Abstract
Asthma is a chronic inflammatory airway disease characterized by airway hyperreactivity, increased mucus production, and reversible airway contraction. Asthma is a complex genetic trait caused by environmental factors in genetically predisposed individuals. The transportation of maternal antigen-specific IgG via amniotic fluid, placenta and breast milk plays an important role in passive immunity. First, to examine whether maternal passive immunity by the transportation of antigen-specific IgG via FcRn regulates allergic airway inflammation, ovalbumin-immunized FcRn+/− female mice were bred with FcRn−/− male mice to evaluate the degree of ovalbumin-induced allergic airway inflammation of FcRn−/− offspring. Maternal passive immunity regulated allergic airway inflammation in an FcRn-dependent manner. Second, to examine the role of maternal antigen-specific IgG1 injection into mothers, we intravenously injected ovalbumin-specific IgG1 into wild-type or FcRn+/− mice immediately after they gave birth. The offspring were sensitized and challenged with ovalbumin. Antigen-specific IgG1 administered to lactating mice reduced allergic airway inflammation in their offspring in an FcRn-dependent manner. Last, to exclude the factor of maternal passive immunity other than ovalbumin-specific IgG1, we administered ovalbumin-specific IgG1 orally to offspring after birth. Oral administration of ovalbu-min-specific IgG1 to offspring during the lactating period prevented the development of allergic airway inflammation in an FcRn-dependent manner. These data show that the transfer of maternal antigen-specific IgG regulates the development of allergic airway inflammation early in life in an FcRn-dependent manner.
Keywords: Allergic airway inflammation, Asthma, IgG, FcRn, Breast milk
1. Introduction
Asthma is a chronic inflammatory airway disease characterized by airway hyperreactivity, increased mucus production, and reversible airway contraction. Lymphocytes, mast cells, eosinophils, and basophils infiltrate the airways of asthma patients. Chemical mediators and cytokines secreted from these cells cause chronic airway inflammation. Th2 cytokines, such as interleukin 4 (IL-4), IL-5, and IL-13, are especially important for airway inflammation [1].
In recent years, the prevalence of asthma has increased in both adults and children [2]. Asthma is a complex genetic trait caused by environmental factors in genetically predisposed individuals [1]. It is reported that atopic asthma is a hereditary disease because it develops from infancy [3]. The development of allergic diseases, such as asthma, atopic dermatitis, and atopic conjunctivitis, during the adolescent period is reduced in children of mothers sensitized with allergens before or during pregnancy and lactation [4–9]. Transportation of Th-2 cells [7], IFN-γ [8] or antigen-specific IgG from mother to offspring reduces the development of allergic diseases. Especially, the transportation of maternal antigen-specific IgG via amniotic fluid, placenta and breast milk plays an important role in passive immunity [7–9]. The neonatal Fc receptor for IgG (FcRn) has been well characterized in the transfer of passive humoral immunity from mother to fetus. Therefore, FcRn expressed on placenta, intestinal epithelia, liver, lung, and vascular endothelium plays an important role in the transportation of maternal IgG [10].
FcRn binds to the Fc domain of IgG at an acidic pH (pH <6.0) but not at a physiological pH (pH >7.0) [11] and functions in the bidirectional transport of IgG across polarized epithelia [12] and in a related cell biologic process that is associated with the protection of monomeric IgG from degradation [13]. FcRn also regulates mucosal immune responses to luminal bacteria and the pathogenesis of colitis [14,15].
First, we confirmed that the development of allergic airway inflammation is reduced in an FcRn-dependent manner by sensitizing mother mice before pregnancy and inducing allergy. Next, we determined whether antigen-specific IgG in breast milk can reduce the development of allergic airway inflammation in offspring which were sensitized and challenged with ovalbumin.
2. Materials and methods
2.1. Animals
Six- to eight-week-old female C57BL/6 mice (WT; specific pathogen free, Nippon CLEA, Tokyo, Japan), FcRn+/− mice, and FcRn−/− mice [16] that weighed 22–25 g were used in these experiments. All animal experiments were performed according to the Guidelines for Animal Experimentation at Kobe University Graduate School of Medicine. Our research was approved by the Institutional Animal Care and Use Committee and performed according to the Kobe University Animal Experimentation Regulations.
2.2. Agents
Ovalbumin (OVA) was purchased from Sigma–Aldrich Japan (Tokyo, Japan). Mouse OVA-specific IgG1 was a kind gift of H. Karasuyama (Department of Immune Regulation, Tokyo Medical and Dental University Graduate School).
2.3. Sensitization and antigen challenge
Six- to eight-week-old female WT, FcRn+/−, and FcRn−/− mice were sensitized with intraperitoneal injections of 50 μg of OVA with 1 mg of alum twice or three times. Then, the mice were exposed to 1% (wt./vol.) OVA diluted in sterile phosphate-buffered saline (PBS) for 30 min on three consecutive days.
2.4. Collection of bronchoalveolar lavage fluid
Twenty-four hours after the last exposure to OVA, bronchoalveolar lavage (BAL) was performed by instilling and aspirating two 0.8-ml aliquots of PBS (recovery >85%). Cells in the lavage fluid were counted by using a hemocytometer. BAL fluid was centrifuged at 1500g for 5 min (at 4 °C).
2.5. Lung histology
Mouse lungs were intratracheally instilled with 10% buffered formalin at a pressure of 20 cm H2O for 24 h and then embedded in paraffin. The lung tissues were stained with hematoxylin and eosin or with periodic acid-Schiff.
2.6. Collection of blood
Blood was collected from the heart via the anterior mediastinum. Blood was centrifuged at 3000g for 10 min (at 4 °C), and supernatants were stored at −20 °C.
2.7. Immunoglobulin assay
Mouse OVA-specific IgG1 serum levels were measured by using a mouse IgG1 ELISA kit (Bethyl). Mouse OVA-specific IgE serum levels were measured by using a DS Mouse IgE ELISA (DS Pharma Biomedical Co., Ltd.).
2.8. Statistical analysis
Results are expressed as means ± SE. Statistical significance between groups was assessed by the Mann–Whitney U test. p Values less than 0.05 were considered statistically significant.
3. Results
FcRn−/− mice have the same susceptibility to allergic airway inflammation as wild-type mice.
FcRn plays an important role in the transportation of maternal antigen-specific IgG [10]. However, the influence of FcRn on allergic airway inflammation is unknown. First, we evaluated the degree of allergic airway inflammation in wild-type mice, FcRn+/− mice, and FcRn−/− mice. Six- to 8-week old mice were sensitized on days 0, 7, and 14 and challenged with OVA on days 21–23. The effects of FcRn in airway inflammation were assessed by cell counts of BALF and histological analysis on day 24 (Fig. 1A). Among WT, FcRn+/− and FcRn−/− mice, there were no significant differences in the numbers of total cells and eosinophils in BALF or the levels of airway inflammation and mucus production (Fig. 1B and C). These results indicate that FcRn+/− and FcRn−/− mice have the same susceptibility to allergic airway inflammation as wild-type mice.
Fig. 1.
FcRn−/− mice have the same susceptibility to allergic airway inflammation as wild-type mice. (A) Six- to 8-week-old C57BL/6 (WT) mice, FcRn+/− mice, and FcRn−/− mice were sensitized on days 0, 7, and 14 and challenged with nebulized OVA on days 21–23. The effects of FcRn on airway inflammation were assessed by cell counts of BALF on day 24. (B) There were no significant differences in the counts of total cells and eosinophils in the BALF of WT, FcRn+/−, and FcRn−/− mice. (C) There were no significant differences in the levels of inflammation (hematoxylin and eosin) and mucus production (PAS) of WT, FcRn+/−, and FcRn−/− mice. Scale bar is 300 μm.
Maternal passive immunity can regulate allergic airway inflammation in an FcRn-dependent manner.
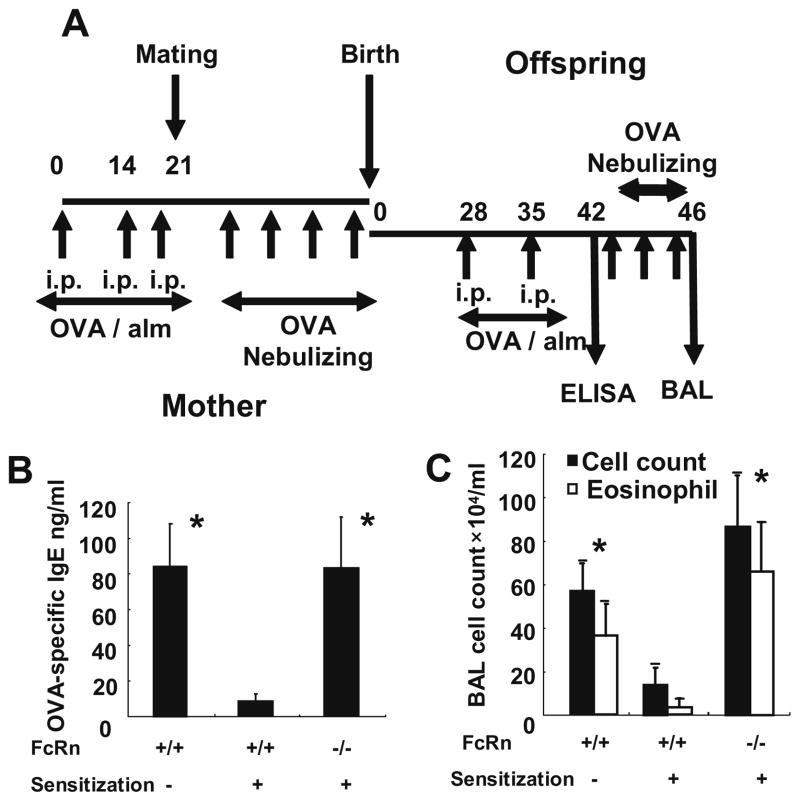
To examine whether maternal passive immunity by the transportation of antigen-specific IgG via FcRn regulates allergic airway inflammation, OVA-immunized FcRn+/− female mice were bred with FcRn−/− male mice to evaluate OVA-induced allergic airway inflammation in the FcRn−/− offspring.
Wild-type and FcRn+/− female mice were immunized with OVA and alum before pregnancy on days 0, 14, and 21. They were bred with wild-type and FcRn−/− male mice, respectively. Mice were challenged with nebulized OVA during pregnancy. Offspring were sensitized with OVA and alum on days 28 and 35. Serum OVA-specific IgE levels were analyzed in offspring on day 42, and they were challenged with nebulized OVA on days 43–45. Airway inflammation was assessed by cell counts of BALF on day 46 (Fig. 2A). By sensitizing the mother before pregnancy and inducing allergic airway inflammation during pregnancy, the serum OVA-specific IgE level was significantly reduced in wild-type offspring but not in FcRn−/− offspring (WT offspring from naïve mothers, 84.32 ± 23.84 ng/ml; WT offspring from immunized mothers, 8.676 ± 3.841 ng/ml; FcRn+/− offspring, 4.052 ± 11.51 ng/ml; FcRn−/− offspring, 83.40 ± 28.67 ng/ml; p < 0.05; Fig. 2B). The cell counts of BALF were significantly reduced in wild-type offspring from immunized mothers but not in FcRn−/− offspring (WT offspring from naïve mothers, total cells; 57.0 ± 12.6 × 104/ml, eosinophils; 36.7 ± 14.4 × 104/ml; WT offspring from immunized mothers, total cells; 14.0 ± 8.04 × 104/l, eosinophils; 3.84 ± 3.93 × 104/μl; FcRn+/− offspring, total cells; 14.5 ± 8.67 × 104/ml, eosinophils; 4.35 ± 1.07 × 104/ml; FcRn−/− offspring, total cells; 86.5 ± 23.9 × 104/ml, eosinophils; 65.9 ± 22.9 × 104/ml; p < 0.05; Fig. 2C). These results indicate that maternal passive immunity regulates allergic airway inflammation in an FcRn-dependent manner.
Fig. 2.
Maternal passive immunity can regulate allergic airway inflammation in an FcRn-dependent manner. (A) Wild-type and FcRn+/− female mice were immunized with OVA and alum before pregnancy on days 0, 14, and 21 and challenged with nebulized OVA during pregnancy. They were bred with wild-type and FcRn−/− male mice, respectively. Offspring were sensitized with OVA and alum on days 28 and 35. Serum OVA-specific IgE levels were analyzed in the offspring on day 42, and the offspring were challenged with nebulized OVA on days 43–45. Airway inflammation was assessed by cell counts of BALF on day 46. (B) By sensitizing the mothers, the serum OVA-specific IgE level was significantly reduced in WT offspring but not in FcRn−/− offspring. (C) The cell counts of BALF were significantly reduced in WT offspring from immunized mothers but not FcRn−/− offspring. Data are expressed as means ± SE. *p < 0.05 vs. WT treated, Mann–Whitney U test.
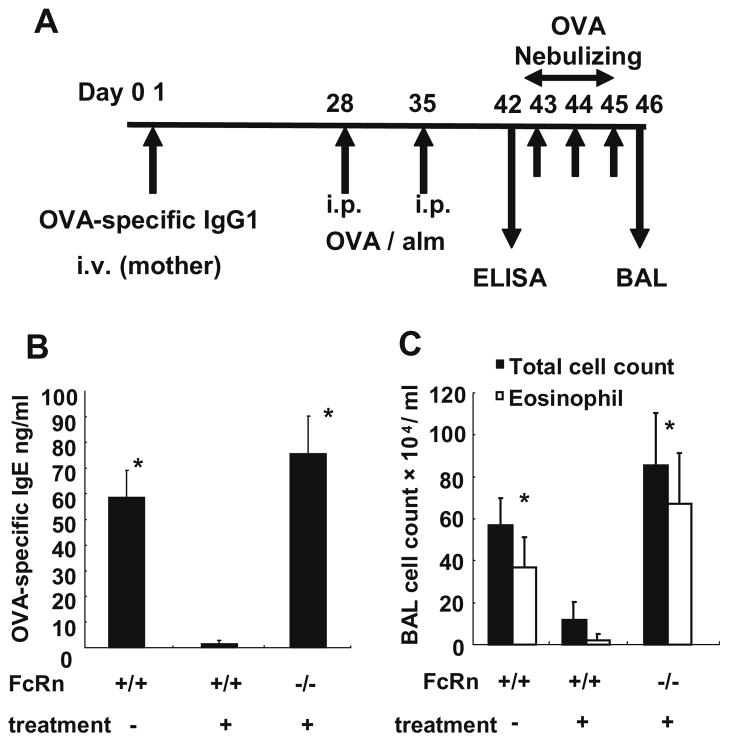
The administration of antigen-specific IgG1 to mother mice during the lactation period can reduce allergic airway inflammation in their offspring in an FcRn-dependent manner.
To examine the role of maternal antigen-specific IgG1 injection into mothers, 1 mg of OVA-specific IgG1 was injected intravenously into wild-type or FcRn+/− mothers immediately after birth (day 1). The offspring were sensitized with OVA and alum on days 28 and 35. Serum OVA-specific IgE levels were analyzed by ELISA on day 42, and offspring were challenged with nebulized OVA on days 43–45, respectively. Airway inflammation was assessed by cell counts of BALF on day 46 (Fig. 3A). By intravenous administration of OVA-specific IgG1 to mother mice, the OVA-specific IgE level was significantly reduced in wild-type offspring but not in FcRn−/− offspring (WT offspring from naïve mothers, 58.65 ± 10.44 ng/ml; WT offspring from treated mothers, 1.590 ± 1.171 ng/ml; FcRn−/− offspring, 75.66 ± 14.60 ng/ml; p < 0.01; Fig. 3B). The cell counts of BALF were also significantly reduced in wild-type offspring from treated mothers but not in FcRn−/− offspring (WT offspring from naïve mothers, total cells; 57.0 ± 12.6 × 104/ml, eosinophils; 36.7 ± 14.4 × 104/ml; WT offspring from treated mothers, total cells; 12.0 ± 8.42 × 104/ml, eosinophils; 2.23 ± 2.76 × 104/ml; FcRn−/− offspring, total cells; 85.3 ± 25.1 × 104/ml, eosinophils; 67.2 ± 24.0 × 104/ml; p < 0.05; Fig. 3C). The OVA-specific IgG1 levels in breast milk were not significantly different between wild-type mothers and FcRn+/− mothers (WT mothers, 1.59 ± 0.60 ng/ml; FcRn+/− mother, 1.27 ± 0.77 ng/ml). By the way, the OVA-specific IgE level of FcRn+/− offspring was 48.89 ± 30.40 ng/ml. This concentration was higher than that of WT offspring from treated mothers and was lower than that of FcRn−/− offspring. The cell counts of BALF of FcRn+/− offspring were also the interval of that of WT offspring from treated mothers and FcRn−/− offspring (total cells; 49.3 ± 15.3 × 104/ml, eosinophils; 35.9 ± 0.88 × 104/ml). These results indicate that antigen-specific IgG1 administered to mother mice during the lactation period was secreted into breast milk and reduced allergic airway inflammation in the offspring in an FcRn-dependent manner. In the following experiment, the OVA-specific IgE levels and the cell counts of BALF were compared in WT and FcRn−/− offspring.
Fig. 3.
The administration of antigen-specific IgG1 to mother mice during the lactation period can reduce allergic airway inflammation in their offspring in an FcRn-dependent manner. (A) OVA-specific IgG1 (1 mg/body) was injected intravenously into wild-type or FcRn+/− mothers immediately after birth (day 1). Offspring were sensitized with OVA and alum on days 28 and 35. Serum OVA-specific IgE levels were analyzed by ELISA on day 42. The offspring were challenged with nebulized OVA on days 43–45, and their levels of airway inflammation were assessed by cell counts of BALF on day 46. (B) By intravenous administration of OVA-specific IgG1 to the mothers, the OVA-specific IgE level was significantly reduced in WT offspring but not in FcRn−/− offspring. (C) The cell counts of BALF were significantly reduced in WT offspring from treated mothers but not in FcRn−/− offspring. Data are expressed as means ± SE. *p < 0.05 vs. WT treated, Mann–Whitney U test.
Oral administration of OVA-specific IgG1 into offspring during the lactation period can prevent the development of allergic airway inflammation in an FcRn-dependent manner.
To exclude the factor of maternal passive immunity other than OVA-specific IgG1, we administered OVA-specific IgG1 (10 μg/ml) orally to offspring according to the following schedule. Serum OVA-specific IgG1 levels of offspring were analyzed on day 19. Wild-type and FcRn−/− offspring were sensitized with OVA and alum on days 28 and 35. OVA-specific IgE levels were analyzed on day 42. Then, the offspring were challenged with nebulized OVA on days 43–45. Airway inflammation was assessed by cell counts of BALF and histological analysis on day 46 (Fig. 4A). By oral administration of OVA-specific IgG1 to offspring, serum OVA-specific IgG1 levels were significantly reduced in FcRn−/− offspring (treated WT, 71.18 ± 14.62 ng/ml; treated FcRn−/− 14.00 ± 0.258 ng/ml; p < 0.01; Fig. 4B). The OVA-specific IgE levels were significantly reduced in wild-type offspring but not in FcRn−/− offspring (untreated WT offspring, 58.65 ± 10.44 ng/ml; treated WT offspring, 12.49 ± 3.79 ng/ml; FcRn−/− offspring, 64.70 ± 28.54 ng/ml; p < 0.05; Fig. 4C). Airway inflammation was also significantly inhibited in treated wild-type offspring but not in FcRn−/− offspring (untreated WT offspring, total cells; 57.0 ± 12.6 × 104/ml, eosinophils; 36.7 ± 14.4 × 104/ml; treated WT offspring, total cells; 21.0 ± 12.7 × 104/ml, eosinophils; 13.8 ± 21.8 × 104/ml; FcRn−/− offspring, total cells; 70.6 ± 29.7 × 104/ml, eosinophils; 54.9 ± 28.7 × 104/ml; p < 0.05; Fig. 4D). Histological analysis of hematoxylin and eosin or periodic acid-Schiff-stained lung sections isolated from the treated wild-type offspring showed less inflammatory infiltrates and mucus production in the airways as compared to the FcRn−/− offspring or untreated wild-type offspring (Fig. 4E). These results indicate that the oral administration of OVA-specific IgG1 to offspring during the lactation period can prevent the development of allergic airway inflammation in an FcRn-dependent manner.
Fig. 4.
Oral administration of OVA-specific IgG1 to offspring during the lactating period can prevent the development of allergic airway inflammation in an FcRn-dependent manner. (A) Serum OVA-specific IgG1 levels in the offspring were analyzed on day 19. Wild-type and FcRn−/− offspring were sensitized with OVA and alum on days 28 and 35. OVA-specific IgE levels were analyzed on day 42. Then, the offspring were challenged with nebulized OVA on days 43–45. Airway inflammation was assessed by cell counts of BALF and histological analysis on day 46. (B) The serum OVA-specific IgG1 levels in offspring were significantly reduced in FcRn−/− offspring. (C) By oral administration of OVA-specific IgG1 to offspring, the OVA-specific IgE level was significantly reduced in WT offspring but not in FcRn−/− offspring. (D) The cell counts of BALF were significantly reduced in treated WT offspring, but not in FcRn−/− offspring. Data are expressed as means ± SE. *p < 0.05 vs. WT treated, #p < 0.01, Mann–Whitney U test. (E) Airway inflammation (hematoxylin and eosin) and mucus production (PAS) were significantly reduced in treated WT offspring but not in FcRn−/− offspring. Scale bar is 600 μm.
4. Discussion
The influence of FcRn on allergic airway inflammation is unknown. We confirmed that the development of allergic airway inflammation was reduced in an FcRn-dependent manner by sensitizing mother mice before pregnancy and inducing allergy. The degree of allergic airway inflammation of FcRn−/− mice was comparable with that of wild-type mice (Fig. 1B). It appears that FcRn itself does not affect the development of allergic airway inflammation by OVA because FcRn is expressed in mouse alveolar epithelial cells at only very low levels and it is not expressed in airway epithelial cells [17].
There are some reports that maternal passive immunity suppresses the development of allergic disease during the adolescent period [4–9]. The transportation of maternal antigen-specific IgG via amniotic fluid, placenta, and breast milk plays an important role in the passive immunity [7–9]. FcRn is critical for the transportation of maternal antigen-specific IgG [10]. So, FcRn must have a suppressive role in passive immunity. First, we confirmed that maternal passive immunity in allergic airway inflammation can be reduced in an FcRn-dependent manner (Fig. 2B and C). In contrast to the findings of the present study, T. Polte reported that maternal tolerance was observed only when both the wild-type and FcRn−/− offspring were nursed by their own mothers and not when nursed by naïve wet-nurses [8]. It was explained that breast-feeding was crucial for maternal passive immunity because OVA-specific IgG1 was not transported from mother to offspring in FcRn−/− mice via placenta. T. Polte also reported that serum OVA-specific IgG1 levels were increased in wild-type fetuses. However, FcRn is not expressed in the placenta of rodents [10]. H. Uth-off reported that OVA was transported from mother to fetus by amniotic fluid or placenta when the mother mice were exposed to OVA during pregnancy [7]. Thus, the increased levels of serum OVA-specific IgG1 in wild-type fetuses might be explained by exposure to OVA in the fetal period. Exposure to OVA during the fetal period might also cause the reduced allergic airway inflammation in FcRn−/− offspring.
To exclude the influence of OVA exposure during the fetal period, we injected OVA-specific IgG1 intravenously into puerperant wild-type and FcRn+/− mothers immediately after delivery. There were similar levels of OVA-specific IgG1 in the breast milk from FcRn+/− mothers and wild-type mothers. We confirmed that antigen-specific IgG1 injected into mother mice during the lactation period was secreted into breast milk, and it reduced allergic airway inflammation in the offspring in an FcRn-dependent manner (Fig. 3B and C).
To exclude the factor of maternal passive immunity other than OVA-specific IgG1, we orally administered OVA-specific IgG1 to offspring. We confirmed that the oral administration of OVA-specific IgG1 to offspring during the lactation period can prevent the development of allergic airway inflammation in an FcRn-dependent manner. By oral administration of OVA-specific IgG1 to offspring, the serum OVA-specific IgG1 level in offspring was significantly reduced in FcRn−/− offspring. The uptake of OVA-specific IgG1 in the breast milk via FcRn expression on the epithelial cells of the intestine must be important for the transportation of OVA-specific IgG1 from mother to offspring.
Previous studies showed that the transportation of IFN-γ [8] or Th-2 cells [7] with OVA-specific IgG is necessary for maternal passive immunity. Our study showed that maternal OVA-specific IgG1 in breast milk transported via FcRn in the epithelial cell layer of the intestine alone can prevent the development of OVA-induced allergic airway inflammation in young mice.
Mice express FcRn on intestinal epithelia but not on placenta. So, in mice, maternal antigen-specific IgG is transported from mother to offspring via breast milk after birth. The other hand, humans express FcRn on both placenta and intestinal epithelia. Maternal antigen-specific IgG is transported from mother to offspring via placenta during pregnancy and via breast milk after birth. Our study showed that exposure to antigen during pregnancy or the administration of antigen-specific IgG after birth can prevent the development of antigen-specific allergic airway inflammation in young mice in an FcRn-dependent manner. In this study, although it is not possible to evaluate the influence of exposure to antigen or the administration of antigen-specific IgG during pregnancy in humans, the administration of antigen-specific IgG during young days can prevent the development of antigen-specific allergic airway inflammation in young humans.
5. Conclusion
In this study, we confirmed that immune therapy in female mice before or during pregnancy and in the lactation period reduces the development of allergic disease in young offspring. It is necessary to pay attention to antigen exposure and immune therapy before conception and during pregnancy and the breast-feeding period to prevent allergy.
Acknowledgments
The authors thank the members of the Division of Respiratory Medicine for their fruitful discussions and technical assistance. This study was supported by a grant from Research Fellows of the Global COE Program “Global Center of Excellence for Education and Research on Signal Transduction Medicine in the Coming Generation” from the Ministry of Education, Culture, Sports, Science, and Technology of Japan [F031 to M.Y]. This study was also supported, in part, by grants KAKENHI from the Japan Society for the Promotion of Science [21590811 to M.Y. and 21790769 to K.K.], the Japan Chemical Industry Association, and the Mother and Child Health Foundation.
References
- 1.Umetsu DT, McIntire JJ, Akbari O, Macaubas C, DeKruyff RH. Asthma: an epidemic of dysregulated immunity. Nat Immunol. 2002;3:715–720. doi: 10.1038/ni0802-715. [DOI] [PubMed] [Google Scholar]
- 2.Cohn L, Elias JA, Chupp GL. Asthma: mechanisms of disease persistence and progression. Annu Rev Immunol. 2004;22:789–815. doi: 10.1146/annurev.immunol.22.012703.104716. [DOI] [PubMed] [Google Scholar]
- 3.Alford SH, Zoratti E, Peterson EL, Maliarik M, Ownby DR, Ownby DR, Johnson CC. Parental history of atopic disease: disease pattern and risk of pediatric atopy in offspring. J Allergy Clin Immunol. 2004;114:1046–1050. doi: 10.1016/j.jaci.2004.08.036. [DOI] [PubMed] [Google Scholar]
- 4.Jarrett EE, Hall E. IgE suppression by maternal IgG. Immunology. 1983;48:49–58. [PMC free article] [PubMed] [Google Scholar]
- 5.Melkild I, Groeng EC, Leikvold RB, Granum B, Løvik M. Maternal allergen immunization during pregnancy in a mouse model reduces adult allergy-related antibody responses in the offspring. Clin Exp Allergy. 2002;32:1370–1376. doi: 10.1046/j.1365-2745.2002.01458.x. [DOI] [PubMed] [Google Scholar]
- 6.Fusaro AE, Brito CA, Victor JR, Rigato PO, Goldoni AL, Duarte AJ, Sato MN. Maternal–fetal interaction: preconception immunization in mice prevents neonatal sensitization induced by allergen exposure during pregnancy and breastfeeding. Immunology. 2007;122:107–115. doi: 10.1111/j.1365-2567.2007.02618.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Uthoff H, Spenner A, Reckelkamm W, Ahrens B, Wölk G, Hackler R, Hardung F, Schaefer J, Scheffold A, Renz H, Herz U. Critical role of preconceptional immunization for protective, nonpathological specific immunity in murine neonates. J Immunol. 2003;171:3485–3492. doi: 10.4049/jimmunol.171.7.3485. [DOI] [PubMed] [Google Scholar]
- 8.Polte T, Hennig C, Hansen G. Allergy prevention starts before conception: maternofetal transfer of tolerance protects against the development of asthma. J Allergy Clin Immunol. 2008;122:1022–1030. doi: 10.1016/j.jaci.2008.09.014. [DOI] [PubMed] [Google Scholar]
- 9.Polte T, Hansen G. Maternal tolerance achieved during pregnancy is transferred to the offspring via breast milk and persistently protects the offspring from allergic asthma. Clin Exp Allergy. 2008;38:1950–1958. doi: 10.1111/j.1365-2222.2008.03096.x. [DOI] [PubMed] [Google Scholar]
- 10.Roopenian DC, Akilesh S. FcRn: the neonatal Fc receptor comes of age. Nat Rev Immunol. 2007;7:715–725. doi: 10.1038/nri2155. [DOI] [PubMed] [Google Scholar]
- 11.Rodewald R. PH-dependent binding of immunoglobulins to intestinal cells of the neonatal rat. J Cell Biol. 1976;71:666–669. doi: 10.1083/jcb.71.2.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yoshida M, Claypool SM, Wagner JS, Mizoguchi E, Mizoguchi A, Roopenian DC, Lencer WI, Blumberg RS. Human neonatal Fc receptor mediates transport of IgG into luminal secretions for delivery of antigens to mucosal dendritic cells. Immunity. 2004;20:769–783. doi: 10.1016/j.immuni.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 13.Junghans RP, Anderson CL. The protection receptor for IgG catabolism is the beta2-microglobulin-containing neonatal intestinal transport receptor. Proc Natl Acad Sci USA. 1996;93:5512–5516. doi: 10.1073/pnas.93.11.5512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kobayashi K, Qiao SW, Yoshida M, Baker K, Lencer WI, Blumberg RS. An FcRn-dependent role for anti-flagellin immunoglobulin G in pathogenesis of colitis in mice. Gastroenterology. 2009;137:1570–1573. doi: 10.1053/j.gastro.2009.07.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yoshida M, Kobayashi K, Kuo TT, Bry L, Glickman JN, Claypool SM, Kaser A, Nagaishi T, Higgins DE, Mizoguchi E, Wakatsuki Y, Roopenian DC, Mizoguchi A, Lencer WI, Blumberg RS. Neonatal Fc receptor for IgG regulates mucosal immune responses to luminal bacteria. J Clin Invest. 2006;116:2142–2151. doi: 10.1172/JCI27821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roopenian DC, Christianson GJ, Sproule TJ, Brown AC, Akilesh S, Jung N, Petkova S, Avanessian L, Choi EY, Shaffer DJ, Eden PA, Anderson CL. The MHC class I-like IgG receptor controls perinatal IgG transport, IgG homeostasis, and fate of IgG-Fc-coupled drugs. J Immunol. 2003;170:3528–3533. doi: 10.4049/jimmunol.170.7.3528. [DOI] [PubMed] [Google Scholar]
- 17.Akilesh S, Christianson GJ, Roopenian DC, Shaw AS. Neonatal FcR expression in bone marrow-derived cells functions to protect serum IgG from catabolism. J Immunol. 2007;179:4580–4588. doi: 10.4049/jimmunol.179.7.4580. [DOI] [PubMed] [Google Scholar]