Abstract
Malaria is a global public health problem, especially in sub-Saharan Africa, where the mosquito Anopheles gambiae Giles serves as the major vector for the protozoan Plasmodium falciparum Welch. One determinant of malaria vector competence is the mosquito's immune system. Hemocytes are a critical component as they produce soluble immune factors that either support or prevent malaria parasite development. However, despite their importance in vector competence, understanding of their basic biology is just developing. Applying novel technologies to the study of mosquito hemocytes, we investigated the effect of blood meal on hemocyte population dynamics, DNA replication and cell cycle progression. In contrast to prevailing published work, the data presented here demonstrate that hemocytes in adult mosquitoes continue to undergo low basal levels of replication. In addition, blood ingestion caused significant changes in hemocytes within 24 h. Hemocytes displayed an increase in cell number, size, granularity and Ras-MAPK signaling as well as altered cell surface moieties. As these changes are well-known markers of immune cell activation in mammals and Drosophila melanogaster Meigen, we further investigated whether a blood meal changes the expression of hemocyte-derived immune factors. Indeed, hemocytes 24 h post-blood meal displayed higher levels of critical components of the complement and melanization immune reactions in mosquitoes. Taken together, this study demonstrates that the normal physiological process of a blood meal activates the innate immune response in mosquitoes. This process is likely in part regulated by Ras-MAPK signaling, highlighting a novel mechanistic link between blood feeding and immunity.
KEY WORDS: Infectious disease, Innate immunity, Immunology, Phenoloxidase, Blood meal
INTRODUCTION
The female Anopheles gambiae mosquito is the major vector of malaria in sub-Saharan Africa. One of the most important determinants of vector competence is the mosquito's humoral immune system (Mitri and Vernick, 2012), which includes the production of reactive oxygen species, melanin synthesis and complement activation. The cellular arm of the mosquito immune system is represented by hemocytes, which are the insect's equivalent to the myeloblast lineage of blood cells. These cells perform phagocytosis and encapsulation, produce free radicals of nitrogen and oxygen, and express the majority of molecules in the melanization and complement-like pathways including prophenoloxidase (PPO) and the thioester-containing protein (TEP)-1, respectively (Hillyer, 2010). In addition, studies suggest hemocytes are recruited to the midgut and dorsal vessel during Plasmodium sp. infection (Volz et al., 2005; Rodrigues et al., 2010; King and Hillyer, 2012), and may mediate innate ‘immune memory’ (Rodrigues et al., 2010). However, despite the multi-faceted contribution of mosquito hemocytes to immunity against malaria parasites, little is known about their basic biology.
Some mosquito hemocytes circulate freely in the hemocoel (Hall, 1983), while others are attached to a variety of tissues including midgut, trachea, muscles, dorsal vessel, Malpighian tubules, cephalic limbs and maxillary palps (Barillas-Mury et al., 1999; Danielli et al., 2000; King and Hillyer, 2012; King and Hillyer, 2013). While true cell lineages have yet to be established, mosquito hemocytes are classified based on morphological and functional characteristics. Granulocytes represent 90% of the adult hemocyte population and are classified based on their granular appearance and their exclusive ability to spread on glass surfaces and phagocytize particles (Castillo et al., 2006). The remaining 10% are split equally into oenocytoids, which express factors required for melanization, and prohemocytes, which are believed to represent progenitor cells (Castillo et al., 2006). While generally it has been accepted that only 500–1000 hemocytes reside in an adult mosquito (reviewed in Hillyer, 2010), a recent study with improved in vivo imaging techniques showed this number to be ~5000 cells per mosquito 2 days post-eclosion (King and Hillyer, 2013). Mosquitoes kept on sugar water exhibited a gradual decrease in hemocyte numbers (Hillyer et al., 2005).
Numerous studies in multiple insects clearly demonstrate the strong correlation between hemocyte numbers and immunity. Drosophila melanogaster larvae parasitized by the parasitic wasp Leptopilina boulardi (Barbotin, Carton and Kelner-Pillault) show an increase in the total number of hemocytes (Russo et al., 2001). Further, distinct genetic lines of D. melanogaster resistant to parasitoid wasps have almost twice the number of hemocytes as susceptible lines (Kraaijeveld et al., 2001). In tsetse flies, removal of their microbiota affects vector competence by decreasing hemocyte numbers, ultimately decreasing expression of important immune factors (Weiss et al., 2011; Weiss et al., 2012). Additionally, inoculation with Dirofilaria immitis (Leidy) microfilariae and Escherichia coli (Migula) results in hemocyte proliferation in Aedes aegypti L. and A. gambiae, respectively (Christensen et al., 1989; King and Hillyer, 2013). In contrast, infection with the rodent malaria parasite Plasmodium berghei Fain does not affect overall hemocyte number (Baton et al., 2009), but induces a number of changes. These include a relative increase in granulocytes compared with oenocytoids and prohemocytes (Baton et al., 2009; Rodrigues et al., 2010), TEP1 secretion (Frolet et al., 2006), changes in lectin binding (Rodrigues et al., 2010), changes in expression patterns (Pinto et al., 2009), and hemocyte aggregation near the ostia of the dorsal vessel of the mosquito (King and Hillyer, 2012). In the absence of infection, some studies show a blood meal induces an increase in circulating hemocyte numbers in A. gambiae and A. aegypti (Castillo et al., 2006; Baton et al., 2009). However, the consequences of a blood meal on different aspects of hemocyte biology have not been addressed.
The purpose of the present study was to determine whether the physiological event of a blood meal causes critical changes in A. gambiae hemocytes. Several highly conserved immune cell activation markers are detectable in hemocytes isolated from female mosquitoes 24 h after blood feeding. In addition, we found upregulation of immune factors critical for the complement-like pathway and the melanization response, demonstrating that a blood meal activates the immune response in mosquitoes.
RESULTS
Blood meal induces hemocyte proliferation
To analyze hemocyte proliferation in A. gambiae we initially determined total hemocyte numbers using an established perfusion protocol (Castillo et al., 2006; Rodrigues et al., 2010). Hemocytes were collected from female mosquitoes that either were maintained on sugar solution or had received a blood meal. Similar to the majority of previous reports in different mosquito species (Christensen et al., 1989; Hillyer et al., 2005; Castillo et al., 2006; Baton et al., 2009; Hillyer, 2010), we consistently observed hemocyte numbers in the range ~500–2500 cells per mosquito. In agreement with published data (Castillo et al., 2006; Baton et al., 2009), hemocyte numbers increased consistently and significantly by 2.1-fold, 24 h post-blood meal (pbm) (U-test, P=0.0058, Fig. 1A).
Fig. 1.
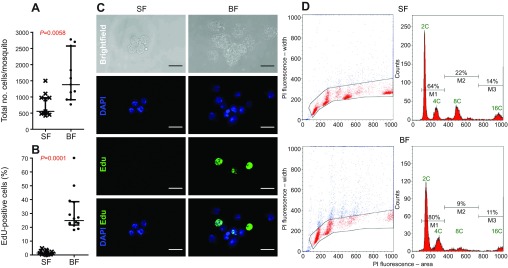
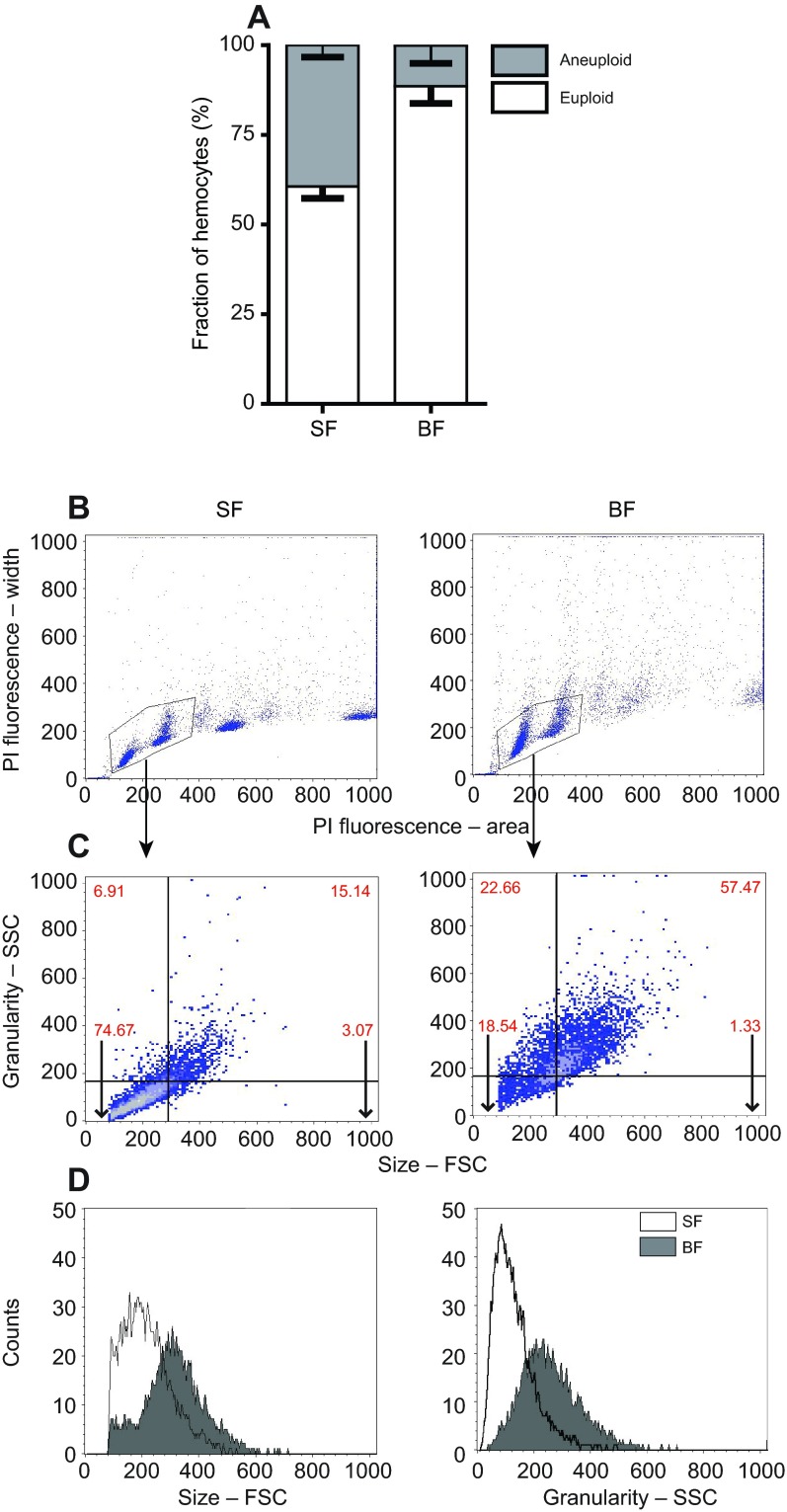
Blood feeding induces hemocyte proliferation. (A) Hemocyte numbers are increased significantly in blood-fed (BF) compared with sugar-fed (SF) mosquitoes (Mann–Whitney U-test). N=11 for each group from two independent biological replicates, shown as median with interquartile range. (B) EdU incorporation is increased significantly in hemocytes from blood-fed females (Mann–Whitney U-test). N=14 for each group, shown as median with interquartile range. For each data point, 300–400 cells were assessed from a hemocyte pool collected from two mosquitoes. (C) Confocal images of representative EdU-positive hemocytes. Blue, DAPI; green, EdU; scale bar, 10 μm. (D) Flow cytometry analysis of PI-stained hemocytes from sugar-fed and blood-fed mosquitoes. Dot plots of propidium iodide (PI) fluorescence signal area over width (expressed in arbitrary units) illustrate DNA content per cell. Gates were drawn to eliminate putative aggregates based on high signal width from further analysis. Histograms show measurements for PI staining and thus DNA content for the gated cells. Markers designate three hemocyte populations based on DNA content, M1 representing euploid, M2 and M3 representing aneuploid cells. Percentages of cells within the three markers are indicated above the brackets. The figure shows a representative result from three independent biological replicates.
To determine whether the increase in hemocyte numbers is due to cell division or remobilization of sessile hemocytes, we assayed DNA replication in hemocytes by monitoring EdU (5-ethynyl-2′-deoxyuridine) incorporation into DNA. Sugar-fed and 20 h pbm mosquitoes were injected with EdU, and their hemocytes were harvested 4 h post-injection. This experimental setup allowed us to label hemocytes for 4 h at the time of proposed hemocyte cell division for the blood-fed group and determine basal levels of DNA replication in the sugar-fed group. The fluorescence signal due to EdU incorporation was striking and easily detected in perfused hemocytes by fluorescence microscopy (Fig. 1C). The percentage of EdU-positive hemocytes obtained from sugar-fed mosquitoes was low, with a median of 1.45% (Fig. 1B). This number increased to 26.2% in hemocytes obtained 24 h pbm, resulting in an 18-fold increase in EdU-positive cells (U-test P<0.0001, Fig. 1B). These data suggest that the increase of hemocytes after a blood meal (Fig. 1A) is due to cell division rather than remobilization of sessile hemocytes.
To determine whether EdU incorporation in hemocytes is a result of mitotic activity and not increasing ploidy levels due to endoreplication, we employed cell cycle analysis by flow cytometry. Propidium iodide (PI) staining was used to analyze the DNA content of hemocyte populations from either sugar-fed or blood-fed mosquitoes. PI staining was displayed as a fluorescence signal in area versus width (Fig. 1D). Cell aggregates were excluded by gating (Givan, 2001), and histograms of distinct cell populations were obtained (Fig. 1D). Experiments were performed in triplicate, and for each treatment group and sample, the fixed number of 20,000 events was counted. Thus, the data represent the relative number of cells and not absolute values. Both treatment groups contained distinct hemocyte populations with varying DNA content ranging from 2C to 16C. Three markers (M1–M3) were placed to delineate these populations based on their ploidy levels, and to determine the percentage of cells within these populations. M1 contained euploid cells within different phases of the canonical cell cycle, defined by the characteristic G0/G1 (2C) and G2 (4C) peaks, with S phase in between. The other two markers highlight distinct populations of aneuploid cells with peaks at 8C (M2) or 16C (M3). In sugar-fed and blood-fed mosquitoes, euploid cells represented the dominant hemocyte population (Fig. 1D, Fig. 2A). In three experimental replicates, an average of 37.8% of hemocytes in sugar-fed mosquitoes were aneuploid, having either an 8C or 16C DNA content. However, in blood-fed mosquitoes, on average only 11.3% of hemocytes were aneuploid, marking a 28% relative increase in euploid cells in blood-fed mosquitoes (Fig. 2A; Fisher's exact test, P<0.0001). Therefore, the increase in EdU incorporation in hemocytes from blood-fed mosquitoes was not due to endoreplication and an increase in aneuploid cells but, rather, was a result of higher mitotic activity in the euploid cell population.
Fig. 2.
Blood feeding induces changes in hemocyte population and morphology. (A) The percentage of euploid and aneuploid cells obtained from the flow cytometry analyses of PI-stained hemocytes (means ± s.e.m.). (B) Euploid cells were gated and analyzed for their size (FSC) and granularity (SSC). Axes are shown in arbitrary units. (C) Density dot plots illustrating size and granularity (expressed as arbitrary units) and their intensity on a blue to white color scale. (D) Overlaying histograms of sugar-fed and blood-fed euploid hemocytes revealing an increase in average cell size and granularity after a blood meal. Data shown are representative of three independent experiments.
Blood meal causes an increase in hemocyte size and granularity
The flow cytometry analyses indicated that cell aggregation was also more prominent in hemocytes isolated from blood-fed mosquitoes. In the dot plots shown in Fig. 1D, hemocytes from sugar-fed mosquitoes fell within the gate, with few cell aggregates outside the gate. In contrast, hemocytes from blood-fed mosquitoes formed large cell aggregates as indicated by the blue dots outside the gate. To investigate these and other potential phenotypic changes in hemocytes after a blood meal, size and granularity properties were assessed. For this analysis, we focused solely on the gated euploid cell population, as they represented the major cell type based on DNA content (Fig. 1D, Fig. 2A).
Backgating analysis revealed the effects of a blood meal on both the size and granularity of hemocytes. In density dot plots, nearly 75% of all circulating hemocytes from sugar-fed mosquitoes were found in the lower left quadrant (small size, low granularity) compared with only 18.5% of hemocytes from blood-fed mosquitoes (Fig. 2B,C). Nearly 60% of hemocytes from blood-fed mosquitoes were found in the upper right quadrant, indicating a larger cell size and increased granularity. This was confirmed by overlaying histograms of either size (forward scatter, FSC) or granularity (side scatter, SSC) (Fig. 2D). Hemocytes from blood-fed mosquitoes showed a significantly increased average and range of size and granularity compared with sugar-fed mosquitoes (Fig. 2D). The combined flow cytometry data clearly demonstrate that a blood meal causes significant changes in morphology of the euploid population of hemocytes.
Blood meal activates hemocytes
The increase in cell number, aggregation, DNA replication, cell size and granularity of hemocytes after a blood meal is highly reminiscent of classical blood cell activation markers used in early immunological studies of mammalian leukocytes (Oppenheim and Rosenstreich, 1976). Based on these findings, we assessed additional potential molecular changes that have been linked to blood cell activation in insects.
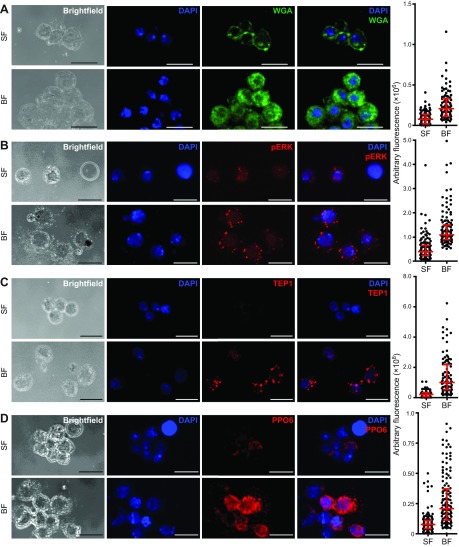
Infection of D. melanogaster larvae by parasitic wasps, an established invertebrate model for blood cell activation, is characterized by increased cell aggregation, wheat germ agglutinin (WGA) lectin binding and Ras-mitogen activated protein kinase (MAPK) signaling. We asked whether a blood meal induces similar changes in A. gambiae. Hemocytes from sugar-fed mosquitoes exhibited low WGA binding, while cells from blood-fed individuals exhibited a much stronger binding signal (Fig. 3A), demonstrating blood meal-induced changes in surface carbohydrate moieties.
Fig. 3.
Blood feeding increases expression of several cell activation markers. Hemocytes from sugar-fed and blood-fed females were analyzed for blood meal-induced activation markers. N=91 for WGA (A), N=126 for pERK (B), N=103 for TEP-1 (C) and N=136 for PPO6 (D). Confocal maximum intensity projections are shown for all stains: blue, DAPI; green, WGA; red, pERK (B), TEP-1 (C) and PPO6 (D). Scale bar, 10 μm. Quantification of activation markers is shown as median with interquartile range. Blood feeding led to a significant increase in fluorescence for all immunofluorescence analyses (Mann–Whitney U-test, P<0.0001). Experiments were performed in triplicate with one representative experiment image and graph shown for each hemocyte activation marker. Results from all replicates are shown in supplementary material Fig. S2.
Phosphorylated ERK, a commonly used and highly conserved marker for Ras-MAPK signaling (pAgERK, AGAP009207), was readily detected in hemocytes isolated from adult female A. gambiae. Immunofluorescence analysis (IFA) showed punctate staining around the perimeter of nuclei as well as diffuse signals within nuclei in hemocytes isolated from sugar-fed mosquitoes (Fig. 3B). Stronger fluorescence signals of pERK were consistently observed in hemocytes isolated 24 h pbm (Fig. 3B), clearly illustrating elevated Ras-MAPK signaling in hemocytes after a blood meal.
In A. gambiae, hemocytes are the only source of several immune factors critical for complement and melanization, including TEP1 and PPO. To determine whether expression of these immune factors was altered by a blood meal, IFA was performed using anti-TEP1- and anti-PPO6-specific antibodies. TEP1 signals were low or undetectable in hemocytes from sugar-fed females, and significantly increased after a blood meal, resulting in punctate/granular staining (Fig. 3C). PPO6 antibody yielded low punctate staining in hemocytes from sugar-fed females, which increased after a blood meal (Fig. 3D). At the same time, the blood meal did not significantly alter global protein production, as measured by incorporation and detection of a methionine analog (supplementary material Fig. S1).
The increase of WGA binding, pERK signal, and TEP1 and PPO6 expression 24 h after a blood meal was highly reproducible (supplementary material Fig. S2) and statistically significant, as compared with control treatments (U-test, P<0.0001, Fig. 3).
DISCUSSION
The innate immune response of the female mosquito is a major obstacle faced by malaria parasites while traveling through and developing in their obligate vector. Within the first 18–48 h after arrival within the mosquito, ookinetes, the motile zygote life stage of the parasite, encounter epithelial immune responses, characterized by the production of reactive oxygen and nitrogen species (Luckhart et al., 1998; Han et al., 2000; Oliveira et al., 2012). At the same time, the mosquito's complement-like system, characterized by TEP1 and leucine-rich repeat proteins, binds to the ookinete surface and ultimately kills and lyses the parasite (Blandin et al., 2004; Fraiture et al., 2009; Povelones et al., 2009). In certain genetic backgrounds, melanization, the production and deposition of eumelanin on the surface of the parasite, further decreases the ookinete population within the first 48 h of infection. In contrast, cellular encapsulation of malaria parasites, a classical anti-parasitic cellular immune response in insects, does not occur. A second cellular immune response, phagocytosis of malaria parasites, has been observed after 10 days post-infection, but does not reduce significantly the parasite population (Hillyer et al., 2007). However, hemocytes contribute significantly to anti-malarial immunity (Ramirez et al., 2013), and they are the only source of many of the critical anti-parasitic humoral immune factors, including phenoloxidase (Müller et al., 1999) and TEP1 (Frolet et al., 2006).
Malaria parasite infection induces a number of significant molecular changes in hemocytes (Baton et al., 2009; Pinto et al., 2009; Rodrigues et al., 2010), which limit parasite development (Pinto et al., 2009; Ramirez et al., 2013). However, all studies to date have evaluated hemocyte responses to infection as compared with a non-infectious blood meal. Given that a blood meal alone increases hemocyte numbers circulating in the hemolymph of A. gambiae (Castillo et al., 2006; Baton et al., 2009; Castillo et al., 2011), focusing on changes induced by infection alone may thus underestimate the contribution of hemocytes to anti-parasitic immunity. Anopheles gambiae females are anautogenous, and must take a blood meal to produce eggs to complete their life cycle. Simultaneously, mosquitoes can encounter many distinct blood-borne pathogens, whose infection they have to overcome for at least the next 2–3 days in order to lay their eggs. Therefore, survival for 48–72 h after blood ingestion is critical to the fitness of the species. We therefore set out to evaluate the putative role of a blood meal on hemocyte stimulation and thus immune system activation.
Previous studies have indicated that hemocytes in circulation increase after a blood meal without determining whether they were dividing or whether sessile hemocytes were detaching (Castillo et al., 2006; Baton et al., 2009; Castillo et al., 2011). The base analog incorporation and flow cytometry data obtained in this study illustrate clearly that the increase in hemocytes after a blood meal was due to mitosis instead of detachment of sessile hemocyte populations. The presence of binucleated hemocytes undergoing mitosis in adult mosquitoes has previously only be demonstrated after bacterial infection (King and Hillyer, 2013). The EdU incorporation assay, which was established in this current study and can be used as a proxy for hemocyte proliferation, proved not only more convenient but also 10-fold more sensitive with a significantly wider dynamic range compared with established cell counting methodologies (Castillo et al., 2006). Surprisingly, flow cytometry analyses also revealed that up to 30% of hemocytes in naive mosquitoes were aneuploid, with DNA content at or above 8C. Aneuploid blood cells are well known in mammals, e.g. megakaryocytes undergo endoreplication upon activation in mice, rats and humans (reviewed in Lee et al., 2009). Aneuploid hemocytes in insects have been reported in Manduca sexta (L.) (Nardi et al., 2003); however, to our knowledge, this is the first description of their existence in dipterans. Future studies will evaluate how DNA content complements existing morphological classifications and how these criteria can be combined to discriminate hemocyte sub-populations.
As hypothesized, the blood meal proved to induce significant changes in A. gambiae hemocytes. The ratio of euploid to aneuploid cells increased significantly, suggesting that cell division of the euploid cell population was stimulated rather than endoreplication. Similarly, changes in the relative abundance of different hemocyte populations have been reported after bacterial and parasite infection in different mosquito species (Christensen et al., 1989; Rodrigues et al., 2010; King and Hillyer, 2013). The only other vector species for which changes in hemocyte populations due to a blood meal have been observed is the kissing bug Rhodnius prolixus Stål, an important vector of Chagas disease (Jones, 1967).
In D. melanogaster, parasitoid wasp infection induces a number of significant changes in larval hemocytes that are required for an encapsulation response. Hemocytes differentiate and increase in numbers in response to the presence of parasitoid wasp eggs, ultimately resulting in hemocyte activation (Russo et al., 2001). Consequently, hemocytes aggregate around the wasp egg to form a tight capsule around the parasite (Nappi and Streams, 1969). This cell adhesion is mediated by significant molecular changes on the hemocyte's cell surface, which is also demonstrated by increased WGA lectin binding (Nappi and Silvers, 1984; Mortimer et al., 2012). Our study shows that blood meal ingestion causes strikingly similar changes in mosquito hemocytes. Not only did the number of A. gambiae hemocytes double but also WGA binding and the propensity of hemocytes to form aggregates increased significantly. These data strongly suggest that a blood meal indeed induces hemocyte activation in mosquitoes and thus elicits an immune response. This is further supported by our observation that TEP1 and PPO6 protein abundance increased significantly in hemocytes isolated from blood-fed mosquitoes. The immune-fluorescence analyses revealed punctuate staining patterns, at least partially explaining the more granular appearance of hemocytes isolated from blood-fed mosquitoes as compared with sugar-fed controls.
The blood meal-induced hemocyte activation data presented here also draw strong parallels to mammalian blood cell activation. Upon activation, mammalian blood cells undergo mitotic replication (Oppenheim and Rosenstreich, 1976), increase in size and granularity (Cohn and Benson, 1965; Cook et al., 2004), and upregulate vital immune factors (Bellingan, 1999; Wynn et al., 2013). The molecular mechanisms underlying blood cell activation are complex and include a number of highly conserved signaling pathways. For example, activation of macrophages can be stimulated by the granulocyte-macrophage colony stimulating factor, which binds to a receptor tyrosine kinase and signals through the Ras-MAPK pathway (Cook et al., 2004). The same pathway also plays a critical role in lymphocyte activation (Downward et al., 1990; Cantrell, 2003). In D. melanogaster, Ras-MAPK signaling is activated in hemocytes after parasitic wasp infection (Sinenko et al., 2012) and plays a vital role in hemocyte homeostasis (Zettervall et al., 2004). Thus, it was not surprising that we observed a consistent and statistically significant increase in Ras-MAPK signaling in mosquito hemocytes after a blood meal. Ras-MAPK signaling is likely not the only pathway required for hemocyte proliferation and differentiation. Other likely candidates are the JAK/STAT, Jun kinase and Toll pathways that control these processes in D. melanogaster (Zettervall et al., 2004). In addition, insulin signaling has recently been implicated in hemocyte proliferation after a blood meal in the yellow fever mosquito, A. aegypti (Castillo et al., 2011). Another candidate is 20-hydroxyecdysone (20-E), which circulates at increased levels in the mosquito's hemolymph after a blood meal (Clements, 2000; Bai et al., 2010). Expression of PPO6 is increased by elevated levels of 20-E in vitro (Müller et al., 1999), suggesting that ecdysone signaling at least partially regulates blood meal-stimulated immune factor expression. Studies are underway to identify the role of these signaling pathways in hemocyte proliferation and activation.
Taken together, this study identifies a blood meal as a significant immune system activator in A. gambiae. The factors that are upregulated upon blood ingestion have broad anti-pathogenic activity. TEP-1 and PPO can limit parasite and bacterial infections (Levashina et al., 2001; Blandin et al., 2004; Volz et al., 2006; Schnitger et al., 2007; Fraiture et al., 2009; Povelones et al., 2009), and PPO further aids in the defense against fungi (Yassine et al., 2012), and filarial worms (Guo et al., 1995). This suggests that the mosquito's immune system is primed to act against a broad range of putative pathogens that may be encountered in a blood meal. Furthermore, we provide a new mechanistic link between blood meal and immunity, which enables future molecular studies on trade-offs between mosquito immunity and fecundity.
MATERIALS AND METHODS
Mosquito rearing and maintenance
The A. gambiae G3 strain was reared according to our standard protocol (An et al., 2011). Mosquitoes were starved for ~6–12 h before blood feeding. Heparinized horse blood (Plasvacc, Templeton, CA, USA) was provided through an artificial membrane feeding system.
Hemocyte collection and counts
Hemocytes were collected by perfusion using a modified protocol (Castillo et al., 2006; Rodrigues et al., 2010). Mosquitoes were injected with ~276 nl anticoagulant buffer [60% of Schneider medium, 10% FBS, 30% citrate buffer (98 mmol l−1 NaOH, 186 mmol l−1 NaCl, 1.7 mmol l−1 EDTA, 41 mmol l−1 citrate), 1 μmol l−1 DAPI] using a Nanoject II system (Drummond Scientific Company, Broomall, PA, USA) and incubated on ice for 10–15 min. Using forceps, a small tear was made in the penultimate abdominal segment and ~6–10 μl of anticoagulant buffer was perfused through the hemocoel at a rate of 1 μl per dispension using a Hamilton syringe system (see supplementary material Fig. S3). The perfused hemolymph was loaded immediately into disposable hemocytometers (Incyto, Chungnam-do, Korea) to determine hemocyte numbers following the manufacturer's instructions.
EdU incorporation assay
EdU assays were performed with the Click-iT EdU Alexa Fluor 488 Imaging kit (Invitrogen, Grand Island, NY, USA) following the manufacturer's instructions. Mosquitoes were injected with 138 nl of 20 mmol l−1 EdU in PBS and allowed to recover under normal rearing conditions. Four hours after injection, hemocytes were collected by perfusion (see above) onto PTFE printed glass slides (Electron Microscopy Sciences, Hatfield, PA, USA). After 1 h incubation at 4°C, perfusion buffer was replaced with fixative (4% formaldehyde in PBS), and incubated for 15 min at room temperature (RT). Cells were washed twice with 3% BSA in PBS, and permeabilized in 0.5% Triton X-100 in PBS for 20 min. Subsequently, cells were washed twice with 3% BSA in PBS, incubated in the dark with the Click-iT Reaction Cocktail for 30 min at RT, and washed again with 3% BSA in PBS. Cells were mounted in VectaShield medium (Vector Laboratories Inc., Burlingame, CA, USA); slides were sealed with nail polish and stored at 4°C. EdU incorporation was determined by fluorescence microscopy using an Axioplan2 fluorescent light microscope (Zeiss, Jena, Germany), and expressed as fraction of positive cells in a pool of at least 300–400 hemocytes.
Cell cycle analysis by flow cytometry
Hemocytes were collected into anticoagulant buffer without DAPI by perfusion as described above. Hemocytes were pooled from 60–75 mosquitoes. Each pool was collected on ice within 1 h and immediately centrifuged at 2350 g at 4°C. Cells were resuspended in 200 μl PBS with 0.1% FBS; 700 μl of 70% ethanol was added drop-wise to the cells and incubated for 1 h at RT. Cells were centrifuged again at 2350 g at 4°C and resuspended in 200 μl of PI solution (50 μg ml−1 propidium iodide, 100 μg ml−1 RNaseA, 0.1% Triton X-100, 0.1 mmol l−1 EDTA in PBS). Cells were incubated for at least 1 h at 4°C, and pushed through a 40 μm nylon filter (Becton Dickinson Falcon, San Jose, CA, USA) to remove large cell aggregates. Cells were analyzed with a FACSCalibur cytometer (Becton Dickinson, Franklin Lakes, NJ, USA), obtaining 20,000 events per sample at a rate not exceeding 150 events s−1. Data were analyzed using WinList software (Verity Software House, Topsham, ME, USA). Events were gated and their corresponding histograms were obtained. Additionally, cell populations with differences in DNA content were backgated to determine size (FSC-H) and granularity (SSC-H). To backgate cells, first a dot plot based on PI (DNA) signal was drawn up for both sugar- and blood-fed mosquitoes. Gated populations of interest were analyzed for size (FSC-H) and granularity (SSC-H) in density dot plots and overlaying histograms.
IFA
Hemocytes from two mosquitoes were collected and pooled onto PTFE-printed glass slides (Electron Microscopy Sciences) as described above. Cells were incubated at 4°C for 1 h then fixed with 4% formaldehyde for 15 min at room temperature. Cells were incubated with blocking buffer (5% BSA, 0.3% Triton X-100 with PBS as diluent) for 1 h at RT. Cells were exposed to primary antibodies in antibody dilution buffer (1% BSA, 0.3% Triton X-100 in PBS) overnight at 4°C. The following primary antibodies were used at the indicated dilutions: rabbit anti-TEP-1, 1:350 (Povelones et al., 2009); rat anti-PPO6, 1:1000 (Müller et al., 1999); rabbit anti-phospho-p44/42 MAPK (Erk1/2) (Thr202/Tyr204), 1:30 (cat. no. 4370, Cell Signaling, Boston, MA, USA). Cells were washed in PBS three times, and incubated with secondary antibody in antibody dilution buffer in the dark for 1–2 h at RT. The following secondary antibodies were used at the indicated dilutions: IgG (H + L) Alexa Fluor 594 (Invitrogen) goat anti-rabbit at 1:500 (TEP1) or 1:100 (pERK); IgG (H + L) Alexa Fluor 594 (Invitrogen) goat anti-rat at 1:1000 (PPO6). Cells were rinsed in PBS three times and mounted in Vectashield (Vector Laboratories, Burlingame, CA, USA). Slides were sealed using nail polish and stored at 4°C until further analysis.
Lectin staining
To determine lectin staining of hemocytes, we followed protocols described previously (Rizki and Rizki, 1983). Hemocytes from two mosquitoes were collected by perfusion and pooled onto PTFE printed glass slides (Electron Microscopy Sciences) as described above. Cells were incubated at 4°C for 1 h, and washed with PBS. Cells were fixed with 4% formaldehyde in PBS for 15 min at room temperature. Fixative was removed and cells were washed twice with PBS. Cells were stained with 10 μg ml−1 of WGA (Vector Laboratories) in PBS for 10 min at room temperature in the dark. Cells were subsequently washed twice with PBS containing 0.3% Triton X-100 to remove unbound lectin, and mounted in Vectashield (Vector Laboratories). Slides were sealed with nail polish and stored at 4°C until further analysis.
Metabolic protein labeling using fluorescent non-canonical amino acid tagging (FUNCAT)
To quantify total protein production in hemocytes in sugar-fed and blood-fed mosquitoes, we labeled proteins through incorporation of the amino acid analog l-azidohomoalanine (AHA) in vivo and detected the incorporated azide by click-it chemistry (Hinz et al., 2012). FUNCAT assays were performed with the Click-iT AHA Alexa Fluor 488 Protein Synthesis Assay (Invitrogen) following the manufacturer's instructions. Mosquitoes were injected with 138 nl of 2.5 mmol l−1 AHA in PBS and allowed to recover under normal rearing conditions. As expected, signal intensity was strongly time dependent (supplementary material Fig. S1). Virtually no AHA incorporation was detectable at 10 min after injection, while a strong signal was observed at 4 h post-injection. All subsequent assays were thus performed using a 4 h labeling period. Hemocytes were collected, fixed and processed as described above for the EdU assays and according to the manufacturer's protocol. Cells were mounted in VectaShield medium (Vector Laboratories); slides were sealed with nail polish, and stored at 4°C until further analysis.
Quantification of immunofluorescence, lectin and AHA staining
To quantify pERK, TEP1, PPO6, WGA and AHA signals of hemocytes from sugar-fed and blood-fed mosquitoes, TIFF images were obtained with an Axioplan2 fluorescent light microscope (Zeiss) equipped with a camera and processed using ImageJ imaging software (http://rsb.info.nih.gov/ij/). All images were taken with identical magnification. In addition, optimal fluorescence intensities and exposure times were empirically determined for each marker and kept constant between the two treatment groups. TIFF files were imported into ImageJ, where circles were drawn around cells and raw intensity values were obtained. To determine background fluorescence, the same circle was used to measure raw intensity values of a blank space in the image, which was subtracted from foreground values. Between 69 and 180 cells were analyzed per biological replicate and treatment group. Experiments were performed in triplicate with three independent biological replicates (supplementary material Figs S1, S2).
Confocal microscopy and image analysis
Representative images were obtained using a LSM700 Confocal Microscope (Zeiss) using identical laser and microscope settings between samples. Images were processed using ZEN 2010 software (Zeiss), and figures were prepared with Photoshop and Illustrator software (Adobe Systems, San Jose, CA, USA).
Supplementary Material
ACKNOWLEDGEMENTS
We thank members of the Michel lab for mosquito rearing. We thank Drs R. Clem and M. Herman (Kansas State University, USA) for use of their microscopes. We further are grateful to Dr Philine Wangemann and Mr Joel Sanneman at the Confocal Microfluorometry & Microscopy, and Molecular Biology & Biochemistry Cores at Kansas State University, T. Koopman and the Fleming Lab at Kansas State University for flow cytometry assistance, and Drs L. Garver and C. Barillas-Mury (NIH-NIAID) for hemocyte perfusion and counting protocols. In addition, we thank Drs M. Povelones and G. Christophides (Imperial College London, UK) for the kind gift of the TEP1 antibody, and Dr H.-M. Mueller (University of Heidelberg, Germany) for the PPO6 antibody.
FOOTNOTES
Competing interests
The authors declare no competing financial interests.
Funding
This study was supported by the National Institutes of Health [P20-RR017686, R01-AI095842 to K.M., F32-AI104154 to W.B.B.]. This is contribution no. 13-167-J from the Kansas Agricultural Experiment Station. Deposited in PMC for release after 12 months.
Supplementary material
Supplementary material available online at http://jeb.biologists.org/lookup/suppl/doi:10.1242/jeb.094573/-/DC1
References
- An C., Budd A., Kanost M. R., Michel K. (2011). Characterization of a regulatory unit that controls melanization and affects longevity of mosquitoes. Cell. Mol. Life Sci. 68, 1929-1939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai H., Gelman D. B., Palli S. R. (2010). Mode of action of methoprene in affecting female reproduction in the African malaria mosquito, Anopheles gambiae. Pest Manag. Sci. 66, 936-943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barillas-Mury C., Han Y. S., Seeley D., Kafatos F. C. (1999). Anopheles gambiae Ag-STAT, a new insect member of the STAT family, is activated in response to bacterial infection. EMBO J. 18, 959-967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baton L. A., Robertson A., Warr E., Strand M. R., Dimopoulos G. (2009). Genome-wide transcriptomic profiling of Anopheles gambiae hemocytes reveals pathogen-specific signatures upon bacterial challenge and Plasmodium berghei infection. BMC Genomics 10, 257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellingan G. (1999). Inflammatory cell activation in sepsis. Br. Med. Bull. 55, 12-29 [DOI] [PubMed] [Google Scholar]
- Blandin S., Shiao S. H., Moita L. F., Janse C. J., Waters A. P., Kafatos F. C., Levashina E. A. (2004). Complement-like protein TEP1 is a determinant of vectorial capacity in the malaria vector Anopheles gambiae. Cell 116, 661-670 [DOI] [PubMed] [Google Scholar]
- Cantrell D. A. (2003). GTPases and T cell activation. Immunol. Rev. 192, 122-130 [DOI] [PubMed] [Google Scholar]
- Castillo J. C., Robertson A. E., Strand M. R. (2006). Characterization of hemocytes from the mosquitoes Anopheles gambiae and Aedes aegypti. Insect Biochem. Mol. Biol. 36, 891-903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo J., Brown M. R., Strand M. R. (2011). Blood feeding and insulin-like peptide 3 stimulate proliferation of hemocytes in the mosquito Aedes aegypti. PLoS Pathog. 7, e1002274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen B. M., Huff B. M., Miranpuri G. S., Harris K. L., Christensen L. A. (1989). Hemocyte population changes during the immune response of Aedes aegypti to inoculated microfilariae of Dirofilaria immitis. J. Parasitol. 75, 119-123 [PubMed] [Google Scholar]
- Clements A. N. (2000). The Biology of Mosquitoes: Development, Nutrition and Reproduction, Vol. 1 Wallingford, UK: CABI Publishing; [Google Scholar]
- Cohn Z. A., Benson B. (1965). The in vitro differentiation of mononuclear phagocytes. 3. The reversibility of granule and hydrolytic enzyme formation and the turnover of granule constituents. J. Exp. Med. 122, 455-466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook A. D., Braine E. L., Hamilton J. A. (2004). Stimulus-dependent requirement for granulocyte-macrophage colony-stimulating factor in inflammation. J. Immunol. 173, 4643-4651 [DOI] [PubMed] [Google Scholar]
- Danielli A., Loukeris T. G., Lagueux M., Müller H. M., Richman A., Kafatos F. C. (2000). A modular chitin-binding protease associated with hemocytes and hemolymph in the mosquito Anopheles gambiae. Proc. Natl. Acad. Sci. USA 97, 7136-7141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downward J., Graves J. D., Warne P. H., Rayter S., Cantrell D. A. (1990). Stimulation of p21ras upon T-cell activation. Nature 346, 719-723 [DOI] [PubMed] [Google Scholar]
- Fraiture M., Baxter R. H., Steinert S., Chelliah Y., Frolet C., Quispe-Tintaya W., Hoffmann J. A., Blandin S. A., Levashina E. A. (2009). Two mosquito LRR proteins function as complement control factors in the TEP1-mediated killing of Plasmodium. Cell Host Microbe 5, 273-284 [DOI] [PubMed] [Google Scholar]
- Frolet C., Thoma M., Blandin S., Hoffmann J. A., Levashina E. A. (2006). Boosting NF-kappaB-dependent basal immunity of Anopheles gambiae aborts development of Plasmodium berghei. Immunity 25, 677-685 [DOI] [PubMed] [Google Scholar]
- Givan A. (2001). Flow Cytometry First Principles. New York, NY: Wiley-Liss; [Google Scholar]
- Guo X., Beerntsen B. T., Zhao X., Christensen B. M. (1995). Hemocyte alterations during melanotic encapsulation of Brugia malayi in the mosquito Armigeres subalbatus. J. Parasitol. 81, 200-207 [PubMed] [Google Scholar]
- Hall D. W. (1983). Mosquito hemocytes: a review. Dev. Comp. Immunol. 7, 1-12 [DOI] [PubMed] [Google Scholar]
- Han Y. S., Thompson J., Kafatos F. C., Barillas-Mury C. (2000). Molecular interactions between Anopheles stephensi midgut cells and Plasmodium berghei: the time bomb theory of ookinete invasion of mosquitoes. EMBO J. 19, 6030-6040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillyer J. F. (2010). Mosquito immunity. Adv. Exp. Med. Biol. 708, 218-238 [DOI] [PubMed] [Google Scholar]
- Hillyer J. F., Schmidt S. L., Fuchs J. F., Boyle J. P., Christensen B. M. (2005). Age-associated mortality in immune challenged mosquitoes (Aedes aegypti) correlates with a decrease in haemocyte numbers. Cell. Microbiol. 7, 39-51 [DOI] [PubMed] [Google Scholar]
- Hillyer J. F., Barreau C., Vernick K. D. (2007). Efficiency of salivary gland invasion by malaria sporozoites is controlled by rapid sporozoite destruction in the mosquito haemocoel. Int. J. Parasitol. 37, 673-681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinz F. I., Dieterich D. C., Tirrell D. A., Schuman E. M. (2012). Non-canonical amino acid labeling in vivo to visualize and affinity purify newly synthesized proteins in larval zebrafish. ACS Chem Neurosci 3, 40-49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones J. C. (1967). Normal differential counts of haemocytes in relation to ecdysis and feeding in Rhodnius. J. Insect Physiol. 13, 1133-1141 [Google Scholar]
- King J. G., Hillyer J. F. (2012). Infection-induced interaction between the mosquito circulatory and immune systems. PLoS Pathog. 8, e1003058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King J. G., Hillyer J. F. (2013). Spatial and temporal in vivo analysis of circulating and sessile immune cells in mosquitoes: hemocyte mitosis following infection. BMC Biol. 11, 55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraaijeveld A. R., Limentani E. C., Godfray H. C. (2001). Basis of the trade-off between parasitoid resistance and larval competitive ability in Drosophila melanogaster. Proc. Biol. Sci. 268, 259-261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H. O., Davidson J. M., Duronio R. J. (2009). Endoreplication: polyploidy with purpose. Genes Dev. 23, 2461-2477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levashina E. A., Moita L. F., Blandin S., Vriend G., Lagueux M., Kafatos F. C. (2001). Conserved role of a complement-like protein in phagocytosis revealed by dsRNA knockout in cultured cells of the mosquito, Anopheles gambiae. Cell 104, 709-718 [DOI] [PubMed] [Google Scholar]
- Luckhart S., Vodovotz Y., Cui L., Rosenberg R. (1998). The mosquito Anopheles stephensi limits malaria parasite development with inducible synthesis of nitric oxide. Proc. Natl. Acad. Sci. USA 95, 5700-5705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitri C., Vernick K. D. (2012). Anopheles gambiae pathogen susceptibility: the intersection of genetics, immunity and ecology. Curr. Opin. Microbiol. 15, 285-291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortimer N. T., Kacsoh B. Z., Keebaugh E. S., Schlenke T. A. (2012). Mgat1-dependent N-glycosylation of membrane components primes Drosophila melanogaster blood cells for the cellular encapsulation response. PLoS Pathog. 8, e1002819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller H. M., Dimopoulos G., Blass C., Kafatos F. C. (1999). A hemocyte-like cell line established from the malaria vector Anopheles gambiae expresses six prophenoloxidase genes. J. Biol. Chem. 274, 11727-11735 [DOI] [PubMed] [Google Scholar]
- Nappi A. J., Silvers M. (1984). Cell surface changes associated with cellular immune reactions in Drosophila. Science 225, 1166-1168 [DOI] [PubMed] [Google Scholar]
- Nappi A. J., Streams F. A. (1969). Haemocytic reactions of Drosophila melanogaster to the parasites Pseudocoila mellipes and P. bochei. J. Insect Physiol. 15, 1551-1554, IN7-IN10, 1555-1566 [Google Scholar]
- Nardi J. B., Pilas B., Ujhelyi E., Garsha K., Kanost M. R. (2003). Hematopoietic organs of Manduca sexta and hemocyte lineages. Dev. Genes Evol. 213, 477-491 [DOI] [PubMed] [Google Scholar]
- Oliveira G. A., Lieberman J., Barillas-Mury C. (2012). Epithelial nitration by a peroxidase/NOX5 system mediates mosquito antiplasmodial immunity. Science 335, 856-859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheim J., Rosenstreich D. (1976). Mitogens in Immunobiology. New York, NY: Academic Press; [Google Scholar]
- Pinto S. B., Lombardo F., Koutsos A. C., Waterhouse R. M., McKay K., An C., Ramakrishnan C., Kafatos F. C., Michel K. (2009). Discovery of Plasmodium modulators by genome-wide analysis of circulating hemocytes in Anopheles gambiae. Proc. Natl. Acad. Sci. USA 106, 21270-21275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Povelones M., Waterhouse R. M., Kafatos F. C., Christophides G. K. (2009). Leucine-rich repeat protein complex activates mosquito complement in defense against Plasmodium parasites. Science 324, 258-261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez J. L., Garver L. S., Brayner F. A., Alves L. C., Rodrigues J., Molina-Cruz A., Barillas-Mury C. (2013). The role of hemocytes in Anopheles gambiae antiplasmodial immunity. J. Innate Immun. 6, 119-128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizki T. M., Rizki R. M. (1983). Blood cell surface changes in Drosophila mutants with melanotic tumors. Science 220, 73-75 [DOI] [PubMed] [Google Scholar]
- Rodrigues J., Brayner F. A., Alves L. C., Dixit R., Barillas-Mury C. (2010). Hemocyte differentiation mediates innate immune memory in Anopheles gambiae mosquitoes. Science 329, 1353-1355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo J., Brehélin M., Carton Y. (2001). Haemocyte changes in resistant and susceptible strains of D. melanogaster caused by virulent and avirulent strains of the parasitic wasp Leptopilina boulardi. J. Insect Physiol. 47, 167-172 [DOI] [PubMed] [Google Scholar]
- Schnitger A. K., Kafatos F. C., Osta M. A. (2007). The melanization reaction is not required for survival of Anopheles gambiae mosquitoes after bacterial infections. J. Biol. Chem. 282, 21884-21888 [DOI] [PubMed] [Google Scholar]
- Sinenko S. A., Shim J., Banerjee U. (2012). Oxidative stress in the haematopoietic niche regulates the cellular immune response in Drosophila. EMBO Rep. 13, 83-89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volz J., Osta M. A., Kafatos F. C., Müller H. M. (2005). The roles of two clip domain serine proteases in innate immune responses of the malaria vector Anopheles gambiae. J. Biol. Chem. 280, 40161-40168 [DOI] [PubMed] [Google Scholar]
- Volz J., Müller H. M., Zdanowicz A., Kafatos F. C., Osta M. A. (2006). A genetic module regulates the melanization response of Anopheles to Plasmodium. Cell. Microbiol. 8, 1392-1405 [DOI] [PubMed] [Google Scholar]
- Weiss B. L., Wang J., Aksoy S. (2011). Tsetse immune system maturation requires the presence of obligate symbionts in larvae. PLoS Biol. 9, e1000619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss B. L., Maltz M., Aksoy S. (2012). Obligate symbionts activate immune system development in the tsetse fly. J. Immunol. 188, 3395-3403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynn T. A., Chawla A., Pollard J. W. (2013). Macrophage biology in development, homeostasis and disease. Nature 496, 445-455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassine H., Kamareddine L., Osta M. A. (2012). The mosquito melanization response is implicated in defense against the entomopathogenic fungus Beauveria bassiana. PLoS Pathog. 8, e1003029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zettervall C. J., Anderl I., Williams M. J., Palmer R., Kurucz E., Ando I., Hultmark D. (2004). A directed screen for genes involved in Drosophila blood cell activation. Proc. Natl. Acad. Sci. USA 101, 14192-14197 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.