Abstract
Host protein synthesis is selectively inhibited in vaccinia virus-infected cells. This inhibition has been associated with the production of a group of small, nontranslated, polyadenylylated RNAs (POLADS) produced during the early part of virus infection. The inhibitory function of POLADS is associated with the poly(A) tail of these small RNAs. To determine the origin of the 5'-ends of POLADS, reverse transcription was performed with POLADS isolated from VV-infected cells at 1 hr and 3.5 hr post infection. The cDNAs of these POLADS were cloned into plasmids (pBS or pBluescript II KS +/-), and their nucleotide composition was determined by DNA sequencing. The results of this investigation show the following: There is no specific gene encoding for POLADS. The 5' ends of POLADS may be derived from either viral or cellular RNAs. Any RNA sequence including tRNAs, small nuclear RNAs and 5'ends of mRNAs can become POLADS if they acquire a poly(A) tail at their 3' ends during infection. This nonspecific polyadenylylation found in vaccinia virus-infected cells is probably conducted by vaccinia virus poly(A)+ polymerase. No consensus sequence is found on the 5' ends of POLADS for polyadenylylation. The 5' ends of POLADS have no direct role in their inhibitory activity of protein synthesis.
Full text
PDF

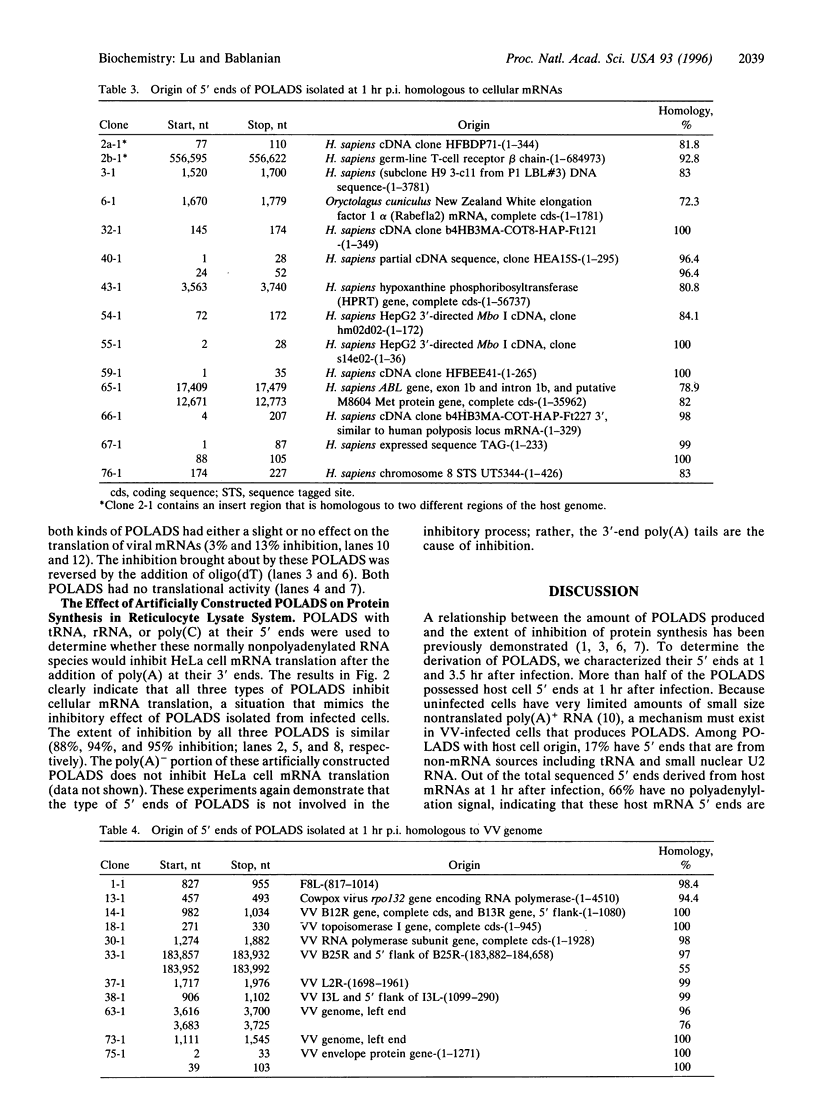

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bablanian R., Banerjee A. K. Poly(riboadenylic acid) preferentially inhibits in vitro translation of cellular mRNAs compared with vaccinia virus mRNAs: possible role in vaccinia virus cytopathology. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1290–1294. doi: 10.1073/pnas.83.5.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bablanian R., Coppola G., Masters P. S., Banerjee A. K. Characterization of vaccinia virus transcripts involved in selective inhibition of host protein synthesis. Virology. 1986 Jan 30;148(2):375–380. doi: 10.1016/0042-6822(86)90334-x. [DOI] [PubMed] [Google Scholar]
- Bablanian R., Coppola G., Scribani S., Esteban M. Inhibition of protein synthesis by vaccinia virus. III. The effect of ultraviolet-irradiated virus on the inhibition of protein synthesis. Virology. 1981 Jul 15;112(1):1–12. doi: 10.1016/0042-6822(81)90606-1. [DOI] [PubMed] [Google Scholar]
- Bablanian R., Coppola G., Scribani S., Esteban M. Inhibition of protein synthesis by vaccinia virus. IV. The role of low-molecular-weight viral RNA in the inhibition of protein synthesis. Virology. 1981 Jul 15;112(1):13–24. doi: 10.1016/0042-6822(81)90607-3. [DOI] [PubMed] [Google Scholar]
- Bablanian R., Goswami S. K., Esteban M., Banerjee A. K., Merrick W. C. Mechanism of selective translation of vaccinia virus mRNAs: differential role of poly(A) and initiation factors in the translation of viral and cellular mRNAs. J Virol. 1991 Aug;65(8):4449–4460. doi: 10.1128/jvi.65.8.4449-4460.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bablanian R., Goswami S. K., Esteban M., Banerjee A. K. Selective inhibition of protein synthesis by synthetic and vaccinia virus-core synthesized poly(riboadenylic acids). Virology. 1987 Dec;161(2):366–373. doi: 10.1016/0042-6822(87)90129-2. [DOI] [PubMed] [Google Scholar]
- Binns M. M., Tomley F. M., Campbell J., Boursnell M. E. Comparison of a conserved region in fowlpox virus and vaccinia virus genomes and the translocation of the fowlpox virus thymidine kinase gene. J Gen Virol. 1988 Jun;69(Pt 6):1275–1283. doi: 10.1099/0022-1317-69-6-1275. [DOI] [PubMed] [Google Scholar]
- Coppola G., Bablanian R. Discriminatory inhibition of protein synthesis in cell-free systems by vaccinia virus transcripts. Proc Natl Acad Sci U S A. 1983 Jan;80(1):75–79. doi: 10.1073/pnas.80.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershon P. D., Ahn B. Y., Garfield M., Moss B. Poly(A) polymerase and a dissociable polyadenylation stimulatory factor encoded by vaccinia virus. Cell. 1991 Sep 20;66(6):1269–1278. doi: 10.1016/0092-8674(91)90048-4. [DOI] [PubMed] [Google Scholar]
- Gershon P. D., Moss B. Stimulation of poly(A) tail elongation by the VP39 subunit of the vaccinia virus-encoded poly(A) polymerase. J Biol Chem. 1993 Jan 25;268(3):2203–2210. [PubMed] [Google Scholar]
- Gershon P. D., Moss B. Uridylate-containing RNA sequences determine specificity for binding and polyadenylation by the catalytic subunit of vaccinia virus poly(A) polymerase. EMBO J. 1993 Dec;12(12):4705–4714. doi: 10.1002/j.1460-2075.1993.tb06159.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goebel S. J., Johnson G. P., Perkus M. E., Davis S. W., Winslow J. P., Paoletti E. The complete DNA sequence of vaccinia virus. Virology. 1990 Nov;179(1):247-66, 517-63. doi: 10.1016/0042-6822(90)90294-2. [DOI] [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- JOKLIK W. K., BECKER Y. THE REPLICATION AND COATING OF VACCINIA DNA. J Mol Biol. 1964 Dec;10:452–474. doi: 10.1016/s0022-2836(64)80066-8. [DOI] [PubMed] [Google Scholar]
- JOKLIK W. K. THE INTRACELLULAR UNCOATING OF POXVIRUS DNA. II. THE MOLECULAR BASIS OF THE UNCOATING PROCESS. J Mol Biol. 1964 Feb;8:277–288. doi: 10.1016/s0022-2836(64)80137-6. [DOI] [PubMed] [Google Scholar]
- JOKLIK W. K. The purification fo four strains of poxvirus. Virology. 1962 Sep;18:9–18. doi: 10.1016/0042-6822(62)90172-1. [DOI] [PubMed] [Google Scholar]
- Karlin S., Altschul S. F. Methods for assessing the statistical significance of molecular sequence features by using general scoring schemes. Proc Natl Acad Sci U S A. 1990 Mar;87(6):2264–2268. doi: 10.1073/pnas.87.6.2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieg P. A., Melton D. A. In vitro RNA synthesis with SP6 RNA polymerase. Methods Enzymol. 1987;155:397–415. doi: 10.1016/0076-6879(87)55027-3. [DOI] [PubMed] [Google Scholar]
- Krystosek A., Cawthon M. L., Kabat D. Improved methods for purification and assay of eukaryotic messenger ribonucleic acids and ribosomes. Quantitative analysis of their interaction in a fractionated reticulocyte cell-free system. J Biol Chem. 1975 Aug 10;250(15):6077–6084. [PubMed] [Google Scholar]
- Lawrence C. E., Altschul S. F., Boguski M. S., Liu J. S., Neuwald A. F., Wootton J. C. Detecting subtle sequence signals: a Gibbs sampling strategy for multiple alignment. Science. 1993 Oct 8;262(5131):208–214. doi: 10.1126/science.8211139. [DOI] [PubMed] [Google Scholar]
- Moss B., Rosenblum E. N., Gershowitz A. Characterization of a polyriboadenylate polymerase from vaccinia virions. J Biol Chem. 1975 Jun 25;250(12):4722–4729. [PubMed] [Google Scholar]
- Nevins J. R., Joklik W. K. Isolation and partial characterization of the poly(A) polymerases from HeLa cells infected with vaccinia virus. J Biol Chem. 1977 Oct 10;252(19):6939–6947. [PubMed] [Google Scholar]
- Padgett R. A., Grabowski P. J., Konarska M. M., Seiler S., Sharp P. A. Splicing of messenger RNA precursors. Annu Rev Biochem. 1986;55:1119–1150. doi: 10.1146/annurev.bi.55.070186.005351. [DOI] [PubMed] [Google Scholar]
- Parsons B. L., Pickup D. J. Transcription of orthopoxvirus telomeres at late times during infection. Virology. 1990 Mar;175(1):69–80. doi: 10.1016/0042-6822(90)90187-v. [DOI] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Poddar S. K., Bauer W. R. Type I topoisomerase activity after infection of enucleated, synchronized mouse L cells by vaccinia virus. J Virol. 1986 Feb;57(2):433–437. doi: 10.1128/jvi.57.2.433-437.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrmann G., Yuen L., Moss B. Transcription of vaccinia virus early genes by enzymes isolated from vaccinia virions terminates downstream of a regulatory sequence. Cell. 1986 Sep 26;46(7):1029–1035. doi: 10.1016/0092-8674(86)90702-6. [DOI] [PubMed] [Google Scholar]
- Schreier M. H., Staehelin T. Initiation of mammalian protein synthesis: the importance of ribosome and initiation factor quality for the efficiency of in vitro systems. J Mol Biol. 1973 Feb 19;73(3):329–349. doi: 10.1016/0022-2836(73)90346-x. [DOI] [PubMed] [Google Scholar]
- Schrom M., Bablanian R. Inhibition of protein synthesis by vaccinia virus. I. Characterization of an inhibited cell-free protein-synthesizing system from infected cells. Virology. 1979 Dec;99(2):319–328. doi: 10.1016/0042-6822(79)90011-4. [DOI] [PubMed] [Google Scholar]
- Schrom M., Bablanian R. Inhibition of protein synthesis by vaccinia virus. II. Studies on the role of virus-induced RNA synthesis. J Gen Virol. 1979 Sep;44(3):625–638. doi: 10.1099/0022-1317-44-3-625. [DOI] [PubMed] [Google Scholar]
- Schuler G. D., Altschul S. F., Lipman D. J. A workbench for multiple alignment construction and analysis. Proteins. 1991;9(3):180–190. doi: 10.1002/prot.340090304. [DOI] [PubMed] [Google Scholar]
- Shuman S., Moss B. Identification of a vaccinia virus gene encoding a type I DNA topoisomerase. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7478–7482. doi: 10.1073/pnas.84.21.7478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuman S., Moss B. Vaccinia virus poly(A) polymerase. Specificity for nucleotides and nucleotide analogs. J Biol Chem. 1988 Jun 15;263(17):8405–8412. [PubMed] [Google Scholar]
- Su M. J., Bablanian R. Polyadenylated RNA sequences from vaccinia virus-infected cells selectively inhibit translation in a cell-free system: structural properties and mechanism of inhibition. Virology. 1990 Dec;179(2):679–693. doi: 10.1016/0042-6822(90)90135-e. [DOI] [PubMed] [Google Scholar]
- Tabor S., Richardson C. C. DNA sequence analysis with a modified bacteriophage T7 DNA polymerase. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4767–4771. doi: 10.1073/pnas.84.14.4767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittek R., Moss B. Tandem repeats within the inverted terminal repetition of vaccinia virus DNA. Cell. 1980 Aug;21(1):277–284. doi: 10.1016/0092-8674(80)90135-x. [DOI] [PubMed] [Google Scholar]