Abstract
The signal transducers and activators of transcription (STAT) family of proteins play a critical role in cytokine signaling required for fine tuning of immune regulation. Previous reports showed that a mutation (L327M) in the Stat5b protein leads to aberrant cytokine signaling in the NOD mice. To further elaborate the role of Stat5b in diabetes, we established a NOD transgenic mouse that over-expresses the wild type Stat5b gene. The incidences of spontaneous diabetes as well as cyclophosphamide-induced diabetes were significantly reduced and delayed in the Stat5b transgenic NOD mice compared to their littermate controls. The total cell numbers of CD4+ T cells and especially CD8+ T cells in the spleen and pancreatic lymph node were increased in the Stat5b transgenic NOD mice. Consistent with these findings, CD4+ and CD8+ T cells from the Stat5b transgenic NOD mice showed a higher proliferation capacity and up-regulation of multiple cytokines including IL-2, IFN-γ, TNF-α and IL-10 as well as anti-apoptotic gene Bcl-xl. Furthermore, the number and proportion of CD4+CD25+ regulatory T cells were significantly increased in transgenic mice although in vitro suppression ability of the regulatory T-cells was not affected by the transgene. Our results suggest that Stat5b confers protection against diabetes in the NOD mice by regulating the numbers and function of multiple immune cell types, especially by up-regulating CD4+CD25+ regulatory T cells.
Keywords: Type-1 diabetes, NOD, Stat5b, Regulatory T cells
1. Introduction
Type-1 diabetes (T1D) develops via a complex process as a result of multiple defects in immune regulation. The non-obese diabetic (NOD) mouse is an excellent model for human T1D since it shares many genetic and pathophysiological characteristics with human patients [1]. Multiple loci including MHC and non-MHC genes control the genetic susceptibility to diabetes in the NOD mice [2]. NOD mice exhibit a number of other immune defects including deficiencies in the CD4+CD25+ regulatory T cell population [3,4], reduction in plasmacytoid dendritic cells [5], defective macrophage maturation and function [6], low levels of nature killer (NK) cell activity [7,8] and defects in NKT cells [9]. The loss of immune homeostasis during diabetes development is a defect in cytokine signaling during differentiation and chronic inflammation [6]. This disruption in cytokine signaling is further linked to the lack of responsiveness at the level of intracellular signaling [6].
We have previously reported a point mutation in the Stat5b gene of NOD mice, which causes a leucine to methionine (L327M) substitution and aberrant intracellular signaling [10]. The mutation leads to reduction of its transcriptional activities and impaired immune modulation in NOD mice [11]. Indeed, loss of Stat5b signaling has been reported in monocytes from human T1D subjects and macrophages obtained from NOD mice [12,13]. Mice that express Stat5b constitutively were found to be glucose tolerant with normal β-cell proliferation and were resistant to streptozotocin induced beta-cell death [14].
These studies suggest that a Stat5b defect may be implicated in the pathogenesis of diabetes in the NOD mice; however, the precise role of Stat5b in preventing diabetes has been elusive. In this study, we created a NOD mouse with wild type Stat5b transgene to explore the exact role of Stat5b in the pathogenesis of T1D.
2. Materials and methods
2.1. Generation and genotyping of transgenic mice
NOD mice were purchased from the Jackson Laboratory (Bar Harbor, ME). Stat5b transgenic C57BL/6 mice were obtained from Dr. Warren J. Leonard (Laboratory of Molecular Immunology, National Heart, Lung, and Blood Institute, National Institutes of Health, Bethesda, MD) and backcrossed onto a NOD background for at least seven generations. Stat5b-transgenic NOD mice (TG) were identified using the polymerase chain reaction (PCR) with the primers: 5′-tac cga gtg gag ctg gct gag-3′ and 5′-atg atg aac gtg ctg gtg acc-3′, which yield a 309 bp fragment.
2.2. Diabetes monitoring
The incidence of spontaneous diabetes onset was monitored in Stat5b-transgenic mice. In the 7th generation of backcross (BC7), 14 transgenic female mice were studied and 23 non-transgenic littermates (LMC) were used as controls. Using Diastix reagent strips (Bayer Corporation, Elkhart, IN), urine glucose was monitored twice per week starting from 12 weeks of age. Mice were considered to be diabetic when glucosuria was observed and confirmed three consecutive times.
2.3. Cyclophosphamide (CY) acceleration of diabetes
Twenty-four Stat5b-transgenic NOD female mice and thirty non-transgenic littermate controls (7- to 8-week-old) were injected by i.p. with CY at a dose of 200 mg/kg twice at an interval of 10 days, starting from day 0. Diabetes onset was monitored for 35 days using Diastix reagent strips three times weekly starting at day 8 after CY injection.
2.4. Flow cytometry
Single-cell suspension from spleen and pancreatic lymph nodes were stained with anti-CD4-FITC, anti-CD8-APC, anti-CD122-FITC, anti-CD25-PE, anti-CD44-PE, anti-B220-FITC, and anti-CD11C-APC, according to manufacturer's recommendations (BD Biosciences, San Jose, CA). Stained cells were analyzed on a FACSort with CellQuest Pro v5.2.1 (BD Biosciences, San Jose, CA).
2.5. Proliferation and suppression assay
CD4+ or CD8+ T cells isolated from fresh splenocytes by FACS sorting (BD Biosciences, San Jose, CA) were cultured in RPMI1640 medium (Cellgro, VA) with 5 × 104/per well in round bottom 96-well plates (Costa, Cambridge, MA), with stimulation of plate-coated anti-CD3 (2 μg/ml) with or without IL-2 (40 U/ml) presence. Each sample was carried out in triplicates for 48 h, and 0.5 μCi 3H-thymidine (Amersham Biosciences, Piscataway, NJ) was added for the final 16 h of culture to assess proliferation.
Mixed leukocyte reaction (MLR), was performed by co-culturing purified CD4+ or CD8+ T cells (5 × 104 cells/per well) from transgenic or control mice with equal numbers of T-cell depleted antigen presenting cells (APC) (Miltenyi Biotech, Germany) from B6 mice. After 96 h, 0.5 μCi 3H-thymidine (Amersham Biosciences, Piscataway, NJ) was added for the final 16 h of culture to assess proliferation.
For testing the suppression function, CD4+CD25+ Treg cells and CD4+CD25− T cells as responder T cells (Tresp) were isolated using CD4+CD25+ regulatory T cells isolation kit (Miltenyi Biotech, Germany). 1 × 105 Tresp cells were co-cultured with Treg cells in a ratio of 0:1, 1:1, 1:4, 1:16 in 96-well plate containing RPMI 1640 and 10% fetal bovine serum. The cells were stimulated with soluble anti-CD3 (1.5 μg/ml). 7.5 × 104 irradiated autologous APC (T-cell depleted splenocytes) were added to a total volume of 200 μl. Cultures were incubated for 48 h at 37 °C, and then 0.5 μCi 3H-thymidine was added for the final 16 h of culture.
2.6. Real-time PCR
Total RNA was extracted from freshly sorted CD4+ and CD8+ T cells using RNAeasy kit (Qiagen, Valencia, CA). RNA was converted to cDNA using the reverse transcription kit from Stratagene (Stratagene, CA, USA). cDNA equivalent of 50 ng of total RNA were used for performing real-time PCR assay, with following settings: 95 °C for 5 min, followed by 35 cycles at 95 °C, 30 s, 58 °C, 30 s and 72 °C, 30 s. The primers used for amplification of Bcl-xl were 5′-ctg ctt gct gtc gcc g-3′ and 5′-tgg gtc tgc tct gtg ttt agc-3′.
2.7. Multiplex cytokine assay
Levels of cytokines, including IL-2, IL-10, IFN-γ and TNF-α, were measured using a bead-based immunofluorescence assay (Luminex Inc. Austin, TX, USA). Fluorokine MAP cytokine arrays were obtained from R&D systems (R&D Systems, MN, USA). Immuno-assay was performed for all the samples in duplicates, as per manufacturer's recommendations. Concentrations of cytokines were determined as per manufacturer's instruction.
2.8. Statistical analyses
All procedures were repeated three times, Mean, SD and SEM were calculated using Microsoft Excel 2010®. Student t-test was used to test the differences between mean of two groups. Differences were identified statistically significant if p < 0.05. All analysis was performed in Microsoft Excel.
3. Results
3.1. Stat5b transgene reduces the incidence of diabetes
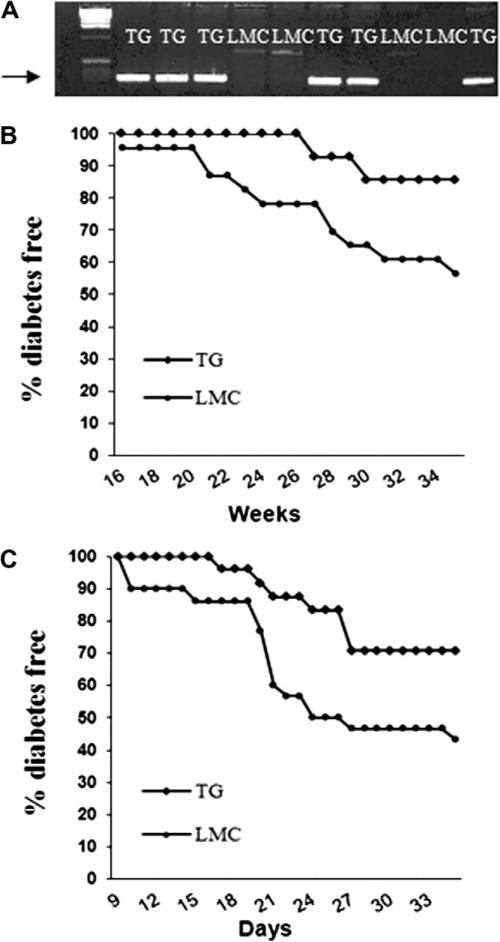
NOD mice expressing wild type Stat5b-transgene (TG) were bred by backcrossing the Stat5b transgenic B6 mouse with NOD. After backcrossing for 7 generations (BC7), transgenic NOD mice were identified by PCR-based genotyping using primers designed to yield a 309 bp fragment in transgenic mice, but not in littermate controls (Fig. 1A).
Fig. 1.
Cumulative incidence of diabetes in Stat5b-transgenic NOD mice. (A) Genotyping of mice by PCR. The 309 bp band indicates the presence of the Stat5b transgene (TG) or absence of the transgene in non-transgenic littermate controls (LMC). (B) Incidence of spontaneous diabetes in TG mice (n = 14) and LMC (n = 23) from the BC7 generation. (C) Incidence of CY induced diabetes in the Sta5b transgenic mice after 15 generations of backcrossing. Diabetes was induced by injecting CY to transgenic mice (n = 24) and their littermates (n = 30).
A total of 14 transgenic BC7 mice and 23 littermate controls were monitored for 35 weeks. At the end of monitoring, 15% (2/14) of transgenic mice and 44% (10/23) of non-transgenic littermates became diabetic (Fig. 1B). We noticed that the diabetes incidence of the control group is lower than the incidence normally observed in NOD mice. One possible explanation is that the BC7 mice may still contain some protective genes from B6. To further confirm the protective role of Stat5b transgene expression in NOD, we compared the incidence of cyclophosphamide (CY)-induced diabetes between transgenic mice that backcrossed for 15 generations (BC15) and their littermate controls. Consistent with the result for spontaneous diabetes incidence, inducible autoimmune diabetes was significantly reduced in Stat5b-transgenic mice (log rank test, p = 0.03, Fig. 1C). By 35 days of monitoring, the disease incidence was 56.7% (17/30) in control group, compared to 29.2% (7/24) in transgenic mice (Fig. 1C).
3.2. Increased total cellularity and decreased CD4/CD8 ratio in Stat5btransgenic NOD mice
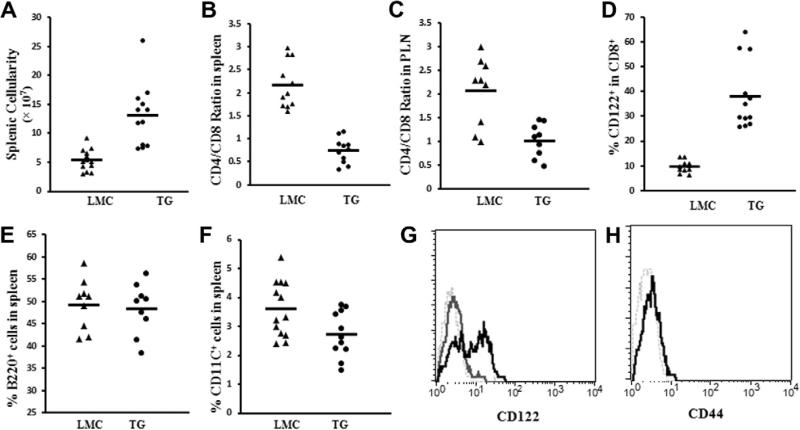
Enlargement in sizes of spleens and pancreatic lymph nodes was observed in Stat5b-transgenic mice than littermate controls (data not shown). In the Stat5b-transgenic B6 mice [15], peripheral total cellularity was increased and CD4/CD8 T cell ratio was decreased in the transgenic mice compared to littermate controls. Similarly, the total cellularity was increased significantly in spleens of transgenic NOD mice (13.06 ± 2.28 × 107) compared to littermate controls (5.34 ± 1.00 × 107) (p < 0.0001, Fig. 2A). We also observed a reduction in CD4/CD8 ratio in spleens (0.74 ± 0.16 vs 2.16 ± 0.30, p < 8.0 × 10−8) (Fig. 2B) and pancreatic lymph nodes (1.02 ± 0.23 vs 2.07 ± 0.47, p < 0.005) (Fig. 2C) in NOD transgenic mice. Reduction in ratio was due to an greater increase of total CD8+ T cell number (roughly 5 folds increase) than CD4+ T cells (roughly 1.7 folds) in the Stat5b transgenic mice. Similar observation accounts for the reduced CD4/CD8 T cell ratio in pancreatic lymph nodes. We analyzed a series of surface markers including CD25, CD69, CD62L, CD122 and CD44. CD122 expression on CD8+ T cells from spleens of transgenic mice was significantly increased in transgenic mice (Fig. 2D and G). Some of these CD122+CD8+ cells (approximately 2%) also expressed T cell memory marker CD44 (Fig. 2H).
Fig. 2.
Overexpression of wild type Stat5b alters total splenic cellularity and CD4+/CD8+ T cell ratio in NOD mice. Splenic cellularity (A), CD4+/CD8+ T cell ratios in spleen (B) and CD4+/CD8+ T cell ratios in pancreatic lymphnodes (C) are shown for TG and LMC. Each point represents a single mouse and means are shown for each group. Expression of CD122 on spleen CD8+ T cells in TG mice (D). Representative flow cytometry profiles of CD122 on gated CD8+ T cells in both TG (56.7%, black line) and LMC mouse spleens (6.6%, dark gray line) (E). CD44 expression (2.44%, black line) on transgenic CD8+CD122+ T cells is shown in (F).
We also examined the frequencies of B cells and dendritic cells in spleen by staining B220 and CD11C markers. No significant difference was observed for B cells between transgenic and control mice (48.34 ± 5.39% vs 49.31 ± 5.36%, p = 0.72) (Fig. 2E). Although a significant decrease of dendritic cell frequency was observed in transgenic mice (2.72 ± 0.73% vs 3.61 ± 0.92%, p = 0.018) (Fig. 2F), the total number of dendritic cells may not be affected due to the increase of total splenocytes.
3.3. Increased CD4+CD25+ regulatory T cells in spleen and PLN in female Stat5b transgenic NOD mice
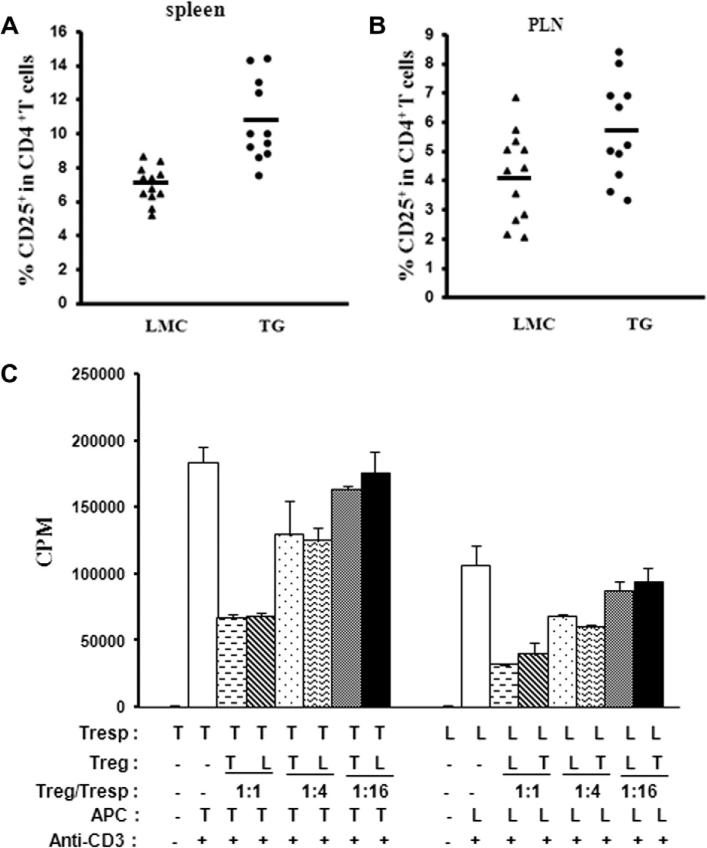
The frequencies of CD4+CD25+ Tregs in CD4+ T cells were significantly increased in spleens (10.69 ± 1.42% vs 6.93 ± 0.60%, p < 0.0001) (Fig. 3A) and PLN (5.72 ± 1.02% vs 4.13 ± 0.87%, p < 0.05) (Fig. 3B) in female transgenic NOD mice compared with controls at 8–12 weeks of age. The absolute numbers of CD4+CD25+ Tregs are also increased as the total number of CD4+ T cells are increased in transgenic mice.
Fig. 3.
Frequency and function of CD4+CD25+ Tregs in transgenic mice. Percentages of CD4+CD25+ Tregs in CD4+ T cells are shown for TG and LMC mice for spleens (A) and pancreatic lymphnodes (PLN) (B). Each point represents a single mouse and means are shown within each group. (C) In vitro suppression assay: CD4+CD25− Tresps are co-cultured with CD4+CD25+ Tregs purified from spleens using different Treg/Tresp ratios. Criss-cross suppression assays are also performed by using Tregs from TG mice co-cultured with Tresp from LMC, and Treg from LMC co-cultured with Tresp from TG mice. Results are expressed as the mean of triplicate culture.
To study the suppressive function of CD4+CD25+ Tregs, we performed in vitro suppression assay, in which CD4+CD25− responder T cells (Tresp) were co-cultured with CD4+CD25+ Tregs purified from spleens using different Treg/Tresp ratios. We also did criss-cross suppression assay using Tregs from transgenic mice co-cultured with Tresps from littermate control mice; and Tregs from control mice co-cultured with Tresps from transgenic mice. The purity of Treg cells isolated from transgenic and control mice were comparable (around 80%). Tregs from both of the two groups showed a dose-dependent suppressive function. We observed a significantly higher rate of proliferation for Tresp cells obtained from Stat5b-transgenic mice than that in littermate control mice (p < 0.005), however no significant difference in terms of Treg suppressive function or sensitivity to suppression of Tresp cells was found between transgenic and control mice (p > 0.05) (Fig. 3C).
3.4. Stat5b increases the proliferative capability for both CD4+ and CD8+ T cells
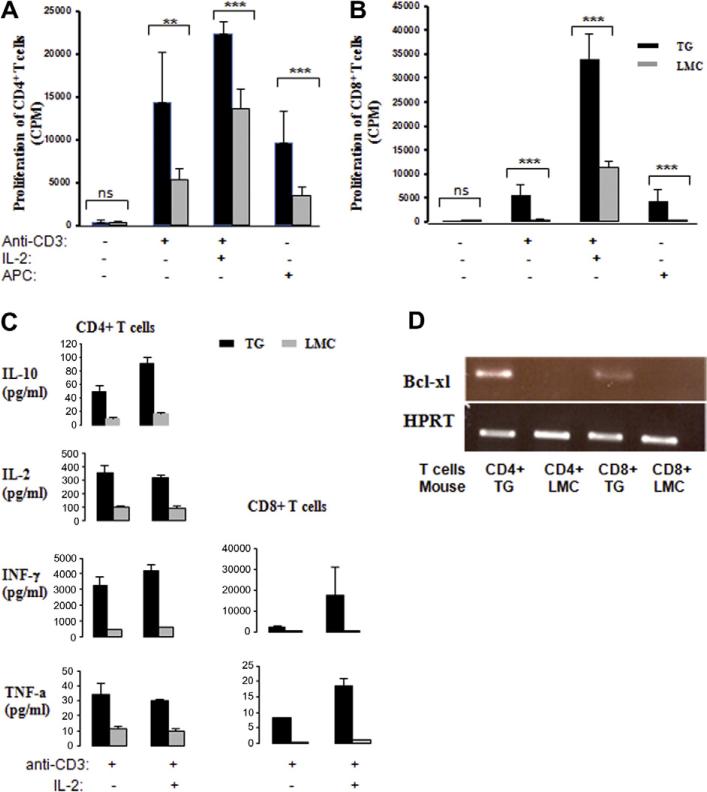
Increased T cell numbers in the periphery of transgenic mice suggested that transgenic Stat5b may influence T cell homeostasis. We evaluated the in vitro proliferation capability of sorted CD4+/CD8+ T cells under different stimulation conditions including anti-CD3 only, anti-CD3 combined with IL-2 and allogeneic APC stimulation. Significantly higher proliferation for both CD4+ and CD8+ T cells were observed in Stat5b-transgenic mice than those in littermate controls under all three simulation conditions (Fig. 4A and B). Expression levels of cytokines including IL-2, IL-10, IFN-γ and TNF-α were measured in culture medium of CD4+ and CD8+ T cells. Under anti-CD3 stimulation with or without IL-2, significantly higher level of IL-10 (p = 0.007, 0.031), IL-2 (p = 0.005, 0.018), IFN-γ (p = 0.008, 0.02) and TNF-α (p = 0.006, 0.048) were observed for transgenic CD4+ T cells and higher level of IFN-γ (p = 0.043, 0.022) and TNF-α (p = 0.011, 9.7E-05) were observed for transgenic CD8+ T cells compared to control cells (Fig. 4C). The expression levels of all the above cytokines were too low to detect for allogeneic APC stimulated CD4+/CD8+ T cells and for unstimulated CD4+/CD8+ T cells (cultured in medium alone).
Fig. 4.
T cell phenotypes in transgenic versus control NOD mice. In-vitro proliferation in response to stimulations is shown for CD4+ T cells (A) and CD8+ T cells (B) from spleens. Results are expressed as the mean of triplicate cultures and representative of three independent experiments. p Values are indicated as: ns, Not Significant, *p < 0.05; **p < 0.01; ***p < 0.001. (C) Cytokine levels in cell culture media. Freshly sorted CD4+ or CD8+ T cells are stimulated with plate-coated anti-CD3 (2 μg/ml) with or without IL-2. After 48 h of culture, supernatant was harvested and used to measure IL-2, IL-10, IFN-γ and TNF-α using Luminex assays. Results are expressed as the mean of triplicate wells and representative of two independent experiments. (D) RT-PCR result for the Bcl-xl gene from freshly sorted CD4+ and CD8+ T cells.
To ascertain the role of Stat5b in cell survival, we measured cellular levels of an anti-apoptotic gene, Bcl-xl, by real-time PCR. Our data shows an up-regulated expression of Bcl-xl gene in freshly isolated transgenic CD4+/CD8+ T cells compared to control cells (Fig. 4D). This observation suggested that Stat5b transgene may regulate T cell proliferation by altering a set of genes involved in cell survival.
4. Discussion
Previously accumulated evidences suggested that an aberrant Stat5b signaling may represent a key defective pathway in NOD mouse. To correct the Stat5b defect, we introduced the wild type Stat5b gene into NOD mice by backcrossing Stat5b-transgenic B6 mouse [15] with NOD mouse. A reduction in diabetes incidence was observed in Stat5b-transgenic NOD mouse, which was further confirmed in CY-induced diabetes models. These results, for the first time, provided direct evidence that aberrant Stat5b is one of the contributors of increased risk of diabetes in NOD mouse. When this deficiency was corrected by supplementing wild type Stat5b into the NOD mouse, the incidence of diabetes was found to be reduced in Stat5b transgenic NOD mouse. Our results presented here suggested that proper correction of the defective Stat5b could reduce and delay the development of diabetes in NOD mice.
We hypothesized that the protective role of Stat5b against diabetes is based on its effects on immune cells, mainly on T cell subsets especially by modulating Treg cells. The deficiency of CD4+CD25+ regulatory T cell population is one of the main immune defect in NOD mice [3,4]. Our observation on increased CD4+CD25+ regulatory T cells may explain the protection conferred by the Stat5b transgene in NOD mice. In fact, transient activation of STAT5 can increase CD25+CD4+ Treg numbers in IL-2 deficient mice, induce Treg cells and maintain self-tolerance in IL-2 knockout mice [16]. We observed increases in both frequency and absolute number of CD4+CD25+ regulatory T cells in transgenic NOD mice compared to their littermate controls. Our results confirm an essential role of Sta5b in maintaining CD4+CD25+ Treg homeostasis and self-tolerance [16–18]. However, Stat5b does not seem to significantly alter the suppressive function of Treg or sensitivity of Tresp cells.
A subset of CD8+ regulatory T cells expressing CD122, CD8+CD122+ T cells was also greatly increased in transgenic mice. It has been reported that CD122 deficient mice exhibit severe hyperimmunity [19] and CD8+CD122+ T cell population contains CD8+ regulatory T cells that can control activated CD8+ or CD4+ T cells both in vivo and in vitro [20]. Although our in vitro suppression assay did not show suppressive function of the CD8+CD122+ T cells on CD8+CD122− T cells (data not shown), additional studies are required to address the specific role of this subset of cells in NOD background. The increased CD8+CD122+ T cells were also reported previously in Stat5b transgenic B6 mice and 55% of these cells were shown to be CD44 high memory T cells [15]. In contrast, only about 2% of the CD8+CD122+ T cells express CD44 in the Stat5b NOD mice.
Increased cellularity in the periphery may be another contributing factor towards the protection against diabetes in transgenic mice since NOD mice are shown to be severely lymphopenic [21]. Indeed, Stat5b deficiency in humans leads to reduced numbers of γδ T-cells and natural killer cells and modest T-cell lymphopenia with a normal CD4/CD8 ratio [22,23]. We hypothesized that expansion of autoreactive lymphocytes are more restricted in transgenic mice than in control mice due to increased peripheral cellularity, or due to antigen induced cell death as reported in Stat5b transgenic B6 mice [15]. Further characterization of CD4+/CD8+ T cell subsets in transgenic NOD mice revealed that Stat5b expression is important for T cell proliferation capability based on the augmentation of in vitro proliferation of both CD4+ and CD8+ T cells from transgenic mice. The production of several cytokines was highly up-regulated in transgenic CD4+ T cells (IL-2, IL-10, INF-γ and TNF-α) and CD8+ T cells (INF-γ and TNF-α) upon in vitro stimulation. IL-2 is the important growth factor for CD4+CD25+ regulatory T cells development and maintenance [24], which may explain the up-regulation of CD4+CD25+ T cells in transgenic mice. The other cytokines are all important in regulation or maintenance of immune response. Besides the enhanced capability of proliferation, the increased splenocyte number also can result from reduction in cell death of T cells. The observation on up-regulation of an anti-apoptosis gene expression, bcl-xl, in both fresh CD4+ and CD8+ T cells in transgenic mice indicates that Stat5b can also regulate a series of survival genes in T cells, which have been observed in survival of other cell types [25].
Although our results mainly shows the effects of Stat5b transgene on Treg cell population, we cannot rule out the potential role of other cell types than T cells including B cells, NK T cells, macrophage and dendritic cells in mediating the protection against diabetes. Since previous reports showed that Stat5b may influence the development of diabetes via its role in macrophage and pancreatic β cells [12–14], future studies focusing on these cells may help elucidate protective role of Stat5b on diabetes.
In summary, this is the first study to provide direct evidence that correcting a Stat5b defect in NOD mice with wild type Stat5b transgene confers protection against diabetes and the protection may be mediated by the up-regulation of CD4+CD25+ regulatory T cells.
Acknowledgments
This work was supported by grants from the National Institutes of Health (4R33HD050196, 4R33-DK069878 and 2RO1HD37800) and JDRF (1-2004-661) to J.-X.S., Sharad Purohit was supported by a JDRF senior postdoctoral fellowship and career development award from JDRF (10-2006-792 and 2-2011-153). J.X.S. is an eminent scholar supported by the Georgia Research Alliance. We thank Dr. Warren J. Leonard for providing Stat5b transgenic C57BL/6 mice.
References
- 1.Anderson MS, Bluestone JA. The NOD mouse: a model of immune dysregulation. Annu. Rev. Immunol. 2005;23:447–485. doi: 10.1146/annurev.immunol.23.021704.115643. [DOI] [PubMed] [Google Scholar]
- 2.Leiter EH. Nonobese diabetic mice and the genetics of diabetes susceptibility. Curr. Diab. Rep. 2005;5:141–148. doi: 10.1007/s11892-005-0042-z. [DOI] [PubMed] [Google Scholar]
- 3.Salomon B, Lenschow DJ, Rhee L, et al. B7/CD28 costimulation is essential for the homeostasis of the CD4+CD25+ immunoregulatory T cells that control autoimmune diabetes. Immunity. 2000;12:431–440. doi: 10.1016/s1074-7613(00)80195-8. [DOI] [PubMed] [Google Scholar]
- 4.Piccirillo CA, Tritt M, Sgouroudis E, et al. Control of type 1 autoimmune diabetes by naturally occurring CD4+CD25+ regulatory T lymphocytes in neonatal NOD mice. Ann. N. Y. Acad. Sci. 2005;1051:72–87. doi: 10.1196/annals.1361.048. [DOI] [PubMed] [Google Scholar]
- 5.Huang Y, Fugier-Vivier IJ, Miller T, et al. Plasmacytoid precursor dendritic cells from NOD mice exhibit impaired function: are they a component of diabetes pathogenesis? Diabetes. 2008;57:2360–2370. doi: 10.2337/db08-0356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Serreze DV, Gaskins HR, Leiter EH. Defects in the differentiation and function of antigen presenting cells in NOD/Lt mice. J. Immunol. 1993;150:2534–2543. [PubMed] [Google Scholar]
- 7.Kataoka S, Satoh J, Fujiya H, et al. Immunologic aspects of the nonobese diabetic (NOD) mouse. Abnormalities of cellular immunity. Diabetes. 1983;32:247–253. doi: 10.2337/diab.32.3.247. [DOI] [PubMed] [Google Scholar]
- 8.Ogasawara K, Hamerman JA, Hsin H, et al. Impairment of NK cell function by NKG2D modulation in NOD mice. Immunity. 2003;18:41–51. doi: 10.1016/s1074-7613(02)00505-8. [DOI] [PubMed] [Google Scholar]
- 9.Wang B, Geng YB, Wang CR. CD1-restricted NK T cells protect nonobese diabetic mice from developing diabetes. J. Exp. Med. 2001;194:313–320. doi: 10.1084/jem.194.3.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davoodi-Semiromi A, Laloraya M, Kumar GP, et al. A mutant Stat5b with weaker DNA binding affinity defines a key defective pathway in nonobese diabetic mice. J. Biol. Chem. 2004;279:11553–11561. doi: 10.1074/jbc.M312110200. [DOI] [PubMed] [Google Scholar]
- 11.Davoodi-Semiromi A, McDuffie M, Litherland S, et al. Truncated pStat5B is associated with the Idd4 locus in NOD mice. Biochem. Biophys. Res. Commun. 2007;356:655–661. doi: 10.1016/j.bbrc.2007.03.028. [DOI] [PubMed] [Google Scholar]
- 12.Litherland SA, Xie TX, Grebe KM, et al. Signal transduction activator of transcription 5 (STAT5) dysfunction in autoimmune monocytes and macrophages. J. Autoimmun. 2005;24:297–310. doi: 10.1016/j.jaut.2005.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Litherland SA, Grebe KM, Belkin NS, et al. Nonobese diabetic mouse congenic analysis reveals chromosome 11 locus contributing to diabetes susceptibility, macrophage STAT5 dysfunction, and granulocyte-macrophage colony-stimulating factor overproduction. J. Immunol. 2005;175:4561–4565. doi: 10.4049/jimmunol.175.7.4561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jackerott M, Moldrup A, Thams P, et al. STAT5 activity in pancreatic beta-cells influences the severity of diabetes in animal models of type 1 and 2 diabetes. Diabetes. 2006;55:2705–2712. doi: 10.2337/db06-0244. [DOI] [PubMed] [Google Scholar]
- 15.Kelly J, Spolski R, Imada K, et al. A role for Stat5 in CD8+ T cell homeostasis. J. Immunol. 2003;170:210–217. doi: 10.4049/jimmunol.170.1.210. [DOI] [PubMed] [Google Scholar]
- 16.Antov A, Yang L, Vig M, et al. Essential role for STAT5 signaling in CD25+CD4+ regulatory T cell homeostasis and the maintenance of self-tolerance. J. Immunol. 2003;171:3435–3441. doi: 10.4049/jimmunol.171.7.3435. [DOI] [PubMed] [Google Scholar]
- 17.Snow JW, Abraham N, Ma MC, et al. Loss of tolerance and autoimmunity affecting multiple organs in STAT5A/5B-deficient mice. J. Immunol. 2003;171:5042–5050. doi: 10.4049/jimmunol.171.10.5042. [DOI] [PubMed] [Google Scholar]
- 18.Burchill MA, Yang J, Vang KB, et al. Interleukin-2 receptor signaling in regulatory T cell development and homeostasis. Immunol. Lett. 2007;114:1–8. doi: 10.1016/j.imlet.2007.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Suzuki H, Kundig TM, Furlonger C, et al. Deregulated T cell activation and autoimmunity in mice lacking interleukin-2 receptor beta. Science. 1995;268:1472–1476. doi: 10.1126/science.7770771. [DOI] [PubMed] [Google Scholar]
- 20.Rifa'i M, Kawamoto Y, Nakashima I, et al. Essential roles of CD8+CD122+ regulatory T cells in the maintenance of T cell homeostasis. J. Exp. Med. 2004;200:1123–1134. doi: 10.1084/jem.20040395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.King C, Ilic A, Koelsch K, et al. Homeostatic expansion of T cells during immune insufficiency generates autoimmunity. Cell. 2004;117:265–277. doi: 10.1016/s0092-8674(04)00335-6. [DOI] [PubMed] [Google Scholar]
- 22.Cohen AC, Nadeau KC, Tu W, et al. Cutting edge: decreased accumulation and regulatory function of CD4+ CD25(high) T cells in human STAT5b deficiency. J. Immunol. 2006;177:2770–2774. doi: 10.4049/jimmunol.177.5.2770. [DOI] [PubMed] [Google Scholar]
- 23.Bernasconi A, Marino R, Ribas A, et al. Characterization of immunodeficiency in a patient with growth hormone insensitivity secondary to a novel STAT5b gene mutation. Pediatrics. 2006;118:e1584–e1592. doi: 10.1542/peds.2005-2882. [DOI] [PubMed] [Google Scholar]
- 24.Burchill MA, Yang J, Vogtenhuber C, et al. IL-2 receptor beta-dependent STAT5 activation is required for the development of Foxp3+ regulatory T cells. J. Immunol. 2007;178:280–290. doi: 10.4049/jimmunol.178.1.280. [DOI] [PubMed] [Google Scholar]
- 25.Socolovsky M, Fallon AE, Wang S, et al. Fetal anemia and apoptosis of red cell progenitors in Stat5a–/–5b–/– mice: a direct role for Stat5 in Bcl-X(L) induction. Cell. 1999;98:181–191. doi: 10.1016/s0092-8674(00)81013-2. [DOI] [PubMed] [Google Scholar]