Background
The prevalence of obesity in the US has increased dramatically over the past three decades, with the greatest increases in the prevalence of class II (body mass index [BMI] 35 to <40 kg/m2) and class III, or extreme, obesity (BMI≥40 kg/m2)(1) . Although lifestyle interventions, which promote a low calorie diet and regular physical activity, are recommended as the foundation for all weight loss treatment (2), the amount of weight loss obtained with lifestyle intervention alone in this population is often insufficient to achieve health goals, such as normalization of blood glucose or remission of hypertension.
In 1991 an NIH Consensus Conference (3) and NIH Clinical Guidelines (2) recognized that bariatric surgical procedures led to substantial weight loss and improvement in comorbidities in adults with extreme obesity, but noted that there were insufficient data upon which to base recommendations for patient selection using objective clinical features alone, and that additional research was needed to determine predictive factors. More than a decade after that Consensus Conference, many questions remained unanswered, including the mechanisms of improvement in comorbid conditions, the safety and efficacy of differing bariatric surgical procedures in varied populations, and the impact on psychosocial outcomes. As a result of a recommendation by the “Working Group on Research in Bariatric Surgery” convened in 2002 (4), the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) established the Longitudinal Assessment of Bariatric Surgery (LABS) consortium in 2003 (5-6). LABS was created to support clinical, epidemiological, and behavioral research in bariatric surgery and to address deficiencies in bariatric surgery research infrastructure, specifically the lack of systematic long-term follow-up across multiple sites and of standardized measures. The LABS consortium, which includes 6 clinical centers encompassing 11 hospitals in the United States, designed studies designated as LABS-1, LABS-2, and LABS-3 to address short and long term safety and efficacy of bariatric surgery. A Data Coordinating Center facilitates the project among the clinical centers, a central laboratory, the NIDDK-supported Biosample Repository, and NIDDK scientists.
LABS-1, designed to address 30-day safety outcomes, included 5,108 participants who had anesthesia induced for a bariatric procedure performed by a LABS-certified surgeon. Short term safety data for the 4,776 LABS-1 participants who had a first-time bariatric surgery have been reported (7). LABS-2, designed to evaluate longer term safety and efficacy of bariatric surgery, collects substantially more information on 2,458 participants who are followed annually. LABS-2 includes assessments of body composition and of cardiovascular, metabolic, pulmonary, kidney, musculoskeletal, urogynecologic, reproductive, gastrointestinal, hepatic, psychosocial, behavioral, and quality of life domains (6). LABS-3 refers to two studies designed to obtain more in depth information on smaller samples; one designed to study mechanisms of diabetes resolution and the other examining psychopathology and eating behaviors (8).
This paper reports select baseline characteristics of the LABS-2 study population to provide a basic description of the health status of a large cohort of patients undergoing bariatric surgery at several, geographically dispersed centers throughout the United States. A description of domains assessed in LABS, including forms and assessment methods, have previously been reported (6), as have some baseline characteristics, such as reproductive health, and follow-up outcomes, such as change in alcohol use disorders (9-16). Given evidence that men are far less likely than women to undergo bariatric surgery (17), this paper also examines whether there is a sex difference in prevalence of comorbidities, and if so, whether differences are independent of age and severity of obesity, as assessed by BMI.
Methods
Recruiting the LABS-2 cohort
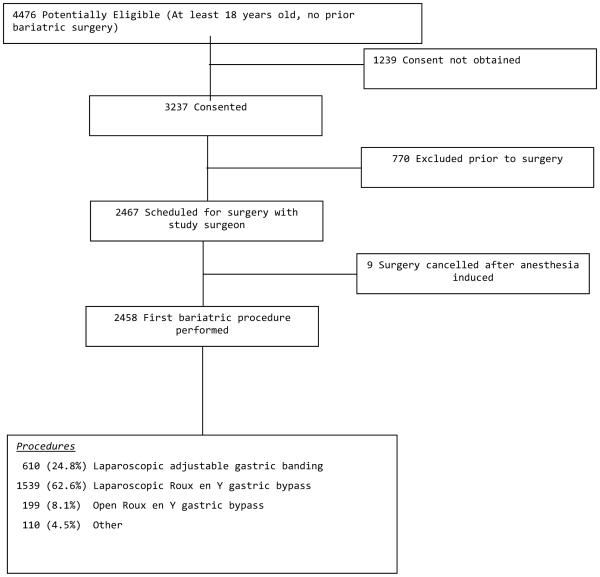
Patients who were at least 18 years old and seeking a first bariatric surgical procedure were recruited between February, 2006 and February, 2009. One of the hospitals that contributed to LABS-1 did not recruit for LABS-2 due to relocation of its principal investigator. By close of enrollment (the last surgery was performed in April 2009), 2,458 participants met inclusion criteria, attended a preoperative research visit and underwent a bariatric surgical procedure (Roux-en-Y gastric bypass, laparoscopic adjustable gastric banding, sleeve gastrectomy, biliopancreatic diversion with duodenal switch, or banded gastric bypass) by one of 33 LABS-certified surgeons (Figure 1).
Figure 1.
LABS-2 Cohort
Data collection
Data collection for the LABS-2 observational, longitudinally followed cohort has been previously described (5-6). LABS-2 utilized standard instruments or established standard definitions of data elements. When feasible, objective measures of patient status and comorbidities were used. Standardized data collection protocols were codified in Manuals of Operations. For example, detailed instructions for obtaining physical measures (e.g., height, weight, neck and waist circumference, blood pressure) were developed. LABS-provided equipment was used to reduce the chances that cross-site differences were due to differences in instrumentation. The Tanita® Body Composition Analyzer (model TBF-310) was used to obtain weight and percentage body fat during research assessments. Certification on data collection protocols was required for all data collectors, including dedicated project coordinators and clinicians, to enhance consistent data collection across sites.
Within 30 days prior to scheduled surgery, participants attended a research visit at which time baseline data were obtained. If the surgery was rescheduled after the research visit, most baseline measures were kept if they were measured within 90 days of the new surgery date. However, physical measures (weight, blood pressure, heart rate, waist and neck circumference), medication use, and selected behavioral questions were redone or readministered within 30 days prior to surgery.
Laboratory Measures
Lipid profile from serum (total cholesterol, high-density lipoprotein [HDL] cholesterol, low-density lipoprotein [LDL] cholesterol, triglycerides), diabetes-related parameters (hemoglobin A1C [HbA1c], glucose [from serum], insulin, proinsulin, C-peptide), an inflammatory marker (high-sensitivity C-reactive protein), a measure of kidney function (estimated glomerular filtration rate [eGFR] using cystatin C) (18), two gut and adipocyte derived hormones (ghrelin, leptin) and liver function (alanine aminotransferases [ALT] and serum aspartate [AST]) were assayed at a central laboratory. Fasting values (at least 8 hours) were reported for LDL, triglycerides, glucose, insulin, proinsulin, C-peptide, high-sensitivity C-reactive protein, ghrelin and leptin. If central laboratory results were not available for ALT or AST and results were available from the center, then those results are reported.
Comorbidities
The presence or absence of comorbid conditions was collected in various ways. Participants self-reported asthma (“have you ever been told by a doctor or other health care professional that you have asthma?”), urinary incontinence (responded at least once per week to the question, “Many people complain that they leak urine accidentally. In the past 3 months, how often have you typically leaked urine, even a small amount?”) and a severe walking limitation (defined as self-reporting the inability to walk 200 feet without assistance).
A LABS-certified clinical researcher used the best available information (medical records, physical exam, patient interview) to determine presence or absence of a history of ischemic heart disease (defined as history of percutaneous coronary intervention, coronary artery bypass surgery, angina or myocardial infarction), congestive heart failure, and venous edema (defined as leg swelling and at least one other symptom such as blistering, infection, discoloration or alteration). History of pulmonary hypertension was defined as mean pulmonary artery pressure greater than 25 mmHg at rest or 30 mmHg with exercise measured by right heart catheterization. The diagnosis of obstructive sleep apnea was based on an Apnea-Hypopnea Index (AHI) of at least 5 from a diagnostic polysomnogram in the 12-months prior to the LABS-2 baseline visit. If results from a past-year diagnostic polysomnogram were unavailable then a LABS-certified researcher used the best available information to determine presence or absence of sleep apnea, e.g., participant-reported use of continuous positive airway pressure (CPAP) or diagnostic AHI result.
Hypertension was defined as having systolic blood pressure (SBP) of at least 140 mm Hg or a diastolic blood pressure (DBP) of at least 90 mm Hg during the baseline visit as measured using the standard LABS protocol, or if the participant self-reported currently taking anti-hypertensive medication. Hyperlipidemia was defined as taking a lipid lowering medication or having LDL cholesterol of at least 160 mg/dL. Dyslipidemia was defined as having hyperlipidemia, HDL<40 mg/dL, or triglycerides of at least 200 mg/dL. Diabetes was defined as taking diabetes medication or having HbA1c of at least 6.5% or, if HbA1c was unavailable, an 8-hour fasting glucose of at least 126 mg/dL (19). An exception was made for participants who reported a diagnosis of polycystic ovary syndrome, did not meet either the HbA1c or fasting glucose requirements, and were taking Metformin but no other diabetes medication; these participants were considered to not have diabetes. Abnormal kidney function was defined as eGFR less than 60 according to the cystatin-C based Dade-Behring formula (18), which is equivalent to stage 3 or greater chronic kidney disease.
Missing data
Definitions of ischemic heart disease, hypertension, hyperlipidemia, and diabetes relied on more than one data element, e.g., laboratory values and medications. Participants had to have values for all the data elements used to determine comorbidity status in order to be included in a prevalence estimate. This was so that estimates would not be biased towards over-reporting comorbidities, which would happen if we considered a comorbidity to be present if any of the criteria were met, in the presence of missing data for other criteria. However, participants were included in analyses investigating potential sex differentials if their comorbidity status could be determined with incomplete data (e.g., reported taking diabetes medication but missing HbA1c) since data completeness was not related to sex.
Quality of life (QOL)
The Impact of Weight on Quality of Life questionnaire-lite (IWQOL-lite) is a validated 31-item measure of the degree to which subjects perceive their weight as impacting their QOL in five domains-work, physical function, public distress, sexual life and self-esteem (20). The IWQOL-lite total score and the specific domain scores range from 0 to 100 with 100 indicating that weight never adversely affects QOL and 0 indicating that it always does. LABS also includes the Medical Outcomes Study 36-item Short-Form (SF-36), a generic QOL instrument with well-established validity and reliability that covers 8 QOL domains (21). Here we report the physical and mental component summary scores, which are scaled such that a general population has a mean of 50 and standard deviation of 10 (22).
Depressive symptoms over the past week was measured with the Beck Depression Inventory (BDI) version 1, for which a higher score (range 0-63) indicates greater severity of depressive symptoms (23). Because many patients are advised to lose weight in preparation for bariatric surgery, no points were assigned to the BDI item that assesses weight loss for participants who indicated that they were purposefully trying to lose weight by eating less. Clinically recognized cutpoints were used to categorize participants according to degree of depressive symptoms (24).
Statistical Methods
Categorical data are presented as frequencies and percentages. Continuous variables are presented as medians and 25th and 75th percentiles. Pearson’s chi-square test of association was used to test equality of categorical values by sex. The Wilcoxon rank-sum test was used to test for differences in continuous measures by sex. The Cochran-Armitage test for trend was used to test whether the distribution of ordinal variables differed by sex. Simple log-binomial regression was used to test for a relationship between sex and each comorbid condition and multiple log-binomial regression models were used to adjust the association between each comorbid condition and sex for age and BMI, which were centered at their respective means. To adjust for potential differences in patient selection or regional characteristics we considered also adjusting for site (i.e. hospital) as a random effect in log-binomial models. However, we determined that the distribution of males and females was similar across sites, and thus site was dropped from the models. To determine the best functional form of age and BMI in log-binomial models, we examined a lowess plot of each variable with each comorbidity. Piece-wise regression with a knot at 40 kg/m2 was considered in models in which the plot indicated that the relationship between BMI and the comorbidity differed below versus at or above 40 kg/m2. We also examined higher-order terms and interaction terms for the models. The Akaike information criterion (AIC) was used as a measure of model fit. Diagnostic plots and an extension of the Hosmer-Lemeshow goodness-of-fit (GOF) test (25) was used to confirm the fit of each model. We present unadjusted and adjusted relative risks (RR) and 95% confidence intervals (CI) for sex at the cohort’s median age and BMI. Statistical analyses were performed using SAS software version 9.3 (SAS Institute Inc.). p-values less than 0.05 were considered to be statistically significant.
Results
Recruitment flow is presented in Figure 1. Of the 4,476 patients identified as potentially eligible for participation, consent was not obtained for 1,239; 672 due to the inability to schedule a baseline research assessment or to contact the patient regarding consent prior to surgery. Of those who consented, 770 were excluded, primarily because they did not proceed to surgery with a LABS-certified surgeon, were unable to schedule a baseline research assessment before surgery, were not scheduled for surgery before recruitment ended, or refused participation after originally agreeing to participate but before attending a baseline research assessment. This left 2,467 participants who attended a research assessment before anesthesia induction for a bariatric surgical procedure with a LABS-certified surgeon. However, 9 had surgery cancelled after anesthesia induction and did not proceed to surgery before recruitment ended. Thus, there were 2,458 LABS-2 participants who had an initial bariatric procedure, the majority of whom (Figure 1) had a laparoscopic Roux en Y gastric bypass (LRYGB).
The majority of participants were female (Table 1). Participants ranged in age from 18 to 78 years with 9% of participants under the age of 30 and 5% at least 65 years of age. 14% were non-white and 5% self-identified as Hispanic. Most were married or living as married; 14% were divorced. A majority was working for pay although 15% reported not working due to disability. The median annual household income was between $50,000 and $74,999. 77% of the participants had some post-high school education and more than 36% had a college degree.
Table 1.
Demographic characteristics
| Total (n=2458) |
Female (n=1931) |
Male (n=527) |
p* | |
|---|---|---|---|---|
| Age (years) median (quartiles) | 46 (37,54) | 45 (36, 54) | 48 (39, 57) | <.0001 |
| Race a | 0.0005 | |||
| White | 2102(86.2) | 1623(84.6) | 479(91.8) | |
| Black | 256(10.5) | 224(11.7) | 32(6.1) | |
| Multi-race | 49(2.0) | 43(2.2) | 6(1.1) | |
| Other | 33(1.4) | 28(1.5) | 5(1.0) | |
| Ethnicity b | 0.09 | |||
| Hispanic | 119(4.8) | 101(5.2) | 18(3.4) | |
| Non-Hispanic | 2337(95.2) | 1829(94.8) | 508 (96.6) | |
| Marital status c | 0.0002 | |||
| Never married or lived as married | 367(16.2) | 295(16.6) | 72(14.8) | |
| Married/living as married | 1444(63.8) | 1096(61.6) | 348(71.6) | |
| Separated/no longer living as married | 82(3.6) | 70(3.9) | 12(2.5) | |
| Divorced | 320(14.1) | 269(15.1) | 51(10.5) | |
| Widowed | 51(2.3) | 48(2.7) | 3(0.6) | |
| Employment status d | <0.0001 | |||
| Work for pay | 1551 (68.7) | 1223(68.9) | 328(67.9) | |
| Homemaker | 108(4.8) | 107(6.0) | 1(0.2) | |
| Disabled | 338(15.0) | 255(14.4) | 83(17.2) | |
| Unemployed | 86(3.8) | 73(4.1) | 13(2.7) | |
| Retired | 148(6.6) | 94(5.3) | 54(11.2) | |
| Other | 27(1.2) | 23(1.3) | 4(0.8) | |
| Household income e | Cumulative % | Cumulative % | Cumulative % | <.0001 |
| Less than $25,000 | 402(18.2) | 328(19.0) | 74(15.5) | |
| $25,000-$49,000 | 568(44.0) | 464(45.9) | 104(37.2) | |
| $50,000-$74,999 | 522(67.7) | 413(69.8) | 109(60.0) | |
| $75,000-$99,999 | 353(93.7) | 278(85.9) | 75(75.7) | |
| $100,000 or more | 359(100.0) | 243(100.0) | 116(100.0) | |
| Education f | Cumulative % | Cumulative % | Cumulative % | 0.84 |
| No high school diploma or GED | 76(3.4) | 61(3.4) | 15(3.1) | |
| High school diploma or GED | 445(23.0) | 351(23.2) | 94(22.5) | |
| Post high school education | 918(63.6) | 729(64.1) | 189(61.4) | |
| College diploma | 486(85.0) | 378(85.4) | 108(83.7) | |
| Graduate or professional degree | 339(100.0) | 260(100.0) | 79(100.0) |
Abbreviations: GED, Graduate Equivalency Diploma
Values are expressed as frequency (%) unless otherwise indicated.
Wilcoxon rank-sum test for continuous variables and Pearson Chi Square for categorical variables.
18 missing
2 missing
194 missing
200 missing
254 missing
194 missing
Men and women differed significantly with respect to age, marital status, employment status, and household income (Table 1). Compared to women, men tended to be older, were more likely to be white, married, retired and to have higher household incomes.
Overall, the BMI ranged from 33.0 to 94.3 kg/m2 with a median of 45.9 kg/m2. All anthropometric measures examined differed significantly by sex (Table 2). As expected, women had a higher percentage of body fat than men, but men tended to have higher BMI, and, as expected, weigh more, be taller and have larger waist and neck circumferences than women. There were statistically significant differences by sex for all laboratory measures reported (Table 3). For 350 participants, at least one assay that required a fasting sample were considered to be missing because the fasting status either was unknown or the fasting period was less than 8 hours. As previously reported for participants without cardiovascular disease (13), men tended to have higher triglyceride values but lower total HDL and LDL cholesterol than women. However, men were more likely to be taking a lipid lowering medication than women. Men tended to have worse diabetes-related parameters than women, with higher values of HbA1c, glucose, insulin, proinsulin and C-peptide, and were more likely to be taking a medication for treating diabetes. Men also had significantly lower eGFR and higher ghrelin levels than women. Reflecting normal sex differences in liver function indicators and hormones (see reference values in table 3), compared to women, men had significantly higher ALT and AST and significantly lower leptin values.
Table 2.
Anthropometric measures
| Female (n=1931) |
Male (n=527) |
p* | |
|---|---|---|---|
| BMI (kg/m2) | 45.7 (41.6,51.0) | 46.9 (42.3,52.8) | .002 |
| Weight (kg) | 124.5 (112.3,139.1) | 151.4 (134.1,172.7) | <.0001 |
| Height (m) | 1.65 (1.60,1.70) | 1.78 (1.75,1.83) | <.0001 |
| Percentage body fat (%) a | 51.8 (49.6,54.1) | 45.1 (38.7,50.6) | <.0001 |
| Waist circumference (cm) b | 127.5 (118.9,137.5) | 146.7 (136.5,156.9) | <.0001 |
| Neck circumference (cm) c | 40.7 (38.5,43.0) | 48.2 (46.0,50.7) | <.0001 |
Abbreviations: BMI, body mass index, kg, kilograms; m, meters; cm, centimeters.
Values are expressed as median (quartiles).
Wilcoxon rank-sum test.
430 missing
167 missing
156 missing
Table 3.
Laboratory values
| Reference Values for healthy individuals unless otherwise noted |
Total (n=2458) |
Female (n=1931) |
Male (n=527) |
p* | |
|---|---|---|---|---|---|
| Lipids | |||||
| Total cholesterol, mg/dla | <200 (“Desirable”) | 184(159,210) | 188(163,214) | 170(145,198) | <.0001 |
| LDL, mg/dlb | <130 (“Optimal/near optimal”) | 108(86,131) | 111(89,132) | 99(77,123) | <.0001 |
| HDL, mg/dla | <40 (“Low”), ≥60 (“High”) | 43(37,51) | 45(38,53) | 37(32,42) | <.0001 |
| Triglycerides, mg/dlc | <150 | 139(101,193) | 136.5(99,190) | 149(110,210) | <.001 |
| Lipid lowering medicationd, n (%) | 735(30.7) | 512(27.2) | 223(43.1) | <.0001 | |
| Diabetes-related parameters | |||||
| Hemoglobin A1C, %e | <5.7% | 5.6(5.2,6.3) | 5.5(5.2,6.1) | 5.8(5.3,6.8) | <.0001 |
| Glucose, mg/dlf | <110 | 98(89,114) | 97(89,112) | 102(92,125) | <.0001 |
| Insulin, uU/mlg | 3-17 | 19.6(13.1,30.0) | 18.3(12.4,28.0) | 24.9(16.3,36.7) | <.0001 |
| Proinsulin, pmoL/Lh | 6.4-9.4 | 21.5(13.0,38.7) | 19.7(12.5,34.4) | 32.3(17.3,56.0) | <.0001 |
| C-peptide, ng/mlf | 0.5-3.0 | 3.8(2.9,4.9) | 3.7(2.8,4.8) | 4.2(3.3,5.4) | <.0001 |
| Diabetes medicationi, n (%) | 760(31.5) | 546(28.8) | 214(41.3) | <.0001 | |
| Inflammatory marker | |||||
| High-sensitivity C-reactive protein, mg/Lg | ≤3.0 mg/L (“Low” or “Average”) |
7.2(4.0,13.2) | 7.7(4.4,14.3) | 5.8(2.9,9.1) | <.0001 |
| Kidney function | |||||
| eGFR, mL/minj | 90-120 | 92.0 (77.0, 109.0) | 93.0 (78.0, 111.0) | 87.0 (72.0, 104.0) | <.0001 |
| Liver function | |||||
| ALT, U/Lk | 13-50 (Female), 17-65 (Male) | 28(21,41) | 26(20,38) | 37(27,55) | <.0001 |
| AST, U/Lk | 14-46 (Female), 15-65 (Male) | 27(22,35) | 26(21,34) | 31(25,41) | <.0001 |
| Hormones | |||||
| Ghrelin, pg/mll | 395.08±125.40m | 714.7(601.7,872.9) | 741.0(627.6,909.0) | 634.6(545.5,746.8) | <.0001 |
| Leptin, ng/mll | 7.4±3.7m (lean women) 3.8±1.8m(lean men) |
56.6(42.6,73.6) | 61.4(47.8,77.8) | 40.0(27.7,54.0) | <.0001 |
Abbreviations: mg, milligrams; dl, deciliter; uU=microunits; ml, milliliters; pmol, picomoles; L, liter; mL, milliliters; min, minute; U, units; pg, picograms; ng, nanograms
LDL, low-density lipoprotein; HDL, high-density lipoprotein; eGFR, estimated Glomerular Filtration Rate, ALT, alanine transaminase; AST, aspartate transaminase
Values are expressed as median (quartiles) unless otherwise noted.
Wilcoxon rank-sum test.
121 missing
450 missing
460 missing
46 missing
123 missing
461 missing
457 missing
480 missing
60 missing
117 missing
22 missing
481 missing
Mean+standard deviation
With respect to QOL, men tended to report that weight had less impact on their QOL than did women, as reflected by a significantly higher total IWQOL-lite score (table 4). Specifically, males tended to score higher than females on the domains of work, sexual life, and self-esteem, whereas there were not statistically significant differences by sex with respect to physical function or public distress.
Table 4.
Quality of life (QOL) measures
| Total (n=2458) |
Female (n=1931) |
Male (n=527) |
p | |
|---|---|---|---|---|
| Impact of Weight Quality Of Life (IWQOL)-Lite | ||||
| Totala | 46.8(33.9,61.3) | 46.0(33.1,59.7) | 51.6(37.1,66.1) | <.0001* |
| Workb | 62.5(43.8,81.3) | 62.5(43.8,81.3) | 68.8(43.8,81.3) | 0.01* |
| Physical functioningc | 40.9(25.0,59.1) | 40.9(25.0,56.8) | 43.2(27.3,61.4) | 0.054* |
| Public distressd | 55.0(30.0,75.0) | 55.0(30.0,75.0) | 55.0(30.0,75.0) | 0.17* |
| Sexual lifee | 56.3(25.0,75.0) | 50.0(25.0,75.0) | 62.5(31.3,87.5) | <.0001* |
| Self-esteemf | 39.3(21.4,60.7) | 35.7(17.9,57.1) | 50.0(32.1,75.0) | <.0001* |
| Short Form 36-item health survey (SF-36) | ||||
| Aggregate Mental Healthg | 51.6(42.8,57.1) | 51.4(42.6,57.1) | 51.9(44.0,57.3) | 0.26* |
| Aggregate Physical Healthg | 36.5(27.8,45.0) | 36.3(27.5,45.1) | 37.1(29.1,44.7) | 0.18* |
| Beck Depression Inventory (BDI)h, n(%) | ||||
| Not depressed (0-9) | 1616(68.7) | 1252(67.7) | 364(72.4) | 0.07** |
| Mild-moderate (10-18) | 572(24.3) | 463(25.0) | 109(21.7) | |
| Moderate-severe (19-29) | 142(6.0) | 117(6.3) | 25(5.0) | |
| Severe (30-63) | 22(0.9) | 17(0.9) | 5(1.0) |
Median (quartiles) presented unless otherwise indicated.
Wilcoxon Rank-sum test
Cochran-Armitage test for trend.
210 missing
291 missing
198 missing
203 missing
297 missing
199 missing
229 missing
106 missing
Neither the SF-36 physical component summary score nor the mental component summary score differed significantly by sex. The mental component summary scores were comparable to the general population (mean=50), with median scores for women and men of 51.4 and 51.9, respectively. However, the median physical component scores were more than 1 standard deviation (10 points) below the mean in the general population (50) indicating that the physical health of this cohort was more impaired than reported by those in the general population. Some depressive symptomatology, as defined by a score on the BDI of at least 10, was reported by 33.3% of women and 27.6% of men, with 7% of women and 6% of men expressing moderate or severe symptomatology, defined as a BDI score of at least 19. There was not a significant difference by sex.
Comorbidities were common with 95% of the cohort reporting at least 1 of the 12 comorbidities (hyperlipidemia was not considered to be a separate comorbidity since it is a subset of dyslipidemia) examined (94% of females and 99% of males; p<.0001). Hypertension, dyslipidemia and sleep apnea were prevalent in the majority of participants (Table 5). Urinary incontinence, hyperlipidemia, diabetes and asthma were each noted in more than a quarter of the cohort. Pulmonary hypertension and severe walking limitations were relatively rare and did not differ significantly by sex. Asthma and urinary incontinence were significantly more prevalent in females than males. All other comorbidities examined were significantly more prevalent in males than females.
Table 5.
Prevalence, unadjusted and adjusted relative risk of comorbidities for males compared to females.
| Total (n=2458) |
Female (n=1931) |
Male (n=527) |
Unadjusted RR (95% CI) |
Adjusted RRa (95% CI) |
|
|---|---|---|---|---|---|
| Comorbidity | |||||
| Hypertensionb | 1596(67.2) | 1187(63.9) | 409(79.3) | 1.23(1.17, 1.30)c | 1.18(1.11, 1.26)c |
| Dyslipidemiad | 1242(63.4) | 885(57.9) | 357(83.0) | 1.37 (1.30, 1.44)c | 1.34 (1.26, 1.41)c |
| Sleep apneae | 1288(52.5) | 904(46.9) | 384(73.0) | 1.56(1.45, 1.67)c | 1.47(1.35, 1.60)c |
| Urinary incontinencef | 962(42.9) | 862(48.9) | 100(21.0) | 0.43(0.36, 0.51)c | 0.32(0.25, 0.41)c |
| Hyperlipidemiag | 719(36.6) | 507(33.1) | 212(49.0) | 1.40(1.26, 1.56)c | 1.20(1.10, 1.31)c |
| Diabetesh | 773(33.4) | 546(30.0) | 227(45.9) | 1.51(1.34, 1.69)c | 1.41(1.26, 1.57)c |
| Asthmai | 607(25.3) | 511(27.2) | 96(18.6) | 0.68(0.56, 0.83)c | 0.68(0.56, 0.83)c |
| Abnormal kidney functionj | 199(8.5) | 137(7.5) | 62(12.4) | 1.66(1.25, 2.20)c | 1.27(0.97, 1.66) |
| Venous edemad | 172(7.0) | 106(5.5) | 66(12.6) | 2.28(1.71, 3.06)c | 1.83(1.40, 2.39)c |
| Severe walking limitationk | 153(6.8) | 120(6.9) | 33(6.8) | 0.99(0.68, 1.44) | 0.75(0.53, 1.06) |
| Ischemic heart diseasel | 151(6.3) | 82(4.4) | 69(13.3) | 2.99(2.21, 4.05)c | 2.44(1.81, 3.29)c |
| Congestive heart failuree | 48(2.0) | 21(1.1) | 27(5.1) | 4.72(2.69, 8.27)c | 6.18(3.09, 12.37)c |
| Pulmonary e, m | 28(1.1) | 22(1.1) | 6(1.1) | 1.00(0.41, 2.46) | - |
Data presented as number of participants, with percentages in parentheses, unless otherwise indicated.
Relative risks presented at median age (46 years) and body mass index (45.9 kg/m2) after adjusting for age, body mass index, and significant interactions.
86 missing from prevalence estimate; 50 missing from models
p<.05.
499 missing from prevalence estimate; 253 missing from models
3 missing
218 missing
491 missing from prevalence estimate; 341 missing from models
145 missing from prevalence estimate; 54 missing from models
62 missing
117 missing
221 missing
58 missing from prevalence estimate; 56 missing from models
Too rare to adjust for covariates.
After adjusting for BMI and age, the sex differences seen in the unadjusted results held for all comorbidities except abnormal kidney function, i.e., at baseline, males as compared to females were significantly more likely to have hypertension, sleep apnea, dyslipidemia, hyperlipidemia, diabetes, venous edema, ischemic heart disease, and congestive heart failure, with the risk in men ranging from 18% (hypertension) to more than 500% (congestive heart failure) higher than in women. Asthma and urinary incontinence remained significantly more common in women than men after adjustment, with urinary incontinence being more than twice as common. The prevalence of pulmonary hypertension was too rare to obtain adjusted estimates.
The multivariable models indicated that older age was significantly associated with higher prevalence of each comorbidity (data not shown). In general, higher BMI was also significantly associated with an increased prevalence of comorbid conditions (data not shown). Exceptions to this association were hyperlipidemia, which was less common in those with a higher BMI, and both dyslipidemia and diabetes which were less common with higher BMI for those with a BMI below 40 kg/m2. Among those with a BMI at least 40 kg/m2, however, the prevalence of both dyslipidemia and of diabetes increased with BMI. The prevalence of ischemic heart disease and of congestive heart failure were not significantly associated with BMI.
Discussion
Despite rapid growth in the number of bariatric surgical procedures in the United States over the past 2 decades, there remains a paucity of long-term data from multiple centers within the United States regarding the effectiveness, durability, and safety of bariatric surgery. LABS was designed to address these deficiencies, focusing on overall effects of surgery and the variability of effects among groups of patients.
Bariatric surgery is an option for well-informed and motivated patients who have class III obesity or class II obesity with serious comorbid conditions(2). Thus, it is not surprising that the prevalence of obesity related medical comorbidities in this preoperative sample was high, with over 90% having at least one of the 13 comorbidities examined, and with the 6 most common comorbidities (hypertension, sleep apnea, urinary incontinence, hyperlipidemia, dyslipidemia, diabetes, and asthma) each having a prevalence between 25% and 68%.
Based on reports from LABS(26) and others(27-29), the prevalence of comorbid conditions among adults undergoing bariatric surgery is related to both BMI and age. Since men tended to be older and have higher BMI than women at the time of surgery in LABS, we adjusted for these characteristics to determine if prevalence of comorbidities differed by sex and found that most of the comorbid conditions remained significantly more common among men than women, though asthma and urinary incontinence were significantly more common among women than men. Furthermore, despite greater use of diabetes medications, men as compared to women had higher hemoglobin A1C and fasting glucose levels. In addition, men had lower eGFR, higher ALT and AST, and a higher percentage of men than women used lipid lowering medications prior to surgery.
In the United States, the prevalence of class III obesity is nearly twice as high in women as in men (30), but approximately 4 times as many women as men undergo bariatric surgery (17). Hence, men in this BMI group are only about half as likely as women to undergo bariatric surgery. Similarly, in Canada men make up only 18% of the surgical population despite the fact that they represent 38% of the eligible patient population(31).This is consistent with use of health care utilization in general, as males utilize fewer health services than females, even after eliminating sex-specific utilization(32). Although reasons for this disparity remain elusive, the results reported here offer some insight.
Inasmuch as the burden of comorbidity was greater in men than women at time of surgery, it may be that men were less likely than women to seek surgery until they perceived themselves as being physically disabled, i.e., their comorbidity “burden” became substantial. Furthermore, women were more negatively affected by their weight compared to men with regards to several aspects of QOL (i.e., work life, sexual life, and self-esteem), the latter two domains also noted in prior work (27). Hence, perceiving greater impact of weight on their QOL, women may be more willing than men to undergo bariatric surgery.
Studies suggest that bariatric surgery may improve survival and reduce the burden of comorbid conditions(33-35). If so, then lower utilization by men than women may have negative consequences on men’s health, including earlier mortality. However, there are also risks of bariatric surgery which include adverse, long term sequelae such as thromboembolic or vascular complications, serious nutritional deficiencies, fluid and electrolyte disorders(36), and surgery-specific complications such as band erosion and anastomotic leaks.
Recognizing that there is variability in weight loss, risk of adverse outcomes and comorbidity response following bariatric surgery, it is important for future research to distinguish patients, male or female, likely to do well from those who may not benefit from weight loss surgery. This includes assessing the relationship of patient characteristics with long-term health benefits and potential adverse effects of bariatric surgery. With its large cohort of patients recruited from several, geographically dispersed centers throughout the United States from whom high-quality data are collected in at least 13 important domains of physical and mental health, LABS is well suited to address these important questions.
Acknowledgments
This clinical study was a cooperative agreement funded by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). Grant numbers: DCC -U01 DK066557; Columbia - U01-DK66667 (in collaboration with Cornell University Medical Center CTSC, Grant UL1-RR024996); University of Washington - U01-DK66568 (in collaboration with CTRC, Grant M01RR-00037); Neuropsychiatric Research Institute - U01-DK66471; East Carolina University – U01-DK66526; University of Pittsburgh Medical Center – U01-DK66585 (in collaboration with CTRC, Grant UL1-RR024153); Oregon Health & Science University – U01-DK66555.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- (1).Sturm R. Increases in morbid obesity in the USA: 2000-2005. Public Health. 2007;121(7):492–96. doi: 10.1016/j.puhe.2007.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).National Institutes of Health . Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults. National Institutes of Health; Jan 9, 1998. pp. 98–4083. [PubMed] [Google Scholar]
- (3).National Institutes of Health Gastrointestinal surgery for severe obesity. NIH Consensus Statement Online. 1991;9(1):1–20. [PubMed] [Google Scholar]
- (4).Kral JG, Brolin RE, Buchwald H, Pories WJ, Sarr MG, Sugerman HJ, et al. Research considerations in obesity surgery. Obesity Research. 2002;10(1):63–4. doi: 10.1038/oby.2002.10. [DOI] [PubMed] [Google Scholar]
- (5).Belle SH. The NIDDK Bariatric Surgery Clinical Research Consortium. Surg Obes Relat Dis. 2005;1(2):145–47. doi: 10.1016/j.soard.2005.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Belle SH, Berk PD, Courcoulas AP, et al. Safety and efficacy of bariatric surgery: longitudinal assessment of bariatric surgery. Surg Obes Relat Dis. 2007;3(2):116–26. doi: 10.1016/j.soard.2007.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Flum D, Belle SH, Berk PD, Chapman W, Courcoulas AP, King WC, et al. Peri-operative safety in the Longitudinal Assessment of Bariatric Surgery. N Engl J Med. 2009;361(5):445–54. doi: 10.1056/NEJMoa0901836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Mitchell JE, Selzer F, Kalarchian MA, et al. Psychopathology prior to surgery in the Longitudinal Assessment of Bariatric Surgery-3 (LABS-3) Psychosocial Study. Surg Obes Relat Dis. 2012;8(5):533–541. doi: 10.1016/j.soard.2012.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Gosman G, King WC, Schrope B, et al. Reproductive health characteristics of women undergoing bariatric surgery. Fertility and Sterility. 2010;94(4):1426–31. doi: 10.1016/j.fertnstert.2009.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).King WC, Chen JY, Mitchell JE, et al. Prevalence of alcohol use disorders before and after bariatric surgery. JAMA. 2012;307(23):2516–25. doi: 10.1001/jama.2012.6147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).King WC, Hsu JY, Belle SH, et al. Pre- to post-operative changes in physical activity: report from the Longitudinal Assessment of Bariatric Sugery-2. Surg Obes Relat Dis. 2012;8(5):522–32. doi: 10.1016/j.soard.2011.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).King WC, Engel SG, Elder KA, et al. Walking capacity of bariatric surgery candidates. Surg Obes Relat Dis. 2012;8(1):48–9. doi: 10.1016/j.soard.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Mackey RH, Belle SH, Courcoulas AP, et al. Distribution of 10-year and lifetime predicted risk for cardiovascular disease prior to surgery in the Longitudinal Assessment of Bariatric Surgery-2 study. Am J Cardiology. 2012;110(8):1130–37. doi: 10.1016/j.amjcard.2012.05.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Khan A, King WC, Patterson EJ, et al. Assessment of obstructives sleep apnea in adults undergoing bariatric surgery in LABS-2. Journal of Clinical Sleep Medicine. 2012 doi: 10.5664/jcsm.2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).King WC, Kalarchian MA, Steffen KJ, et al. Associations between physical activity and mental health among bariatric surgical candidates. J Psychosom Res. 2012 doi: 10.1016/j.jpsychores.2012.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).King WC, Belle SH, Eid GM, et al. Physical activity levels of patients undergoing bariatric surgery in the Longitudinal Assessment of Bariatric Surgery study. Surg Obes Rel Dis. 2008;4(6):721–8. doi: 10.1016/j.soard.2008.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Nguyen NT, Masoomi H, Magno CP, et al. Trends in use of bariatric surgery, 2003 - 2008. J Am Coll Surg. 2011;213(2):261–66. doi: 10.1016/j.jamcollsurg.2011.04.030. [DOI] [PubMed] [Google Scholar]
- (18).Larsson A, Malm J, Grubb A, Hansson L-O. Calculation of glomerular filtration rate expressed in mL/min from plasma cystatin C values in mg/L. Scandinavian Journal of Clinical and Laboratory Investigation. 2004;64:25. doi: 10.1080/00365510410003723. 0. [DOI] [PubMed] [Google Scholar]
- (19).International Expert Committee report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care. 2009 Jan 7;32(7):1327–34. doi: 10.2337/dc09-9033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Kolotkin RL, Crosby RD, Kosloski KD, Williams GR. Development of a brief measure to assess quality of life in obesity. Obesity Research. 2001;9:102–11. doi: 10.1038/oby.2001.13. [DOI] [PubMed] [Google Scholar]
- (21).Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30(6):473–83. [PubMed] [Google Scholar]
- (22).Ware JE, Kosinski M, Keller SK. SF-36 physical and mental health summary scales: a user’s manual. The Health Institute; Boston: 1994. [Google Scholar]
- (23).Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–71. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- (24).Beck AT, Steer RA, Garbin MG. Psychometric properties of the Beck Depression Inventory: Twenty-five years of evaluation. Clin Psych Rev. 1988;8(1):77–100. [Google Scholar]
- (25).Blizzard L, Hosmer DW. Parameter estimation and goodness-of-fit in log binomial regression. Biometrical. 2006;48:5–22. doi: 10.1002/bimj.200410165. [DOI] [PubMed] [Google Scholar]
- (26).Belle SH, Chapman W, Courcoulas AP, et al. The relationship of BMI with demographic, clinical, and procedure characteristics: results from the Longitudinal Assessment of Bariatric Surgery. Surg Obes Relat Dis. 2008;4(4):474–80. doi: 10.1016/j.soard.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Kolotkin RL, Crosby RD, Gress RE, et al. Health and health-related quality of life: differences between men and women who seek gastric bypass surgery. Surgery for Obesity and Related Diseases. 2008;4(5):651–59. doi: 10.1016/j.soard.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Residori L, Garcia-Lordi P, Flancbaum L, Pi-Sunyer FX, Laferrere B. Prevalence of comorbidities in obese patients before surgery. Obesity Surgery. 2003;13:333–40. doi: 10.1381/096089203765887615. [DOI] [PubMed] [Google Scholar]
- (29).Tymitz K, Kerlakian G, Engel A, Bollmer C. Gender differences in early outcomes following hand-assisted laparoscopic Roux-en-Y gastric bypass surgery: gender differences in bariatric surgery. Obesity Surgery. 2007;17:1588–91. doi: 10.1007/s11695-007-9296-7. [DOI] [PubMed] [Google Scholar]
- (30).Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999-2010. JAMA. 2012;307(5):491–497. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- (31).Samuel I, Mason EE, Renquist KE HY, Zimmerman MB, Jamal M. Bariatric surgery trends: an 18-year report from the International Bariatric Surgery Registry. Am J Surg. 2006;192:657–62. doi: 10.1016/j.amjsurg.2006.07.006. [DOI] [PubMed] [Google Scholar]
- (32).Green CA, Pope CR. Gender, psychological factors and the use of medical services: a longitudinal analysis. Social Science and Medicine. 1999;48:1363–72. doi: 10.1016/s0277-9536(98)00440-7. [DOI] [PubMed] [Google Scholar]
- (33).Adams TD, Gress RE, Smith SC, et al. Long-term mortality after gastric bypass surgery. N Engl J Med. 2007;357(8):753–61. doi: 10.1056/NEJMoa066603. [DOI] [PubMed] [Google Scholar]
- (34).Adams TD, Davidson LE, Litwin SE, et al. Health benefits of gastric bypass surgery after 6 years. JAMA. 2012;308(11):1122–31. doi: 10.1001/2012.jama.11164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Sjostrom L, Narbro K, Sjostrom CD, et al. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med. 2007;357(8):741–52. doi: 10.1056/NEJMoa066254. [DOI] [PubMed] [Google Scholar]
- (36).Bolen SD, Chang H-Y, Weiner JP, et al. Clinical outcomes after bariatric surgery: A five-year matched cohort analysis in seven US states. Obesity Surgery. 2012;22:749–63. doi: 10.1007/s11695-012-0595-2. [DOI] [PMC free article] [PubMed] [Google Scholar]