Abstract
Objectives: Phase 1 studies were conducted to determine the sensitization (PEP005-005; NCT00357916; http://clinicaltrials.gov/ct2/show/NCT00357916), photoirritation (PEP005-023; NCT00850811; http://clinicaltrials.gov/ct2/show/NCT00850811?term=PEP005-023&rank=1), and photoallergic (photosensitizing) potential (PEP005-024; NCT00850681; http://clinicaltrials.gov/ct2/show/NCT00850681?term=PEP005-024&rank=1) of ingenol mebutate gel 0.01% versus vehicle on normal skin. Design, setting, participants, and measurements: Healthy volunteers were enrolled in single-center, randomized, controlled, within-subject comparison trials. PEP005-005 was designed as a repeat-insult patch test study. In PEP005-023, treatment areas were examined after irradiation for photoirritation potential; dermal reactions were evaluated. In PEP005-024, irradiation was performed to determine the photoallergic (photosensitizing) potential of the medication. All treatment areas were graded immediately prior to irradiation and 24, 48, and 72 hours following irradiation. In all studies, local tolerability was assessed visually using an ordinal scoring system at set intervals before and after medication application/irradiation. Results: In PEP005-005 (n=238), a significant difference (p<0.001) was seen between ingenol mebutate and vehicle for mean and total cumulative irritation scores. In PEP005-023 (n=34), mild erythema in all irradiated treatment areas was as expected for the ultraviolet dose. There was no clinically significant irritation in response to ingenol mebutate or vehicle, irrespective of irradiation. In PEP005-024 (n=60), there was no significant irritation in response to either ingenol mebutate or vehicle at their irradiated treatment areas. Conclusion: Results from three pharmacology studies in healthy volunteers indicate a favorable topical safety profile for ingenol mebutate gel, with no evidence seen of skin sensitization, photoirritation, or photoallergic potential.
Actinic keratosis (AK) is a field disease that manifests as multiple clinical and subclinical dysplastic skin lesions. It is common in fair-skinned people with a history of long-term ultraviolet (UV) radiation exposure.1-4 Coupled with its rising incidence and subsequent risk factors, the burden of AK is substantial as AK lesions have the potential to progress and transform into invasive squamous cell carcinoma.5 Effective field therapy is therefore important.6-8
Ingenol mebutate gel is a novel field therapy indicated for the topical treatment of adults with AK; specific dosing regimens have been developed for treating AK lesions on the face and scalp and on the trunk and extremities.9 Ingenol mebutate has been extensively refined from the sap of the plant Euphorbia peplus to create an active pharmaceutical ingredient, which is an AK lesion-directed cell death inducer and immune response modifier.10-14
For lesions on the face and scalp, ingenol mebutate 0.015% is self-applied once daily for three consecutive days; for lesions on the trunk and extremities, ingenol mebutate 0.05% is self-applied once daily for two consecutive days. Treatment with ingenol mebutate has a short local skin response duration and was effective and well tolerated in Phase 2 and 3 clinical studies.15,16
Ingenol mebutate does not absorb light in the UV range. During the clinical development program, the potential of ingenol mebutate to induce skin sensitization, photoirritation, and photoallergy was investigated in three early Phase 1 clinical studies. In this paper, the authors present for the first time in full, the collective results from these three randomized controlled studies with 0.01% ingenol mebutate gel in healthy volunteers.
METHODS
Three randomized, Phase 1 studies of ingenol mebutate gel (Picato®, LEO Pharma) versus vehicle control were conducted from 2006 to 2009. The study objectives are outlined below:
PEP005-005: The primary objective was to determine the sensitization potential of ingenol mebutate on normal skin. The secondary objective was to evaluate cumulative skin irritation.
PEP005-023: The primary objective was to determine the photoirritation potential of ingenol mebutate on normal skin when application was followed by light exposure.
PEP005-024: The primary objective was to determine the photoallergic (photosensitizing) potential of ingenol mebutate on normal skin.
All subjects provided written informed consent, and the studies were approved by ethical review boards of the participating centers. These studies were conducted in compliance with United States federal regulations, United States Food and Drug Administration guidelines, the principles of the International Conference on Harmonisation, Good Clinical Practice, and the Declaration of Helsinki.
PEP005-005 (sensitization potential). Subject population. Eligible subjects were healthy volunteers aged 18 to 65 years with a Fitzpatrick skin type of I, II, III, or IV. Exclusion criteria included subjects with Fitzpatrick skin types of V or VI, those with excessive hair on their back, or subjects with a visible skin condition that would interfere with the evaluation of the treatment area reaction.
Trial design. Study PEP005-005 (NCT00357916) was a single-center, randomized, controlled, within-subject comparison, repeat-insult patch test study. A pilot study was conducted with 10 subjects prior to the initiation of the main study.
In total, 10 gel applications were made over 6 to 8 weeks. Ingenol mebutate gel 0.01% or vehicle control gel was applied three times weekly for three weeks (9 applications) during the induction phase to treatment areas on the infrascapular region of the back (sufficient to cover a 4cm2 area of skin). Following a rest period of 10 to 14 days, a single challenge application was performed.
Assessments. Local tolerability was assessed visually using an ordinal scoring system. Treatment areas were assessed nine times during the induction phase, four times following challenge, and, if applicable, four times following rechallenge. A rechallenge was only undertaken if a cutaneous response was observed in the challenge phase (suggestive of possible sensitization) or at the discretion of the investigator.
All local and systemic adverse events (AEs) observed by or reported to the investigators were evaluated; the intensity, duration, and causal relationship to the investigational product were rated for all AEs.
Statistical analyses. Cumulative irritancy during induction was quantified by analysis of the mean and total cumulative irritancy scores recorded during the induction phase (nine readings). These parameters were tested pairwise for product differences using a two-way analysis of variance (ANOVA; subject, product). Narrative descriptions of reactions in the challenge and rechallenge phases were provided and, together with the opinion of the investigator, these determined whether such reactions were considered to be indicative of contact sensitization.
PEP005-023 (photoirritation potential). Study population. Eligible subjects were healthy volunteers aged 18 to 65 years with a Fitzpatrick skin type of I, II, or III. Subjects were excluded if they had a Fitzpatrick skin type of IV, V, or VI or had a visible skin condition that would interfere with the evaluation of the treatment area reaction or had damaged skin around the treatment area.
Trial design. Study PEP005-023 (NCT00850811) was a single-center, randomized, controlled, open application, within-subject comparison, four-day study to evaluate the photoirritation potential of ingenol mebutate gel in healthy volunteers, using a phototoxicity test design.
Prior to determining the photoirritation potential of the ingenol mebutate and vehicle gel, each subject’s minimal erythemal dose (MED) was determined. A defined area (approximately 50cm2 divided into 6 equal treatment areas) on the subscapular region of each subject’s back was irradiated to determine the MED of UV light. Following MED determination, for each subject, a total of four treatment areas (2 irradiated and 2 nonirradiated), separate to the six areas used for MED determination, were designated for study medication application and/or irradiation. Study medication was applied to these assigned treatment areas under open conditions. Subjects received a single administration of ingenol mebutate gel 0.01% or vehicle gel in an amount sufficient to cover a 4cm2 area of skin and were then monitored for four days. A fifth area was not treated but was irradiated. Therefore, control conditions included an untreated irradiated control area and a treated non-irradiated control area. Irradiation (16J/cm2 of UVA light followed by ½ MED of UVA/UVB [290-400nm]) of the designated treatment areas took place 24 hours after gel application.
Assessments. Treatment areas were examined 24 and 48 hours after irradiation to determine the phototoxicity irritation potential. Study assessors who evaluated responses were blinded to the randomization of treatments to the specific treatment areas. Dermal reactions at the treatment areas were evaluated using a visual scale that rated the degree of erythema, edema, and other signs of cutaneous irritation. If a cutaneous response observed was associated with phototoxicity, the product was reapplied as soon as the original reactions had resolved or at the discretion of the investigator.
Local tolerability was assessed visually immediately before irradiation using an ordinal scoring system and at 24 and 48 hours after irradiation. All local and systemic AEs observed by or reported to the investigators were evaluated; intensity, duration, and causal relationship to the investigational product were rated for all AEs.
Statistical analysis of local tolerability assessments. Selected pairwise comparisons were performed in the context of the ANOVA. Pairs to be compared were investigational products on irradiated versus nonirradiated treatment areas and investigational products on irradiated areas versus untreated irradiated control areas.
PEP005-024 (photoallergic potential). Study population. Eligible subjects were healthy volunteers aged 18 to 65 years with uniformly colored skin on the lower thoracic area of the back to allow discernment of erythema, and a Fitzpatrick skin type of I, II, or III. Exclusion criteria included Fitzpatrick skin types of IV, V, or VI, visible skin conditions that could interfere with the evaluation of the treatment area reaction, or damaged skin around the treatment area.
Trial design. PEP005-024 (NCT00850681) was a single-center, randomized, controlled, open application, within-subject comparison study. A defined area (approximately 50cm2 divided into 6 equal treatment areas) on the subscapular region of each subject’s back was irradiated to determine the MED of UV light. All subjects had four application areas (2 irradiated and 2 nonirradiated), separate to the six areas used for MED determination, designated for study medication application and/or irradiation.
Subjects received ingenol mebutate gel 0.01% or vehicle gel twice weekly for three weeks (induction phase) and once more (challenge phase) 10 to 14 days after the last induction treatment. During the induction phase, approximately 24 hours after each application, the irradiated areas were exposed to double the subject’s MED of UVA/UVB (290-400nm). Treatment areas were irradiated during the challenge phase, 24 hours after gel application, with 6J/cm2 of UVA (320-400nm) using a filtered light source and ½ MED of UVA/UVB (290-400nm) at the designated areas. For control purposes, an additional area was also irradiated using the same irradiation conditions.
Assessments. All treatment areas were graded immediately prior to irradiation and 24, 48, and 72 hours following irradiation. Furthermore, all were assessed visually during the challenge phase for local reactions using an ordinal scoring system approximately 24 hours following study medication and 48 and 72 hours after irradiation. If photosensitization was suspected following a cutaneous response in the challenge phase, a rechallenge was undertaken as soon as the reaction resolved or at the discretion of the investigator.
Statistical analyses of local tolerability assessments. Photosensitization was assessed by summarizing all assigned scores during induction and challenge by frequency counts according to time point and treatment. The incidence of photosensitization reactions was summarized by frequency counts for each treatment. Local tolerability was measured by analyzing the mean numerical equivalent score by subject and treatment, including all scores assigned during induction, using an ANOVA. All possible pairwise comparisons of treatments were performed.
RESULTS
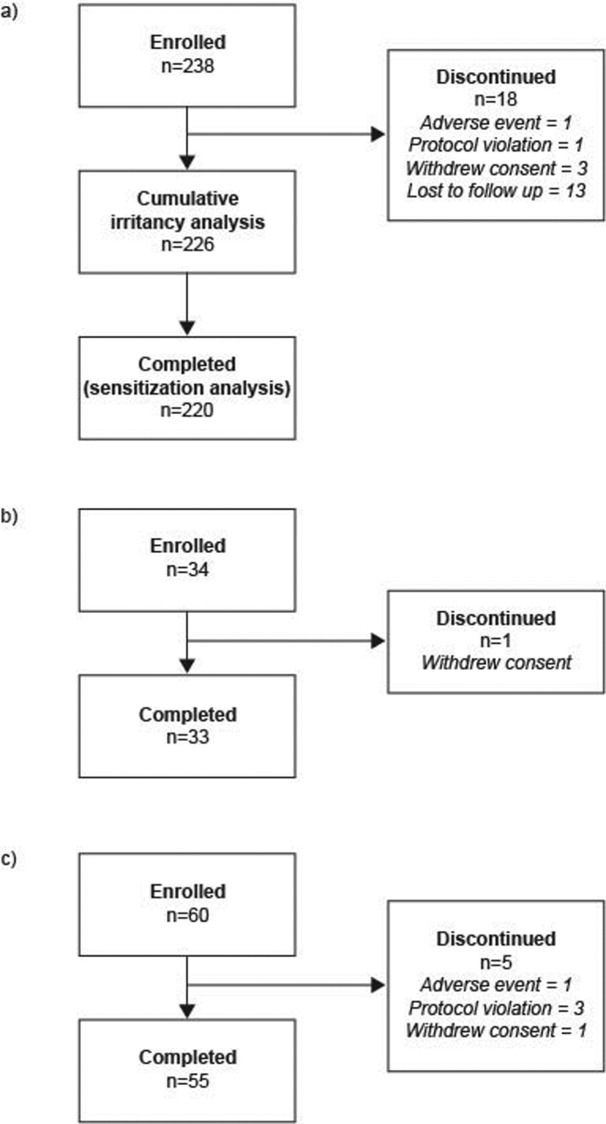
PEP005-005 (sensitization potential). Subjects. Seven subjects were enrolled in the pilot study, and six completed the study; the single discontinuation was due to a protocol violation. Following the results of the pilot study, the decision was taken to continue onto the full panel. Subject disposition for the entire study is summarized in Figure 1A; of 238 enrolled subjects, 226 subjects were included in the cumulative irritancy analysis and 220 in the sensitization analysis. The majority of subjects were women (82.4%) and had Fitzpatrick skin type III (51.3%). The mean age was 43.7 years (Table 1).
Figures 1A-C.
Subject disposition: (a) PEP005-005 (sensitization potential); (b) PEP005-023 (photoirritation potential); (c) PEP005-024 (photoallergic potential)
TABLE 1.
Demographics and baseline characteristics for all three studies
| PEP005-005 | PEP005-023 | PEP005-024 | |
|---|---|---|---|
| AGE | |||
| N | 238 | 34 | 60 |
| Mean | 43.7 | 45.7 | 47.9 |
| Standard deviation | 11.8 | 14.7 | 11.3 |
| Median | 44.8 | 49.4 | 48.8 |
| Range | 19.4-65.9 | 18.5-65.2 | 19.5-64.6 |
| GENDER, n (%) | |||
| Male | 42 (17.6) | 4 (11.8) | 14 (23.3) |
| Female | 196 (82.4) | 30 (88.2) | 46 (76.7) |
| RACE, n (%) | |||
| Caucasian | 165 (69.3) | 34 (100.0) | 54 (90.0) |
| African American | 21 (8.8) | 0 | 0 |
| Hispanic | 49 (20.6) | 0 | 6 (10.0) |
| Asian | 2 (0.8) | 0 | 0 |
| Other* | 1 (0.4) | 0 | 0 |
| FITZPATRICK SKIN TYPE, n (%) | |||
| I | 5 (2.1) | 0 | 6 (10.0) |
| II | 37 (15.5) | 8 (23.5) | 16 (26.7) |
| III | 122 (51.3) | 26 (76.5) | 38 (63.3) |
| IV | 74 (31.1) | — | — |
| MINIMAL ERYTHEMAL DOSE | |||
| Mean | — | 1.41 | 1.11 |
| Standard deviation | — | 0.40 | 0.37 |
| Range | — | 0.80-2.41 | 0.62-1.91 |
= Mixed race.
= Data not appropriate for specific study
Safety results. Dermal sensitization. The six subjects who completed the challenge during the pilot study had no reactions indicative of a possible sensitization response or any that required rechallenge. Overall, 220 subjects (pilot study inclusive) completed the challenge phase of the study and were included in the sensitization analysis. A summary of the repeated-insult patch test responses during the induction phase is provided in Table 2.
TABLE 2.
PEP005-005 (sensitizing potential) repeated insult patch test responses
| TIME POST-CHALLENGE | ||||
|---|---|---|---|---|
| RESPONSE | IMMEDIATE (n=220) | 24 HOURS (n=220) | 48 HOURS (n=220) | 72 HOURS (n=220) |
| No reaction | 210 | 212 | 217 | 218 |
| Minimal or doubtful response, slightly different from surrounding normal skin | 9 | 6 | 1 | 0 |
| Definite erythema; no edema. Damage to epidermis; oozing, crusting and/or superficial erosions | 1 | 2 | 2 | 2 |
Two subjects experienced significant irritation reactions at challenge. One subject had a minimal/doubtful response at the first challenge reading that increased to erythema with damage to the epidermis (i.e., oozing, crusting, and/or superficial erosions) at the 24-hour challenge reading. This was sustained through the 72-hour reading and resolved two days later. Another subject had erythema with damage to the epidermis at all four challenge readings. The subject was advised to return for a follow-up visit two days after the 72-hour evaluation, but did not return. Both subjects had experienced minimal erythema during the induction phase.
Cumulative skin irritancy. Within the pilot phase of the study, two subjects experienced mild irritation (minimal erythema) at the active product treatment area during the induction phase. Both subjects had erythema that resolved by the fifth and sixth induction evaluations, respectively. No reactions were observed at challenge.
Overall, 226 subjects (pilot phase inclusive) were included in the cumulative irritancy analysis. A significant difference (p<0.001) was seen between ingenol mebutate gel and vehicle gel for both mean (standard deviation) cumulative irritation score (0.04 [0.15] vs. 0) and total cumulative irritation score (0.34 [1.31] vs. 0).
Adverse events. The AE profile is presented in Table 3. AEs were pregnancy, mild headache, and gum abscess; none was considered related to study treatment. There were no serious or severe AEs and no deaths during this study.
TABLE 3.
Adverse event summary for all three studies
| EVENT, n (%) | PEP005-005 | PEP005-023 | PEP005-024 |
|---|---|---|---|
| Subjects with at least one adverse event | 3 (1.3) | 0 | 13 (21.7) |
| Adverse events | 3 | 0 | 22 |
| Subjects with at least one treatment-related adverse event | 0 | 0 | 0 |
| Subjects with at least one serious adverse event | 0 | 0 | 0 |
| Subjects with at least one severe adverse event | 0 | 0 | 0 |
| Subjects who discontinued due to adverse event | 1 (0.4) | 0 | 1 (1.7) |
| Pregnancy, puerperium, and perinatal conditions | 1 (0.4) | 0 | 0 |
| • Pregnancy | 1 (0.4) | 0 | 0 |
| Injury, poisoning, and procedural complications | 0 | 0 | 4 (6.7) |
| • Contusion | 0 | 0 | 1 (1.7) |
| • Facial bones fracture | 0 | 0 | 1 (1.7) |
| • Joint injury | 0 | 0 | 1 (1.7) |
| • Laceration | 0 | 0 | 1 (1.7) |
| • Muscle injury | 0 | 0 | 1 (1.7) |
| Gastrointestinal disorders | 0 | 0 | 3 (5.0) |
| • Vomiting | 0 | 0 | 4 (6.7) |
| • Abdominal pain | 0 | 0 | 2 (3.3) |
| • Diarrhea | 0 | 0 | 1 (1.7) |
| • Nausea | 0 | 0 | 1 (1.7) |
| Infections and infestations | 1 (0.4) | 0 | 3 (5.0) |
| • Nasopharyngitis | 0 | 0 | 2 (3.3) |
| • Bronchitis | 0 | 0 | 1 (1.7) |
| • Gingival abscess | 1 (0.4) | 0 | 0 |
| Musculoskeletal and connective tissue disorders | 0 | 0 | 2 (3.3) |
| • Back pain | 0 | 0 | 2 (3.3) |
| Skin and subcutaneous tissue disorders | 0 | 0 | 2 (3.3) |
| • Pruritus | 0 | 0 | 1 (1.7) |
| • Rash | 0 | 0 | 1 (1.7) |
| • Rash papular | 0 | 0 | 1 (1.7) |
| Eye disorders | 0 | 0 | 1 (1.7) |
| • Eye swelling | 0 | 0 | 1 (1.7) |
| Nervous system disorders | 1 (0.4) | 0 | 1 (1.7) |
| • Headache | 1 (0.4) | 0 | 1 (1.7) |
| Respiratory, thoracic, and mediastinal disorders | 0 | 0 | 1 (1.7) |
| • Cough | 0 | 0 | 1 (1.7) |
PEP005-023 (photoirritation potential). Subjects. Subject disposition is summarized in Figure 1B; 34 subjects were enrolled, and 33 completed the study. One subject withdrew their consent and discontinued from the study. The majority of subjects were women (88.2%) and had Fitzpatrick skin type III (76.5%). The mean subject age was 45.7 years (Table 1).
Safety results. The observations of mild erythema at all treatment areas irradiated were within the expected range for the UV dose. There was no clinically significant irritation seen in response to either ingenol mebutate gel or vehicle, irrespective of irradiation (Table 4). However, for both ingenol mebutate gel and vehicle, there were statistically significant differences between the irradiated (p<0.001) and nonirradiated (p=0.002) treatment areas; this irritation was mild in nature and none of these reactions were at a level indicative of photoirritation. There were no statistically significant differences between any of the other comparisons undertaken. No subjects met the prespecified criteria for a reaction indicative of phototoxicity and no AEs were reported in this study (Table 3).
TABLE 4.
PEP005-023 (photoirritation potential) mean irritation scores and p-values
| IRRADIATED | |||||
|---|---|---|---|---|---|
| Ingenol mebutate gel, 0.01% | Vehicle gel | Untreated | |||
| Average of Day 3 and 4, mean (standard deviation) | 0.20 (0.35) | 0.17 (0.35) | 0.21 (0.38) | ||
| IRRADIATED | Ingenol mebutate gel, 0.01% | 0.20 (0.35) | - | 0.558 | 0.769 |
| Vehicle gel | 0.17 (0.35) | 0.558 | - | 0.38 | |
| Untreated | 0.21 (0.38) | 0.769 | 0.38 | - | |
| NONIRRADIATED | Ingenol mebutate gel, 0.01% | 0.02 (0.09) | <0.001 | - | - |
| Vehicle gel | 0 | - | 0.002 | - | |
| Untreated | N/A | - | - | - | |
Shading indicates significant differences.
PEP005-024 (photoallergic potential). Subjects. Subject disposition is summarized in Figure 1C; 60 subjects were enrolled and 55 completed the study. There were five discontinuations (3 protocol violations, 1 AE, and 1 voluntary withdrawal). The majority of subjects were women (76.7%) and had Fitzpatrick skin type III (63.3%). The mean age in the study was 47.9 years (Table 1).
Safety results. A summary of mean scores and p-values for irradiated and nonirradiated treatment areas is shown in Table 5. Statistically significant differences were observed between all comparisons tested; irritation was greater with ingenol mebutate gel versus vehicle and greater when skin was irradiated versus non-irradiated.
TABLE 5.
PEP005-024 (photoallergic potential) irradiated and nonirradiated mean scores and p-values
| IRRADIATED | NONIRRADIATED | |||||
|---|---|---|---|---|---|---|
| Ingenol mebutate gel, 0.01% | Vehicle gel | Ingenol mebutate gel, 0.01% | Vehicle gel | |||
| Mean (standard deviation) | 0.47 (0.27) | 0.39 (0.28) | 0.11 (0.17) | 0.00 (0.02) | ||
| IRRADIATED | Ingenol mebutate gel, 0.01% | 0.47 (0.27) | - | 0.035 | <0.001 | <0.001 |
| Vehicle gel | 0.39 (0.28) | 0.035 | - | <0.001 | <0.001 | |
| NONIRRADIATED | Ingenol mebutate gel, 0.01% | 0.11 (0.17) | <0.001 | <0.001 | - | 0.003 |
| Vehicle gel | 0.00 (0.02) | <0.001 | <0.001 | 0.003 | - | |
Shading indicates significant differences.
Mean scores showed mod-erate erythema at the treatment areas irradiated after the application of either ingenol mebutate gel or vehicle. Non-irradiated treatment areas showed mild erythema for ingenol mebutate gel and no reaction for vehicle. The most common AEs are presented in Table 3; there were no serious AEs or deaths.
DISCUSSION
Data from three Phase I studies in healthy volunteers suggest that ingenol mebutate gel has a favorable topical safety profile. Potential for photosensitization was not observed in subjects receiving ingenol mebutate gel or vehicle gel. A repeat insult test design was used to test the potential of ingenol mebutate gel to induce skin sensitization. No reactions greater than minimal erythema were reported during the induction phase; during the challenge phase, no reactions were indicative of a possible sensitization response, nor were any reactions that required rechallenge observed. Therefore, no evidence of skin-sensitizing potential was seen following repeated applications of ingenol mebutate gel.
Furthermore, neither ingenol mebutate gel nor vehicle gel showed any potential for photoirritation in this particular treatment environment, based on pre-specified criteria. When testing for photoallergic potential, moderate erythema was observed at the treatment areas that were irradiated post-application of ingenol mebutate gel and vehicle gel. Nonirradiated treatment areas showed mild erythema for ingenol mebutate gel and no irritation for vehicle. Mild erythema is considered a local skin response. As this is an expected consequence of treatment with ingenol mebutate gel, it is not recorded as an AE. This favorable safety and tolerability profile of ingenol mebutate was confirmed in later phase studies in adult patients with AK.15,17,18
Other treatments are also licensed for the treatment of AK, and the photosensitization, phototoxicity, and photoallergic potential of these agents have also been assessed in clinical studies. First, Ortonne et al19 published data from a study with 3% diclofenac in 2.5% hyaluronic acid, which evaluated photosensitivity or phototoxicity in conjunction with skin irradiation. The impact of sunscreens was also assessed in this study. Data show that areas treated with diclofenac plus sunscreen had the lowest incidence of erythema in both irradiated and nonirradiated test patches. Similar to ingenol mebutate studies, no phototoxicity or photosensitization was observed with 3% diclofenac in 2.5% hyaluronic acid when used alone. Furthermore, no phototoxicity or photosensitization was observed for diclofenac in combination with sunscreen. Second, safety studies have been conducted with imiquimod 5% to determine its tolerability when used in conjunction with irradiation.20 As with ingenol mebutate gel, no evidence of photoallergic or phototoxic responses was detected. Furthermore, no significant differences were observed between imiquimod and the control in terms of sunburn cell counts and deoxyribonucleic acid (DNA) pyrimidine dimer formation, which are markers of potential photodamage. Third, and in contrast with the findings from both the above studies and the ingenol mebutate studies, in vitro data suggest that 5fluorouracil is associated with both photosensitization effects and photodegradation when exposed to UVB light.21
Although the ingenol mebutate studies included more than the regulatory required 30 subjects to assess phototoxicity and photosensitization, they are not without limitations. The first point to note is that the dose used in these studies, where treatments were applied to the subject’s back, was 0.01% compared with the approved doses of 0.05% (trunk/extremities; 2 days) and 0.015% (face/scalp; 3 days). Therefore, higher doses of ingenol mebutate may not be as well-tolerated compared with these studies. Moreover, the data from these studies cannot simply be combined to create a full tolerability picture because different clinical trial designs with varied dosing regimens and UV exposure were used; this may have biased the study conclusions. However, as the majority of subjects in all three studies had Fitzpatrick skin type III, this bias was probably not substantial.
In conclusion, a considerable body of evidence from three clinical pharmacology studies in healthy volunteers indicates that ingenol mebutate gel has a favorable topical safety profile, with no evidence seen of skin sensitization, photoirritation, or photoallergic potential.
ACKNOWLEDGMENTS
These studies (PEP005-005; PEP005-023; PEP005-024) were funded by Peplin Operations Pty Ltd (subsequently acquired by LEO Pharma). Janelle Katsamas, an employee of LEO Pharma Pty Ltd, Brisbane, Queensland, Australia, provided input on study conduct and data analysis. Medical writing services were provided by Dr. Paula Michelle del Rosario of iMed Comms, Macclesfield, UK, and were funded by LEO Pharma. The authors would like to thank the investigators who participated in these studies.
Footnotes
DISCLOSURE:Jonathan S. Dosik was principal investigator for these studies (PEP005-005; PEP005-023; PEP005-024) of ingenol mebutate. Maureen Damstra and Carol Udell provided clinical and statistical support, respectively. Peter Welburn, an employee of LEO Pharma Pty Ltd, Brisbane, Queensland, Australia, was responsible for study design. These studies (PEP005-005; PEP005-023; PEP005-024) were funded by Peplin Operations Pty Ltd (subsequently acquired by LEO Pharma). Medical writing services were provided by Dr. Paula Michelle del Rosario of iMed Comms, Macclesfield, United Kingdom, and were funded by LEO Pharma.
REFERENCES
- 1.Goldberg LH, Mamelak AJ. Review of actinic keratosis. Part I: etiology, epidemiology and clinical presentation. J Drugs Dermatol. 2010;9:1125–1132. [PubMed] [Google Scholar]
- 2.de Berker D, McGregor JM, Hughes BR. Guidelines for the management of actinic keratoses. Br J Dermatol. 2007;156:222–230. doi: 10.1111/j.1365-2133.2006.07692.x. [DOI] [PubMed] [Google Scholar]
- 3.Fenske NA, Spencer J, Adam F. Actinic keratoses: past, present and future. J Drugs Dermatol. 2010;9(5 Suppl ODAC Conf Pt 1):s45–s49. [PubMed] [Google Scholar]
- 4.Hensen P, Mtiller ML, Haschemi R, et al. Predisposing factors of actinic keratosis in a North-West German population. Eur J Dermatol. 2009;19:345–354. doi: 10.1684/ejd.2009.0706. [DOI] [PubMed] [Google Scholar]
- 5.Berman B, Amini S, Valins W, Block S. Pharmacotherapy of actinic keratosis. Expert Opin Pharmacother. 2009;10:3015–3031. doi: 10.1517/14656560903382622. [DOI] [PubMed] [Google Scholar]
- 6.Bonerandi JJ, Beauvillain C, Caquant L, et al. Guidelines for the diagnosis and treatment of cutaneous squamous cell carcinoma and precursor lesions. J Eur Acad Dermatol Venereol. 2011;25(Suppl 5):1–51. doi: 10.1111/j.1468-3083.2011.04296.x. [DOI] [PubMed] [Google Scholar]
- 7.Cohen JL. Actinic keratosis treatment as a key component of preventive strategies for nonmelanoma skin cancer. J Clin Aesthet Dermatol. 2010;3:39–44. [PMC free article] [PubMed] [Google Scholar]
- 8.Criscione VD, Weinstock MA, Naylor MF, et al. Actinic keratoses: natural history and risk of malignant transformation in the Veterans Affairs Topical Tretinoin Chemoprevention Trial. Cancer. 2009;115:2523–2530. doi: 10.1002/cncr.24284. [DOI] [PubMed] [Google Scholar]
- 9. Picato® [package insert]. LEO Pharma; 2012.
- 10.Challacombe JM, Suhrbier A, Parsons PG, et al. Neutrophils are a key component of the antitumor efficacy of topical chemotherapy with ingenol-3-angelate. J Immunol. 2006;177:8123–8132. doi: 10.4049/jimmunol.177.11.8123. [DOI] [PubMed] [Google Scholar]
- 11.Hampson P, Chahal H, Khanim F, et al. PEP005, a selective small-molecule activator of protein kinase C has potent antileukemic activity mediated via the delta isoform of PKC. Blood. 2005;106:1362–1368. doi: 10.1182/blood-2004-10-4117. [DOI] [PubMed] [Google Scholar]
- 12.Ogbourne SM, Suhrbier A, Jones B, et al. Antitumor activity of 3-ingenyl angelate: plasma membrane and mitochondrial disruption and necrotic cell death. Cancer Res. 2004;64:2833–2839. doi: 10.1158/0008-5472.can-03-2837. [DOI] [PubMed] [Google Scholar]
- 13.Rosen RH, Gupta AK, Tyring SK. Dual mechanism of action of ingenol mebutate gel for topical treatment of actinic keratoses: rapid lesion necrosis followed by lesion-specific immune response. J Am Acad Dermatol. 2012;66:486–493. doi: 10.1016/j.jaad.2010.12.038. [DOI] [PubMed] [Google Scholar]
- 14.Kedei N, Lundberg DJ, Toth A, et al. Characterization of the interaction of ingenol 3-angelate with protein kinase C. Cancer Res. 2004;64:3243–3255. doi: 10.1158/0008-5472.can-03-3403. [DOI] [PubMed] [Google Scholar]
- 15.Lebwohl M, Swanson N, Anderson LL, et al. Ingenol mebutate gel for actinic keratosis. N Engl J Med. 2012;366:1010–1019. doi: 10.1056/NEJMoa1111170. [DOI] [PubMed] [Google Scholar]
- 16.Martin G, Swanson N. Clinical findings using ingenol mebutate gel to treat actinic keratoses. J Am Acad Dermatol. 2013;68(1 Suppl 1):S39–S48. doi: 10.1016/j.jaad.2012.09.050. [DOI] [PubMed] [Google Scholar]
- 17.Spencer J. Multicenter, randomized, double-blind, vehicle-controlled, dose-ranging study to evaluate the efficacy and safety of PEP005 (ingenol mebutate) gel 0.005%, 0.01%, and 0.015% when used to treat actinic keratoses on the head. J Am Acad Dermatol. 2010;62(3 Suppl 1) AB105. [Google Scholar]
- 18.Anderson L, Schmieder GJ, Werschler WP, et al. Randomized, double-blind, double-dummy, vehicle-controlled study of ingenol mebutate gel 0.025% and 0.05% for actinic keratosis. J Am Acad Dermatol. 2009;60:934–943. doi: 10.1016/j.jaad.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 19.Ortonne JP, Queille-Roussel C, Duteil L. 3% diclofenac in 2.5% hyaluronic acid (Solaraze™) does not induce photosensitivity or phototoxicity alone or in combination with sunscreens. Eur J Dermatol. 2006;16:385–390. [PubMed] [Google Scholar]
- 20.Kaidbey K, Owens M, Liberda M, Smith M. Safety studies of topical imiquimod 5% cream on normal skin exposed to ultraviolet radiation. Toxicology. 2002;178:175–182. doi: 10.1016/s0300-483x(02)00320-7. [DOI] [PubMed] [Google Scholar]
- 21.Miolo G, Marzano C, Gandin V, et al. Photoreactivity of 5-fluorouracil under UVB light: photolysis and cytotoxicity studies. Chem Res Toxicol. 2011;24:1319–1326. doi: 10.1021/tx200212z. [DOI] [PubMed] [Google Scholar]