Abstract
Background
Oxidative stress (OS) and its biomarkers are the biochemical end point of the imbalance between reactive oxygen species (ROS) production and the ability of the antioxidant (AOX) biological systems to fight against oxidative injury.
Objective
We reviewed the role of OS and its downstream signaling in aging eyes.
Methods
A search of the literature and current knowledge on the physiological and pathological mechanisms of OS were revisited in relation to the eyes and the aging process. Most prevalent ocular diseases have been analyzed herein in relation to OS and nutraceutic supplements, such as dry-eye disorders, glaucoma, age-related macular degeneration, and diabetic retinopathy.
Results
Clinical, biochemical, and molecular data from anterior and posterior eye segment diseases point to OS as the common pathogenic mechanism in the majority of these ocular disorders, many of which are pathologies causing visual impairment, blindness, and subsequent loss of life quality. Studies with nutraceutic supplements in aging eye-related pathologies have also been reviewed.
Conclusion
OS, nutritional status, and nutraceutic supplements have to be considered within the standards of care of older ophthalmologic patients. OS biomarkers and surrogate end points may help in managing the aging population with ocular diseases.
Keywords: oxidative stress, eyes, aging, ophthalmic diseases, antioxidants, epidemiology
Introduction
Oxygen (O2) is essential for life, as it plays a major role in the energy cycle of living organisms and is fundamental for the aerobic breathing of cells and tissues. From a chemical point of view, any reaction ending with a gain of electrons results in a reduction process, and in contrast, when concluding with losing electrons results in an oxidation reaction.1
These concepts of reduction–oxidation (redox) are based on electron-transfer events. Compounds capable of accepting electrons are oxidizing agents, and those able to assign electrons are reducing agents (Figure 1). Under normal conditions (during aerobic metabolism), the O2 undergoes tetravalent reduction to water (H2O). However, in situations that induce an incomplete reduction, very unstable and reactive species are formed, the so-called reactive oxygen species (ROS). In general, ROS are classified into two groups: radical species and nonradical species.2
Figure 1.
Redox reactions and Electron-transfer. Reducing and oxidizing agents.
A free radical (FR) is an atom or molecule that contains an unpaired electron in its outer orbital. This situation confers them instability and the need to lose/gain an electron in order to get stable. Among the nonradical oxygen species are hydrogen peroxide (H2O2), ozone (O3), and nitric oxide (NO). There is a typical sequential order in ROS generation, characterized by the production in the first place of the superoxide anion (O2•), followed by H2O2 and the hydroxyl radical (OH•).3–6
O2• is formed in multiple processes, and is the most abundant among the ROS. It possesses a half-life of milliseconds. Although O2• is not reactive enough to attack macromolecules directly, it can start an ROS chain formation. H2O2, a non-radical oxygen species, has the ability to generate other ROS. It is a small molecule lacking electrochemical charge, diffuses easily, and can pass through cell membranes, propagating its effects farther than other species. OH• is the most powerful and harmful of all oxygen species. However, its short half-life (10 seconds) is a limiting condition to the diffusion capacity. It is formed from O2 and H2O2 in the presence of transition metals (Fe/iron) during the sequential reactions of Haber–Weiss and Fenton:
Oxidative stress
All biomolecules can be attacked by ROS: lipids, proteins, and nucleic acids. Among them, lipids are probably the most susceptible to undergo oxidation.7 All cell membranes are rich in polyunsaturated fatty acids (PUFAs), which are easily injured by oxidizing agents. There are endogenous and exogenous defense mechanisms against oxidative attack. Oxidative stress (OS) and its biomarkers are the biochemical end point of the imbalance between ROS production and the ability of antioxidant (AOX) biological systems to counteract the effects of ROS metabolites.6,7
Cells maintain a reducing environment by the corresponding enzymes and the constant supply of metabolic energy. The OS effects depend on the intensity of such abnormalities and the cellular response. If the cell is unable to overcome and to recover its function, the exogenous and endogenous AOX defenses cannot counter it, and the cell can become extinct (Figure 2). However, ROS have also been shown to function as signaling molecules.8 This underlies relevant mechanisms that are essential for the integrity of living organisms and their aging process. Knowledge of the pathways that regulate ROS homeostasis is pivotal for relieving the deleterious effects of ROS in cells and tissues. Further research in this topic can provide strong evidence about specificity in ROS signaling in health and disease.8
Figure 2.
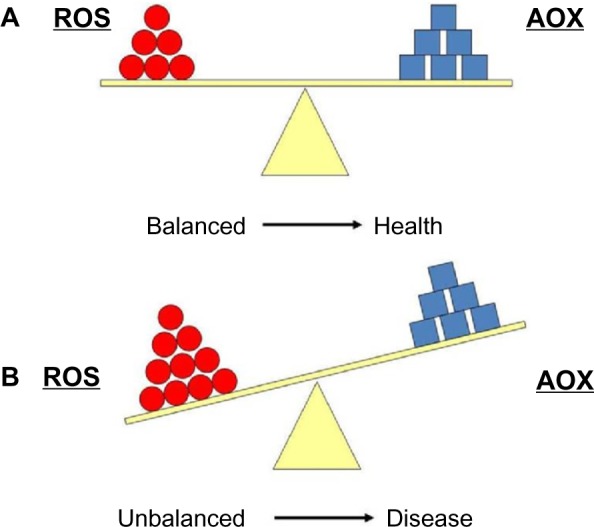
(A) Equilibrium between reactive oxygen species (ROS) production and antioxidant defense (AOX). (B) Imbalanced situation between ROS and AOX, which is associated with many pathologies.
Among the best known AOXs are the enzymes superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GSH-Px).9 Since the discovery of SOD in 1969,10 it has been assumed that ROS participate in the pathogenic mechanisms of various diseases and abnormal conditions. In 1956, Harman proposed the theory of FRs and aging,11 which was supported by the basic theory of the relationship between the duration of life, metabolic rate, and O2 consumption by organisms. Gerschman and Fenn12 and Gerschman et al13,14 demonstrated that FRs are toxic to our body.
OS has been associated with such pathologies as atherosclerosis, Parkinson’s and Alzheimer’s diseases, aging processes, dermatitis, cancer, asthma, neurodegeneration, infertility, atherosclerosis, hypertension, diabetes, and rheumatoid arthritis, among others.7,9,15–20 OS has also been implicated in ophthalmic processes, including ocular surface disorders, cataracts, glaucoma, diabetic retinopathy (DR), retinitis pigmentosa, toxic neuropathies, uveitis, and age-related macular degeneration (AMD).13,21–28 However, the exact signaling pathways regulated by ROS, especially in age-related eye diseases, need further investigation. Delineating the specific signals modulated by ROS is of great diagnostic and therapeutic interest.
Biomarkers and surrogate end points of OS and its downstream effectors have been extensively studied in relation to ocular diseases. Ocular oxidative damage can be determined by direct/indirect methods in biological samples, including such techniques as enzymatic colorimetric assay or high-performance liquid chromatography. With these assays, oxidative activity can be measured: 1) malondialdehyde (MDA), 4-hydroxynonenal, oxidation of nucleic acids 8-hydroxy-2′-deoxyguanosine or 8-oxo-7,8-dihydro-2′-deoxyguanosine; 2) the isoprostanes and the prostaglandin-like compounds that are produced in vivo independently of cyclooxygenase enzymes; and 3) AOX capacity (total AOX capacity [TAC]) can also be determined, as well as enzymatic activities (SOD, CAT, GSH-Px, vitamins) and FR scavengers (GSH). All these oxidative and AOX activities can be assayed on various ophthalmological samples, including aqueous humor, vitreous body, and tear biopsies, and in the blood, urine, or saliva.23–26 Comparing the results of determinations of oxidative and AOX status may reflect the balance between them and the degree of OS by laboratory tests performed in samples from our ophthalmic patients.
Antioxidant mechanisms
The human body has some natural AOX defense systems to diminish the harmful action of continuous ROS production.7,9,10,21 These defenses are located in the cytoplasm, cellular membrane, and extracellular space, and can be classified as follows.
Enzymatic defense systems
These consist of intracellular mechanisms in which SOD, CAT, and GSH-Px eliminate ROS once formed. SODs are metalloproteins that accelerate the dismutation of superoxide to hydrogen peroxide. Protective action is associated with CATs and peroxidases in order to avoid the accumulation of hydrogen peroxide that would otherwise be converted into OH•. There are two main molecular types of SOD in humans: the cytosolic one (CuZnSOD), which contains copper and zinc, and the mitochondrial one (MnSOD), which contains manganese. CAT is located in the peroxisome and mitochondria, and its function consist of catalyzing the dismutation of hydrogen peroxide to water and molecular oxygen. It has been demonstrated that high concentrations of hydrogen peroxide stimulate CAT activity, while low concentrations are preferentially catalyzed by peroxidases. GSH-Px is located in cytosol and mitochondria. It is known that this works by eliminating hydroperoxides, transforming them into water. This reaction is associated with the transformation of reduced glutathion (GSH) into oxidized GSH (GSSG).29,30
Free radical scavengers
FR scavengers slow down the reactions of oxidation or “catch” FRs, transforming them into less aggressive compounds. They can be hydrosoluble and cytosolic (GSH and C vitamin) or liposoluble, located in the membrane (vitamin E and carotenoids). Vitamin C is the main hydrosoluble AOX, and may also regenerate tocopherol (vitamin E), which is one of the main liposoluble AOXs in cellular membranes, together with carotenoids (β-carotene, lutein, zeaxanthin, and lycopenes).31 GSH is considered the most abundant AOX in cytosol, nucleus, and mitochondria, making the ratio of GSH:GSSG a good way to measure OS.
Chelating agents of transition metals
These are molecules that bind iron and copper, like the flavonoids. In this way, they avoid these metals to act in Fenton and Haber–Weiss reactions.3,4 Some authors prefer another classification according to the AOX source. In this manner, AOXs can be endogenous (synthesized by the own body), such as GSH, SOD, and CAT. But they also can be exogenous (only obtained from food), such as vitamins C and E, carotenoids, flavonoids and oligoelements. Besides these AOX defense systems, there are also repairing and exchanging mechanisms of damaged biomolecules.9,29,31
Aging eyes as targets of oxidative stress
The predicted gain in life span in most countries is expected to lead progressively to an increase in the number of older people suffering age-related chronic diseases in the next decade. Moreover, within the cycle of life, aging can be defined as a progressive loss of the efficacy of physiological and biochemical processes occurring until death. Several theories have postulated the link between OS and aging, of which the most famous is the FR theory of aging,11 which maintains that production of FRs and the subsequent FR damage increases with age.
The eye is an organ that is continuously exposed to ionizing radiation, pollutants, industrial smoke, and driving fumes, which makes the eyeballs extremely susceptible to oxidative attack. The retina is a photosensitive tissue that is affected by light rays and undergoes high metabolic activity. Furthermore, the retina contains a higher concentration of PUFAs than other body tissues.32 Because of this, our retinas are vulnerable to the action of ROS. Also, those endogenous oxygen species generated into the eyes can induce ROS-related ocular disorders, as mentioned earlier. OS processes have been associated with a wide variety of eye diseases, such as ocular surface diseases,21 cataracts,22 glaucoma,23 uveitis,33 retinopathies, and AMD.25,26,28,33,34 All of these pathologies are related to age, which is in agreement with the Harman theory of FRs in aging.11
In this context, it is conceivable that the concentration of endogenous AOX sources suffers a significant decrease with age, and likewise, ROS production increases with aging. Moreover, OS processes associated with ocular pathologies contribute to their progression. Many of these diseases are prevalent pathologies leading to blindness. We review these visual impairment-inducing disorders in relation to OS, nutrition, and nutraceutic formulations.
Oxidative stress-related ocular pathologies
Ocular surface diseases
Dry-eye disorder (DED) is a recently introduced term that defines the ocular surface dysfunction leading to tear-film impairment and dry eyes. DEDs are complex diseases involving the lacrimal glands, eyelids, and tear film, as well as a variety of ocular surface tissues, including epithelial, inflammatory, immune, and goblet cells.35–38
DED displays high prevalence rates worldwide,35,39 and usually affects people aged 65 years and above. Moreover, DED is more common in women, as emphasized by the Women’s Eye Health Organization and other large studies,40–42 with an estimation of about 3.23 million American women suffering from DED.
OS plays a significant role in a wide spectrum of ocular conditions, including ocular surface pathologies.43,44 As a result of the imbalance between prooxidant and AOX sources, the ocular surface structures can be damaged. It has also been reported that tears provide key molecules for preventing OS injury to the cornea.45 Evidence is growing that constant exposure to OS is involved in the initiation and progression of cellular injury that leads to ocular surface pathology in DED, conjunctivochalasis, ultraviolet light-induced ocular surface epithelial injury, and tobacco smoke-induced ocular surface epithelial damage. OS and its downstream signaling are linked to a series of cellular events. This strongly indicates that OS activates cell regulatory molecules that may alter the regenerative capacity of the corneal epithelial cell layer under dry-eye conditions, as reported by Nakamura et al in a blink-suppressed dry-eye rat model.46 The authors speculated that an imbalance in the tear-film status, a synergistic effect between prolonged exposure to atmospheric oxygen, and insufficient supplementation of AOX, agents may have induced the overexpression of ROS production on the ocular surface. In this context, AOX supplements may help in counteracting the oxidative status in the altered ocular surface.
A significant increase in oxidative activity and a decrease in AOX defenses in ocular fluids and tissues, as well as in plasma samples, have been associated with DED patients.43–45 In fact DED has been recognized as an OS-induced process. In addition, it has recently been reported that chronic inflammation plays a crucial role in DED, as measured by the release of cytokines and chemokines that initiate or amplify the body’s immune and inflammatory response.46–48 Significantly increased levels of cytokines/chemokines have been detected in the tear fluid and conjunctiva of people with DED compared to normal eyes.46–50 Certain cytokines, such as interleukin 6, may induce cells to produce other compounds that play a role in the development of inflammation, such as prostaglandins, enzymes, and ROS.51 Tsubota et al52 recently suggested a new approach to the aging process and DED through controlling levels of calories and/or ROS in order to prevent DED.
OS can harm the ocular surface by inducing serious tissue damage to the cornea and conjunctiva, leading to visual impairment that may be counteracted, at least in part, by appropriate diet and nutritional supplements.
Glaucoma
Glaucoma is the second-most relevant cause of blindness worldwide. The disease is a progressive optic neuropathy, often caused by elevated intraocular pressure (IOP) consequent to abnormal high resistance to aqueous humor drainage via the trabecular meshwork and Schlemm’s canal.53 Regulation of IOP depends on the balance of complex mechanisms involved in the production and outflow of aqueous humor, including the stability and survival of cellular phenotypes involved in this process and the correct maintenance of homeostasis.54,55 The most frequent glaucoma type is the chronic clinical manifestation known as the primary open-angle glaucoma (POAG), mainly affecting individuals aged 40 years or more.53 In spite of elevated IOP, apoptosis has been recognized as the end point for retinal ganglion cell (RGC) death in any glaucoma type.54–56
Oxidative damage was found in the deoxyribonucleic acid (DNA) of POAG patients.57 Moreover, a significant correlation between oxidative DNA damage and IOP was described in glaucomatous patients. It has also been shown that POAG patients display a genetic background rendering them susceptible to ROS-induced damage because of a more frequent deletion, compared to control individuals, of the gene encoding for GSH S-transferase, pivotal for the AOX defense mechanism.57 It has to be considered that OS occurs in the trabecular meshwork and Schlemm’s canal, as well as in the posterior eye-segment structures (RGCs and optic nerve fibers). OS has also been linked to POAG by increasing flow resistance in the anterior chamber of the eye in the presence of high levels of hydrogen peroxide. Abundant AOX activity of the trabecular meshwork and aqueous humor of these patients has also been reported in POAG individuals.23 Recent reports implicate OS, inflammation, ischemia, and excitotoxicity among the proapoptotic factors for RGCs in glaucomatous patients.58 Corresponding markers to these etiopathogenic mechanisms have been subsequently described.59 The most probable OS biomarkers for POAG are shown in Table 1.
Table 1.
Oxidative stress biomarkers in primary open-angle glaucoma
| Reference | Biomarker | Sample | Levels in POAG |
|---|---|---|---|
| Ames et al19 | AOXT | Dietary antioxidants | ↓ |
| Zanón-Moreno et al23 | MDA | Aqueous humor | ↑ |
| AOXT | Aqueous humor | ↓ | |
| Galbis-Estrada et al51 | IL-6 | Human tears | ↑ |
| TNF-a | Human tears | ↑ | |
| Huang et al55 | CASPASES 8, 9 | Rat retinal lysates and retinal whole mounts | ↑ |
| PARP 1 | Rat retinal lysates and retinal whole mounts and cross-sections | ↑ | |
| Pinazo-Durán et al56 | MDA | Aqueous humor | ↑ |
| AOXT | Aqueous humor | ↓ | |
| CASPASE 3 | Aqueous humor | ↑ | |
| PARP 1 | Aqueous humor | ↑ | |
| Saccá and Izzotti57 | NO | Human trabecular meshwork | ↑ |
| Endothelin 1 | Aqueous humor | ↓ | |
| Tezel et al58 | GAPDH | Retinal lysates | ↑ |
| HSP72 | Retinal lysates | ↑ | |
| Glutamine synthetase | Retinal lysates | ↑ | |
| Pinazo-Durán et al59 | MDA | Aqueous humor | ↑ |
| AOXT | Aqueous humor | ↓ | |
| SOD | Aqueous humor | ↑ | |
| Bazán et al60 | NPD 1 | ARPE 19 cells | ↓ |
| Galbis-Estrada et al77 | MDA | Rat retinal cross-sections, and lysates | ↑ |
| AOXT | Rat retinal cross-sections and lysates | ↓ | |
| GSH | Rat retinal cross-sections and lysates | ↓ | |
| Zanón-Moreno et al102 | Vitamin E | Serum | ↓ |
| Vitamin C | Serum | ↓ | |
| GSH Px | Serum | ↓ |
Abbreviations: AOXT, total antioxidant activity; MDA, malondyaldehyde; IL-6, Interleukin-6; TNF-a, tumor necrosis factor-alpha; PARP-1, poly adenyl rybose polymerase-1; CS 3, caspase 3; NO, nitric oxide; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; HSP72, heat shock protein 72; GSH, glutathione; SOD, superoxide dismutase; NPD 1, 10,17S-docosatriene; GSH Px, glutathione peroxidase.
However, RGC loss in glaucoma is still a mystery to scientists. In response to glaucomatous stimuli, cross talk between proapoptotic signals (caspase activation and mitochondrial dysfunction) and survival promoters (neurotrophins) determines the fate of RGCs.54–56 From a molecular viewpoint, it is difficult to address the role of OS in glaucoma standards of care. Future studies in OS and its downstream signaling may be useful for better managing this insidious disease
Retinal diseases
The retina is extremely vulnerable to ROS damage.21,32 Major reasons for this susceptibility are constant exposure to visible light, causing photooxidation, and high oxygen consumption, due to the great energy demanded.32 In addition, the extraordinary concentration of PUFAs makes the retina prone to lipid oxidation.60
Also, the retinal pigment epithelium (RPE) is subjected to OS. The ROS formed from phagocytosis and lipid peroxidation and/or focal light, together with high oxygen tension in the macula, point to the fact that OS is extremely important in the RPE. Moreover, the RPE phagocytic function provides an additional oxidative burden because of the constant shedding of the outer segments of photoreceptors.61,62
The major PUFA in the retina is the docosahexaenoic acid (DHA), highly concentrated in the outer segment of the photoreceptors. Retinal DHA is a target for ROS, but at the same time it plays a key role in the homeostasis and function of the photoreceptors and RPE under physiologic conditions.63–65 When OS increases, DHA activates prosurvival signaling via neuroprotectin D1, which is its major mediator.63–65 The presence of lipofuscin, a product of the degradation of photoreceptors, may also act as a photosensitizer,66 leading to an increase of the risk of oxidative injury. However, studies in this field are steadily growing. Curcio et al67 emphasized that the RPE secretes apolipoprotein B particles into Bruch’s membrane, and these accumulate with age, which in turn may form a lipid wall, a precursor of the basal linear deposit. Then, certain constituents of the described aggregates may interact with ROS, resulting in proinflammatory peroxidised lipids leading to upregulation of cytokines/chemokines, as well as provoking neovascularization.
However, in the RPE, OS is also capable of inducing protective pathways, such as the phosphatidylinositide 3-kinase/Akt and nuclear factor erythroid-2-related factor 2 pathways. Vascular endothelial growth factor and neuroprotectin D1 signaling acts also by protecting cells and tissues, as reported by Faghir and Bazan68 and Klettner69 in recent publications.
Many retinal disorders have been related to oxidative damage. The pathophysiology of some of them includes oxidative damage to the RPE, as in AMD or Stargardt disease.64,65 The mechanism of RPE injury has not been clearly described, but it may be related to A2E (a lipofuscin fluorophore).69,70–74 AMD, a leading cause of severe visual loss, is a multifactorial disease, and thus its pathogenesis remains poorly understood. Nevertheless, the important role of OS in the development of AMD has been demonstrated. In normal conditions, the RPE constantly phagocytizes the photoreceptor outer-segment membranes, but in AMD an RPE dysfunction leads to the accumulation of undigested material, called lipofuscin.69–73 Lipofuscin is deposited into insoluble aggregates in the RPE cells, displaying potent photoinducible properties, contributing to an enhancement and propagation of OS.
Other retinal dystrophies, such as retinitis pigmentosa, have been related to oxidative damage and progressive cell loss.74 Furthermore, the role of OS in mitochondrial neuroretinal diseases, such as Leber hereditary optic neuropathy, has also been documented.75
Increased intraocular iron results in oxidative damage to the retina. The retinal localization of lipid peroxidation (LPO) sites induced by iron or nicotine adenine dinucleotide phosphate (NADPH) have been described by histochemical and immunocytochemical assays.76,77 Also, increased superoxide anions were found in photoreceptor inner segments of adult C57Bl/6 mice given intravitreous injections of FeSO4, and higher concentrations of the injected FeSO4 induced photoreceptor damage.78 Iron overload was identified “postmortem” in the RPE as well as in the photoreceptors of a 72-year-old patient with advanced macular geographic atrophy.79 Whether iron contributes to AMD pathogenesis or it is an AMD by-product remains unclear. Anyway, it can be speculated that AOX and iron chelators may be effective in preventing the development and progression of AMD.80 In fact, it has been recently demonstrated that iron chelators protect experimental animals from OS.81
The role of OS in diabetics and their eyes warrants particular attention. DR is the leading cause of blindness in developed countries. The presence of inappropriate glucose levels in the blood for years produces changes in retinal vessels that cause damage to the cells and progressively to the vision. These complications caused by the hyperglycemia are increased by excess generation of ROS and nitrogen species.82 This occurs mainly when diabetes is not properly controlled. FRs may function as signaling molecular and intra/extracellular defense mechanisms, as well as phagocytosis regulators. They may also act as molecules involved in biogenic vascular regulation.8 FR overproduction and/or insufficient removal results in vascular dysfunction, causing damage to cellular protein fragmentation, cross-linking and aggregation of amino acids, change in membrane lipids, and nucleic acid alteration by fragmentation, deletions, and mutations in the DNA chain. All these changes may trigger a nonphysiological cell death (necrosis), and constitute important OS biomarkers that can be measured in an appropriate and accurate form in diabetic patients, especially in the presence of chronic complications.83 Many types of ROS have been implicated in diabetes (both of mitochondrial and nonmitochondrial origin), among them NADPH oxidase, xanthine oxidase, uncoupled endothelial nitric oxide synthase, lipoxygenase, cyclooxygenase, cytochrome P450 enzymes, and other hemoproteins.84 Mitochondria are thought to be the main source of ROS generation in diabetics. Lipid peroxidation is one major pathogenic mechanism through which OS may damage the retina.25,32,84,85 Endothelial cells of retinal vessels and the RPE are constituents of the blood–retinal barrier. These vessels are stabilized by the presence of pericytes. During DR development, these pericytes disappear progressively, and the microvasculature undergoes progressive changes, such as altered endothelial cells, presence of capillary occlusions by endothelial cell proliferation, and neovascularization.86 The hyperglycemia also causes biochemical alterations through OS, leading to an increase in apoptosis of the retinal endothelial cells.87
Independently of manifestations of the ocular complications of diabetes, the main objectives for managing diabetics include the following challenges: 1) maximum nutritional and metabolic control (Tables 2 and 3), 2) normal blood glycemia levels to reduce the risk of complications, 3) adequate lipid and lipoprotein profiles to reduce the risk of cardiovascular disease, and 4) normal blood pressure levels to reduce cardiac and brain stroke risk.
Table 2.
Biochemical parameters for optimal control in diabetic patients
| Parameter | DM managing | Healthy population |
|---|---|---|
| Prepandrial plasmatic glycemia | 90–130 mg/dL | 70–90 mg/dL |
| Postpandrial plasmatic glycemia | <180 mg/dL | 70–135 mg/dL |
| HbA1c (%) | <7% | 6% |
| Arterial pressure | <130/80 mmHg | <140/90 mmHg |
| Cholesterol | <175 m/dL | <200 mg/dL |
| LDL | <100 mg/dL | <130 mg/dL |
| HDL | Man: >40 mg/dL Women: 50 mg/dL |
Man: >40 mg/dL Women: >50 mg/dL |
| Triglycerides | <150 mg/dL | <150 mg/dL |
Abbreviations: DM, diabetes mellitus; HbA1c, glycosilated haemoglobin; LDL, low density cholesterol; HDL, high density cholesterol.
Table 3.
References of glycemic index respect to the glucose (100) in main nutrients.
| Bread | Glycemic index | Cereals | Glycemic index | Fruits | Glycemic index | Vegetables | Glycemic index | Dairy | Glycemic index |
|---|---|---|---|---|---|---|---|---|---|
| White bread | 70 | Rice | 55 | Watermelon | 72 | Carrot | 71 | Milk | 34 |
| Rye bread | 50 | Corn | 55 | Mango | 64 | Pumpkin | 75 | Yogurt | 36 |
| Oat bread | 47 | Wheat | 40 | Banana | 53 | Sweet potato | 51 | Ice cream | 36 |
| Macaroni | 45 | Chickpeas | 33 | Kiwi | 52 | Peans | 48 | White sugar | 59 |
| Spaghetti | 35 | Lentils | 29 | Apple | 36 | Potatoes | 70 | Fructose | 20 |
| White beans | 31 | Special K® | 54 | Pear | 33 | Tomatoes | 38 | Honey | 87 |
| Fresh beans | 29 | Cornflakes | 85 | Plum | 24 | Beet | 64 | Soja milk | 18 |
Note: The glycemic index estimates how much each gram of carbohydrate in a particular food raises the blood glucose level following food consumption (in relation to consumption of pure glucose, which is used as the benchmark, with glucose absorption being 100% or 100).
Nutritional intervention in diabetes mellitus is essential for early identification and treatment of chronic diabetes complications by modifying the diet and to introduce adequate changes in lifestyle to prevent obesity, hypertension, dyslipidemia, neuropathy, cardiovascular disease, nephropathy, and retinopathy (see Table 2). It is widely recommended to gather data from individual nutrition facts by considering anthropometry, social and cultural demands, working conditions, and lifestyle of the diabetic patient (see Table 3).
Currently, OS is considered an important factor in the pathogenesis of DR.81–88 OS is measured indirectly by determining the oxidation of by-products of proteins and lipids, which reflects the extent of cellular damage. Most popular and frequently used is the determination of MDA by the thiobarbituric acid-reactive substance test and the detection of F2-isoprostanes that reflect oxidative damage to lipids.25,84,86,87 Also, concentration measurements of AOX enzymes and other compounds with the same functions (GHS, NADPH, coenzyme Q, and vitamins A, C, and E), are performed in some cases by means of high-performance liquid chromatography.89
In Italy, Mancino et al25 examined 19 patients with type 2 diabetes mellitus with DR and 14 nondiabetic, age-matched subjects forming a control group. Blood, aqueous humor, and vitreous body samples were collected at the time of surgery (cataract, vitrectomy). MDA concentrations and blood extracorporeal photopheresis were measured with high-performance liquid chromatography. The TAC of the samples was estimated with the oxygen radical absorbance-capacity method. The authors reported that the level of blood and vitreous MDA in the proliferative DR (PDR) subgroup was significantly higher compared to controls and to the no-PDR subgroup of patients. The PDR patients also had lower levels of TAC at the vitreous body and aqueous humor levels, but not at the blood level, compared to controls and nonproliferative DR (NPDR) patients. In all diabetic patients, the blood extracorporeal photopheresis values were significantly lower, compared to control subjects. These results support the hypothesis that OS and the decreased AOX defenses are associated with the progression of DR to its proliferative form, suggesting that AOX supply may be helpful for correcting OS and for blocking disease progression.
Gene expression can be influenced by the environment, including the exogenous and the endogenous world, which includes such factors as chemicals, light, temperature, metabolism, and hormones that can determine which genes are turned on and off, thereby impacting the way an organism develops and functions. Dolinoy et al90 supplemented the diet of rat mothers with methyl-donating substances, such as folic acid and vitamin B12, and it was found that this type of intervention counteracted the reduction in DNA methylation caused by bisphenol A (a compound that is usually present in plastic drink bottles, including baby bottles). This epigenetic mechanism clearly shows how profoundly environment can affect gene expression in a long-lasting way.
Although diabetes mellitus is a multifactorial disease, ethnicity, sex, genomic factors, age, nutritional characteristics, lifestyle, and even environmental factors can change the genetics. Therefore, measuring solely on glycemic biomarkers may not be sufficient for controlling DR.
Studies on the role of nutritional supplements in eye diseases
For an optimal nutritional status, as well as for having a healthy body and for being at lesser risk of developing diseases, the dietary guidelines for Americans from the US Department of Health and Human Services91 describe a healthy diet as one that: 1) emphasizes fruits, vegetables, whole grains, and fat-free (or low-fat) milk and milk products, 2) includes lean meats, poultry, fish, beans, eggs, and nuts, and 3) is low in saturated fats, trans fats, cholesterol, salt (sodium), and added sugars. However, if additional supplements are needed, these have to be appropriately traded and labeled to display full information about composition, nutritional properties, and safety.
The Mediterranean diet has been strongly associated with a reduction in overall risk for cardiovascular diseases, cancer, and neurodegenerative disorders.92 Surprisingly, there are no studies specifically looking at an association between the Mediterranean diet and a presumptive benefit for aging eyes. However, the Mediterranean diet,92–95 with variations reflecting influences of the culture and landscape, which has proven to be rich in vitamins, carotenoids, and ω-3 FAs, seems to be a good tool for counteracting oxidative damage linked to age-related eye diseases.
The eyes are at risk of suffering oxidative damage due to a great deal of FAs in the retina, their high exposure to oxygen and light, environmental pollutants, and ultraviolet rays. In addition, the rate of ROS formation in the mitochondria increases through aging. Given this scenario, the most prevalent ocular diseases closely related to OS and the role of diet and nutritional supplements are now revisited.
Dry-eye disorders
The ocular surface epithelial cell layers (the conjunctiva and cornea) are the most important tissues for protecting the eyes from external injuries.35,37 Several studies44–46,50–52,96,97 have been published on the role of OS and nutraceutic supplements in DED.
Nutraceutic formulations containing specific AOXs and ω-3 FAs have been assayed in relation to DED, with positive results on subjective symptoms as well as the clinical evolution of the disease, as confirmed by the Ocular Surface Disease Index (OSDI), a personal questionnaire of DED severity, the ophthalmologic examination, expression patterns of immune mediators in tear samples of mild-to-moderate dry eyes with respect to individuals with healthy ocular surface,50 and in patients undergoing chronic glaucomatous therapy with antihypertensive eyedrops compared to control eyes.51
In another European study, the French ALIENOR (Antioxydants, Lipides Essentiels, Nutrition et Maladies Oculaires) study96 analyzed several characteristics of DED, among other eye diseases, such as self-reported dry eye, current use of artificial tears, OSDI score, and the tear breakup-time test. In addition, the authors recorded some nutritional data and performed plasmatic determinations of vitamins and FAs. The results confirmed a slight increase with age of use of artificial tears and OSDI score, as well as the association of eye diseases with history of nutritional status. An update of this study has been recently published.97
Glaucoma
Recent evidence demonstrates the important role of OS in glaucoma pathogenesis.21,23 Nutritional supplementation, including Ginkgo biloba, curcumin (derived from a spice), and resveratrol has been recommended for patients with normotensive glaucoma for its beneficial effect on the processes of inflammation and reduction of vascular flow and OS.98,99 A correlation was also found between AOX supplementation in patients with POAG (in the intermediate stage), a significant percentage of stabilization in the visual field, and in the analysis of optical fibers in relation to the progression of glaucoma.
Coleman et al100 showed that a diet with a low intake of AOXs, particularly retinol and vitamin B1, is associated with a high risk of glaucoma. From a total of 1,155 women participating in a study of osteoporotic fractures, Kang et al101 diagnosed 95 women with glaucoma. These patients were evaluated (by analysis of optic disk photographs and visual field) for the risk of glaucomatous progression according to the consumption of fruits and vegetables. The study showed less progression of glaucoma in women who frequently consumed carrots, cabbage, green leafy vegetables, and apricots versus those that did not. These results are really interesting, and make us look to the importance of nutrition and OS, as well as the AOX defenses in glaucoma. Moreover, dietary fat consumption in relation to POAG was analyzed in 76,199 women from the Nurses’ Health Study and 40,306 men from the Health Professionals Follow-up Study101 that were POAG-free. A high ratio of n-3 to n-6 PUFAs appears to increase the risk of POAG.
Zanon-Moreno et al102,103 proposed that genetic factors may play a role in modulating the effect of dietary AOX intake on glaucoma. The researchers firstly studied the association between selected polymorphisms in key transporter proteins related to vitamin C and vitamin A concentrations and POAG. A total of 300 subjects (150 POAG cases and 150 controls) from a Mediterranean population were recruited to determine the plasmatic concentrations of vitamins A and C and the single-nucleotide polymorphisms (SNPs) in genes related to these vitamin concentrations (rs176990 and rs190910 in the RBP1 gene, and rs10063949 and rs1279683 in the Na+-dependent L-ascorbic acid transporters 1 and 2, respectively [encoded by the SLC23A1 and SLC23A2 genes]). A significant association between the rs1279386 (A>G) SNP in SLC23A2 and POAG risk was detected. It was also found that POAG patients had lower plasma vitamin A and C concentrations than the controls (Figure 3A and B). SNPs in RBP1 were not associated with vitamin A concentrations or POAG risk. A significant association between the rs1279386 SNP in SLC23A2 and plasma vitamin C concentrations was found in relation to glaucoma risk. The rs10063949 SNP in SLC23A1 was not associated with either plasma vitamin C concentrations or POAG risk.102 The same group analyzed the association of selected SNPs in genes related to vitamins C and E, and GSH-Px activity and POAG risk. In this study, 250 POAG cases and 250 controls were selected, also from the same Mediterranean area. Plasma concentrations of vitamins C and E were measured (Figure 3C), and GSH-Px activity was also determined. POAG patients had statistically significant (after correction for multiple testing) lower plasma vitamin C (P<0.001) and vitamin E (P<0.001) concentrations than the nonglaucomatous subjects. Higher plasma GSH-Px activity in POAG cases than in controls (P<0.001) was found. Also analyzed were specific SNPs (rs1279683 in the Na+-dependent L-ascorbic acid transporter 2 gene [SLC23A2], rs6994076 in the TTPA gene, rs737723 in the SEC14L2/TAP gene, and rs757228 in the GPX4 gene) in relation to glaucoma risk. A novel association between the rs737723 polymorphism and POAG risk was found that remained statistically significant after adjustment for multiple comparisons. A higher glaucoma risk in GG homozygous individuals was statistically confirmed in association with the rs1279683 polymorphism. Furthermore, a significant (P<0.05) gene–gene interaction between the SEC14L2/TAP and SLC23A2 polymorphisms in determining POAG risk was detected in those subjects who had both risk genotypes at the same time (P<0.01). Any association with POAG risk for the rs6994076 or rs757228 polymorphisms was identified. The rs1279683 and rs6994076 polymorphisms were significantly (P<0.001) associated with plasma vitamins C and E, respectively. However, the rs757228 polymorphism in the GPX4 gene was not associated with plasma GSH-Px activity.103 In these two studies, novel associations between the specific SNPs and higher POAG risk were confirmed. The results also suggested a gene–gene interaction between both polymorphisms that increases POAG risk. These works support the role of vitamin-transporter proteins in the expression and availability of antioxidant vitamins, from both the natural food and nutraceutics supplementation.
Figure 3.
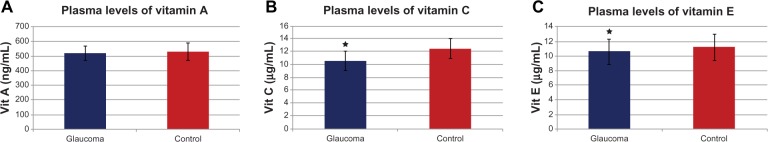
(A–C) Plasma vitamin levels in primary open-angle glaucoma patients.
Age-related macular degeneration
AMD is the leading cause of severe vision loss in developed nations. AMD is caused by complex interactions between aging, genetics,27,28,34 environmental factors, and comorbid diseases.66,67 Numerous studies have analyzed the relationship between the intake of nutritional supplements and AMD, and the most relevant are revisited herein. For a summary on these studies in AMD see Table 4.
Table 4.
Relation of clinical-epidemiological studies on the role of micronutritional supplements in age-related macular degeneration
| Cited reference | Study | Findings in AMD |
|---|---|---|
| 104 | The Beaver Dam Eye Study | Inverse association between vitamins A, E and zinc intake and incidence of large drussen and macro-pigmentary changes |
| 105 | The Rotterdam Eye Study | High dietary intake of β-carotene and vitamins C, E and zinc was associated with reduced risk of AMD in the elderly |
| 106 | The Age-related Eye Disease Study (AREDS 1) | Daily intake of tablets with vitamins C, E and β-carotene, zinc and copper resulted in a moderate reduction of the risk of acquiring advanced AMD |
| 113 | The Carotenoids Age-Related Eye Disease Study (CAREDS) | Lutein- and zeaxanthin-rich diets may protect against intermediate AMD in female patients aged <75 years |
| 114 | The Australian Blue Mountains Eye Study | Intake of ω-3 fatty acids in the diet, as well as higher dietary lutein and zeaxanthin intake induced a lower incidence of developing AMD initial forms and AMD advanced forms |
| 118 | The Dietary Ancillary Study of the Eye Disease Case-Control Study (DSEDCS) | Participants with higher food intake with lutein and zeaxanthin showed a lower risk of AMD |
| 117 | The French POLA Study | Analysed the levels of antioxidant nutrients in relation to AMD. A lower rate of progression from moderate AMD to advanced AMD in one eye was found. Authors concluded that, vitamin E may provide protection against AMD |
| 118,122 | The North American National Health and Nutrition Examination Survey (NHANES) | Main goal was to analyze the blood levels of vitamin E and AMD and the results showed a lesser risk of AMD initial stages with elevated levels of this vitamin |
| 119 | The Lutein and Antioxidant Supplementation study Trial (The veterans LAST) | Found a relationship between the intake of carotenoids and subjective signs of improvement of vision in 90 patients with AMD |
| 120 | The Age-Related Eye Diseases Study (AREDS 2) | Concluded and reported data on the intake of carbohydrates and the risk of suffering AMD and progression of disease, probably by oxidative damage to protein |
Abbreviations: AMD, age-related macular degeneration; AREDS I, Age-Related Eye Disease Study 1; AREDS 2, Age-Related Eye Disease Study 2; CAREDS, The Carotenoids Age-Related Eye Disease Study; DSEDCS, The Dietary Ancillary Study of the Eye Disease Case-Control Study; LAST, the Lutein and Antioxidant Supplementation study Trial (The veterans LAST); NHANES, the North American National Health and Nutrition Examination Survey; POLA, the Pathologies Oculaires Liées à l’Age study.
The Beaver Dam Eye Study104 found an inverse association between vitamin A and E intake and the incidence of large drusen, and between zinc intake and the incidence of macropigmentary changes in early AMD. The Rotterdam Eye Study105 concluded that a high dietary intake of β-carotene, vitamins C and E, and zinc was associated with a substantially reduced risk of AMD in elderly individuals. Clinical trials like the Age-Related Eye Disease Study (AREDS)106 randomly assigned patients to daily oral tablets containing AOXs (vitamin C 500 mg, vitamin E 400 IU, and β-carotene 15 mg), zinc 80 mg, and copper 2 mg, or placebo. This clinical trial showed a statistically significant benefit of the combination of high-dose AOX vitamins and zinc, providing a moderate risk reduction of developing advanced AMD over a median of 6.3 years of follow-up in those at high risk of developing advanced AMD. Increased intake of ω-3 FAs, especially long-chain ω-3 FAs, such as DHA and eicosapentaenoic acid (EPA), has been associated with improving a number of chronic diseases, including AMD.107,108 Strong evidence for a beneficial role of ω-3 fatty acids in eye health was found in two cross-sectional studies: the US Twin Study of Age-Related Macular Degeneration (US Twin)108 and AREDS.106 Also, lutein and zeaxanthin seems to have a protective effect on the outer retina and RPE by absorbing short-wavelength light and minimizing oxidative damage, thus aiding cell-membrane stability. The Carotenoids in Age-Related Eye Disease Study (CAREDS)109–113 concluded that lutein- and zeaxanthin-rich diets may protect against intermediate AMD in female patients aged 75 years or more. The Australian Blue Mountains Eye Study114 analyzed the intake of ω-3 fatty acids in diet, as well as higher dietary lutein and zeaxanthin intake. Results displayed a lower incidence of developing forms of initial and advanced AMD. Furthermore, the AREDS reported in 2007 that dietary lutein and zeaxanthin intake was inversely associated with neovascular AMD, geographic atrophy, and large or extensive intermediate drusen.115 Thus, the Dietary Ancillary Study of the Eye Disease Case-Control Study (DSEDCS)116 revealed a relative risk for AMD estimated according to dietary indicators of AOX status, controlling for smoking and other risk factors, by using multiple logistic regression analyses in which participants with higher food intake with lutein and zeaxanthin showed a lower risk of AMD. In the French POLA Study,117 levels of AOX nutrients in relation to AMD were analyzed, and data showed a lower rate of progression from moderate AMD to advanced AMD in one eye. The authors concluded that vitamin E may provide protection against AMD. Current data processing of this study strongly indicates that AMD develops mainly in subjects aged 80 years or older, and in subjects with early age-related maculopathy signs and symptoms. The North American National Health and Nutrition Examination Survey (NHANES)118 was a cross-sectional nested case-control study matching subjects on age, sex, and race that was performed using data on adult participants in the third NHANES. Study outcomes included late AMD, defined as neovascular disease or geographic atrophy (5:1 matching), and a composite of both early AMD, defined as soft drusen or pigment irregularities with or without any drusen, and late AMD (1:1 matching). The main goal of this study was to analyze the blood levels of vitamin E and AMD. Data showed a lesser risk of AMD at initial stages with elevated levels of this vitamin. The Lutein and Antioxidant Supplementation Trial (Veterans LAST)119 found a relationship between the intake of carotenoids and subjective signs of improvement of vision in 90 patients with AMD. Finally, the most recent results from the AREDS2,120 a multicenter, randomized, double-masked, placebo-controlled Phase III study, merit special consideration in this review. It was conducted through 2006–2012 with 4,203 participants aged 50–85 years at risk for progression to advanced AMD. The clinical characteristics of the suitable participants were bilateral large drusen, or large drusen in one eye and advanced AMD in the fellow eye. The main purpose of the AREDS2 was to determine whether adding lutein + zeaxanthin, DHA + EPA, or both to the initial AREDS formulation decreases the risk of developing advanced AMD or not. After a median follow-up of 5 years, 1,940 study eyes (1,608 participants) progressed to advanced AMD. Statistical probabilities of progression to advanced AMD by 5 years of follow-up were 31% for placebo, 29% for lutein + zeaxanthin, 31% for DHA + EPA, and 30% for lutein + zeaxanthin and DHA + EPA. When compared with placebo in the primary analyses, no statistically significant reduction in the progression to advanced AMD was found. The evaluation of the effects of eliminating β-carotene from the original AREDS formulation (500 mg vitamin C, 400 IU vitamin E, and 15 mg β-carotene) and/or lowering the zinc (previously 80 mg zinc as zinc oxide, and 2 mg copper as cupric oxide) is an important point to consider. In fact, zinc decreased copper levels and caused copper-deficiency anemia. β-carotene elimination from the utilized formulation or the incorporation of a lower dose of zinc had no apparent effect of progression to advanced AMD. Important data reflected that more lung cancers were identified between the β-carotene versus no-β-carotene group (2.0%) versus 11 (0.9%) (nominal P=0.04), mostly in former smokers.
The main conclusion of the AREDS2120 was that addition of lutein + zeaxanthin, DHA + EPA, or both to the primary formulation did not further reduce risk of progression to advanced AMD. Moreover, AREDS2120 also concluded that lutein + zeaxanthin could appropriately substitute beta-carotene in AMD interventional studies with nutraceutics (to avoid the described risk of lung cancer in smokers).
Diabetic retinopathy
DR is the most common microvascular complication of diabetes mellitus and one of the leading causes of blindness worldwide. Among the studies on the role of AOXs and ω-3 fatty acids in the prevention of DR, the San Luis Valley Diabetes Study121 found no protective effect between the AOX nutrients β-carotene and vitamin C or E and DR. The Third National Health and Nutrition Examination124 reported that serum levels of α-tocopherol and vitamin C were not associated with DR. It was also concluded in the Diabetes Control and Complications Trial123 (which included over 1,000 type 1 diabetics) that those patients who followed a low-fat diet decreased the rate of progression of DR a third more than those patients not following the low-fat diet. As for type 2 diabetes, another study found less frequency of DR in subjects who took multivitamins or supplements that included vitamins C and E.124 ω-3 PUFAs may also play a beneficial role in DR. In a recent study, the antiangiogenic properties of edible berries were shown.125 Brownlee126 proposed hyperglycemia as responsible for superoxide anion overproduction (occurring in the mitochondria). This may provide a new concept for future research and drug discovery.
Garcia-Medina et al127 designed a follow-up study in the Mediterranean area of Spain with the main goal of evaluating the effect of AOX supplementation on 105 type 2 diabetics with NPDR over a 5-year period, in a similar manner to the follow-up schedule designed by the AREDS. A complete ophthalmic examination and plasmatic determinations of oxidative (MDA) and AOX parameters (total AOX status) were obtained as the baseline. One part of the cohort was randomly assigned to oral AOX supplementation at nutritional doses with the formulation defined in Table 5. The same examinations were performed with 97 diabetic patients who completed the 5-year follow-up period. Best-corrected visual acuity, DR score, MDA, and total AOX status values were compared at the beginning and the end of the follow-up. The authors found that best-corrected visual acuity did not change during the follow-up, irrespective of supplementation. However, the DR stage showed a retardation of progression in the subgroup with supplementation, but worsened in the subgroup with no AOX supplementation. Furthermore, the AOX-supplementation group maintained its AOX plasma status levels, which was related to decreased oxidative plasma activity. The authors concluded that oral AOX supplementation could be a useful adjunctive long-term therapy in the treatment of NPDR. Currently, a new study (the Valencia Diabetic Retinopathy Study [VDRS]) is being carried out to analyze the role of a combined formulation of AOX and ω-3 fatty acids in the risk and progression of DR by evaluating clinical manifestations, biochemical parameters, nutritional facts, and lifestyle, as well as subjective sensations in 350 participants made up of type 1 diabetics, type 2 diabetics, and healthy controls, through a 3-year follow-up. Preliminary data confirmed previous results from our group and others, with a significant increase in plasmatic MDA levels (P<0.01) and decreased total AOX activity (P<0.001) in both diabetic types with respect to the controls.
Table 5.
Combined formulation of antioxidants utilized in the 5-year follow-up study on type 2 diabetic patients and diabetic retinopathy
| Vitalux forte® | ώ-3 fatty acids | Lutein/zeaxanthin | Vitamin C | Vitamin E | Zinc | Copper |
|---|---|---|---|---|---|---|
| Per capsule | 160 mg | 10 mg/1 mg | 60 mg | 20 mg | 10 mg | 0.25 mg |
| % recomended daily allowance | 100% | 100% | 75% | 167% | 100% | 25% |
Note: Data from Garcia-Medina JJ, Pinazo-Duran MD, Garcia-Medina M, Zanon-Moreno V, Pons-Vazquez S. A 5-year follow-up of antioxidant supplementation in type 2 diabetic retinopathy. Eur J Ophthalmol. 2011;21:637–643.127
Adverse effects of vitamins and oligoelements in human health
It is of maximum interest to ascertain if nutraceutic formulations with vitamins, minerals, and PUFAs may avoid, delay, or modify age-related ocular diseases. All nutraceutic formulations have to be balanced and based on scientific evidence. With the megadoses of vitamins and minerals that have been extensively suggested by different authors, it has to be considered that secondary effects can appear.
Firstly, it has to be said that vitamin E supplementation has been associated with increased risk of heart failure.128 In addition, β-carotene and vitamin A intake have been reported in relation with a significant increased incidence of cancer and mortality rate in smokers and workers exposed to asbestos.129–131 Zinc sulfate supplementation was associated with an increased risk of genitourinary disorders during the AREDS.96,120 Zinc decreased copper levels and copper deficiency induced anemia. For this reason, copper should be taken with high-dose zinc.
Liposoluble vitamins, particularly vitamin A and vitamin K and some of its by-products, can induce toxicity. Excess in vitamin A has been associated with birth defects and increased risk of bleeding. Patients with liver disease and high alcohol intake may be at risk for hepatotoxicity from vitamin A supplementation.132 Severe toxicity induced by hypervitaminosis A includes eye and liver damage, irritability, headache, visual disturbances, skin redness and peeling, hypercalcemia, delirium, and coma.
Vitamin C mediates the absorption of iron, a prooxidant that can be stored by the organism, and that has been associated with a major risk of cardiovascular diseases and formation of hydroxyl radicals through the reactions of Haber–Weiss and Fenton.3,4 Previous reports confirm that a high intake of vitamin C and vitamin E slowed the process of cellular aging and increased life expectancy. However, new findings in experimental animals suggest that high-dose supplementation may actually reduce lifespan by 11% and 26% for vitamins E and C respectively for voles in the cold, and by 17% and 18% for vitamins E and C respectively for voles in the warm. This study also confirms that major differences exist in the effects of high-dose AOXs on oxidative damage and life span across species.133
Appropriate vitamin E dosing can be confusing. Current guidelines provide recommended dietary allowances and upper tolerable threshold for vitamin E in milligrams. It is usually recognized that most commercial products remain labeled in international units.134 It was suggested that vitamin E supplementation may potentiate the effects of warfarin (Coumadin); therefore, taking vitamin E along with the latter can increase the risk of bruising and bleeding.
In spite of these reports, it is important to say that the Food Standards Agency135 showed that there were only eleven reported reactions from nutritional supplements over an 11 year follow-up period. The majority was in the lowest category of harm. When compared with other foods or medicines, food supplements in general displayed better results.
Final comments
Micronutrient supplementation remains a subject of debate worldwide. Still, many ophthalmologists remain skeptical about nutraceutic supplements and their role at the initiation or progression of age-related eye diseases. However, it is becoming scientifically more evident that optimal nutrition status helps in preventing OS and subsequent OS-related ocular disorders.
The main epidemiological studies have focused basically on formulations including vitamins C and E, carotenoids, flavonoids, selenium, and zinc. Perhaps we have to include other micronutrients that could influence certain processes related to eye health. More data from studies in different populations may improve our knowledge about the specific changes in diet and lifestyle that could positively influence the development of ocular conditions that are becoming more common as the population ages. A greater number of epidemiological studies on diet and the use of nutraceutics in Spain and other European countries, as well as the repercussion for on ocular diseases, have to be done to maximize eye health in the elderly.
Today, there is general agreement that ROS are involved in the physical, biochemical, molecular, and pathological changes associated with aging. Oxidative damage to lipids, proteins, and nucleic acids accumulates and increases with age, whereas AOX defenses decrease in parallel. These facts are closely related to such age-related diseases as DED, POAG, AMD, and DR. As one of the modifiable lifestyle factors is nutrition, elderly people should be informed about the importance of a healthy diet and the exact role of micronutritional supplement intake, since it has a positive effect on the body and may help to decrease the risk of ocular diseases and visual impairment associated with aging.
Footnotes
Disclosure
Roberto Gallego-Pinazo is a consultant for Novartis, Alcon, and Zeiss. Maria I Lopez-Galvez is a consultant for Allergan, Alimera, Novartis. The other authors report no conflicts of interest in this work.
References
- 1.Krebs HA. Some aspects of the regulation of fuel supply in omnivorous animals. Adv Enzyme Regul. 1972;10:397–420. doi: 10.1016/0065-2571(72)90025-8. [DOI] [PubMed] [Google Scholar]
- 2.Cheeseman KH, Slater TF. An introduction to free radical biochemistry. Br Med Bull. 1993;49:481–493. doi: 10.1093/oxfordjournals.bmb.a072625. [DOI] [PubMed] [Google Scholar]
- 3.Haber F, Weiss J. Über die Katalyse des Hydroperoxydes [On the catalysis of hydroperoxides] Naturwissenschaften. 1932;20:948–950. [Google Scholar]
- 4.Fenton HJ. The oxidation of tartaric acid in presence of iron. J Chem Soc Trans. 1894;65:899–910. [Google Scholar]
- 5.Goldstein S, Meyerstein D, Czapski G. The Fenton reagents. Free Radic Biol Med. 1993;15:435–445. doi: 10.1016/0891-5849(93)90043-t. [DOI] [PubMed] [Google Scholar]
- 6.Ames BN, Shigenaga MK. Oxidants are a major contributor to aging. Ann N Y Acad Sci. 1992;663:85–96. doi: 10.1111/j.1749-6632.1992.tb38652.x. [DOI] [PubMed] [Google Scholar]
- 7.Sies H. Oxidative stress: introductory remarks. In: Sies H, editor. Oxidative Stress. London: Academic Press; 1985. pp. 1–7. [Google Scholar]
- 8.D’Autréaux B, Toledano MB. ROS as signalling molecules: mechanisms that generate specificity in ROS homeostasis. Nat Rev Mol Cell Biol. 2007;8:813–824. doi: 10.1038/nrm2256. [DOI] [PubMed] [Google Scholar]
- 9.Halliwell B. Free radicals and antioxidants: updating a personal view. Nutr Rev. 2012;70:257–265. doi: 10.1111/j.1753-4887.2012.00476.x. [DOI] [PubMed] [Google Scholar]
- 10.McCord JM, Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein) J Biol Chem. 1969;244:6049–6055. [PubMed] [Google Scholar]
- 11.Harman D. Ageing: a theory based on free radical and radiation chemistry. J Gerontol. 1956;2:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- 12.Gerschman R, Fenn W. Ascorbic acid content of adrenal glands of rat in oxygen poisoning. Am J Physiol. 1952;176:6–8. doi: 10.1152/ajplegacy.1953.176.1.6. [DOI] [PubMed] [Google Scholar]
- 13.Gerschman R, Nadig PW, Snell AC, Nye SW. Effect of high oxygen concentrations on eyes of newborn mice. Am J Physiol. 1954;179:639–639. doi: 10.1152/ajplegacy.1954.179.1.115. [DOI] [PubMed] [Google Scholar]
- 14.Gerschman R, Gilbert DL, Nye SW, Fenn W. Influence of x-irradiation on oxygen poisoning in mice. Proc Soc Exp Biol Med. 1954;86:27–29. doi: 10.3181/00379727-86-21002. [DOI] [PubMed] [Google Scholar]
- 15.Agil A, Durán R, Barrero F, et al. Plasma lipid peroxidation in sporadic Parkinson’s. Role of the L-dopa. J Neurol Sci. 2006;240:31–36. doi: 10.1016/j.jns.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 16.Choi J, Sullards MC, Olzmann JA, et al. Oxidative damage of DJ-1 is linked to sporadic Parkinson and Alzheimer diseases. J Biol Chem. 2006;281:10816–10824. doi: 10.1074/jbc.M509079200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alaluf S, Muir-Howie H, Hu HL, Evans A, Green MR. Atmospheric oxygen accelerates the induction of a post-mitotic phenotype in human dermal fibroblasts: the key protective role of glutathione. Differentiation. 2000;66:147–155. doi: 10.1046/j.1432-0436.2000.660209.x. [DOI] [PubMed] [Google Scholar]
- 18.Alexandrova M, Bochev P, Markova V, et al. Dynamics of free radical processes in acute ischemic stroke: influence on neurological status and outcome. J Clin Neurosci. 2004;11:501–506. doi: 10.1016/j.jocn.2003.10.015. [DOI] [PubMed] [Google Scholar]
- 19.Ames BN, Shigenaga MK, Hagen TM. Oxidants, antioxidants and degenerative diseases of aging. Proc Natl Acad Sci U S A. 1993;90:7915–7922. doi: 10.1073/pnas.90.17.7915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Conner EM, Grisham MB. Inflammation, free radicals, and antioxidants. Nutrition. 1996;12:274–277. doi: 10.1016/s0899-9007(96)00000-8. [DOI] [PubMed] [Google Scholar]
- 21.Williams DL. Oxidative stress and the eye. Vet Clin North Am Small Anim Pract. 2008;38:179–192. doi: 10.1016/j.cvsm.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 22.Spector A. Oxidative stress-induced cataract: mechanism of action. FASEB J. 1995;9:1173–1182. [PubMed] [Google Scholar]
- 23.Zanon-Moreno V, Marco-Ventura P, Lleo-Perez A, et al. Oxidative stress in primary open angle glaucoma. J Glaucoma. 2008;17:263–268. doi: 10.1097/IJG.0b013e31815c3a7f. [DOI] [PubMed] [Google Scholar]
- 24.Pinazo-Durán MD, Azorin I, Montoliu C, Guerri C, Renau-Piqueras J. Free radicals are involved in the alcoholic optic neuropathy. Alcohol Clin Exp Res. 1998;22:A84. [Google Scholar]
- 25.Mancino R, Di Pierro D, Varesi C, et al. Lipid peroxidation and total antioxidant capacity in vitreous, aqueous humor, and blood samples from patients with diabetic retinopathy. Mol Vis. 2011;17:1298–1304. [PMC free article] [PubMed] [Google Scholar]
- 26.Melo P, Zanon-Moreno V, Alves CJ, et al. Oxidative stress response in the adult rat retina and plasma after repeated administration of methamphetamine. Neurochem Int. 2010;56:431–436. doi: 10.1016/j.neuint.2009.11.017. [DOI] [PubMed] [Google Scholar]
- 27.Beatty S, Koh H, Phil M, Henson D, Boulton M. The role of oxidative stress in the pathogenesis of age-related macular degeneration. Surv Ophthalmol. 2000;45:115–134. doi: 10.1016/s0039-6257(00)00140-5. [DOI] [PubMed] [Google Scholar]
- 28.Margrain TH, Boulton M, Marshall J, Sliney DH. Do blue light filters confer protection against age-related macular degeneration? Prog Retin Eye Res. 2004;23:523–531. doi: 10.1016/j.preteyeres.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 29.Rahman K. Studies on free radicals, antioxidants and co-factors. Clin Interv Aging. 2007;2:219–236. [PMC free article] [PubMed] [Google Scholar]
- 30.Carocho M, Ferreira IC. A review on antioxidants, prooxidants and related controversy: natural and synthetic compounds, screening and analysis methodologies and future perspectives. Food Chem Toxicol. 2013;51:15–25. doi: 10.1016/j.fct.2012.09.021. [DOI] [PubMed] [Google Scholar]
- 31.Birben E, Sahiner UM, Sackesen C, Erzurum S, Kalayci O. Oxidative stress and antioxidant defense. World Allergy Organ J. 2012;5:9–19. doi: 10.1097/WOX.0b013e3182439613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Doly M, Droy-Lefaix MT, Braquet P. Oxidative stress in diabetic retina. EXS. 1992;62:299–307. doi: 10.1007/978-3-0348-7460-1_30. [DOI] [PubMed] [Google Scholar]
- 33.Yadav UC, Kalariya NM, Ramana KV. Emerging role of antioxidants in the protection of uveitis complications. Curr Med Chem. 2011;18:931–942. doi: 10.2174/092986711794927694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Prashar S, Pandav SS, Gupta A, Nath R. Antioxidant enzymes in RBCs as a biological index of age related macular degeneration. Acta Ophthalmol (Copenh) 1993;71:214–218. doi: 10.1111/j.1755-3768.1993.tb04993.x. [DOI] [PubMed] [Google Scholar]
- 35.Perry HD. Dry eye disease: pathophysiology, classification, and diagnosis. Am J Manag Care. 2008;14:S79–S87. [PubMed] [Google Scholar]
- 36.Tavares FP, Fernandes RS, Bernardes TF, Bonfioli AA, Soares EJ. Dry eye disease. Semin Ophthalmol. 2010;25:84–93. doi: 10.3109/08820538.2010.488568. [DOI] [PubMed] [Google Scholar]
- 37.Labbé A, Brignole-Baudouin F, Baudouin C. [Ocular surface investigations in dry eye] J Fr Ophtalmol. 2007;30:76–97. doi: 10.1016/s0181-5512(07)89557-x. French. [DOI] [PubMed] [Google Scholar]
- 38.Schein OD, Muñoz B, Tielsch JM, Bandeen-Roche K, West S. Prevalence of dry eye among the elderly. Am J Ophthalmol. 1997;124:723–728. doi: 10.1016/s0002-9394(14)71688-5. [DOI] [PubMed] [Google Scholar]
- 39.Muñoz B, West SK, Rubin GS, et al. Causes of blindness and visual impairment in a population of older Americans: the Salisbury Eye Evaluation Study. Arch Ophthalmol. 2000;118:819–825. doi: 10.1001/archopht.118.6.819. [DOI] [PubMed] [Google Scholar]
- 40.Moss SE, Klein R, Klein BE. Long-term incidence of dry eye in an older population. Optom Vis Sci. 2008;85:668–674. doi: 10.1097/OPX.0b013e318181a947. [DOI] [PubMed] [Google Scholar]
- 41.Schaumberg DA, Sullivan DA, Buring JE, Dana MR. Prevalence of dry eye syndrome among US women. Am J Ophthalmol. 2003;136:318–326. doi: 10.1016/s0002-9394(03)00218-6. [DOI] [PubMed] [Google Scholar]
- 42.Gipson IK, Spurr-Michaud S, Argüeso P, Tisdale A, Ng TF, Russo CL. Mucin gene expression in immortalized human corneal-limbal and conjunctival epithelial cell lines. Invest Ophthalmol Vis Sci. 2003;44:2496–2506. doi: 10.1167/iovs.02-0851. [DOI] [PubMed] [Google Scholar]
- 43.Zuclich JA, Connolly JS. Ocular damage induced by near-ultraviolet laser radiation. Invest Ophthalmol Vis Sci. 1976;15:760–764. [PubMed] [Google Scholar]
- 44.Higuchi A, Takahashi K, Hirashima M, Kawakita T, Tsubota K. Selenoprotein P controls oxidative stress in cornea. PLoS One. 2010;5:e9911. doi: 10.1371/journal.pone.0009911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Uchino Y, Kawakita T, Ishii T, Ishii N, Tsubota K. A new mouse model of dry eye disease: oxidative stress affects functional decline in the lacrimal gland. Cornea. 2012;31(Suppl 1):S63–S67. doi: 10.1097/ICO.0b013e31826a5de1. [DOI] [PubMed] [Google Scholar]
- 46.Nakamura S, Shibuya M, Nakashima H, et al. Involvement of oxidative stress on corneal epithelial alterations in a blink-suppressed dry eye. Invest Ophthalmol Vis Sci. 2007;48:1552–1558. doi: 10.1167/iovs.06-1027. [DOI] [PubMed] [Google Scholar]
- 47.VanDerMeid KR, Su SP, Krenzer KL, Ward KW, Zhang JZ. A method to extract cytokines and matrix metalloproteinases from Schirmer strips and analyze using Luminex. Mol Vis. 2011;17:1056–1063. [PMC free article] [PubMed] [Google Scholar]
- 48.Enríquez-de-Salamanca A, Castellanos E, Stern ME, et al. Tear cytokine and chemokine analysis and clinical correlations in evaporative-type dry eye disease. Mol Vis. 2010;16:862–873. [PMC free article] [PubMed] [Google Scholar]
- 49.Baudouin C, Brignole F, Becquet F, Pisella PJ, Goguel A. Flow cytometry in impression cytology specimens. A new method for evaluation of conjunctival inflammation. Invest Ophthalmol Vis Sci. 1997;38:1458–1464. [PubMed] [Google Scholar]
- 50.Pinazo-Durán MD, Galbis-Estrada C, Pons-Vázquez S, Cantú-Dibildox J, Marco-Ramírez C, Benítez-del-Castillo J. Effects of a nutraceutical formulation based on the combination of antioxidants and ω-3 essential fatty acids in the expression of inflammation and immune response mediators in tears from patients with dry eye disorders. Clin Interv Aging. 2013;8:139–148. doi: 10.2147/CIA.S40640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Galbis-Estrada C, Pinazo-Durán MD, Cantú-Dibildox J, Marco-Ramírez C, Diaz-Llopis M, Benítez-del-Castillo J. Patients undergoing antihypertensive treatment with antihypertensive eye drops responded positively with respect to their ocular surface disorder to oral supplementation with antioxidants and essential fatty acids. Clin Interv Aging. 2013;8:711–719. doi: 10.2147/CIA.S43191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tsubota K, Kawashima M, Inaba T, et al. The antiaging approach for the treatment of dry eye. Cornea. 2012;31(Suppl 1):S3–S8. doi: 10.1097/ICO.0b013e31826a05a8. [DOI] [PubMed] [Google Scholar]
- 53.Weinreb RN, Khaw PT. Primary open-angle glaucoma. Lancet. 2004;363:1711–1720. doi: 10.1016/S0140-6736(04)16257-0. [DOI] [PubMed] [Google Scholar]
- 54.Osborne NN, Wood JP, Chidlow G, Bae JH, Melena J, Nash MS. Ganglion cell death in glaucoma: what do we really know? Br J Ophthalmol. 1999;83:980–986. doi: 10.1136/bjo.83.8.980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Huang W, Dobberfuhl A, Filippopoulos T, et al. Transcriptional up-regulation and activation of initiating caspases in experimental glaucoma. Am J Pathol. 2005;167:673–681. doi: 10.1016/S0002-9440(10)62042-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pinazo-Durán MD, Gallego-Pinazo R, Zanón-Moreno V, Serrano M. Glaucoma genetics – regulation of cell surviving and death in the retina. In: Rumelkt S, editor. Glaucoma: Basic and Clinical Concepts. Rijeka, Croatia: InTech; 2011. pp. 207–224. [Google Scholar]
- 57.Saccà SC, Izzotti A. Oxidative stress and glaucoma: injury in the anterior segment of the eye. Prog Brain Res. 2008;173:385–407. doi: 10.1016/S0079-6123(08)01127-8. [DOI] [PubMed] [Google Scholar]
- 58.Tezel G. Oxidative stress in glaucomatous neurodegeneration: mechanisms and consequences. Prog Retin Eye Res. 2006;25:490–513. doi: 10.1016/j.preteyeres.2006.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pinazo-Durán MD, Zanón-Moreno V, García-Medina JJ, Gallego-Pinazo R. Evaluation of presumptive biomarkers of oxidative stress, immune response and apoptosis in primary open-angle glaucoma. Curr Opin Ophthalmol. 2013;13:98–107. doi: 10.1016/j.coph.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 60.Bazan NG. Survival signaling in retinal pigment epithelial cells in response to oxidative stress: significance in retinal degenerations. Adv Exp Med Biol. 2006;572:531–540. doi: 10.1007/0-387-32442-9_74. [DOI] [PubMed] [Google Scholar]
- 61.Kennedy CJ, Rakoczy PE, Constable IJ. Lipofuscin of the retinal pigment epithelium: a review. Eye (Lond) 1995;9:763–771. doi: 10.1038/eye.1995.192. [DOI] [PubMed] [Google Scholar]
- 62.Cai J, Nelson KC, Wu M, Sternberg P, Jr, Jones DP. Oxidative damage and protection of the RPE. Prog Retin Eye Res. 2000;19:205–221. doi: 10.1016/s1350-9462(99)00009-9. [DOI] [PubMed] [Google Scholar]
- 63.Mukherjee PK, Marcheselli VL, Serhan CN, Bazan NG. Neuroprotectin D1: a docosahexaenoic acid-derived docosatriene protects human retinal pigment epithelial cells from oxidative stress. Proc Natl Acad Sci U S A. 2004;101:8491–8496. doi: 10.1073/pnas.0402531101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bazan NG. Neuroprotectin D1 (NPD1): a DHA-derived mediator that protects brain and retina against cell injury-induced oxidative stress. Brain Pathol. 2005;15:159–166. doi: 10.1111/j.1750-3639.2005.tb00513.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Calandria JM, Mukherjee PK, de Rivero Vaccari JC, Zhu M, Petasis NA, Bazan NG. Ataxin-1 poly(Q)-induced proteotoxic stress and apoptosis are attenuated in neural cells by docosahexaenoic acid-derived neuroprotectin D1. J Biol Chem. 2012;287:23726–23739. doi: 10.1074/jbc.M111.287078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Klein R, Peto T, Bird A, Vannewkirk MR. The epidemiology of age related macular degeneration. Am J Ophthalmol. 2004;137:486–495. doi: 10.1016/j.ajo.2003.11.069. [DOI] [PubMed] [Google Scholar]
- 67.Curcio CA, Johnson M, Rudolf M, Huang JD. The oil spill in ageing Bruch membrane. Br J Ophthalmol. 2011;95:1638–1645. doi: 10.1136/bjophthalmol-2011-300344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Faghir Z, Bazan NG. PI3K/Akt and mTOR/p70S6K pathways mediate neuroprotectin D1-induced retinal pigment epithelial cell survival during oxidative stress-induced apoptosis. Exp Eye Res. 2010;90:718–725. doi: 10.1016/j.exer.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Klettner A. Oxidative stress induced cellular signaling in RPE cells. Front Biosci (Schol Ed) 2012;4:392–411. doi: 10.2741/s275. [DOI] [PubMed] [Google Scholar]
- 70.Koenekoop R. The gene for Stargardt disease, ABCA4, is a major retinal gene: a mini-review. Ophthalmic Genet. 2003;24:75–80. doi: 10.1076/opge.24.2.75.13996. [DOI] [PubMed] [Google Scholar]
- 71.Nowak JZ. Oxidative stress, polyunsaturated fatty acids-derived oxidation products and bisretinoids as potential inducers of CNS diseases: focus on age-related macular degeneration. Pharmacol Rep. 2013;65:288–304. doi: 10.1016/s1734-1140(13)71005-3. [DOI] [PubMed] [Google Scholar]
- 72.Castilho A, Aveleira CA, Leal EC, et al. Heme oxygenase-1 protects retinal endothelial cells against high glucose- and oxidative/nitrosative stress-induced toxicity. PLoS One. 2012;7:e42428. doi: 10.1371/journal.pone.0042428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sparrow JR, Zhou J, Ben-Shabat S, Vollmer H, Itagaki Y, Nakanishi K. Involvement of oxidative mechanisms in blue-light-induced damage to A2E-laden RPE. Invest Ophthalmol Vis Sci. 2002;43(4):1222–1227. [PubMed] [Google Scholar]
- 74.Maleki S, Gopalakrishnan S, Ghanian Z, et al. Optical imaging of mitochondrial redox state in rodent model of retinitis pigmentosa. J Biomed Opt. 2013;18:16004. doi: 10.1117/1.JBO.18.1.016004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhuo Y, Luo H, Zhang K. Leber hereditary optic neuropathy and oxidative stress. Proc Natl Acad Sci U S A. 2012;109:19882–19883. doi: 10.1073/pnas.1218953109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pinazo-Durán MD, Verdejo C, Azorín I, Renau-Piqueras J, Iborra FJ. Colocalization of aldehyde dehydrogenases and Fe/NADPH-induced lipid peroxidation in tissue sections of rat retina. Ophthalmic Res. 2000;32:61–68. doi: 10.1159/000055591. [DOI] [PubMed] [Google Scholar]
- 77.Galbis-Estrada C, Pons-Vázquez S, Gallego-Pinazo R, et al. Glutathione-dependent formaldehyde dehydrogenase (ADH3) and low km mitochondrial aldehyde dehydrogenase (ALDH2). New evidence for differential expression in the rat retina in response to oxidative stress. Free Radic Res. 2012;46:77–84. doi: 10.3109/10715762.2011.640324. [DOI] [PubMed] [Google Scholar]
- 78.Rogers BS, Symons RC, Komeima K, et al. Differential sensitivity of cones to iron-mediated oxidative damage. Invest Ophthalmol Vis Sci. 2007;48:438–445. doi: 10.1167/iovs.06-0528. [DOI] [PubMed] [Google Scholar]
- 79.Dentchev T, Hahn P, Dunaief JL. Strong labeling for iron and the iron-handling proteins ferritin and ferroportin in the photoreceptor layer in age-related macular degeneration. Arch Ophthalmol. 2005;123:1745–1746. doi: 10.1001/archopht.123.12.1745. [DOI] [PubMed] [Google Scholar]
- 80.He X, Hahn P, Iacovelli J, et al. Iron homeostasis and toxicity in retinal degeneration. Prog Retin Eye Res. 2007;26:649–673. doi: 10.1016/j.preteyeres.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Song D, Song Y, Hadziahmetovic M, Zhong Y, Dunaief JL. Systemic administration of the iron chelator deferiprone protects against light-induced photoreceptor degeneration in the mouse retina. Free Radic Biol Med. 2012;53:64–71. doi: 10.1016/j.freeradbiomed.2012.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Arden GB, Sivaprasad S. Hypoxia and oxidative stress in the causation of diabetic retinopathy. Curr Diabetes Rev. 2011;7:291–304. doi: 10.2174/157339911797415620. [DOI] [PubMed] [Google Scholar]
- 83.Son SM. Role of vascular reactive oxygen species in development of vascular abnormalities in diabetes. Diabetes Res Clin Pract. 2006;77(Suppl 1):S65–S70. doi: 10.1016/j.diabres.2007.01.036. [DOI] [PubMed] [Google Scholar]
- 84.Gupta MM, Chari S. Lipid peroxidation and antioxidant status in patients with diabetic retinopathy. Indian J Physiol Pharmacol. 2005;49:187–192. [PubMed] [Google Scholar]
- 85.Verdejo C, Marco P, Renau-Piqueras J, Pinazo-Duran MD. Lipid peroxidation in proliferative vitreoretinopathies. Eye (Lond) 1999;13:183–188. doi: 10.1038/eye.1999.48. [DOI] [PubMed] [Google Scholar]
- 86.Robison WG, Jr, Jacot JL, Katz ML, Glover JP. Retinal vascular changes induced by the oxidative stress of alpha-tocopherol deficiency contrasted with diabetic microangiopathy. J Ocul Pharmacol Ther. 2000;16:109–120. doi: 10.1089/jop.2000.16.109. [DOI] [PubMed] [Google Scholar]
- 87.Martín-Gallán P, Carrascosa A, Gussinyé M, Domínguez C. Biomarkers of diabetes-associated oxidative stress and antioxidant status in young diabetic patients with or without subclinical complications. Free Radic Biol Med. 2003;34:1563–1574. doi: 10.1016/s0891-5849(03)00185-0. [DOI] [PubMed] [Google Scholar]
- 88.Valko M, Leibfritz D, Moncol J, Cronin MT, Mazuz M, Telsen J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 89.Pácal L, Varvařovská J, Rušavý Z, et al. Parameters of oxidative stress, DNA damage and DNA repair in type 1 and type 2 diabetes mellitus. Arch Physiol Biochem. 2011;117:222–230. doi: 10.3109/13813455.2010.551135. [DOI] [PubMed] [Google Scholar]
- 90.Dolinoy DC, Huang D, Jirtle RL. Maternal nutrient supplementation counteracts bisphenol A-induced DNA hypomethylation in early development. Proc Natl Acad Sci U S A. 2007;104:13056–13061. doi: 10.1073/pnas.0703739104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.US Department of Health and Human Services Dietary guidelines for Americans, 2010. [Accessed December 19, 2013]. Available from: http://www.dietaryguidelines.gov.
- 92.Keys A, Menotti A, Karvonen MJ, et al. The diet and 15-year death rate in the seven countries study. Am J Epidemiol. 1986;124:903–915. doi: 10.1093/oxfordjournals.aje.a114480. [DOI] [PubMed] [Google Scholar]
- 93.Keys A. Mediterranean diet and public health: personal reflections. Am J Clin Nutr. 1995;61:1321S–1323S. doi: 10.1093/ajcn/61.6.1321S. [DOI] [PubMed] [Google Scholar]
- 94.Sofi F, Cesari F, Abbate R, Gensini GF, Casini A. Adherence to Mediterranean diet and health status: meta-analysis. BMJ. 2008;377:a1344. doi: 10.1136/bmj.a1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Serra-Majem L, Roman B, Estruch R. Scientific evidence of interventions using the Mediterranean diet: a systematic review. Nutr Rev. 2006;64:S27–S47. doi: 10.1111/j.1753-4887.2006.tb00232.x. [DOI] [PubMed] [Google Scholar]
- 96.Delcourt C, Korobelik JF, Barberger-Gateau P, et al. Nutrition and age-related eye diseases: the ALIENOR (Antioxydants, LIpides Essentiels, Nutrition et Maladies OculaiRes) study. J Nutr Health Aging. 2009;14:854–861. doi: 10.1007/s12603-010-0131-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Malet F, Le Goff M, Colin J, et al. Dry eye disease in French elderly subjects: the Alienor Study. Acta Ophthalmol. 2013 Jun 7; doi: 10.1111/aos.12174. Epub. [DOI] [PubMed] [Google Scholar]
- 98.Quaranta L, Bettelli S, Uva MG, Semeraro F, Turano R, Gandolfo E. Effect of Ginkgo biloba extract on preexisting visual field damage in normal tension glaucoma. Ophthalmology. 2003;110(2):359–362. doi: 10.1016/S0161-6420(02)01745-1. [DOI] [PubMed] [Google Scholar]
- 99.Ritch R. Potential role for Ginko biloba extract in the treatment of glaucoma. Med Hypotheses. 2000;54:221–235. doi: 10.1054/mehy.1999.0025. [DOI] [PubMed] [Google Scholar]
- 100.Coleman AL, Stone KL, Kodjebacheva G, et al. Glaucoma risk and the consumption of fruits and vegetables among older women in the study of osteoporotic fractures. Am J Ophthalmol. 2008;145:1081–1089. doi: 10.1016/j.ajo.2008.01.022. [DOI] [PubMed] [Google Scholar]
- 101.Kang JH, Pasquale LR, Willett WC, et al. Dietary fat consumption and primary open-angle glaucoma. Am Soc Clin Nutr. 2004;79:5755–5764. doi: 10.1093/ajcn/79.5.755. [DOI] [PubMed] [Google Scholar]
- 102.Zanon-Moreno V, Asensio-Marquez EM, Ciancotti-Oliver L, et al. Effects of polymorphisms in vitamin E-, vitamin C-, and glutathione peroxidase-related genes on serum biomarkers and associations with glaucoma. Mol Vis. 2013;19:231–242. [PMC free article] [PubMed] [Google Scholar]
- 103.Zanon-Moreno V, Ciancotti-Olivares L, Asencio J, et al. Association between a SLC23A2 gene variation, plasma vitamin C levels, and risk of glaucoma in a Mediterranean population. Mol Vis. 2011;17:2997–3004. [PMC free article] [PubMed] [Google Scholar]
- 104.VandenLangenberg GM, Mares-Perlman JA, Klein R, Klein BE, Brady WE, Palta M. Associations between antioxidant and zinc intake and the 5-year incidence of early age-related maculopathy in the Beaver Dam Eye Study. Am J Epidemiol. 1998;148:204–214. doi: 10.1093/oxfordjournals.aje.a009625. [DOI] [PubMed] [Google Scholar]
- 105.Van Leeuwen R, Boekhoorn S, Vingerling JR, et al. Dietary intake of antioxidants and risk of age-related macular degeneration. JAMA. 2005;294:3101–3107. doi: 10.1001/jama.294.24.3101. [DOI] [PubMed] [Google Scholar]
- 106.Age-Related Eye Disease Study Research Group A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no 8. Arch Ophthalmol. 2001;119:1417–1436. doi: 10.1001/archopht.119.10.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Connor KM, SanGiovanni JP, Lofqvist C, et al. Increased dietary intake of omega-3-polyunsaturated fatty acids reduces pathological retinal angiogenesis. Nat Med. 2007;13:868–873. doi: 10.1038/nm1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Seddon JM, George S, Rosner B. Cigarette smoking, fish consumption, omega-3 fatty acid intake, and associations with age-related macular degeneration: the US Twin Study of Age-Related Macular Degeneration. Arch Ophthalmol. 2006;124:995–1001. doi: 10.1001/archopht.124.7.995. [DOI] [PubMed] [Google Scholar]
- 109.SanGiovanni JP, Chew EY, Agrón E, et al. The relationship of dietary omega-3 long-chain polyunsaturated fatty acid intake with incident age-related macular degeneration. AREDS report no. 23. Arch Ophthalmol. 2008;126:1274–1279. doi: 10.1001/archopht.126.9.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Krinsky NI, Landrum JT, Bone RA. Biologic mechanisms of the protective role of lutein and zeaxanthin in the eye. Annu Rev Nutr. 2003;23:171–201. doi: 10.1146/annurev.nutr.23.011702.073307. [DOI] [PubMed] [Google Scholar]
- 111.Semba RD, Dagnelie G. Are lutein and zeaxanthin conditionally essential nutrients for eye health? Med Hypotheses. 2003;61:465–472. doi: 10.1016/s0306-9877(03)00198-1. [DOI] [PubMed] [Google Scholar]
- 112.Schweigert FJ, Reimann J. Micronutrients and their relevance for the eye – function of lutein, zeaxanthin and omega-3 fatty acids. Klin Monbl Augenheilkd. 2011;228:537–543. doi: 10.1055/s-0029-1245527. German. [DOI] [PubMed] [Google Scholar]
- 113.Moeller SM, Parekh N, Tinker L, et al. Associations between intermediate age-related macular degeneration and lutein and zeaxanthin in the Carotenoids in Age-Related Eye Disease study (CAREDS): ancillary study of the Women’s Health Initiative. Arch Ophthalmol. 2006;124:1151–1162. doi: 10.1001/archopht.124.8.1151. [DOI] [PubMed] [Google Scholar]
- 114.Tan JS, Wang JJ, Flood V, Rochtchina E, Smith W, Mitchell P. Dietary antioxidants and the long-term incidence of age-related macular degeneration: the Blue Mountains Eye Study. Ophthalmology. 2008;115:334–341. doi: 10.1016/j.ophtha.2007.03.083. [DOI] [PubMed] [Google Scholar]
- 115.Age-Related Eye Disease Study Research Group. SanGiovanni JP, Chew EY, et al. The relationship of dietary carotenoid and vitamin A, E, and C intake with age-related macular degeneration in a case control study: AREDS report no 22. Arch Ophthalmol. 2007;125:1225–1232. doi: 10.1001/archopht.125.9.1225. [DOI] [PubMed] [Google Scholar]
- 116.Seddon JM, Ajani UA, Sperduto RD, et al. Dietary carotenoids, vitamins A, C, and E, and advanced age-related macular degeneration. Eye Disease Case-Control Study Group. JAMA. 1994;272:1413–1420. [PubMed] [Google Scholar]
- 117.Delcourt C, Cristol JP, Tessier F, Léger CL, Descomps B, Papoz L. Age-related macular degeneration and antioxidant status in the POLA study. POLA Study Group. Pathologies Oculaires Liées à l’Age. Arch Ophthalmol. 1999;117:1384–1390. doi: 10.1001/archopht.117.10.1384. [DOI] [PubMed] [Google Scholar]
- 118.Mares-Perlman JA, Fisher AI, Klein R, et al. Lutein and zeaxanthin in the diet and serum and their relation to age-related maculopathy in the third national health and nutrition examination survey. Am J Epidemiol. 2001;153:424–432. doi: 10.1093/aje/153.5.424. [DOI] [PubMed] [Google Scholar]
- 119.Richer S, Stiles W, Statkute L. Double-masked, placebo-controlled, randomized trial of lutein and antioxidant supplementation in the intervention of atrophic age-related macular degeneration: the Veterans LAST study (Lutein Antioxidant Supplementation Trial) Optometry. 2004;75:216–230. doi: 10.1016/s1529-1839(04)70049-4. [DOI] [PubMed] [Google Scholar]
- 120.Age-Related Eye Disease Study 2 Research Group Lutein + zeaxanthin and omega-3 fatty acids for age-related macular degeneration: the Age-Related Eye Disease Study 2 (AREDS2) randomized clinical trial. JAMA. 2013;309:2005–2015. doi: 10.1001/jama.2013.4997. [DOI] [PubMed] [Google Scholar]
- 121.Mayer-Davis EJ, Bell RA, Reboussin BA, Rushing J, Marshall JA, Hamman RF. Antioxidant nutrient intake and diabetic retinopathy: the San Luis Valley Diabetes Study. Ophthalmology. 1998;105:2264–2270. doi: 10.1016/S0161-6420(98)91227-1. [DOI] [PubMed] [Google Scholar]
- 122.Weiner DE, Tighiouart H, Reynolds R, Seddon JM. Kidney function, albuminuria and age-related macular degeneration in NHANES III. Nephrol Dial Transplant. 2011;26:3159–3165. doi: 10.1093/ndt/gfr022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Diabetes Control and Complications Trial (DCCT): results of feasibility study. The DCCT Research Group. Diabetes Care. 1987;10:1–19. doi: 10.2337/diacare.10.1.1. No authors listed. [DOI] [PubMed] [Google Scholar]
- 124.Millen AE, Gruber M, Klein R, Klein BE, Palta M, Mares JA. Relations of serum ascorbic acid and alpha-tocopherol to diabetic retinopathy in the Third National Health and Nutrition Examination Survey. Am J Epidemiol. 2003;158:225–233. doi: 10.1093/aje/kwg116. [DOI] [PubMed] [Google Scholar]
- 125.Roy S, Khanna S, Alessio HM, et al. Anti-angiogenic property of edible berries. Free Radic Res. 2002;36(9):1023–1031. doi: 10.1080/1071576021000006662. [DOI] [PubMed] [Google Scholar]
- 126.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2011;414:813–820. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 127.Garcia-Medina JJ, Pinazo-Duran MD, Garcia-Medina M, Zanon-Moreno V, Pons-Vazquez S. A 5-year follow-up of antioxidant supplementation in type 2 diabetic retinopathy. Eur J Ophthalmol. 2011;21:637–643. doi: 10.5301/EJO.2010.6212. [DOI] [PubMed] [Google Scholar]
- 128.Lonn E, Bosch J, Yusuf S, et al. Effects of long-term vitamin E supplementation on cardiovascular events and cancer: a randomized controlled trial. JAMA. 2005;293:1338–1347. doi: 10.1001/jama.293.11.1338. [DOI] [PubMed] [Google Scholar]
- 129.Rodríguez-Castilla V, Cuadrado C, Pozo S, Moreiras O. Concentraciones plasmáticas de carotenos y vitaminas antioxidantes en personas de edad avanzada: influencia del tabaquismo. Proyecto HALE [Plasmatic concentrations of carotenoids and antioxidant vitamins in elder people: influence of smoking. Hale Project] Clin Investig Arterioscler. 2005;17:101–111. [Google Scholar]
- 130.The effect of vitamin E and beta carotene on the incidence of lung cancer and other cancers in male smokers. The Alpha Tocopherol, Beta Carotene Cancer Prevention Study Group. N Engl J Med. 1994;330:1029–1035. doi: 10.1056/NEJM199404143301501. No authors listed. [DOI] [PubMed] [Google Scholar]
- 131.Omenn GS, Goodman GE, Thornquist MD, et al. Effects of a combination of beta carotene and vitamin A on lung cancer and cardiovascular disease. N Engl J Med. 1996;334:1150–1155. doi: 10.1056/NEJM199605023341802. [DOI] [PubMed] [Google Scholar]
- 132.Fosmire GJ. Zinc toxicity. Am J Clin Nutr. 1990;51:225–227. doi: 10.1093/ajcn/51.2.225. [DOI] [PubMed] [Google Scholar]
- 133.Block KI, Koch AC, Mead MN, Tothy PK, Newman RA, Gyllenhaal C. Impact of antioxidant supplementation on chemotherapeutic toxicity: a systematic review of the evidence from randomized controlled trials. Int J Cancer. 2008;15(123):1227–1239. doi: 10.1002/ijc.23754. [DOI] [PubMed] [Google Scholar]
- 134.Selman C, McLaren JS, Collins AR, Duthie GG, Speakman JR. Deleterious consequences of antioxidant supplementation on lifespan in a wild-derived mammal. Biol Lett. 2013;9:1–5. doi: 10.1098/rsbl.2013.0432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Food and Nutrition Board, Institute of Medicine . Copper. Dietary reference intakes for vitamin A, vitamin K, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. Washington, D.C.: National Academy Press; 2001. pp. 224–257. [PubMed] [Google Scholar]


