Abstract
Introduction:
Intensive care units (ICUs) are associated with a greater risk of developing nosocomial infections (NIs) than other departments.
Aim:
The aim of this study was to determine the rate, the site and causative organisms of NIs in the surgical ICU at University Clinical Center Tuzla.
Methods:
All patients admitted to the surgical ICU were followed prospectively, for the development of NIs (January-December 2010). Determination of NIs was performed using standardized the Centers for Disease Control and Prevention (CDC) criteria.
Results:
94 out of 834 patients (11.27%) developed NIs. Respiratory tract infections were seen in 56 (60%), urinary tract infections in 15 (16%) and gastrointestinal tract infections in 8 (9%) patients. Other infections identified were surgical site, bloodstream and skin infections. Gram-negative organisms were reported in approximately 75% of cases (78.7% extended-spectrum beta-lactamase (ESBL)-producers). Klebsiella pneumoniae was the commonest (51.0%), followed by Proteus mirabilis (21.3%) and Pseudomonas aeruginosa (10.6%). Methicillin-resistant Staphylococcus aureus (MRSA) (16%), and Clostridium difficile (9.6%) were the commonest among gram-positive bacteria.
Conclusion:
Respiratory and urinary tract infections made up the great majority of NIs. ICU patients are more susceptible to NIs, emphasizing the importance of continuous surveillance and enforcement of specific infection control measures.
Keywords: nosocomial infections, surgical intensive care unit, ESBL-producing strains, surveillance
1. INTRODUCTION
The risk of developing nosocomial infections (NIs) is not evenly distributed throughout the hospital. Intensive care units (ICUs) are associated with a much greater risk than other departments (1-3). The risk of nosocomial infection in ICU is 5–10 times greater than those acquired in general medical and surgical wards (3). In many units, 40 to 50 percent of patients develop an infection, and it is often the ultimate cause of death (1). The pathophysiology of nosocomial infections differs from community-acquired infections. Nosocomial infection is defined as an infection which develops 48 hours after hospital admission or within 48 hours after being discharged (3). The development of nosocomial infection is dependent on two key pathophysiological factors: decreased host defences and colonization by pathogenic, or potentially pathogenic, bacteria. Due to their underlying diseases conditions, ICU patients are at unusually high risk of infection for they tend to be more susceptible (2). Also, critically ill ICU patients frequently require invasive medical devices such as urinary catheters, central venous and arterial catheters and endotracheal tubes which compromise normal skin and mucosal barriers, predisposing them to infection (1, 4).
Excellent therapies are available, and in general, mortality has fallen drastically, paralleling improvements in socioeconomic conditions. The organisms responsible for these infections remain susceptible to a wide variety of antimicrobials. Simultaneously, nosocomial infections are a product of advances in medical technology. The use of invasive diagnostic and therapeutic maneuvers, as well as the frequent alteration of the host immune system, makes patients susceptible to infection (3, 4). In addition, these maneuvers make therapy more difficult. Despite the constant development of new antimicrobials, nosocomial infections will continue to complicate technologic advances. Diagnosis and treatment of nosocomial infections are very costly and present an additional economic burden to health insurance funds (2). Even a relatively uncomplicated infection prolongs the length of hospitalization (1).
Locating the source of infection is often a matter of critical importance in infection prevention, because one may be able either to eliminate the source or take measures to segregate the source from susceptible patients (5). Patients admitted to ICU are at risk of acquiring nosocomial infection from different sources. The organisms causing most nosocomial infections usually come from the patient’s normal flora of the skin and mucous membranes (endogenous flora), when host factors that alter susceptibility to infection permit these organisms to behave as pathogens (6). In general, endogenous infection is best prevented by attention to individual patient susceptibility factors such as use of good surgical technique, preservation of the integrity of mechanical barriers to infection, and judicious use of immunosuppressive agents. Nosocomial infections can also be transmitted by direct contact by the hands of hospital staff members (cross-contamination), other patients, contaminated instruments and needles, and the inanimate environment (exogenous flora) (1, 6).
Patients admitted to ICU are frequently transferred to such units from other wards or other hospitals, where they may have become infected or colonized with the endemic nosocomial bacterial pathogens. Bacterial colonization is strongly associated with hospital stay and is especially common in the critically ill (3). Infected or colonized patients frequently serve as reservoirs of bacterial infection for other patients, and occasionally for staff as well (5). The presence of certain indwelling devices such as urinary catheters, endotracheal tubes, and vascular catheters appears to predispose to the establishment of such patient-associated reservoirs. The introduction of these endemic bacterial strains, which are often resistant to multiple antibiotics, into an area with a high density of susceptible patients can lead to an outbreak of serious infection. Furthermore, the inanimate environment can also serve as an important reservoir for agents, such as the gram-negative bacteria, that have the ability to survive and multiply apart from the human host (5).
The purpose of our research was to establish an active monitoring over a phenomenon of bacterial nosocomial infections in the surgical ICU at the University Clinical Center (UCC) Tuzla, Bosnia and Herzegovina. By identifying the characteristics of the nosocomial infections in the surgical ICU, we can more effectively direct and prioritize our prevention and control efforts, and also more closely monitor the trends of these infections. We aimed to identify: rates of bacterial nosocomial infections; types of infections according to anatomical location; types of organisms causing NIs, over a 12 months period (January to December 2010).
2. METHODS
During January-December 2010, 834 patients were treated in the surgical intensive care unit at UCC Tuzla. All the patients were prospectively studied since the day they were admitted until the end of the episode by the Infection Control Team. Any infection in these patients during the studied period was registered. Patient samples were tested using standard methods at the Institute of Microbiology, UCC Tuzla.
This study was conducted by using the method of the National Nosocomial Infections Surveillance (NNIS) criteria from the United States (U.S.) (7-9). The research used the application made on the base of recommendations of the Health Infection Control Practice Advisory Committee (HICPAC) which exists within the U.S. Centers for Disease Control and Prevention (CDC). Determination of nosocomial infections was done according to criteria defined by the CDC. The overall rate of patients with nosocomial infection was calculated by dividing the number of patients with nosocomial infections by the total number of patients in the surgical ICU.
3. RESULTS
During the 12 months study period, 834 patients were admitted to the surgical ICU at UCC Tuzla. Nosocomial infections were identified in 94 (11.27%) patients. A comparison between the number of patients with NIs and the number of discharged patients by time, for different months in 2010, is shown in Table 1. Distribution of nosocomial infections by time during 2010 is also shown in Figure 1.
Table 1.
Nosocomial infection rates in the surgical ICU by time (January-December 2010)
Figure 1.
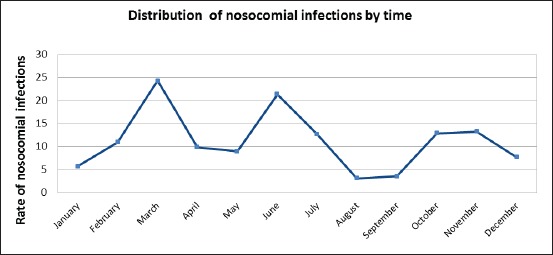
Distribution rates of nosocomial infections in the surgical intensive care unit by months during 2010.
Out of the total number of nosocomial infections registered, the respiratory tract infections (RTIs) were the most frequent (60%), than the urinary tract infections (UTIs) (16%), gastrointestinal tract (GIT) infections (9%), surgical site infections (SSIs) (7%), bloodstream infections (BSIs) (6%) and skin infections (2%) (Figure 2).
Figure 2.
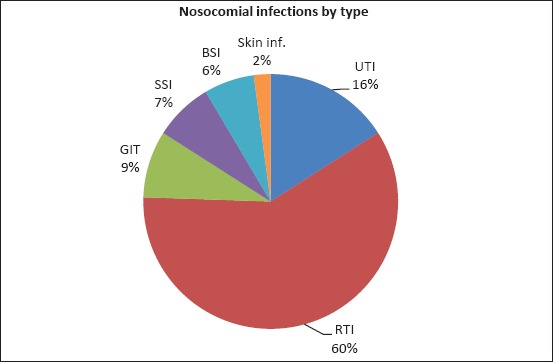
Major sites of nosocomial infections in the surgical intensive care unit. Abbreviations: UTI, urinary tract infection; RTI, respiratory tract infection; BSI, bloodstream infection; SSI, surgical site infection; GIT, gastrointestinal tract; inf.; infections.
Microbiological data for patients with nosocomial infections is given in Table 2. From 94 patient samples, we isolated and identified 123 bacteria, and almost all polymicrobial infections occurred in patients with RTIs (Table 2). Out of the 56 respiratory tract infections, Klebsiella pneumoniae (K. pneumoniae) was isolated in 30 patients. Overall, the majority of respiratory tract infections (60 (78.9%) out of 76 organism isolates identified from 56 patients) involved gram-negative bacteria including: K. pneumoniae, Pseudomonas aeruginosa (P. aeruginosa), Escherichia coli (E. coli), Proteus mirabilis (P. mirabilis), and Acinetobacter species (spp.). On the other hand, methicillin-resistant Staphylococcus aureus (MRSA) was responsible for the greatest number of RTIs caused by gram-positive bacteria. MRSA was also responsible for one bloodstream infection, along with K. pneumoniae which was detected in 3 patients, and En-terobacter cloacae and a coagulase-negative Staphylococcus spp., each causing one infection (Table 2).
Table 2.
Microbiological findings in 94 cases of different site specific nosocomial infections. Abbreviations: UTIs, urinary tract infection; RTIs, respiratory tract infection; BSIs, bloodstream infection; SSIs, surgical site infection; GIT, gastrointestinal tract infection; MRSA: Methicillin-resistant Staphylococcus aureus; CoNS, coagulase-negative Staphylococcus species, ESBL, extended–spectrum beta-lactamase (producing strain). * Organism isolates (n=123) were identified from 94 patients.
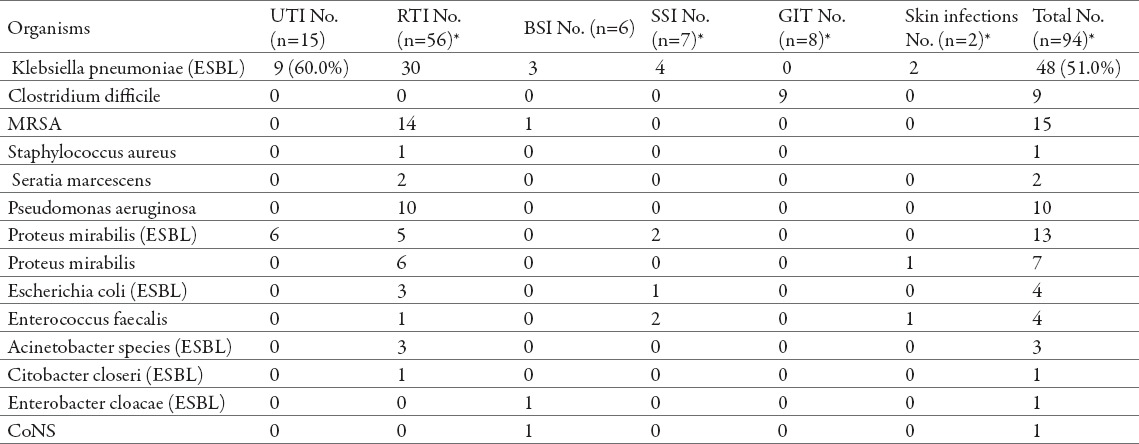
All the respiratory tract infections caused by K. pneumoniae, E. coli and Acinetobacter spp. were caused by extended-spectrum beta-lactamase (ESBL)-producing strains, while of the 11 infections cause by P. mirabilis, 5 were caused by ESBL-producing strains. Likewise, ESBL-producing K. pneumoniae and P. mira-bilis strains were responsible for the 15 urinary tract infections.
Similar to RTIs, in our study the surgical site infections (SSIs) were primarily caused by gram-negative bacteria (Table 2). Again, K. pneumoniae were the most frequent resulting in 4 infections, P. mirabilis 2 infections and one infection caused by E. coli, all ESBL-producing strains. Of the gram-positive bacteria, Enterococcus faecalis was isolated and identified in two patients as a causative agent of the surgical site infection.
Furthermore, the gastrointestinal tract (GIT) infections were detected in 9 (9%) patients (Table 2). Diarrhoea is a common problem in hospitalized patients (1), and not surprisingly Clostridium difficile (C. difficile)was identified to be responsible for these infections.
Finally, the four skin infections (2%) reported were caused by K. pneumoniae (2 NIs), P. mirabilis (1 NI) and Enterococcus faecalis (1 NI).
4. DISCUSSION
Surveillance of nosocomial infections is widely recognized as an important instrument of infection control programs. Surveillance refers to the routine and consistent collection of information regarding the occurrence of a disease (1). It is used to define endemic rates and identify problems and allows for recognition of clusters and epidemics. The Study on the Efficacy of Nosocomial Infection Control (SENIC) project provided the strongest scientific basis to date for the assertion that surveillance is an essential element of an infection control program and improves the outcomes of patients; it is strongly associated with reduction rates of all nosocomial infections (10). Infection rates are compiled by identifying the number of infections and the total number of patients at risk, usually the number of patients admitted or discharged. The results of this study are confirming our expectations that the intensive care units are at high risk for the occurrence of nosocomial infections. Thus, in the surgical ICU at UCC Tuzla in 2010, the rate of NIs was 11.27% (range 3.08 – 24.32). It has been previously shown that patients admitted to the ICU are at a particular high risk of acquiring nosocomial infections, 5-10 times greater than those in general wards, with prevalence rate as high as 30% (3, 11).
Our finding that the most frequent site of infection was the respiratory tract coincides with the literature (3, 12, 13). In our study, if all the respiratory tract infections were included they accounted for 60% of nosocomial infections, which is similar to previous reports showing the figure of 65% (3). Predisposition to nosocomial RTI is dependent on many factors. Using univariate analysis, it was found that patients undergoing a surgical procedure had 3.91 fold higher risk of developing pneumonia than did nonsurgical patients (14). The highest rates of respira-tory tract infections, especially pneumonia, occur in mechanically ventilated patients with an endotracheal or tracheostomy tube (12). Compared with non-ventilated patients, the risk of pneumonia is increased at least 7- to 10 fold. Contrary to our finding, the studies from the U.S. report the respiratory tract infections as the second most common after UTIs (2, 15, 16). Thus, Richards and colleagues have reported to the NNIS system that UTIs were the most frequent, responsible for 31% of NIs in medical/surgical ICU, followed by pneumonia (27%) and primary BSIs (19%) (15).
Although recent years have also seen a swing in the pattern of infecting organisms toward gram-positive infections (3), data from the literature suggest that the most nosocomial infections are still caused by gram-negative organisms (2, 13, 14). Similarly, our research also showed that gram-negative organisms were predominant, accounting for almost three-fourths of all nosocomial infections. The two large international European studies of the prevalence and outcomes of infection in ICUs (EPIC and EPIC II), including data from different countries/regions in the world, identified P. aeruginosa, E. coli, Acinetobacter spp. and Klebsiella spp. as the most common gram-negative pathogens overall, while S. aureus, CoNS and enterococci were predominant among gram-positive organisms (3, 13). However, there was a variation in the rates of infection of specific organisms depending on geographic regions. In our current study, K. pneumonia was the most frequently isolated gram-negative pathogen (38% of all NIs), followed by P. aeruginosa, P. mirabilis, E. coli and Acinetobacter spp., respectively; while MRSA was the most common among gram-positive bacteria. These organisms were also the primary causative agents of respiratory tract infections. Remarkably high prevalence of K. pneumoniae among our patients with RTIs was somewhat surprising and contrary to the previous studies, which report a much lower number of RTIs caused by K. pneumoniae (2, 6, 16, 17). It is noteworthy that the distribution of reported causative organisms of nosocomial infections may vary across countries and even between units, according to patient case mix, sites of infection, antibiotic protocols, infection, control practices, and local ecology and resistance patterns (3).
MRSA are important medical pathogens in many larger medical centres and once they have become endemic, extraordinary efforts may be required to stop nosocomial transmission (2, 3). UCC Tuzla is a tertiary-care teaching hospital, and one of the largest medical centres in the region. Hence, not surprisingly, MRSA was isolated from a considerable number of our patient samples. More than 90% of MRSA infections occurred in the respiratory tract, while MRSA accounted for 18.4% of all respiratory tract isolates. Along with drug-resistant ESBL-producing gram-negative bacteria, which accounted for approximately three-fourths of all nosocomial infections in our study (78.7% of isolated gram-negative bacteria were ESBL-producing strains), MRSA infections are prevalent worldwide and pose considerable therapeutic problems (2). In recent years, several reports have emphasized the increase in antibiotic resistance in hospitals, particular in ICUs (2, 3, 18). NNIS report, over a 5 year period, showed an increase in percentage of antimicrobial-resistant pathogens associated with nosocomial infection in ICU patients;11% increase in MRSA and a nearly 50% increase in ESBL-producing K. pneumoniae,isolated non-susceptible to 3rd generation cephalosporines (19). All of K. pneumoniae isolates in our study were ESBL-producing, which could be one of possible reasons for such high prevalence of this organism in the ICU in our hospital.
According to our study, and in the agreement with the European reports, the second most common site of nosocomial infections was the urinary tract (3). Most nosocomial urinary tract infections are preceded by instrumentation. Because many of the causative organisms of UTIs can be found as part of the endogenous gastrointestinal flora, many UTIs associated with indwelling catheters considered autogenously acquired, are caused by organisms ascending from the rectum to the urethra and bladder; although they can also be transmitted by cross-contamination or from exogenous environment (20). Urinary tract infections in intensive care patients are often associated with serious secondary complications. Prevention, therefore, is the key to minimizing morbidity and mortality. Meticulous attention to the maintenance of the closed sterile system of urinary drainage is the cornerstone of infection control. The two organisms isolated from our UTI patients were Klebsiella spp. (60%) and P. mirabilis. These pathogens played a lesser role in nosocomial urinary tract infections in some previously published studies. According to the NNIS study sponsored by the CDC, Klebsiella spp. accounted for 7.6% of the 13,000 nosocomial UTI reported, while E. coli was the most common (31.7%) (20). Shaikh and colleagues also detected E. coli as the most frequent pathogen (26.3%), and Klebsiella spp. was responsible for only 5.2 % of all UTIs (21). However, a study conducted among general-ward patients revealed Klebsiella spp. as the predominant organism isolated from nosocomial UTIs, but at a lower frequency (25%) (22). Additionally, these researchers identified E. coli (17.7%), Streptococcus faecalis (10.6%) and Pseudomonas spp. (8.6%). Overall, it has been reviewed that although contribution of a certain pathogen to a total number of UTIs may vary, urinary tract infections are most often associated with Enterococcus spp., E. coli, Klebsiella spp., and P. aeruginosa (3, 23).
Other infections, identified in the remaining one-fourth of our patients, included: GIT infections, SSIs, BSIs and skin infections. Interestingly, while other studies report higher incidence of BSI and SSI, we observed GIT infection to be the third most common infection among our ICU patients. Diarrhoea is a common problem in critically ill patients. Non-infectious causes of diarrhoea in the ICU include tube feeding, hypoalbuminemia, intestinal ischemia, and antibiotics-induced (1, 24). C. difficile has been recognized as the most common cause of nosocomial infectious diarrhoea in the ICU, causing antibiotic-induced pseudomembranous colitis. The incidence of C. difficile infection (CDI) is increasing in the ICU, as well as the hospitalized population as a whole. The nosocomial transmission rate of C. difficile varies between centres according to different studies, from 8.5% to 32.2% (25). Barbut and colleagues also show that the incidence of CDI may vary between hospital wards (range 6%–15%), with the prevalence of 11% in the ICU (25). At UCC Tuzla, we reported 9% incidence of CDI. Critically ill patients remain at high risk for C. difficile pathogen, and preventive measures, such as meticulous contact precautions, hand hygiene, environmental disinfection, and, most importantly, antibiotic stewardship, are the cornerstones of mitigation in the intensive care unit (26). Causative organisms of SSIs identified in our study were Klebsiella spp., P. mirabilis, E. coli and E. faecalis. In fact, K. pneumoniae was the most common, associated with more than half of surgical site infections. The incidence of SSIs (7%) in this study was somewhat lower from previous reports that showed the figures of 12.9%, 14%, 15% (2, 6, 16). As most surgical site infections become manifested after patient has been discharged from the hospital (6), one of possible reasons for such a low recorded incidence could be a poor or a lack of post-discharge reporting. Furthermore, the incidence of bloodstream infections was also lower (6%) than in other medical centres (13%, 18.6%), and again K. pneumoniae was the predominant isolate (6, 16). We have previously discussed that the incidence rates of nosocomial infections by body site may vary.
5. CONCLUSION
Nosocomial infections are common in the surgical ICU at UCC Tuzla. Respiratory and urinary tract infections were responsible for three-fourths of all infections and often associated with microbiological isolates of resistant organisms. The most prevalent organism was ESBL-producing K. pneumoniae, responsible for approximately a half of all nosocomial infections. The potential effect of resistant organisms on treatment outcome of primary diseases in critically ill patients emphasizes the importance of continuous surveillance, enforcement of specific infection control measures, as well as the assessment of appropriateness of antibiotic use. Surveillance performance of nosocomial infections in the surgical ICUs enables the observations endemic appearance of nosocomial infections, which is the main precondition in finding the measures for reduction and prevention of nosocomial infections. Attempts to understand and control nosocomial infections require a basic understanding of epidemiology, microbiology, infectious diseases, and hospital administration. Because of the enormous burden of nosocomial infections, every hospital must have an organized infection control program.
Footnotes
CONFLICT OF INTEREST: NONE DECLARED.
REFERENCES
- 1.Farber BF. Nosocomial infections: An introduction. In: Farber BF, editor. Infection Control in Intensive Care. New York: Churchill Livingstone; 1987. pp. 1–7. [Google Scholar]
- 2.Emori TG, Gaynes RP. An overview of nosocomial infections, including the role of the microbiology laboratory. Clin Microbiol Rev. 1993 Oct;6(4):428–442. doi: 10.1128/cmr.6.4.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vincent JL. Nosocomial infections in adult intensive-care units. Lancet. 2003 Jun 14;361(9374):2068–2077. doi: 10.1016/S0140-6736(03)13644-6. [DOI] [PubMed] [Google Scholar]
- 4.Weinstein RA. Nosocomial infection update. Emerg Infect Dis. 1998 Jul-Sep;4(3):416–420. doi: 10.3201/eid0403.980320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Muder RR, Lumish RM. Isolation in the intesive care unit. In: Farber BF, editor. Infection Control in Intensive Care. New York: Churchill Livingstone; 1987. pp. 59–75. [Google Scholar]
- 6.Abdel-Fattah MM. Surveillance of nosocomial infections at a Saudi Arabian military hospital for a one-year period. Ger Med Sci. 2005;3 Doc06. [PMC free article] [PubMed] [Google Scholar]
- 7.Emori TG, Culver DH, Horan TC, Jarvis WR, White JW, Olson DR, et al. National nosocomial infections surveillance system (NNIS): description of surveillance methods. Am J Infect Control. 1991 Feb;19(1):19–35. doi: 10.1016/0196-6553(91)90157-8. [DOI] [PubMed] [Google Scholar]
- 8.Garner JS, Jarvis WR, Emori TG, Horan TC, Hughes JM. CDC definitions for nosocomial infections 1988. Am J Infect Control. 1988 Jun;16(3):128–140. doi: 10.1016/0196-6553(88)90053-3. [DOI] [PubMed] [Google Scholar]
- 9.Horan TC, Gaynes RP, Martone WJ, Jarvis WR, Emori TG. CDC definitions of nosocomial surgical site infections 1992: a modification of CDC definitions of surgical wound infections. Am J Infect Control. 1992 Oct;20(5):271–274. doi: 10.1016/s0196-6553(05)80201-9. [DOI] [PubMed] [Google Scholar]
- 10.Haley RW, Culver DH, White JW, Morgan WM, Emori TG, Munn VP, et al. The efficacy of infection surveillance and control programs in preventing nosocomial infections in US hospitals. Am J Epidemiol. 1985 Feb;121(2):182–205. doi: 10.1093/oxfordjournals.aje.a113990. [DOI] [PubMed] [Google Scholar]
- 11.Craven DE, Kunches LM, Lichtenberg DA, Kollisch NR, Barry MA, Heeren TC, et al. Nosocomial infection and fatality in medical and surgical intensive care unit patients. Arch Intern Med. 1988 May;148(5):1161–1168. [PubMed] [Google Scholar]
- 12.Craven DE, Steger KA. Nosocomial pneumonia in the intubated patients. In: Moellering RC, editor. Infectious Disease Clinics of North America. Philadephia: W. B. Saunders Company; 1989. pp. 843–866. [PubMed] [Google Scholar]
- 13.Vincent JL, Rello J, Marshall J, Silva E, Anzueto A, Martin CD, et al. International study of the prevalence and outcomes of infection in intensive care units. JAMA. 2009 Dec 2;302(21):2323–2329. doi: 10.1001/jama.2009.1754. [DOI] [PubMed] [Google Scholar]
- 14.Bamberger DM. Nosocomial pneumonia. In: Farber BF, editor. Infection control in intensive care. New York: Churchiil Livingstone; 1987. pp. 9–23. [Google Scholar]
- 15.Richards MJ, Edwards JR, Culver DH, Gaynes RP. Nosocomial infections in medical intensive care units in the United States. National Nosocomial Infections Surveillance System. Crit Care Med. 1999 May;27(5):887–892. doi: 10.1097/00003246-199905000-00020. [DOI] [PubMed] [Google Scholar]
- 16.Richards MJ, Edwards JR, Culver DH, Gaynes RP. Nosocomial infections in combined medical-surgical intensive care units in the United States. Infect Control Hosp Epidemiol. 2000 Aug;21(8):510–515. doi: 10.1086/501795. [DOI] [PubMed] [Google Scholar]
- 17.Ćosić G, Đekić J, Rajčević S, Ristić M, Ikonić N. Nosocomial infections and microbiological agents in an intensive care unit. Arch Biol Sci. 2012;64(4):1357–1362. [Google Scholar]
- 18.Archibald L, Phillips L, Monnet D, McGowan JE, Jr, Tenover F, Gaynes R. Antimicrobial resistance in isolates from inpatients and outpatients in the United States: increasing importance of the intensive care unit. Clin Infect Dis. 1997 Feb;24(2):211–215. doi: 10.1093/clinids/24.2.211. [DOI] [PubMed] [Google Scholar]
- 19.National Nosocomial Infections Surveillance (NNIS) System Report, data summary from January 1992 through June 2004, issued October 2004. Am J Infect Control. 2004 Dec;32(8):470–485. doi: 10.1016/S0196655304005425. [DOI] [PubMed] [Google Scholar]
- 20.Wong ES. New aspects of urinary tract infections. In: Farber BF, editor. Infection Control in Intenstive Care. New York: Churchill Livingstone; 1987. pp. 25–38. [Google Scholar]
- 21.Shaikh JM, Devrajani BR, Shah SZ, Akhund T, Bibi I. Frequency, pattern and etiology of nosocomial infection in intensive care unit: an experience at a tertiary care hospital. J Ayub Med Coll Abbottabad. 2008 Oct-Dec;20(4):37–40. [PubMed] [Google Scholar]
- 22.Chan RK, Lye WC, Lee EJ, Kumarasinghe G. Nosocomial urinary tract infection: a microbiological study. Ann Acad Med Singapore. 1993 Nov;22(6):873–877. [PubMed] [Google Scholar]
- 23.Bouza E, San Juan R, Munoz P, Voss A, Kluytmans J. A European perspective on nosocomial urinary tract infections II. Report on incidence, clinical characteristics and outcome (ESGNI-004 study). European Study Group on Nosocomial Infection. Clin Microbiol Infect. 2001 Oct;7(10):532–542. doi: 10.1046/j.1198-743x.2001.00324.x. [DOI] [PubMed] [Google Scholar]
- 24.Riddle DJ, Dubberke ER. Clostridium difficile infection in the intensive care unit. Infect Dis Clin North Am. 2009 Sep;23(3):727–743. doi: 10.1016/j.idc.2009.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barbut F, Corthier G, Charpak Y, Cerf M, Monteil H, Fosse T, et al. Prevalence and pathogenicity of Clostridium difficile in hospitalized patients. A French multicenter study. Arch Intern Med. 1996 Jul 8;156(13):1449–1454. [PubMed] [Google Scholar]
- 26.Zilberberg MD, Shorr AF. Preventing clostridium difficile infection in the intensive care unit. Crit Care Clin. 2013 Jan;29(1):11–18. doi: 10.1016/j.ccc.2012.10.006. [DOI] [PubMed] [Google Scholar]


