Selected matrix metalloproteinases (MMPs), including gelatinase MMP-9 and collagenases MMP-8 and -13, and tissue inhibitor of metalloproteinase (TIMP)-1 increase during the onset and progression of experimental posttraumatic fungal keratitis.
Abstract
purpose. This study was designed to investigate the expression of matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs) during the inception and progression of experimental keratomycosis.
methods. Scarified corneas of adult BALB/c mice were topically inoculated with Candida albicans strain SC5314 and monitored for disease severity. Infected and mock-infected corneas were compared at 1 day post inoculation (p.i.) with a murine gene microarray. Real-time reverse transcriptase–polymerase chain reaction (RT-PCR) determined MMP and TIMP levels at 1, 3, and 7 days p.i. for infected, mock-infected, and normal corneas. Immunostaining localized target proteins at 1 day p.i.
results. Eyes inoculated with C. albicans developed corneal infection with a mean clinical score of 8.2 ± 0.8 at 1 day p.i. Compared to controls at 1 day p.i., MMP-8, -9, -10, -12, -13, -19, and TIMP-1 were significantly upregulated from fivefold to 375-fold by microarray and from threefold to 78-fold by real-time RT-PCR. Upregulated MMPs and TIMP-1 in the corneal epithelium and stroma of infected eyes correlated with the influx of acute inflammatory cells. Neither MMP-8 nor -13 expression was affected by mechanical trauma, but both increased >100-fold during the week after the onset of fungal keratitis. TIMP-1 expression rose from 21-fold more than controls at 1 day to 46-fold at 7 days p.i. by RT-PCR.
conclusions. Transcriptional and translational levels of MMP-8, -9, -13, and TIMP-1 increase during the early stages of C. albicans keratitis, confirming findings for MMP-9 and TIMP-1 in other infectious keratitis models and suggesting roles for MMP-8 and -13.
Fungal infections of the eye are epidemiologically important diseases.1 Fungi such as Candida albicans that are commensals in the conjunctival flora2 3 can become pathogenic with ocular surface injury or dysfunction. Ophthalmic candidiasis is an opportunistic infection of the eye acquired through trauma, surgery, contact lens wear, and chronic keratopathy.4
A murine model of experimental keratitis using a human isolate of C. albicans to induce corneal infection is helpful in understanding the pathogenesis of oculomycosis.5 6 A key early event in posttraumatic C. albicans keratitis involves fungal morphogenesis and invasion that trigger inflammatory and wounding responses.7 We used this model to examine matrix metalloproteinases (MMPs) in the development of keratomycosis.
MMPs are proteolytic enzymes involved in multiple physiological and pathologic processes. This family includes collagenases, gelatinases, stromelysins, and matrilysins that are grouped according to their structure and substrate and that are modulated, in part, by tissue inhibitors of metalloproteinases (TIMPs).8 TIMPs consist of a family of four glycoproteins that inhibit MMP activation or activity. TIMPs differ in their affinity for various MMPs; for example, TIMP-1 prevents activation of MMP-9 and can bind to the catalytic site of MMP-9 and other MMPs. Coordinated actions of MMPs and TIMPs are pivotal in maintaining structural homeostasis, and altered regulation disrupts connective tissue integrity through degradation of the extracellular matrix.9 Determining the roles of MMPs in infection and inflammation may lead to new opportunities for controlling corneal ulceration.10
Changes in MMPs occur after corneal trauma and during corneal infection.11 12 13 MMP-9 increases in acute Pseudomonas aeruginosa keratitis and potentiates the severity of bacterial keratitis by degrading corneal stroma and by stimulating the release of proinflammatory cytokines and chemokines that attract polymorphonuclear leukocytes.12 14 15 16 Studies of experimental and human fungal keratitis have also found increased levels of MMP-9 during corneal infection by yeasts and filamentous fungi.17 18 19 20 Hypothesizing that MMP-9 and other MMPs contribute to the initial manifestation of keratomycosis, we systematically examined the expression patterns of 18 MMPs and 4 TIMPs in murine C. albicans keratitis.
Materials and Methods
Fungi
C. albicans strain SC5314 is a clinical isolate capable of producing experimental keratomycosis.5 21 Yeasts were grown on glucose-peptone medium (Sabouraud Dextrose Agar; Difco, Detroit, MI) for 3 days at 25°C. Colonies were harvested and diluted in sterile phosphate-buffered saline (PBS) to yield 2 × 105 colony-forming units/μL based on optical density (OD) at 600 nm, using a conversion factor of 1 OD600 unit equal to approximately 3 × 107 CFU/mL.21
Animals
Naïve female BALB/c mice 6 to 8 weeks of age (Harlan Sprague–Dawley, Houston, TX) were anesthetized intraperitoneally with rodent combination anesthesia, and the corneas of the right eyes were superficially scarified.6 A 5-μl inoculum (1 × 106 CFU) of C. albicans was applied to the scarified cornea, while sterile PBS dilution buffer was applied to scarified corneas of mock-infected controls. All animals were treated in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research, and the protocols were approved by the Baylor College of Medicine Institutional Animal Care and Use Committee. Mice were monitored daily for up to 7 days post inoculation (p.i.) using a dissecting microscope to determine the severity of keratomycosis by criteria that assigned grades of 0 to 4 for inflammatory area, density, and surface irregularity, respectively.6 7 Mice were killed 1, 3, and 7 days p.i., and eyes were enucleated for analysis.
RNA Extraction
Corneas were dissected from freshly enucleated eyes, and surrounding conjunctiva, Tenon capsule, uvea, and lens were removed. Five-cornea pools randomly grouped were prepared in triplicate from C. albicans-infected and mock-infected control animals at days 1, 3, and 7 p.i. and from normal unmanipulated mouse corneas. Total RNA was immediately extracted (RNeasy MicroKit; Qiagen, Valencia, CA). Samples were treated with DNase (Qiagen) to exclude DNA contamination and stored at -80°C until use.
Gene Microarray
Microarray analysis was performed in the Microarray Core Facility of Baylor College of Medicine. Microarray protocols (Affymetrix GeneChip, Santa Clara, CA) were applied to all qualified samples for two cycles of amplification (Affymetrix Two-Cycle Kit) that included the standard primer protocol (Affymetrix T7 oligo) followed by reverse transcription with a kit (MegaScript; Applied Biosystems, Foster City, CA) to produce cRNA. The unlabeled cRNA product was used as template for a second cycle of amplification. The double-stranded cDNA end product was processed with an in vitro transcription kit (Affymetrix) to produce biotin-labeled cRNA that was quantified with a spectrophotometer (ND-1000; NanoDrop Technologies, Wilmington, DE). A hybridization mixture containing spike-in controls and fragmented labeled cRNA was loaded onto an array (GeneChip). The array was hybridized overnight, then stained with a streptavidin-phycoerythrin conjugate. After signal amplification with biotinylated antistreptavidin, stained arrays were scanned on a GeneChip Scanner 3000 (Affymetrix), and raw signal intensity data were adjusted and analyzed with BioConductor software. The criterion for significance of differentially regulated genes was established as more than a twofold change with adjusted P < 0.05.
Reverse Transcription and Quantitative Real-Time RT-PCR
Total RNA isolated from corneas at 1, 3, and 7 days p.i. was quantified by absorption at 260 nm. The first-strand cDNA was synthesized from 0.4 μg of total RNA with beads (Ready-To-Go You-Prime First-Strand Beads; GE Healthcare, Princeton, NJ) and random hexamers (Applied Biosystems). Real-time PCR was performed using assays (TaqMan Assays and TaqMan Gene Expression Master Mix and Assays; Applied Biosystems) with primers specific for the various MMP and TIMP transcripts (Applied Biosystems). The threshold cycle (CT) for each target mRNA was normalized to glyceraldehyde 3-phosphate dehydrogenase (GAPDH) mRNA and averaged. Normalized CT results were used to calculate gene expression levels, and mean results were used to determine relative fold changes between experimental groups. Two-group comparisons were done using the Student’s t-test or Mann–Whitney rank sum test. Three-group comparisons used one-way analysis of variance (ANOVA) or Kruskal-Wallis analysis of variance on ranks. For kinetic analysis of MMP and TIMP transcriptional levels, mean results were compared with ANOVA applying the Holm-Sidak method for pairwise multiple comparison procedures. P < 0.05 was considered statistically significant.
Histology and Immunostaining
Eyes from mice 1 day p.i. were embedded in OCT compound (Sakura Finetek, Torrance, CA) and snap-frozen in liquid nitrogen. Frozen tissues were sectioned on a cryostat at 15-μm thickness and processed with Gill hematoxylin and eosin or periodic acid-Schiff (PAS) stains (Sigma-Aldrich, St. Louis, MO).
For immunofluorescent staining frozen corneal sections were thawed, dehydrated, and fixed in 2% paraformaldehyde at 4°C for 10 minutes. Sections were blocked with 10% normal donkey serum (Jackson ImmunoResearch Laboratories, Philadelphia, PA) in PBS for 1 hour to decrease nonspecific binding. The following primary antibodies were diluted 1:100, applied to the blocked sections, and incubated overnight at 4°C: polyclonal rabbit antibody MMP-2 (29575, AnaSpec, San Jose, CA), MMP-8 (29578, AnaSpec), MMP-9 (AB19016, Millipore, Billerica, MA), and TIMP-1 (sc-5538, Santa Cruz Biotechnology, Santa Cruz, CA), or goat antibody MMP-13 (sc-12363, Santa Cruz Biotechnology). Secondary Alexa-Fluor 488-conjugated donkey anti-rabbit or anti-goat (Invitrogen, Carlsbad, CA) antibodies were then applied and incubated in a dark chamber for 1 hour at room temperature followed by washing and counterstaining with propidium iodine (Invitrogen) in gel (Gel/Mount; Biomeda, Foster City, CA), and a coverslip was applied. Sections were observed with a laser-scanning confocal microscope (LSM 510 with krypton-argon and He-Ne laser; Zeiss, Thornwood, NY) with 488- and 543-nm excitation and emission filters (LP 505 and LP 560). Images were acquired with a 40× oil-immersion objective and processed using Zeiss LSM-PC software.
Immunohistochemistry was performed using a similar protocol to immunofluorescent staining. After fixation with 2% paraformaldehyde, corneal sections were serially treated with 0.3% hydrogen peroxide and 10% normal donkey serum. Primary antibodies were applied (MMP-2, 1:200; MMP-8, 1:200; MMP-9, 1:200; MMP-13, 1:100; and TIMP-1, 1:200). After incubation, biotin-conjugated donkey anti-rabbit or anti-goat (Jackson ImmunoResearch Laboratories, West Grove, PA) secondary antibodies were applied and incubated, followed by reagent (Vectastain Elite ABC Kit, Vector Laboratories, Burlingame, CA). The samples were incubated with diaminobenzidine (DAB) as a chromogen (Vector Laboratories) followed by counterstaining with hematoxylin. Sections were dehydrated, coverslipped, and photographed with an epifluorescent microscope.
Results
Experimental Fungal Keratitis
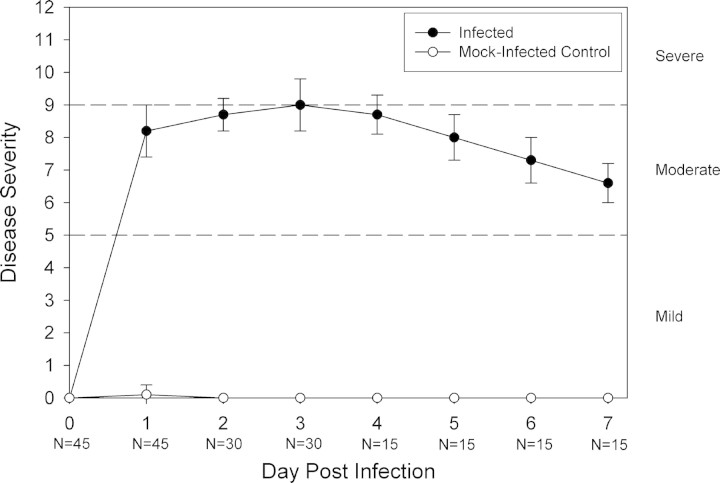
All eyes inoculated with C. albicans developed clinical signs of keratitis (Fig. 1) . Corneal inflammation began 1 day p.i. (mean ± SD score, 8.2 ± 0.8), peaked 3 days p.i. (score, 9.0 ± 0.8), then diminished by 7 days p.i. (6.6 ± 0.6). No significant differences occurred among daily severity scores at 1 day, 3 days, and 7 days p.i. No inflammation was found in eyes from mock-infected controls. Histopathologic evaluation of infected eyes revealed partial loss of epithelial integrity and acute inflammatory cells invading the stroma and anterior chamber. PAS staining showed pseudohyphae and hyphae invading into the mid to deep stroma.
Figure 1.
Clinical evaluation of C. albicans keratitis. Scarified corneas of immunocompetent BALB/c mice were infected with C. albicans or mock-infected with diluent. Disease severity was considered mild for a total score less than 5, moderate for scores 5 to 9, and severe for scores greater than 9. Each point represents the mean score (±SD) of indicated sample sizes (N).
Gene Expression Profile of MMPs and TIMPs
Gene array analysis of C. albicans-infected corneas (n = 3 five-cornea pools) and mock-infected control corneas (n = 3 five-cornea pools) on day 1 p.i. detected 18 MMPs and 4 TIMPs (Table 1) . Compared to mock-infected controls, the normalized signals of 6 MMPs (MMP-8, -9, -10, -12, -13, and -19) changed significantly in infected corneas on day 1 p.i., with MMP-13, -8, and -9 demonstrating relative increases in infected-mock ratios of 375-fold, 102-fold, and 74-fold, respectively. TIMP-1 was upregulated 22-fold while TIMP-2 and -3 were downregulated twofold and threefold, respectively. Transcript levels detected by quantitative real-time RT-PCR for five-cornea pools of infected or mock-infected corneas (n = 3/group) were consistent with microarray findings for upregulated genes (Table 2) . Whereas MMP-2 was not significantly downregulated based on microarray methodology (P = 0.38), real-time RT-PCR found a twofold downregulation (P = 0.01) in C. albicans-infected corneas. Table 3 shows the average real-time RT-PCR CT values among the three experimental groups of corneas.
Table 1.
Microarray Analysis of MMP and TIMP Gene Expression Ratios Comparing C. albicans Keratitis to Mock-Infected Controls
| Gene | Mean Ratio* | P |
|---|---|---|
| MMP-1 | 1.7 | 0.22 |
| MMP-2 | −1.3 | 0.38 |
| MMP-3 | 2.9 | 0.07 |
| MMP-7 | −1.0 | 0.95 |
| MMP-8, † | 102.5 | 0.00028 |
| MMP-9, † | 74.3 | 0.0027 |
| MMP-10, † | 21.0 | 0.0035 |
| MMP-11 | −1.3 | 0.25 |
| MMP-12, † | 24.8 | 0.011 |
| MMP-13, † | 375.4 | 0.001 |
| MMP-14 | 1.4 | 0.021 |
| MMP-15 | −1.2 | 0.80 |
| MMP-16 | −1.0 | 0.57 |
| MMP-17 | −1.2 | 0.29 |
| MMP-19, † | 5.2 | 0.005 |
| MMP-20 | −1.0 | 0.79 |
| MMP-23 | −1.7 | 0.067 |
| MMP-24 | 1.1 | 0.50 |
| TIMP-1, † | 22.2 | 0.009 |
| TIMP-2, † | −2.0 | 0.01 |
| TIMP-3, † | −3.2 | 0.0044 |
| TIMP-4 | −1.1 | 0.56 |
Determined from samples of five-cornea pools at 1 day p.i.
Significant (P < 0.05) twofold or greater upregulation or downregulation.
Table 2.
Real-Time RT-PCR Confirmation of MMP and TIMP Gene Expression Ratios Comparing C. albicans Keratitis to Mock-Infected Controls
| Gene | Mean Ratio* | P |
|---|---|---|
| MMP-1 | 4.2 | 0.15 |
| MMP-2, † | −2.2 | 0.01 |
| MMP-3 | 2.4 | 0.23 |
| MMP-7 | 2.23 | 0.22 |
| MMP-8, † | 68.1 | 0.0067 |
| MMP-9, † | 40.7 | 0.0001 |
| MMP-10, † | 8.0 | 0.0001 |
| MMP-12, † | 8.0 | 0.014 |
| MMP-13, † | 78.3 | 0.0001 |
| MMP-19, † | 2.7 | 0.013 |
| TIMP-1, † | 20.8 | 0.0096 |
| TIMP-2 | −1.9 | 0.37 |
| TIMP-3, † | −4.3 | 0.0023 |
| TIMP-4 | −3.2 | 0.072 |
Determined from triplicate samples of five-cornea pools at 1 day p.i.
Significant (P < 0.05) upregulation or downregulation.
Table 3.
Quantitative Gene Expression Levels
| Gene | Normal Cornea | Mock-Infected Cornea | Infected Cornea | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Day 1 | Day 3 | Day 7 | Day 1 | Day 3 | Day 7 | ||||||
| MMP-1 | 17.5 ± 0.7 | 14.0 ± 1.5 | 16.2 ± 1.6 | 16.8 ± 0.9 | 11.9 ± 1.3 | 12.9 ± 1.0 | 11.9 ± 0.7 | ||||
| MMP-2 | 3.3 ± 0.8 | 4.9 ± 0.2 | 3.7 ± 0.3 | 3.5 ± 0.7 | 6.1 ± 0.4 | 3.7 ± 0.6 | 1.2 ± 0.4 | ||||
| MMP-3 | 3.5 ± 0.7 | 1.8 ± 1.0 | 3.9 ± 0.2 | 5.2 ± 0.8 | 0.5 ± 1.2 | 1.0 ± 0.3 | 0.9 ± 0.6 | ||||
| MMP-7 | 16.4 ± 0.7 | 16.1 ± 1.3 | 15.0 ± 0.7 | 17.9 ± 0.7 | 15.0 ± 0.7 | 15.3 ± 0.4 | 14.3 ± 1.0 | ||||
| MMP-8 | 16.3 ± 0.2 | 13.5 ± 2.0 | 15.5 ± 0.9 | 17.0 ± 0.2 | 7.4 ± 0.4 | 9.2 ± 0.6 | 10.1 ± 0.7 | ||||
| MMP-9 | 10.0 ± 0.3 | 8.8 ± 0.5 | 9.2 ± 0.4 | 10.4 ± 1.6 | 3.5 ± 0.1 | 4.5 ± 0.5 | 5.3 ± 1.3 | ||||
| MMP-10 | 12.0 ± 0.7 | 10.7 ± 0.2 | 13.1 ± 0.7 | 13.6 ± 0.8 | 7.7 ± 0.3 | 10.5 ± 0.5 | 11.5 ± 0.8 | ||||
| MMP-12 | 6.7 ± 0.6 | 6.9 ± 1.1 | 7.8 ± 2.1 | 8.3 ± 0.49 | 3.9 ± 0.6 | 3.6 ± 1.0 | 4.2 ± 0.7 | ||||
| MMP-13 | 10.5 ± 0.7 | 10.1 ± 0.7 | 11.8 ± 0.7 | 10.9 ± 1.9 | 3.8 ± 0.2 | 7.2 ± 0.5 | 3.6 ± 0.3 | ||||
| MMP-19 | 8.3 ± 0.6 | 9.2 ± 0.2 | 8.7 ± 0.6 | 9.6 ± 0.5 | 7.7 ± 0.5 | 7.3 ± 0.3 | 6.5 ± 0.6 | ||||
| TIMP-1 | 9.9 ± 0.4 | 6.7 ± 1.4 | 8.6 ± 0.5 | 9.7 ± 0.9 | 2.3 ± 0.8 | 3.8 ± 0.4 | 4.2 ± 1.0 | ||||
| TIMP-2 | 3.4 ± 0.2 | 4.3 ± 0.4 | 3.4 ± 0.04 | 4.2 ± 0.3 | 5.3 ± 1.6 | 4.3 ± 0.9 | 2.3 ± 0.4 | ||||
| TIMP-3 | 3.0 ± 0.3 | 3.1 ± 0.2 | 3.3 ± 0.1 | 3.1 ± 0.7 | 5.3 ± 0.5 | 5.1 ± 0.7 | 2.8 ± 0.1 | ||||
| TIMP-4 | 14.9 ± 0.1 | 14.2 ± 0.6 | 14.7 ± 0.7 | 15.0 ± 0.5 | 15.9 ± 1.0 | 15.7 ± 0.8 | 14.2 ± 1.6 | ||||
Triplicate samples of five-cornea pools of normal, mock-infected, or C. albicans–infected corneas at 1, 3, or 7 days p.i. with mean threshold cycle number ± SD normalized to GAPDH by real-time RT-PCR.
Protein Expression Pattern of MMPs and TIMPs
The in situ protein expression pattern determined by immunofluorescent staining and immunohistochemistry were consistent with transcript levels measured by real-time RT-PCR (Fig. 2) . Mock-infected corneas demonstrated moderate epithelial staining and minor stromal staining for MMP-8, -9, -13, and TIMP-1 while corneas from infected animals had increased staining for these proteins throughout the epithelium and stroma. MMP-2 staining was primarily localized to the epithelial layers in infected and mock-infected corneas. Negative controls in which no primary antibody was used demonstrated no detectable staining in immunofluorescent or immunohistochemical assays.
Figure 2.
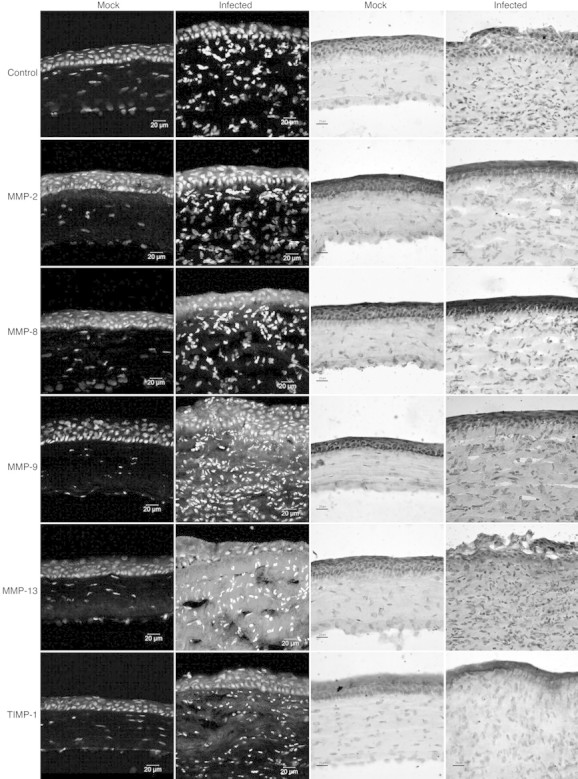
Molecular expression patterns in situ in C. albicans keratitis (Infected) or mock-infected controls (Mock), using monoclonal antibodies against selected MMPs or TIMP-1. Negative controls included no primary antibody (Control). The two left columns were stained using indirect immunofluorescence in which a cyan-green labeled secondary antibody was used along with propidium iodine as a nuclear counterstain. The two right columns were stained using an immunohistochemistry protocol in which diaminobenzidine was used as chromogen and hematoxylin as counterstain.
Kinetic Analysis of MMPs and TIMPs
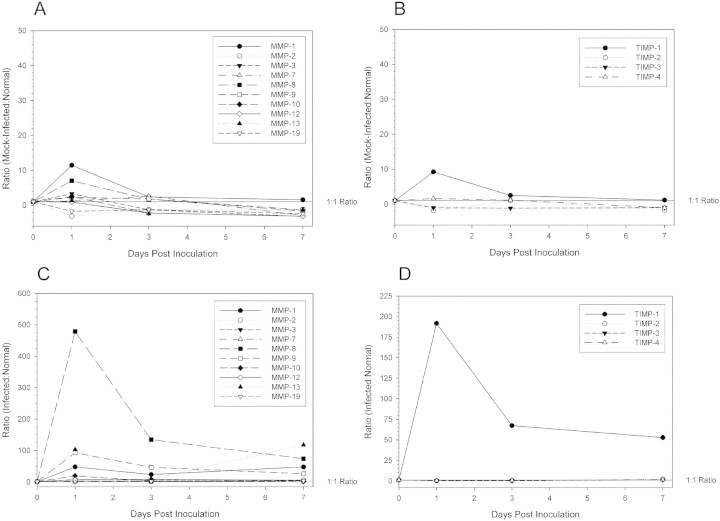
Real-time RT-PCR was conducted on total RNA extracted from five-cornea pools (n = 3/group) for C. albicans-infected and mock-infected at 1, 3, and 7 days p.i. and in normal, nonscarified corneas (Table 3) . MMP genes that were upregulated on day 1 p.i. remained elevated relative to levels in mock-infected corneas (Fig. 3) and, except for MMP-9 and -10, relative levels were higher at day 7 than on day 1 p.i. Comparison of MMP and TIMP transcript levels in mock-infected corneas and in normal corneas (Fig. 4A 4B) and comparison in infected corneas and normal corneas (Fig. 4C and 4D) revealed that transcript levels declined toward baseline levels.
Figure 3.
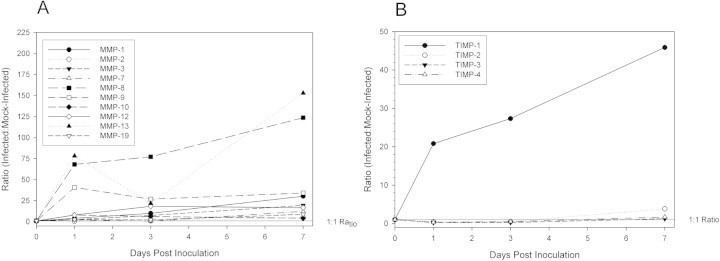
Differential gene expression ratios of MMPs and TIMPS in C. albicans keratitis compared to control. Total RNA was quantified by real-time RTPCR, with CT values normalized to GAPDH to show the relative amounts of MMPs (A) or TIMPs (B) of infection compared to controls over time.
Figure 4.
Differential gene expression ratios of MMPs and TIMPS over one week in scarified, mock-infected controls compared to normal corneas and in posttraumatic C. albicans keratitis compared to normal mouse corneas. (A) MMP ratios of mock-infected compared to normal mouse corneas. (B) TIMP ratios of mock-infected compared to normal mouse corneas. (C) MMP ratios of C. albicans-infected compared to normal mouse corneas. (D) TIMP ratios of C. albicans keratitis compared to normal mouse corneas.
Discussion
Metalloproteinases are part of the cornea’s response to injury and infection,22 23 and these proteolytic enzymes are intimately involved in the onset and outcome of fungal keratitis. Findings from microarray, real-time RT-PCR, and immunostaining showed that MMP-8, -9, -10, -12, -13, and -19 were substantially upregulated in C. albicans-infected corneas compared with controls. By inciting inflammation and disrupting corneal structure MMPs affect the course of keratomycosis.
Leukocyte-derived metalloproteinases that are involved in fungal keratitis include a collagenase (MMP-8), gelatinase (MMP-9), stromelysin (MMP-10), and elastase (MMP-12) that are released from polymorphonuclear leukocytes or macrophages soon after microbial inoculation. Our study confirms that MMP-8 and -9 have increased expression in fungal keratitis and suggests that these MMPs signal leukocyte extravasation and chemotaxis.18 MMP-2 did not increase, consistent with previous findings in P. aeruginosa keratitis and Fusarium solani keratitis.14 19 MMPs that are part of the innate immune response mediate the initial reaction to microbial keratitis.
MMPs can also be released by corneal epithelial cells and keratocytes to potentiate the degradation of corneal epithelial basement membrane and stroma that occurs with fungal adherence and invasion. MMP-9 increases immediately after superficial corneal wounding,13 and fungal infection perpetuates its upregulation. MMP-9 may also promote necrotizing inflammation and neovascularization with progressive corneal infection.10 11 24 Inhibiting the expression and activity of MMP-9 might reduce corneal destruction and angiogenesis caused by infectious keratitis.25
MMP-13 expression is an early corneal response of corneal cells to fungal exposure, and its expression rises over the week following exposure. This interstitial collagenase influences corneal reepithelialization and stromal thinning.26 27 By digesting collagen and proteoglycan, MMP-13 may be involved in reparative processes of ulcerative keratomycosis.
MMP-13 appears to have a role in fungal keratitis independent from scarification, but corneal trauma affects the differential expression of other MMPs. Superficial scarification increased the expression of MMP-9 and -10, and fungal infection augmented their upregulation. Compared to normal eyes, MMP-1 was upregulated the day after scarification but was not further affected by fungal infection. MMP-19 was slightly downregulated by scarification, but its expression more than doubled when injury and infection were combined. Posttraumatic microbial keratitis involves an interplay of inflammatory and wounding responses.
Corneal homeostasis depends on interactions between MMPs and TIMPs. Adequate TIMP expression dampens undue damage of the corneal stroma during infection.16 TIMP-1 is rapidly upregulated during C. albicans keratitis, somewhat faster than in P. aeruginosa keratitis.14 Though increased by corneal trauma, the expression of TIMP-1 is amplified by fungal infection within one day to >20 times that of scarified controls. The expression of TIMP-2, -3, and -4, on the other hand, was mildly downregulated or unchanged. Efforts are underway to study whether MMP or TIMP antagonists could alter the cornea’s susceptibility to infection or dampen the severity of microbial keratitis.28
In summary, the pathogenesis of C. albicans infection begins with adherence of yeasts to the traumatized mucosal surface followed by a morphogenic transition to invading hyphae.29 Early in the course of candidiasis, C. albicans induces tissue and inflammatory responses with upregulation of certain metalloproteinases.30 MMP-9 and other MMPs may contribute to ulcerative keratitis and facilitate fungal growth and extension.20 By understanding the molecular processes of mycotic keratitis, antifungal drug discovery efforts can explore new compounds that interreact with specific mediators involved in the initial stages of fungal infection.
Acknowledgments
The authors thank De-Quan Li and Stephen Pflugfelder for real-time RT-PCR assistance and Samuel Wu for support in immunofluorescent staining.
Footnotes
Supported by Core Grant EY02520 from the National Institutes of Health, Research to Prevent Blindness, and Sid W. Richardson Foundation.
Submitted for publication June 5, 2008; revised August 28, 2008; accepted December 11, 2008.
Disclosure: X. Yuan, None; B.M. Mitchell, None; K.R. Wilhelmus, None
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be marked “advertisement” in accordance with 18 U.S.C. §1734 solely to indicate this fact.
Corresponding author: Kirk R. Wilhelmus, Department of Ophthalmology, 6565 Fannin Street, NC205, Houston, TX 77030; kirkw@bcm.tmc.edu.
References
- 1.Leck AK, Thomas PA, Hagan M, et al. Aetiology of suppurative corneal ulcers in Ghana and south India, and epidemiology of fungal keratitis. Br J Ophthalmol . 2002;86:1211–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kercher L, Wardwell SA, Wilhelmus KR, Mitchell BM. Molecular screening of donor corneas for fungi before excision. Invest Ophthalmol Vis Sci . 2001;42:2578–2583. [PubMed] [Google Scholar]
- 3.Wu T, Mitchell BM, Carothers T, et al. Molecular analysis of the pediatric ocular surface for fungi. Curr Eye Res . 2003;2003:33–36. [DOI] [PubMed] [Google Scholar]
- 4.Sun RL, Jones DB, Wilhelmus KR. Clinical characteristics and outcome of Candida keratitis. Am J Ophthalmol . 2007;143:1043–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O'Day DM, Head WS, Csank C, et al. Differences in virulence between two Candida albicans strains in experimental keratitis. Invest Ophthalmol Vis Sci . 2000;41:1116–1121. [PubMed] [Google Scholar]
- 6.Wu TG, Wilhelmus KR, Mitchell BM. Experimental keratomycosis in a mouse model. Invest Ophthalmol Vis Sci . 2003;44:210–216. [DOI] [PubMed] [Google Scholar]
- 7.Jackson BE, Wilhelmus KR, Mitchell BM. Genetically regulated filamentation contributes to Candida albicans virulence during corneal infection. Microb Pathog . 2007;42:88–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Malemud CJ. Matrix metalloproteinases (MMPs) in health and disease: an overview. Front Biosc . 2006;11:1696–1701. [DOI] [PubMed] [Google Scholar]
- 9.Sivak JM, Fini ME. MMPs in the eye: emerging roles for matrix metalloproteinases in ocular physiology. Prog Retin Eye Dis . 2002;21:1–14. [DOI] [PubMed] [Google Scholar]
- 10.Ma JJ, Dohlman CH. Mechanisms of corneal ulceration. Ophthalmol Clin North Am . 2002;15:27–33. [DOI] [PubMed] [Google Scholar]
- 11.Yang YN, Bauer D, Wasmuth S, Steuhl KP, Heiligenhaus A. Matrix metalloproteinases (MMP-2 and 9) and tissue inhibitors of matrix metalloproteinases (TIMP-1 and 2) during the course of experimental necrotizing herpetic keratitis. Exp Eye Res . 2003;77:227–237. [DOI] [PubMed] [Google Scholar]
- 12.McClellan SA, Huang X, Barrett RP, et al. Matrix metalloproteinase-9 amplifies the immune response to Pseudomonas aeruginosa corneal infection. Invest Ophthalmol Vis Sci . 2006;47:256–264. [DOI] [PubMed] [Google Scholar]
- 13.Carter RT, Kambampati R, Murphy CJ, Bentley E. Expression of matrix metalloproteinase 2 and 9 in experimentally wounded canine corneas and spontaneous chronic corneal epithelial defects. Cornea . 2007;26:1213–1219. [DOI] [PubMed] [Google Scholar]
- 14.Ikema K, Matsumoto K, Inomata Y, et al. Induction of matrix metalloproteinases (MMPs) and tissue inhibitors of MMPs correlates with outcome of acute experimental pseudomonal keratitis. Exp Eye Res . 2006;83:1396–1404. [DOI] [PubMed] [Google Scholar]
- 15.Xue ML, Wakefield D, Willcox MD, et al. Regulation of MMPs and TIMPs by IL-1beta during corneal ulceration and infection. Invest Ophthalmol Vis Sci . 2003;44:2020–2025. [DOI] [PubMed] [Google Scholar]
- 16.Kernacki KA, Chunta JL, Barrett RP, Hazlett LD. TIMP-1 role in protection against Pseudomonas aeruginosa-induced corneal destruction. Exp Eye Res . 2004;78:1155–1162. [DOI] [PubMed] [Google Scholar]
- 17.Dong X, Shi W, Zeng Q, Xie L. Roles of adherence and matrix metalloproteinases in growth patterns of fungal pathogens in cornea. Curr Eye Res . 2005;30:613–620. [DOI] [PubMed] [Google Scholar]
- 18.Rohini G, Murugeswari P, Prajna NV, Lalitha P, Muthukkaruppan V. Matrix metalloproteinases (MMP-8, MMP-9) and the tissue inhibitors of metalloproteinases (TIMP-1, TIMP-2) in patients with fungal keratitis. Cornea . 2007;26:207–211. [DOI] [PubMed] [Google Scholar]
- 19.Mitchell BM, Wu TG, Chong EM, Pate JC, Wilhelmus KR. Expression of matrix metalloproteinases 2 and 9 in experimental corneal injury and fungal keratitis. Cornea . 2007;26:589–593. [DOI] [PubMed] [Google Scholar]
- 20.Zhai HL, Xie LX, Dong XG. The role of gelatinases in pathological changes of fungal keratitis in experimental rabbits. Zhonghua Yan Ke Za Zhi . 2007;43:817–822. [PubMed] [Google Scholar]
- 21.Mitchell BM, Wu TG, Jackson BE, Wilhelmus KR. Candida albicans strain-dependent virulence and Rim13p-mediated filamentation in experimental keratomycosis. Invest Ophthalmol Vis Sci . 2007;48:774–780. [DOI] [PubMed] [Google Scholar]
- 22.Wong TT, Sethi C, Daniels JT, Limb GA, Murphy G, Khaw PT. Matrix metalloproteinases in disease and repair processes in the anterior segment. Surv Ophthalmol . 2002;47:239–256. [DOI] [PubMed] [Google Scholar]
- 23.Ollivier FJ, Gilger BC, Barrie KP, et al. Proteinases of the cornea and preocular tear film. Vet Ophthalmol . 2007;10:199–206. [DOI] [PubMed] [Google Scholar]
- 24.Lee S, Zheng M, Kim B, Rouse BT. Role of matrix metalloproteinase-9 in angiogenesis caused by ocular infection with herpes simplex virus. J Clin Invest . 2002;110:1105–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Azkur AK, Kim B, Suvas S, Lee Y, Kumaraguru U, Rouse BT. Blocking mouse MMP-9 production in tumor cells and mouse cornea by short hairpin (sh) RNA encoding plasmids. Oligonucleotides . 2005;15:72–84. [DOI] [PubMed] [Google Scholar]
- 26.Ye HQ, Maeda M, Yu FSX, Azar DT. Differential expression of MT1-MMP (MMP-14) and collagenase III (MMP-13) genes in normal and wounded rat corneas. Invest Ophthalmol Vis Sci . 2000;41:2894–2899. [PubMed] [Google Scholar]
- 27.Li D-Q, Shang TY, Kim HS, Solomon A, Lokeshwar BL, Pflugfelder SC. Regulated expression of collagenases MMP-1, -8, and -13 and stromelysines MMP-3, -10, and -11 by human corneal epithelial cells. Invest Ophthalmol Vis Sci . 2003;44:2928–2936. [DOI] [PubMed] [Google Scholar]
- 28.Lee MM, Yoon BJ, Osiewicz K, et al. Tissue inhibitor of metalloproteinase 1 regulates resistance to infection. Infect Immun . 2005;73:661–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jackson BE, Mitchell BM, Wilhelmus KR. Corneal virulence of Candida albicans strains deficient in Tup1-regulated genes. Invest Ophthalmol Vis Sci . 2007;48:2535–2539. [DOI] [PubMed] [Google Scholar]
- 30.Claveau I, Mostefaoui Y, Rouabhia M. Basement membrane protein and matrix metalloproteinase deregulation in engineered human oral mucosa following infection with Candida albicans Matrix Biol . 2004;23:477–486. [DOI] [PubMed] [Google Scholar]