Abstract
The Periostin Cre (Postn-Cre) lineage includes endocardial and neural crest derived mesenchymal cells of the cardiac cushions, neural crest-derived components of the sympathetic and enteric nervous systems, and cardiac fibroblasts. In this study, we use the Postn-Cre transgenic allele to conditionally ablate Hand2 (H2CKO). We find that Postn-Cre H2CKOs die shortly after birth despite a lack of obvious cardiac structural defects. To ascertain the cause of death, we performed a detailed comparison of the Postn-Cre lineage and Hand2 expression at mid and late stages of embryonic development. Gene expression analyses demonstrate that Postn-Cre ablates Hand2 from the adrenal medulla as well as the sphenopalatine ganglia of the head. In both cases, Hand2 loss-of-function dramatically reduces expression of Dopamine Beta Hydroxylase (Dbh), a gene encoding a crucial catecholaminergic biosynthetic enzyme. Expression of the genes Tyrosine Hydroxylase (Th) and Phenylethanolamine N-methyltransferase (Pnmt), which also encode essential catecholaminergic enzymes, were severely reduced in postnatal adrenal glands. Electrocardiograms demonstrate that 3-day postnatal Postn-Cre H2CKO pups exhibit sinus bradycardia. In conjunction with the aforementioned gene expression analyses, these results strongly suggest that the observed postnatal lethality occurs due to a catecholamine deficiency and subsequent heart failure.
Keywords: Hand2, basic Helix-loop-Helix (bHLH) transcription factor, heart development, sympathetic neurogenesis, bradycardia, heart failure
Introduction
Hand2 is a member of the Twist family of bHLH transcription factors, exhibiting essential roles in both cardiogenesis and sympathetic neuronal development (Hendershot et al., 2008; Howard, 2005; Vincentz et al., 2011). Loss-of-function experiments in mice demonstrate the necessity of Hand2 for proper cardiac neural crest cell migration, ventricular chamber expansion, epicardial differentiation, cell type specific gene expression within both sympathetic and enteric neurons, craniofacial development, and digit formation (Barnes et al., 2011; Firulli et al., 2005; Galli et al., 2010; Hendershot et al., 2008; Holler et al., 2010; Lei and Howard, 2011; McFadden et al., 2005; Morikawa et al., 2007; Srivastava et al., 1997; Tsuchihashi et al., 2011). Although these previous studies have done much to improve our understanding of the role of Hand2 in these tissues, the dynamic spatiotemporal expression profile of Hand2 suggests the existence of additional functions, which remain unexplored.
Hand2 is strongly expressed within both the endocardium of the heart tube (Barnes et al., 2011), and the cardiac neural crest (Holler et al., 2010). The early heart tube consists of an inner endocardial layer and an outer myocardial layer separated by extracellular matrix referred to as cardiac jelly (Abu-Issa and Kirby, 2007). As development proceeds the heart tube loops, expands, and septates to form the distinct ventricular and atrial chambers. A subset of endocardial cells simultaneously undergo an epithelial to mesenchymal transition (EMT) and migrate into the atrioventricular (AV) cushions, which will subsequently remodel into the tricuspid and mitral valves of the heart (VanDusen and Firulli, 2012). Concurrently, cardiac neural crest ectomesenchyme invades the outflow tract (OFT) cushions, which, along with some endodermally-derived cells, will remodel into the aortic and pulmonary valves (Keyte and Hutson, 2012). Thus, Hand2 is strongly expressed in all of the cellular progenitors of all cardiac valves.
Hand2 is also strongly expressed within the neurons of the sympathetic chain, where it is required to both induce and maintain expression of genes encoding the biosynthetic enzymes that produce nor-epinephrine (Hendershot et al., 2008; Howard et al., 1999). Additionally, Hand2 expression within the catecholaminergic cells of the adrenal medulla has been reported (Wildner et al., 2008), but the role of Hand2 in this tissue remains unknown.
To determine the role, if any, that Hand2 plays during later stages of embryonic development, we employed the Postn-Cre transgenic allele (Lindsley et al., 2007; Takeda et al., 2010) to conditionally delete Hand2. Postn-Cre lineage overlaps with Hand2 expression within populations of post-migratory cardiac neural crest and some neural crest-derived components of the autonomic nervous system, as well as the endocardial derived mesenchymal cells of the endocardial cushions (Lindsley et al., 2007; Takeda et al., 2010; VanDusen and Firulli, 2012). Interestingly, these H2CKOs do not exhibit detectable cardiac phenotypes within the OFT, and exhibit normally formed tricuspid and mitral valves. Despite the lack of cardiac phenotypes, H2CKOs die within 10 days of birth. A low incidence of Postn-Cre–independent cleft palate in Hand2fx/fx offspring contributes to some of the observed H2CKO lethality, but this hypomorphic phenotype does not account for the complete penetrance of neonatal lethality observed. To better understand the mechanisms which underlie the major causes of H2CKO lethality, we performed detailed Postn-Cre lineage-trace analyses and directly compared these findings with Hand2 expression during mid-(E12.5) and late-stage (E16.5) embryonic development. These studies reveal that H2CKOs retain Hand2 and Dopamine-β Hydroxylase (Dbh) expression within the ganglia of the sympathetic chain; however, Postn-Cre efficiently ablates Hand2 expression within the sphenopalatine ganglia and the catecholaminergic cells of the adrenal medulla, an organ that, via its catecholamine production, regulates cardiac homeostatic functions such as blood pressure, metabolism, and heart rate (Axelrod and Reisine, 1984; Fung et al., 2008). Loss of Hand2 function in the adrenal medulla and sphenopalatine ganglia results in a corresponding large decrease in Dbh levels, as well as a drop in levels of Th and Pnmt expression within adrenal glands. We show that this downregulation of genes encoding enzymes crucial for catecholamine synthesis has a functional effect on the heart rates of 3-day postnatal (P3) H2CKO pups. In addition, we demonstrate that H2CKOs suffer from impaired gastrointestinal motility, which may also contribute to neonatal lethality.
Materials and methods
Mice
Postn-Cre(+) mice (Lindsley et al., 2007) were crossed with Hand2fx/fx (Morikawa et al., 2007) mice to generate Postn-Cre(+);Hand2fx/+ males. These males were then crossed with Hand2fx/fx;ROSA26R lacZ (R26Rz/z) reporter mice to generate conditionally null Hand2 embryos. Genotyping of ROSA26R and Hand2 conditional alleles was carried out as previously described (Barnes et al., 2011). Similarly, Nestin-Cre(+);Hand2+/− males were crossed with Hand2fx/fx;ROSA26R YFP reporter females to generate conditionally null Hand2 embryos. Nestin-Cre mice were genotyped as previously described (Tronche et al., 1999). Postn-Cre mice were genotyped by southern blot with a probe corresponding to an EcoRI fragment of the pTurbo-Cre cDNA.
Section RNA In Situ Hybridization and quantitative RT-PCR
Antisense digoxygenin labeled riboprobes were transcribed with T7, SP6, or T3 (Roche). Section in situ hybridization was performed as previously described (Vincentz et al., 2008). Analysis was performed on a minimum of 3 somite-matched embryos for each probe and genotype. For quantitative RT-PCR (qRT-PCR) total RNA was isolated from flash-frozen adrenal glands using the High Pure RNA Isolation Kit (Roche). This RNA served as a template to generate cDNA using the Transcriptor First Strand cDNA Synthesis Kit (Roche). cDNA was amplified using Taqman Probe-Based Gene Expression Assays (Applied Biosystems). Relative gene expression was determined after normalization to GAPDH. Three samples were collected per genotype. The Student’s t-test was used to detect significant differences between sample groups, with P-values ≤ 0.05 considered significant.
Histological Preparations
Histology and X-gal staining were conducted essentially as previously described for paraffin embedded embryos (Vincentz et al., 2008). For Alcian Blue staining of endocardial cushions, xylene cleared paraffin embedded embryos were sectioned at 10μm and stained using 0.15mg/ml Alcian Blue in 5% glacial acetic acid. Results reflect an n of 3 or more.
Electrocardiograms
P3 pups were anesthetized with 4% isoflurane before electrode placement. Four percent isoflurane was continuously supplied via facemask and environmental temperature was controlled via heat lamp. ECGs were collected and processed as described previously (Shen et al., 2008). Results reflect n = 4; as previous studies have established that catecholamine insufficiency would only be expected to result in a decreased heart rate, p-value was calculated using a student’s one tailed t-test.
Results
H2CKOs exhibit normal cardiac valve development
Timed matings between Postn-Cre;Hand2fx/+ males and Hand2fx/fx;R26Rz/z females were conducted and the embryos collected at various time points from mid-gestation to birth. As Postn-Cre activity is evident by E11.5 (Lindsley et al., 2007), hearts from E12.5 embryos were analyzed for β-galactosidase activity to compare Cre lineage in relation to Hand2 expression. The enhancer controlling Postn-Cre activity is reportedly induced at approximately E10.5 in mesenchymal cells of the cardiac cushions (Lindsley et al., 2007). As expected, β-galactosidase staining within the E12.5 heart robustly marks the AV cushion (black arrow Fig. 1A) and OFT cushion mesenchyme (black arrow Fig. 1D), as well as a small population of cells within the epicardium from which a population of cardiac myofibroblasts are derived (Fig. 1A, D). Hand2 in situ hybridizations (ISH) similarly confirm that Hand2 expression overlaps with Postn-Cre lineage in each of these cardiac cell populations, but Postn-Cre lineage does not overlap with Hand2 expression within the endocardium (Fig. 1A–C). Hand2 ISH of H2CKO embryos shows significant Postn-Cre-mediated deletion of Hand2 within both the cardiac OFT and AV cushions (asterisks in Fig. 1C, F), in contrast to unaffected Hand2 expression within the endocardium and epicardium.
Figure 1. Postn-Cre mediated deletion of Hand2 from AV and OFT cushions.
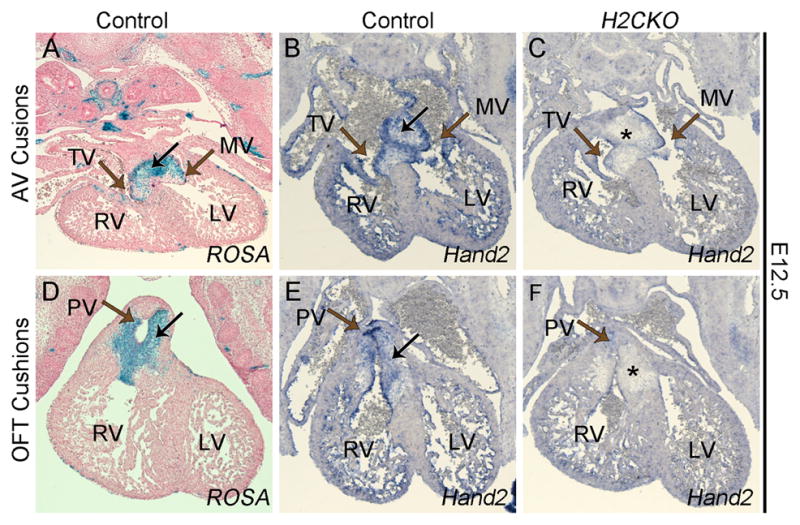
Transverse section of Postn-Cre(+) lacZ stained control AV cushion at E12.5 (A). Hand2 ISH of control (B) and H2CKO (C) AV cushion. Transverse section of Postn-Cre(+) β-galactosidase stained control OFT cushions at E12.5 (D). Hand2 ISH of control (E) and H2CKO (F) OFT cushion. Black arrows indicate cushion mesenchyme, brown arrows indicate the primitive tricuspid valve (TV), mitral valve (MV), and pulmonary valve (PV). Asterisks indicate deletion of Hand2, right ventricle, RV; left ventricle, LV.
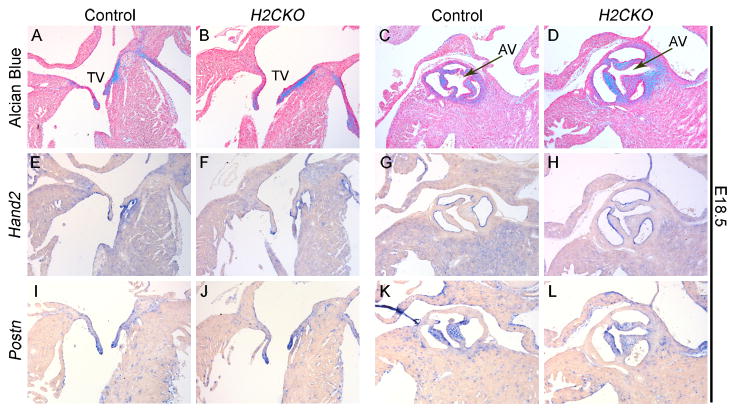
H2CKOs are viable at birth, but die within the first 10 days of life (Table 1). A small portion of Hand2fx/fx pups die from cleft palate (Sup. Fig. 1, Table 2); however, the occurrence of the palate defect is independent of the presence of the Cre allele, and results from hypomorphic expression, which has been previously reported with this Hand2 conditional allele (Morikawa et al., 2007). To determine whether H2CKOs exhibit aortic or AV valve phenotypes, E18.5 hearts were sectioned and analyzed by Alcian Blue staining, which marks proteoglycans within the heart valves (Fig. 2A–D). No significant differences in size or shape between control and H2CKO cardiac valves are observed. Alcian Blue staining reveals comparable proteoglycan levels and distribution in control (Fig. 2A, C) and H2CKO valves (Fig. 2B, D). E18.5 Hand2 ISH reveals that Hand2 expression is restricted to the endocardium overlying both OFT and AV canal valves, and is not detectable within the valve mesenchyme of either control (Fig. 2E, G) or H2CKO (Fig. 2F, H) leaflets. Postn ISH shows no significant difference in Postn expression within the mesenchymal cells of the cushions or within the cardiac fibroblasts between control (Fig. 2I, K) and H2CKO (Fig. 2J, L) embryos. These data indicate that Hand2, within the Postn-Cre lineage, has no obvious cell-autonomous role in OFT or AV cushion remodeling.
Table 1. H2CKOs die shortly after birth.
Genotypes of embryos and pups collected at various stages of development. Expected number in parentheses.
| Postn-Cre(−) | Postn-Cre(+);Hand2fx/+ | Postn-Cre(+);Hand2fx/fx | |
|---|---|---|---|
| E12.5 | 21 (22) | 11 (11) | 13 (11) |
| E14.5 | 7 (5) | 1 (3) | 3 (3) |
| E16.5 | 9 (7) | 4 (4) | 1 (4) |
| E18.5 | 30 (29) | 15 (15) | 13 (15) |
| P10 | 29 (23) | 16 (11) | 0 (11) |
Table 2. Penetrance of Cleft Palate in Hand2fx/fx embryos.
Embryos assessed from E14.5 to E18.5.
| Postn-Cre(−); Hand2fx/+ | Postn-Cre(−); Hand2fx/fx | Postn-Cre(+);Hand2fx/+ | Postn-Cre(+);Hand2fx/fx | |
|---|---|---|---|---|
| Total Analyzed | 10 | 12 | 10 | 10 |
| # with Cleft Palate | 0 | 2 | 0 | 2 |
Figure 2. Postn-Cre H2CKO heart valves are normal at E18.5.
Alcian blue stained section of control (A) and H2CKO (B) tricuspid valve. Alcian blue stained section of control (C) and H2CKO (D) aortic valve. Hand2 ISH of control (E) and H2CKO (F) tricuspid valve. Hand2 ISH of control (G) and H2CKO (H) aortic valve. Postn ISH of control (I) and H2CKO (J) tricuspid valve. Postn ISH of control (K) and H2CKO (L) aortic valve. TV, tricuspid valve; AV, aortic valve.
Postn-Cre ablates Hand2 from the enteric nervous system
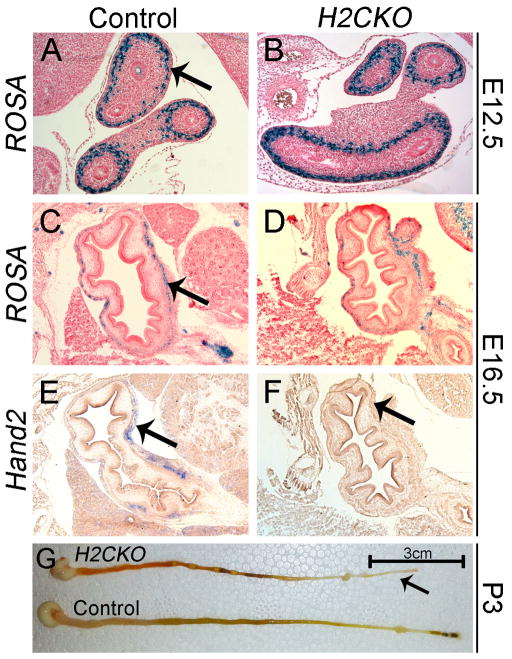
It has been demonstrated that Hand2 function is required for many aspects of proper enteric nervous system development, including neurogenesis, cell type specification, proliferation of enteric precursor cells, and gangliogenesis (D’Autreaux et al., 2011; Hendershot et al., 2007; Lei and Howard, 2011). As these functions may be impaired in H2CKOs, we next looked for defects within the developing gut. Lineage tracing at E12.5 and E16.5 shows that Postn-Cre indeed marks the myenteric plexus in the stomachs and intestines of control (Fig. 3A, C) and H2CKO embryos (Fig. 3B, D). Similarly, ISH at E16.5 confirms previous reports (Hendershot et al., 2008; Lei and Howard, 2011; Wu and Howard, 2002) that Hand2 is also expressed within these enteric neurons (Fig. 3E). Furthermore, this expression is ablated in H2CKOs (Fig. 3F). To determine if this loss of Hand2 affects viability, we dissected out and visually assessed the gastrointestinal tracts of P3 pups (Fig. 3G). While deletion of Hand2 in enteric neural precursor cells results in gut obstruction and severe bowel distention by P20 (Lei and Howard, 2011), P3 Postn-Cre H2CKOs displayed a lack of fecal matter posterior to the cecal appendages (arrow Fig. 3E). This is indicative of impaired bowel motility and may contribute to the observed early neonatal death in H2CKOs.
Figure 3. Postn-Cre is co-expressed with Hand2 within the enteric nervous system.
B-galactosidase stained sections of control (A) and H2CKO (B) intestines at E12.5. B-galactosidase stained sections of control (C) and H2CKO (D) stomachs at E16.5. Hand2 section ISH of control (E) and H2CKO (F) stomachs at E16.5. Dissected out gastrointestinal tract of control and H2CKO P3 pups (G). Arrows in (A, C, E) indicate myenteric plexus, arrow in (F) indicates deletion of Hand2, arrow in (G) indicates lack of fecal matter posterior to the cecal appendage in H2CKOs.
H2CKOs display a loss of Dbh expression within sphenopalatine ganglia
We next carefully compared Postn-Cre lineage with Hand2 expression in the sympathetic chain. It has been previously demonstrated that Hand2 is required for early development of sympathetic neurons. Neural crest-specific H2CKO embryos die at approximately E11.0 from low levels of catecholamines, and can be pharmacologically rescued by administration of catecholamine intermediates (Hendershot, Liu et al. 2008). Wholemount analysis at E12.5 reveals robust Cre activity in components of the peripheral nervous system, as well as in and around the developing nasal cavity and maxilla (data not shown). As the Postn-Cre lineage is reported to include Schwann cells surrounding the sympathetic ganglia (Lindsley et al., 2007), we examined the sympathetic ganglia of H2CKOs at E12.5, when Wnt1-Cre generated H2CKOs die from lack of catecholamines (Hendershot, Liu et al. 2008). Postn-lineage analysis in transverse sections at the level of the heart confirms activation of the ROSA reporter within the cells surrounding the sympathetic ganglia (Fig. 4B, C). Although the cells enveloping the trunk sympathetic ganglia were clearly marked, ISH demonstrates that expression of Hand2 (Fig. 4D, E) as well as Dbh (Fig. 4F, G), a gene known to be directly regulated by Hand2 (Rychlik et al., 2003; Xu et al., 2003), is unaffected when control and H2CKO embryos are compared. Further immunohistochemical analyses using the Nestin-Cre (Tronche et al., 1999) and a YFP reporter allele at E14.5 indicate that the Postn-Cre(+) population of cells surrounding the trunk ganglia are also selectively marked by the Nestin-Cre lineage (Sup. Fig. 2A–I). ISH shows that ablation of Hand2 within the Nestin-Cre lineage does not result in loss of Hand2 within trunk ganglia cells (Sup. Fig. 2J, M, P), while expression of the pan-neuronal marker Hu is also not affected (Sup. Fig. 2K, N, Q).
Figure 4. Postn-Cre mediates deletion of Hand2 within cells surrounding the sympathetic trunk and within the sphenopalatine ganglia at E12.5.
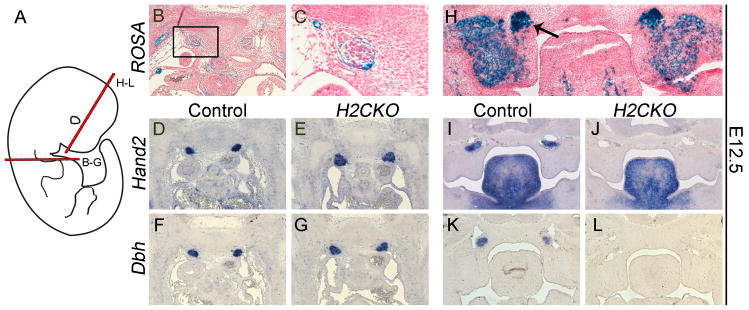
Schematic showing planes of section (A). B-galactosidase stained sections of control sympathetic ganglia (B, C). Hand2 ISH sections of control (D) and H2CKO (E) sympathetic ganglia. Dbh section ISH of control (F) and H2CKO (G) sympathetic ganglia. B-galactosidase stained frontal sections of sphenopalatine ganglia (arrow, H). Hand2 ISH of sphenopalatine ganglia in control (I), and H2CKO (J) embryos. Dbh ISH in control (K), and H2CKO (L) embryos. B-galactosidase staining and ISH was conducted at E12.5
Interestingly, more detailed analyses of E12.5 heads reveal that the Postn-Cre lineage is not restricted to cells on the outer surface, but also marks the neurons within parasympathetic sphenopalatine ganglia (arrow; Fig. 4H). Despite being commonly considered parasympathetic and having a parasympathetic root, the sphenopalatine ganglia have an additional sympathetic root derived from the cervical sympathetic ganglia (Coppola et al., 2010). The sphenopalatine ganglia are known to express Dbh, with levels peaking at E12.5 and gradually being downregulated (Hirsch et al., 1998). In the rat, at least a subpopulation of sphenopalatine cells express TH and produce catecholamines, while most of the cells produce low levels of TH but do not produce catecholamines (Leblanc and Landis, 1989). Projections from the sphenopalatine ganglia are known to innervate the lacrimal gland, and regulate blood flow to the nasal mucosa, while additional evidence suggests a role in regulating cerebral blood flow (Suzuki et al., 1990; Ter Laan et al., 2013). ISH of frontal sections reveals that Hand2 is robustly expressed within the sphenopalatine ganglia of control embryos (Fig. 4I), and its expression is ablated in H2CKOs (Fig. 4J). This loss of Hand2 is accompanied by a loss of Dbh, which is likely dependent on Hand2 function (Fig. 4K, L). This data suggested that, despite normal Hand2 expression within the mid-gestation sympathetic trunk, neonatal H2CKOs may exhibit a reduction in catecholamine biosynthesis outside the sympathetic chain, or possibly within trunk ganglia due to a later stage deletion. We thus sought to examine additional tissues at later embryonic time points, to better define all of the sources of norepinephrine and epinephrine that might be compromised in H2CKOs.
Late-stage Cre activity does not affect the sympathetic chain, but mediates the deletion of Hand2 from the adrenal medulla
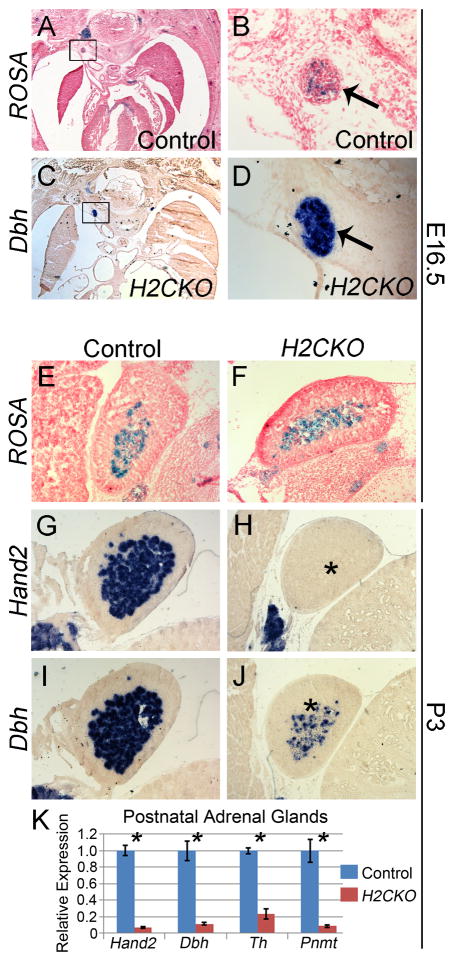
To determine whether the Postn-Cre lineage, which is initially restricted to cells surrounding the trunk ganglia, later expands expression to include the ganglia neurons, ROSA reporter staining was conducted at E16.5. β-galactosidase staining indicates that the Postn-Cre lineage includes only a small subpopulation of neurons within sympathetic trunk ganglia (Fig. 5A, B). Importantly, Hand2 (data not shown) and Dbh (Fig. 5C, D) expression within the sympathetic trunk remains robust in H2CKOs. In the neonate and the adult, the adrenal medulla is the primary site for synthesis of circulating catecholamines (Malmejac, 1964). ROSA reporter staining at E16.5 indicates that Postn-Cre lineage includes cells of control (Fig. 5E) and H2CKO (Fig. 5F) adrenal medulla. Control and H2CKO adrenal glands were then collected from P3 pups, and analyzed by ISH. Hand2 (Fig. 5G) and Dbh (Fig. 5I) are both robustly expressed within control adrenal medulla at P3, whereas Postn-Cre efficiently ablates Hand2 (Fig. 5H), resulting in downregulation of Dbh, which would be expected to reduce catecholamine biosynthesis (Fig. 5J) in H2CKOs. Furthermore, we confirmed these results by collecting additional P3 adrenal glands, isolating RNA, and conducting qRT-PCR. In H2CKOs Hand2 expression was reduced to approximately 7% of control levels (p-value = 0.004), while Dbh levels were reduced to approximately 11% (p-value = 0.023). Expression of Th, which encodes the enzyme responsible for conversion of L-tyrosine to a dopamine precursor, and Pnmt, which encodes the enzyme responsible for converting norepinephrine to epinephrine, was reduced to approximately 23% (p-value = 0.001) and 9% (p-value = 0.016) respectively (Fig. 5K)
Figure 5. Expression of Dbh within sympathetic ganglia of H2CKOs is maintained at late stages of embryonic development, but Postn-Cre does ablate Hand2 expression within the adrenal medulla, resulting in downregulation of Dbh, Th, and Pnmt.
B-galactosidase stained sections of control sympathetic trunk ganglia (A) and magnification (B) at E16.5. Dbh ISH of H2CKO sympathetic trunk ganglia (C), and magnification (D) at E16.5. Arrows in (B, D) indicate ganglia. B-galactosidase stained sections of control (E), and H2CKO (F) adrenal medulla at E16.5. Hand2 ISH of control (G) and H2CKO (H) adrenal medulla at P3. Dbh ISH of control (I) and H2CKO (J) adrenal medulla at P3. qRT-PCR of Hand2, Dbh, Th, and Pnmt in isolated P3 adrenal glands (K). Asterisks in (H, J) indicate reduction of Hand2 and Dbh respectively. Asterisks in (K) indicate p-values less than 0.05.
Postn-Cre H2CKO Pups exhibit bradycardia
When observing H2CKO pups, it is clear that the animals are significantly smaller by P3 and exhibit a failure to thrive (Fig. 6A). Given the lack of cardiac structural defects, and the identification of multiple catecholaminergic Hand2-expressing cell populations that overlap with the Postn-Cre lineage, a catecholamine deficiency in conjunction with gastrointestinal dysfunction is the most likely cause of the lethal failure to thrive phenotype. As catecholaminergic stimulation is essential for proper cardiac function, we tested this hypothesis by conducting electrocardiograms (ECGs) on P3 pups, and thereby assessing heart rates. After assessing four mutants, and an equal number of littermate controls (Fig. 6B and C), we determined that Postn-Cre H2CKOs had significantly slower sinus node rates (P-value = 0.019, Fig. 6D), a condition commonly referred to as bradycardia. Control pups averaged 466 beats per minute (bpm), while Postn-Cre H2CKOs averaged only 337 bpm. In addition to heart rate, the ECG traces were used to calculate the average amount of time between the start of atrial activation and the start of ventricular activation (PR interval), but no significant difference was noted (Fig. 6E). Similarly, the duration of atrial depolarization (P-wave duration), and duration of ventricular depolarization (QRS complex duration) were measured, with no significant difference found between controls and H2CKOs (Fig. 6E). To determine if H2CKOs were experiencing heart failure we examined expression of Nppa (atrial natriuretic factor; Anf), which becomes upregulated in ventricular muscle when undergoing stress (Edwards et al., 1988). Compared to the highly restricted atrial expression of Anf in control hearts (Fig. 6F) a clear marked increase in ventricular Anf expression is observed in H2CKO hearts (Fig. 6G; see Supplemental Fig. 3 for additional sections), supporting heart failure as a cause of H2CKO neonatal lethality.
Figure 6. Postn-Cre H2CKO pups have significantly slower heart rates and expanded Anf expression.
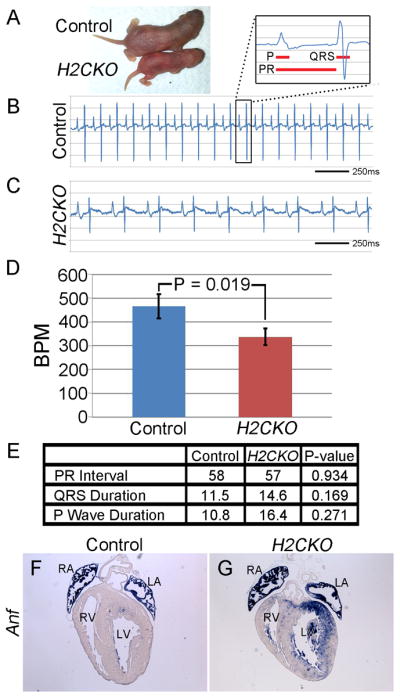
H2CKO pups that survive to P3 are noticeably smaller than littermates (A). Representative ECG trace for P3 control (B) and H2CKO (C) pups. Panel (B) insert represents one cycle of cardiac contraction. Red bars denote the portion of the trace measured for P-wave duration, PR interval, and QRS complex duration as labeled. Control pups averaged 466 bpm, while H2CKO pups averaged 337 bpm (D). P = p-value. Differences in average PR interval (ms), QRS complex duration (ms), and P wave duration (ms) between control and H2CKO P3 pups were not statistically significant (E). Anf ISH in P3 control (F) and H2CKO (G) hearts.
Discussion
This study demonstrates that, in addition to the established early embryonic roles Hand2 plays in cardiac morphogenesis and the development and function of the sympathetic ganglia, Hand2 plays important homeostatic post-embryonic functions in the production of catecholamines. Deletion of Hand2 from mesenchymal cells of the cardiac cushions at E11.5, well after initiation of Hand2 expression in endocardial precursors, does not result in any cardiac valve phenotypic abnormalities. This data supports a model in which essential Hand2 function likely lies within the ventricular endocardium. Hand2 expression within the early stage cushion mesenchyme is gradually downregulated, and by E18.5, Hand2 expression within the valves is restricted to only the Postn-Cre-negative endothelium covering the valve surface. We conclude that Hand2 does not play a critical role in endocardial- or neural crest-derived cushion cells post EMT. It is interesting that Hand2 expression is robustly sustained in overlying valve endocardium, particularly on the backside of valves (ventricular side in AV canal, non-ventricular side in OFT; Fig. 2E–H). These data do not rule out a possible non cell-autonomous role in cushion remodeling, through endothelial to mesenchymal signaling.
Hand2 expression and Postn-Cre lineage also overlap within components of the enteric nervous system. Previous studies of enteric function show that ablation of Hand2 within enteric neural precursors via the Nestin-Cre, which is initiated by E11.5, results in a severe bowel distention by P20 (Lei and Howard, 2011). The data in the current study show that Postn-Cre is initiated by E12.5, making it possible that Postn-Cre H2CKOs share the same gastrointestinal dysfunction as Nestin-Cre H2CKOs. Indeed, a lack of fecal matter posterior to cecal appendages of Postn-Cre H2CKOs indicates that decreased gastrointestinal motility could be contributing to neonatal lethality. Interestingly, this lack of motility resembles a human congenital motility disorder called Hirschsprung’s disease, which is pathologically characterized by a lack of enteric ganglia in a variable stretch of the distal bowel wall. While Postn-Cre H2CKOs do not appear to suffer from a complete loss of enteric ganglia (Fig. 3D), it is possible that a reduction in gangliogenesis within specific regions of the gut has evaded our detection. Indeed, as Nestin-Cre H2CKOs suffer from a functional aganglionosis (Lei and Howard, 2011), and the Postn-Cre enteric lineage closely resembles that of the Nestin-Cre, this would not be surprising; however, as the Nestin-Cre H2CKOs survive until P20, while H2CKOs generated by the Postn-Cre do not, the involvement of additional non-enteric phenotypes is likely.
It is well established that Hand2 expression within the sympathetic chain is required for neurons to acquire and maintain a catecholaminergic phenotype (Hendershot et al., 2008; Schmidt et al., 2009). Loss of Hand2 within the sympathetic chain results in downregulation of the crucial biosynthetic enzymes Tyrosine Hydroxylase and Dbh (Hendershot et al., 2008). For a more complete description of Hand2’s role within the sympathetic nervous system, as established by various manipulations of Hand2 expression within multiple genetic systems, see Table 3. Our results confirm previous data showing that Postn-Cre expression is restricted to the cells surrounding sympathetic chain ganglia at E12.5 (Lindsley et al., 2007). As expected, Hand2 expression within the trunk sympathetic ganglia is unaffected in H2CKOs, at both E12.5 and E16.5. Dbh expression is similarly unaffected, indicating that trunk sympathetic ganglia in Postn-Cre H2CKOs produce sufficient concentrations of norepinephrine and epinephrine for embryonic survival to birth. However, we observed that Hand2 expression and the Postn-Cre lineage overlap within the sphenopalatine ganglia at E12.5, and ablation of Hand2 results in a dramatic decrease in Dbh expression in these ganglia. Given that the sphenopalatine ganglia is reported to have a sympathetic root derived from the superior cervical sympathetic ganglion, in addition to parasympathetic and sensory roots, the observation of Dbh downregulation is not surprising, and suggests that dysfunction within these ganglia could be contributing to the early neonatal death in H2CKOs.
Table 3.
Hand2 function within the sympathetic nervous system.
| Study | Model | Phenotype/Results | Conclusions |
|---|---|---|---|
| (Morikawa et al., 2005) | P19-embryonic carcinoma (P19-EC) cells stably expressing Hand2 (P19-H2) | Retinoic acid treated P19-H2 cells but not P19-EC cells express peripherin (a peripheral nervous system marker), and a subset of these co-express Th. | Ectopic Hand2 expression is able to activate the sympathetic nervous system developmental program within P19-EC cells. |
| (Lucas et al., 2006) | Zebrafish hand2 deletion mutant (hands off) | Sympathetic precursor cells aggregate to form normal sympathetic ganglion primordial, but th and dbh expression is strongly reduced | Generic neuronal differentiation is unaffected, but noradrenergic differentiation of sympathetic neurons is impaired |
| (Morikawa et al., 2007) | Mouse Wnt1-Cre Hand2 CKO | Death at E12.5 with cardiovascular and craniofacial defects. Sites of sympathetic development are populated by neural crest cells, which express pan-neuronal markers. Th and Dbh expression is dramatically reduced. | Hand2 permits sympathetic neurons to acquire a catecholaminergic phenotype |
| (Hendershot et al., 2008) | Mouse Wnt1-Cre Hand2 CKO | See above; H2CKOs exhibit a significant and progressive loss of sympathetic neurons. | Hand2 affects generation of the neural precursor pool by affecting proliferative capacity of progenitors and by regulating expression of transcription factors necessary for noradrenergic neuronal differentiation |
| (Morikawa and Cserjesi, 2008) | Mouse Wnt1-Cre Hand2 CKO | See above; early embryonic lethality could be rescued by administration of isoproterenol, a β-adrenoceptor agonist | Noradrenergic deficiency alone accounts for early embryonic lethality of Wnt1-Cre H2CKOs |
| (Schmidt et al., 2009) | Reduction/ablation of Hand2 within differentiated sympathetic neurons by siRNA in cultured chick sympathetic neurons, and Dbh-Cre in Hand2 conditional mice | Large decrease in Th and Dbh expression within Hand2 siRNA treated chick sympathetic neurons. Pan-neuronal genes were not affected, while expression of cholinergic marker genes was enhanced. Dbh-Cre H2CKO mice showed decreased numbers of sympathetic neurons, and large reduction in Th expression | Hand2 plays a key role in maintaining noradrenergic properties in differentiated neurons |
Interestingly, Hand2 expression and the Postn-Cre lineage overlap within the catecholaminergic cells of the adrenal medulla. Similar to what is observed in sphenopalatine ganglia, ablation of Hand2 within the adrenal medulla also results in a dramatic reduction of Dbh. ISH of H2CKO adrenal glands indicates that a small population of cells maintains Dbh expression, while the vast majority of expression within the adrenal medulla is lost. This is consistent with Hand2 loss of function studies within the sympathetic chain, where Dbh is known to be a direct Hand2 target (Rychlik et al., 2003; Vincentz et al., 2013; Xu et al., 2003). Furthermore, qRT-PCR shows that H2CKO adrenal glands exhibit significant decreases in Th and Pnmt, which like Dbh, encode enzymes that catalyze essential steps in the synthesis of the catecholamine adrenalin. In a study of Dbh−/− embryos, it was stated that while most die in utero, approximately 12% survive until birth. The 12% survival was attributed to a flow of catecholamines across the placenta, thus also explaining why of those that survive to birth, 40% die by P2 (Thomas et al., 1995). These data make it tempting to consider that Postn-Cre H2CKOs may have enough catecholaminergic biosynthetic capability to survive to birth as a result of unaffected sympathetic chain ganglia and supplementation from the mother, but at birth this supplementation ceases, leading to lethal catecholamine deficiency. It is well established that the adrenal medulla is a particularly important source of catecholamines that regulate heart rate, blood pressure, blood vessel constriction, and other critical aspects of cardiovascular function (Axelrod and Reisine, 1984; Fung et al., 2008). These data suggest that catecholamine deficiency, in conjunction with an enteric phenotype, causes H2CKO postnatal lethality. Heart rates in H2CKOs are significantly slower than control littermates. This phenotype is consistent with previous publications on genetic models of catecholamine deficiency, such as Epas1 null mice, which are reported to have low catecholamine levels and a pronounced bradycardia (Tian et al., 1998), as well as Th null embryos, which are reported to have a 28% reduction in heart rate (Ream et al., 2008). In contrast, no difference in PR interval was observed between controls and H2CKOs, and while P-wave duration and QRS complex duration both trended toward an increase, no significant differences were observed, indicating that conditional ablation of Hand2 does not alter dromotropic properties of the P3 heart. The reason for the differential effects of Hand2 deficiency on chronotropy versus dromotropy is unclear. It may involve differential expression of beta-adrenergic receptors and/or their downstream targets in sinoatrial nodal cells versus cells of the cardiac specific conduction system. The decreased heart rates observed in P3 H2CKOs support the conclusion that a catecholaminergic insufficiency, in addition to enteric dysfunction, results in the neonatal H2CKO failure to thrive. In further support of this conclusion, upregulation of ventricular Anf expression in H2CKO hearts indicates pathological changes within these cardiomyocytes that ultimately lead to cardiac failure.
Conclusion
This study establishes that in post-migratory/post-EMT cushion mesenchyme Hand2 function is not required for proper valve development. Postn-Cre H2CKOs survive till birth, but fail to thrive and die soon after. While our analyses reveal an absence of cardiac or sympathetic chain phenotypes, Hand2 is clearly ablated from the adrenal medulla and sphenopalatine ganglia where, similar to the sympathetic trunk, Hand2 plays an important role in regulating Dbh expression. The reduction of Dbh, Th, and Pnmt in H2CKOs indicates that pups likely have a decreased ability to synthesize catecholamines, resulting in the observed postnatal lethality. Both ECG data showing that P3 H2CKOs have slower heart rates than control littermates and upregulation of Anf within the ventricles of H2CKOs supports this conclusion, as catecholaminergic sympathetic stimulation is essential for proper cardiac function.
Supplementary Material
Highlights.
Postn-Cre(+);Hand2fx/fx mice die neonatally
Mesenchymal Hand2 function is dispensable in the formation of the cardiac valves
Postn-Cre ablates Hand2 from sphenopalatine ganglia and the adrenal medulla
Hand2 ablation results in a loss of Dopamine Beta Hydroxylase expression
Postn-Cre(+);Hand2fx/fx mice exhibit bradycardia and fail to thrive, resulting in death.
Acknowledgments
We thank Danny Carney and Nichole Northrop for technical assistance and support, and Simon J. Conway (IUPUI) for supplying Postn-Cre mice. We also thank the Riley Heart Research Center Group for discussion and helpful feedback. Infrastructural support at the Herman B Wells Center is partially supported by the Riley Children’s Foundation and Division of Pediatric Cardiology. Grant support for this work was provided by: NIH R01 AR061392-03, R01 HL122123, and R01 HL120920 (ABF) and R01NS040644 (MJH)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- Abu-Issa R, Kirby ML. Heart field: from mesoderm to heart tube. Annu Rev Cell Dev Biol. 2007;23:45–68. doi: 10.1146/annurev.cellbio.23.090506.123331. [DOI] [PubMed] [Google Scholar]
- Axelrod J, Reisine TD. Stress hormones: their interaction and regulation. Science. 1984;224:452–459. doi: 10.1126/science.6143403. [DOI] [PubMed] [Google Scholar]
- Barnes RM, Firulli BA, VanDusen NJ, Morikawa Y, Conway SJ, Cserjesi P, Vincentz JW, Firulli AB. Hand2 Loss-of-Function in Hand1-Expressing Cells Reveals Distinct Roles in Epicardial and Coronary Vessel Development. Circulation Research. 2011;108:940–949. doi: 10.1161/CIRCRESAHA.110.233171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppola E, Rallu M, Richard J, Dufour S, Riethmacher D, Guillemot F, Goridis C, Brunet JF. Epibranchial ganglia orchestrate the development of the cranial neurogenic crest. Proc Natl Acad Sci U S A. 2010;107:2066–2071. doi: 10.1073/pnas.0910213107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Autreaux F, Margolis KG, Roberts J, Stevanovic K, Mawe G, Li Z, Karamooz N, Ahuja A, Morikawa Y, Cserjesi P, Setlick W, Gershon MD. Expression level of Hand2 affects specification of enteric neurons and gastrointestinal function in mice. Gastroenterology. 2011;141:576–587. 587 e571–576. doi: 10.1053/j.gastro.2011.04.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards BS, Ackermann DM, Lee ME, Reeder GS, Wold LE, Burnett JC., Jr Identification of atrial natriuretic factor within ventricular tissue in hamsters and humans with congestive heart failure. J Clin Invest. 1988;81:82–86. doi: 10.1172/JCI113314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firulli BA, Krawchuk D, Centonze VE, Vargesson N, Virshup DM, Conway SJ, Cserjesi P, Laufer E, Firulli AB. Altered Twist1 and Hand2 dimerization is associated with Saethre-Chotzen syndrome and limb abnormalities. Nat Genet. 2005;37:373–381. doi: 10.1038/ng1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung MM, Viveros OH, O’Connor DT. Diseases of the adrenal medulla. Acta Physiol (Oxf) 2008;192:325–335. doi: 10.1111/j.1748-1716.2007.01809.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galli A, Robay D, Osterwalder M, Bao X, Benazet JD, Tariq M, Paro R, Mackem S, Zeller R. Distinct roles of Hand2 in initiating polarity and posterior Shh expression during the onset of mouse limb bud development. PLoS Genet. 2010;6:e1000901. doi: 10.1371/journal.pgen.1000901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendershot TJ, Liu H, Clouthier DE, Shepherd IT, Coppola E, Studer M, Firulli AB, Pittman DL, Howard MJ. Conditional deletion of Hand2 reveals critical functions in neurogenesis and cell type-specific gene expression for development of neural crest-derived noradrenergic sympathetic ganglion neurons. Dev Biol. 2008;319:179–191. doi: 10.1016/j.ydbio.2008.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendershot TJ, Liu H, Sarkar AA, Giovannucci DR, Clouthier DE, Abe M, Howard MJ. Expression of Hand2 is sufficient for neurogenesis and cell type-specific gene expression in the enteric nervous system. Dev Dyn. 2007;236:93–105. doi: 10.1002/dvdy.20989. [DOI] [PubMed] [Google Scholar]
- Hirsch MR, Tiveron MC, Guillemot F, Brunet JF, Goridis C. Control of noradrenergic differentiation and Phox2a expression by MASH1 in the central and peripheral nervous system. Development. 1998;125:599–608. doi: 10.1242/dev.125.4.599. [DOI] [PubMed] [Google Scholar]
- Holler KL, Hendershot TJ, Troy SE, Vincentz JW, Firulli AB, Howard MJ. Targeted deletion of Hand2 in cardiac neural crest-derived cells influences cardiac gene expression and outflow tract development. Dev Biol. 2010;341:291–304. doi: 10.1016/j.ydbio.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard M, Foster DN, Cserjesi P. Expression of HAND gene products may be sufficient for the differentiation of avian neural crest-derived cells into catecholaminergic neurons in culture. Dev Biol. 1999;215:62–77. doi: 10.1006/dbio.1999.9450. [DOI] [PubMed] [Google Scholar]
- Howard MJ. Mechanisms and perspectives on differentiation of autonomic neurons. Dev Biol. 2005;277:271–286. doi: 10.1016/j.ydbio.2004.09.034. [DOI] [PubMed] [Google Scholar]
- Keyte A, Hutson MR. The neural crest in cardiac congenital anomalies. Differentiation. 2012;84:25–40. doi: 10.1016/j.diff.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leblanc GG, Landis SC. Differentiation of noradrenergic traits in the principal neurons and small intensely fluorescent cells of the parasympathetic sphenopalatine ganglion of the rat. Dev Biol. 1989;131:44–59. doi: 10.1016/s0012-1606(89)80037-5. [DOI] [PubMed] [Google Scholar]
- Lei J, Howard MJ. Targeted deletion of Hand2 in enteric neural precursor cells affects its functions in neurogenesis, neurotransmitter specification and gangliogenesis, causing functional aganglionosis. Development. 2011;138:4789–4800. doi: 10.1242/dev.060053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsley A, Snider P, Zhou H, Rogers R, Wang J, Olaopa M, Kruzynska-Frejtag A, Koushik SV, Lilly B, Burch JB, Firulli AB, Conway SJ. Identification and characterization of a novel Schwann and outflow tract endocardial cushion lineage-restricted periostin enhancer. Dev Biol. 2007;307:340–355. doi: 10.1016/j.ydbio.2007.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas ME, Muller F, Rudiger R, Henion PD, Rohrer H. The bHLH transcription factor hand2 is essential for noradrenergic differentiation of sympathetic neurons. Development. 2006;133:4015–4024. doi: 10.1242/dev.02574. [DOI] [PubMed] [Google Scholar]
- Malmejac J. Activity of the Adrenal Medulla and its Regulation. Physiol Rev. 1964;44:186–218. doi: 10.1152/physrev.1964.44.2.186. [DOI] [PubMed] [Google Scholar]
- McFadden DG, Barbosa AC, Richardson JA, Schneider MD, Srivastava D, Olson EN. The Hand1 and Hand2 transcription factors regulate expansion of the embryonic cardiac ventricles in a gene dosage-dependent manner. Development. 2005;132:189–201. doi: 10.1242/dev.01562. [DOI] [PubMed] [Google Scholar]
- Morikawa Y, Cserjesi P. Cardiac neural crest expression of Hand2 regulates outflow and second heart field development. Circ Res. 2008;103:1422–1429. doi: 10.1161/CIRCRESAHA.108.180083. [DOI] [PubMed] [Google Scholar]
- Morikawa Y, D’Autreaux F, Gershon MD, Cserjesi P. Hand2 determines the noradrenergic phenotype in the mouse sympathetic nervous system. Dev Biol. 2007;307:114–126. doi: 10.1016/j.ydbio.2007.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morikawa Y, Dai YS, Hao J, Bonin C, Hwang S, Cserjesi P. The basic helix-loop-helix factor Hand 2 regulates autonomic nervous system development. Dev Dyn. 2005;234:613–621. doi: 10.1002/dvdy.20544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ream MA, Chandra R, Peavey M, Ray AM, Roffler-Tarlov S, Kim HG, Wetsel WC, Rockman HA, Chikaraishi DM. High oxygen prevents fetal lethality due to lack of catecholamines. Am J Physiol Regul Integr Comp Physiol. 2008;295:R942–953. doi: 10.1152/ajpregu.00860.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rychlik JL, Gerbasi V, Lewis EJ. The interaction between dHAND and Arix at the dopamine beta-hydroxylase promoter region is independent of direct dHAND binding to DNA. J Biol Chem. 2003;278:49652–49660. doi: 10.1074/jbc.M308577200. [DOI] [PubMed] [Google Scholar]
- Schmidt M, Lin S, Pape M, Ernsberger U, Stanke M, Kobayashi K, Howard MJ, Rohrer H. The bHLH transcription factor Hand2 is essential for the maintenance of noradrenergic properties in differentiated sympathetic neurons. Dev Biol. 2009;329:191–200. doi: 10.1016/j.ydbio.2009.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen WH, Chen Z, Shi S, Chen H, Zhu W, Penner A, Bu G, Li W, Boyle DW, Rubart M, Field LJ, Abraham R, Liechty EA, Shou W. Cardiac restricted overexpression of kinase-dead mammalian target of rapamycin (mTOR) mutant impairs the mTOR-mediated signaling and cardiac function. J Biol Chem. 2008;283:13842–13849. doi: 10.1074/jbc.M801510200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava D, Thomas T, Lin Q, Kirby ML, Brown D, Olson EN. Regulation of cardiac mesodermal and neural crest development by the bHLH transcription factor, dHAND. Nat Genet. 1997;16:154–160. doi: 10.1038/ng0697-154. [DOI] [PubMed] [Google Scholar]
- Suzuki N, Hardebo JE, Kahrstrom J, Owman C. Selective electrical stimulation of postganglionic cerebrovascular parasympathetic nerve fibers originating from the sphenopalatine ganglion enhances cortical blood flow in the rat. J Cereb Blood Flow Metab. 1990;10:383–391. doi: 10.1038/jcbfm.1990.68. [DOI] [PubMed] [Google Scholar]
- Takeda N, Manabe I, Uchino Y, Eguchi K, Matsumoto S, Nishimura S, Shindo T, Sano M, Otsu K, Snider P, Conway SJ, Nagai R. Cardiac fibroblasts are essential for the adaptive response of the murine heart to pressure overload. J Clin Invest. 2010;120:254–265. doi: 10.1172/JCI40295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ter Laan M, van Dijk JM, Elting JW, Staal MJ, Absalom AR. Sympathetic regulation of cerebral blood flow in humans: a review. Br J Anaesth. 2013 doi: 10.1093/bja/aet122. [DOI] [PubMed] [Google Scholar]
- Thomas SA, Matsumoto AM, Palmiter RD. Noradrenaline is essential for mouse fetal development. Nature. 1995;374:643–646. doi: 10.1038/374643a0. [DOI] [PubMed] [Google Scholar]
- Tian H, Hammer RE, Matsumoto AM, Russell DW, McKnight SL. The hypoxia-responsive transcription factor EPAS1 is essential for catecholamine homeostasis and protection against heart failure during embryonic development. Genes Dev. 1998;12:3320–3324. doi: 10.1101/gad.12.21.3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tronche F, Kellendonk C, Kretz O, Gass P, Anlag K, Orban PC, Bock R, Klein R, Schutz G. Disruption of the glucocorticoid receptor gene in the nervous system results in reduced anxiety. Nat Genet. 1999;23:99–103. doi: 10.1038/12703. [DOI] [PubMed] [Google Scholar]
- Tsuchihashi T, Maeda J, Shin CH, Ivey KN, Black BL, Olson EN, Yamagishi H, Srivastava D. Hand2 function in second heart field progenitors is essential for cardiogenesis. Dev Biol. 2011;351:62–69. doi: 10.1016/j.ydbio.2010.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanDusen NJ, Firulli AB. Twist factor regulation of non-cardiomyocyte cell lineages in the developing heart. Differentiation. 2012;84:79–88. doi: 10.1016/j.diff.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincentz JW, Barnes RM, Firulli AB. Hand factors as regulators of cardiac morphogenesis and implications for congenital heart defects. Birth Defects Res A Clin Mol Teratol. 2011;91:485–494. doi: 10.1002/bdra.20796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincentz JW, Barnes RM, Rodgers R, Firulli BA, Conway SJ, Firulli AB. An absence of Twist1 results in aberrant cardiac neural crest morphogenesis. Dev Biol. 2008;320:131–139. doi: 10.1016/j.ydbio.2008.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincentz JW, Firulli BA, Lin A, Spicer DB, Howard MJ, Firulli AB. Twist1 controls a cell-specification switch governing cell fate decisions within the cardiac neural crest. PLoS Genet. 2013;9:e1003405. doi: 10.1371/journal.pgen.1003405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wildner H, Gierl MS, Strehle M, Pla P, Birchmeier C. Insm1 (IA-1) is a crucial component of the transcriptional network that controls differentiation of the sympatho-adrenal lineage. Development. 2008;135:473–481. doi: 10.1242/dev.011783. [DOI] [PubMed] [Google Scholar]
- Wu X, Howard MJ. Transcripts encoding HAND genes are differentially expressed and regulated by BMP4 and GDNF in developing avian gut. Gene Expr. 2002;10:279–293. doi: 10.3727/000000002783992361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Firulli AB, Zhang X, Howard MJ. HAND2 synergistically enhances transcription of dopamine-beta-hydroxylase in the presence of Phox2a. Dev Biol. 2003;262:183–193. doi: 10.1016/s0012-1606(03)00361-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.