Abstract
The 76-element Geochemical Mapping (76 GEM) Project was undertaken in southwestern China in 2000 and in southeastern China in 2008. In this project, 5244 composite samples of stream sediment at a density of one composite sample for each 1:50,000-scale map sheet were prepared from sample archives of the China Regional Geochemistry-National Reconnaissance (RGNR) Project, which have been available since 1978. The 76 elements were analyzed by using inductively coupled plasma mass spectrometry (ICP-MS), X-ray fluorescence (XRF), and inductively coupled plasma atomic emission spectroscopy (ICP-AES). In the present study, a new quality-control method known as the visualized standard map method was applied to the results of the 76 GEM project. Mean value and background value, which indicate the average concentration of the 76 elements in southern China, were derived from statistical data. Moreover, geochemical maps were compiled to demonstrate the distribution of the 76 elements in southern China.
Keywords: 76 elements, Geochemical mapping, Analytical scheme
Highlights
-
•
76 element analytical scheme was developed based largely on multi-method.
-
•
The detection limits of all elements are less than their crustal abundances.
-
•
We introduce the analytical methods of 76 elements in stream sediments.
-
•
The geochemical maps were compiled to demonstrate the distribution of the 76 elements.
-
•
A standard code for the samples was used to control the analytical quality.
1. Introduction
All elements in the periodic table appear in the Earth's environment and provide nutrients necessary for sustaining all life forms, ecology, and the environment. These elements and their isotopes are the smallest units used in geoscience research and can be compared to genes studied in biological disciplines. Mapping of the spatial distribution of nearly all elements in the periodic table will offer an updated knowledge of the construction of the Earth's surface for better stewardship of sustainable environmental management and mineral resource development.
In 1973, Webb et al. (1973, 1978) published Provisional Geochemical Atlas of Northern Ireland, which was the first geochemical atlas. Since that time, more than 40 regional and national geochemical mapping projects have been conducted (Bolivar, 1980; Bölviken et al., 1986; Bowie and Plant, 1978a, 1978b; De Caritat and Cooper, 2011; Chiprés et al., 2008; Cloete et al., 2009; De Vos and Tarvainen, 2006; Fauth et al., 1985; Friske and Hornbrook, 1991; Geological Survey of Canada, 1981; Imai et al., 2004; Koljonen, 1992; Laszlo et al., 1997; Prieto, 2009; Reedman, 1973; Reimann et al., 1998; Salminen, 2005; Shin, 2002; Simpson, 1993; Smith, 2009; Stephenson et al., 1982; Varna et al., 1997; Weaver et al., 1983).
During International Geological Correlation Programme (IGCP) 259/360 (1989–98), production of a global geochemical atlas of Earth's land surface was recommended in which 5000 Geochemical Reference Network (GRN) sampling cells were to be arranged to cover the entire planet, and 71 elements were to be analyzed (Darnley et al., 1995). To meet the requirements of this recommendation, a new national mapping program was initiated in 2000 after the development of a new 76-element analytical scheme that included Os, Ir, Ru, Rh, and Re in addition to the 71 suggested elements.
In 1978, the Regional Geochemistry-National Reconnaissance (RGNR) Project was initiated in China (Xie, 1977, 1978, 1979; Xie and Cheng, 1997; Xie et al., 1989a) in which various methods were used to analyze 39 elements; in 1993, the Environmental Geochemical Monitoring Network Project analyzed 54 elements (Xie and Cheng, 1997, 2001; Xie et al., 1997). In 2000, a project that plotted the geochemical maps for 76 elements, known as 76 GEM, was undertaken in southwestern China (Xie et al., 2008). In this project, approximately 100 samples within each 1:50,000-scale map sheet were combined and composited into one sample. A successor project that covered the rest of southern China was undertaken in 2008. Approximately 5244 composite samples were submitted for analysis. Several laboratories in China have collaborated on projects for developing analytical methods for platinum group elements (PGEs), rare earth elements (REEs), Re, and Te. The analytical scheme used was based largely on inductively coupled plasma mass spectrometry (ICP-MS), inductively coupled plasma-atomic emission spectrometry (ICP-AES), and X-ray fluorescence (XRF), supplemented with other techniques. In addition, the detection limits of all elements analyzed were less than their crustal abundance values. The geochemical maps produced through these projects show the distributions of most of the elements in the periodic table.
2. Sample collection
The RGNR program was conducted in China the late 1970s (Xie et al., 1989a, 1997). Stream sediment was used as the main sampling medium (Xie, 1979). Samples were collected from second-grade drainage or the mouth of first-grade drainage at a density of one to two samples per square kilometer. The minimum and maximum areas of the basin controlled by the samples in the uppermost region were 1/3 km2 and 3 km2, respectively. Samples were air-dried and sieved with a 60-mesh screen to obtain particles smaller than 0.22 mm. Parts of the original samples were used for analysis of 39 elements, and the remaining samples were archived for future applications. More than 3 million stream sediment samples have been collected in southern China. The present study utilized RGNR samples from 12 provinces in southern China including Sichuan, Yunnan, Guizhou, Guangxi, Guangdong, Hunan, Hubei, Jiangxi, Fujian, Anhui, Zhejiang, and Jiangsu in addition to several autonomous regions (Fig. 1).
Fig. 1.
Location of study area for the 76 element geochemical mapping in southern China.
3. Sample preparation and processing
The RGNR project conducted in the aforementioned 12 provinces in southern China commenced in 1980 and was completed in 1995. Approximately 100 RGNR samples from each 1:50,000-scale map sheet were composited into a single sample for reanalysis in 76 GEM. Each analytical sample contained all of the collected samples within each 1:50,000-scale map sheet included in an area of 410–460 km2 from the RGNR sample bank. 20 g of sediment was collected every 4 km2 to provide a composite sample. Blank spaces were not considered in this sample preparation plan. Overall, 5244 composite samples were prepared during this program, covering an area of 2,300,000 km2.
The composite samples were ground by using an agate jar ball mill and sieved through a 200-mesh screen to obtain particles smaller than 74 μm. The processed samples were then analyzed by various laboratories.
4. Sample analysis and quality control
Analytical technology has advanced rapidly in recent decades. Since the analysis of 39 elements in the RGNR program, technological advances have enabled analysis of 54 elements through the National Environment Monitoring Network Program of China and through multi-purpose ecological geochemical survey. The precision and accuracy of testing have improved significantly, as demonstrated by the advanced analysis through 76 GEM (Xie et al., 2008).
The fundamental requirement (Darnley et al., 1995; Xie, 1995) of the present study was to ensure that the detection limits of trace and sub-trace elements are lower than their crustal abundance values. A multi-method–multi-instrument analytical approach was adopted, and the visualized standard maps method was established for strict data-monitoring.
According to the aforementioned requirements, ICP-MS, XRF, and ICP-AES were adopted to analyze most of the elements; alternative methods were used to analyze those with low crustal abundance values. Table 1 shows the analytical method adopted for each element.
Table 1.
The analytical method, detection limits and precision of 76 elements.
| Component | Detection limit | Method | RSD % | Component | Detection limit | Method | RSD % |
|---|---|---|---|---|---|---|---|
| Ag | 0.01 mg/kg | AES | 3.82 | Mo | 0.02 mg/kg | DF-ICPMS | 4.76 |
| Al2O3 | 0.01% | XRF | 1.11 | N | 20 mg/kg | VOL | 2.22 |
| As | 0.2 mg/kg | HG-AFS | 2.68 | Na2O | 0.01% | DF-ICPES | 5.65 |
| Au | 0. 1 μg/kg | DA-GFAAS | 6.40 | Nb | 1 mg/kg | XRF | 0.99 |
| B | 1 mg/kg | AES | 8.18 | Nd | 0.05 mg/kg | FU-I-ICPMS | 3.76 |
| Ba | 9 mg/kg | XRF | 4.30 | Ni | 0.6 mg/kg | DF-ICPMS | 4.54 |
| Be | 0.2 mg/kg | DF-ICPES | 1.77 | Os | 0.01 μg/kg | FU-ICPMS | 10.2 |
| Bi | 0.015 mg/kg | DF-ICPMS | 8.85 | P | 10 mg/kg | XRF | 1.21 |
| Br | 0.8 mg/kg | XRF | 5.80 | Pb | 2 mg/kg | XRF | 0.62 |
| C | 0.04% | VOL | 1.60 | Pd | 0.2 μg/kg | DA-ICPMS | 9.97 |
| CaO | 0.01% | XRF | 0.91 | Pt | 0.2 μg/kg | DA-ICPMS | 6.50 |
| Cd | 0.02 mg/kg | DF-ICPMS | 5.58 | Pr | 0.01 mg/kg | FU-I-ICPMS | 4.03 |
| Ce | 0.12 mg/kg | FU-I-ICPMS | 4.19 | Rb | 1 mg/kg | XRF | 0.64 |
| Cl | 7 mg/kg | XRF | 3.25 | Re | 0.05 μg/kg | FU-I-ICPMS | 2.23 |
| Co | 0.02 mg/kg | DF-ICPMS | 4.41 | Rh | 0.01 μg/kg | FA-ICPMS | 4.76 |
| Cr | 3 mg/kg | XRF | 1.77 | Ru | 0.01 μg/kg | FU-ICPMS | 8.20 |
| Cs | 0.003 mg/kg | DF-ICPMS | 3.71 | S | 7 mg/kg | XRF | 0.65 |
| Cu | 1 mg/kg | DF-ICPMS | 4.85 | Sb | 0.02 mg/kg | DF-ICPMS | 9.62 |
| Dy | 0.015 mg/kg | FU-I-ICPMS | 4.08 | Sc | 0.6 mg/kg | DF-ICPMS | 5.27 |
| Er | 0.015 mg/kg | FU-I-ICPMS | 3.65 | Se | 0.01 mg/kg | HG-AFS | 3.27 |
| Eu | 0.004 mg/kg | FU-I-ICPMS | 2.86 | SiO2 | 0.01% | XRF | 0.18 |
| F | 20 mg/kg | ISE | 6.12 | Sm | 0.015 mg/kg | FU-I-ICPMS | 3.21 |
| Fe2O3 | 0.01% | XRF | 0.65 | Sn | 0.2 mg/kg | AES | 5.18 |
| Ga | 2 mg/kg | XRF | 1.28 | Sr | 1.5 mg/kg | XRF | 1.07 |
| Gd | 0.015 mg/kg | FU-I-ICPMS | 3.22 | Ta | 0.005 mg/kg | DF-ICPMS | 11.69 |
| Ge | 0.02 mg/kg | HG-AFS | 2.72 | Tb | 0.01 mg/kg | FU-I-ICPMS | 3.83 |
| Hf | 0.015 mg/kg | DF-ICPMS | 5.42 | Te | 5 μg/kg | DF-ICPMS | 5.00 |
| Hg | 0.3 μg/kg | CV-AFS | 2.18 | Th | 0.003 mg/kg | DF-ICPMS | 8.36 |
| Ho | 0.004 mg/kg | FU-I-ICPMS | 5.42 | Ti | 10 mg/kg | XRF | 1.18 |
| I | 0.5 mg/kg | CF-COL | 3.53 | Tl | 0.003 mg/kg | DF-ICPMS | 6.96 |
| In | 0.002 mg/kg | DF-ICPMS | 5.34 | Tm | 0.003 mg/kg | FU-I-ICPMS | 4.62 |
| Ir | 0.01 μg/kg | FA-ICPMS | 6.76 | U | 0.01 mg/kg | DF-ICPMS | 6.03 |
| K2O | 0.01% | DF-ICPES | 2.2 | V | 5 mg/kg | XRF | 2.11 |
| La | 0.12 mg/kg | FU-I-ICPMS | 4.77 | W | 0.02 mg/kg | DF-ICPMS | 4.94 |
| Li | 0.06 mg/kg | DF-ICPES | 1.77 | Y | 0.12 mg/kg | FU-I-ICPMS | 3.86 |
| Lu | 0.004 mg/kg | FU-I-ICPMS | 4.56 | Yb | 0.015 mg/kg | FU-I-ICPMS | 4.5 |
| MgO | 0.01% | DF-ICPES | 1.55 | Zn | 1 mg/kg | XRF | 0.71 |
| Mn | 10 mg/kg | XRF | 0.49 | Zr | 1 mg/kg | XRF | 1.72 |
Decomposition method: DA: Digestion with aqua regia; DF: Digestion with HCl, HNO3, HClO4 and HF; FU: Alkaline fusion or fusion with Eschka mixture, FA: enrichment of assay, I: ion exchange resin preconcentration.
Determination method: ICP-MS: inductively coupled plasma mass spectrometry; ICP-ES: Inductively couple plasma atomic emission spectrometry; XRF: X-ray fluorescence spectrometry; GFAAS: Graphite furnace atomic absorption spectrometry; AFS: Atomic fluorescence spectrometry; VOL: Volumetry; COL: Colorimetry; ISE: Ionic selection electrometry; AES: Atomic emission spectrometry.
A standard code for samples and duplicate samples was used to control the analytical quality and to ensure that both internal and external qualities are in good agreement with the results obtained in the present study (Xie et al., 2003; Ye, 2002; Ye and Yao, 2004). Parameters of average value , maximum value (Xmax), minimum value (Xmin), median, and standard deviation (S) were calculated for determined and certified values of the coded samples. The coded samples were taken as a statistical unit to calculate the ΔlgC between the determined and recommended values of each element. Content higher than three times the detection limit required ΔlgC ≤ 0.10 to fulfill the quality control requirement; that less than three times the detection limit required ΔlgC ≤ 0.12. For the statistical unit, the acceptance rate should be ≥ 90% to fulfill the quality control requirement. In the calculation of the correlation coefficient of the analytical data and the recommended values of the coded samples, the correlation coefficient should be ≥ 0.90. The coded samples were used as a statistical unit to apply the F-test on the analytical data and the recommended values of the code samples. FD < F0 (F0 is the critical F values at the 95 wt.% confidence level) should be ensured for all elements. Rather than using the standard reference sample concept to monitor analytical data quality, a new, advanced concept of reference mapping was used in the present research. 150 reference samples were prepared by mixing various proportions of Geochemical Reference Samples of Drainage Sediment (GSD) and Geochemical Reference Samples of Soil (GSS) samples (Xie et al., 1985a, 1985b, 1989b). These samples were arranged by virtual spatial distribution, and their element values were calculated by the certified values of various proportions of original standard reference samples of GSD and GSS series. Reference maps were then prepared and compared to those prepared by element values of the reference samples analyzed by laboratories that participated in 76 GEM. Similarities in both map types were determined through visual observation or by calculating the correlation coefficient.
Fig. 2 shows a comparison of the Cl reference map and the same Cl map prepared by Laboratory A. Fig. 3 shows the same kind of comparison but with the Laboratory B map. Visual comparison revealed that the map quality of Laboratory B is poor; comparison of statistical parameters indicated the same result.
Fig. 2.

Visualized Cl maps produced by reference data and analysis data by Lab A.
Fig. 3.

Visualized Cl maps produced by reference data and analysis data by Lab B.
5. Data statistics and map compilation
Table 2 shows statistical data of stream sediments in southern China including minimum, median, arithmetic mean, and standard deviation.
Table 2.
Statistical data on the 76 elements in southern China.
| Element | Minimum | 5% percentile | 25% percentile | Median | 75% percentile | 95% percentile | Maximum | Before removing X ± 3S |
After removing X ± 3S |
||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Average | Standard deviation | Average | Standard deviation | ||||||||
| Ag | 24 | 46 | 65 | 81 | 109 | 198 | 2429 | 103 | 99 | 83 | 28 |
| As | 0.4 | 3.2 | 7.9 | 12.5 | 19 | 37.3 | 535 | 16.4 | 19.1 | 13.1 | 7.4 |
| Au | 0.04 | 0.83 | 1.3 | 1.8 | 2.5 | 5.0 | 889 | 3.2 | 21.2 | 1.8 | 0.75 |
| B | 3.3 | 15 | 45 | 62 | 77 | 101 | 333 | 61 | 28 | 60 | 25 |
| Ba | 61 | 216 | 332 | 430 | 531 | 795 | 7146 | 468 | 268 | 429 | 142 |
| Be | 0.34 | 1.2 | 1. 8 | 2.2 | 2.6 | 4.0 | 18.6 | 2.35 | 1.1 | 2.2 | 0.62 |
| Bi | 0.05 | 0.2 | 0.3 | 0.39 | 0.6 | 1.63 | 33.1 | 0.75 | 1.75 | 0.39 | 0.15 |
| Br | 0.2 | 1.5 | 2.5 | 3.8 | 5.5 | 8.1 | 24.6 | 4.2 | 2.3 | 4.1 | 2 |
| C | 0.04 | 0.62 | 1.09 | 1.6 | 2.23 | 3.24 | 8.18 | 1.75 | 0.89 | 1.69 | 0.78 |
| Cd | 24 | 76 | 139 | 219 | 398 | 1247 | 21,954 | 431 | 828 | 230 | 134 |
| Ce | 24 | 55 | 72 | 85 | 101 | 140 | 352 | 90 | 29 | 87 | 22 |
| Cl | 30 | 45 | 56 | 69 | 88 | 135 | 5609 | 86 | 130 | 71 | 20 |
| Co | 0.8 | 5.6 | 9.9 | 13.5 | 17.2 | 27.8 | 79.1 | 14.6 | 7.6 | 13.3 | 5.1 |
| Cr | 8.8 | 23 | 51 | 70 | 87 | 162 | 608 | 78 | 51 | 67 | 27 |
| Cs | 1 | 4 | 6.2 | 7.9 | 10.3 | 15.8 | 77.4 | 8.8 | 4.4 | 8.2 | 3 |
| Cu | 2.8 | 9 | 18 | 25 | 34 | 72 | 423 | 31.3 | 25.9 | 25 | 10.8 |
| Dy | 1.4 | 3.7 | 4.7 | 5.3 | 6.3 | 9.0 | 20.5 | 5.8 | 1.8 | 5.5 | 1.3 |
| F | 112 | 294 | 403 | 515 | 657 | 995 | 5581 | 569 | 276 | 527 | 173 |
| Er | 0.9 | 2.1 | 2.7 | 3.0 | 3.6 | 5.2 | 13.1 | 3.3 | 1.1 | 3.1 | 0.7 |
| Eu | 0.3 | 0.78 | 1.03 | 1.2 | 1.47 | 2.15 | 4 | 1.3 | 0.5 | 1.2 | 0.3 |
| Ga | 5.6 | 11.8 | 14.9 | 17 | 19.2 | 24 | 37.1 | 17.3 | 3.8 | 17.1 | 3.4 |
| Gd | 1.4 | 3.7 | 4.8 | 5.6 | 6.7 | 9.5 | 18.6 | 6 | 1.9 | 5.8 | 1.5 |
| Ge | 0.67 | 1.11 | 1.29 | 1.42 | 1.55 | 1.76 | 12.8 | 1.43 | 0.26 | 1.42 | 0.19 |
| Hf | 2 | 5.3 | 6.8 | 8.1 | 10.4 | 17.8 | 60 | 9.4 | 4.4 | 8.3 | 2.4 |
| Hg | 8.4 | 21 | 45 | 72 | 116 | 264 | 15,537 | 131 | 500 | 75 | 43 |
| Ho | 0.3 | 0.73 | 0.93 | 1.05 | 1.26 | 1.82 | 4.39 | 1.15 | 0.38 | 1.08 | 0.26 |
| I | 0.10 | 0.81 | 1.46 | 2.3 | 3.52 | 6.22 | 17.7 | 2.8 | 1.9 | 2.5 | 1.3 |
| Ir | 0.005 | 0.017 | 0.029 | 0.044 | 0.066 | 0.13 | 1.2 | 0.056 | 0.048 | 0.047 | 0.025 |
| In | 0.017 | 0.042 | 0.056 | 0.066 | 0.081 | 0.117 | 2.18 | 0.075 | 0.064 | 0.068 | 0.018 |
| La | 14 | 29 | 37 | 42 | 51 | 71 | 187 | 46 | 15 | 43 | 10 |
| Li | 5.5 | 17 | 27 | 34 | 42 | 58 | 247 | 36 | 14 | 34 | 11 |
| Lu | 0.15 | 0.32 | 0.41 | 0.47 | 0.55 | 0.85 | 2.33 | 0.51 | 0.19 | 0.47 | 0.1 |
| Mn | 55 | 339 | 570 | 739 | 967 | 1545 | 4894 | 821 | 400 | 766 | 300 |
| Mo | 0.19 | 0.42 | 0.67 | 1.02 | 1.61 | 3.0 | 33.5 | 1.35 | 1.25 | 1.12 | 0.59 |
| N | 47 | 552 | 935 | 1309 | 1689 | 2280 | 5415 | 1351 | 559 | 1328 | 518 |
| Nb | 1.4 | 11.9 | 15.0 | 17.7 | 22.5 | 34.9 | 172 | 20 | 8.2 | 18.6 | 5.3 |
| Nd | 9.7 | 23 | 30 | 35 | 41 | 58 | 137 | 37.1 | 11.5 | 35.7 | 8.9 |
| Ni | 3.6 | 9 | 20 | 29 | 38 | 66 | 348 | 32.5 | 22.7 | 28.6 | 13.2 |
| Os | 0.01 | 0.028 | 0.040 | 0.054 | 0.084 | 0.169 | 0.77 | 0.074 | 0.06 | 0.059 | 0.027 |
| P | 130 | 297 | 420 | 555 | 769 | 1180 | 14,098 | 636 | 365 | 601 | 245 |
| Pb | 8.5 | 18.9 | 25 | 31 | 41 | 67 | 2544 | 38.9 | 58.2 | 32.3 | 10.7 |
| Pd | 0.1 | 0.21 | 0.35 | 0.54 | 0.80 | 1.96 | 16.1 | 0.74 | 0.7 | 0.54 | 0.27 |
| Pr | 2.8 | 6.5 | 8.3 | 9.5 | 11.4 | 15.8 | 40 | 10.2 | 3.2 | 9.8 | 2.4 |
| Pt | 0.03 | 0.12 | 0.29 | 0.46 | 0.71 | 1.85 | 15.5 | 0.68 | 0.88 | 0.46 | 0.25 |
| Rb | 11.6 | 63 | 85.4 | 103 | 127.8 | 196.1 | 487 | 113 | 47.9 | 105 | 30 |
| Re | 0.04 | 0.05 | 0.07 | 0.1 | 0.19 | 1.39 | 19.9 | 0.25 | 0.69 | 0.1 | 0.049 |
| Rh | 0 | 0.013 | 0.022 | 0.031 | 0.045 | 0.095 | 1.13 | 0.041 | 0.041 | 0.032 | 0.014 |
| Ru | 0.011 | 0.038 | 0.05 | 0.065 | 0.089 | 0.191 | 1.2 | 0.087 | 0.087 | 0.065 | 0.022 |
| S | 42 | 107 | 175 | 250 | 367 | 614 | 3278 | 304 | 213 | 271 | 130 |
| Sb | 0.03 | 0.29 | 0.62 | 1.05 | 1.93 | 6.83 | 290 | 2.41 | 7.22 | 1.07 | 0.65 |
| Sc | 1.3 | 6.6 | 8.8 | 10.9 | 13.4 | 19.1 | 35.6 | 11.6 | 4.1 | 11.1 | 3.2 |
| Se | 0.05 | 0.11 | 0.20 | 0.31 | 0.45 | 0.72 | 3.85 | 0.35 | 0.23 | 0.33 | 0.17 |
| Sm | 1.7 | 4.33 | 5.51 | 6.4 | 7.7 | 10.81 | 20 | 6.9 | 2.2 | 6.6 | 1.7 |
| Sn | 1 | 2 | 2.8 | 3.5 | 5.5 | 13.5 | 4.2 | 5.8 | 10.6 | 3.6 | 1.4 |
| Sr | 15 | 30 | 49 | 71 | 109 | 180 | 1513 | 88 | 66 | 77 | 38 |
| Ta | 0.31 | 0.85 | 1.09 | 1.34 | 1.85 | 3.3 | 21.2 | 1.66 | 1.08 | 1.42 | 0.48 |
| Tb | 0.23 | 0.62 | 0.8 | 0.92 | 1.09 | 1.56 | 3.4 | 0.98 | 0.31 | 0.94 | 0.23 |
| Te | 10 | 27 | 39 | 49 | 65 | 108 | 1410 | 58 | 44 | 51 | 17 |
| Th | 3.5 | 8.4 | 11.2 | 13.3 | 16.8 | 31.6 | 87.8 | 15.9 | 8.7 | 13.3 | 3.5 |
| Ti | 1439 | 3224 | 4028 | 4646 | 5491 | 10,117 | 29,201 | 5385 | 2788 | 4600 | 950 |
| Tl | 0.039 | 0.33 | 0.52 | 0.64 | 0.82 | 1.24 | 2.96 | 0.7 | 0.31 | 0.67 | 0.24 |
| Tm | 0.14 | 0.33 | 0.42 | 0.48 | 0.57 | 0.84 | 2.3 | 0.52 | 0.18 | 0.49 | 0.11 |
| U | 0.8 | 1.86 | 2.57 | 3.30 | 4.58 | 7.65 | 45.6 | 4 | 2.5 | 3.5 | 1.3 |
| V | 20 | 46 | 71 | 93 | 116 | 208 | 580 | 105 | 58 | 91 | 31 |
| W | 0.4 | 1.05 | 1.55 | 2 | 3.07 | 7.82 | 151 | 3.6 | 7.8 | 2.1 | 0.8 |
| Y | 8.3 | 19 | 25 | 29 | 33 | 51 | 128 | 31.2 | 11.5 | 28.8 | 6.5 |
| Yb | 0.95 | 2.1 | 2.7 | 3.0 | 3.6 | 5.4 | 15.3 | 3.3 | 1.2 | 3.1 | 0.7 |
| Zn | 18.4 | 44 | 65 | 81 | 99 | 155 | 2558 | 91 | 65 | 81 | 24 |
| Zr | 134 | 214 | 255 | 303 | 386 | 572 | 1837 | 340 | 128 | 320 | 90 |
| Al2O3 | 6.37 | 10.4 | 12.5 | 13.7 | 15 | 17.7 | 25.2 | 13.9 | 2.33 | 13.8 | 2.1 |
| CaO | 0.06 | 0.17 | 0.37 | 0.97 | 1.99 | 4.65 | 22.8 | 1.56 | 1.9 | 1.1 | 0.95 |
| Fe2O3 | 1.4 | 3.09 | 4.03 | 4.88 | 5.81 | 9.31 | 22.0 | 5.32 | 2.17 | 4.8 | 1.2 |
| MgO | 0.1 | 1.29 | 1.91 | 1.03 | 2.75 | 3.44 | 7.22 | 1.23 | 0.81 | 1.1 | 0.63 |
| K2O | 0.18 | 0.34 | 0.64 | 2.33 | 1.63 | 2.53 | 5.53 | 2.35 | 0.68 | 2.3 | 0.63 |
| Na2O | 0.04 | 0.12 | 0.24 | 0.48 | 0.99 | 1.68 | 4.41 | 0.69 | 0.63 | 0.6 | 0.45 |
| SiO2 | 31.9 | 50.0 | 60.4 | 65.6 | 69.9 | 75.3 | 85.5 | 64.7 | 7.76 | 64.9 | 7.39 |
Note: The unit for Ag, Au, Cd, Hg, Ir, Os, Pd, Pt, Rh, Ru, Re, and Te is μg kg− 1; the unit for SiO2, Al2O3, Fe2O3, MgO, CaO, Na2O, K2O, and C is %; and the unit for other elements is mg kg− 1.
The geochemical map was compiled with the same 20-km grid spacing as that for the original data and included a search radius 2.5 times the grid spacing, and an index factor of five. In addition, exponential inverse distance weighting was applied as the interpolation scheme.
Contouring and coloring of all elements were performed on the basis of content at various cumulative element frequencies of 0.5%, 1.5%, 4.0%, 8.0%, 15.0%, 25.0%, 40.0%, 60.0%, 75.0%, 85.0%, 92.0%, 96.0%, 98.5%, and 99.5%. The contour colors were consistent with the corresponding filled colors, and the contour values were unmarked.
6. Geochemical maps in southern China
High Ag values occur in the border areas of Sichuan and Yunnan provinces as well as in Tibet (Fig. 4). Specifically such values are observed in Gejiu in Yunnan, Hechi in northern Guangxi, Chenzhou in Hunan, Shaoguan in Guangdong, and Longyan in Fujian. Ag enrichment has been facilitated by mineralization in the well-known Pb–Zn–Ag districts.
Fig. 4.
Geochemical map of Ag in southern China.
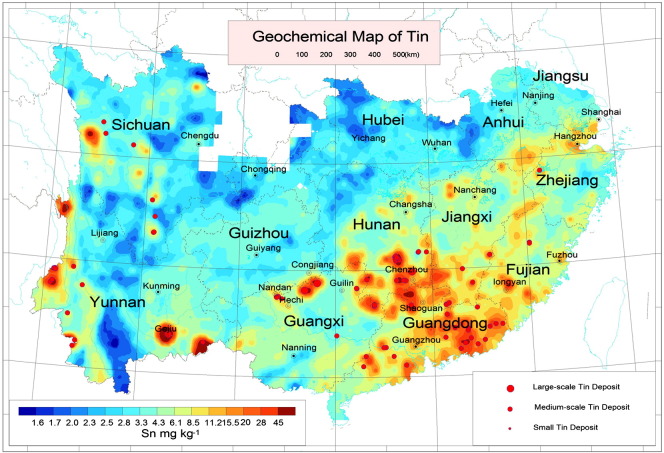
Sn anomalies are located in west Yunnan province, Gejiu, Nandan, and Congjiang, as well as in southern Hunan and northern Guangdong province (Fig. 5). Such anomalies are associated with Sn mineralization. Large Sn deposits are within the scope of Sn geochemical anomalies.
Fig. 5.
Geochemical map of Sn in southern China.
Figs. 6 and 7 show distribution of rare earth elements Nd and Tm, respectively. Light rare earth element anomalies are present at the junction of Jiangxi and Fujian provinces, northeastern Guangdong province, southwestern Guangxi province, western Yunnan province, and northwestern Sichuan province. Heavy rare earth element anomalies in stream sediments are well represented in Variscan and Yanshanian granitic basement rocks. Anomalies of light rare earth elements La, Ce, Pr, Nd, and Sm are stronger than those of heavy rare earth elements in the junction of Jiangxi and Fujian provinces and in northeastern Guangdong province.
Fig. 6.
Geochemical map of Nd in southern China.
Fig. 7.
Geochemical map of Tm in southern China.
Elements abundant in north Yunnan, southwest Sichuan, and west Guizhou provinces such as Pt, Pd, Os, Ru, Ir, and Rh of the platinum group and Cu, Co, Cr, Ni, V, Ti, Fe, and Mg are associated with mafic and ultramafic rocks. Os, Ir, Rh, Ru, Ni, and Mg anomalies are relatively stronger than Pt and Pd anomalies in Lijiang in Yunnan province (Figs. 8, 9). Pt and Pd anomalies are stronger than those of other platinum group elements in eastern Yunnan, western Guizhou, and southern Sichuan provinces. Such trends are associated with Permian Emeishan basalts (Fig. 10).
Fig. 8.
Geochemical map of Pt in southern China.
Fig. 9.
Geochemical map of Ir in southern China.
Fig. 10.
Location of Emeishan basalt in southern China.
The distribution map of In (Fig. 11) shows four large geochemical anomalies in Gejiu in Yunnan province, Hechi in Guangxi province, Chenzhou and Shaoguan in the junction of Hunan and Guangdong provinces, and Longyan in Fujian province. These obvious In anomalous areas are associated with the mineralization of Sn and Pb–Zn. A strong Te anomaly is delineated in Chenzhou and Shaoguan in the junction of Hunan and Guangdong provinces (Fig. 12), which is associated with the mineralization of Sn and Pb–Zn.
Fig. 11.
Geochemical map of In in southern China.
Fig. 12.
Geochemical map of Te in southern China.
7. Conclusions
Samples composed from archived specimens of a highly detailed geochemical mapping program in South China were used in the present multi-element geochemical mapping project to identify regional anomalies that may be related to economic areas of mineralization. Such regional programs illustrate the usage of low-density geochemistry as an important mineral prediction tool at a national scale. Furthermore, the analysis of a wide variety of elements at low levels proves that the data are suited for environmental management as well as mineral exploration.
Acknowledgments
This study was made possible by the cooperation of many people with the Bureau of Geology and Mineral Resources in 12 provinces in southern China. We thank J. Z. Feng, L. S. Zhang, J. Q. Gu, B. L. Wang, N. F. Cheng, Y. Xie, K. L. Chen, Y. N. Luo, Q. H. Lai, M. H. Xu, M. Z. Ceng, X. L. Qin, and H. G. Chen, who combined samples for composite projects. We acknowledge the analytical support provided by the following laboratories: Institute of Geophysical and Geochemical Exploration Laboratory, Henan Analytical and Testing Centre for Minerals and Rocks, and Geoanalysis Laboratory of Hubei Province. We also thank the Journal of Geochemical Exploration reviewer in addition to Dr. Patrice de Caritat and the two anonymous reviewers for providing valuable comments and suggestions, which have improved this paper.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-No Derivative Works License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
References
- Bolivar S.L. Los Alamos Scientific Laboratory; 1980. An Overview of the National Uranium Resource Evaluation Hydrogeochemical and Stream Sediment Reconnaissance Program. (LA-8457-MS, 24 pp.) [Google Scholar]
- Bølviken, B., Bergstorm, J., Bjorklund, A., Kontio, M., Lehmuspelto, P., Lindholm, T., Magnussson, J., Ottesen, R.T., Steenfelt, A., Volden, T., 1986. Geochemical Atlas of Northern Fennoscandia, Scale 1:4000000, Mapped by Geological Surveys of Finland. Norway and Sweden with Swedish Geological Co. and the Geological Survey of Greenland. Nordic Council of Ministers.
- Bowie S.H.E., Plant J. Institute of Geological Science; London: 1978. Regional Geochemical Atlas, Shetland. [Google Scholar]
- Bowie S.H.E., Plant J. Institute of Geological Science; London: 1978. Regional Geochemical Atlas, Orkney. [Google Scholar]
- Chiprés J.A., Salinas J.C., Castro-Larragoitia J., Monroy M.G. Geochemical mapping of major and trace elements in soils from the Altiplano Potosino, Mexico: a multi-scale comparison. Geochemistry: Exploration, Environment, Analysis. 2008;8:279–290. [Google Scholar]
- Cloete M., Elsenbroek J.H., Strauss S.W. Global Geochemical Mapping Symposium, Abstracts, China Geological Survey. 2009. Regional geochemical mapping in South Africa and future planning; pp. 20–23. [Google Scholar]
- Darnley A.G., Björklund A., Bølviken B., Gustavsson N., Koval V., Plant J.A., Steenfelt A., Tauchid M., Xie X.J. Vol. 19. UNESCO Publishing; 1995. A global geochemical database for environmental and resource management-recommendations for international geochemical mapping—final report of IGCP Project 259. (Earth Sciences). (122 pp.) [Google Scholar]
- De Caritat P., Cooper M. Geoscience Australia, Record 2011/20. 2011. National Geochemical Survey of Australia: the geochemical atlas of Australia. (2 Volumes, 557 pp.) [Google Scholar]
- De Vos, W., Tarvainen, T., (chief editors), 2006. FOREGS Geochemical Atlas of Europe, Part II: interpretation of geochemical maps, additional tables, figures, maps and related publications. ESPOO, Geology Survey of Finland.
- Fauth H., Hindel R., Siewers U., Zinner J. Schweizerbart'sche Verlagsbuchhandlung; Stuttgart: BGR Hannover: 1985. Geochemischer Atlas Bundesrepublik Deutschland. (79 pp.) [Google Scholar]
- Friske P.W.B., Hornbrook E.H.W. Canada's National Geochemical Reconnaissance Programme. Transaction of Institute of Mining and Metallurgy. 1991;B100:B47–B56. [Google Scholar]
- Geological Survey of Canada, 1981. National geochemical reconnaissance, 1:2,000,000, colored compilation series. Geological Survey of Canada, Open File Report, 730-749.
- Imai N., Terashima S., Ohta A., Mikoshiba M., Okai T., Tachibana Y., Togashi S., Matsuhisa Y., Kanai Y., Kamioka H. Geological Survey of Japan, National Institute of Advanced Science and Technology; Tokyo: 2004. Geochemical Map of Japan. (209 pp.) [Google Scholar]
- Koljonen, T., (chief editor), 1992. The Geochemical Atlas of Finland, Part 2: till. Geological Survey of Finland, Espoo, 218 pp.
- Laszlo O., Istvan H., Ubul F. Low density geochemical mapping in Hungaria. Journal of Geochemical Exploration. 1997;60:55–66. [Google Scholar]
- Prieto G. Global Geochemical Mapping Symposium, Abstracts, China Geological Survey. 2009. Geochemical atlas of Colombia, exploring the Colombian territory; pp. 13–14. [Google Scholar]
- Reedman A.J. Geological Survey of Uganda; Entebbe: 1973. Geochemical Atlas of Uganda. (42 pp.) [Google Scholar]
- Reimann C., Ayras M., Chekushin V., Bogatyrev I., Boyd R., Caritat P., de Dutter R., Finne T.E., Halleraker J.H., Jæger Ø., Kashulina G., Lehto O., Niskavaara H., Pavlov V., Räisänen M.L., Strand T., Volden T. NGU-GTK-CKE Special publication, Geological Survey of Norway; Trondheim, Norway: 1998. Environmental Geochemical Atlas of the Central Barents Region. (743 pp.) [Google Scholar]
- Salminen, R., (chief editor), 2005. FOREGS Geochemical Atlas of Europe, Part I: background information, methodology, and maps. ESPOO, Geology Survey of Finland.
- Shin, S.C., 2002, Geochemical atlas of Korea, scale 1:700000. Korean Institute of Geoscience and Mineral Resources.
- Simpson P.R. Institute of Geological Sciences; Nottingham: 1993. Regional Geochemical Atlas, Southern Scotland. [Google Scholar]
- Smith D.B. Preface: geochemical studies of North American soils: results from the pilot study phase of the North American Soils Geochemical Landscapes Project. Applied Geochemistry. 2009;24:1355–1356. [Google Scholar]
- Stephenson B., Ghazali S.S., Widjaja H. Institute of Geological Sciences, Keyworth, Nottingham; Northern Sumatra: 1982. Regional Geochemical Atlas Series of Indonesia: 1. [Google Scholar]
- Varna, K., Rapant, S., Marsina, K., 1997. Geochemical atlas of Slovak Republic at scale of 1:1,000,000. Journal of Geochemical Exploration 60, 7–37.
- Weaver, T.A., Freeman, S.H., Broxton, D.E., Bolivar, S.L., 1983. Geochemical atlas of Alaska, 1:6 000 000. 1A9897-MS, Los Alamos National Laboratory, N. M. USA, 57 pp.
- Webb J.S., Nichol I., Foster R., Lowenstein P.L., Howarth R.J. Applied Geochemistry Research Group. Imperial College of Science and Technology; London: 1973. Provisional geochemical atlas of Northern Ireland. (35 pp.) [Google Scholar]
- Webb J.S., Thornton I., Thompson M., Howarth R.J., Lowenstein P.L. Oxford University Press; Oxford: 1978. The Wolfson Geochemical Atlas of England and Wales. (70 pp.) [Google Scholar]
- Xie X.J. Current problems in regional geochemistry. Geophysical and Geochemical Exploration. 1977;2:1–10. (In Chinese) [Google Scholar]
- Xie X.J. Regional geochemistry-retrospect, present status and prospect. Institute Geophysical and Geochemical Exploration Research Report. 1978;3:1–10. (In Chinese) [Google Scholar]
- Xie X.J. Geological Publishing House; Beijing: 1979. Regional Geochemical Exploration. (192 pp., in Chinese) [Google Scholar]
- Xie X.J. Analytical requirements in international geochemical mapping. Analyst. 1995;120:1497–1504. [Google Scholar]
- Xie X.J., Cheng H.X. The suitability of floodplain sediments as a global sampling medium: evidence from China. Journal of Geochemical Exploration. 1997;58:51–62. [Google Scholar]
- Xie X.J., Cheng H.X. Global geochemical mapping and its implementation in the Asia–Pacific region. Applied Geochemistry. 2001;16:1309–1321. [Google Scholar]
- Xie X.J., Yan M.C., Li L.Z., Shen H.J. Geochemical Reference Samples, Drainage Sediment GSD1-8 from China. Geostandard Newsletter. 1985;14:83–159. [Google Scholar]
- Xie X.J., Yan M.C., Li L.Z., Shen H.J. Usable values for Chinese standard reference samples of stream sediments, soils and rocks: GSD9-12, GSS1-8 and GSR1-6. Geostandard Newsletter. 1985;14:277–280. [Google Scholar]
- Xie X.J., Sun H.Z., Ren T.X. Regional Geochemistry-National Reconnaissance Project in China. Journal of Geochemical Exploration. 1989;33:1–9. [Google Scholar]
- Xie X.J., Yan M.C., Wang C.S., Li L.Z., Shen H.J. Geochemical standard reference samples GSD9-12, GSS1-8 and GSR1-6. Geostandard Newsletter. 1989;18:83–179. [Google Scholar]
- Xie X.J., Mu X.Z., Ren T.X. Geochemical mapping in China. Journal of Geochemical Exploration. 1997;60:99–113. [Google Scholar]
- Xie X.J., Ye J.Y., Yan M.C., Zhou G.H. New proficiency test for the analytical laboratories involved in the environmental geochemical mapping. Geological Bulletin of China. 2003;22:1–11. (In Chinese) [Google Scholar]
- Xie X.J., Cheng Z.Z., Zhang L.S., Feng J.Z., Zhu Y.S. Geological Publishing House; Beijing: 2008. 76 Geochemical Elements Atlas of Southwest China. (219 pp., In Chinese) [Google Scholar]
- Ye J.Y. Quality monitoring and quality control of samples analysis in regional geochemical survey. Geophysical and Geochemical Exploration. 2002;26:6–11. (In Chinese) [Google Scholar]
- Ye J.Y., Yao L. Discussion of quality control method for the analysis of samples in regional geochemical survey. Rock and Mineral Analysis. 2004;23:137–142. (In Chinese) [Google Scholar]