Abstract
Objectives: To evaluate the safety, efficacy, and durability of treating axillary hyperhidrosis with high-intensity micro-focused ultrasound plus visualization. Design: Two randomized double-blind, sham-controlled pilot studies. Measurements: For Study 1, the primary endpoint was response defined as ≥50-percent reduction in baseline sweat production as measured gravimetrically. For Study 2, the primary endpoint was response defined as a reduction of Hyperhidrosis Disease Severity Scale scores from 3 or 4 to 1 or 2. Secondary endpoints included changes in gravimetric and starch-iodine testing and patient satisfaction. Results: In Study 1, ≥50 percent of patients achieved a positive treatment response. In Study 2, the response rate at post-treatment Day 60 for micro-focused ultrasound plus visualization- (N=12) and sham-treated (N=8) patients was 67 and zero percent, respectively (p=0.005). Patients evaluated 12 months after treatment (N=11) demonstrated the long-lasting effectiveness of micro-focused ultrasound plus visualization for treating axillary hyperhidrosis. All but one patient in the micro-focused ultrasound plus visualization group were satisfied with their results while all sham group patients were dissatisfied (p=0.0001). Subjective reports of greatest improvement were sweat production (92%) and social embarrassment (83%). Adverse events were found to be mild and were resolved within a short timeframe. Conclusion: Micro-focused ultrasound plus visualization appears to be safe, effective, well-tolerated, and a long-lasting means for treating axillary hyperhidrosis.
Hyperhidrosis is characterized by perspiration in excess of the physiological amount necessary to maintain thermal homeostasis.1 Primary hyperhidrosis is idiopathic while secondary hyperhidrosis may be the result of any one of a large number of medical conditions, such as endocrine or neurological disorders.2,3 In both forms of the condition, excessive sweating may be restricted to local areas of the body, such as the soles, palms, or axillae (focal hyperhidrosis), the entire body (generalized hyperhidrosis), or in different combinations of areas, all with varying degrees of severity. Although patients with hyperhidrosis have the same size and number of sweat glands as normal individuals, they are hyperfunctional resulting in a higher-than-normal basal levels of sweat production and an increased response to emotional or physical stimuli.4
All forms of hyperhidrosis are associated with physical discomfort and social embarrassment and have an overall negative impact on quality of life.5 Nonsurgical treatment options for patients with primary hyperhidrosis include topical antiperspirants, iontophoresis, and systemic medications. Surgical treatments include endoscopic thoracic sympathectomy or excision of axillary tissue.1
Botulinum toxin type A has become an effective and minimally invasive treatment option6,7 for patients who fail to respond to conservative treatment prior to resorting to surgery8; however, botulinum injections are painful and must be periodically repeated.9,10 Several energy-based treatments are also under investigation for treating axillary hyperhidrosis. The use of neodymium-doped yttrium aluminum garnet (Nd:YAG) lasers was reported to be safe and effective in one study11 while another study reported unconvincing results.12 The use of microwaves also appears to be effective,13 but is sometimes associated with persistent adverse events.14
A novel device has been developed that uses high-intensity micro-focused ultrasound plus visualization (MFU-V) to produce small (∼1mm3) thermal lesions or thermal coagulation points (TCPs) within the dermis. The use of ultrasound ensures the focused ultrasound energy is delivered to specific soft tissue layers to depths of up to 4.5mm within subcutaneous tissue layers beneath the superficial dermis.15,16 The visualization component of the device ensures the device is well-coupled to the dermis for consistent energy delivery as well as allowing the user to avoid anatomical structures, such as large blood vessels or bone. This device has been cleared by the U.S. Food and Drug Administration (FDA) for noninvasive eyebrow lift and to noninvasively lift lax tissue on the neck and the submental areas (Ulthera® System; Ulthera, Inc., Mesa, Arizona).17
The authors hypothesize that creating TCPs at the depth of sweat glands will effectively damage them without surface effects. Because sweat glands have limited to no capacity for regeneration, this effect is expected to be long lasting. The following pilot studies were performed to assess the safety and long-term efficacy of MFU-V for treating patients with axillary hyperhidrosis.
METHODS
Patient population. Both studies recruited male and female patients who were 18 to 75 years of age with moderate-to-severe bilateral axillary hyperhidrosis that was refractory to prior topical therapies. The diagnosis of hyperhidrosis included ≥50mg of spontaneous resting axillary sweat production in each axilla measured gravimetrically over a five-minute period at normal room conditions (20-25.6°C, 20-80% relative humidity) and a Hyperhidrosis Disease Severity Scale (HDSS) score of 3 or 4.
Each participant was otherwise healthy and agreed to forgo any other procedures in the planned treatment areas during the course of the study. Women of childbearing potential were required to provide a negative urine pregnancy test at the start of the study and agree to use an acceptable method of birth control.
Patients were excluded from the study if they had any skin disorder in the planned treatment areas; undergone treatment with botulinum toxin during the previous year or anticipated treatment with botulinum toxin during the study period; a known allergy to starch powder or iodine; secondary hyperhidrosis due to an underlying disease, such as hyperthyroidism, lymphoma, or malaria; prior surgical treatment of hyperhidrosis including sympathectomy, surgical debulking of the sweat glands, subcutaneous tissue curettage, or ultrasonic surgery; a history of chronic drug or alcohol abuse or autoimmune disease; concurrent therapy that might interfere with the evaluation of the study endpoints; a psychiatric or developmental disorder that could prevent the patient from providing informed consent or might compromise the objectives of the study; or were currently enrolled in another study involving the use of an investigational drug or device.
Study design. These were prospective, randomized, double-blind, sham-controlled pilot studies. Study 1 consisted of the following two groups: Group A received two MFU-V treatments on one randomly chosen axilla and two sham treatments on the other axilla 30 days apart. Group B received two MFU-V treatments on both axilla 30 days apart. This group was used to study the effect of subcutaneous lidocaine on pain management and possible effects on treatment efficacy. Patients in Study 2 were randomized to undergo two bilateral MFU-V treatments or bilateral sham treatments 30 days apart.
Treatment procedure, Studies 1 and 2. Using a template provided by the manufacturer, a 100x75mm grid that was divided into 12 25x25mm squares was applied to the treatment area. An example of the target treatment area is shown in Figure 1. Ultrasound gel was applied to each treatment square and an ultrasound image was obtained prior to treatment to ensure proper coupling between the transducer and skin. Patients undergoing MFU-V were initially treated using a 4MHz transducer with a focal depth of 4.5mm followed by a 7MHz transducer with a focal depth of 3mm. The energy was set at the maximal level for the particular transducer being used while the energy level for sham treatments was set a 0 Joules. Using the 4MHz transducer, each 25mm2 square was exposed to 20 lines of MFU-V placed 2 to 3mm apart, each line requiring about two seconds to treat. The investigator continued treatment by making a second pass using the 7MHz transducer in the same manner.
Figure 1.
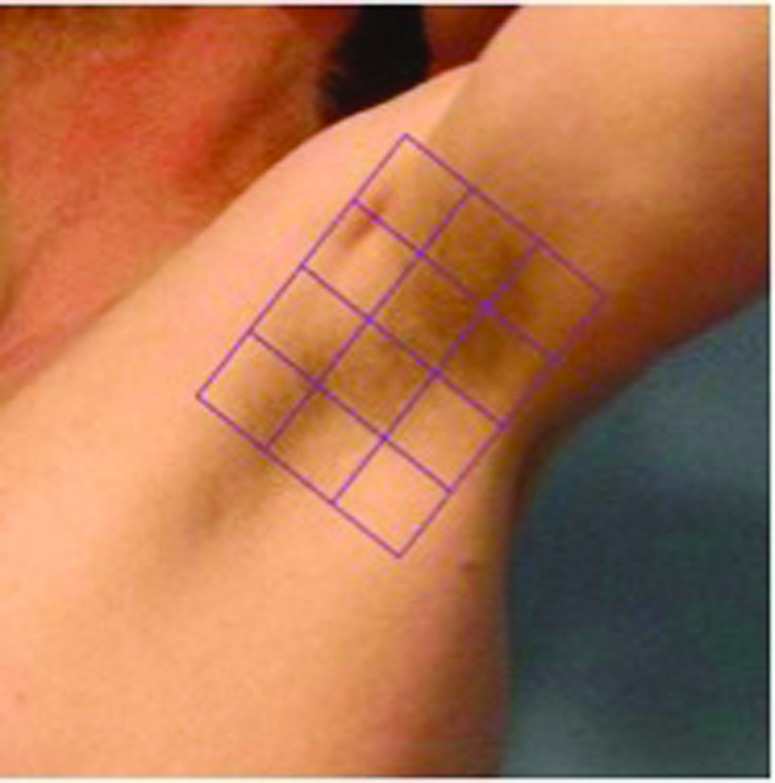
Representative treatment area for Study 1 and Study 2. Each 2.5cm2 square received 20 lines of treatment using 4MHz at a depth of 4.5mm followed by 20 lines of treatment using 7MHz at a depth of 3.0mm.
Efficacy assessments. Study 1. After the initial treatment on Day 0, efficacy assessments using gravimetric testing and starch-iodine testing with digital images were completed on Days 7, 14, and 28 or 30 (at the time of second treatment). After the second treatment, additional assessments were made on Days 60, 90, and 120.
Study 2. After the initial treatment, efficacy assessments consisting of the HDSS, gravimetric measurements and starch iodine tests were made on Days 7, 14, and 30. After the second treatment, efficacy assessments were made on Days 37, 44, 60, 90, 120, and 365. Following the second treatment, patients also completed a Patient Satisfaction Questionnaire (PSQ) on Days 60, 90, 120, and 365.
Study 1: Treatment endpoints. The primary endpoints were 1) a positive treatment response defined as a ≥50-percent reduction in baseline spontaneous axillary sweat production in the MFU-V-treated axilla measured gravimetrically18 on Day 120 (90 days following the second treatment) and 2) the difference in gravimetric tests between the MFU-V- and sham-treated axillae on Day 120. A subgroup (N=3) comparison of treatment efficacy and patient discomfort was made by comparing one axilla of each subject pretreated with subcutaneous lidocaine with epinephrine or lidocaine alone. For patients in Group B, gravimetric test measurements for pretreated axilla were compared to axilla that did not receive pretreatment.
Study 2: Treatment endpoints. The primary endpoint was a positive treatment response defined as a reduction in the HDSS score from 3 or 4 to 1 or 2 on Day 60 (30 days following the second treatment).
Secondary endpoints included changes in HDSS scores at each follow-up visit, at least a 50-percent reduction in gravimetric measurement at each follow-up visit, and changes in the starch iodine test and PSQ parameters.
Safety assessments. Reported adverse events (AEs) were recorded at each follow-up visit during both studies. Patients were asked to rate the severity of discomfort during each treatment procedure using a validated 11-point Numerical Rating Scale (NRS) where pain severity ranged from 0 (no pain) to 10 (worst possible pain). During each treatment session, an overall pain score was calculated as the mean of both axillae, both passes, and both focal depths.
Statistical analysis. Fisher’s exact tests were used to test for differences in proportions. For Study 2, rank-based mixed models were used to test for group differences in gravimetric test measurements over time, including the evaluation of the relationship between gravimetric test measurement and HDSS. Rank-based mixed models and signed rank tests for location were used to test for differences in NRS scores by time point, number of passes, focal depth, and gender. All tests were two-sided with 0.05 significance levels. Analyses were done using SAS v9.2 (SAS Institute, Inc., Cary, North Carolina).
Ethics. The protocols used in this study were approved by an independent institutional review board (Asentral, Inc., Newburyport, Massachusetts). Each participant provided informed consent prior to participating in any study-related procedures. ClinicalTrials.gov Identifier: NCT01713959.
RESULTS
Patient demographics. Most subjects (79%) in Study 1 were female with a mean age of 38 years. Subjects described themselves as Caucasian (36%), Hispanic/Latino (36% each), African American (21%), and Asian (7%). Most subjects (65%) in Study 2 were male, with a mean age of 34 years. Subjects described themselves as Caucasian (30%), Hispanic/Latino (50%), African American (15%), and Asian (5%). The demographics of both study populations are summarized in Table 1.
TABLE 1.
Demographic characteristics
| STUDY 1 (N=14) | |
| Age (years), mean (SD) | 38.2 (12.65) |
| Gender, N (%) | |
| • Female | 11 (78.6) |
| • Male | 3 (21.4) |
| Race/ethnicity, N (%) | |
| • Caucasian | 5 (35.7) |
| • African American/Black | 3 (21.4) |
| • Hispanic/Latino | 5 (35.7) |
| • Asian | 1 (7.1) |
| BMI (kg/m2), mean (SD) | 24.798 (3.2262) |
| STUDY 2 (N=20) | |
| Age (years), mean (SD) | 33.9 (9.16) |
| Gender, N (%) | |
| • Female | 7 (35.0) |
| • Male | 13 (65.0) |
| Race/ethnicity, N (%) | |
| • Caucasian | 6 (30.0) |
| • African American/Black | 10 (50.0) |
| • Hispanic/Latino | 3 (15.0) |
| • Asian | 1 (5.0) |
| BMI (kg/m2), mean (SD) | 25.992 (3.3932) |
Study 1. The study enrolled 16 patients; however, one was excluded due to a device malfunction and another was lost to follow-up after two weeks. Thus, 11 patients were assigned to Group A and three patients were assigned to Group B. For one patient, no data were obtained on Day 7 or 14.
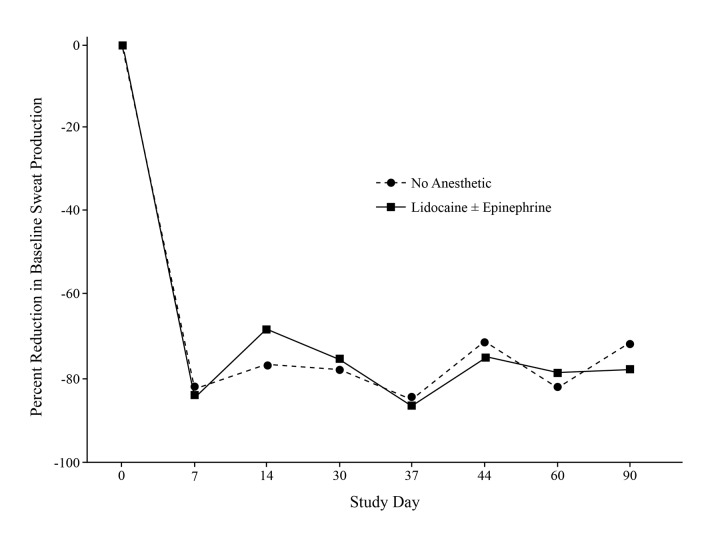
There was no difference in the percent reduction in baseline sweat production among axillae that were pretreated with subcutaneous lidocaine with or without epinephrine and those receiving no pre-treatment anesthetic. lidocaine with epinephrine was better at controlling discomfort than lidocaine alone (Figure 2). More than 50 percent of patients achieved a positive treatment response. The only nonresponder was a female patient with a baseline gravimetric measurement below the median value of 150.
Figure 2.
Effects of anesthetic pretreatment on multi-focused ultrasound. The use of subcutaneous anesthetic injection prior to treatment did not alter the effect of MFU on sweat reduction.
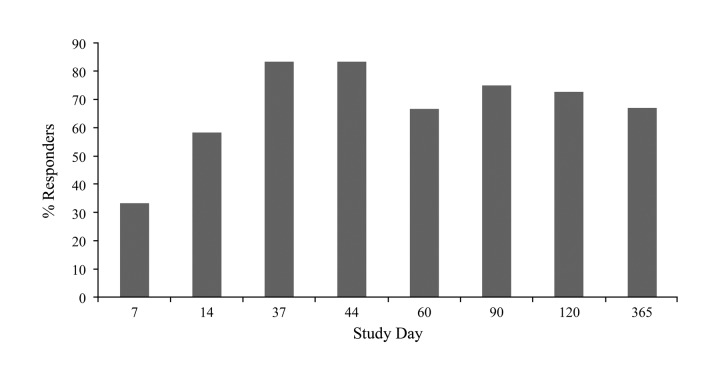
Study 2: Primary efficacy. Study 2 enrolled 20 patients who were randomized to either bilateral MFU-V (N=12) or sham treatment (N=8). one MFU-V patient was lost to follow-up at Day 90. At Day 60 (30 days after the second treatment), a positive HDSS response rate of 67 percent was achieved by the MFU-V group versus zero percent in the sham group (p=0.005) (Figure 3).
Figure 3.
Percent HDSS responders in study 2. On Study 60 (30 days after second treatment), there was a 67% response rate in the MFU-V treatment group vs. 0% in the sham group (p<0.005). The treatment response peaked 7 days after the second treatment (83%). *Responders were defined as patients whose scores HDSS changed from 3 or 4 to 1 or 2.
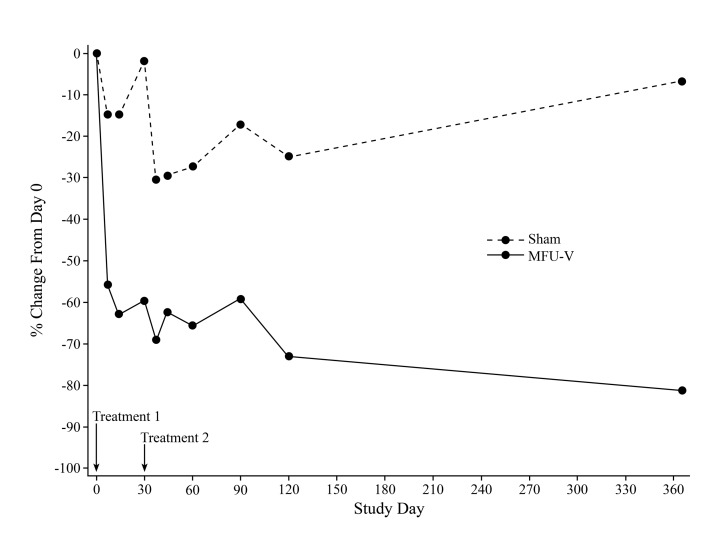
Study 2: Secondary efficacy. HDSS response rate was ≥50 percent in the MFU-V group at all time points except Day 7 (Figure 2). The HDSS response rate was zero percent in the sham group at all time points. Median percent reduction from baseline in gravimetric test measurements for both MFU-V and sham groups are shown by time point in Figure 4. Baseline sweat production decreased by ≥50 percent at all time points in the MFU-V group, but not the sham group. Regardless of study visit, percent change from baseline in gravimetric test measurement was significantly greater in the MFU-V group than in the sham group (p>0.0001). Considering the median gravimetric test measurement over all time points after the second treatment, 10 of 12 (83%) patients in the MFU-V group and no patients in the sham group experienced a ≥50-percent sweat reduction from baseline. The percent change in gravimetric test measurement was strongly related to HDSS (p=0.005), and this relationship was independent of time point.
Figure 4.
Percent change in gravimetric testing in Study 2. Baseline sweat production decreased by ≥50% in the MFU-V-treated but not sham-treated axillae. Among patients in the MFU-V treatment group, the greatest change from baseline (73.1%) occurred on Study Day 90 (120 days after the second treatment). The percent change from baseline in gravimetric test measurement was significantly greater in the MFU-V group than in the sham group at each visit (p<0.0001).
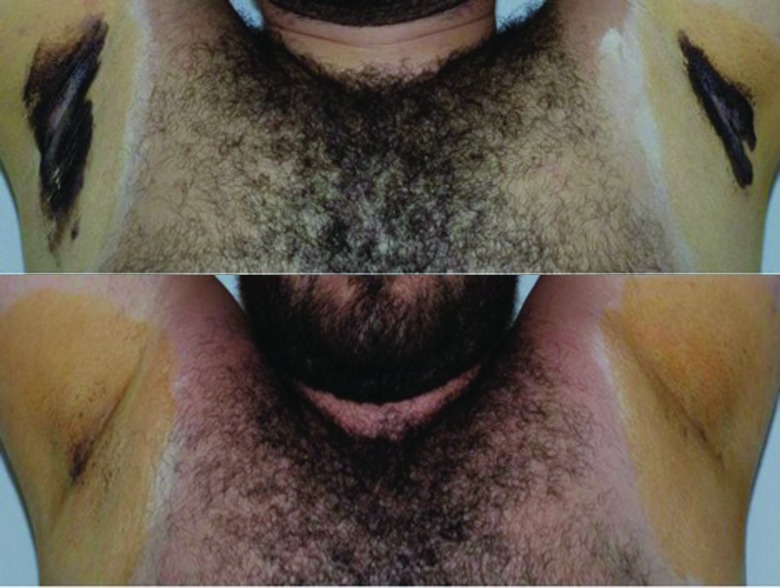
The results of the starch iodine test revealed substantial differences between baseline and follow-up visits for the treated axillae while no differences were observed among sham-treated axillae (Figure 5).
Figure 5.
Effect of MFU on sweat production, Study 2. Starch iodine test at baseline (top) and 120 days following MFU-V treatment (bottom).
All but one patient in the MFU-V group and none of the patients in the sham group reported clinical improvements at Day 60, 90, and 120 (30, 60, and 90 days after the second treatment; p<0.0001 for the Day 60 comparison). At Day 60, the areas of greatest improvement were sweat production (92%) and social embarrassment (83%) (Table 2).
TABLE 2.
Perceived improvements after second treatment, MFU-V treatment group, Study 2a
| DAY 30 | DAY 60 | DAY 90 | DAY 365 | |
|---|---|---|---|---|
| PARAMETER, N (%) | ||||
| Improved | 11 (91.7) | 11 (91.7) | 10 (90.9)b | 5 (100.0) |
| Areas of improvement | ||||
| • Sweating | 11 (91.7) | 4 (33.3) | 7 (58.3) | 4 (80) |
| • Discomfort | 5 (41.7) | 4 (33.3) | 7 (58.3) | 3 (60) |
| • Embarrassment | 10 (83.3) | 8 (66.7) | 7 (58.3) | 2 (40) |
| • Psychological | 5 (41.7) | 4 (33.3) | 5 (41.7) | 1 (20) |
None of the sham-treated patients reported improvements;
N=11
PSQ results are shown in Table 3. At all time points, most patients in the MFU-V group were satisfied or very satisfied while all patients in the sham group were dissatisfied or very dissatisfied with their treatment results (p=0.0001 for the Day 60 comparison). Similarly, all but one patient in the MFU-V group and no patients in the sham group would recommend MFU-V treatment to their family and friends (p<0.0001 for the Day 60 comparison).
TABLE 3.
Patient satisfaction after the second treatment, Study 2
| DAY 30 | DAY 60 | DAY 90 | ||||
|---|---|---|---|---|---|---|
| MFU-V N=12 | SHAM N=8 | MFU-V N=12 | SHAM N=8 | MFU-V N=11 | SHAM N=8 | |
| PARAMETER, N (%) | ||||||
| Satisfaction level | ||||||
| • Very dissatisfied | - | 5 (62.5) | - | 5 (62.5) | - | 5 (62.5) |
| • Dissatisfied | 1 (8.3) | 3 (37.5) | 2 (16.7) | 3 (37.5) | 1 (9.1) | 3 (37.5) |
| • Satisfied | 7 (58.3) | - | 6 (50.0) | - | 6 (54.5) | - |
| • Very satisfied | 4 (33.3) | - | 4 (33.3) | - | 4 (36.4) | - |
| Would recommend | ||||||
| • No | 1 (8.3) | 8 (100.0) | 1 (8.3) | 8 (100.0) | 1 (9.1) | 8 (100.0) |
| • Yes | 11 (91.7) | - | 11 (91.7) | - | 10 (90.9) | - |
Study 2: Long-term efficacy. Long-term follow-up assessments were made in a subset of patients 12 months after undergoing MFU-V (N=6) or sham (N=5) treatment (Table 4). Of the six MFU-V-treated patients, five continued to report noticeable clinical improvement, five remained satisfied with their treatment results, four maintained positive response based on HDSS, and five maintained a positive response based on gravimetric testing.
TABLE 4.
Long-term efficacy 12 months after treatment, Study 2
| NOTICED IMPROVEMENT | SATISFIED, VERY SATISFIED | DISSATISFIED, VERY DISSATISFIED | % RESPONDER S (HDSS) | % RESPONDERS (GRAVIMETRIC) | |
|---|---|---|---|---|---|
| Sham, N=5 | 0% | 0% | 100% | 0% | 0% |
| MFU-V, N=6 | 83% | 83% | 17% | 67% | 83% |
Study 1: Safety endpoints. The incidence of treatment-emergent AEs is shown in Table 5. All patients experienced at least one AE that was probably related to the treatment procedure. Significantly more patients reported AEs associated with MFU-V treatment versus sham treatment (93% vs. 9%; p<0.0001) and significantly more MFU-V patients reported treatment-related AEs after the first treatment session than after the second session (86% vs. 54%; p=0.03). Most AEs were mild and there were no serious or unexpected AEs. Individual AEs are summarized in Table 6. The most commonly reported AE was axilla tenderness or soreness reported by 86 percent of patients after MFU-V treatment with a median duration of 11 days (range, 2 to 41 days). Bruising of the axilla was reported by one patient in the sham treatment group. One patient required a single dose of ibuprofen one day after MFU-V treatment.
TABLE 5.
Incidence of adverse events, Study 1
| MFU-V (N=14) | SHAM (N=11) | N/A* (N=14) | TOTAL (N=14) | |
|---|---|---|---|---|
| Overall, N (%) | 13 (92.9) | 1 (9.1) | 3 (21.4) | 14 (100.0) |
| • After 1st treatment | 12 (85.7) | 1 (9.1) | 1 (7.1) | 13 (92.9) |
| • After 2nd treatment† | 7 (53.8) | - | 3 (23.1) | 9 (69.2) |
| Severity, N (%) | ||||
| • Mild | 12 (85.7) | 1 (9.1) | 3 (21.4) | 13 (92.9) |
| • Moderate | 2 (14.3) | - | 1 (7.1) | 3 (21.4) |
| • Severe | 1 (7.1) | - | - | 1 (7.1) |
| Related to Device, N (%) | ||||
| • Unrelated/unlikely | 1 (7.1) | - | 3 (21.4) | 3 (21.4) |
| • Possibly | 13 (92.9) | - | 1 (7.1) | 14 (100.0) |
| • Probably | 1 (7.1) | - | - | 1 (7.1) |
| Related to Procedure, N (%) | ||||
| • Unrelated/unlikely | 1 (7.1) | - | 3 (21.4) | 3 (21.4) |
| • Possibly | 1 (7.1) | - | - | 1 (7.1) |
| • Probably | 13 (92.9) | 1 (9.1) | 1 (7.1) | 14 (100.0) |
Not specific to MFU-V or sham treatment;
For MFU-V, N/A, and total columns, N=13; for sham, N=10
TABLE 6.
Reported adverse events, Study 1
| ADVERSE EVENT, N (%)† | MFU-V (N=14) | SHAM (N=11) | N/A* (N=14) | TOTAL (N=14) |
|---|---|---|---|---|
| Arm paresthesia | 1 (7.1) | - | - | 1 (7.1) |
| Axilla bruising | 4 (28.6) | 1 (9.1) | - | 5 (35.7) |
| Axilla folliculitis | 1 (7.1) | - | - | 1 (7.1) |
| Axilla paresthesia | 2 (14.3) | - | - | 2 (14.3) |
| Axilla redness | 1 (7.1) | - | - | 1 (7.1) |
| Axilla tenderness/soreness | 12 (85.7) | - | 1 (7.1) | 13 (92.9) |
| Bowel preparation for colonoscopy | - | - | 1 (7.1) | 1 (7.1) |
| Mechanical fall while jogging | - | - | 1 (7.1) | 1 (7.1) |
| Sinusitis | - | - | 1 (7.1) | 1 (7.1) |
Not specific to MFU-V or sham.
For active, N/A, and total columns, N=13; for sham, N=10
Study 2: Safety endpoints. The incidence of treatment-emergent AEs is summarized in Table 7. AE incidence was higher in the MFU-V group compared to the sham group (92% vs. 50%; p=0.32) and, in the MFU-V group, higher after the second treatment than after the first (92% vs. 42%, p=0.03); however, most AEs were mild and none were unexpected. There were no serious AEs. Most AEs were possibly or probably related to the procedure in both treatment groups. Individual AEs are summarized in Table 8. The most commonly reported AE was axilla soreness or tenderness reported by 83 percent of MFU-V-treated patients with a median duration of 11 days (range, 4-32 days) and 25 percent of sham-treated patients with a median duration of two days (range, 2-2 days).
TABLE 7.
Incidence of adverse events, Study 2
| MFU-V N=12 | SHAM N=8 | TOTAL (N=20) | |
|---|---|---|---|
| GROUP, N (%) | |||
| Overall | 11 (91.7) | 4 (50.0) | 15 (75.0) |
| • After treatment #1 | 11 (91.7) | 2 (25.0) | 13 (65.0) |
| • After treatment #2 | 5 (41.7) | 3 (37.5) | 8 (40.0) |
| • Mild | 9 (75.0) | 4 (50.0) | 13 (65.0) |
| • Moderate | 4 (33.3) | 1 (12.5) | 5 (25.0) |
| • Severe | 1 (8.3) | - | 1 (5.0) |
| Relationship to device | |||
| • Unrelated/unlikely | 3 (25.0) | 1 (12.5) | 4 (20.0) |
| • Possibly | 10 (83.3) | 3 (37.5) | 13 (65.0) |
| • Probably | - | - | - |
| Relationship to procedure | |||
| • Unrelated/unlikely | 3 (25.0) | 1 (12.5) | 4 (20.0) |
| • Possibly | - | - | - |
| • Probably | 10 (83.3) | 3 (37.5) | 13 (65.0) |
TABLE 8.
Reported adverse events, Study 2
| ADVERSE EVENT, N (%) | MFU-V (N=12) | SHAM (N=8) | TOTAL (N=20) |
|---|---|---|---|
| Back pain | 1 (8.3) | - | 1 (5.0) |
| Bruising | 3 (25.0) | - | 3 (15.0) |
| Chills/URI | - | 1 (12.5) | 1 (5.0) |
| Eye ulcer | 1 (8.3) | - | 1 (5.0) |
| Insomnia | 1 (8.3) | - | 1 (5.0) |
| Palpitation | - | 1 (12.5) | 1 (5.0) |
| Paresthesia/numbness | 4 (33.3) | 1 (12.5) | 5 (25.0) |
| Soreness/tenderness | 10 (83.3) | 2 (25.0) | 12 (60.0) |
DISCUSSION
The results of these pilot studies demonstrate the efficacy of micro-focused ultrasound for the treatment of axillary hyperhidrosis. In Study 1, ≥50 percent of patients achieved a positive treatment response. One patient did not respond (in either axilla) for unknown reasons although this patient’s baseline level of sweat production was relatively low. In Study 2, improved HDSS scores were reported by the majority of MFU-V-treated patients at the end of the study. The substantial differences in sweat production between MFU-V- and sham-treated patients in Study 2 were confirmed by starch iodine testing.
The subjective measures in Study 2 were also very positive. All but one MFU-V-treated patients reported clinical improvements in their condition compared to none of the sham-treated patients. Importantly, the areas showing greatest improvements were decreased sweat production and decreased social embarrassment. Not surprisingly, most MFU-V-treated patients reported being satisfied or very satisfied with the results they achieved throughout the trial while sham-treated patients reported being dissatisfied or very dissatisfied. All but one MFU-V-treated patient but none of the sham-treated patients would recommend MFU-V to their family and friends for treating axillary hyperhidrosis.
These results compare favorably with the response rate reported following the use of systemic drug therapy for the treatment of axillary hyperhidrosis, such as glycopyrrolate or clonidine19 and oxybutynin.20 Although reported to be effective in many patients, orally administered medications have the disadvantage of requiring chronic use and possible systemic and treatment-limiting adverse events.
Botulinum toxins (onabotulinumtoxinA and incobotulinumtoxinA) have emerged as a treatment option for some patients who do not respond to more conservative treatment prior to resorting to surgical methods.21,22 Injecting botulinum toxin is clinically effective and considered minimally invasive6,7; however, the onset of effect may require a week to occur,23 the injections must be periodically repeated as the effect diminishes,7 and they are painful. Efforts to overcome injection pain include cryotherapy9,10 and diluting botulinum toxin with lidocaine.24
These results also compare favorably with other energy-based devices that have been used for treating axillary hyperhidrosis. A Nd-YAG laser has been shown to effectively reduce sweating in two studies,11,25 but a randomized, sham-controlled study resulted in no significant difference in sweat reduction between treated and untreated axilla.12 A microwave device has also been evaluated for treating axillary hyperhidrosis.13 It has been reported to produce long-lasting results, but the incidence of adverse events is high.14 Local edema and discomfort were reported as short-term events reported in 90 and 87 percent of patients, respectively, while more persistent events that occurred in the treatment area included altered sensation (65%), lasting a median of 37 days, and palpable bumps (71%), lasting for a median duration of 41 days. In another study, a patient treated with microwaves developed altered sensation in their arms that persisted for three months.26
The majority of MFU-V-treated patients in the current study reported some axillary discomfort during treatment; however, it was generally mild and described as paresthesia or numbness (N=2) or soreness or tenderness (N=12), but not pain. The mild discomfort experienced during treatment did not persist and immediately stopped when MFU-V treatment ended. The degree of discomfort was not substantially different for the first and second treatment pass although the pain rating, albeit low, was slightly higher for the 4.5mm focal depth.
Preliminary results suggest that MFU-V provides long-lasting improvements in patients with axillary hyperhidrosis. In contrast with systemic medications or injections of botulinum toxins, the use of micro-focused ultrasound may possibly represent a permanent solution to axillary hyperhidrosis. Larger clinical trials are clearly warranted to establish the role of MFU-V for the treatment of axillary hyperhidrosis.
CONCLUSION
The results of these two pilot studies indicate micro- focused ultrasound is an effective method of treating patients with axillary hyperhidrosis. In addition to significant reductions in baseline sweat production, patients reported high levels of satisfaction with the results they achieved. MFU-V was generally well tolerated, and patients reported only minor transient discomfort during treatment. Preliminary data indicates the effect of MFU-V on axillary sweat glands is very durable as the beneficial effects persist for at least 12 months. Further studies are needed to determine whether MFU-V represents a permanent means for treating axillary hyperhidrosis.
ACKNOWLEDGMENT
The authors acknowledge the statistical support of Dr. Janice M. Pogoda and the editorial assistance of Dr. Carl S. Hornfeldt during the preparation of this manuscript. This study was sponsored by Ulthera Inc., Mesa, Arizona.
Footnotes
DISCLOSURE:Dr. Nestor is a consultant and received research grants for this study from Ulthera Corporation, Mesa, Arizona. Dr. Park reports no relevant conflicts of interest. ClinicalTrials.gov Identifier: NCT01713959.
REFERENCES
- 1.Atkins JL, Butler PE. Hyperhidrosis: a review of current management. Plast Reconstr Surg. 2002;110:222–228. doi: 10.1097/00006534-200207000-00039. [DOI] [PubMed] [Google Scholar]
- 2.Adar R, Kurchin A, Zweig A, et al. Palmar hyperhidrosis and its surgical treatment: a report of 100 cases. Ann Surg. 1977;186:34–41. doi: 10.1097/00000658-197707000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Böni R. Generalized hyperhidrosis and its systemic treatment. Curr Probl Dermatol. 2002;30:44–47. doi: 10.1159/000060676. [DOI] [PubMed] [Google Scholar]
- 4.Heckmann M. Hyperhidrosis of the axilla. Curr Probl Dermatol. 2002;30:149–155. doi: 10.1159/000060688. [DOI] [PubMed] [Google Scholar]
- 5.Glaser DA, Hebert AA, Pariser DM, et al. Primary focal hyperhidrosis: scope of the problem. Cutis. 2007;79:5–17. [PubMed] [Google Scholar]
- 6.Naumann M, Lowe NJ, Kumar CR, et al. Botulinum toxin type a is a safe and effective treatment for axillary hyperhidrosis over 16 months: a prospective study. Arch Dermatol. 2003;139:731–736. doi: 10.1001/archderm.139.6.731. [DOI] [PubMed] [Google Scholar]
- 7.Scamoni S, Valdatta L, Frigo C, et al. Treatment of primary axillary hyperhidrosis with botulinum toxin type a: our experience in 50 patients from 2007 to 2010. ISRN Dermatol. doi: 10.5402/2012/702714. 2012 October 17 [Epub]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Collin J, Whatling P. Treating hyperhidrosis. Surgery and botulinum toxin are treatments of choice in severe cases. BMJ. 2000;320:1221–1222. doi: 10.1136/bmj.320.7244.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bechara FG, Sand M, Altmeyer P, et al. Skin cooling for botulinum toxin A injection in patients with focal axillary hyperhidrosis: a prospective, randomized, controlled study. Ann Plast Surg. 2007;58:299–302. doi: 10.1097/01.sap.0000238338.61631.e0. [DOI] [PubMed] [Google Scholar]
- 10.Skiveren J, Kjaerby E, Nordahl Larsen H. Cooling by frozen gel pack as pain relief during treatment of axillary hyperhidrosis with botulinum toxin A injections. Acta Derm Venereol. 2008;88:366–369. doi: 10.2340/00015555-0465. [DOI] [PubMed] [Google Scholar]
- 11.Letada PR, Landers JT, Uebelhoer NS, et al. Treatment of focal axillary hyperhidrosis using a long-pulsed Nd:YAG 1064 nm laser at hair reduction settings. J Drugs Dermatol. 2012;11:59–63. [PubMed] [Google Scholar]
- 12.Bechara FG, Georgas D, Sand M, et al. Effects of a long-pulsed 800-nm diode laser on axillary hyperhidrosis: a randomized controlled half-side comparison study. Dermatol Surg. 2012;38:736–740. doi: 10.1111/j.1524-4725.2012.02339.x. [DOI] [PubMed] [Google Scholar]
- 13.Jacob C. Treatment of hyperhidrosis with microwave technology. Semin Cutan Med Surg. 2013;32:2–8. [PubMed] [Google Scholar]
- 14.Hong HC, Lupin M, O’Shaughnessy KF. Clinical evaluation of a microwave device for treating axillary hyperhidrosis. Dermatol Surg. 2012;38:728–735. doi: 10.1111/j.1524-4725.2012.02375.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Laubach HJ, Makin IR, Barthe PG, et al. Intense focused ultrasound: evaluation of a new treatment modality for precise microcoagulation within the skin. Dermatol Surg. 2008;34:727–34. doi: 10.1111/j.1524-4725.2008.34196.x. [DOI] [PubMed] [Google Scholar]
- 16.White WM, Makin IR, Barthe PG, et al. Selective creation of thermal injury zones in the superficial musculoaponeurotic system using intense ultrasound therapy: a new target for noninvasive facial rejuvenation. Arch Facial Plast Surg. 2007;9:22–29. doi: 10.1001/archfaci.9.1.22. [DOI] [PubMed] [Google Scholar]
- 17.Brobst RW, Ferguson M, Perkins SW. Ulthera: initial and six month results. Facial Plast Surg Clin North Am. 2012;20:163–176. doi: 10.1016/j.fsc.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 18.Noël F, Piérard-Franchimont C, Piérard GE, et al. Sweaty skin, background and assessments. Int J Dermatol. 2012;51:647–655. doi: 10.1111/j.1365-4632.2011.05307.x. [DOI] [PubMed] [Google Scholar]
- 19.Walling HW. Systemic therapy for primary hyperhidrosis: a retrospective study of 59 patients treated with glycopyrrolate or clonidine. J Am Acad Dermatol. 2012;66:387–392. doi: 10.1016/j.jaad.2011.01.023. [DOI] [PubMed] [Google Scholar]
- 20.Wolosker N, de Campos JR, Kauffman P, et al. The use of oxybutynin for treating axillary hyperhidrosis. Ann Vasc Surg. 2011;25:1057–1062. doi: 10.1016/j.avsg.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 21. BOTOX® (onabotulinumtoxinA) [package insert]. Irvine, CA: Allergan, Inc.; 2011.
- 22. XEOMIN® (incobotulinumtoxinA) [package insert]. Greensboro, NC: Merz Pharma GmbH & Co KGaA; 2011.
- 23.Vergilis-Kalner IJ. Same-patient prospective comparison of Botox versus Dysport for the treatment of primary axillary hyperhidrosis and review of literature. J Drugs Dermatol. 2011;10:1013–1015. [PubMed] [Google Scholar]
- 24.Güileç AT. Dilution of botulinum toxin A in lidocaine vs. in normal saline for the treatment of primary axillary hyperhidrosis: a double-blind, randomized, comparative preliminary study. J Eur Acad Dermatol Venereol. 2012;26:314–318. doi: 10.1111/j.1468-3083.2011.04066.x. [DOI] [PubMed] [Google Scholar]
- 25.Goldman A, Wollina U. Subdermal Nd-YAG laser for axillary hyperhidrosis. Dermatol Surg. 2008;34:756–762. doi: 10.1111/j.1524-4725.2008.34143.x. [DOI] [PubMed] [Google Scholar]
- 26.Lee SJ, Chang KY, Suh DH, et al. The efficacy of a microwave device for treating axillary hyperhidrosis and osmidrosis in Asians: A preliminary study. J Cosmet Laser Ther. doi: 10.3109/14764172.2013.807114. 2013 May 29 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]