Abstract
Circadian rhythms in social insects are highly plastic and are modulated by multiple factors. In addition, complex behaviors such as sun-compass orientation and time learning are clearly regulated by the circadian system in these organisms. Despite these unique features of social insect clocks, the mechanisms as well as the functional and evolutionary relevance of these traits remain largely unknown. Here we show a modification of the Drosophila activity monitoring (DAM) system that allowed us to measure locomotor rhythms of the honey bee, Apis mellifera (three variants; gAHB, carnica and caucasica), and two paper wasps (Polistes crinitus and Mischocyttarus phthisicus). A side-by-side comparison of the endogenous period under constant darkness (free-running period) led us to the realization that these social insects exhibit significant deviations from the Earth's 24 h rotational period as well as a large degree of inter-individual variation compared with Drosophila. Experiments at different temperatures, using honey bees as a model, revealed that testing the endogenous rhythm at 35°C, which is the hive's core temperature, results in average periods closer to 24 h compared with 25°C (23.8 h at 35°C versus 22.7 h at 25°C). This finding suggests that the degree of tuning of circadian temperature compensation varies among different organisms. We expect that the commercial availability, cost-effectiveness and integrated nature of this monitoring system will facilitate the growth of the circadian field in these social insects and catalyze our understanding of the mechanisms as well as the functional and evolutionary relevance of circadian rhythms.
KEY WORDS: Circadian rhythms, Locomotor activity, Honey bees, Apis mellifera, Wasps, Mischocyttarus, Polistes, Temperature compensation
INTRODUCTION
Circadian rhythms are biological oscillations with a period of ~24 h that serve as endogenous timers to synchronize internal physiological processes, such as sleep and metabolism, with environmental conditions (Green et al., 2008). At the molecular level, a set of proteins (e.g. period, clock, cryptochrome) cycle as a result of negative feedback loops formed by the interactions of these proteins at the transcriptional and translational levels (Bell-Pedersen et al., 2005; Hardin, 2011). At the level of the organism, various pacemakers in the brain, as well as the periphery, work together to integrate multiple predictable changes of the environment (e.g. light, temperature, etc.) to orchestrate a wide range of physiological processes (Helfrich-Förster et al., 2011; Mohawk and Takahashi, 2011). Despite the wealth of knowledge contributing to our understanding of the neural and molecular bases of circadian clocks, much less is known about the evolution of the circadian systems and how a wide range of biological processes including sleep, age, social interactions and reproductive status regulate the circadian machinery and vice versa (Bloch, 2010; Johnson et al., 2010; Helfrich-Förster et al., 2011). Therefore, comparative studies of the circadian rhythms are needed.
The circadian system of the honey bee and other social insects exhibits a great degree of plasticity and provides an excellent model with which to understand the mechanisms underlying sleep, social and reproductive regulation, as well as the development of circadian rhythms. In addition, the honey bee circadian system regulates complex physiological and behavioral processes such as sleep stages, spatiotemporal learning, sun-compass navigation, time perception, division of labor, mating and reproduction (von Frisch, 1967; Moore and Rankin, 1985; Goodwin and Lewis, 1987; Bloch et al., 2001; Harano et al., 2007; Shemesh et al., 2007; Moore and Doherty, 2009; Johnson et al., 2010; Shemesh et al., 2010; Cheeseman et al., 2012; Eban-Rothschild and Bloch, 2012; Galindo-Cardona et al., 2012). Both the wide range of biological processes under circadian regulation and the extraordinary plasticity of the circadian network of these organisms potentially represent unique evolutionary challenges and solutions. Despite the potential insight into the functional and evolutionary relevance of circadian rhythms that could be uncovered from studying different social insects, only a few representatives (honey bees and bumble bees) have been studied in depth (Spangler, 1972; Moore and Rankin, 1985; Toma et al., 2000; Bloch and Robinson, 2001; Bloch et al., 2001; Bloch, 2010; Eban-Rothschild et al., 2011).
In Drosophila, the existence of a simple, affordable and commercially available system for measurements of circadian locomotor activity has facilitated genome-wide genetic screens of thousands of mutant lines and catalyzed the growth of the field (Reddy et al., 1984; Zehring et al., 1984; Kjærsgaard et al., 2010; Hardin, 2011; Peschel and Helfrich-Förster, 2011). In addition, Bahrndorff et al. (Bahrndorff et al., 2012) has recently adopted the Trikinetics system to measure locomotor activity in the housefly, Musca domestica. Given the success of this system, our goal was to adapt the Drosophila activity monitoring system (DAM) (Trikinetics Inc., Waltman, MA, USA) for use in honey bees, wasps and other similar-sized insects.
Although there are currently various methods to record locomotor activity, such as video recordings combined with video-tracking systems (Colomb et al., 2012; Donelson et al., 2012; Gilestro, 2012), infrared-based systems such as the Trikinetics system are still the most commonly used. In general, infrared-based systems for measuring locomotor activity require the following components: (1) a chamber with an infrared beam that detects activity as the number of beam interruptions, (2) an interphase that will collect the data from the chamber and send it to a computer, (3) data acquisition software to translate the interphase data into readable files, and (4) data analysis software to calculate the different circadian parameters (period, phase, rhythm strength, etc.).
The Trikinetics system includes the infrared-containing chambers, the interphase and the data acquisition software as a unified system (for a diagram of the complete system, see catalog at http://www.trikinetics.com/Downloads/Catalog1105.pdf). It is commercially available, easily modified and has been used in more than 600 publications. In addition, its adaptation for use in honey bees and other similar-sized insects would enable the use of multiple sophisticated data analysis tools that have been developed and validated throughout the years (Levine et al., 2002b; Gilestro and Cirelli, 2009; Schmid et al., 2011).
In contrast, other available monitoring systems are not unified in the sense that the infrared chambers, interphase and acquisition software come from different sources (Moore and Rankin, 1985; Toma et al., 2000; Shimizu et al., 2001). For example, one of the most commonly used systems, described in Toma et al. (Toma et al., 2000), uses custom-made infrared chambers (see Moore and Rankin, 1985) with an interphase originally designed for a rodent infrared system. This adaptation makes the setup and optimization process troublesome, as the output of the infrared cage requires substantial adjustments to meet the interphase specifications. Finally, without considering the cost of the custom-made infrared chambers, this system requires an initial investment of ~8610.00 USD to measure locomotor activity of a maximum of 24 individuals, which is the limit of the interphase unit. This is in sharp contrast with the Trikinetics system, which, including the infrared chambers, requires an investment of ~1200.00 USD for 32 individuals and the interphase limit is 3480 individuals.
In this study, we modified the DAM from Trikinetics and optimized experimental conditions for measuring locomotor activity rhythms in larger insects (Fig. 1; see Materials and methods for a detailed description). Using this system, we measured the locomotor activity rhythms of three honey bee (Apis mellifera L.) variants, the gentle Africanized Honey Bee (gAHB) (Rivera-Marchand et al., 2012), and the subspecies carnica, a honey bee ecotype from Kırklareli, and caucasica, discriminated by morphology and microsatellite analyses (Kandemir et al., 2000; Bodur et al., 2007). In addition, we measured for the first time the locomotor rhythms of two paper wasps, Mischocyttarus phthisicus and Polistes crinitus. Using this system, we found that the free-running period of honey bees and wasps at 25°C significantly deviates from 24 h and exhibits a large degree of inter-individual variation when compared with Drosophila. Moreover, we found that testing the endogenous rhythm at 35°C, which is the hive's core temperature, results in average periods closer to 24 h.
Fig. 1.
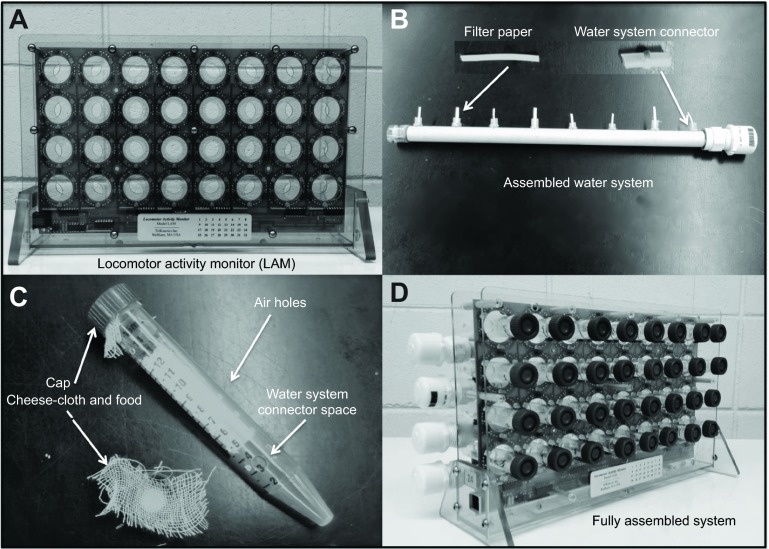
Locomotor activity monitoring system. (A) Locomotor activity monitor (LAM16) as sold by Trikinetics Inc. The LAM16 monitor contains 32 channels. Each channel contains three infrared beams and their corresponding receptor. The wiring of the internal board of the monitors is arranged such that the interruption of one or more beams on each channel counts as a single activity event. (B) Water system assembly ensures that every individual has a constant supply of water. This assembly minimizes handling and refilling time compared with individual water supplies. A detailed description of this system has been provided in the Materials and methods. (C) Sampling tubes and honey candy. The sampling tube is a standard 15 ml centrifuge tube with holes for air and water system connection. The honey candy is first placed directly into the tube cap and then a piece of cheese-cloth is placed on top of the food. The cloth prevents spillage and spreading of the food throughout the tube, and thus prevents individuals from becoming stuck. (D) Fully assembled LAM system including the water system assembly and the sampling tubes.
RESULTS
Previous studies have shown that the endogenous period of honey bee foragers under constant darkness (DD) is less than 24 h and ranges from 21.5 to 23.3 h (Spangler, 1972; Moore and Rankin, 1985; Toma et al., 2000). To test whether our activity monitoring system would produce comparable results, we examined the endogenous period of individual colonies of three different honey bees sampled under constant darkness: gAHB from Gurabo Experimental Agricultural Station, Puerto Rico (Rivera-Marchand et al., 2012), and carnica and caucasica subspecies from a common garden apiary in Ankara, Turkey (Giray et al., 2010).
Consistent with previous results, we found that the endogenous period under DD of these bee groups is less than 24 h (Fig. 2). Interestingly, our results show that the gAHB hybrid has a significantly longer period (mean ± s.e.m., 22.5±0.13 h, n=58) than either the carnica (21.3±0.24 h, n=11, P<0.0013) or caucasica (21.7±0.36 h, n=13, P<0.0339) subspecies. Given the large colony–colony variation in period length, we interpret that the observed differences among these honey bees is associated with this variation, rather than actual species differences. However, further studies using a representative number of colonies from each subspecies and gAHB are needed to determine the statistical significance of these differences.
Fig. 2.
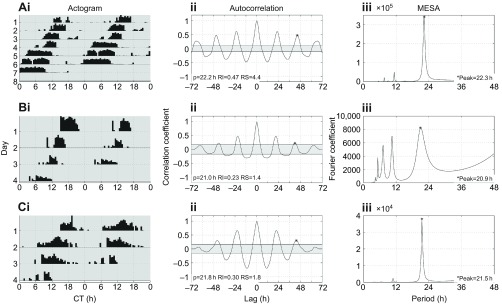
Honey bee foragers exhibit short period phenotype under constant darkness (<24 h endogenous circadian rhythm). (i) Double-plotted actograms showing the locomotor activity pattern of representative individuals of the different Apis mellifera groups sampled: (A) gAHB, (B) carnica and (C) caucasica. Each row contains the locomotor activity (counts per 30 min) of two consecutive days and the last day is repeated such that the second day is always the beginning of the next row. The x-axis shows the time of day under constant darkness expressed as circadian time (CT). (ii) Autocorrelation plots used to determine the period (p), rhythm index (RI) and rhythm strength (RS), as described previously (Levine et al., 2002b). In general, the oscillation of this function shows periodicity. The asterisk shown on the third peak of the autocorrelation plot indicates the specific time point used for the determination of the rhythm parameters. (iii) The maximum entropy spectral analysis (MESA) plot is an independent algorithm used to determine period (t) (Levine et al., 2002b).
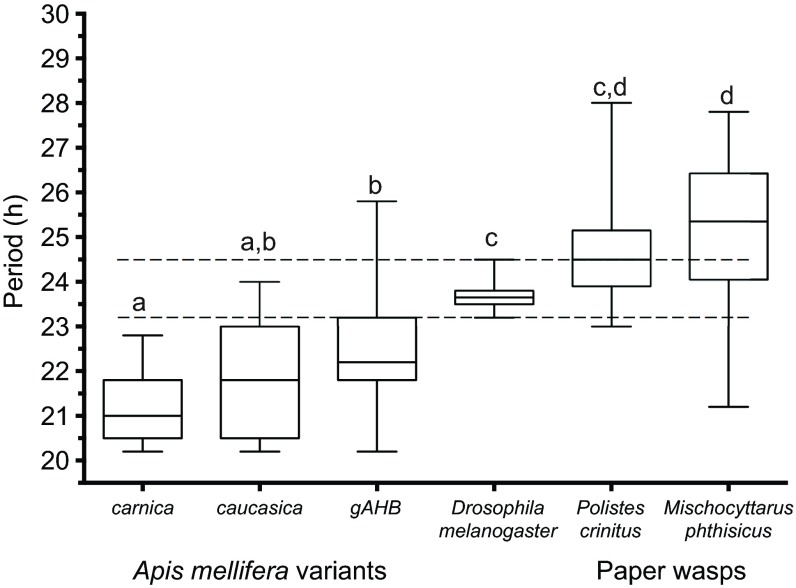
To test whether the conditions of our monitoring system are suitable for other similarly sized insects, locally available wasps from different genera within the Polistinae subfamily (P. crinitus and M. phthisicus) were collected and examined under DD. Analysis of the circadian locomotor behavior revealed that both species of wasp have an endogenous rhythm greater than 24 h under DD (P. crinitus, 24.6±0.29 h, n=13; and M. phthisicus, 25.3±0.22 h, n=24; Fig. 3). When comparing the locomotor pattern of individual honey bees, wasps and fruit flies (as a reference), we noticed that not only is the period different among these insects, but also that the degree of inter-individual variation dramatically changes (Fig. 4).
Fig. 3.
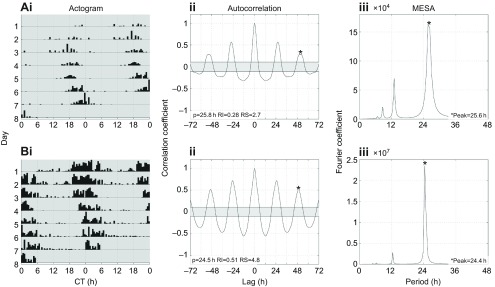
Female wasps exhibit long period phenotype under constant darkness (>24 h endogenous circadian rhythm). (i) Double-plotted actograms showing the locomotor activity pattern of representative individuals of (A) Mischocyttarus phthisicus and (B) Polistes crinitus. (ii) Autocorrelation plots used to determine the period (p), rhythm index (RI) and rhythm strength (RS). (iii) MESA plot.
Fig. 4.
The period of honey bees and wasps significantly deviates from 24 h and exhibits a large degree of inter-individual variation. Box plots of the endogenous period distribution in constant darkness for individuals of the A. mellifera subspecies carnica (n=11) and caucasica (n=13) and the hybrid gAHB (n=58); Drosophila melanogaster (n=32); Polistes crinitus (n=13); and Mischocyttarus phthisicus (n=24). The dashed lines represent the calculated minimum and maximum values for circadian period measured from D. melanogaster individuals. A Kruskal–Wallis test revealed significant differences across all of the sampled groups (F5=94.80, P<0.0001). Box plots with different letters are significantly different at P<0.05 level in a post hoc Tukey's HSD tests.
Given the adaptive role of having a period that matches the environment (Pittendrigh and Bruce, 1959; Saunders, 1972; Ouyang et al., 1998; Dodd et al., 2005; Emerson et al., 2008; Vaze and Sharma, 2013), we were intrigued by the finding that the endogenous clock of honey bees and paper wasps significantly deviates from 24 h (Fig. 4). Honey bees tightly regulate the hive's temperature so that the center of the hive is 35°C±0.5 h (Winston, 1987; Jones et al., 2004). We wondered whether the fact that our experiments were performed at 25°C rather than 35°C was associated with the observed deviation. We hypothesized that if the period deviation is due to the difference between the natural and the test temperature, then testing the endogenous rhythm at 35°C will result in periods closer to 24 h. To test this hypothesis, we collected foragers from six different colonies of gAHB and divided them into two groups that were tested at either 25°C or 35°C.
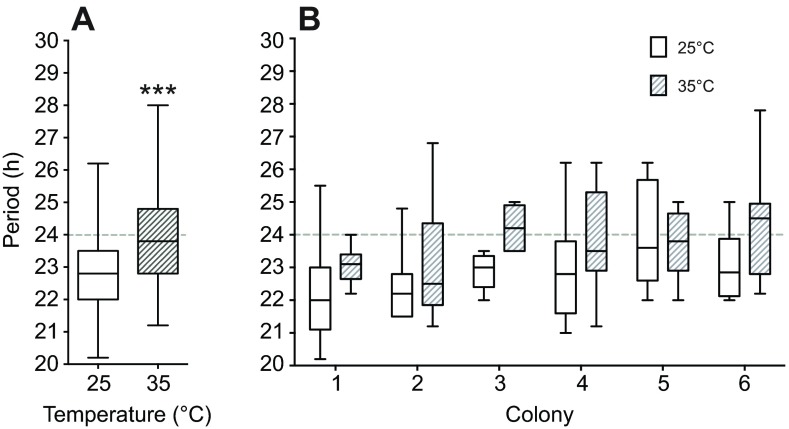
Consistent with our hypothesis, we found that the overall average period at 35°C is closer to 24 h (23.8±0.19 h, n=54) than at 25°C (22.7±0.19 h, n=50) (Fig. 5A). A two-way ANOVA using temperature and colony as factors revealed that an increase in temperature increases period length, regardless of the colony sampled (Fig. 5B).
Fig. 5.
Testing the endogenous rhythm at 35°C decreases period deviations from 24 h. (A) Box plots of the endogenous rhythm distribution under constant darkness of honey bee foragers at 25°C (white; n=51) and 35°C (shaded; n=54) reveal significant differences (Kruskal–Wallis test, F5=26.41, P<0.01). (B) Examining the endogenous rhythm at different temperatures of foragers in six different colonies reveals significant differences of the period distributions at different temperatures for colonies 1 (P=0.04), 3 (P<0.01) and 6 (P=0.04), while comparison of these conditions in colonies 2, 4 and 5 did not yield significant differences.
DISCUSSION
Here we demonstrate for the first time that the Trikinetics LAM system, originally developed for measuring locomotor activity in fruit flies, allows high-throughput measurements of circadian rhythms in different honey bee groups and paper wasp genera. Using this system, we show that the endogenous period of honey bees and wasps significantly deviates from 24 h under constant darkness (Figs 2, 3 and 4). Using the honey bee as our model, we found that testing the endogenous rhythm at 35°C reduces the observed deviations from 24 h (Fig. 5). In addition, we show that honey bees and paper wasps exhibit a large degree of inter-individual variation (Fig. 4).
We found that honey bees exhibit periods shorter than 24 h while both P. crinitus and M. phthisicus wasps show long periods (Figs 2, 3 and 4). The short period phenotype of honey bees has been shown by multiple groups around the world using different subspecies and various monitoring systems (Spangler, 1972; Moore and Rankin, 1985; Toma et al., 2000). Both honey bee subspecies from Turkey (carnica and caucasica) show shorter periods when compared with honey bees from Puerto Rico. Because bees were placed directly into DD, differences in the environment may affect their endogenous rhythm.
The sunrise and sunset at the time of collection for the bees in Puerto Rico was 05:57 and 19:05 h, respectively, while for the bees in Turkey they were 04:30 and 19:18 h. These sunrise/sunset time schedules represent light:dark (LD) cycles of ~13 h:11 h and 15 h:9 h, respectively. Previous studies in a wide range of species have shown that entrainment with different photoperiods can lead to changes in the free-running period as well as in the relative length of the activity and rest phases (Pittendrigh and Daan, 1976). Whether these changes occur and their direction largely depends on the species (Pittendrigh and Daan, 1976). For example, the free-running period of the mouse Peromyscus leucopus is reduced by long photoperiods (e.g. 18 h:6 h LD), while that in the hamster Mesocricetus auratus is not altered by the same treatment. In contrast, long photoperiods increase the free-running period of the white-crowned sparrow Zonotrichia leucophrys as well as the fruit fly Drosophila pseudoobscura (Gwinner, 1975; Pittendrigh and Daan, 1976). Even though the locomotor pattern of honey bees under short and long photoperiods has been studied (Moore and Rankin, 1993), whether these photoperiods alter circadian locomotor behavior under constant conditions remains to be elucidated.
Our findings indicate that the free-running period of the two strains assayed in Turkey tend to be shorter than of honey bees from Puerto Rico (Fig. 4). Although these findings suggest that long photoperiods may shorten the free-running period of honey bees, it is important to note that aside from being exposed to different photoperiods, the individuals shown in Fig. 4 come from single hives and are genetically distinct subspecies (Kandemir et al., 2000; Bodur et al., 2007; Rivera-Marchand et al., 2012). Therefore, further studies controlling these factors are required to determine the potential effects of photoperiod on the free-running period of honey bees.
Although the circadian locomotor rhythms of M. phthisicus and P. crinitus wasps have never been reported, circadian studies using the solitary wasp Nasonia vitripennis have shown that its endogenous period under DD is also greater than 24 h (Bertossa et al., 2010). Whether these deviations from the Earth's rotational period are related to the specific laboratory conditions or whether they are observed under more natural conditions became another of our research questions.
We found that testing the endogenous rhythm at the hive's core temperature (35°C) results in periods closer to 24 h (Fig. 5), highlighting the importance of simulating natural conditions in laboratory experiments. The importance of simulating the natural environment for circadian studies was recently underlined in Drosophila (Vanin et al., 2012). Although this is the first time that circadian temperature responses have been reported in A. mellifera, similar findings have been observed in A. cerana japonica (Fuchikawa and Shimizu, 2007a; Fuchikawa and Shimizu, 2007b). This finding is surprising because the ability to maintain a fixed endogenous period throughout a range of physiological temperatures (temperature compensation) is a defining feature of circadian rhythms (Zehring et al., 1984). Moreover, population genetic studies in Drosophila indicate that temperature compensation is under natural selection and is important for adapting to high latitudes (Sawyer et al., 1997; Sawyer et al., 2006). A possible explanation to the observed alterations of temperature compensation in honey bees could be that because they tightly regulate their hive temperature (Winston, 1987; Jones et al., 2004), the selective pressure to maintain this trait has been eliminated or reduced. If this were the case, we would expect that the clock's response to temperature changes would greatly vary among different honey bee species. However, the fact that A. m. ligustica, A. m. carnica and A. cerana japonica exhibit short periods at temperatures below 35°C suggests that the circadian clock's response to temperature is at least conserved within the Apis genus. This pattern is inconsistent with relaxed selection and therefore suggests that the tendency to present shorter periods in temperatures lower than 35°C may be an adaptive trait in honey bees. At this moment, we do not know what the functional relevance of this trait could be, but one might speculate that having a fast clock during cold seasons may allow bees to take full advantage of the shorter light periods associated with these seasons. Further studies are needed to ascertain whether altered temperature compensation is also associated with long period in the wasps used in this study, and whether it extends to other social insects.
Beyond the differences in the endogenous period under constant darkness, we observed a large degree of inter-individual variation among honey bees and paper wasps when compared with fruit flies (Fig. 4). Among the possible factors that could explain this inter-individual variation are age, genetic differences in the core circadian genes, reproductive status and division of labor. Moreover, the sources of variability may differ in each of these organisms.
In the case of Drosophila, even though the phase and strength of circadian locomotor rhythms is influenced by age and social interactions, the endogenous period length under constant darkness is highly robust and appears to be resistant to these factors (Levine et al., 2002a; Koh et al., 2006; Billeter et al., 2012). Thus, the small variation observed in Drosophila may be related to the robustness of the endogenous period of this organism combined with the fact that age, sex and genetic background were controlled. In addition, even when the whole genome is blasted with mutagenic agents, introducing a large number of random mutations and dramatically increasing genetic variability, the period length only changes significantly when core circadian genes are mutated (Konopka and Benzer, 1971; Zehring et al., 1984).
In honey bees, the large inter-individual variation among foragers (Fig. 4) could be associated with age differences or foraging specialization. Although all of the bees collected were foragers, their age could range anywhere from 15 to 28 days (Winston, 1987). Given that age is a major regulator of the circadian system in honey bees (Moore et al., 1998), this may be another source of variation. An additional factor could be that specialized water-, pollen-, nectar- and/or propolis-foraging individuals may be present within our sample (Page et al., 2006; Giray et al., 2007). Despite the fact that foraging specialization and age may be different, no studies have dissected the effect of forager age and specialization on circadian periodicity. Thus, whether these factors contribute to the observed variation requires further study.
In the paper wasp species P. crinitus and M. phthisicus, the large variation in period length, compared with Drosophila, could also be associated with age differences, division of labor or reproductive status (Giray et al., 2005). In this study, only females from both P. crinitus and M. phthisicus were used in our samples, regardless of age. Although neither circadian periodicity nor its modulation by age has been previously reported for these wasp genera, it has been shown that their division of labor is dependent on age (Giray et al., 2005; Torres et al., 2012). Therefore, age and its associated division of labor could be contributing factors to the observed period variation. Further studies are required to determine whether there is a relationship between age, division of labor and circadian rhythms in these eusocial wasps.
In our wasp samples, reproductive status may differ among individuals (i.e. there could be workers as well as mated queens and/or unmated queens). Although it is unknown whether the reproductive status alters the periodicity of circadian rhythms in these wasps, studies in honey bees and bumble bees have shown that rhythmic behavior in unmated and egg-laying queen bees is dramatically different across colony and isolation conditions (Koeniger and Koeniger, 2000; Harano et al., 2007; Johnson et al., 2010; Eban-Rothschild et al., 2011). Furthermore, it is known that females of both of these paper wasp species can leave their parental nest, develop their reproductive system and become a nest foundress (Ross and Matthews, 1991). This raises the possibility that isolated individuals in this study could have been undergoing processes related to separation from the colony and therefore contributing to the observed variability. In fact, we observed individual wasps that changed their period over time (data not shown), and we are currently exploring whether these changes are associated with the development of their reproductive system.
In conclusion, this study shows that the Trikinetics LAM system is suitable for measuring circadian rhythms in honey bees, paper wasps (P. crinitus and M. phthisicus) and potentially other similar-sized insects. The use of this system facilitates the setup and optimization processes and we expect this will increase the number of laboratories doing circadian research on social insects. Understanding the mechanisms underlying period deviations and the adaptive role of inter-individual variation are novel research avenues that could provide insights into the evolution of circadian rhythms as well as their relation to sociality.
MATERIALS AND METHODS
Activity monitoring system
Locomotor activity monitor
Locomotor activity monitors (Fig. 1A) were developed by Trikinetics Inc. (Waltham, MA, USA) at the request of the investigators (model number LAM16). Each unit has 32 independent activity channels, which measure activity by using three infrared beams and sensors to ensure that recordings are accurate. One of the main challenges of adapting the Trikinetics system for use with bees and wasps was the food and water delivery system. We designed a water system (see Water system, below) to be capable of providing water ad libitum for several days, while food was provided in the form of honey candy.
Water system
The water system (Fig. 1B) was built from a 30-cm-long plastic tube with eight 6.35 mm and one 1.6 mm hole between the seventh and eighth hole for water refilling. The left side of the tube was sealed using a clear custom-made plastic cap, which served to gauge the water level. Both the screw cap attachment and the clear cap were permanently sealed using plumber's cement (Oatey Rain-R-Shine no. 308933). Each sampling tube was connected to the water system by plastic refrigeration tubing 6.35 mm in diameter and 25 mm in length. Thinly cut (10×2 mm) Trans-Blot® filter paper (Bio-Rad Laboratories Inc.) was placed inside each water system connector to provide water ad libitum to each individual.
Sampling tubes and food
VWR® high-performance 15 ml centrifuge tubes (Fig. 1C) were modified to be used as sampling tubes by adding a 6.35 mm hole for the water system connector and 16 parallel 1.6 mm holes for air flow in each tube. Bee candy was prepared (in the laboratory) using a 60:40 mixture of pulverized cane sugar and honey and was provided ad libitum in the cap of each sampling tube. The cane sugar was pulverized using a coffee grinder and mixed with the honey slowly to ensure homogenization. Cheese-cloth was put on top of the food to ensure that only the mouthparts of the insect would have access to the food, thus preventing the insect from getting stuck to the food.
Animals
Apis mellifera gAHB (A. m. scutellata hybrid from Puerto Rico)
During July 2011, honey bee foragers were collected at the entrance of a healthy colony, located at the University of Puerto Rico (UPR) Gurabo Experimental Station in Gurabo, Puerto Rico. To ensure the capture of foragers, an 8-mesh wire screen (3.2×3.2 mm mesh size) was placed in the entrance of the hive and incoming foragers were allowed to climb inside plastic vials where they were captured, in a similar fashion as in Giray et al. (Giray et al., 2007). After capture, foragers were provided food and water during transportation to the UPR Rio Piedras campus, which took between 30 and 45 min. Upon arrival to the UPR Rio Piedras campus, bees were briefly anesthetized using CO2, placed in the activity monitors and transferred to a light-, temperature- and humidity-controlled incubator (Percival, I-30BLL). Bees were placed in constant darkness from the beginning of the experiment; their circadian rhythms were entrained to the sunrise and sunset at the Gurabo experimental station, which prior to collection were ~05:50 to 19:05 h local time (−04:00 GMT) (United States Naval Observatory et al., 2011). For all experiments, environmental conditions in the incubator were kept at 25°C, 80% relative humidity and constant darkness (DD). These conditions were continuously monitored using an Environmental Monitor (Trikinetics Inc., DEnM). Water system levels were checked daily and refilled as needed.
Temperature experiments
To determine the sample size required to detect a temperature effect of at least 1 h in the free-running period, we ran a power analysis test using the mean and standard deviation from the experiments shown in Fig. 4 for gAHB. The power analysis indicated that a total sample size of 60 individuals (30 per treatment) would be sufficient to have an actual power of 0.94 using an α-level of 0.05. Because we planned to use six colonies, in theory we needed 10 bees per colony. To account for potential colony-to-colony differences, as well as the high mortality of foragers (>50% at day 7) during circadian assays (Moore and Rankin, 1985), a sample size of 40 foragers per colony was needed.
Groups of 40 foragers from six healthy colonies were collected, transported and anesthetized in the same fashion as described above, in January 2013. These groups were equally divided (20 per colony) and placed inside different incubators to record their endogenous periodicity in constant darkness at either 25°C or 35°C. Bees were entrained to the environmental light:dark cycles prior to collection, which began at 06:56 h and ended at 18:08 h local time (−04:00 GMT) (United States Naval Observatory et al., 2013). From these experiments, rhythmic individuals (>70%) were considered for circadian period analysis.
Apis mellifera carnica and A. mellifera caucasica
Foragers were collected from the entrance of one colony of A. m. caucasica Gorbachev 1916 and one colony of A. m. carnica Pollman 1879 located in a common garden in the Middle East Technical University, Ankara, Turkey, during July 2011. After capture, foragers were placed in the LAMs with commercially available honey candy, which eliminates the necessity of adding water because it contains sorbitol and retains greater amount of water than regular bee candy (up to 25% water, Konya Seker™). Bees were entrained to the environmental light:dark cycles prior to collection, which began at 04:30 h and ended at 19:18 h local time (+02:00 GMT) (United States Naval Observatory et al., 2011). For all experiments, incubator conditions were the following: 25°C, 80% relative humidity and constant darkness.
Polistes crinitus and Mischocyttarus phthisicus
Two nests of P. crinitus (Felton 1764) were collected in February 2012 from abandoned houses in the municipality of Cidra and in the Maricao State Forest in Puerto Rico and brought to our vivarium. At the time of collection, nests were post-emergence with a dominant queen, her adult offspring, eggs, larvae and pupae present (Ross and Matthews, 1991). The wasps were anesthetized using CO2 to allow for the fixing of the nest pedicel to the back wall of fly cages from BioQuip® (Gibo, 1977; Hunt, 1984; Tibbetts and Curtis, 2007; Daugherty et al., 2011).
Two nests of M. phthisicus (Fabricius 1793) were collected in February 2012 from Cambalache State Forest near Manatí, Puerto Rico. Nests contained approximately 30–70 workers and ~50–100 cells at the time of collection. Collection, placement, rearing and housing of M. phthisicus nests were performed in the same fashion as described for P. crinitus (Gibo, 1977; Hunt, 1984; Tibbetts and Curtis, 2007; Daugherty et al., 2011).
Both M. phthisicus and P. crinitus nests were kept for 3 weeks in the vivarium before behavioral assays. During the housing period, we used honey bee worker pupae (white eye stage) as a protein source and bee candy as a carbohydrate source for the wasps. Both food items and water were provided ad libitum. During the 3-week period, individuals from nests inevitably died, escaped or were used in preliminary studies; the remaining individuals were used in this study. Individuals from both M. phthisicus and P. crinitus were collected in February 2012 and placed directly in constant darkness; they were entrained to their environmental light:dark cycles prior to collection, which began at 06:38 h and ended at 18:33 h local time (−04:00 GMT) (United States Naval Observatory et al., 2012). Although all M. phthisicus (nest 1 n=13, nest 2 n=14) and P. crinitus (Cidra nests=8, Maricao nests=6) individuals used were females, age and reproductive status were not accounted for. Thus the presence of gynes, workers and queens within our sample is likely with respect to their natural history (Ross and Matthews, 1991).
Drosophila melanogaster
Circadian assays were performed as previously described (Stoleru et al., 2004). Briefly, 3- to 5-day-old female flies were anesthetized with CO2 and placed in individual tubes containing fly food (5% sucrose, 2% agar). Tubes were then placed in Drosophila activity monitors (DAMs) within an environmentally controlled incubator (25°C, 60% humidity and constant darkness) and connected to the monitoring system (TriKinetics, Waltham, MA, UA).
Statistical analyses
Circadian rhythm and locomotor activity for all the individuals were analyzed using the MATLAB toolboxes developed in Jeffrey Hall's laboratory (Levine et al., 2002b). The output of these toolboxes provides data on the individual's locomotor activity throughout the experiment in the form of an actogram, an autocorrelation and maximum entropy spectral analysis (MESA), which calculates the periodicity of the circadian rhythms for each individual, and the strength of their rhythms using circular statistics.
To compare variability among and between each of the groups sampled, non-parametric Kruskal–Wallis rank of sums test was followed by a post hoc Tukey's honestly significant difference test. A two-way ANOVA was used to verify the effects of colony and temperature in period length of foragers. All statistical analyses were performed using the JMP™ software package from SAS (SAS Institute Inc., 2009); graphs and figures were created in MATLAB (MathWorks, Inc., Natick, MA, USA) and GraphPad Prism 6.00 (GraphPad Software, La Jolla, CA, USA).
Troubleshooting
Were there any problems encountered adapting a fruit fly apparatus to honey bees and wasps?
In terms of adapting the apparatus to honey bees and wasps, the main changes compared with the fruit fly system were the addition of two more photocells, the type of food, the necessity of an independent water supply and air holes to the sampling tubes. For example, in fruit flies only one photocell is used and water and food come from a single food source containing 5% sucrose and 2% agar. This is sufficient to obtain reliable locomotor recordings for 2–3 weeks.
Why only three photocells?
The decision on the number of photocells was based on empirical evidence. We first tried different sized tubes and found that workers from our local honey bee (gAHB) could easily turn around to obtain food and water in each side of a tube with an inside diameter of 14 mm. Using the tube diameter and the average size of our worker bees (5–7 mm), we discussed the issues with the engineers from Trikinetics and decided to test three photocells in our initial prototype. We tested this prototype by comparing the number of beam crosses observed on video with those recorded by the infrared system. Surprisingly, our findings indicated that even one photocell resulted in a 100% correspondence between the video recording and the infrared system. However, we decided to continue with three photocells anticipating the eventuality of using smaller specimens.
How can the apparatus be changed to accommodate various sizes of bees and wasps?
The important parameters to change in order to accommodate organisms of different sizes are the size of the tube and the number of photocells. The number of photocells required depends on the relative size difference between the monitoring tube and the organism. The larger the tube with respect to the organism size, the higher the number of photocells required. Currently, the Trikinetics catalog has monitor sizes to accommodate tubes of 5, 7, 10, 16, 25 and 60 mm with various options in the number of photocells. In addition, they provide custom services to accommodate the specific needs of the researcher.
Why was the water system needed?
The water system was needed for various reasons. Initially, we supplied water to the bees by placing a 1.5 ml micro tube on a hole in the top of the sampling tube. However, this system required daily refilling, which was time consuming and required extensive handling. With the water system described in the present study, we ensure that every bee has a constant supply of water and decrease handling by dramatically decreasing the frequency (daily versus every 4 days) and duration (15 min versus 1 min) of refilling.
As this is a new apparatus, what might go wrong and how can the experimenter detect it?
Because the DAM has been available for more than 20 years, a troubleshooting section with the different problems that we have encountered with the system is already available in the DAM User's Guide (http://www.trikinetics.com/Downloads/DAMSystem%20User's%20Guide%203.0.pdf). Some of these problems are computer/software errors, communication errors, skipped readings and power outage. In addition, Trikinetics' technical support is excellent.
ACKNOWLEDGEMENTS
We thank honey bee specialists Rene Bonano and Mustafa Cirik for caring for the colonies used for these experiments; Gabriel Diaz for honey bee colony care and the collection of one Polistes nest; Carlos Jimenez and Raisa Rosado for establishing the initial conditions for the honey bee recordings; and Nicole Valle-Raimundi, Yamil Ortiz, Liz Hernandez, Ozge Tozkar and Okan Can Arslan for helping with the experiments. We would also like to recognize the director, Manuel Diaz and the personnel of the Gurabo Experimental Agriculture Station of the University of Puerto Rico at Mayaguez for use of facilities at ‘Casa Amarilla’. We also thank Dr Darrell Moore, Dr Katherine M. Parisky, Dr Ada Haiman and the BIOL6990-Behavioral Plasticity Seminar course members for taking the time to provide revisions and critiques on this manuscript.
FOOTNOTES
Competing interests
The authors declare no competing financial interests.
Funding
This work was sponsored by the Toyota FCPR Award [2009-0180], TUBITAK grant [109T547], the METU Research Fund [BAP-08-11-DPT-2002-K120510], the National Science Foundation BDP-LSAMP Award [1026560] and the National Institutes of Health RISE Graduate Fellowship Award [2R25GM061151]. Deposited in PMC for release after 12 months.
References
- Bahrndorff S., Kjærsgaard A., Pertoldi C., Loeschcke V., Schou T. M., Skovgård H., Hald B. (2012). The effects of sex-ratio and density on locomotor activity in the house fly, Musca domestica. J. Insect Sci. 12, 71 [Google Scholar]
- Bell-Pedersen D., Cassone V. M., Earnest D. J., Golden S. S., Hardin P. E., Thomas T. L., Zoran M. J. (2005). Circadian rhythms from multiple oscillators: lessons from diverse organisms. Nat. Rev. Genet. 6, 544-556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertossa R. C., van Dijk J., Beersma D. G., Beukeboom L. W. (2010). Circadian rhythms of adult emergence and activity but not eclosion in males of the parasitic wasp Nasonia vitripennis. J. Insect Physiol. 56, 805-812 [DOI] [PubMed] [Google Scholar]
- Billeter J. C., Jagadeesh S., Stepek N., Azanchi R., Levine J. D. (2012). Drosophila melanogaster females change mating behaviour and offspring production based on social context. Proc. Biol. Sci. 279, 2417-2425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloch G. (2010). The social clock of the honeybee. J. Biol. Rhythms 25, 307-317 [DOI] [PubMed] [Google Scholar]
- Bloch G., Robinson G. E. (2001). Chronobiology. Reversal of honeybee behavioural rhythms. Nature 410, 1048 [DOI] [PubMed] [Google Scholar]
- Bloch G., Toma D. P., Robinson G. E. (2001). Behavioral rhythmicity, age, division of labor and period expression in the honey bee brain. J. Biol. Rhythms 16, 444-456 [DOI] [PubMed] [Google Scholar]
- Bodur C., Kence M., Kence A. (2007). Genetic structure of honeybee, Apis mellifera L. (Hymenoptera: Apidae) populations of Turkey inferred from microsatellite analysis. J. Apic. Res. 46, 50-56 [Google Scholar]
- Cheeseman J. F., Winnebeck E. C., Millar C. D., Kirkland L. S., Sleigh J., Goodwin M., Pawley M. D., Bloch G., Lehmann K., Menzel R., et al. (2012). General anesthesia alters time perception by phase shifting the circadian clock. Proc. Natl. Acad. Sci. USA 109, 7061-7066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colomb J., Reiter L., Blaszkiewicz J., Wessnitzer J., Brembs B. (2012). Open source tracking and analysis of adult Drosophila locomotion in Buridan's paradigm with and without visual targets. PLoS ONE 7, e42247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daugherty T. H., Toth A. L., Robinson G. E. (2011). Nutrition and division of labor: effects on foraging and brain gene expression in the paper wasp Polistes metricus. Mol. Ecol. 20, 5337-5347 [DOI] [PubMed] [Google Scholar]
- Dodd A. N., Salathia N., Hall A., Kévei E., Tóth R., Nagy F., Hibberd J. M., Millar A. J., Webb A. A. (2005). Plant circadian clocks increase photosynthesis, growth, survival, and competitive advantage. Science 309, 630-633 [DOI] [PubMed] [Google Scholar]
- Donelson N. C., Kim E. Z., Slawson J. B., Vecsey C. G., Huber R., Griffith L. C. (2012). High-resolution positional tracking for long-term analysis of Drosophila sleep and locomotion using the ‘tracker’ program. PLoS ONE 7, e37250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eban-Rothschild A., Bloch G. (2012). Circadian rhythms and sleep in honey bees. In Honeybee Neurobiology and Behavior: A Tribute to Randolf Menzel (ed. Galizia C. G., Eisenhardt D., Giurfa M.), pp. 31-44 Dordrecht: Springer Science+Business Media BV; [Google Scholar]
- Eban-Rothschild A., Belluci S., Bloch G. (2011). Maternity-related plasticity in circadian rhythms of bumble-bee queens. Proc. Biol. Sci. 278, 3510-3516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerson K. J., Bradshaw W. E., Holzapfel C. M. (2008). Concordance of the circadian clock with the environment is necessary to maximize fitness in natural populations. Evolution 62, 979-983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabricius J. C. F. (1793). Entomologia Systematica Emendata et Aucta. Copenhagen: C. G. Proft; [Google Scholar]
- Felton S. (1764). An account of a singular wasp and locust. Philos. Trans. R. Soc. B 1764, 53-56 [Google Scholar]
- Fuchikawa T., Shimizu I. (2007a). Circadian rhythm of locomotor activity in the Japanese honeybee, Apis cerana japonica. Physiol. Entomol. 32, 73-80 [DOI] [PubMed] [Google Scholar]
- Fuchikawa T., Shimizu I. (2007b). Effects of temperature on circadian rhythm in the Japanese honeybee, Apis cerana japonica. J. Insect Physiol. 53, 1179-1187 [DOI] [PubMed] [Google Scholar]
- Galindo-Cardona A., Monmany A. C., Moreno-Jackson R., Rivera-Rivera C., Huertas-Dones C., Caicedo-Quiroga L., Giray T. (2012). Landscape analysis of drone congregation areas of the honey bee, Apis mellifera. J. Insect Sci. 12, 1-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibo D. L. (1977). A method for rearing various species of social wasps of the genus Polistes (Hymenoptera: Vespidae) under controlled conditions. Can. Entomol. 109, 1013-1015 [Google Scholar]
- Gilestro G. F. (2012). Video tracking and analysis of sleep in Drosophila melanogaster. Nat. Protoc. 7, 995-1007 [DOI] [PubMed] [Google Scholar]
- Gilestro G. F., Cirelli C. (2009). pySolo: a complete suite for sleep analysis in Drosophila. Bioinformatics 25, 1466-1467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giray T., Giovanetti M., West-Eberhard M. J. (2005). Juvenile hormone, reproduction, and worker behavior in the neotropical social wasp Polistes canadensis. Proc. Natl. Acad. Sci. USA 102, 3330-3335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giray T., Galindo-Cardona A., Oskay D. (2007). Octopamine influences honey bee foraging preference. J. Insect Physiol. 53, 691-698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giray T., Kence M., Oskay D., Döke M. A., Kence A. (2010). Scientific note: colony losses survey in Turkey and causes of bee deaths. Apidologie (Celle) 41, 451-453 [Google Scholar]
- Goodwin R. M., Lewis D. (1987). Honeybees use a biological clock to incorporate sun positions in their waggle dances after foraging under heavy overcast skies. N. Z. Entomol. 10, 138-140 [Google Scholar]
- Green C. B., Takahashi J. S., Bass J. (2008). The meter of metabolism. Cell 134, 728-742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwinner V. E. (1975). [The circannual rhythm of reproductive activity in the starling (Sturnus vulgaris) under the influence of homosexual and heterosexual mates of the same species]. Z. Tierpsychol. 38, 34-43 [PubMed] [Google Scholar]
- Harano K., Sasaki M., Sasaki K. (2007). Effects of reproductive state on rhythmicity, locomotor activity and body weight in the european honeybee, Apis mellifera queens (Hymenoptera, Apini). Sociobiology 50, 189-200 [Google Scholar]
- Hardin P. E. (2011). Molecular genetic analysis of circadian timekeeping in Drosophila. Adv. Genet. 74, 141-173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helfrich-Förster C., Nitabach M. N., Holmes T. C. (2011). Insect circadian clock outputs. Essays Biochem. 49, 87-101 [DOI] [PubMed] [Google Scholar]
- Hunt J. H. (1984). Rearing conditions influence quality signals but not individual identity signals in Polistes wasps. Insectes Soc. 31, 452-460 [Google Scholar]
- Johnson J. N., Hardgrave E., Gill C., Moore D. (2010). Absence of consistent diel rhythmicity in mated honey bee queen behavior. J. Insect Physiol. 56, 761-773 [DOI] [PubMed] [Google Scholar]
- Jones J. C., Myerscough M. R., Graham S., Oldroyd B. P. (2004). Honey bee nest thermoregulation: diversity promotes stability. Science 305, 402-404 [DOI] [PubMed] [Google Scholar]
- Kandemir I., Kence M., Kence A. (2000). Genetic and morphometric variation in honeybee (Apis mellifera L.) populations of Turkey. Apidologie (Celle) 31, 343-356 [Google Scholar]
- Kjærsgaard A., Demontis D., Kristensen T. N., Le N., Faurby S., Pertoldi C., Sørensen J. G., Loeschcke V. (2010). Locomotor activity of Drosophila melanogaster in high temperature environments: plastic and evolutionary responses. Clim. Res. 43, 127-134 [Google Scholar]
- Koeniger N., Koeniger G. (2000). Reproductive isolation among species of the genus Apis. Apidologie (Celle) 31, 313-339 [Google Scholar]
- Koh K., Evans J. M., Hendricks J. C., Sehgal A. (2006). A Drosophila model for age-associated changes in sleep:wake cycles. Proc. Natl. Acad. Sci. USA 103, 13843-13847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konopka R. J., Benzer S. (1971). Clock mutants of Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 68, 2112-2116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine J. D., Funes P., Dowse H. B., Hall J. C. (2002a). Resetting the circadian clock by social experience in Drosophila melanogaster. Science 298, 2010-2012 [DOI] [PubMed] [Google Scholar]
- Levine J. D., Funes P., Dowse H. B., Hall J. C. (2002b). Signal analysis of behavioral and molecular cycles. BMC Neurosci. 3, 1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohawk J. A., Takahashi J. S. (2011). Cell autonomy and synchrony of suprachiasmatic nucleus circadian oscillators. Trends Neurosci. 34, 349-358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore D., Doherty P. (2009). Acquisition of a time-memory in forager honey bees. J. Comp. Physiol. A 195, 741-751 [DOI] [PubMed] [Google Scholar]
- Moore D., Rankin M. A. (1985). Circadian locomotor rhythms in individual honey bees. Physiol. Entomol. 10, 191-197 [Google Scholar]
- Moore D., Rankin M. A. (1993). Light and temperature entrainment of a locomotor rhythm in honeybees. Physiol. Entomol. 18, 271-278 [Google Scholar]
- Moore D., Angel J. E., Cheeseman I. M., Fahrbach S. E., Robinson G. E. (1998). Timekeeping in the honey bee colony: integration of circadian rhythms and division of labor. Behav. Ecol. Sociobiol. 43, 147-160 [Google Scholar]
- Ouyang Y., Andersson C. R., Kondo T., Golden S. S., Johnson C. H. (1998). Resonating circadian clocks enhance fitness in cyanobacteria. Proc. Natl. Acad. Sci. USA 95, 8660-8664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page R. E., Jr, Scheiner R., Erber J., Amdam G. V. (2006). 8. The development and evolution of division of labor and foraging specialization in a social insect (Apis mellifera L.). Curr. Top. Dev. Biol. 74, 253-286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peschel N., Helfrich-Förster C. (2011). Setting the clock – by nature: circadian rhythm in the fruitfly Drosophila melanogaster. FEBS Lett. 585, 1435-1442 [DOI] [PubMed] [Google Scholar]
- Pittendrigh C. S., Bruce V. G. (1959). Daily rhythms as coupled ocillator systems and their relation to thermoperiodism and photoperiodism. In Photoperiodism and Related Phenomenon in Plants and Animals, Vol. 475-505 (ed. Withrow R. B.). Washington, DC: American Association of the Advancement of Sciences; [Google Scholar]
- Pittendrigh C., Daan S. (1976). A functional analysis of circadian pacemakers in nocturnal rodents I. The stability and lability of spontaneous frequency. J. Comp. Physiol. 106, 223-252 [Google Scholar]
- Reddy P., Zehring W. A., Wheeler D. A., Pirrotta V., Hadfield C., Hall J. C., Rosbash M. (1984). Molecular analysis of the period locus in Drosophila melanogaster and identification of a transcript involved in biological rhythms. Cell 38, 701-710 [DOI] [PubMed] [Google Scholar]
- Rivera-Marchand B., Oskay D., Giray T. (2012). Gentle Africanized bees on an oceanic island. Evol. Appl. 5, 746-756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross K. G., Matthews R. W. (1991). The Social Biology of Wasps. Ithaca, NY: Cornell University Press; [Google Scholar]
- SAS Institute Inc. (2009). JMP® 8 User Guide. Cary, NC: SAS Institute; [Google Scholar]
- Saunders D. S. (1972). Circadian control of larval growth rate in Sarcophaga argyrostoma. Proc. Natl. Acad. Sci. USA 69, 2738-2740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawyer L. A., Hennessy J. M., Peixoto A. A., Rosato E., Parkinson H., Costa R., Kyriacou C. P. (1997). Natural variation in a Drosophila clock gene and temperature compensation. Science 278, 2117-2120 [DOI] [PubMed] [Google Scholar]
- Sawyer L. A., Sandrelli F., Pasetto C., Peixoto A. A., Rosato E., Costa R., Kyriacou C. P. (2006). The period gene Thr-Gly polymorphism in Australian and African Drosophila melanogaster populations: implications for selection. Genetics 174, 465-480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid B., Helfrich-Förster C., Yoshii T. (2011). A new ImageJ plug-in “ActogramJ” for chronobiological analyses. J. Biol. Rhythms 26, 464-467 [DOI] [PubMed] [Google Scholar]
- Shemesh Y., Cohen M., Bloch G. (2007). Natural plasticity in circadian rhythms is mediated by reorganization in the molecular clockwork in honeybees. FASEB J. 21, 2304-2311 [DOI] [PubMed] [Google Scholar]
- Shemesh Y., Eban-Rothschild A., Cohen M., Bloch G. (2010). Molecular dynamics and social regulation of context-dependent plasticity in the circadian clockwork of the honey bee. J. Neurosci. 30, 12517-12525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu I., Kawai Y., Taniguchi M., Aoki S. (2001). Circadian rhythm and cDNA cloning of the clock gene period in the honeybee Apis cerana japonica. Zoolog. Sci. 18, 779-789 [Google Scholar]
- Spangler H. G. (1972). Daily activity rhythms of individual worker and drone honey bees. Ann. Entomol. Soc. Am. 65, 1073-1076 [Google Scholar]
- Stoleru D., Peng Y., Agosto J., Rosbash M. (2004). Coupled oscillators control morning and evening locomotor behaviour of Drosophila. Nature 431, 862-868 [DOI] [PubMed] [Google Scholar]
- Tibbetts E. A., Curtis T. R. (2007). Rearing conditions influence quality signals but not individual identity signals in Polistes wasps. Behav. Ecol. 18, 602-607 [Google Scholar]
- Toma D. P., Bloch G., Moore D., Robinson G. E. (2000). Changes in period mRNA levels in the brain and division of labor in honey bee colonies. Proc. Natl. Acad. Sci. USA 97, 6914-6919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres V. O., Montagna T. S., Raizer J., Antonialli-Junior W. F. (2012). Division of labor in colonies of the eusocial wasp, Mischocyttarus consimilis. J. Insect Sci. 12, 21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- United States Naval Observatory. Nautical Almanac Office, Great Britain. Nautical Almanac Office, Science and Engineering Research Council, Great Britain. Science Research Council, Great Britain. Rutherford Appleton Laboratory, Council for the Central Laboratory of the Research Councils, Great Britain. United States Department of the Navy. United States Congress. Hydrographic Office, Great Britain (2011). Astronomical Almanac for the Year and its Companion, the Astronomical Almanac Online. Washington, DC; London: US GPO; HMSO; [Google Scholar]
- United States Naval Observatory. Nautical Almanac Office, Great Britain. Nautical Almanac Office, Science and Engineering Research Council, Great Britain. Science Research Council, Great Britain. Rutherford Appleton Laboratory, Council for the Central Laboratory of the Research Councils, Great Britain. United States Department of the Navy. United States Congress. Hydrographic Office, Great Britain (2012). Astronomical Almanac for the Year and its Companion, the Astronomical Almanac Online. Washington, DC; London: US GPO; HMSO; [Google Scholar]
- United States Naval Observatory. Nautical Almanac Office, Great Britain. Nautical Almanac Office, Science and Engineering Research Council, Great Britain. Science Research Council, Great Britain. Rutherford Appleton Laboratory, Council for the Central Laboratory of the Research Councils, Great Britain. United States Department of the Navy. United States Congress. Hydrographic Office, Great Britain (2013). Astronomical Almanac for the Year and its Companion, the Astronomical Almanac Online. Washington, DC; London: US GPO; HMSO; [Google Scholar]
- Vanin S., Bhutani S., Montelli S., Menegazzi P., Green E. W., Pegoraro M., Sandrelli F., Costa R., Kyriacou C. P. (2012). Unexpected features of Drosophila circadian behavioural rhythms under natural conditions. Nature 484, 371-375 [DOI] [PubMed] [Google Scholar]
- Vaze K. M., Sharma V. K. (2013). On the adaptive significance of circadian clocks for their owners. Chronobiol. Int. 30, 413-433 [DOI] [PubMed] [Google Scholar]
- von Frisch K. (1967). The Dance Language and Orientation of Bees. Cambridge, MA: Harvard University Press; [Google Scholar]
- Winston M. L. (1987). The Biology of the Honey Bee. Cambridge, MA: Harvard University Press; [Google Scholar]
- Zehring W. A., Wheeler D. A., Reddy P., Konopka R. J., Kyriacou C. P., Rosbash M., Hall J. C. (1984). P-element transformation with period locus DNA restores rhythmicity to mutant, arrhythmic Drosophila melanogaster. Cell 39, 369-376 [DOI] [PubMed] [Google Scholar]