Abstract
Reporting in Developmental Cell, Forster et al (2014) show that the basal myoepithelial cell layer (via p63) directs the final maturation of the adjacent luminal cell sheet during pregnancy (Forster et al., 2014). Do all mammary epithelial cells both give and take instructions from others to create the milk machine?
Though some epithelial layers may look homogeneous, no cell works autonomously – we know this from myriad examples, ranging from the development of fly eyes to mouse limbs. However, the analysis of breast epithelial cell communities is beginning to reveal the remarkable degree of teamwork that enables mammary morphogenesis. Breast tissues have evolved relatively recently in evolutionary time as the defining feature of mammals; they respond to developmental cues with growth and colonization of a subcutaneous fat pad, multiplying and differentiating during pregnancy to enable the assembly of milk secretions and milk ejection on demand.
There are relatively simple design principles that could work effectively to perform this task; for example, cells could be predetermined with an on-off functionality. Instead, the mammalian breast comprises a robustly interactive and functionally heterogeneous team of cells, which is supremely adaptable to the local and systemically defined environment. Included in this population are cells that retain the blueprint for breast development, such that one single basal epithelial cell implanted into a fat pad divides to regenerate a balanced population comprising one to two luminal cells per basal cell (Shackleton et al., 2006), where the progeny self-organize into bi-layered ductal units with spacing exact enough to enable proliferation of lobulo-alveolar units and milk production. This is remarkable.
The molecular basis for teamwork and intercommunication of breast epithelial cells was implied many years ago by the observation that key endocrine factors such as estrogen work indirectly to induce growth in breast tissues; the epithelial cells that divide do not necessarily express the estrogen receptor (Clarke, 2003). Typically the sensory cells, expressing nuclear hormone receptors, and the responder cells are not one and the same (Brisken and O'Malley, 2010; Joshi et al., 2010). In breast cancer tissues, these sensory and effector functions are often combined; indeed the basis of this disease is likely to rely on this gain of autonomy.
A study from Ellisen and colleagues (Dev. Cell, this issue) describes a novel collaboration between the two principal mammary epithelial cell types, basal and luminal cells. This study aimed to evaluate the function of p63, a basal cell-specific transcription factor, in mammary gland. This protein is a member of the p53 super-family, indeed, it may be more ancient than p53. Like p53, it is a hub and a master regulator of cellular growth and responses; specifically it is key to the specification of epidermal appendages, and to the growth and differentiation of basal cell compartments of epithelial tissues. This molecule is the target for almost all known post-transcriptional regulator mechanisms (including splicing), and these modifications alter its function (Su et al., 2013), making it difficult to predict the effect of enhancing or inhibiting this molecule during any given process.
This new study shows that a loss of function of p63 in basal cells causes a failure of lactation. Given prior studies on the role of p63 in differentiation, intuitively, this phenotype might arise from a failure of terminal differentiation in the basal/myoepithelial community. Thus inadequate development of the myogenic program that lends this cell type its name and principal function might lead to a failure of contraction, and lack of milk ejection upon suckling. However, this outcome was not what this study found. Using in silico datasets that describe p63 target genes, Forster et al found that p63 regulated a gene previously implicated in the differentiation associated with the lactogenic switch. Thus, the EGF family member, neuregulin, was reduced in p63-low glands, which in turn induced a profound change in the associated luminal cells, with little activation of the principal lactogenic signaling pathway (Stat5). This study therefore proposes the activation of the p63-NRG-erbB4-Stat5 pathway during pregnancy as a novel paracrine circuit in the lactogenic program.
There are interesting questions that remain to be answered. Presumably this pathway is specifically activated during pregnancy, suggesting that one of the pathway components is regulated by a pregnancy-associated factor, perhaps p63 itself. Furthermore, the exact identification of the sensor and responder cells awaits a more thorough interrogation. New methods have increased the accuracy and specificity of luminal progenitor cell identification (Shehata et al., 2012), updating prior phenotyping that relied on CD61 expression. Indeed, a sub-population of luminal cells appears to be directly related to the process of alveologenesis. These so-called “alveolar progenitors” comprise approximately one out of four luminal cells (EpCAMhi CD49flo CD49b+ Sca1-), and have a somewhat mixed basal/luminal expression profile. How will the examination of this population integrate with the story presented here?
This story adds to a larger theme of breast epithelial cell interactions; these interactions govern all aspects of breast biology, including estrogen-driven ductal outgrowth, progesterone-mediated alveologenesis and stem cell dynamics. Some examples of known circuit mediators are illustrated in Fig. 1. It is not yet clear how individual cells become differentiated within epithelia; for example how does an ER positive cell evolve in a community of ER negative cells? (Approximately 1 cell in 8 expresses ER, and these are often evenly spaced in mature ductal populations). Are basal cells induced to acquire stem-ness by the proximity of Wnt-expressing stromal cells, or by a partner cell in the epithelial population? How are alveolar progenitor cells related to other luminal progenitors? Connections between differing neighbors and microenvironments make each combination of cells a unique and flexible entity. Together, this community of cells can balance form and function, even when the epithelium is initiated from non-canonical origins, such as single cells, or cells from different tissues.
Figure 1. Examples of known interactions that govern breast epithelial form and function.
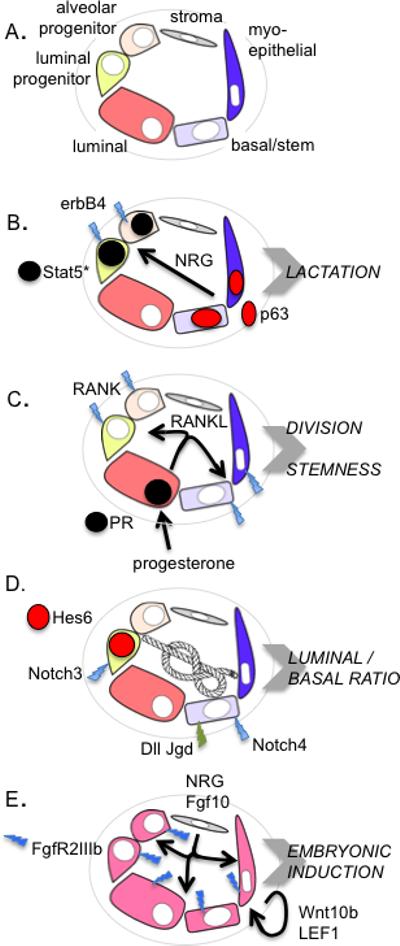
A. Different types of mammary epithelial cell are color coded as indicated. B. The example described here by Forster et al (2014). C. Progesterone impacts the epithelial population by exerting paracrine effects; one of the effects of progesterone is to induce RANKL, with effects on RANK-expressing luminal and basal cells (Joshi et al., 2010). D. Loss of function for Notch signaling decreases luminal/basal cell ratios, where gain of function increases the proportion of luminal cells; these lineages are tied together but the specifics that govern the interaction are unknown. The relative enrichment of expression of Notch receptors and Notch cell-surface ligands (Dll and Jgd) (together with Notch reporters, Hes6/Hey1) is illustrated for specific mammary epithelial cell types E. At least two paracrine factors are required to specify the mammary placodes; the survival of the placodes depends upon the subsequent induction of a Wnt signal (Alexander et al., 2012; Robinson, 2007).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alexander CM, Goel S, Fakhraldeen SA, Kim S. Wnt Signaling in Mammary Glands: Plastic Cell Fates and Combinatorial Signaling. Cold Spring Harbor perspectives in biology. 2012 doi: 10.1101/cshperspect.a008037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brisken C, O'Malley B. Hormone action in the mammary gland. Cold Spring Harbor perspectives in biology. 2010;2:a003178. doi: 10.1101/cshperspect.a003178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke RB. Steroid receptors and proliferation in the human breast. Steroids. 2003;68:789–794. doi: 10.1016/s0039-128x(03)00122-3. [DOI] [PubMed] [Google Scholar]
- Forster N, Saladi SV, van Bragt M, Sfondouris SE, Jones FE, Ellisen LW. Basal cell signaling by p63 controls luminal progenitor function and lactation via NRG1. Dev Cell. 2014 doi: 10.1016/j.devcel.2013.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi PA, Jackson HW, Beristain AG, Di Grappa MA, Mote PA, Clarke CL, Stingl J, Waterhouse PD, Khokha R. Progesterone induces adult mammary stem cell expansion. Nature. 2010;465:803–807. doi: 10.1038/nature09091. [DOI] [PubMed] [Google Scholar]
- Robinson GW. Cooperation of signalling pathways in embryonic mammary gland development. Nat Rev Genet. 2007;8:963–972. doi: 10.1038/nrg2227. [DOI] [PubMed] [Google Scholar]
- Shackleton M, Vaillant F, Simpson KJ, Stingl J, Smyth GK, Asselin-Labat ML, Wu L, Lindeman GJ, Visvader JE. Generation of a functional mammary gland from a single stem cell. Nature. 2006;439:84–88. doi: 10.1038/nature04372. [DOI] [PubMed] [Google Scholar]
- Shehata M, Teschendorff A, Sharp G, Novcic N, Russell A, Avril S, Prater M, Eirew P, Caldas C, Watson CJ, et al. Phenotypic and functional characterization of the luminal cell hierarchy of the mammary gland. Breast cancer research : BCR. 2012;14:R134. doi: 10.1186/bcr3334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su X, Chakravarti D, Flores ER. p63 steps into the limelight: crucial roles in the suppression of tumorigenesis and metastasis. Nature reviews Cancer. 2013;13:136–143. doi: 10.1038/nrc3446. [DOI] [PMC free article] [PubMed] [Google Scholar]


