Abstract
Background
It is unclear whether physical activity in later life is beneficial for maintenance of cognitive function. We performed a systematic review examining the effects of exercise on cognitive function in older individuals, and present possible mechanisms whereby physical activity may improve cognition.
Methods
Sources consisted of PubMed, Medline, CINAHL, the Cochrane Controlled Trials Register, and the University of Washington, School of Medicine Library Database, with a search conducted on August 15, 2012 for publications limited to the English language starting January 1, 2000. Randomized controlled trials including at least 30 participants and lasting at least 6 months, and all observational studies including a minimum of 100 participants for one year, were evaluated. All subjects included were at least 60 years of age.
Results
Twenty-seven studies met the inclusion criteria. Twenty-six studies reported a positive correlation between physical activity and maintenance or enhancement of cognitive function. Five studies reported a dose-response relationship between physical activity and cognition. One study showed a nonsignificant correlation.
Conclusion
The preponderance of evidence suggests that physical activity is beneficial for cognitive function in the elderly. However, the majority of the evidence is of medium quality with a moderate risk of bias. Larger randomized controlled trials are needed to clarify the association between exercise and cognitive function and to determine which types of exercise have the greatest benefit on specific cognitive domains. Despite these caveats, the current evidence suggests that physical activity may help to improve cognitive function and, consequently, delay the progression of cognitive impairment in the elderly.
Keywords: exercise, cognitive function, elderly
Introduction
An unprecedented growth of the aging population is taking place. For example, in 2000, 28% of adults aged 65 and older were expected to reach at least 90 years; this number is projected to rise to 47% by 2050, representing a near-doubling of the elderly population to 80 million.1 The economic impact of an aging population on health care systems is potentially overwhelming, in particular for age-related disorders such as dementia. In 2005, approximately 29.3 million individuals with dementia incurred a cost of US$315 billion worldwide, with the highest costs in North America and Europe.2 Since then, the global prevalence of dementia has increased to more than 34 million, and the bulk of disease burden is shifting from developed to developing countries.3 As such, effective interventions to help reduce the prevalence of cognitive disability in the elderly are needed. One possible intervention that deserves consideration is physical activity, an adjunct that has many well established health benefits and may serve to enhance quality of life.4 However, the effect of exercise on cognitive function remains controversial. A National Institutes of Health conference review of age-related cognitive decline reported a marginal benefit of exercise in one small randomized controlled trial (RCT) and eight observational studies showing a possible decrease in cognitive decline with exercise.4 A Cochrane review of eleven randomized clinical trials reported that aerobic exercise improved cognition in a few domains, including cognitive speed and auditory/visual attention, in subjects without cognitive impairment.5 Another Cochrane review of exercise in patients with dementia found only two relevant studies and concluded that there was insufficient evidence of benefit from exercise in these patients.6 These reviews did not find sufficient evidence to endorse exercise as beneficial to cognition, but were, overall, narrow in scope. However, other reviews have determined different results; for example, a more recent review concluded that an exercise regimen of one hour at least 3 times per week for 6 weeks was beneficial in subjects with or without cognitive impairment.7 We have performed a systematic review to assess the validity of the current data, including more recent randomized clinical trials and observational studies that provide a broad-based view of the effect of exercise on cognition in elderly persons.
Materials and methods
Studies
All RCTs with at least 30 participants and lasting at least 6 months, and all observational studies (prospective cohort studies, case-control studies, and longitudinal studies) with at least 100 participants and lasting at least one year, which were published in the English language on or after January 1, 2000 until August 15, 2012, and met the inclusion criteria were considered (Table 1).
Table 1.
Characteristics of included studies
| Parameters | Number of studies | References |
|---|---|---|
| Type of included studies | ||
| Randomized controlled trial | 10 | 8–10,17–21,26,27 |
| Prospective cohort | 15 | 11–16,22–24,55–60 |
| Case-control | 1 | 61 |
| Observational | 1 | 62 |
| Overall quality of included studies | ||
| Good | 9 | 9,10,14,17–22 |
| Fair | 15 | 8,11–13,15,23,26,27, 55–58,60–62 |
| Poor | 3 | 16,24,59 |
| Overall risk of bias in included studies | ||
| Low | 8 | 9,10,14,17,18,20–22 |
| Moderate | 16 | 8,11–13,15,19,23,26, 27,55–58,60–62 |
| High | 3 | 16,24,59 |
Participants
Only participants who were 60 years or older were included in this review. Studies examining the effects of physical activity in elderly individuals with or without mild cognitive impairment or cognitive disease (such as Alzheimer’s disease or other dementia) were included. Studies including participants with systemic disorders such as chronic obstructive pulmonary disease or diabetes, those with traumatic brain injury, or comorbidities that precluded participation in exercise programs were excluded.
Interventions
Physical activity was considered to be any aerobic or isometric exercise of any intensity, duration, or frequency that aimed to improve overall physical fitness. For randomized clinical trials, active interventions such as aerobic exercise, isometric exercise, health education programs with monitored exercise sessions, or physical therapy-driven exercise treatments were compared with control groups that received no intervention (Table 2).
Table 2.
Design, methods, interventions and assessment, and outcome measures in included studies
| Source and study design | Methods | Participants | Interventions | Cognitive function measurements |
|---|---|---|---|---|
| Buchman et al22 Prospective study |
Total daily physical activity was measured with actigraphs at baseline. Late-life physical, social, and cognitive activities were assessed by self-report and by the 1985 National Health Interview Survey questions at baseline and follow-up. Follow-up: annual assessment for 3.5±1.54 years |
716 subjects 114 males and 602 females 81.6±7.12 years Inclusion criteria: eligible participants of Rush Memory and Aging Project. Exclusion criteria: Presence of clinical AD dementia and non-AD dementia; unable to have at least one follow-up cognitive testing |
None | A computer-scoring battery of 19 tests Diagnosis of AD and non-AD was performed by clinicians using National Institute of Neurological and Communicative Disorders, Stroke-Alzheimer’s Disease and Related Disorders Association Criteria |
| Busse et al17 RCT |
Participants were randomized to a control group (n=14) or a treatment group (n=17). Cognitive status was assessed at baseline and follow-up using a neurocognitive test battery. Neither the participants nor the outcome assessors were blinded, and the use of allocation concealment is unclear. Follow-up: 3, 6, and 9 months |
31 subjects 8 males and 23 females 62–86 years Inclusion criteria: No programmed physical exercise 6 months prior to selection; subjective memory complaints; normal GDS and MMSE; changes in objective memory test; preserved function in instrumental and basic activities of daily living. Exclusion criteria: dementia, depression, anxiety disorders, head trauma or stroke within one year, substance abuse, unstable cardiovascular disease |
Treatment group: a one-hour biweekly training session for 9 months with 6 resistance-training exercises per session. Loads progressively increased in series of 12, 10, and 8 repetitions. Control group: no intervention |
Rivermead Behavioral Memory Test Wechsler Adult Intelligence Scale Direct and Indirect Digit Span Memory Complaints Scale Cambridge Cognitive Test |
| Bixby et al62 Observational |
Participants were recruited from a retirement community through posted flyers, closed-circuit television announcements, and investigator presentations. Physical activity levels were assessed using the YPAS. Cognitive and inhibitory executive function was assessed by the Stroop Color and Word Test. Follow-up: not applicable |
120 subjects 38 males and 82 females 65–92 years Retirement community residents recruited through posted flyers, closed-circuit television announcements, and investigator presentations. Inclusion criteria: above average intelligence; stable patterns of physical activity during a 3–5-year period before the study. Exclusion criteria: depression, dementia |
None Physical fitness assessment: YPAS and weekly energy expenditure (beyond basal metabolic rate). Stability in physical activity levels for 3–5 years prior to the study assessment: a health history questionnaire. Cognitive function assessment: Kaufman Brief Intelligence Test and Stroop Color and Word Test |
Kaufman Brief Intelligence Test Stroop Color and word Test |
| Cassilhas et al18 RCT |
Participants were randomized to 3 groups: a control group (n=23), moderate exercise group (n=19), and high exercise group (n=20). Use of blinding and allocation concealment in the study is unclear. Physical fitness was assessed at baseline and follow-up by the one RM test. Cognitive status was assessed at baseline and follow-up using a neuropsychological test battery. Follow-up: 24 weeks |
62 subjects All males 65–75 years Inclusion criteria: not described. Exclusion criteria: cardiovascular disease; psychiatric conditions; use of psychotropic drugs; <8 years of schooling; dementia (MMSE score <23) |
Moderate exercise group: Three one-hour sessions/week (10-minute cycling warm-up, stretching exercises and weight training using loads of 50% of one RM and alternating segments with two series of 8 repetitions for each segment). High exercise group: Three one-hour sessions/week (10-minute cycling warm-up, stretching exercises, and weight training using loads of 80% of one RM and alternating segments with two series of 8 repetitions for each segment). Control group: one weekly training session consisting of warm-up and stretching exercises, but no overload training |
Wechsler Adult Intelligence Scale III Wechsler Memory Scale-Revised Toulouse-Pieron concentration attention test Ray-Osterrieth complex figure |
| Geda et al61 Case-control |
Participants underwent stratified random sampling in to case or control group. Physical fitness was assessed through self-reporting at baseline. Cognitive status was assessed at baseline and follow-up using a neuropsychological test battery and visuospatial skills. Follow-up: 4 years |
1,324 subjects 681 males and 633 females 70–89 years Participants of the Mayo Clinic Study of Aging Cases: (n=198) cases with mild cognitive impairment based on: concern expressed by a physician or nurse; cognitive impairment in one or more tested domains; ability to participate in normal functioning activities; and free of dementia. Controls: (n=1,126) cases with normal cognitive function according to published normative criteria for the community |
None Physical fitness assessment: a self-reported questionnaire derived from the 1985 National Health Interview Survey and the Minnesota Heart Survey intensity codes. Cognitive function assessment: Mayo Clinic criteria for mild cognitive impairment |
Mayo Clinic criteria for mild cognitive impairment |
| Klusmann et al10 RCT |
This study enrolled German-speaking women from Berlin. Eligible subjects were randomized into 2 intervention groups (n=91 for exercise group, n=92 for computer group), and a control group (n=76). 12 participants (5 in the exercise group and 7 in the computer group) refused to participate after being informed about their group assignment and withdrew consent before treatment started. A complete neuropsychological assessment and physical evaluation at baseline and 6 months. Follow-up: 6 months |
259 subjects All female Age >70 years Eligibility criteria: being unfamiliar with the computer and exercising less than one hour per week. Exclusion criteria: severe visual or hearing impairment; a previous or current diagnosis of depression or psychosis; any other neurological or medical disorder that would interfere with cognitive performance or preclude successful participation in the intervention programs |
Exercise group: exercise program consisted of aerobic endurance, strength, and flexibility training, as well as practice of balance and coordination. Computer group: heterogeneous and multifaceted themes including creative matters, coordinative and memory tasks, eg, operating with the common software and hardware, writing, playing, calculating, surfing on the Internet, emailing, drawing, image editing, and videotaping. Control group: continued their habitual life |
Neuropsychological assessment RBMT, FCSRT, TMT, and Stroop Test |
| Ku et al11 Prospective study |
Physical activity and activities of daily living were assessed through questionnaires. The survey was conducted every 3–4 years from 1996 to 2007. Cognitive performance was assessed using the 10-item SPMSQ. Follow-up: 11 years |
1,160 subjects 586 males and 574 females, ≥67 years Inclusion criteria: participants of the longitudinal Survey of Health and Living Status of the Elderly (Taiwan Department of Health) aged ≥67 years. Exclusion criteria: not stated |
None Physical activity assessment: questionnaire survey at baseline. Cognitive performance assessment: SPMSQ that was validated for the Chinese version of the MMSE |
Ten-item SPMSQ |
| Larson et al55 Prospective study |
The current study was to examine the temporal relationship of physical exercise preceding development of dementia. Physical activity was assessed at baseline by an interview. Cognitive function was assessed at baseline and follow-up using the CASI. Follow-up: biennially for 6.2 years |
1,740 subjects 693 males and 1,047 females, ≥65 years Participants of the ACT study Inclusion criteria: ACT study participant with CASI score above the 25th percentile; residing in the Seattle area at time of study. Exclusion criteria: pre-existing dementia or cognitive impairment |
None Physical exercise assessment: subject interview (number of days per week and number of hours per session) during the past year. Participants who exercised at least 3 times a week were classified as regular exercisers. Cognitive status and incident dementia assessment: CASI |
CASI (score <86 resulted in a neuropsychological clinical evaluation) DSM-IV criteria for dementia (25 criteria in total) |
| Laurin et al12 Prospective study |
Physical activity and cognitive status were assessed at baseline and follow-up. Follow-up: 5 years |
4,615 subjects 1,831 males and 2,784 females, ≥65 years Participants of the 1991–1992 Canadian Study of Health and Aging, a prospective cohort study of dementia. Inclusion criteria: age ≥65 years and registered in the 1991–1992 Canadian Study of Health and Aging. Exclusion criteria: dementia |
None Physical fitness assessment: a self-administered questionnaire by mail. Cognitive function assessment: 3MS |
3MS score (a reduction of ≥5 points indicative of cognitive decline) |
| Lytle et al13 Prospective study |
Physical activity was assessed at baseline through self-reporting and cognitive function was assessed at baseline and at follow-up using the MMSE. Follow-up: 2 years |
1,146 participants 722 males versus 424 females, ≥65 years Participants of MoVIES Inclusion criteria: Residing in the community of Monongahela Valley (not in a skilled care facility) at time of recruitment; fluent in English and having at least a 6th grade education. Exclusion criteria: not described |
None Physical activity assessment: a standardized questionnaire. Exercise was classified as aerobic (high level) or anaerobic (low level). Cognitive status assessment: MMSE |
MMSE score (drop of at least 3 points between assessments was indicative of cognitive decline) |
| Middleton et al14 Prospective study |
Physical fitness was assessed at baseline and follow-up by using a battery of fitness and metabolism tests, and cognitive function was assessed at baseline and follow-up by using the 3MS. Follow-up: 2 or 5 years |
197 subjects Sex not stated 70–79 years Participants of the Health ABC study. Inclusion criteria: ability to walk 0.4 km, climb ten stairs, and perform basic activities of daily living without difficulty; no plans to leave the area for the next 3 years. Exclusion criteria: life-threatening illness; mobility limitations; cognitive impairment (3MS score <80) |
None Physical fitness assessment: doubly-labeled water techniques for total energy expenditure measurement between baseline and follow-up; a respiratory gas analyzer for measuring resting metabolic rate; an interviewer-administered questionnaire at first visit to ascertain physical activity habits over the past 7 days. Cognitive status assessment: 3MS score |
3MS score (a decline of at least one standard deviation or 9 points from baseline to the most recent follow-up visit indicated cognitive decline) |
| Miu et al9 RCT |
Participants were randomized to a control group (n=49) or a physical activity treatment group (n=36). Physical fitness was assessed at baseline and follow-up by using a battery of physical function tests. Cognitive status was assessed at baseline and follow-up using the MMSE and the ADAS-Cog. Follow-up: 3, 6, 9, and 12 months |
85 participants Sex not clearly stated ≥65 years Participants of the memory clinic at a regional hospital in Hong Kong Inclusion criteria: mild-to-moderate dementia; MMSE 10–26; age >60 years; community-dwelling; ambulatory, having a caregiver willing to participate and escort the patient to the hospital for training and assessment. Exclusion criteria: severe dementia or MMSE score <10 |
Treatment group: aerobic exercise training supervised by a physiotherapist, including treadmill, bicycle, and arm ergometry, and 10-minute flexibility training prior to each session. Training sessions occurred biweekly and lasted 45–60 minutes each. Total duration of treatment was 3 months. Control group: no intervention |
MMSE ADAS-Cog |
| Mortimer et al19 RCT |
Participants were randomized into 4 groups: group 1, Tai Chi (n=30); group 2, walking (n=30); group 3, social (n=30); and group 4, no intervention (n=30) for a total of 40 weeks. | 120 subjects 40 males and 80 females 60–79 years Inclusion criteria: participants of Jingansi Temple Community of Shanghai, China aged 60–79 years. Exclusion criteria: history of stroke, Parkinson’s disease, or other neurological disease; inability to walk unassisted for 2 km or maintain balance with feet side-by-side or semi-tandem for 10 seconds each; education-adjusted Chinese MMSE <26; cardiovascular disease; musculoskeletal conditions; contraindication for MRI; unable to participate in the full study and regular vigorous exercise or Tai Chi practice |
Tai Chi: practising 3 times per week (20 minutes warm-up, 30 minutes of Tai Chi practice, and 10 minutes cool-down). Walking: a 400 m circular walking (10 minutes of warm-up, 30 minutes of brisk walking, and 10 minutes of cool-down exercise). Social interaction: meeting with group leader for one hour 3 times per week. Brain volume assessment: MRI at baseline and at the end of intervention (40 weeks). Cognitive assessment: neuropsychological battery at baseline, 20 weeks, and 40 weeks |
Brain volume using MRI Neuropsychological battery test |
| Muscari et al20 RCT |
Subjects were randomized into a control group (n=60) and a physical activity treatment group (n=60). Cognitive status was assessed at baseline and follow-up by using the MMSE. Follow-up: 12 months |
120 subjects 62 males and 58 females 65–74 years Inclusion criteria: participants of the Pianoro Study, aged 65–74 years. Exclusion criteria: Presence of any cardiovascular disease and the followings; MMSE score <24; BMI <18 or >32; systolic BP >180 or <110 mmHg; diastolic BP <110 mmHg; malignancy; moderate or severe respiratory insufficiency; severe arthrosis; recent fractures, palsy or relevant neuromotor deficits; hemoglobin <11 g/dL; aortic aneurysm >3.5 cm |
Treatment group: 12 months of 3 one hour-long sessions per week of supervised endurance exercise training in a community group. Control group: education to improve lifestyle and self-administered programs to increase physical activity |
MMSE score (decrease of greater than one point was indicative of cognitive decline) |
| Nagamatsu et al26 RCT |
Subjects were randomized, single-blinded into: twice-weekly resistant training (n=28); twice-weekly aerobic training (n=30); or twice-weekly balance and tone training (control) group (n=28). Follow-up: 6 months |
86 subjects All females 70–80 years Inclusion criteria: female, aged 70–80 years, with probable mild cognitive impairment. Exclusion criteria: not described |
Resistant training group: a Keiser pressurized air system and free weights were used. Aerobic training group: an outdoor walking program. Balance and tone training (control) group: stretching, range of motion, balance exercises, and relaxation technique |
Primary outcome measure: Stroop Test performance Secondary outcome measures: TMT, Verbal Digits Test Memorizing face-scene pairs Everyday Problem Test |
| Nguyen et al27 RCT |
Participants were randomly divided into 2 groups; Tai Chi (n=48) and control (n=48) group. Experienced Tai Chi instructors were selected by investigators to teach classes. Outcome measures were assessed at baseline and the end of 6-month Tai Chi training. Follow-up: 6 months |
102 subjects 48 males and 48 males 60–79 years Inclusion criteria: MMSE score >25; having no experience in Tai Chi. Exclusion criteria: serious diseases such as symptomatic coronary diseases, angina, arrhythmia, orthostatic hypotension, and dementia |
Treatment group: a 60-minute Tai Chi session twice a week for 6 months. The session consisted of a 15-minute warm-up and cool-down period. Control group: no intervention, maintained daily routine activities and not to begin any new exercise program |
TMT for motor speed and visual attention |
| Podewils et al56 Prospective study |
Physical activity information was assessed at baseline and follow-up by interview and the 3MS, respectively. Follow-up: annually for 5.4 years |
3,375 subjects 1,350 males and 2,025 females ≥65 years Inclusion criteria: enrollment in Cardiovascular Health Cognition Study; residing in Sacramento County, CA, Washington County, MD, Forsyth County, NC, or Pittsburgh, PA, USA. Exclusion criteria: dementia |
None Physical activity assessment: modified Minnesota Leisure Time Activity Questionnaire. Cognitive status assessment: 3MS or the Telephone Interview for Cognitive Status for participants who did not receive a clinical evaluation |
3MS score (<80 within the last 2 visits; decline of at least 5 points within the follow-up period; Telephone Interview for Cognitive Status score of <28; diagnosis of dementia that was documented in medical records |
| Ravaglia et al23 Prospective study |
Physical activity was self-reported at baseline by using a questionnaire and cognitive status was measured at baseline and follow-up by using a neuropsychological test battery. Participants were screened for incident dementia using an extensive neuropsychological test battery. Follow-up: 4 years |
749 subjects 348 males and 401 females, ≥65 years Inclusion criteria: individuals ≥65 years in the Conselice Study of Brain Ageing. Exclusion criteria: per the Conselice Study of Brain Ageing |
None Physical fitness assessment: Paffenbarger Physical Activity Questionnaire. Cognitive status assessment: GDS, MMSE, and MDB for use in rural and poorly educated subjects |
MMSE (cognitive impairment defined as a score of <24) MDB |
| Scarmeas et al57 Prospective study |
Physical activity was self-reported at baseline, and cases of incident dementia were identified at each follow-up using a neuropsychological test battery in conjunction a consensus diagnosis among an expert panel based on the DSM-IV criteria. Follow-up: every 1.5 years for 15 years |
1,880 participants 587 males and 1,293 females 70–82 years Participants were recruited through the WHICAP from a sample of Medicare beneficiaries in northern Manhattan. Inclusion criteria: WHICAP participants. Exclusion criteria: not described |
None Physical activity assessment: two versions of the Godin leisure time exercise questionnaire. Cognitive status assessment: a neuropsychological test battery testing the domains of memory, language, reasoning, processing speed, and visual-spatial ability |
The decision of expert panel composed of neurologists and neuropsychologists in according to DSM-IV criteria |
| Schuit et al24 Prospective study |
Physical activity was assessed at baseline through self reporting. Cognitive function was assessed at baseline and follow-up using the MMSE. Follow-up: 3 years |
347 participants All males 70–80 years Inclusion criteria: participants of the Zutphen Elderly Study, the Netherlands. Exclusion criteria: per the Zutphen Elderly Study |
None Physical fitness assessment: a self-administered questionnaire at baseline. Physical activity was categorized as either “maximal 1 hour/day” or “more than 1 hour/day”. Cognitive status assessment: MMSE at baseline and follow-up |
MMSE (drop in 3 points indicative of cognitive decline) |
| Smiley-Oyen et al21 RCT |
Participants were randomized to an aerobic physical activity (Cardio, n=28) group or a strength-and-flexibility (Flex-Tone, n=29) training group. Physical fitness was assessed at baseline and follow-up using a battery of fitness tests, and cognitive status was assessed at baseline and follow-up by using a battery of neurocognitive tests. Follow-up: at 4 and 10 months |
62 subjects 16 males and 41 females 65–79 years Residents of the mid-western US Inclusion criteria: living independently; able to exercise safely. Exclusion criteria: various health-related reasons (autoimmune disease, cancer diagnosis within the previous 5 years, or conditions which may be exacerbated by strenuous exercise); being “too-fit” (participation in exercise >3 times/week at >40% of their heart rate reserve), or participant’s aerobic fitness level above the 75th percentile for their age and sex by a time walking test) |
Cardio group: a 10-month of tri-weekly training sessions (10-minute warm-up, 25–30 minutes of aerobic exercise on the equipment of the participant’s choice (treadmill, stair-stepping machine, stationary cycle, and elliptical machine), and a 10-minute cool down. Flex-Tone group: A 10-month tri-weekly training sessions (10-minute warm-up, 25–30 minutes of strength, flexibility, and balance exercises (yoga, Tai Chi, Flex bands, free hand weights, resistance weight training machines, and stability balls), and a 10-minute cool down; 8–10 exercises of 1–15 repetitions each were performed |
Reaction time tests including simple reaction time, 8-choice reaction time, 8-choice incompatible reaction time, and Go/No-Go reaction time Stroop Color and Word Test Wisconsin Card Sort Test |
| Taaffe et al58 Prospective study |
Physical activity was assessed at baseline by self-reporting and performance. The incident dementia was assessed at baseline and follow-up using CASI. Follow-up: at 3 and 6 years |
2,263 subjects All males 71–92 years Inclusion criteria: enrollment in the Honolulu-Asia Aging Study; Japanese-American men born between 1900 and 1919 living on the island of Oahu, Hawaii. Exclusion criteria: dementia |
None Physical activity assessment: a self-reported questioner. Physical function assessment: four performance tasks, ie, timed walk, sitting-to-standing time, grip strength, and balance. Cognitive status assessment: CASI used as the initial assessment. For CASI <74, subjects underwent a second phase of screening (a repeat CASI and administration of the IQCODE). Men with an IQCODE score of >3.6 underwent a third phase assessment (a standardized interview and neuropsychological battery, neurological examination, neuroimaging, and blood testing) |
CASI (scores <74 indicative of possible dementia) IQCODE (scores <3.6 indicative of probable dementia) Diagnoses were finally decided by consensus of an appointed expert panel |
| Van Gelder et al59 Prospective study |
Physical activity was assessed at baseline and at follow-up by using a self-administered questionnaire, and cognitive status was assessed at baseline and at follow-up by using MMSE. Follow-up: at 5 and 10 years |
295 subjects All males 70–90 years Inclusion criteria: participants of the Surviving cohorts of the Seven Countries Study in Europe. Exclusion criteria: poor health (myocardial infarction, stroke, diabetes, or cancer); severe cognitive impairment (MMSE <18) |
None Physical fitness assessment: a self-administered questionnaire. Physical activity was categorized into four groups: <30, 31–60, 61–120, and >120 minutes per day. Cognitive status assessment: MMSE |
MMSE (scores <18 indicative of cognitive decline) |
| Wang et al60 Prospective study |
Physical fitness was assessed at baseline by using a physical function test battery and cognitive function was assessed at baseline and follow-up by using a neurocognitive test battery. Follow-up: biennially through October 2003 (8–10 years) |
2,228 subjects 863 males versus 1,365 females ≥65 years Inclusion criteria: participants of the Adult Changes in Thought study (1994–1996) exclusion criteria: CASI <86; dementia; invalid measurements on the cognitive performance test or physical performance test at baseline; persons without a follow-up examination |
None Physical fitness assessment: Four different physical performance tests (timed walk, seating-to-standing time, standing balance, grip strength). Cognitive function assessment: CASI |
CASI (score ≥86 were categorized as dementia-free) |
| Wang et al15 Prospective study |
Leisure activity levels and cognitive status assessment were performed at baseline and follow-up. Follow-up: mean 2.4 (2.3–2.6) years |
1,463 subjects 744 males and 719 females ≥65 years Participants of a longitudinal population-based study of aging in the People’s Republic of China between 2003 and 2005. Inclusion criteria: residents aged ≥65 years in the study regions. Exclusion criteria: baseline global cognitive score in the bottom 10%; physical disability |
None Leisure activities assessment: a self-reported questionnaire in predefined list of mental, physical and social activities. Cognitive assessment: face-to-face interviews at the home of subjects using the followings; CSID, Word List Learning, Word List Recall, IU Story Recall, Animal Fluency Test, IU Token Test |
Global cognitive function: CSID Episodic memory: Word List Learning, Word List Recall, IU Story Recall Language: Animal Fluency Test Executive function: IU Token Test |
| Williamson et al8 RCT |
Subjects were randomized to a control group (n=52) or a physical activity treatment group (n=50). Physical fitness was assessed at baseline and follow-up using a battery of performance tests for balance, walking speed, and sitting-to-standing time. Cognitive function was assessed at baseline and follow-up by using a neuropsychological test battery. Follow-up: 12 months |
102 participants 50 males and 72 females 70–89 years Participants of the LIFE-P study Inclusion criteria: sedentary lifestyle; ability to walk 400 m in 15 minutes without resting or assistance; SPPB score ≤9. Exclusion criteria: MMSE <21; life expectancy of <12 months; heart disease; severe neurological conditions such as Parkinson’s disease during time of study |
Treatment group: physical activity intervention consisting of a combination of aerobic, strength, balance, and flexibility exercises divided into 3 phases: adoption (weeks 1–8), transition (weeks 9–24), and maintenance (week 25 to end of study). Control group: health education intervention designed to provide attention and health education to participants. Participants met in small groups weekly for the first 26 weeks and then monthly to the end of the study |
Digit Symbol Substitution Test Modified Stroop Test MMSE The Rey Auditory Verbal Learning Test |
| Yaffe et al16 Prospective study |
Physical activity was self-reported at baseline through both interview and questionnaire. Cognitive function was assessed at baseline and follow-up using the 3MS. Follow-up: 6–8 years |
5,925 subjects All females ≥65 years Inclusion criteria: participants of the Study of Osteoporotic Fractures (a prospective study for risk factors of fractures in Baltimore, MD, Minneapolis, MN, Pittsburgh, PA, or Portland, OR, USA). Exclusion criteria: black women; unable to walk without assistance; bilateral hip replacements; baseline cognitive impairment; baseline physical limitations |
None Physical fitness assessment: a self-reported questionnaire and a modified Paffenbarger scale (to quantify frequency and duration of weekly participation in 33 different physical activities) administered by trained interviewees. Cognitive function assessment: 3MS |
3MS score (cognitive decline defined as a decrease in 3 or more points from baseline to follow-up) |
Abbreviations: 3MS, Modified Mini-Mental State; ACT, Adult Changes in Thought; ADAS-Cog, Alzheimer’s Disease Assessment Scale-Cognitive subscale; BMI, body mass index; BP, blood pressure; CASI, Cognitive Abilities Screening Instrument; CSID, Community Screening for Dementia; DSM-IV, Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition; Health ABC, Health, Aging, and Body Composition; GDS, Geriatric Depression Scale; YPAS, Yale Physical Activity Survey; LIFE-P, Lifestyle Interventions and Independence for Elders Pilot; MDB, Mental Deterioration Battery; MMSE, Mini-Mental State Examination; RBMT, Rivermead Behavioural Memory Test; FCSRT, Free and Cued Selective Reminding Test; MRI, magnetic resonance imaging; TMT, Trail Making Tests; SPMSQ, Short Portable Mental Status Questionnaires; 3GM, Modified Mini-Mental Status Examination; MoVIES, Monongahela Valley Independent Elders Survey; SPPB, Short Physical Performance Battery; RCT, randomized controlled trial; WHICAP, Washington Heights-Inwood Columbia Aging Project; IQCODE, Informant Questionnaire on Cognitive Decline in the Elderly; AD, Alzheimer’s disease; RM, repetition maximum; IU, Indiana University.
Outcome measures
The primary outcome measurement was cognitive function. The most commonly used tests included the Mini-Mental State Examination (MMSE) or Modified Mini-Mental State Examination (3MS), both of which give a global measure of cognitive function, and the Cognitive Ability Screening Instrument (CASI), which indicates the presence or absence of dementia (Table 2). A neuropsychological test battery with published criteria (such as the Mayo Clinic Criteria for dementia) utilized by an expert panel in diagnosing the presence or absence of dementia was also accepted.
Search methods for study identification
We searched PubMed, Medline, CINAHL, the Cochrane Controlled Trials Register, and the University of Washington School of Medicine Library database on August 15, 2012 for studies published in English on or after January 1, 2000. We used MeSH terms to find studies of physical activity including: adaptation, physiological/physiology*, exercise/physiology*, and physical fitness/physiology*. To reduce our findings to studies that measured cognition or incidence of cognitive disease and physical fitness, we searched using the following MeSH terms: cognition, cognitive disease, cognition disorders/prevention and control, cognition/physiology*, brain/physiology*, memory/physiology*, motor activity/physiology*, neuropsychological tests, dementia, and Alzheimer’s disease. To further reduce our findings to studies that focused on elderly human subjects, we searched using the MeSH terms: humans, elderly, aged, aging, old, older, and geriatric.
Data collection
Two reviewers screened the titles and abstracts of all studies identified by the search (71 studies) and irrelevant studies were excluded. Relevant papers were then assessed in full for inclusion eligibility.
Quality assessment
Two reviewers assessed the methodological quality of the selected studies. The Agency for Healthcare Research and Quality Methods Reference Guide for Effectiveness and Comparative Effectiveness Reviews was used to perform quality assessment of the trials. These criteria include information on sampling method, outcome measurement, intervention, and reporting of biases and limitations. A summary of these criteria is presented in Table S1.
Results
Description of studies
Seventy-one studies were identified using the database as described in the Materials and methods section. After removal of duplicates, 57 full-text articles were assessed for eligibility (Figure 1). Thirty studies were excluded, leaving 27 studies that were eligible for review according to the prespecified criteria (Figure 1). A total of 30,572 subjects over 60 years of age were included in the 27 studies that met our inclusion criteria. The characteristics of the studies included in this review are described in Tables 1, 2, and 3. The 30 excluded studies are described in Table S2.
Figure 1.
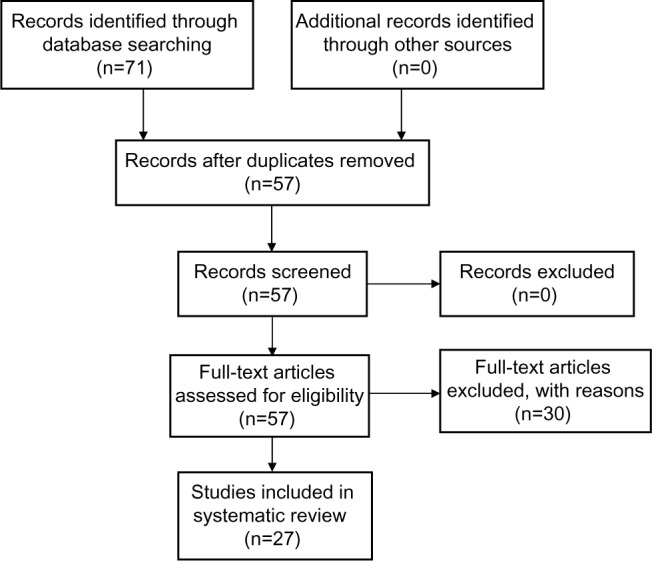
Description of studies which were identified, screened, and included in the systematic review.
Table 3.
Results of included studies
| Source and study design | Results |
|---|---|
| Buchman et al22 Prospective study |
Total daily physical activities were associated with incident Alzheimer’s disease (hazard ratio 0.477, 95% confidence interval 0.273–0.832). |
| Busse et al17 RCT |
After 9 months, the physical activity group showed a significant increase in RBMT score from pre-test to post-test, while the control group showed no increase. |
| Bixby et al62 Observational |
A small but significant association between physical fitness and executive function in the sample of older men and women. |
| Cassilhas et al18 RCT |
Both moderate-intensity and high-intensity resistance exercise programs had equally beneficial effects on cognitive functioning. However, the study was not able to identify a dose-response relationship between level of exercise and level of cognitive functioning. |
| Geda et al61 Case control |
The odds ratio for any frequency of exercise of at least a moderate level in late life was 0.68, suggesting that any frequency of moderate-intensity exercise performed in late life is associated with a reduced odds ratio of mild cognitive impairment. |
| Klusmann et al10 RCT |
Both the exercise group (mean ± SD change 2.09±2.66, P<0.001) and the computer group (mean ± SD change 1.89±2.88, P<0.001) showed improved delayed story recall. They maintained performance in delayed word recall and working memory (time measure) as opposed to the control group that showed a decline (mean ± SD change −0.91±2.15, P=0.001, and mean ± SD change 0.24±0.68, P=0.04, respectively). In conclusion, in older healthy women, exercise and computer classes seem to generate equivalent beneficial effects. |
| Ku et al11 Prospective study |
Using the multivariate adjustment (controlling for sociodemographic variables, lifestyle behavior, and health status), higher initial levels of physical activity were significantly associated with better initial cognitive performance (standardized coefficient β=0.17). A higher level of physical activity at baseline was significantly related to slower decline in cognitive performance, as compared with a lower level of activity (β=0.22). The authors conclude that physical activity in later life is associated with slower age-related cognitive decline. |
| Larson et al55 Prospective study |
During the follow-up period, 158 participants developed dementia while 107 developed Alzheimer’s disease. The interaction between exercise and incident dementia or Alzheimer disease was found to be statistically significant. The incidence rate of dementia was 13.0 per 1,000 person-years for participants who exercised 3 or more times per week, compared with 19.7 per 1,000 person-years for those who exercised less than 3 times per week. Similar results were observed in analyses for incident Alzheimer’s disease. |
| Laurin et al12 Prospective study |
The results showed that, compared with no exercise, physical activity was significantly associated with lower risks of cognitive impairment of all types, including dementia and Alzheimer’s disease. Furthermore, a significant dose-response relationship was observed whereby greater physical activity was associated with increased protection for cognitive decline and disease. |
| Lytle et al13 Prospective study |
A significant negative association (positive effect of exercise) between both low and high exercise and cognitive decline was observed. |
| Middleton et al14 Prospective study |
The results showed that older adults in the highest level of activity energy expenditure had lower odds of incident cognitive impairment than those in the lowest levels of activity energy expenditure. Furthermore, a significant dose-response relationship was observed between incident activity energy expenditure and incidence of cognitive impairment. |
| Miu et al9 RCT |
Eighty-two patients were available for analysis. The results showed no statistically significant difference between the treatment or control groups in terms of cognitive function. |
| Mortimer et al19 RCT |
One hundred and twenty subjects were analyzed. In comparison with a no intervention group, Tai Chi and social intervention showed an increase of brain volume via magnetic resonance imaging (P<0.05) and improvement in several neuropsychological measures (P<0.05). No difference was observed between the walking and the no intervention group. This result differs from the previous trials in that the increase of brain volume and cognitive function in the current study is associated with nonaerobic exercise and social interaction. |
| Muscari et al20 RCT |
One hundred and nine patients were available for analysis. A significant decrease in MMSE score in the control group was observed, and the odds ratio for treated older adults having stable cognitive status one year later (as compared with the control group) was 2.74, suggesting that a 12-month endurance exercise training program may delay the onset of age-related cognitive decline in the elderly. |
| Nagamatsu et al26 RCT |
Resistance training participants had significantly improved performance on the Stroop Test, an executive cognitive test of selective attention/conflict resolution and the associated memory task compared with subjects in a balance and tone training group (P=0.04 and P=0.03, respectively). This study suggests that twice-weekly resistance training could alter the trajectory of cognitive decline in seniors with mild cognitive impairment. |
| Nguyen et al27 RCT |
There were no significant differences between balance, sleep quality, and cognitive performance test. At the end of the study, participants in the Tai Chi training group showed a significantly (P<0.001) higher Trail Making Test score in part A (44.2±4.5 versus 35±4.3) and part B (118.3±6.4 versus 102±5). |
| Podewils et al56 Prospective study |
Participants in the highest quartile of physical energy expenditure had a relative risk of dementia of 0.85 compared with those in the lowest quartile, and participants who participated in more than four physical activities had a relative risk of dementia of 0.51 as compared with those who participated in 0 or 1 physical activities. Similar results were observed with risk of Alzheimer’s disease. |
| Ravaglia et al23 Prospective study |
Physical activity is associated with a lowered risk of vascular dementia, but not of Alzheimer’s disease. |
| Scarmeas et al57 Prospective study |
During a mean of 5.4 years of follow-up, a total of 282 incident Alzheimer’s disease cases occurred. The hazard ratio for some physical activity (compared with no physical activity) was 0.67, and for much physical activity was 0.67. This study suggests that physical activity is associated with a reduced risk for Alzheimer’s disease. |
| Schuit et al24 Prospective study |
Subjects with one hour or less of daily physical activity were at doubly increased risk of cognitive decline as compared with subjects who participated in more than one hour of physical activity daily. This study suggests that promotion of physical activity at an advance aged may reduce the risk of cognitive decline. |
| Smiley-Oyen et al21 RCT |
The results showed improvements in performance on the Stroop Color and Word Test only in the aerobic exercise group, and the study failed to show a dose-response relationship. |
| Taaffe et al58 Prospective study |
For men with low physical function at baseline, high levels of exercise were associated with half the dementia risk as compared with men who were the least active. A moderate level of physical activity was found to be protective, because the risk of dementia and Alzheimer’s disease decreased significantly with higher levels of physical activity. However, the study was not able to identify a correlation between dementia and Alzheimer’s disease risk and physical activity in men with moderate or high levels of physical activity at baseline. |
| Van Gelder et al59 Prospective study |
While there was no difference in the rates of cognitive decline between men with a high or low duration of physical activity at baseline, it was observed that a decrease in physical activity duration >60 minutes per day over 10 years resulted in a decline of 1.7 points in the MMSE. Further, men in the lowest physical activity intensity quartile had a 10-year cognitive decline 1.8 times greater than that observed in men in the higher physical activity intensity quartiles. This study suggests that participation in physical activities of at least low-medium intensity in old age may delay the onset of cognitive decline. |
| Wang et al60 Prospective study |
During the 10-year period, 319 participants developed dementia and 221 developed Alzheimer’s disease. The results showed that a one-point decrease in performance-based physical function test scores was associated with an increased risk of dementia and Alzheimer’s disease. This study suggests that poor physical function may lead to onset of dementia and Alzheimer’s disease, while higher levels of physical fitness may delay onset of cognitive decline and disease. |
| Wang et al15 Prospective study |
A high level of physical activity was related to less decline in episodic memory (P<0.05) and language (P<0.01). When mental, physical, and social activities were integrated into a composite activity index, a dose-response pattern was observed. |
| Williamson et al8 RCT |
Ninety participants were available for analysis at the end of the study. The results did not show a significant difference between the groups; however, improvements in cognitive test scores on the Digit Symbol Substitution Test, Rey Auditory and Verbal Learning Test, and modified Stroop Test were associated with improvements in physical function. |
| Yaffe et al16 Prospective study |
Women with a greater baseline physical fitness level were less likely to undergo cognitive decline during the 6–8-year follow-up period. A dose-response relationship was observed whereby cognitive decline occurred in 17%, 18%, 22%, and 24% of women in the highest, third, second, and lowest quartiles of physical activity as measured by blocks walked per week. Similar results were obtained when analyzing quartiles of kilocalorie expenditure. This study suggests that women with higher levels of baseline physical activity and fitness are less likely to develop cognitive decline. |
Abbreviations: RBMT, Rivermead Behavioural Memory Test; RCT, randomized controlled trial; MMSE, Mini-Mental State Examination.
Type of included studies, and quality and bias
Fifteen prospective cohorts, ten RCTs, one case-control study, and one observational study met the criteria for review (Table 1). Eight studies were considered to be of high quality. One study,8 an RCT, was considered to be of fair quality, rather than high, because approximately 12% of patients dropped out of the study and could not be assessed. The majority of studies (15/27) were of fair quality while three were considered poor quality. The overall risk of bias was moderate for the majority of the studies (16/27) with eight considered low risk and three at high risk of bias (Table 1). Seven of ten RCTs included in the review were generally of higher quality and exhibited lower bias overall (Table 1). However, the RCT evidence included in this review displayed potential bias in the form of lack of allocation concealment and lack of assessor blinding, as well as lack of participant blinding, since participants were randomized into either a physical activity group or an education/noninterventional control group. In these studies, the evidence tended to be of lower quality with potentially higher bias due to possible unreliable self-reporting, potential influence of interviewers, and use of questionnaires and interviews to assess physical activity rather than direct measurements.
Selection bias
Most of the studies included in this review were at risk of selection bias, because the participants were largely drawn from specific population samples (hospital, city, region). Selection bias may also have occurred due to the fact that the decision to partake in physical activity may be linked to potentially confounding lifestyle choices. Further, follow-up visits were required for most of the studies, and would require the ability to commute to study centers. Potential participants were excluded if they had chronic disease, such as cardiovascular disease, pulmonary disease, diabetes, physical disability, or depression. Therefore, the study data cannot be extrapolated to such individuals.
Effect of physical activity intervention
Twenty-seven studies (Table 1) met the inclusion criteria for this review. Of these, 26 studies reported a significant association between physical activity and cognitive function in late life (Tables 3 and 4). Of the ten RCTs, nine showed a positive correlation and one showed a nonsignificant correlation.9 Although these studies included both male and female subjects, one RCT by Klusmann et al10 enrolled only elderly healthy female subjects. As compared with controls, the authors also found a significant benefit of physical exercise (aerobic training with a bicycle ergometer or treadmill) in this elderly female population. Therefore, the majority of the studies concluded that physical activity in later life confers a protective effect on cognition in elderly subjects. Additionally, there were five studies11–16 that reported a dose-response association between physical activity and cognitive function.
Table 4.
Association between physical activity and cognitive function in selected studies
| Level of association | Number of studies | References |
|---|---|---|
| Significant | 26 | 8,10–24,26,27,55–62 |
| Insignificant | 1 | 9 |
| No association | 0 | N/A |
| Total | 27 | (See above references) |
Abbreviation: N/A, not applicable.
Discussion
This review examined the effect of physical activity in late life on age-related cognitive decline in older individuals with normal cognitive function or mild cognitive impairment at baseline. When selecting studies for review, the assumption was made that there is no difference in effect on cognition between different physical activities, ranging from aerobic to isometric exercises. As such, all types of physical activity program interventions were accepted in the study selection process. The data indicate that this assumption was generally correct; 26 of 27 studies showed a significant association between physical activity and cognitive decline, whereby an increased level of physical activity resulted in attenuation of cognitive decline and cognitive disease (Tables 3 and 4). In the ten RCTs evaluated in this review, the different interventions included aerobic and isometric exercise, weight training, and Tai Chi. Eight of these studies showed a significant outcome benefit, with one study showing a nonsignificant correlation (Table 4). Although these trials were not designed to determine a threshold effect or dose-response effect, there were five prospective studies suggesting a dose-response relationship in the level of benefit found with exercise,11–16 thus providing additional credence to the specificity of the effects of exercise on cognitive function.
Implications of evidence quality
Despite the preponderance of positive studies, only nine of the 27 studies were considered to be of high quality and the overall risk of bias was moderate in 16 studies (Table 1). Many studies included in this review relied on self-reporting to assess exercise habits, rather than using a more objective means of measuring physical activity.
In the studies evaluated in this review, outcome measures of cognitive performance were wide-ranging and measured different aspects of cognitive function (Table S3). Several studies used a neuropsychological test battery to test multiple aspects of cognitive function, while other studies used only one or two cognitive tests. Data heterogeneity may have confounded identification of the domains of cognitive function that were most affected by exercise. We standardized the cognitive metrics, inclusion criteria, and outcomes in this review as much as possible, which may have enabled us to ascertain an association between exercise and a few specific domains of cognitive function, such as the MMSE and Cognitive Inhibition (Stroop Color and Word Test). In the nine studies considered to be of higher quality, seven were RCTs9,10,17–21 and two were prospective studies.14,22 In these studies, a positive correlation was evident between physical activity in later life and cognition in the elderly subjects evaluated.
Similarly, the types of physical activity interventions used in the studies reviewed were wide-ranging, from aerobic or isometric physical activity, or combinations of both. Given the variability in physical activity interventions and the measures of cognitive function, it was not possible to determine a distinct relationship between specific types of physical activity and improvements in specific cognitive domains. As such, better standardization of the types of physical activity interventions could have clarified the specific causal relationships more effectively.
The durations of the included studies ranged from 6 months to several years. It is possible that improvements in some aspects of cognitive function occur shortly after completion of an exercise program, while improvements in other aspects may take several months or years to develop. For example, when using the 3MS or MMSE as one of the outcome measures, there was only one study showing a positive effect at 12 months20 whereas the other two did not;8,9 and five studies demonstrated the positive impact of exercise on cognitive function over the course of more than 12 months.12,16,23–25 The positive effect of exercise on cognitive speed26,27 and cognitive inhibitory function21 can be observed as early as 6 months. These observations highlight the time-specific effect of physical activity on each cognitive domain. Of interest, a study by Segal et al28 found that the acute effects of exercise enhance learning ability in patients with mild cognitive impairment and subjects with normal cognition. These investigators postulated that exercise could function as a stimulus for memory consolidation due to its stimulatory effects on the locus coeruleus and consequent release of norepinephrine. They found that exercise, conducted acutely after a period of learning, significantly increased the release of endogenous norepinephrine in both types of study subjects and resulted in retrograde enhancement of memory.28 As such, acute exercise, associated with periods of learning, may be a positive therapeutic intervention for cognitive decline in elderly subjects. In future research, it would be important to determine which forms of exercise affect specific domains of cognition and, also, the latency and duration of effect.
An exclusion criterion for this review was the presence of specific underlying conditions or diseases in the study population (such as chronic obstructive pulmonary disease, diabetes, traumatic head injury, cardiovascular disease, or depression). Therefore, our findings cannot be extrapolated to individuals with chronic underlying conditions in whom improvements in cognitive performance following a program of physical activity may be diminished or not apparent. For example, Hoffman et al29 published an RCT in which a program of physical activity failed to improve neurocognition in elderly subjects with clinical depression. This also has implications when assessing the overall effectiveness of physical activity in later life on cognitive performance in the very elderly, since a significant proportion of this population suffers from chronic conditions that may impede improvements in cognitive function following a physical activity regimen.
Neural plasticity: possible mechanisms for effect of exercise on cognition
Decline in cognitive function is one of the hallmarks of the aging process. The concept of neuronal structural plasticity in learning and memory processes30,31 suggests that cognitive decline in aging may be associated with dysregulation of brain plasticity.32 Mahncke et al33 demonstrated that elderly subjects with normal cognitive function had enhancement of memory following an intensive, plasticity-based computer training program. Physical exercise34,35 promotes positive neuroplasticity, increases cognitive reserve and higher neuronal connection density, and results in improved cognitive function. On the contrary, negative neuroplasticity results from physical inactivity, poor nutrition, substance abuse, and social isolation, decreases cognitive reserve, and inhibits formation of neuronal connections, leading to reduced cognitive function.1,36,37
Cerebral blood flow
While both aerobic and isometric physical activity are thought to confer improved cognition, studies suggest that aerobic exercise may be more effective in slowing degenerative neurological processes that lead to age-related cognitive decline and dementia.38 How might aerobic exercise contribute to neuroprotection? Many processes leading to cognitive decline stem from atherosclerotic or cerebrovascular conditions that produce cerebral hypoperfusion.39 Ruitenberg et al found that higher cerebral blood flow velocity was significantly associated with less cognitive decline and lower velocity was related to Alzheimer’s disease.40 The capacity of long-term aerobic exercise to mitigate the effects of vascular disease is well established,41 and may be an important mechanism of cognitive preservation due to exercise. Other mechanisms of neuronal enhancement with exercise include the role of neurotransmitters, changes in brain vasculature, and effects of neurotrophins.42,43 These processes, individually or together, may attenuate neurodegeneration and confer neuroprotective benefits, resulting in improved cognitive function.
Angiogenesis
Angiogenesis, the formation of vasculature by pre-existing endothelial cells, occurs in the brain during development but declines with age. Animal models have shown that exercise induces angiogenesis of small-vessel vasculature in the cerebellum, motor cortex, and hippocampus. Animal studies have shown that the hippocampus, which is essential for memory formation, is highly oxygen-dependent. Consequently, hippocampal angiogenesis may explain improvements in learning and memory following sustained, moderate-level physical activity. Maximal oxygen consumption increases with aerobic exercise, which is thought to be effective in promoting brain angiogenesis in experimental animals (rodents43 and monkeys42). Therefore, aerobic exercise may have more impact on cognitive performance than isometric exercise.
Effects of cytokines, neurotrophins, and brain volume
Neurotrophins are endogenous brain proteins that serve to promote neuroplasticity, and are thought to play a central role in response to physical activity.44 Granulocyte colony-stimulating factor (G-CSF) and brain-derived neurotrophic factor (BDNF) are implicated in mediating increases in cerebral gray matter volume and hippocampal volume, respectively,45 and enhancing cognitive performance by optimizing cognitive reserve, increasing learning capacity, and streamlining memory processes.45 The effect of G-CSF in subjects undergoing exercise protocols has been evaluated in several studies; plasma levels of G-CSF have been found to increase significantly after short bursts of aerobic exercise46 as well as following periods of endurance exercise.47 The role of G-CSF on neutrophil activation, proliferation, and survival is important for the immune response, thus illustrating the possible correlation with exercise in immunomodulation. BDNF is integral to differentiation, extension, and survival of neurons in the hippocampus, cortex, and cerebellum during brain development,48–50 and increases levels of synaptophysin and synaptobrevin, substances that aid transport of neurotransmitter vesicles. Support for this mechanism comes from animal studies showing that regulation of BDNF is associated with physical activity, as demonstrated by increased BDNF gene expression in rats as a result of running,25,51 with diminished or nonapparent effects when BDNF production is blocked.52 The role of BDNF in cognitive impairment remains inconclusive, with studies reporting different results.53,54 Nevertheless, the importance of BDNF in preservation and enhancement of cognitive function in humans was demonstrated by Erickson et al,45 who found that decreased levels were associated with age-related decline in hippocampal volume, and that aerobic exercise increased BDNF, hippocampal and temporal lobe volumes, and spatial memory. The association between BDNF level, hippocampal volume, and dementia was also established at the molecular level in subjects with BDNF gene polymorphism.52
Conclusion
There is evidence suggesting that physical activity in later life is beneficial for cognitive function in elderly persons. These benefits include enhancement of existing cognitive function and maintenance of optimal cognitive function, as well as prevention or delayed progression of cognitive diseases, such as Alzheimer’s dementia or other neurocognitive disorders. However, the majority of the evidence included in this review was of medium quality, and the overall risk of bias in the studies used in this review is moderately high. Despite the variable quality of the evidence, most of the data supports the concept that moderate-level physical activity in late life may improve cognitive function and delay the onset of debilitating cognitive disease in older persons. More evidence obtained from larger RCTs, preferably lasting for at least one year, is needed to confirm the association between physical activity in late life and improvements in cognitive function. Future research should focus on whether aerobic or isometric physical activity has a greater effect on cognition in the elderly, and which cognitive domains are most affected by physical activity. Additionally, research should be directed toward identifying and implementing exercise programs that would produce extended results on cognitive function in elderly patients.
Supplementary material
Table S1.
Agency for Healthcare Research and Quality Methods summary ratings of quality of individual studies
| Good (low risk of bias) | These studies have the least bias and results are considered valid. A study that adheres mostly to the commonly held concepts of high quality including the following: a formal randomized controlled design; clear description of the population, setting, interventions, and comparison groups; appropriate measurement of outcomes; appropriate statistical and analytic methods and reporting; no reporting errors; low dropout rate and clear reporting of dropouts. |
| Fair | These studies are susceptible to some bias, but it is not sufficient to invalidate the results. They do not meet all the criteria required for a rating of good quality because they have some deficiencies, but no flaw is likely to cause major bias. The study may be missing information, making it difficult to assess limitations and potential problems. |
| Poor (high risk of bias) | These studies have significant flaws that imply biases of various types that may invalidate the results. They have serious errors in design, analysis, or reporting; large amounts of missing information; or discrepancies in reporting. |
Source: Agency for Healthcare Research and Quality Methods Reference Guide for Effectiveness and Comparative Effectiveness Reviews (http://www.ahrq.gov/).
Table S2.
Characteristics of excluded studies
| Andel et al1 | The study examines the effect of mid-life, not late-life, physical activity on cognition. |
| Baker et al2 | Participants were too young to meet the given inclusion criteria of this review. |
| Barnes et al3 | Participants were too young to meet the given inclusion criteria of this review. |
| Brown et al4 | The study contained too few participants and participants were too young to meet the given inclusion criteria of this review. |
| Chang et al5 | Examines the effect of mid-life, not late-life, physical activity on cognition. |
| Colcombe et al6 | The study contained too few participants and participants were too young to meet the given inclusion criteria of this review. |
| Devore et al7 | Participants were too young to meet the given inclusion criteria of this review. |
| Etgen et al8 | Participants were too young to meet the given inclusion criteria of this review. |
| Fabre et al9 | The duration of the study was too short to meet the given inclusion criteria of this review. |
| Floel et al10 | Participants were too young to meet the given inclusion criteria of this review. |
| Gillum et al11 | The outcome measure was death; this does not meet the given inclusion criteria for this review. |
| Hassett et al12 | Participants were patients who had suffered traumatic brain injury; this was an exclusion criterion for the review. |
| Kasai et al13 | The study contained too few participants to meet the given inclusion criteria of this review. |
| Lautenschlager et al14 | Participants were too young to meet the given inclusion criteria of this review. |
| Liu-Ambrose et al15 | Participants were elderly patients who had specifically suffered falls; this was an exclusion criterion for the review. |
| McAuley et al16 | The study contained too few participants and participants were too young to meet the given inclusion criteria of this review. |
| McAuley et al17 | Outcome measures included social-relation capacity and well-being; this does not meet the given inclusion criteria for this review. |
| Netz et al18 | Participants were too young to meet the given inclusion criteria of this review. |
| O’Dwyer et al19 | The duration of the trial was too short to meet the given inclusion criteria of this review. |
| Ojofeitimi et al20 | Participants were too young to meet the given inclusion criteria for this review. |
| Parekh et al21 | Participants were patients with lung disease; this was an exclusion criterion for this review. |
| Rovio et al22 | The study examines the effect of mid-life, not late-life, physical activity. |
| Rovio et al23 | The study examines the effect of mid-life, not late-life, physical activity. |
| Scherder et al24 | The duration of the trial was too short to meet the given inclusion criteria for this review. |
| Shubert et al25 | The duration of the trial was too short to meet the given inclusion criteria for this review. |
| Vercambre et al26 | Participants were patients specifically with vascular disease; this was an exclusion criterion for the review. |
| Verghese et al27 | The study examines the effect of cognitive, not physical, leisure activities on late-life cognition. |
| Voelcker-Rehage et al28 | The study contained too few participants to meet the given inclusion criteria for this review. |
| Weuve et al29 | Participants were too young to meet the given inclusion criteria for this review. |
| Wolinsky et al30 | The study examines the effect of cognitive, not physical, activities on late-life cognition. |
Table S3.
Grouping of cognitive tests and studies of cognitive function
| Cognitive domain | Name of test | References |
|---|---|---|
| Cognitive speed | Simple reaction time 8-Choice reaction time Go/no-go reaction time Digit symbol substitution test Trail making test |
Smiley-Oyen et al31 Smiley-Oyen et al31 Smiley-Oyen et al31 Williamson et al32 Klusmann et al,33 Nguyen et al,34 Nagamatsu et al35 |
| Immediate verbal memory function | Wechsler Adult Intelligence Scale The Rey Auditory Verbal Learning Test |
Busse et al36 Williamson et al32 |
| Global cognitive function | Mini-Mental State Examination Modified Mini-Mental State Examination |
Lytle et al,37 Miu et al,38 Muscari et al,39 Ravaglia et al,40 Schuit et al,41 Van Gelder et al,42 Williamson et al,32 Laurin et al,43 Klusmann et al33 Middleton et al,44 Podewils et al,45 Yaffe et al46 |
| Cognitive inhibition | Stroop Color and Word Test | Bixby et al,47 Smiley-Oyen et al,31 Williamson et al,32 Nagamatsu et al35 |
| Working memory | Direct and Indirect Digit Span Rivermead Behavioral Memory Test Memory Complaints Scale |
Busse et al36 Busse et al36 Cassilhas et al,48 Busse et al36 |
| Differentiation between dementia and Alzheimer’s disease | Cambridge Cognitive Test | Busse et al36 |
| Verbal/nonverbal intelligence Sustained attention Presence of dementia |
Kaufman Brief Intelligence Test Toulouse-Pieron Concentration Attention Test Cognitive Abilities Screening Instrument Informant Questionnaire on Cognitive Decline in the Elderly Community Screening for Dementia |
Bixby et al47 Cassilhas et al48 Taaffe et al,49 Wang et al51 Taaffe et al49 Wang et al50 |
| Presence of Alzheimer’s disease | Alzheimer’s Disease Assessment Scale-Cognitive Subscale Mental Deterioration Battery |
Miu et al38 Ravaglia et al40 |
| Executive function | Wisconsin Card Sort Test Rey-Osterrieth Complex Figure Test |
Smiley-Oyen et al31 Williamson et al,32 Wang et al51 |
References
- 1.Andel R, Crowe M, Pedersen NL, Fratiglioni L, Johansson B, Gatz M. Physical exercise at midlife and risk of dementia three decades later: a population-based study of Swedish twins. J Gerontol A Biol Sci Med Sci. 2008;63(1):62–66. doi: 10.1093/gerona/63.1.62. [DOI] [PubMed] [Google Scholar]
- 2.Baker LD, Frank LL, Foster-Schubert K. Effects of aerobic exercise on mild cognitive impairment: a controlled trial. Arch Neurol. 2010;67(1):71–79. doi: 10.1001/archneurol.2009.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barnes DE, Yaffe K, Satariano WA, Tager IB. A longitudinal study of cardiorespiratory fitness and cognitive function in healthy older adults. J Am Geriatr Soc. 2003;51:459–465. doi: 10.1046/j.1532-5415.2003.51153.x. [DOI] [PubMed] [Google Scholar]
- 4.Brown AD, McMorris CA, Longman RS, et al. Effects of cardiorespiratory fitness and cerebral blood flow on cognitive outcomes in older women. Neurobiol Aging. 2010;31:2047–2057. doi: 10.1016/j.neurobiolaging.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 5.Chang M, Jonsson PV, Snaedal J, et al. The effect of midlife physical activity on cognitive function among older adults: AGES–Reykjavik Study. J Gerontol A Biol Sci Med Sci. 2010;65:1369–1374. doi: 10.1093/gerona/glq152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Colcombe SJ, Kramer AF, Erickson KI, et al. Cardiovascular fitness, cortical plasticity, and aging. Proc Natl Acad Sci USA. 2004;101:3316–3321. doi: 10.1073/pnas.0400266101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Devore EE, Kang JH, Okereke O, Grodstein F. Physical activity levels and cognition in women with type 2 diabetes. Am J Epidemiol. 2009;170:1040–1047. doi: 10.1093/aje/kwp224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Etgen T, Sander D, Huntgeburth U, Poppert H, Forstl H, Bickel H. Physical activity and incident cognitive impairment in elderly persons: the INVADE study. Arch Intern Med. 2010;170:186–193. doi: 10.1001/archinternmed.2009.498. [DOI] [PubMed] [Google Scholar]
- 9.Fabre C, Chamari K, Mucci P, Masse-Biron J, Prefaut C. Improvement of cognitive function by mental and/or individualized aerobic training in healthy elderly subjects. International Journal of Sports Medicine. 2002;23:415–421. doi: 10.1055/s-2002-33735. [DOI] [PubMed] [Google Scholar]
- 10.Floel A, Ruscheweyh R, Kruger K, et al. Physical activity and memory functions: are neurotrophins and cerebral gray matter volume the missing link? NeuroImage. 2010;49:2756–2763. doi: 10.1016/j.neuroimage.2009.10.043. [DOI] [PubMed] [Google Scholar]
- 11.Gillum RF, Obisesan TO. Physical activity, cognitive function, and mortality in a US national cohort. Ann Epidemiol. 2010;20:251–257. doi: 10.1016/j.annepidem.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hassett LM, Moseley AM, Tate R, Harmer AR. Fitness training for cardiorespiratory conditioning after traumatic brain injury. Cochrane Database Syst Rev. 2008:CD006123. doi: 10.1002/14651858.CD006123.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kasai JYT, Busse AL, Magaldi RM, et al. Effects of Tai Chi Chuan on cognition of elderly women with mild cognitive impairment. Einstein (16794508) 2010;8:40–45. doi: 10.1590/S1679-45082010AO1470. [DOI] [PubMed] [Google Scholar]
- 14.Lautenschlager NT, Cox KL, Flicker L, et al. Effect of physical activity on cognitive function in older adults at risk for Alzheimer disease: a randomized trial. JAMA. 2008;300:1027–1037. doi: 10.1001/jama.300.9.1027. [DOI] [PubMed] [Google Scholar]
- 15.Liu-Ambrose T, Donaldson MG, Ahamed Y, et al. Otago home-based strength and balance retraining improves executive functioning in older fallers: a randomized controlled trial. J Am Geriatr Soc. 2008;56:1821–1830. doi: 10.1111/j.1532-5415.2008.01931.x. [DOI] [PubMed] [Google Scholar]
- 16.McAuley E, Szabo AN, Mailey EL, et al. Non-exercise estimated cardiorespiratory fitness: associations with brain structure, cognition, and memory complaints in older adults. Ment Health Phys Act. 2011;4:5–11. doi: 10.1016/j.mhpa.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McAuley E, Blissmer B, Marquez DX, Jerome GJ, Kramer AF, Katula J. Social relations, physical activity, and well-being in older adults. Prev Med. 2000;31:608–617. doi: 10.1006/pmed.2000.0740. [DOI] [PubMed] [Google Scholar]
- 18.Netz Y, Argov E, Inbar O. Fitness’s moderation of the facilitative effect of acute exercise on cognitive flexibility in older women. J Aging Phys Act. 2009;17:154–166. doi: 10.1123/japa.17.2.154. [DOI] [PubMed] [Google Scholar]
- 19.O’Dwyer ST, Burton NW, Pachana NA, Brown WJ. Protocol for Fit Bodies, Fine Minds: a randomized controlled trial on the affect of exercise and cognitive training on cognitive functioning in older adults. BMC Geriatr. 2007;7:23. doi: 10.1186/1471-2318-7-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ojofeitimi EO, Ijadunola KT, Jegede VA, et al. Nutritional status and physical activity in relation to cognitive function in a group of elderly in Nigeria. Journal of Nutrition For the Elderly. 2002;22:49–62. [Google Scholar]
- 21.Parekh PI, Blumenthal JA, Babyak MA, et al. Gas exchange and exercise capacity affect neurocognitive performance in patients with lung disease. Psychosom Med. 2005;67:425–432. doi: 10.1097/01.psy.0000160479.99765.18. [DOI] [PubMed] [Google Scholar]
- 22.Rovio S, Kåreholt I, Helkala EL, et al. Leisure-time physical activity at midlife and the risk of dementia and Alzheimer’s disease. Lancet Neurol. 2005;4:705–711. doi: 10.1016/S1474-4422(05)70198-8. [DOI] [PubMed] [Google Scholar]
- 23.Rovio S, Kåreholt I, Viitanen M, et al. Work-related physical activity and the risk of dementia and Alzheimer’s disease. Int J Geriatr Psychiatry. 2007;22:874–882. doi: 10.1002/gps.1755. [DOI] [PubMed] [Google Scholar]
- 24.Scherder EJ, Van Paasschen J, Deijen JB, et al. Physical activity and executive functions in the elderly with mild cognitive impairment. Aging Ment Health. 2005;9:272–280. doi: 10.1080/13607860500089930. [DOI] [PubMed] [Google Scholar]
- 25.Shubert TE, McCulloch K, Hartman M, Giuliani CA. The effect of an exercise-based balance intervention on physical and cognitive performance for older adults: a pilot study. J Geriatr Phys Ther. 2010;33:157–164. [PubMed] [Google Scholar]
- 26.Vercambre MN, Grodstein F, Manson JE, Stampfer MJ, Kang JH. Physical activity and cognition in women with vascular conditions. Arch Intern Med. 2011;171:1244–1250. doi: 10.1001/archinternmed.2011.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Verghese J, Lipton RB, Katz MJ, et al. Leisure activities and the risk of dementia in the elderly. N Engl J Med. 2003;348:2508–2516. doi: 10.1056/NEJMoa022252. [DOI] [PubMed] [Google Scholar]
- 28.Voelcker-Rehage C, Godde B, Staudinger UM. Physical and motor fitness are both related to cognition in old age. Euro J Neurosci. 2010;31:167–176. doi: 10.1111/j.1460-9568.2009.07014.x. [DOI] [PubMed] [Google Scholar]
- 29.Weuve J, Kang JH, Manson JE, Breteler MM, Ware JH, Grodstein F. Physical activity, including walking, and cognitive function in older women. JAMA. 2004;292:1454–1461. doi: 10.1001/jama.292.12.1454. [DOI] [PubMed] [Google Scholar]
- 30.Wolinsky FD, Unverzagt FW, Smith DM, Jones R, Wright E, Tennstedt SL. The effects of the ACTIVE cognitive training trial on clinically relevant declines in health-related quality of life. J Gerontol B Psychol Sci Soc Sci. 2006;61:S281–S287. doi: 10.1093/geronb/61.5.s281. [DOI] [PubMed] [Google Scholar]
- 31.Smiley-Oyen AL, Lowry KA, Francois SJ, Kohut ML, Ekkekakis P. Exercise, fitness, and neurocognitive function in older adults: the “selective improvement” and “cardiovascular fitness” hypotheses. Ann Behav Med. 2008;36:280–291. doi: 10.1007/s12160-008-9064-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Williamson JD, Espeland M, Kritchevsky SB, et al. Changes in cognitive function in a randomized trial of physical activity: results of the lifestyle interventions and independence for elders pilot study. J Gerontol A Biol Sci Med Sci. 2009;64:688–694. doi: 10.1093/gerona/glp014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Klusmann V, Evers A, Schwarzer R, et al. Complex mental and physical activity in older women and cognitive performance: a 6-month randomized controlled trial. J Gerontol A Biol Sci Med Sci. 2010;65:680–688. doi: 10.1093/gerona/glq053. [DOI] [PubMed] [Google Scholar]
- 34.Nguyen MH, Kruse A. A randomized controlled trial of Tai chi for balance, sleep quality and cognitive performance in elderly Vietnamese. Clin Interv Aging. 2012;7:185–190. doi: 10.2147/CIA.S32600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nagamatsu LS, Handy TC, Hsu CL, Voss M, Liu-Ambrose T. Resistance training promotes cognitive and functional brain plasticity in seniors with probable mild cognitive impairment. Arch Intern Med. 2012;172:666–668. doi: 10.1001/archinternmed.2012.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Busse AL, Filho WJ, Magaldi RM, et al. Effects of resistance training exercise on cognitive performance in elderly individuals with memory impairment: results of a controlled trial. Einstein. 2008;6:402–407. [Google Scholar]
- 37.Lytle ME, Vander Bilt J, Pandav RS, Dodge HH, Ganguli M. Exercise level and cognitive decline: the MoVIES project. Alzheimer Dis Assoc Disord. 2004;18:57–64. doi: 10.1097/01.wad.0000126614.87955.79. [DOI] [PubMed] [Google Scholar]
- 38.Miu DKY, Szeto SL, Mak YF. A randomized controlled trial on the effect of exercise on physical, cognitive, and affective function in dementia subjects. Asian J Gerontol Geriatr. 2008;3:8–16. [Google Scholar]
- 39.Muscari A, Giannoni C, Pierpaoli L, et al. Chronic endurance exercise training prevents aging-related cognitive decline in healthy older adults: a randomized controlled trial. Int J Geriatr Psychiatry. 2010;25:1055–1064. doi: 10.1002/gps.2462. [DOI] [PubMed] [Google Scholar]
- 40.Ravaglia G, Forti P, Lucicesare A, et al. Physical activity and dementia risk in the elderly: findings from a prospective Italian study. Neurology. 2008;70:1786–1794. doi: 10.1212/01.wnl.0000296276.50595.86. [DOI] [PubMed] [Google Scholar]
- 41.Schuit AJ, Feskens EJ, Launer LJ, Kromhout D. Physical activity and cognitive decline, the role of the apolipoprotein e4 allele. Med Sci Sports Exerc. 2001;33:772–777. doi: 10.1097/00005768-200105000-00015. [DOI] [PubMed] [Google Scholar]
- 42.van Gelder BM, Tijhuis MA, Kalmijn S, Giampaoli S, Nissinen A, Kromhout D. Physical activity in relation to cognitive decline in elderly men: the FINE Study. Neurology. 2004;63:2316–2321. doi: 10.1212/01.wnl.0000147474.29994.35. [DOI] [PubMed] [Google Scholar]
- 43.Laurin D, Verreault R, Lindsay J, MacPherson K, Rockwood K. Physical activity and risk of cognitive impairment and dementia in elderly persons. Arch Neurol. 2001;58:498–504. doi: 10.1001/archneur.58.3.498. [DOI] [PubMed] [Google Scholar]
- 44.Middleton LE, Manini TM, Simonsick EM, et al. Activity energy expenditure and incident cognitive impairment in older adults. Arch Intern Med. 2011;171:1251–1257. doi: 10.1001/archinternmed.2011.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Podewils LJ, Guallar E, Kuller LH, et al. Physical activity, APOE genotype, and dementia risk: findings from the Cardiovascular Health Cognition Study. Am J Epidemiol. 2005;161:639–651. doi: 10.1093/aje/kwi092. [DOI] [PubMed] [Google Scholar]
- 46.Yaffe K. A prospective study of physical activity and cognitive decline in elderly women: Women who walk. Archives of Internal Medicine. 2001;161:1703–1708. doi: 10.1001/archinte.161.14.1703. [DOI] [PubMed] [Google Scholar]
- 47.Bixby WR, Spalding TW, Haufler AJ, et al. The unique relation of physical activity to executive function in older men and women. Med Sci Sports Exerc. 2007;39:1408–1416. doi: 10.1249/mss.0b013e31806ad708. [DOI] [PubMed] [Google Scholar]
- 48.Cassilhas RC, Viana VA, Grassmann V, et al. The impact of resistance exercise on the cognitive function of the elderly. Med Sci Sports Exerc. 2007;39:1401–1407. doi: 10.1249/mss.0b013e318060111f. [DOI] [PubMed] [Google Scholar]
- 49.Taaffe DR, Irie F, Masaki KH, et al. Physical activity, physical function, and incident dementia in elderly men: the Honolulu-Asia Aging Study. J Gerontol A Biol Sci Med Sci. 2008;63:529–535. doi: 10.1093/gerona/63.5.529. [DOI] [PubMed] [Google Scholar]
- 50.Wang L, Larson EB, Bowen JD, van Belle G. Performance-based physical function and future dementia in older people. Arch Intern Med. 2006;166:1115–1120. doi: 10.1001/archinte.166.10.1115. [DOI] [PubMed] [Google Scholar]
- 51.Wang HX, Jin Y, Hendrie HC, et al. Late life leisure activities and risk of cognitive decline. J Gerontol A Biol Sci Med Sci. 2013;68:205–213. doi: 10.1093/gerona/gls153. [DOI] [PMC free article] [PubMed] [Google Scholar]
Acknowledgments
The authors acknowledge the Department of Veterans Affairs, Queen’s University Belfast and The John Butler Lung Foundation for their support of this work.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Vance D, Wright M. Positive and negative neuroplasticity: implications for age-relative cognitive declines. J Gerontol Nurs. 2009;35:11–17. doi: 10.3928/00989134-20090428-02. [DOI] [PubMed] [Google Scholar]
- 2.Kinsella K, He W. An Aging World: 2008. US Census Bureau, International Population Reports; [Accessed January 6, 2009]. Available from: http://www.census.gov/prod/2009pubs/p95-09-1.pdf. [Google Scholar]
- 3.Wimo A, Winblad B, Johnsson L. The worldwide societal costs of dementia: estimates for 2009. Alzheimers Dement. 2010;6:98–103. doi: 10.1016/j.jalz.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 4.Plassman BL, Williams J, Burke JR, Holsinger T, Benjamin S. Systematic review: factors associated with risk for and possible prevention of cognitive decline in later life. Ann Intern Med. 2010;153:182–193. doi: 10.7326/0003-4819-153-3-201008030-00258. [DOI] [PubMed] [Google Scholar]
- 5.Maaike A, Geert A, Verhaar H, Aleman A, Luc V. Physical activity and enhanced fitness to improve cognitive function in older people without known cognitive impairment. Cochrane Database Syst Rev. 2008;2:1–37. doi: 10.1002/14651858.CD005381.pub3. [DOI] [PubMed] [Google Scholar]
- 6.Forbes D, Forbes S, Morgan DG, Markle-Reid M, Wood J, Culum I. Physical activity programs for persons with dementia. Cochrane Database Syst Rev. 2008;3:1–26. doi: 10.1002/14651858.CD006489.pub2. [DOI] [PubMed] [Google Scholar]
- 7.Tseng CN, Gau BS, Lou MF. The effectiveness of exercise on improving cognitive function in older people: a systematic review. J Nurs Res. 2011;19:119–131. doi: 10.1097/JNR.0b013e3182198837. [DOI] [PubMed] [Google Scholar]
- 8.Williamson JD, Espeland M, Kritchevsky SB, et al. Changes in cognitive function in a randomized trial of physical activity: results of the lifestyle interventions and independence for elders pilot study. J Gerontol A Biol Sci Med Sci. 2009;64A:688–694. doi: 10.1093/gerona/glp014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miu DKY, Szeto SL, Mak YF. A randomized controlled trial on the effect of exercise on physical, cognitive, and affective function in dementia subjects. Asian J Gerontol Geriatr. 2008;3:8–16. [Google Scholar]
- 10.Klusmann V, Evers A, Schwarzer R, et al. Complex mental and physical activity in older women and cognitive performance: a 6-month randomized controlled trial. J Gerontol A Biol Sci Med Sci. 2010;65A:680–688. doi: 10.1093/gerona/glq053. [DOI] [PubMed] [Google Scholar]
- 11.Ku PW, Stevinson C, Chen LJ. Prospective associations between leisure-time physical activity and cognitive performance among older adults across an 11-year period. J Epidemiol. 2012;22:230–237. doi: 10.2188/jea.JE20110084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Laurin D, Verreault R, Lindsay J, MacPherson K, Rockwood K. Physical activity and risk of cognitive impairment and dementia in elderly persons. Arch Neurol. 2001;58:498–504. doi: 10.1001/archneur.58.3.498. [DOI] [PubMed] [Google Scholar]
- 13.Lytle ME, Vander Bilt J, Pandav RS, Dodge HH, Ganguli M. Exercise level and cognitive decline: the MoVIES project. Alzheimer Dis Assoc Disord. 2012;18:57–64. doi: 10.1097/01.wad.0000126614.87955.79. [DOI] [PubMed] [Google Scholar]
- 14.Middleton LE, Manini TM, Simonsick EM, et al. Activity energy expenditure and incident cognitive impairment in older adults. Arch Intern Med. 2011;171:1251–1257. doi: 10.1001/archinternmed.2011.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang HX, Jin Y, Hendrie HC, et al. Late life leisure activities and risk of cognitive decline. J Gerontol A Biol Sci Med Sci. 2012;68:205–213. doi: 10.1093/gerona/gls153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yaffe K. A prospective study of physical activity and cognitive decline in elderly women: women who walk. Arch Intern Med. 2001;161:1703–1708. doi: 10.1001/archinte.161.14.1703. [DOI] [PubMed] [Google Scholar]
- 17.Busse AL, Filho W, Magaldi R, et al. Effects of resistance training exercise on cognitive performance in elderly individuals with memory impairment: results of a controlled trial. Einstein. 2008;6:402–407. [Google Scholar]
- 18.Cassilhas R, Viana V, Grassmann V, et al. The impact of resistance exercise on the cognitive function of the elderly. Med Sci Sports Exerc. 2007;39:1401–1407. doi: 10.1249/mss.0b013e318060111f. [DOI] [PubMed] [Google Scholar]
- 19.Mortimer JA, Ding D, Borenstein AR, et al. Changes in brain volume and cognition in a randomized trial of exercise and social interaction in a community-based sample of non-demented Chinese elders. J Alzheimers Dis. 2012;30:757–766. doi: 10.3233/JAD-2012-120079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Muscari A, Giannoni C, Pierpaoli L, et al. Chronic endurance exercise training prevents aging-related cognitive decline in healthy older adults: a randomized controlled trial. Int J Geriatr Psychiatry. 2010;25:1055–1064. doi: 10.1002/gps.2462. [DOI] [PubMed] [Google Scholar]
- 21.Smiley-Oyen A, Lowry K, Francois S, Kohut M, Ekkekakis P. Exercise, fitness, and neurocognitive function in older adults: the selective improvement and cardiovascular fitness hypotheses. Ann Behav Med. 2008;36:280–291. doi: 10.1007/s12160-008-9064-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Buchman AS, Boyle PA, Yu L, Shah RC, Wilson RS, Bennett DA. Total daily physical activity and the risk of AD and cognitive decline in older adults. Neurology. 2012;78:1323–1329. doi: 10.1212/WNL.0b013e3182535d35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ravaglia G, Forti P, Lucicesare APN, Rietti E, Bianchin M, Dalmonte E. Physical activity and dementia risk in the elderly: findings from a prospective Italian study. Neurology. 2008;70:1786–1794. doi: 10.1212/01.wnl.0000296276.50595.86. [DOI] [PubMed] [Google Scholar]
- 24.Schuit AJ, Feskens EJ, Launer LJ, Kromhout D. Physical activity and cognitive decline, the role of the apolipoprotein e4 allele. Med Sci Sports Exerc. 2001;33:772–777. doi: 10.1097/00005768-200105000-00015. [DOI] [PubMed] [Google Scholar]
- 25.van Praag H, Shubert T, Zhao C, Gage FH. Exercise enhances learning and hippocampal neurogenesis in aged mice. J Neurosci. 2005;25:8680–8685. doi: 10.1523/JNEUROSCI.1731-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nagamatsu LS, Ma H, Hsu T. Resistance training promotes cognitive and functional brain plasticity in seniors with probable mild cognitive impairment. Arch Intern Med. 2012;172:666–668. doi: 10.1001/archinternmed.2012.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nguyen MH, Kruse A. A randomized controlled trial of Tai chi for balance, sleep quality and cognitive performance in elderly Vietnamese. Clin Interv Aging. 2012;7:185–190. doi: 10.2147/CIA.S32600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Segal SK, Cotman CW, Cahill LF. Exercise-induced noradrenergic activation enhances memory consolidation in both normal aging and patients with amnestic mild cognitive impairment. J Alzheimers Dis. 2012;32:1011–1018. doi: 10.3233/JAD-2012-121078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hoffman B, Blumenthal J, Babyak M, et al. Exercise fails to improve neurocognition in depressed middle-aged and older adults. Med Sci Sports Exerc. 2008;40:1344–1352. doi: 10.1249/MSS.0b013e31816b877c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Caroni P, Donato F, Muller D. Structural plasticity upon learning: regulation and functions. Nat Rev Neurosci. 2012;13:478–490. doi: 10.1038/nrn3258. [DOI] [PubMed] [Google Scholar]
- 31.DeCarli C, Kawas C, Morrison JH, Reuter-Lorenz PA, Sperling RA, Wright CB. Session II: Mechanisms of age-related cognitive change and targets for intervention: neural circuits, networks, and plasticity. J Gerontol A Biol Sci Med Sci. 2012;67:747–753. doi: 10.1093/gerona/gls111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Desai AK. Revitalizing the aged-brain. Med Clin North Am. 2011;95:463–475. doi: 10.1016/j.mcna.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 33.Mahncke HW, Connor BB, Appelman J, et al. Memory enhancement in healthy older adults using a brain plasticity-based training program: a randomized, controlled study. Proc Natl Acad Sci U S A. 2006;103:12523–12528. doi: 10.1073/pnas.0605194103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Colcombe SJ, Kramer AF, Erickson KI, et al. Cardiovascular fitness, cortical plasticity, and aging. Proc Natl Acad Sci U S A. 2004;101:3316–3321. doi: 10.1073/pnas.0400266101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pang TYC, Hannan AJ. Enhancement of cognitive function in models of brain disease through environmental enrichment and physical activity. Neuropharmacology. 2013;64:515–528. doi: 10.1016/j.neuropharm.2012.06.029. [DOI] [PubMed] [Google Scholar]
- 36.Hall CB, Lipton RB, Sliwinski M, Katz MJ, Derby CA, Verghese J. Cognitive activities delay onset of memory decline in persons who develop dementia. Neurology. 2009;73:365–361. doi: 10.1212/WNL.0b013e3181b04ae3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Howes MJ, Perry E. The role of phytochemicals in the treatment and prevention of dementia. Drugs Aging. 2011;28:439–468. doi: 10.2165/11591310-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 38.Ahlskog JE, Geda YE, Graff-Radford NR, Petersen RC. Physical exercise as a preventive or disease-modifying treatment of dementia and brain aging. Mayo Clin Proc. 2011;86:876–884. doi: 10.4065/mcp.2011.0252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lojovich JM. The relationship between aerobic exercise and cognition: is movement medicinal? J Head Trauma Rehabil. 2010;25:184–192. doi: 10.1097/HTR.0b013e3181dc78cd. [DOI] [PubMed] [Google Scholar]
- 40.Ruitenberg A, den Heijer T, Bakker SLM, et al. Cerebral hypoperfusion and clinical onset of dementia: The Rotterdam study. Ann Neurol. 2005;57:789–794. doi: 10.1002/ana.20493. [DOI] [PubMed] [Google Scholar]
- 41.Heran BS, Chen JM, Ebrahim S, et al. Exercise-based cardiac rehabilitation for coronary heart disease. Cochrane Database Syst Rev. 2011;7:CD001800. doi: 10.1002/14651858.CD001800.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ploughman M. Exercise is brain food: the effects of physical activity on cognitive function. Dev Neurorehabil. 2008;11:236–240. doi: 10.1080/17518420801997007. [DOI] [PubMed] [Google Scholar]
- 43.Rhyu IJ, Bytheway JA, Kohler SJ, et al. Effects of aerobic exercise training on cognitive function and cortical vascularity in monkeys. Neuroscience. 2010;167:1239–1248. doi: 10.1016/j.neuroscience.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Erickson KI, Voss MW, Prakash RS, et al. Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci U S A. 2011;108:3017–3022. doi: 10.1073/pnas.1015950108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Erickson KI, Prakash RS, Voss M, et al. Brain-derived neurotrophic factor is associated with age-related decline in hippocampal volume. J Neurosci. 2010;30:5368–5375. doi: 10.1523/JNEUROSCI.6251-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yamada M, Suzuki K, Kudo S, Totsuka M, Nakaji S, Sugawara K. Raised plasma G-CSF and IL-6 after exercise may play a role in neutrophil mobilization into the circulation. J Appl Physiol (1985) 2002;92:1789–1794. doi: 10.1152/japplphysiol.00629.2001. [DOI] [PubMed] [Google Scholar]
- 47.Suzuki K, Yamada M, Kurakake S, et al. Circulating cytokines and hormones with immunosuppressive but neutrophil-priming potentials rise after endurance exercise in humans. Eur J Appl Physiol. 2000;81:281–287. doi: 10.1007/s004210050044. [DOI] [PubMed] [Google Scholar]
- 48.Johnson RA, Rhodes JS, Jeffrey SL, Garland T, Jr, Mitchell GS. Hippocampal brain-derived neurotrophic factor but not neurotrophin-3 increases more in mice selected for increased voluntary wheel running. Neuroscience. 2003;121:1–7. doi: 10.1016/s0306-4522(03)00422-6. [DOI] [PubMed] [Google Scholar]
- 49.Richter-Schmidinger T, Alexopoulos P, Horn M, et al. Influence of brain-derived neurotrophic-factor and apolipoprotein E genetic variants on hippocampal volume and memory performance in healthy young adults. J Neural Transm. 2011;118:249–257. doi: 10.1007/s00702-010-0539-8. [DOI] [PubMed] [Google Scholar]
- 50.Webster MJ, Herman MM, Kleinman JE, Shannon Weickert C. BDNF and trkB mRNA expression in the hippocampus and temporal cortex during the human lifespan. Gene Expr Patterns. 2006;6:941–951. doi: 10.1016/j.modgep.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 51.Vaynman S, Ying Z, Gomez-Pinilla F. Hippocampal BDNF mediates the efficacy of exercise on synaptic plasticity and cognition. Eur J Neurosci. 2004;20:2580–2590. doi: 10.1111/j.1460-9568.2004.03720.x. [DOI] [PubMed] [Google Scholar]
- 52.Borroni B, Bianchi M, Premi E, et al. The brain-derived neurotrophic factor Val66Met polymorphism is associated with reduced hippocampus perfusion in frontotemporal lobar degeneration. J Alzheimers Dis. 2012;31:243–251. doi: 10.3233/JAD-2012-120226. [DOI] [PubMed] [Google Scholar]
- 53.Coen RF, Lawlor BA, Kenny R. Failure to demonstrate that memory improvement is due either to aerobic exercise or increased hippocampal volume. Proc Natl Acad Sci U S A. 2011;108:E89. doi: 10.1073/pnas.1102593108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Driscoll I, Martin B, An Y, et al. Plasma BDNF is associated with age-related white matter atrophy but not with cognitive function in older, non-demented adults. PLoS One. 2012;7:e35217. doi: 10.1371/journal.pone.0035217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Larson EB, Wang L, Bowen JD, et al. Exercise is associated with reduced risk for incident dementia among persons 65 years of age and older. Ann Intern Med. 2006;144:73–81. doi: 10.7326/0003-4819-144-2-200601170-00004. [DOI] [PubMed] [Google Scholar]
- 56.Podewils LJ, Guallar E, Kuller LH, et al. Physical activity, APOE genotype, and dementia risk: findings from the Cardiovascular Health Cognition Study. Am J Epidemiol. 2005;161:639–651. doi: 10.1093/aje/kwi092. [DOI] [PubMed] [Google Scholar]
- 57.Scarmeas N. Physical activity, diet, and risk of Alzheimer disease. JAMA. 2009;302:627–637. doi: 10.1001/jama.2009.1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Taaffe DR, Irie F, Masaki KH, et al. Physical activity, physical function, and incident dementia in elderly men: the Honolulu-Asia Aging Study. J Gerontol A Biol Sci Med Sci. 2008;63:529–535. doi: 10.1093/gerona/63.5.529. [DOI] [PubMed] [Google Scholar]
- 59.van Gelder BM, Tijhuis MAR, Kalmijn S, Giampaoli S, Nissinen A, Kromhout D. Physical activity in relation to cognitive decline in elderly men: the FINE study. Neurology. 2004;63:2316–2321. doi: 10.1212/01.wnl.0000147474.29994.35. [DOI] [PubMed] [Google Scholar]
- 60.Wang L. Performance-based physical function and future dementia in older people. Arch Intern Med. 2006;166:1115–1120. doi: 10.1001/archinte.166.10.1115. [DOI] [PubMed] [Google Scholar]
- 61.Geda YE, Roberts R, Knopman D, et al. Physical exercise, aging, and mild cognitive impairment: a population-based study. Arch Neurol. 2010;67:80–86. doi: 10.1001/archneurol.2009.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bixby WR, Spalding TW, Haufler A, et al. The unique relation of physical activity to executive function in older men and women. Med Sci Sports Exerc. 2007;39:1408–1416. doi: 10.1249/mss.0b013e31806ad708. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1.
Agency for Healthcare Research and Quality Methods summary ratings of quality of individual studies
| Good (low risk of bias) | These studies have the least bias and results are considered valid. A study that adheres mostly to the commonly held concepts of high quality including the following: a formal randomized controlled design; clear description of the population, setting, interventions, and comparison groups; appropriate measurement of outcomes; appropriate statistical and analytic methods and reporting; no reporting errors; low dropout rate and clear reporting of dropouts. |
| Fair | These studies are susceptible to some bias, but it is not sufficient to invalidate the results. They do not meet all the criteria required for a rating of good quality because they have some deficiencies, but no flaw is likely to cause major bias. The study may be missing information, making it difficult to assess limitations and potential problems. |
| Poor (high risk of bias) | These studies have significant flaws that imply biases of various types that may invalidate the results. They have serious errors in design, analysis, or reporting; large amounts of missing information; or discrepancies in reporting. |
Source: Agency for Healthcare Research and Quality Methods Reference Guide for Effectiveness and Comparative Effectiveness Reviews (http://www.ahrq.gov/).
Table S2.
Characteristics of excluded studies
| Andel et al1 | The study examines the effect of mid-life, not late-life, physical activity on cognition. |
| Baker et al2 | Participants were too young to meet the given inclusion criteria of this review. |
| Barnes et al3 | Participants were too young to meet the given inclusion criteria of this review. |
| Brown et al4 | The study contained too few participants and participants were too young to meet the given inclusion criteria of this review. |
| Chang et al5 | Examines the effect of mid-life, not late-life, physical activity on cognition. |
| Colcombe et al6 | The study contained too few participants and participants were too young to meet the given inclusion criteria of this review. |
| Devore et al7 | Participants were too young to meet the given inclusion criteria of this review. |
| Etgen et al8 | Participants were too young to meet the given inclusion criteria of this review. |
| Fabre et al9 | The duration of the study was too short to meet the given inclusion criteria of this review. |
| Floel et al10 | Participants were too young to meet the given inclusion criteria of this review. |
| Gillum et al11 | The outcome measure was death; this does not meet the given inclusion criteria for this review. |
| Hassett et al12 | Participants were patients who had suffered traumatic brain injury; this was an exclusion criterion for the review. |
| Kasai et al13 | The study contained too few participants to meet the given inclusion criteria of this review. |
| Lautenschlager et al14 | Participants were too young to meet the given inclusion criteria of this review. |
| Liu-Ambrose et al15 | Participants were elderly patients who had specifically suffered falls; this was an exclusion criterion for the review. |
| McAuley et al16 | The study contained too few participants and participants were too young to meet the given inclusion criteria of this review. |
| McAuley et al17 | Outcome measures included social-relation capacity and well-being; this does not meet the given inclusion criteria for this review. |
| Netz et al18 | Participants were too young to meet the given inclusion criteria of this review. |
| O’Dwyer et al19 | The duration of the trial was too short to meet the given inclusion criteria of this review. |
| Ojofeitimi et al20 | Participants were too young to meet the given inclusion criteria for this review. |
| Parekh et al21 | Participants were patients with lung disease; this was an exclusion criterion for this review. |
| Rovio et al22 | The study examines the effect of mid-life, not late-life, physical activity. |
| Rovio et al23 | The study examines the effect of mid-life, not late-life, physical activity. |
| Scherder et al24 | The duration of the trial was too short to meet the given inclusion criteria for this review. |
| Shubert et al25 | The duration of the trial was too short to meet the given inclusion criteria for this review. |
| Vercambre et al26 | Participants were patients specifically with vascular disease; this was an exclusion criterion for the review. |
| Verghese et al27 | The study examines the effect of cognitive, not physical, leisure activities on late-life cognition. |
| Voelcker-Rehage et al28 | The study contained too few participants to meet the given inclusion criteria for this review. |
| Weuve et al29 | Participants were too young to meet the given inclusion criteria for this review. |
| Wolinsky et al30 | The study examines the effect of cognitive, not physical, activities on late-life cognition. |
Table S3.
Grouping of cognitive tests and studies of cognitive function
| Cognitive domain | Name of test | References |
|---|---|---|
| Cognitive speed | Simple reaction time 8-Choice reaction time Go/no-go reaction time Digit symbol substitution test Trail making test |
Smiley-Oyen et al31 Smiley-Oyen et al31 Smiley-Oyen et al31 Williamson et al32 Klusmann et al,33 Nguyen et al,34 Nagamatsu et al35 |
| Immediate verbal memory function | Wechsler Adult Intelligence Scale The Rey Auditory Verbal Learning Test |
Busse et al36 Williamson et al32 |
| Global cognitive function | Mini-Mental State Examination Modified Mini-Mental State Examination |
Lytle et al,37 Miu et al,38 Muscari et al,39 Ravaglia et al,40 Schuit et al,41 Van Gelder et al,42 Williamson et al,32 Laurin et al,43 Klusmann et al33 Middleton et al,44 Podewils et al,45 Yaffe et al46 |
| Cognitive inhibition | Stroop Color and Word Test | Bixby et al,47 Smiley-Oyen et al,31 Williamson et al,32 Nagamatsu et al35 |
| Working memory | Direct and Indirect Digit Span Rivermead Behavioral Memory Test Memory Complaints Scale |
Busse et al36 Busse et al36 Cassilhas et al,48 Busse et al36 |
| Differentiation between dementia and Alzheimer’s disease | Cambridge Cognitive Test | Busse et al36 |
| Verbal/nonverbal intelligence Sustained attention Presence of dementia |
Kaufman Brief Intelligence Test Toulouse-Pieron Concentration Attention Test Cognitive Abilities Screening Instrument Informant Questionnaire on Cognitive Decline in the Elderly Community Screening for Dementia |
Bixby et al47 Cassilhas et al48 Taaffe et al,49 Wang et al51 Taaffe et al49 Wang et al50 |
| Presence of Alzheimer’s disease | Alzheimer’s Disease Assessment Scale-Cognitive Subscale Mental Deterioration Battery |
Miu et al38 Ravaglia et al40 |
| Executive function | Wisconsin Card Sort Test Rey-Osterrieth Complex Figure Test |
Smiley-Oyen et al31 Williamson et al,32 Wang et al51 |


