD88-dependent signaling pathway[28]. The activation of MyD88-dependent signaling pathway leads to the induction of inflammatory cytokines[28]. There are studies showing delayed dermal wound healing in nondiabetic MyD88-deficient mice[29], suggesting that alternate TLR pathways may be active in diabetic milieu (for example, TLR4/MD2). Here, we provide the first evidence, that in human DM wounds, there is increased TLR2 and TLR4 expression, with corresponding increased NF-κB activity, increased expression of downstream adapter proteins such as MyD88 and IRAK-1, resulting in increased local pro-inflammatory cytokines. Similar findings were found when cells were treated in vitro under hyperglycemic, dyslipidemia, and increased oxidative stress conditions[4,6,27]. Thus, we suggest that abrogating inflammation in human DM wounds using TLR2/4 as a target appears to be a reasonable approach to alleviate inflammation accelerating DM wound-healing process.
Collectively, these findings are best valued when recognizing that TLR activation, signaling, and inflammation may be undesirable for proper healing of wounds in DM patients. The limitations of the current study include the lack of correlative evidence between hyperglycemia, duration of diabetes, wound size, and TLR expression due to small sample size. Future and ongoing studies are focussed on collecting sequential wound debridement specimens, infected wound tissues to record the relationship between TLR activation and wound healing as this will aid in establishing the timing of the receptor expression and activation and the relationship between innate immunity and infection in manifesting the impaired wound healing phenotype. At the same time, TLR expression and activation may be used as a cue for healing. Prolonged and exacerbated cytokine production leads to sustained inflammatory responses and impaired healing, causing extensive tissue damage (amputations in case of diabetic wounds). Therefore, it is important to understand local inflammatory mechanisms that might be useful in developing therapeutic strategies for the management of difficult wounds burdened by excessive inflammation. Our findings suggest a role for TLRs in the human DM wound pathology and emphasize the importance of understanding the various pathogenic mechanisms involved in a complicated wound-healing process.
ACKNOWLEDGMENTS
This article grant support by ADA Junior Faculty Award to MRD.
COMMENTS
Background
Toll-like receptors (TLRs) are sentinel pathogen recognition receptors with a pivotal role in inflammation, tissue injury, diabetes and its complications.
Innovations and breakthroughs
Increased TLR expression, signaling, and activation may contribute to the hyper inflammation in the human diabetic wounds.
Peer review
This manuscript is well written and shows results of potential interest.
Footnotes
P- Reviewers: Martin-Villa JM, Slomiany BL, Zhang Q S- Editor: Wen LL L- Editor: A E- Editor: Wu HL
References
- 1. Available from: http://www.cdc.gov/diabetes/pubs/pdf/ndfs_2011.pdf.
- 2.Danaei G, Finucane MM, Lu Y, Singh GM, Cowan MJ, Paciorek CJ, Lin JK, Farzadfar F, Khang YH, Stevens GA, et al. National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2·7 million participants. Lancet. 2011;378:31–40. doi: 10.1016/S0140-6736(11)60679-X. [DOI] [PubMed] [Google Scholar]
- 3.Sen CK, Gordillo GM, Roy S, Kirsner R, Lambert L, Hunt TK, Gottrup F, Gurtner GC, Longaker MT. Human skin wounds: a major and snowballing threat to public health and the economy. Wound Repair Regen. 2009;17:763–771. doi: 10.1111/j.1524-475X.2009.00543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dasu MR, Ramirez S, Isseroff RR. Toll-like receptors and diabetes: a therapeutic perspective. Clin Sci (Lond) 2012;122:203–214. doi: 10.1042/CS20110357. [DOI] [PubMed] [Google Scholar]
- 5.Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 6.Rosa Ramirez S, Ravi Krishna Dasu M. Toll-like receptors and diabetes complications: recent advances. Curr Diabetes Rev. 2012;8:480–488. doi: 10.2174/157339912803529887. [DOI] [PubMed] [Google Scholar]
- 7.Acosta JB, del Barco DG, Vera DC, Savigne W, Lopez-Saura P, Guillen Nieto G, Schultz GS. The pro-inflammatory environment in recalcitrant diabetic foot wounds. Int Wound J. 2008;5:530–539. doi: 10.1111/j.1742-481X.2008.00457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dasu MR, Thangappan RK, Bourgette A, DiPietro LA, Isseroff R, Jialal I. TLR2 expression and signaling-dependent inflammation impair wound healing in diabetic mice. Lab Invest. 2010;90:1628–1636. doi: 10.1038/labinvest.2010.158. [DOI] [PubMed] [Google Scholar]
- 9.Dasu MR, Jialal I. Amelioration in wound healing in diabetic toll-like receptor-4 knockout mice. J Diabetes Complications. 2013;27:417–421. doi: 10.1016/j.jdiacomp.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Devaraj S, Tobias P, Jialal I. Knockout of toll-like receptor-4 attenuates the pro-inflammatory state of diabetes. Cytokine. 2011;55:441–445. doi: 10.1016/j.cyto.2011.03.023. [DOI] [PubMed] [Google Scholar]
- 11.Anderson I. Debridement methods in wound care. Nurs Stand. 2006;20:65–66, 68, 70 passim. doi: 10.7748/ns2006.02.20.24.65.c4077. [DOI] [PubMed] [Google Scholar]
- 12.Jialal I, Devaraj S. Low-density lipoprotein oxidation, antioxidants, and atherosclerosis: a clinical biochemistry perspective. Clin Chem. 1996;42:498–506. [PubMed] [Google Scholar]
- 13.Gill R, Tsung A, Billiar T. Linking oxidative stress to inflammation: Toll-like receptors. Free Radic Biol Med. 2010;48:1121–1132. doi: 10.1016/j.freeradbiomed.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dasu MR, Devaraj S, Zhao L, Hwang DH, Jialal I. High glucose induces toll-like receptor expression in human monocytes: mechanism of activation. Diabetes. 2008;57:3090–3098. doi: 10.2337/db08-0564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schwartz EA, Zhang WY, Karnik SK, Borwege S, Anand VR, Laine PS, Su Y, Reaven PD. Nutrient modification of the innate immune response: a novel mechanism by which saturated fatty acids greatly amplify monocyte inflammation. Arterioscler Thromb Vasc Biol. 2010;30:802–808. doi: 10.1161/ATVBAHA.109.201681. [DOI] [PubMed] [Google Scholar]
- 16.Galkowska H, Wojewodzka U, Olszewski WL. Chemokines, cytokines, and growth factors in keratinocytes and dermal endothelial cells in the margin of chronic diabetic foot ulcers. Wound Repair Regen. 2006;14:558–565. doi: 10.1111/j.1743-6109.2006.00155.x. [DOI] [PubMed] [Google Scholar]
- 17.Eming SA, Krieg T, Davidson JM. Inflammation in wound repair: molecular and cellular mechanisms. J Invest Dermatol. 2007;127:514–525. doi: 10.1038/sj.jid.5700701. [DOI] [PubMed] [Google Scholar]
- 18.Jeffcoate WJ, Game F, Cavanagh PR. The role of proinflammatory cytokines in the cause of neuropathic osteoarthropathy (acute Charcot foot) in diabetes. Lancet. 2005;366:2058–2061. doi: 10.1016/S0140-6736(05)67029-8. [DOI] [PubMed] [Google Scholar]
- 19.Mohammad MK, Morran M, Slotterbeck B, Leaman DW, Sun Y, Grafenstein Hv, Hong SC, McInerney MF. Dysregulated Toll-like receptor expression and signaling in bone marrow-derived macrophages at the onset of diabetes in the non-obese diabetic mouse. Int Immunol. 2006;18:1101–1113. doi: 10.1093/intimm/dxl045. [DOI] [PubMed] [Google Scholar]
- 20.Kim HS, Han MS, Chung KW, Kim S, Kim E, Kim MJ, Jang E, Lee HA, Youn J, Akira S, et al. Toll-like receptor 2 senses beta-cell death and contributes to the initiation of autoimmune diabetes. Immunity. 2007;27:321–333. doi: 10.1016/j.immuni.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 21.Dasu MR, Devaraj S, Park S, Jialal I. Increased toll-like receptor (TLR) activation and TLR ligands in recently diagnosed type 2 diabetic subjects. Diabetes Care. 2010;33:861–868. doi: 10.2337/dc09-1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Devaraj S, Dasu MR, Park SH, Jialal I. Increased levels of ligands of Toll-like receptors 2 and 4 in type 1 diabetes. Diabetologia. 2009;52:1665–1668. doi: 10.1007/s00125-009-1394-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Creely SJ, McTernan PG, Kusminski CM, Fisher fM, Da Silva NF, Khanolkar M, Evans M, Harte AL, Kumar S. Lipopolysaccharide activates an innate immune system response in human adipose tissue in obesity and type 2 diabetes. Am J Physiol Endocrinol Metab. 2007;292:E740–E747. doi: 10.1152/ajpendo.00302.2006. [DOI] [PubMed] [Google Scholar]
- 24.Song MJ, Kim KH, Yoon JM, Kim JB. Activation of Toll-like receptor 4 is associated with insulin resistance in adipocytes. Biochem Biophys Res Commun. 2006;346:739–745. doi: 10.1016/j.bbrc.2006.05.170. [DOI] [PubMed] [Google Scholar]
- 25.Davis JE, Gabler NK, Walker-Daniels J, Spurlock ME. Tlr-4 deficiency selectively protects against obesity induced by diets high in saturated fat. Obesity (Silver Spring) 2008;16:1248–1255. doi: 10.1038/oby.2008.210. [DOI] [PubMed] [Google Scholar]
- 26.Devaraj S, Jialal I, Yun JM, Bremer A. Demonstration of increased toll-like receptor 2 and toll-like receptor 4 expression in monocytes of type 1 diabetes mellitus patients with microvascular complications. Metabolism. 2011;60:256–259. doi: 10.1016/j.metabol.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Drage MG, Pecora ND, Hise AG, Febbraio M, Silverstein RL, Golenbock DT, Boom WH, Harding CV. TLR2 and its co-receptors determine responses of macrophages and dendritic cells to lipoproteins of Mycobacterium tuberculosis. Cell Immunol. 2009;258:29–37. doi: 10.1016/j.cellimm.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kawai T, Akira S. TLR signaling. Cell Death Differ. 2006;13:816–825. doi: 10.1038/sj.cdd.4401850. [DOI] [PubMed] [Google Scholar]
- 29.Macedo L, Pinhal-Enfield G, Alshits V, Elson G, Cronstein BN, Leibovich SJ. Wound healing is impaired in MyD88-deficient mice: a role for MyD88 in the regulation of wound healing by adenosine A2A receptors. Am J Pathol. 2007;171:1774–1788. doi: 10.2353/ajpath.2007.061048. [DOI] [PMC free article] [PubMed] [Google Scholar]


