Abstract
The genotoxin cisplatin is commonly used in chemotherapy to treat solid tumors, yet our understanding of the mechanism underlying the drug response is limited. In a focused siRNA screen, using an siRNA library targeting genes involved in ubiquitin and ubiquitin-like signaling, we identified the E3 ubiquitin ligase HOIP as a key regulator of cisplatin-induced genotoxicity. HOIP forms, with SHARPIN and HOIL-1L, the linear ubiquitin assembly complex (LUBAC). We show that cells deficient in the HOIP ligase complex exhibit hypersensitivity to cisplatin. This is due to a dramatic increase in caspase 8/caspase 3 mediated apoptosis that is strictly dependent on ATM-, but not ATR-mediated DNA damage checkpoint activation. Moreover, basal and cisplatin-induced activity of the stress response kinase JNK is enhanced in HOIP-depleted cells and, conversely, JNK inhibition can increase cellular resistance to cisplatin and reverse the apoptotic hyper-activation in HOIP-depleted cells. Furthermore, we show that HOIP depletion sensitizes cancer cells, derived from carcinomas of various origins, through an enhanced apoptotic cell death response. We also provide evidence that ovarian cancer cells classified as cisplatin-resistant can regain sensitivity following HOIP down-regulation. Cumulatively, our study identifies a HOIP-regulated anti-apoptotic signaling pathway, and we envisage HOIP as a potential target for the development of combinatorial chemotherapies to potentiate the efficacy of platinum-based anti-cancer drugs.
Keywords: siRNA screen, cisplatin, HOIP, apoptosis, ubiquitin-signaling
INTRODUCTION
Platinum-based agents such as cisplatin and carboplatin are commonly used in chemotherapy. They display therapeutic activity against a wide variety of solid tumors such as testicular, ovarian and non-small cell lung cancers (1). Cisplatin is a potent genotoxin that initially interferes with proliferation of the tumor cell (anti-proliferative activity) but ultimately induces apoptosis in the tumor cell (cytotoxic activity). Despite the successful application of this drug over the last 30 years, side effects, in particular myelosuppression, nephrotoxicity and neurotoxicity, remain the limiting factors for its therapeutic efficacy. Furthermore, efficient therapy is frequently challenging because tumor cells are either intrinsically resistant, or acquire resistance, to platinum drugs (2). In particular, resistance to platinum-based drugs remains a major problem in the management of advanced ovarian cancer. High-grade serous ovarian cancers frequently exhibit a complete clinical response to platinum-based drugs after initial chemotherapy. However, the majority of these patients will relapse with a progressive development of platinum drug resistance, resulting in overall poor prognosis. Hence the underlying resistance mechanisms to these chemotherapeutic drugs are subject to intense studies (3).
Cisplatin reacts directly with DNA, inducing DNA inter- and intra-strand crosslink lesions (ICLs) in proliferating tissues, and thereby triggering a DNA damage response (1). ICL repair is complex, involving multiple DNA repair pathways, and their coordination and regulation relies heavily upon the interplay between phosphorylation and ubiquitin-signaling (4-6). Initial recognition and removal of the ICL is coordinated by the Fanconi anemia (FA) tumor suppressor pathway (7), which targets the FANCI/FANCD2 complex for mono-ubiquitylation (8). Subsequently, the mono-ubiquitylated FANCI/FANCD2 complex promotes the removal of ICL-lesions by translesion synthesis (TLS), as well as homologous recombination-dependent repair mechanisms (7). Moreover, DNA double strand breaks (DSBs) have been identified as intermediates in ICL repair during S phase, and their formation involves the activity of the Mus81–Eme1 endonuclease (9). DSB repair is initiated by the ATM (ataxia-telangiectasia mutated) checkpoint kinase-dependent phosphorylation of MDC1 (mediator of DNA damage checkpoint), and the subsequent assembly of an ubiquitin-signaling complex, consisting of the E3 ubiquitin ligases RNF8 and RNF168 (4, 10). RNF168 and RNF8 cooperate with BRCA1 to promote repair of DNA lesions (4, 11). Cancers with defects in the ICL-repair pathway, which are frequently associated with BRCA1 and BRCA2/FANCD1 deficiency, are hypersensitive to DNA crosslinking agents, including cisplatin. Hence, this provides a strong rationale to use cisplatin for the treatment of BRCA1/2 mutated tumors.
Less is known about the determinants and pathways that sense excessive DNA crosslink damage, and that activate the apoptotic cell death pathway that eliminates irreversibly damaged cells. The tumour suppressor p53, a transcription factor, is stabilized and activated by genotoxic stress and triggers multiple effector pathways, including the intrinsic (also known as mitochondrial) apoptotic pathway [reviewed in (12)]. To induce apoptosis, activated homo-tetrameric p53 directly transcriptionally up-regulates the expression of several pro-apoptotic BCL-2 family members such as PUMA, NOXA and BAX. There is increasing evidence that excessive DNA damage, including cisplatin-induced lesions, can also activate the extrinsic death receptor apoptosis pathway [reviewed in (13, 14)]. This pathway is triggered by the binding of death ligands of the TNF (tumor necrosis factor) family to their cognate receptors, and the subsequent assembly of the receptor-associated DISC (death-inducing signaling complex). Initiator caspases 8 and 10 are activated within the DISC, and the death signal is amplified by the subsequent proteolytic activation of downstream effector caspases 3 and 7. Cisplatin treatment can also induce sustained activation of JNK (c-Jun N-terminal kinase), which triggers high levels of the transcription factor AP-1 (15). This leads to the expression of the FAS-L (FAS ligand) and consequently results in FAS receptor-mediated apoptosis.
The identification of novel determinants of the cellular response to cisplatin and other platinum-based cancer drugs might extend their clinical application, as well as provide potential insight into drug resistance mechanisms. The use of RNA interference as a tool for specifically silencing genes has opened up the possibility of performing high throughput loss-of-function and synthetic lethality screens. We have employed a collection of siRNA pools targeting the expression of genes involved in ubiquitin and ubiquitin-like (UBL) signaling to address whether ablation of expression of these proteins can cause cisplatin hypersensitivity. The screen was carried out using human osteosarcoma U2OS cells, which are p53-proficient and exhibit a robust DNA damage response (16). We identified the RING-in-between-RING (RBR) E3 ubiquitin ligase HOIP as a novel anti-apoptotic regulator in response to cisplatin-induced genotoxicity.
MATERIALS AND METHODS
U2OS cells used in the siRNA screen were grown in phenol red-free DMEM supplemented with 10% fetal calf serum and 2 mM L-glutamine. The cell lines: A2780, ZR.75.1, MDA-MB-231, PEA1, PEA2, PEO14, and PEO23 were maintained in RPMI supplemented with 10% fetal calf serum, 2 mM L-glutamine, 100 units/ml penicillin and 100 μg/ml streptomycin. Cisplatin-resistant A2780 cells were maintained as A2780 cells but supplemented with 1 μM cisplatin. HEK293, HeLa, HCT116, PC3 and U2OS cells were maintained in DMEM supplemented with 10 % fetal calf serum, 2 mM L-glutamine, 100 units/ml penicillin and 100 μg/ml streptomycin. MEFs were obtained from Prof. Philip Cohen (University of Dundee) and maintained as for HEK293 cells, with the addition of 1% sodium pyruvate and 1% non-essential amino acids. Stably transfected Flp-In T-Rex-293 cells were generated according to the manufacturer’s instructions (Invitrogen). A2780 and A2780 cisplatin-resistant cell lines were a kind gift from Dr. Gillian Smith (University of Dundee). PEA1, PEA2, PEO14 and PEO23 cell lines were a kind gift from Dr. Simon Langdon (University of Edinburgh). These cell lines were regularly examined for morphology and tested for cisplatin sensitivity status. All other cell lines were obtained from ATCC, where they were authenticated using short tandem repeat analysis, and passaged for a maximum of 2 months post resuscitation. All cell lines used were tested regularly for mycoplasma contamination.
Cell proliferation assay
HEK293 cells were seeded at 25–30% confluence in 10 cm2 dishes and treated with the indicated siRNA for 48 hours before being seeded at 5000 cells per well in a 96-well plate; five wells seeded for each dose of cisplatin. Cells were allowed to adhere for a minimum of 8 hours before addition of 0–5 μg/ml cisplatin. Cells were grown for 72–96 hours before MTS assays (Promega) were performed according to manufacturer’s instructions. Proliferation assays for all other cells were carried out as for HEK293 cells.
Clonogenic survival assays
Clonogenic survival assays were performed in triplicate. Cells were seeded at 25% confluence and allowed to adhere before being transfected with the indicated siRNA for 48 hours. Cells were then seeded in 10 cm2 dishes at 5000 cells per dish and allowed to adhere before being treated with the indicated concentrations of genotoxin for 24 hours. Cells were incubated in fresh medium for 10 days and colonies containing more than 50 cells were counted.
Cell lysis and immunoblot analysis
Whole cell extracts were prepared from mammalian cells by lysis in 40 mM HEPES pH 7.4, 120 mM NaCl, 1 mM EDTA, 1% Triton X-100 and HALT protease and phosphatase inhibitor cocktail (Pierce). Clarified protein lysates were separated by SDS-PAGE and transferred to a nitrocellulose membrane. ImageJ software was used to quantify immunoblots, and at least three independent experiments were used to obtain results.
Caspase activity in vitro assay
Cells were seeded at 25–30% confluence in 10 cm2 dishes and treated with the indicated siRNA for 48 hours prior to plating 10,000 cells per well of a 96-well plate, in triplicate for each condition. Cells were grown in phenol red-free DMEM and were replica plated on a 96-well plate for MTS assays. Caspase activity was measured 48 hours after treatment with the indicated dose of cisplatin, using the Caspase-Glo 3/7 or Caspase-Glo 8 assays (Promega), according to manufacturer’s instructions. Caspase activity was normalized with respect to cell number per well, as calculated by an MTS assay (Promega).
Immunofluorescence analysis
Cells were treated with the indicated dose of genotoxin for the times indicated in the figure legends, and processed as described previously (17).
JNK in vitro kinase assay
JNK kinases were immunoprecipitated from 100 μg of HEK293 cell whole cell lysate, treated as indicated in the figure legend, using 3 μg of anti-JNK1 antibody coupled to 10 μl protein G Sepharose. Immunoprecipitates were washed thoroughly in cell lysis buffer and then equilibrated in kinase assay buffer (50 mM Tris/HCl pH 7.5, 0.1 mM EGTA and 0.1% 2-mercaptoethanol). Assays were performed as described previously (18) using a peptide corresponding to GST-ATF2 amino acids 19–96, at a concentration of 0.2 mg/ml as a substrate.
NF-κB luciferase reporter assay
Cells to be analyzed for NF-κB activation were seeded at 25–30% confluence in 6-well plates and treated with the indicated siRNA for 24 hours, or induced with tetracycline for 24 hours, prior to transfection with 3 × NF-κB ConA luciferase reporter plasmid. After 24 hours, the indicated concentrations of cisplatin were added to cells and luciferase activity was measured 24 or 48 hours later, as indicated. Luciferase activity was measured using the Dual-Luciferase Reporter Assay System (Promega) according to manufacturer’s instructions. Assays were performed in triplicate and luciferase signals were normalized with respect to the cell lysate protein concentration.
RESULTS
siRNA screen identifies HOIP as an enhancer of cisplatin-induced cytotoxicity
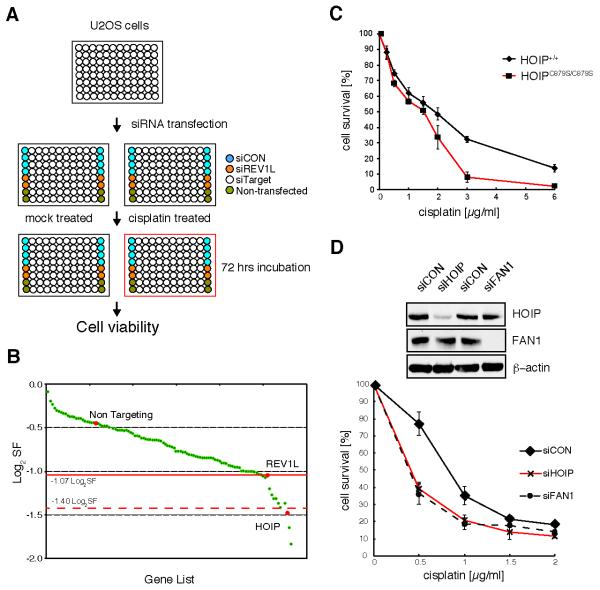
We designed a robust, high throughput RNA interference (RNAi) platform to screen for enhancement of cisplatin-induced cell death in the human osteosarcoma cell line U2OS. We used an siRNA library targeting 1067 human genes, which are either validated, or computationally predicted, components related to the ubiquitin- and ubiquitin-like (UBL)- signaling machinery (Supplementary Material). These include: ubiquitin, SUMO, NEDD8, E1s, E2s, E3s, UBL-specific proteases, and UBL-binding domain-containing proteins (siRNA “ubiquitome” library). The design of our enhancer screen is outlined in Figure 1A. Briefly, U2OS cells were reverse transfected in replicas with a library of siRNA pools (SMARTPools). Each plate contained non-transfected cells, negative control (siCON, non-target), and cells transfected with siRNA against the positive control REV1L (siREV1L), a TLS polymerase required for ICL repair (19). Sixteen hours after transfection, one replica was treated with 3 μM cisplatin and the other replica with the vehicle, DMSO. Cells were incubated for further 72 hours and viability of cells was assayed using an ATP-dependent cell viability assay. To quantify the robustness of this assay system, we calculated the Z′ factor. The average factor for our entire screen was Z′ = 0.58, indicating an excellent assay performance. The screen was completed in duplicate. Firstly, data were filtered for lethal siRNA by calculating cell growth (CGE) and rejecting siRNAs causing CGE ≤50%. Secondly, we used a log2 surviving fraction (log2SF) threshold of −1.47 or less to identify 112 siRNAs that significantly sensitized cells to cisplatin (Supplemental Material).
Figure 1.
siRNA screen identifies HOIP as an enhancer of cisplatin-induced genotoxicity. A, schematic illustration of the primary siRNA screen, using the siRNA “ubiquitome” library in U2OS cells. B, maximum log2SF is plotted for each gene that was classed as cisplatin-hypersensitive in the primary screen. C, paired wild-type HOIP+/+ and HOIPC879S/C879S knock-in mouse embryonic fibroblasts; cell viability was assayed 72 hrs after cisplatin treatment by MTS cell proliferation assay. D, HEK293 cells were transfected with the indicated siRNAs and cell lysates were assayed for efficient knockdown by immunoblotting with the indicated antibodies (top panel). Cell viability was assayed as in (C). Data in (C) and (D) are represented as mean ± SEM from three independent experiments.
siRNAs from the primary screen were subjected to a secondary screen in which cells were treated with 0–6 μM of cisplatin (Supplementary Fig. S1A). This was performed in triplicate, and dose dependent log2SF functions were plotted to determine the maximum log2SF for each siRNA (Fig. 1B). Setting the threshold of the maximum log2SF values to −1.07 we identified, with high confidence, 14 siRNAs that sensitized U2OS cells to cisplatin.
To test for off-target effects, the 14 siRNAs were assayed separately with each of the four different siRNA species from the SMARTPool that target the same gene. Such siRNAs were considered to function on-target when at least three of the four individual siRNAs caused enhanced cisplatin sensitivity. By applying these strict criteria we identified the E3 ubiquitin ligase HOIP (RNF31) as being required for cellular resistance to cisplatin (Supplementary Fig. S1B). HOIP is the catalytic subunit of the E3 ubiquitin ligase LUBAC, which also consists of the components HOIL-1L and SHARPIN (20, 21). LUBAC is a critical regulator of the canonical NF-κB signaling pathway, and has been implicated in inflammatory diseases and immune regulation (22). No role, however, has been suggested for HOIP in response to cisplatin. We therefore chose to examine the biological significance of HOIP depletion, and the mechanisms impacting cisplatin hypersensitivity.
As further validation, we analyzed cisplatin sensitivity in mouse embryonic fibroblast (MEF) cell lines obtained from paired wild-type and HOIPC879S/C879S E3 ligase-dead knock-in mice, in which there is no LUBAC activity and no detectable linear ubiquitin chain formation (21). The HOIPC879S/C879S MEFs exhibited cisplatin hypersensitivity, indicating that HOIP ligase activity is required for cellular resistance to cisplatin (Fig. 1C). In addition, we repeated cisplatin sensitivity assays using transformed human embryonic kidney 293 (HEK293) cells. HOIP depletion (siHOIP) was confirmed by immunoblot analysis and resulted in enhanced cisplatin sensitivity when compared to cells transfected with vehicle only (siCON) (Fig. 1D and Supplementary Fig. S1C). Consistent with published data, we noted that depletion of HOIP in HEK293 and U2OS cells resulted in destabilization of the LUBAC complex members HOIL-1L and SHARPIN (Supplementary Fig. S1D and S1E) (23-25). siRNA targeting FAN1, a structure specific nuclease required for interstrand crosslink repair, was used as control for cisplatin hypersensitivity (26).
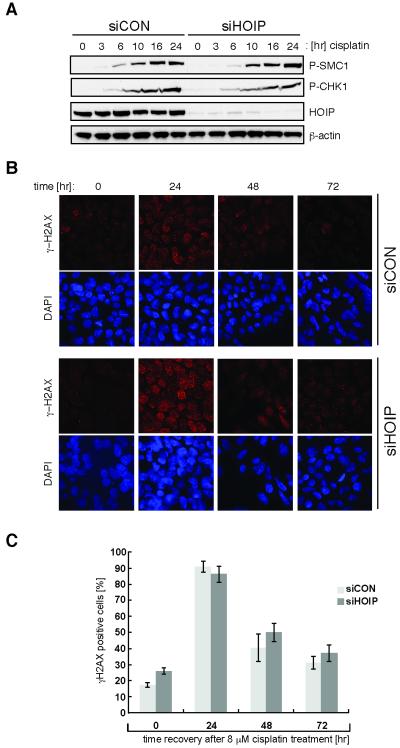
HOIP is not required for DNA damage checkpoint activation
To uncover the mechanisms underlying cisplatin hypersensitivity of HOIP-depleted cells, we first analysed the integrity of early DNA damage signaling mediated by the checkpoint kinases ATM and ATR. Cisplatin-induced phosphorylation of SMC1 on serine 966, a target of ATM, and phosphorylation of CHK1 kinase on serine 345, a substrate of ATR, were unaffected by HOIP depletion (siHOIP) in HEK293 cells (Fig. 2A). These results indicate a robust and timely DNA damage checkpoint activation. The presence of cisplatin-induced DNA lesions triggers the checkpoint kinase-dependent phosphorylation of the histone variant H2AX (γ-H2AX). Distinct nuclear foci of γ-H2AX can be visualized at sites of damage, allowing monitoring of DNA repair (27, 28). To test the possibility that accumulation of persistent DNA damage is the cause of cisplatin hypersensitivity, we monitored γ-H2AX foci formation in mock (siCON) and HOIP-depleted (siHOIP) HEK293 cells, following cisplatin exposure and recovery over 72 hours (Fig. 2B and 2C). HOIP-depleted cells show kinetics of accumulation and resolution of γ-H2AX foci similar to those of mock transfected cells, suggesting that cisplatin hypersensitivity after HOIP depletion is unlikely to be caused by an accumulation of persistent cisplatin-induced DNA lesions.
Figure 2.
HOIP is not required for DNA damage checkpoint activation. A, HEK293 cells were transfected with siCON or siHOIP and treated with 5 μM cisplatin for the indicated times. Cell lysates were analyzed by immunoblotting with the antibodies indicated. B, siCON or siHOIP transfected HEK293 cells were treated with 8 μM cisplatin for 2 hours before being allowed to recover for the times indicated. Cells were then fixed and stained with DAPI to visualize nuclei and γ-H2AX antibody to visualize γ-H2AX foci. C, quantitation of the data in (B): cells with more than 6 foci were classed as γ-H2AX positive. Data in (C) is presented as mean ± SEM from three independent experiments.
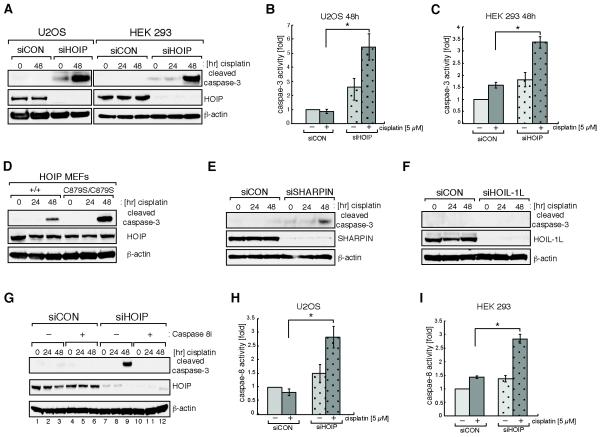
HOIP depletion sensitizes cells to genotoxin-induced apoptotic cell death
Cells trigger apoptotic cell death when genotoxic stress exceeds a certain threshold or upon the accumulation of persistent DNA lesions. It was recently shown that loss of the LUBAC component SHARPIN increased susceptibility to TNF-α-induced caspase-8/caspase-3 mediated apoptosis (24). Given this, we hypothesized that HOIP depletion might sensitize cells to apoptotic cell death following cisplatin-induced damage. We treated U2OS and HEK293 cells with 5 μM cisplatin and observed, in both cell lines, that after 48 hours the proteolytic cleavage of the effector caspase, caspase-3, was almost undetectable in siCON control cells but greatly increased when HOIP was depleted (Fig. 3A). Similar results were obtained following treatment of cells with other genotoxins such as etoposide, hydroxyurea and ionizing radiation, or stress stimuli, including the broad-spectrum kinase inhibitor staurosporine and the mitotic inhibitor taxol (Supplementary Fig. S2A-E). We assayed caspase-3 activity in lysates of either siCON- or siHOIP-depleted cells treated with cisplatin and noted a modest elevation of caspase-3 activity in untreated siHOIP cells. However, 48 hours after cisplatin exposure caspase-3 activity was significantly enhanced in siHOIP cells, compared to siCON cells (Fig. 3B and 3C). Importantly, we also observe increased cisplatin-induced caspase-3 cleavage in HOIPC879S/C879S MEFs when compared to control wild-type MEFs (Fig. 3D). We tested whether the phenotype of HOIP depletion could be mimicked by siRNA silencing of the LUBAC subunits SHARPIN and HOIL-1L. Indeed, SHARPIN, but not HOIL-1L, depletion causes a cisplatin-induced accumulation of cleaved caspase-3, suggesting that HOIP and SHARPIN cooperate in an anti-apoptotic cell death response (Fig. 3E and 3F). Caspase-8 is one of the critical caspases upstream of caspase-3 and has been previously implicated in genotoxin-induced apoptosis (29-31). To test whether caspase-8 mediates the observed cisplatin-induced caspase-3 activation, cells were pre-treated with the specific caspase-8 inhibitor Z-IETD-FMK before cisplatin treatment. Caspase-8 inhibition completely abolished siHOIP-enhanced caspase-3 activation (Fig. 3G and S2F). In agreement with this, we observed a significant elevation of caspase-8 activity in HOIP-depleted U2OS, as well as HEK293, cells after cisplatin treatment (Fig. 3H and 3I). These data suggest that HOIP protects cells from cisplatin-induced caspase-8-mediated apoptotic cell death.
Figure 3.
HOIP depletion sensitizes cells to cisplatin-induced apoptotic cell death. A, U2OS (left panel) or HEK293 (right panel) cells were transfected with mock siRNA (siCON) or siRNA targeting HOIP (siHOIP) and treated with 5 μM cisplatin for the indicated time. Cell lysates were analyzed by immunoblotting with the antibodies indicated. B, siCON or siHOIP transfected U2OS cells were treated with 5 μM cisplatin for 48 hours before caspase-3 activity was measured using a caspase-3 Glo assay; * indicates a p-value of 0.0028 C, HEK293 cells were treated as described in (B); * indicates a p-value of 0.0222. D, paired wild-type HOIP+/+ and HOIPC879S/C879S knock-in mouse embryonic fibroblasts were treated with 3 μM cisplatin for the indicated time. Cell lysates were analyzed by immunoblotting. E and F, as (A) except that cells were transfected with siSHARPIN or siHOIL-1L. G, siCON or siHOIP transfected HEK293 cells were treated with vehicle only (−) or 20 μM Z-IETD-FMK caspase-8 inhibitor (+) one hour before treatment with 5 μM cisplatin for the indicated times. Cell lysates were analyzed by immunoblotting. H, as (B) except that a caspase-8 Glo assay was used to measure caspase-8 activity; * indicates a p-value of 0.0076. I, HEK293 cells were treated as described in (H). * indicates a p-value of 0.014. Data in (B), (C), (H) and (I) are represented as mean ± SEM from three independent experiments and Student’s t-test was used to calculate significance.
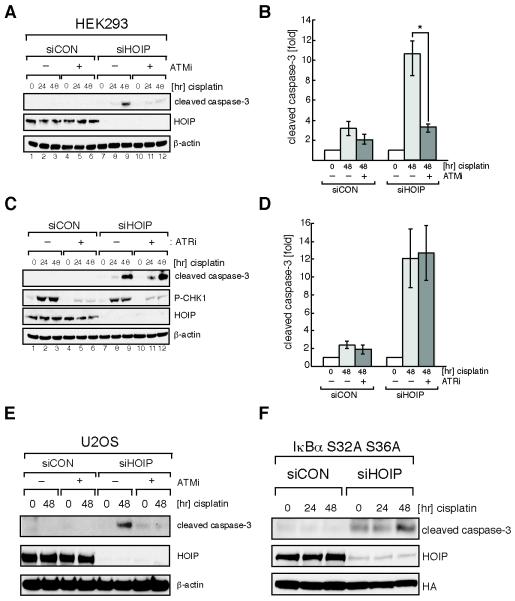
Apoptotic sensitization of HOIP-depleted cells requires ATM but occurs in the absence of NF-κB activation
Recent evidence indicates that ATM drives LUBAC-dependent linear ubiquitin chain formation on NEMO, which promotes NF-κB activation in cells exposed to topoisomerase inhibitors, such as etoposide (32). We show that the highly specific ATM kinase inhibitor KU55933 (Fig. 4, A, B and E) (33), but not the ATR kinase inhibitor ETP-46464 (Fig. 4, C and D) (34) blocks enhanced proteolytic caspase-3 cleavage in HOIP-depleted HEK293 and U2OS cells. Next we analyzed whether NF-κB signaling is implicated in the hyper-apoptotic cisplatin response observed in HOIP-depleted cells. We generated a HEK293 cell overexpressing a dominant mutant version of the NF-κB inhibitory protein IκBα ( IκBα S32A/S36A), which cannot be phosphorylated by IKK and thus cannot be targeted for proteasomal degradation (35). Overexpression of IκBα S32A/S36A in HEK293 cells completely abolished cisplatin-induced NF-κB activation (Supplementary Fig. S3). We then transfected IκBα S32A/S36A expressing HEK293 cells with either siCON or siHOIP followed by cisplatin treatment for 48 hours. HOIP depletion in IκBα S32A/S36A cells resulted in an elevation of caspase-3 activation in untreated cells (t = 0 h), and this was enhanced following cisplatin exposure (t = 48 h) (Fig. 4F). No significant caspase-3 activation was detected in siCON silenced IκBα S32A/S36A cells following cisplatin exposure. Cumulatively, this suggests that cisplatin-induced apoptotic cell death in HOIP-depleted cells occurs independently of NF-κB activation.
Figure 4.
Apoptotic sensitization of HOIP-depleted cells requires ATM but is independent of ATR activity. A, siRNA transfected HEK293 cells were treated with vehicle only (−) or 10 μM KU-55933 ATM inhibitor (+) one hour before treatment with 5 μM cisplatin for the indicated times. Cell lysates were analyzed by immunoblotting. B, quantitation of the data shown in (A). Image J software was used to quantify immunoblots; * indicates a p-value of 0.0078. C, as (A) except that 5 μM ETP-46464 ATR inhibitor was used. D, quantitation of the data shown in (C). E, As (A) except that U2OS cells were used. F, HA-IκBa(S32A,S36A)-expressing HEK293 cells were transfected either with siCON or siHOIP and treated with 5 μM cisplatin for the indicated times. Cell lysates were subjected to immunoblot analyses. Data in (B) and (D) are represented as mean ± SEM from three independent experiments and Student’s t-test was used to calculate significance.
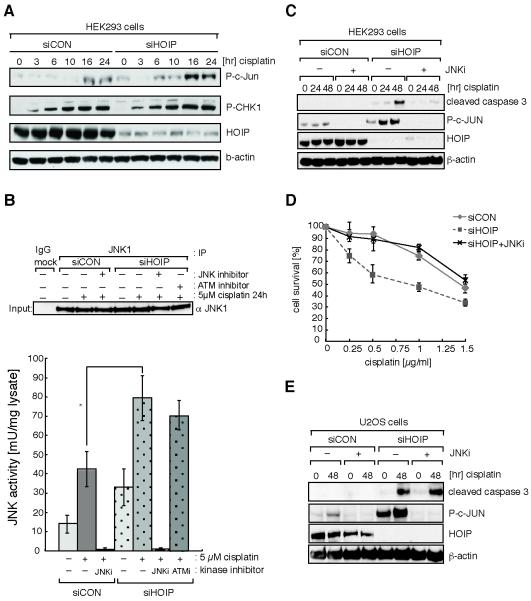
JNK activity is elevated in HOIP-depleted cells
In addition to NF-κB activation, extensive DNA damage triggers activation of the stress-responsive Jun kinase (JNK) and induces FADD-mediated pro-caspase-8 activation (36). We therefore assayed JNK activity by monitoring JNK-targeted phosphorylation of c-Jun (P-c-Jun) in response to cisplatin. We observed that HOIP-depleted cells respond with enhanced c-Jun phosphorylation, suggesting increased JNK activity (Fig. 5A). To assess JNK kinase activity directly we immunoprecipitated JNK from cell lysates of mock (−) or cisplatin (+) treated cells that were either siCON or siHOIP transfected, and assayed the kinase in vitro. In agreement with increased P-c-JUN we observed a significantly enhanced basal, as well as cisplatin-induced, JNK activity in HOIP-depleted cells (Fig. 5B). To test whether ATM is required for cisplatin-induced JNK activation, we pre-treated cells with ATM kinase inhibitor KU55933 (ATMi) following cisplatin treatment. ATM inhibition had no effect on JNK activation, suggesting that ATM checkpoint activation is not regulating JNK activity in response to cisplatin (Fig. 5B). Utilizing the highly specific JNK inhibitor JNK-IN-8 (37) we next showed that caspase-3 processing in HOIP-depleted HEK293 cells was significantly suppressed (Fig. 5C), and cisplatin hypersensitivity reversed (Fig. 5D), following JNK inhibition. Consistent with HEK293 cells, HOIP depletion in U2OS cells triggered elevation of both basal and cisplatin-induced JNK activity (Fig 5E). However, JNK inhibition did not alleviate caspase-3 hyper-activation in HOIP-depleted U2OS cells, suggesting a cell type-specific requirement of JNK in apoptotic cell death.
Figure 5.
JNK activity is elevated and essential for apoptotic cell death in HOIP-depleted cells. A, siRNA transfected HEK293 cells were treated with 25 μM cisplatin for the indicated times. Cell lysates were analyzed by immunoblotting. B, siCON or siHOIP transfected HEK293 cells were pre-treated with 3 μM JNK inhibitor or 10 μM ATM inhibitor before vehicle only (−) or 5 μM cisplatin treatment (+) as indicated. JNK kinases were immunoprecipitated from cell lysates, and kinase activity was measured using an in vitro kinase assay. Activity is presented as mU/mg cell lysate; * Student’s t-test was used to calculate significance: p=0.0396. C, siCON- or siHOIP-transfected HEK293 cells were pre-treated with vehicle only (−) or 3 μM JNK inhibitor (+) before treatment with 5 μM cisplatin for the indicated times. Cell lysates were analyzed by immunoblotting. D, Cell viability of siCON or siHOIP-transfected cells pre-treated with JNK inhibitor in response to cisplatin was measured by MTS cell proliferation assay. E, As (C) except that U2OS cells were used. Data in (B) and (D) are represented as mean ± SEM from three independent experiments.
HOIP depletion induces cisplatin hypersensitivity in different types of cancer cells
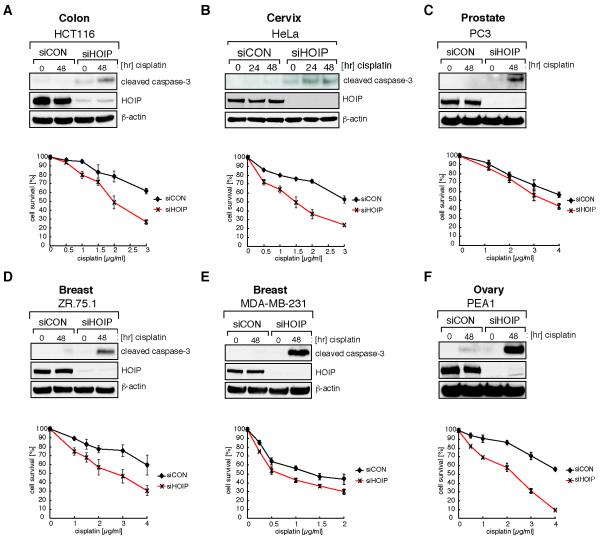
We demonstrate here that HOIP depletion sensitizes U2OS osteosarcoma cells to cisplatin-induced apoptotic cell death. These findings suggest potential strategies to sensitize cancer cells or, even more relevantly, to re-sensitize cancer cells that have become resistant following prolonged cisplatin exposure. To test this concept, we studied the effect of HOIP depletion in a panel of cancer cell lines derived from different tissues. HCT116 (colon), HeLa (cervix), PC3 (prostate), ZR.75.1 (breast), MDA-MB-231 (breast) and PEA1 (ovary) were transfected with either siCON or siHOIP and analyzed for cisplatin-induced caspase-3 activation. In addition, cell viability was determined following exposure to a dose range of cisplatin (Fig. 6). Consistently, all siHOIP-transfected cancer cell lines exhibited, to varying degrees, elevated cleaved caspase-3 after cisplatin exposure, which coincided with increased cisplatin sensitivity.
Figure 6.
HOIP-depletion sensitizes a range of cancer cell lines to cisplatin-induced apoptotic cell death. HCT116 (A), HeLa (B), PC3 (C), ZR.75.1 (D), MDA-MB-231 (E) or PEA1 (F) cells were transfected with mock siRNA (siCON) or siRNA targeting HOIP (siHOIP) and treated with 5 μM cisplatin for the indicated time before cell lysates were analyzed by immunoblotting with the antibodies indicated (upper panels). Cell viability of siCON- or siHOIP-transfected cells in response to the indicated concentration range of cisplatin was measured by MTS cell proliferation assays (lower panels). Data shown are represented as mean ± SEM from three independent experiments.
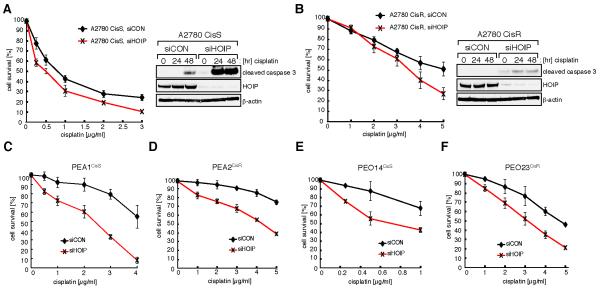
The PEA1 ovarian cancer cell line exhibits particularly striking cisplatin hypersensitivity upon HOIP-depletion. During platinum drug chemotherapy, ovarian cancers frequently develop drug resistance. Hence we next addressed whether cisplatin-resistant ovarian cancer cells can be re-sensitized by HOIP-depletion. We analyzed paired platinum sensitive (A2780CisS) and resistant (A2780CisR) ovarian tumor cell lines. A2780CisR cells are in vitro derivatives of the sensitive A2780CisS cells that have acquired resistance through continuous treatment with cisplatin (38). A2780CisS and A2780CisR cells were transfected with either siCON or siHOIP and cellular viability was determined following exposure to cisplatin. HOIP depletion modestly sensitized A2780CisS cells (Fig. 7A), however, depletion resulted in a significant re-sensitization of A2780CisR cells (Fig. 7B). Importantly, A2780CisS and A2780CisR cells also showed increased apoptosis following HOIP depletion (Fig. 7, A and B). Next, we investigated high-grade serous ovarian cancers (HGS) derived from cases of HGS carcinomas before and after the development of platinum resistance (39). HOIP depletion in the matched pair of PEA1CisS and PEA2CisR triggered enhanced cisplatin sensitivity in both cell lines (Fig 7, C and D). Similar results were obtained from the matching pair of PEO14CisS and PEO23CisR, suggesting that HOIP protects these cells from cisplatin genotoxicity (Fig 7, E and F).
Figure 7.
HOIP depletion sensitizes cisplatin-resistant ovarian cancer cell lines. A and B (left), A2780CisS and A2780CisR cells were transfected with siCON or siHOIP as indicated and cell viability assessed by MTS cell proliferation assays. A and B (right), A2780CisS and A2780CisR cells were transfected with siCON or siHOIP and treated with 5 μM cisplatin for the indicated time. Cell lysates were analyzed by immunoblotting. C–F, cell lines were transfected with siCON or siHOIP as indicated and cell viability assessed by MTS cell proliferation assays. Data shown are presented as mean ± SEM from three independent experiments.
DISCUSSION
Managing resistance that occurs during platinum-based drug chemotherapy remains a major challenge in the treatment of ovarian and other cancers, and results in poor prognosis for successful eradication of the cancer. Cells respond to platinum drugs in several ways and the resistance mechanisms modulating these responses, to protect cells from platinum genotoxicity, are equally complex. Typically for platinum-based drugs, cancer cells frequently gain resistance by reduced cellular drug uptake, increased drug efflux, increased DNA repair and MHL1 hypermethylation (3). In this study we identified HOIP, the catalytic E3 ubiquitin ligase component of LUBAC, as an anti-apoptotic factor attenuating the genotoxic effect of genotoxic agents including cisplatin. We provide evidence that HOIP-depletion in several cancer cell lines can potentiate cellular genotoxicity of cisplatin due to enhanced apoptotic cell death response.
We show that two subunits of LUBAC: HOIP and SHARPIN, are required to suppress cisplatin-induced apoptosis, whereas we have no evidence that the subunit HOIL-1L participates in the cisplatin response. Studies in vitro have demonstrated that HOIP on its own has only weak E3 ubiquitin ligase activity, but that its catalytic activity is strongly enhanced in the presence of either SHARPIN or HOIL-1L, assembling a HOIP/SHARPIN or HOIP/HOIL-1L complex respectively (40, 41). It is debateable whether these subcomplexes co-exist in vivo, however genetic evidence supports the idea that these LUBAC subcomplexes have separable functions (22). SHARPIN-deficient mice exhibit chronic proliferative dermatitis (cpdm), a pathology not observed in HOIL-1L-deficient mice. Moreover, these studies show that SHAPRIN-deficient MEFs have far higher levels of TNF-α-induced FADD and caspase-8-dependent apoptotic cell death than cells from HOIL-1L-deficient mice (24). TNF-α-induced apoptosis is triggered by the formation of a TRADD–TRAF2–RIP1–FADD–caspase-8 death-promoting complex (TNFR1 complex II), whereas in response to genotoxic stress an analogous ripoptosome complex, consisting of RIP1–FADD–capase-8, is formed (42). Moreover, CUL3 RING ligase-mediated poly-ubiquitylation of caspase-8 stabilizes and promotes caspase-8 activity (43). It is tempting to speculate that a HOIP/SHARPIN ligase complex regulates cisplatin-induced apoptosis via ripoptosome assembly and activity. Indeed this idea is supported by data indicating that the ripoptosome component RIP1 is modified with linear ubiquitin chains (23, 44). Whether the ripoptosome components are direct targets for HOIP/SHARPIN-mediated linear ubiquitylation following genotoxic stress will be the subject of future investigations.
Besides an essential function for ATM in promoting the apoptotic cell death response in response to cisplatin, we found that basal and cisplatin-induced JNK kinase activity is significantly up-regulated in HOIP-depleted cells. It has been shown that sustained activation of JNK in ovarian cancer cells, in response to cisplatin, leads to activation of the AP-1 transcription factor and AP-1-induced FAS-L expression leading, in turn, to Fas receptor-triggered apoptosis (15). Moreover, JNK activation has previously been linked to the degradation of the anti-apoptotic factor c-FLIPL, suggesting that JNK hyperactivation would lead to enhanced ripoptosome/caspase-8 activity (45). The importance of the JNK/FAS apoptosis pathway is further demonstrated by the observation that several independently derived cisplatin-resistant cancer cell lines exhibit attenuated JNK activation (46).
The design of the RNA interference approach applied in this study allowed us to screen directly for targets whose down-regulation might either sensitize cancer cells for cisplatin or might reverse cisplatin resistance in tumours. In all cancer cells tested, siRNA depletion of HOIP reduced their viability and enhanced apoptotic cell death following cisplatin exposure. Enhanced cisplatin-induced apoptosis was also observed in HOIPC879S/C879S MEFs that express catalytically inactive HOIP. We therefore propose that HOIP E3 ligase activity plays a critical role in limiting apoptotic cell death upon cisplatin exposure. Indeed our data show that HOIP-depleted cells exhibit enhanced apoptosis in response to treatment with a wide range of genotoxins, as well as non-genotoxic cellular stress-inducers. This suggests that a HOIP-dependent anti-apoptotic mechanism is likely to be conserved in the response to cellular stress in general. Hence, blocking or easing the anti-apoptotic function of HOIP will render cancer cells more susceptible to apoptosis, and low levels of genotoxic stress will trigger apoptotic cell death.
Although it is clear that HOIP-depletion renders cells hypersensitive to cisplatin treatment, based on our current knowledge it is not clear what, if any, contribution HOIP has to drug resistance. Some evidence that HOIP overexpression may contribute to cisplatin-resistance in cancer comes from large-scale gene–drug association studies (https://www.oncomine.com) (47). Expression profile data from a panel of several hundred cancer cell lines derived from tumours classified as cisplatin-resistant or cisplatin-sensitive has revealed that cisplatin-resistant cancer cell lines (n=335) show a significantly higher expression of HOIP (p=0.011) and SHARPIN (p=0.016) when compared to cisplatin-sensitive cancer cell lines (n=29) (Supplementary Fig. S4). Oncomine™ analysis also revealed that both HOIP and SHARPIN are overexpressed in serous ovarian carcinomas patient samples compared to normal tissue (Supplementary Fig. S5). These data suggest that HOIP/SHARPIN expression is altered in cancer cell lines, and that an elevation of HOIP and HOIP’s anti-apoptotic function may promote cisplatin resistance.
Enhancing or re-establishing the apoptotic cell death programme in tumour cells is a promising strategy for cancer therapy. Based on our findings, we propose that small molecule inhibitory drugs targeting HOIP E3 ligase activity, used in combination with cisplatin chemotherapy, might potentiate caspase-8-mediated death in cancer cells. This concept is supported by recent studies that describe Smac mimetics, i.e. small molecules that mimic the antagonistic activity of Smac towards IAPs (inhibitor of apoptosis proteins), as efficient potentiators of cancer cell apoptosis. They stimulate IAP auto-ubiquitylation and proteasomal degradation, leading to TNFα signalling which, in turn, induces ripotosome activation and caspase-8-dependent cell death in multiple cancer cell lines (48-50). We predict that HOIP inhibitors, although acting through mechanisms different from those of the Smac mimetics, would significantly up-regulate cisplatin-induced caspase-8 activity. HOIP depletion or deficiency does not kill transformed cells and hence HOIP inhibitors are unlikely to be toxic when used alone. However, HOIP plays a critical role in the innate immune response and the application of HOIP inhibitors bear the potential risk of stimulating inflammatory and/or auto-inflammatory side effects (22). An in-depth analysis of the impact of HOIP inhibition is needed in order to fully understand how HOIP can best be exploited as an anti-cancer drug target. This study, however, provides the rationale for the development of HOIP inhibitors to increase not only the efficacy of first-line cisplatin chemotherapy but also to achieve a better clinical outcome of cisplatin-based chemoptherapy in drug-resistant cancer.
Supplementary Material
Acknowledgements
We gratefully acknowledge the technical support provided by the Division of Signal Transduction Therapy (DSTT). We would like to thank Philip Cohen and his laboratory for sharing reagents and useful discussions. We would also like to thank Gillian Smith and Simon Langdon for providing cancer cell lines.
Funding: This work was supported by the Scottish Institute for Cell Signalling and the pharmaceutical companies supporting DSTT (AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Janssen Pharmaceutica, Merck–Serono and Pfizer), and, in part, by the Wellcome Trust Strategic Award grant 097945/B/11/Z.
Footnotes
Disclosure of Potential Conflicts of Interest: No potential conflicts of interest were disclosed.
REFERENCES
- 1.Kelland L. The resurgence of platinum-based cancer chemotherapy. Nat Rev Cancer. 2007;7:573–84. doi: 10.1038/nrc2167. [DOI] [PubMed] [Google Scholar]
- 2.Koberle B, Tomicic MT, Usanova S, Kaina B. Cisplatin resistance: preclinical findings and clinical implications. Biochimica et biophysica acta. 2010;1806:172–82. doi: 10.1016/j.bbcan.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 3.Holohan C, Van Schaeybroeck S, Longley DB, Johnston PG. Cancer drug resistance: an evolving paradigm. Nature reviews Cancer. 2013;13:714–26. doi: 10.1038/nrc3599. [DOI] [PubMed] [Google Scholar]
- 4.Al-Hakim A, Escribano-Diaz C, Landry MC, O’Donnell L, Panier S, Szilard RK, et al. The ubiquitous role of ubiquitin in the DNA damage response. DNA repair. 2010;9:1229–40. doi: 10.1016/j.dnarep.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ulrich HD, Walden H. Ubiquitin signalling in DNA replication and repair. Nat Rev Mol Cell Biol. 2010;11:479–89. doi: 10.1038/nrm2921. [DOI] [PubMed] [Google Scholar]
- 6.Deans AJ, West SC. DNA interstrand crosslink repair and cancer. Nat Rev Cancer. 2011;11:467–80. doi: 10.1038/nrc3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moldovan GL, D’Andrea AD. How the fanconi anemia pathway guards the genome. Annu Rev Genet. 2009;43:223–49. doi: 10.1146/annurev-genet-102108-134222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alpi AF, Patel KJ. Monoubiquitylation in the Fanconi anemia DNA damage response pathway. DNA Repair (Amst) 2009;8:430–5. doi: 10.1016/j.dnarep.2009.01.019. [DOI] [PubMed] [Google Scholar]
- 9.Hanada K, Budzowska M, Modesti M, Maas A, Wyman C, Essers J, et al. The structure-specific endonuclease Mus81-Eme1 promotes conversion of interstrand DNA crosslinks into double-strands breaks. The EMBO journal. 2006;25:4921–32. doi: 10.1038/sj.emboj.7601344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oestergaard VH, Pentzold C, Pedersen RT, Iosif S, Alpi A, Bekker-Jensen S, et al. RNF8 and RNF168 but not HERC2 are required for DNA damage-induced ubiquitylation in chicken DT40 cells. DNA repair. 2012;11:892–905. doi: 10.1016/j.dnarep.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 11.Mattiroli F, Vissers JH, van Dijk WJ, Ikpa P, Citterio E, Vermeulen W, et al. RNF168 ubiquitinates K13-15 on H2A/H2AX to drive DNA damage signaling. Cell. 2012;150:1182–95. doi: 10.1016/j.cell.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 12.Vousden KH, Lane DP. p53 in health and disease. Nature reviews Molecular cell biology. 2007;8:275–83. doi: 10.1038/nrm2147. [DOI] [PubMed] [Google Scholar]
- 13.Basu A, Krishnamurthy S. Cellular responses to Cisplatin-induced DNA damage. Journal of nucleic acids. 2010;2010 doi: 10.4061/2010/201367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roos WP, Kaina B. DNA damage-induced cell death: from specific DNA lesions to the DNA damage response and apoptosis. Cancer letters. 2013;332:237–48. doi: 10.1016/j.canlet.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 15.Mansouri A, Ridgway LD, Korapati AL, Zhang Q, Tian L, Wang Y, et al. Sustained activation of JNK/p38 MAPK pathways in response to cisplatin leads to Fas ligand induction and cell death in ovarian carcinoma cells. The Journal of biological chemistry. 2003;278:19245–56. doi: 10.1074/jbc.M208134200. [DOI] [PubMed] [Google Scholar]
- 16.Zenvirt S, Kravchenko-Balasha N, Levitzki A. Status of p53 in human cancer cells does not predict efficacy of CHK1 kinase inhibitors combined with chemotherapeutic agents. Oncogene. 2010;29:6149–59. doi: 10.1038/onc.2010.343. [DOI] [PubMed] [Google Scholar]
- 17.MacKay C, Declais AC, Lundin C, Agostinho A, Deans AJ, MacArtney TJ, et al. Identification of KIAA1018/FAN1, a DNA repair nuclease recruited to DNA damage by monoubiquitinated FANCD2. Cell. 2010;142:65–76. doi: 10.1016/j.cell.2010.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hastie CJ, McLauchlan HJ, Cohen P. Assay of protein kinases using radiolabeled ATP: a protocol. Nature protocols. 2006;1:968–71. doi: 10.1038/nprot.2006.149. [DOI] [PubMed] [Google Scholar]
- 19.Niedzwiedz W, Mosedale G, Johnson M, Ong CY, Pace P, Patel KJ. The Fanconi anaemia gene FANCC promotes homologous recombination and error-prone DNA repair. Mol Cell. 2004;15:607–20. doi: 10.1016/j.molcel.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 20.Kirisako T, Kamei K, Murata S, Kato M, Fukumoto H, Kanie M, et al. A ubiquitin ligase complex assembles linear polyubiquitin chains. EMBO J. 2006;25:4877–87. doi: 10.1038/sj.emboj.7601360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Emmerich CH, Ordureau A, Strickson S, Arthur JS, Pedrioli PG, Komander D, et al. Activation of the canonical IKK complex by K63/M1-linked hybrid ubiquitin chains. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:15247–52. doi: 10.1073/pnas.1314715110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Walczak H, Iwai K, Dikic I. Generation and physiological roles of linear ubiquitin chains. BMC biology. 2012;10:23. doi: 10.1186/1741-7007-10-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gerlach B, Cordier SM, Schmukle AC, Emmerich CH, Rieser E, Haas TL, et al. Linear ubiquitination prevents inflammation and regulates immune signalling. Nature. 2011;471:591–6. doi: 10.1038/nature09816. [DOI] [PubMed] [Google Scholar]
- 24.Ikeda F, Deribe YL, Skanland SS, Stieglitz B, Grabbe C, Franz-Wachtel M, et al. SHARPIN forms a linear ubiquitin ligase complex regulating NF-kappaB activity and apoptosis. Nature. 2011;471:637–41. doi: 10.1038/nature09814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tokunaga F, Nakagawa T, Nakahara M, Saeki Y, Taniguchi M, Sakata S, et al. SHARPIN is a component of the NF-kappaB-activating linear ubiquitin chain assembly complex. Nature. 2011;471:633–6. doi: 10.1038/nature09815. [DOI] [PubMed] [Google Scholar]
- 26.Sengerova B, Wang AT, McHugh PJ. Orchestrating the nucleases involved in DNA interstrand cross-link (ICL) repair. Cell cycle. 2011;10:3999–4008. doi: 10.4161/cc.10.23.18385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rogakou EP, Boon C, Redon C, Bonner WM. Megabase chromatin domains involved in DNA double-strand breaks in vivo. The Journal of cell biology. 1999;146:905–16. doi: 10.1083/jcb.146.5.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rogakou EP, Pilch DR, Orr AH, Ivanova VS, Bonner WM. DNA double-stranded breaks induce histone H2AX phosphorylation on serine 139. The Journal of biological chemistry. 1998;273:5858–68. doi: 10.1074/jbc.273.10.5858. [DOI] [PubMed] [Google Scholar]
- 29.Bertrand MJ, Vandenabeele P. The Ripoptosome: death decision in the cytosol. Molecular cell. 2011;43:323–5. doi: 10.1016/j.molcel.2011.07.007. [DOI] [PubMed] [Google Scholar]
- 30.Feoktistova M, Geserick P, Kellert B, Dimitrova DP, Langlais C, Hupe M, et al. cIAPs block Ripoptosome formation, a RIP1/caspase-8 containing intracellular cell death complex differentially regulated by cFLIP isoforms. Molecular cell. 2011;43:449–63. doi: 10.1016/j.molcel.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tenev T, Bianchi K, Darding M, Broemer M, Langlais C, Wallberg F, et al. The Ripoptosome, a signaling platform that assembles in response to genotoxic stress and loss of IAPs. Molecular cell. 2011;43:432–48. doi: 10.1016/j.molcel.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 32.Niu J, Shi Y, Iwai K, Wu ZH. LUBAC regulates NF-kappaB activation upon genotoxic stress by promoting linear ubiquitination of NEMO. The EMBO journal. 2011;30:3741–53. doi: 10.1038/emboj.2011.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hickson I, Zhao Y, Richardson CJ, Green SJ, Martin NM, Orr AI, et al. Identification and characterization of a novel and specific inhibitor of the ataxia-telangiectasia mutated kinase ATM. Cancer research. 2004;64:9152–9. doi: 10.1158/0008-5472.CAN-04-2727. [DOI] [PubMed] [Google Scholar]
- 34.Toledo LI, Murga M, Zur R, Soria R, Rodriguez A, Martinez S, et al. A cell-based screen identifies ATR inhibitors with synthetic lethal properties for cancer-associated mutations. Nature structural & molecular biology. 2011;18:721–7. doi: 10.1038/nsmb.2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brockman JA, Scherer DC, McKinsey TA, Hall SM, Qi X, Lee WY, et al. Coupling of a signal response domain in I kappa B alpha to multiple pathways for NF-kappa B activation. Molecular and cellular biology. 1995;15:2809–18. doi: 10.1128/mcb.15.5.2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Biton S, Ashkenazi A. NEMO and RIP1 control cell fate in response to extensive DNA damage via TNF-alpha feedforward signaling. Cell. 2011;145:92–103. doi: 10.1016/j.cell.2011.02.023. [DOI] [PubMed] [Google Scholar]
- 37.Zhang T, Inesta-Vaquera F, Niepel M, Zhang J, Ficarro SB, Machleidt T, et al. Discovery of potent and selective covalent inhibitors of JNK. Chemistry & biology. 2012;19:140–54. doi: 10.1016/j.chembiol.2011.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lu Y, Han J, Scanlon KJ. Biochemical and molecular properties of cisplatin-resistant A2780 cells grown in folinic acid. The Journal of biological chemistry. 1988;263:4891–4. [PubMed] [Google Scholar]
- 39.Langdon SP, Lawrie SS, Hay FG, Hawkes MM, McDonald A, Hayward IP, et al. Characterization and properties of nine human ovarian adenocarcinoma cell lines. Cancer research. 1988;48:6166–72. [PubMed] [Google Scholar]
- 40.Smit JJ, Monteferrario D, Noordermeer SM, van Dijk WJ, van der Reijden BA, Sixma TK. The E3 ligase HOIP specifies linear ubiquitin chain assembly through its RING-IBR-RING domain and the unique LDD extension. The EMBO journal. 2012;31:3833–44. doi: 10.1038/emboj.2012.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stieglitz B, Morris-Davies AC, Koliopoulos MG, Christodoulou E, Rittinger K. LUBAC synthesizes linear ubiquitin chains via a thioester intermediate. EMBO reports. 2012;13:840–6. doi: 10.1038/embor.2012.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Imre G, Larisch S, Rajalingam K. Ripoptosome: a novel IAP-regulated cell death-signalling platform. Journal of molecular cell biology. 2011;3:324–6. doi: 10.1093/jmcb/mjr034. [DOI] [PubMed] [Google Scholar]
- 43.Jin Z, Li Y, Pitti R, Lawrence D, Pham VC, Lill JR, et al. Cullin3-based polyubiquitination and p62-dependent aggregation of caspase-8 mediate extrinsic apoptosis signaling. Cell. 2009;137:721–35. doi: 10.1016/j.cell.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 44.Tokunaga F, Sakata S, Saeki Y, Satomi Y, Kirisako T, Kamei K, et al. Involvement of linear polyubiquitylation of NEMO in NF-kappaB activation. Nat Cell Biol. 2009;11:123–32. doi: 10.1038/ncb1821. [DOI] [PubMed] [Google Scholar]
- 45.Chang L, Kamata H, Solinas G, Luo JL, Maeda S, Venuprasad K, et al. The E3 ubiquitin ligase itch couples JNK activation to TNFalpha-induced cell death by inducing c-FLIP(L) turnover. Cell. 2006;124:601–13. doi: 10.1016/j.cell.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 46.Brozovic A, Fritz G, Christmann M, Zisowsky J, Jaehde U, Osmak M, et al. Long-term activation of SAPK/JNK, p38 kinase and fas-L expression by cisplatin is attenuated in human carcinoma cells that acquired drug resistance. International journal of cancer Journal international du cancer. 2004;112:974–85. doi: 10.1002/ijc.20522. [DOI] [PubMed] [Google Scholar]
- 47.Garnett MJ, Edelman EJ, Heidorn SJ, Greenman CD, Dastur A, Lau KW, et al. Systematic identification of genomic markers of drug sensitivity in cancer cells. Nature. 2012;483:570–5. doi: 10.1038/nature11005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Petersen SL, Wang L, Yalcin-Chin A, Li L, Peyton M, Minna J, et al. Autocrine TNFalpha signaling renders human cancer cells susceptible to Smac-mimetic-induced apoptosis. Cancer cell. 2007;12:445–56. doi: 10.1016/j.ccr.2007.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Varfolomeev E, Blankenship JW, Wayson SM, Fedorova AV, Kayagaki N, Garg P, et al. IAP antagonists induce autoubiquitination of c-IAPs, NF-kappaB activation, and TNFalpha-dependent apoptosis. Cell. 2007;131:669–81. doi: 10.1016/j.cell.2007.10.030. [DOI] [PubMed] [Google Scholar]
- 50.Vince JE, Wong WW, Khan N, Feltham R, Chau D, Ahmed AU, et al. IAP antagonists target cIAP1 to induce TNFalpha-dependent apoptosis. Cell. 2007;131:682–93. doi: 10.1016/j.cell.2007.10.037. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.