Abstract
Epithelial ovarian cancer (OvCa) is associated with high mortality and, as the majority (>75%) of women with OvCa have metastatic disease at the time of diagnosis, rates of survival have not changed appreciably over 30 years. A mechanistic understanding of OvCa initiation and progression is hindered by the complexity of genetic and/or environmental initiating events and lack of clarity regarding the cell(s) or tissue(s) of origin. Metastasis of OvCa involves direct extension or exfoliation of cells and cellular aggregates into the peritoneal cavity, survival of matrix-detached cells in a complex ascites fluid phase, and subsequent adhesion to the mesothelium lining covering abdominal organs to establish secondary lesions containing host stromal and inflammatory components. Development of experimental models to recapitulate this unique mechanism of metastasis presents a remarkable scientific challenge and many approaches used to study other solid tumors (lung, colon, and breast, for example) are not transferable to OvCa research given the distinct metastasis pattern and unique tumor microenvironment. This review will discuss recent progress in the development and refinement of experimental models to study OvCa. Novel cellular, three-dimensional organotypic, and ex vivo models are considered and the current in vivo models summarized. The review critically evaluates currently available genetic mouse models of OvCa, the emergence of xenopatients, and the utility of the hen model to study OvCa prevention, tumorigenesis, metastasis, and chemoresistance. As these new approaches more accurately recapitulate the complex tumor microenvironment, it is predicted that new opportunities for enhanced understanding of disease progression, metastasis and therapeutic response will emerge.
Keywords: ovarian cancer, mouse model, cell lines, hen, 3D models, organotypic cultures, metastasis
1 INTRODUCTION
Ovarian cancer (OvCa) continues to be a poorly understood disease with an extremely poor prognosis. Its cell of origin is still unknown and researchers have only begun to elucidate its molecular mechanisms. Several molecular changes have been confirmed in human tumors and functionally characterized in cell and animal models (e.g. p53, PI3 kinase, ARID1, K-ras, BRCA1, 2).1–6 However, no genetic alteration has, as yet, been found to be a clinically “actionable” genetic change. One reason for the slow progress made in understanding the biology of OvCa and translating that knowledge into substantial clinical benefits has been a lack of clinically representative model systems that mimic the progression of the human disease. Since the majority of women diagnosed with epithelial OvCa (>75%) have intra-abdominal metastasis at the time of diagnosis, models that replicate the molecular events that underlie dissemination are a crucial tool to gain the knowledge necessary for the development of new treatments.
Several excellent reviews discuss the current knowledge of OvCa biology.1–3,7 This review offers a comprehensive overview of experimental models available for the study of ovarian cancer. By reviewing the current state of model systems, the hope is to stimulate discussions on how these models may be improved, as well as provide a needed reference. The biology of OvCa poses special challenges to the development of model systems. The disease commonly referred to as ‘ovarian cancer’ is not a single entity, but consists of several histological subtypes (serous, endometrioid, clear cell, and mucinous) with distinct molecular aberrations.1,3 The site of origin for high-grade serous ovarian cancer, the major histological subtype, is still unknown and is intensely debated. Possible sites include the abdominal peritoneum and the fallopian tube fimbriae, as well as the ovarian surface epithelium.7,8 Moreover, OvCa is distinct from most other solid tumors in that hematogenous metastasis is rare.9 The initial early event in epithelial OvCa metastasis is proteinase-mediated shedding into the peritoneal cavity.10–12 Intra-peritoneal metastatic implants are initiated by single cells and spheroids adhering to peritoneal mesothelial cells and anchoring in the sub-mesothelial matrix to establish secondary lesions.13–15 Accumulated peritoneal ascites (often > 500ml) contains a unique non-adherent population of individual tumor cells and multicellular aggregates (spheroids) exposed to a set of microenvironmental cues that promote metastatic implantation.1,13,14 Since the mortality of OvCa is directly attributed to disseminated peritoneal metastasis, this distinctive microenvironment provides a marked set of therapeutic challenges and necessitates research that makes use of a suite of model systems to recapitulate specific cellular and molecular events in metastasis.
2 THREE-DIMENSIONAL CULTURE MODELS OF OVARIAN CANCER TO RECAPITULATE THE TUMOR MICROENVIRONMENT
Ovarian tumors are not a solid matrix of epithelial cells, but rather a mixture of epithelial, stromal, immune, and endothelial cells.1,3,9 This complex tumor microenvironment (TME) includes (a) primary tumor with tumor associated stromal cells (fibroblasts, mesothelial cells) and inflammatory cells (macrophages, leukocytes); (b) non-adherent cells suspended in ascites with inflammatory and mesothelial cells; and (c) metastases to various parts of peritoneal cavity that contact mesothelial cells, adipocytes, and fibroblasts at the metastatic site.9 This heterogeneity of cell types likely impacts tumor histology, growth potential and ability to evade chemotherapy. Optimal treatment for ovarian cancer patients consists of tumor debulking (removing all tumors >1mm) followed by chemotherapy. As ovarian cancer is often widely disseminated with many microscopic implants that cannot be surgically removed, leading to disease recurrence within the first 2–3 years, there is a critical need for a more detailed understanding of early events in (re)colonization of metastatic implants. These experimental questions require new model systems that have been designed to recapitulate events occurring in the OvCa TME.
While our knowledge of OvCa biology has largely been derived from experiments using cancer cells grown in two-dimensions on plastic, studying OvCa initiation, progression and metastasis in a three-dimensional (3D) system has many advantages. In particular, epithelial cells grown in 3D with extracellular and/or cellular components of the TME exhibit a number of features similar to those of cells in the clinical microenvironment. Therefore, 3D models that incorporate extracellular matrices (ECM) and stromal cells provide an opportunity for understanding the crosstalk between OvCa and stromal cells; for detailed analysis of the contribution of stromal elements to cell adhesion, invasion, and nutrient access; and to assess the influence of the TME on tumor cell survival, proliferation and differentiation.
2.1 Ovarian cancer cells and three-dimensional matrices
Initial strategies for creating 3D cultures16,17 began with mixing OvCa cell lines or primary cells with different forms of ECM. The ECM used was typically either a purified series of proteins, such as collagen, or an extract derived from a more complex mixture, such as Matrigel®. The early events that follow sub-mesothelial matrix contact are modeled by culturing cells on top of 3D collagen gels, allowing the evaluation of changes in gene expression that accompany metastatic anchoring [Figure 1Aa–c].2,18 Matrix penetration, survival and proliferation within the mechanically constrained 3D matrix environment can be modeled by seeding single cells within 3D collagen gels [Figure 1Ad–e]. Indeed, successful growth of a metastatic aggregate requires proliferation within a mechanically constrained matrix microenvironment, a process facilitated by the expression of matrix-degrading proteinases such as membrane type 1 matrix metalloproteinase (MT1-MMP) that promotes proliferation within an interstitial collagen mileu.19,20 OvCa cells grown in 3D culture on growth factor-reduced (GFR)-Matrigel exhibit a central hypoxic region, leading to the decreased diffusion and perfusion of drugs.21 The GFR-Matrigel OvCa model has been used to screen for drugs that are able to penetrate deep into the hypoxic and acidic region of the 3D culture.22
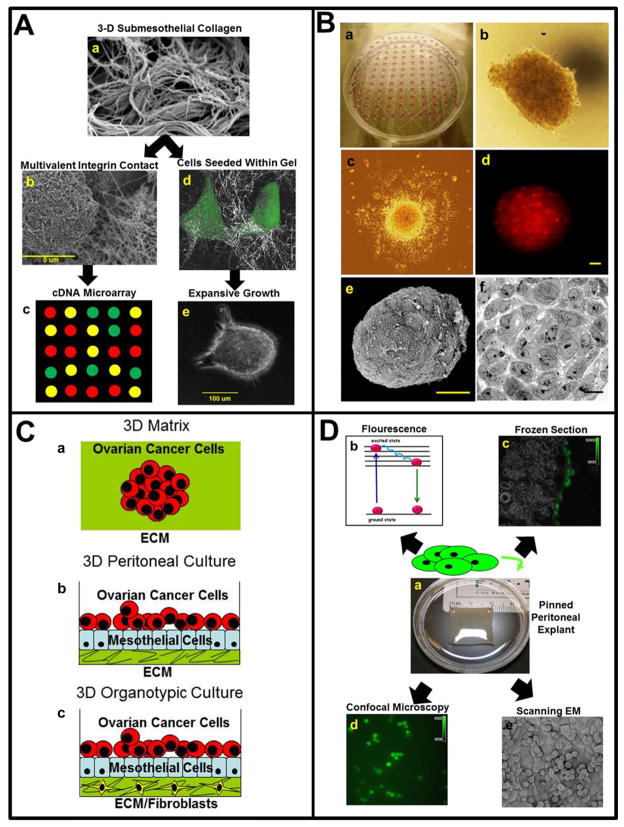
FIGURE 1. Three-dimensional organotypic and ex vivo models of ovarian cancer metastasis.
A) Three-dimensional (3D) culture models of cellular contact with sub-mesothelial collagen. (a) Following initial intra-peritoneal adhesion, mesothelial cell retraction exposes the sub-mesothelial interstitial collagen-rich matrix to which ovarian cancer (OvCa) cells avidly adhere. Scanning electron micrograph of murine submesothelial collagen matrix. (b) Initial multivalent cell-matrix contact of OvCa cell (left) with collagen (right) is visualized by SEM. (c) Culturing cells atop 3D collagen (as in b) followed by cDNA microarray analysis can reveal changes in gene expression that result from the initial adhesive contact. (d) Metastasizing cells migrate into the 3D collagen matrix to anchor metastatic lesions. Confocal reflectance microscopy overlaid with fluorescence microscopy visualizes cells within the 3D matrix. (e) Cells seeded into a 3D matrix proliferate in a matrix metalloproteinase-dependent manner to form expansive matrix-anchored multicellular aggregates. Micrographs shown in a, b are courtesy of Yueying Liu, University of Notre Dame and Dr. Katarina Wolf, Radboud University Nijmegen, The Netherlands. Photographs and micrographs shown in e are courtesy of Dr. Natalie Moss, Northwestern University.
B) Multicellular aggregate (MCA) cultures mimic non-adherent OvCa cells in ascites. (a) To generate MCA cultures, cells are suspended at a concentration of 100,000 cells/ml and seeded as 20 μl droplets on the inner surface of a tissue culture dish lid. After addition of PBS to the culture dish to maintain humidity, the lid is gently inverted. Following aggregation, individual MCAs may be subcultured for use in additional assays. (b) Light micrograph of individual MCA generated using DOV13 cells. (c) Dispersal of MCA generated from DOV13 cells. An individual MCA was subcultured onto a coverslip coated with type I collagen and photographed after 12 hours. Dispersal can be quantified by DAPI staining and measuring inter-nuclear distance.2 (d) Fluorescence micrograph of individual MCA generated using CellTracker Red-labeled DOV13 cells. (Scale bar 30 μm) (e) Scanning electron micrograph of MCA generated using DOV13 cells. MCAs were placed in primary fixative (2% glutaraldehyde, 2% paraformaldehyde in 0.1 M Cacodylate buffer pH 7.35) followed by fixation with osmium tetroxide, dehydration in ethanol, and critical point drying. Platinum coated samples were examined using a Hitachi S-4700 Field Emission Scanning Electron Microscope (scale bar 50 μm). (f) Transmission electron micrograph of MCA generated using DOV13 cells. Following primary fixation as described in e, samples were encapsulated in HistoGel, fixed with osmium tetroxide, dehydrated in ethanol and acetone, infiltrated with Epon/Spurr’s resin and cut into ultrathin sections. Sections were mounted on nickel grids, stained with uranyl acetate and Sato’s Triple Lead stain and examined using a JEOL 1400 transmission electron microscope (scale bar 10 μm). Micrographs shown are courtesy of Yuliya Klymenko, University of Notre Dame.
C) Ovarian cancer in vitro models recapitulate peritoneal metastasis. (a) Ovarian cancer growing as spheroids in a 3D matrix. (b) A 3D peritoneal culture using primary human peritoneal mesothelial cells and extracellular matrix to investigate OvCa cell adhesion. (c) A 3D organotypic “meso-mimetic” culture of the peritoneal cavity. Primary human omental fibroblasts are embedded in extracellular matrix and a layer of primary human omental mesothelial cells plated on top. Ovarian cancer cells are added to the culture and adhesion, invasion, and proliferation of the cancer cells is investigated. Using fluorescently-labeled OvCa cells, changes in gene and protein expression can be individually evaluated in the cancer and stromal cells.
D) Ex vivo peritoneal explant model of early events in intra-peritoneal adhesion. (a) Optically clear silastic resin is generated using a Sylgard® 184 silicone elastomer kit (Fisher), using approximately 6 ml per well of a 6-well culture plate. Dissected murine peritoneal tissue obtained from a midline incision is pinned mesothelial-side up to the silastic resin and immersed in PBS. Tissue integrity is maintained for up to 48 h. Addition of fluorescently tagged tumor cells to the explant enables monitoring by (b) relative fluorescence of cell lysate, (c) fluorescence microscopy of frozen sections, (d) confocal microscopy, or (e) scanning electron microscopy. Beneath the tumor cells in (e, round), the cobblestone mesothelium is visible. Note that images in panels (b–d) are representative of a 2 h time point following addition of OvCa cells to the explant. Photographs and micrographs shown are courtesy of Yueying Liu, University of Notre Dame.
2.2 Spheroids or multi-cellular aggregates
Ovarian cancer spheroids that range in size from 30–200 μm can be isolated from patient-ascites23 or produced by growing OvCa cells on non-adherent plates24, spinner-flasks,25 or by using the hanging drop culture method [Figure 1B].26 Multi-cellular aggregate formation may facilitate metastasis, since spheroids resist anoikis and can adhere to both omentum and exposed ECM.13,14 Adhesion of spheroids initiates a transition from a floating cell population to a peritoneally-anchored metastatic lesion. Following adhesion to a collagen surface, the cells acquire unique mRNA profiles that more closely resemble in vivo tumor gene expression profiles.26
2.3 Co-culture of mesothelial with ovarian cancer cells
The main sites of OvCa metastasis are mesothelial cell-lined peritoneal surfaces, including the peritoneum, omentum and pleural surface.15 Notably, in 1985 the first 3D peritoneal culture was used to investigate the adhesion of primary human OvCa cells to primary human mesothelial cells grown on a bovine corneal endothelial cell-derived extracellular matrix.27 Other 3D OvCa co-cultures followed, with cancer cell lines or primary cells grown as monolayers on plastic or as tissue-like multi-cellular aggregates [Figure 1B], which were co-cultured with mesothelial cell monolayers.28–33 A derivation of this strategy was the utilization of conditioned medium secreted by mesothelial cells to monitor the influence of secreted factors on cancer cell invasion through ECM.34 Following adhesion of tumor cells to peritoneal mesothelium, rapid mesothelial cell retraction is initiated, resulting in exposure of the interstitial collagen-rich sub-mesothelial matrix to which OvCa cells avidly adhere via integrin-mediated interactions [Figure 1A]. 33,35,36 The resulting integrin engagement and clustering modify gene expression profiles, altering the expression of genes that potentiate matrix penetration, anchoring, and proliferation.2,18
2.4 Complex three-dimensional organotypic models with multiple cell types and extracellular matrices
A recent model of OvCa metastasis provides an environment that more closely mimics the in vivo human peritoneal microenvironment, as it includes the superficial layer of the peritoneum (“mesothelium”) to include both mesothelial cells and the sub-mesothelial ECM interspersed with primary human peritoneal fibroblasts.15 The 3D organotypic model uses tumor-derived primary human OvCa cells or cancer cell lines cultured with omentum-derived primary human mesothelial cells, primary human fibroblasts and patient-derived extracellular matrix [Figure 1C].37 This model recapitulates key events in OvCa metastasis including adhesion, proliferation, and invasion, thereby re-establishing morphological and functional features of the corresponding tissue in vivo.37 By using fluorescently labeled cells and fluorescence-activated cell sorting, changes in gene or protein levels and post-translational modification can be individually investigated in the cancer cells, mesothelial cells or fibroblasts after co-culture. Thus far, the 3D “mesothelium-mimetic” organotypic culture has contributed to our understanding of the role of c-Met38, MMP-239, HOXA940, E-cadherin12, β3-integrin41 and α5-integrin12 in early OvCa metastasis. The 3D organotypic culture of peritoneal metastasis is particularly suited for use as a predictive preclinical model for drug testing, since drug sensitivity in ex vivo and in vivo studies can be recapitulated in the 3D model.38,39 Furthermore, this model can be expanded to use other cell types (e.g. adipocytes42, macrophages) and provides a system to test the effects of treatments on both normal peritoneal cells and tumor cells, and may be modified for use in high-throughput screening (HAK, EL unpublished).
2.5 Peritoneal and organ explants
Three dimensional systems using ex vivo human or mouse peritoneal or omental explants are used to evaluate OvCa tumor cell interactions with intact peritoneal tissue (MSS, unpublished).39,43 Dissected peritoneal explants can be pinned to optically clear silastic resin and incubated with OvCa cells or multi-cellular aggregates [Figure 1D]. Use of fluorescently-tagged OvCa cells enables optical monitoring of adherent cells as well as visualization by confocal microscopy. Scanning electron microscopy can also be used to visualize the morphology of early heterogeneous cell-cell adhesive interactions. Furthermore, explants can be histologically sectioned and used to quantify depth of tumor cell penetration under various conditions. This model confers the ability to monitor the extent and kinetics of adhesion, observe early events in peritoneal anchoring, and test potential anti-adhesive therapeutics. Similarly, tissue explants can also be used to evaluate pre-neoplastic changes in potential progenitors from the ovary and fallopian tube. For example 3D alginate scaffolds have been used to support the ex vivo growth of ovarian surface cells, normal mouse oviducts and baboon fimbria.44–46
In the current iteration, these models are limited by the lack of vasculature, immune and endothelial cells, and other extracellular components present in vivo as well as by the ability to provide reproducible conditions only for a short period of time (<1 week). Nevertheless, the development and use of advanced 3D and ex vivo OvCa models can bridge the gap between in vitro studies, OvCa mouse models, and the in vivo human disease. The choice of a model system for a given experiment should reflect the experimental question to be addressed and the stage of OvCa metastasis under investigation.
3 CELL LINES AND XENOGRAFTS
Although OvCa cell lines and primary tissues that recapitulate the molecular diversity, cellular heterogeneity, and histology seen in patient tumors are available, there is no uniform collection or structured overview of these valuable reagents. Here we summarize the most frequently used OvCa cell lines, including those used as xenografts (Supplementary Table S1). The positive attributes of cell culture include ease of propagation, relatively low cost, ability to control culture conditions, less heterogeneous cell populations, and the presence of side population cells which represent a renewable source of OvCa stem cells. Cell culture is limited, however, by the absence of the tumor microenvironment, artifacts from prolonged culture conditions in unphysiological culture medium on plastic, high passage number, and the lack of patient-matched normal cell counterparts. When these cells are used to generate xenografts, positive attributes include recapitulation of tumor heterogeneity with recruitment of host stromal cells and maintenance of both tumor stroma and tumor/non-tumor vasculature interactions. This is balanced by the time and expense of these models and the maintenance of only a small portion of niche cells which do not represent the entire tumor.
3.1 Serous ovarian cancer epithelial cell lines and intraperitoneally derived xenografts
Most high-grade serous OvCa cell lines (Supplementary Table S1) have been derived from ascites of patients ranging from chemotherapy naïve to heavily treated, with various stage malignancies47. Cell lines established from patient whole tumor tissue (rather than ascites), that are considered platinum-sensitive, include HEY48, derived from the peritoneal deposit of a moderately differentiated papillary cystadenocarcinoma49, and the cisplatin-resistant derivations HeyA8 and HeyC2, both generated by passaging HEY in athymic nude mice.48 OVCAR-450 was developed from a patient refractory to platinum-based chemotherapy, and Caov-351 from the solid components of a serous OvCa prior to treatment with cyclophosphamide, adriamycin, and 5-fluorouracil. Given that taxanes are the second cornerstone of OvCa treatment, a paclitaxel-resistant subline (SKOV3TR) was derived from SKOV-3 cells through selection in incrementally increasing paclitaxel concentrations.52,53
Some cell lines have been treated with chemotherapeutic agents to produce resistant sub-lines.54 Drug resistant sub-lines of A2780, which are all cisplatin resistant at the corresponding micro molar values, include CP70, C30, and C200. An adriamycin-resistant A2780 cell line, A2780ADR (100nM resistance and cross-resistant to melphalan and vinblastine)55 and paclitaxel-resistant A2780 sub-lines, PTX10 and PTX22 (5ng/mL and 15ng/mL, respectively)56, are also available. The IGROV-1 cell line was derived from the ovarian tumor of a patient with stage III human ovarian adenocarcinoma who had not received chemotherapy. By subjecting the chemotherapy-naïve cells to 0.5μg/mL and 1.0μg/mL cisplatin, IGROV-1/Pt 0.5 and IGROV-1/Pt 1 sub-lines were developed. The chemo-resistant 2008 cell line was established from a serous cystadenocarcinoma of the ovary. Monthly treatment of 2008 with 1μM cisplatin with subsequent selection resulted in the 2008 *C13 subline57. Deep sequencing data for IGROV-1 cells are available58 and the line is also included in the NCI 60 cell line panel. Indeed, 6 OvCa cell lines (OVCAR-3, OVCAR-4, OVCAR-5, OVCAR8, IGROV-1, SKOV3) are included in the NCI 60 cell lines and are used by the NCI for in vitro drug screening. Therefore, they can be considered as the most extensively characterized set of OvCa cell lines, with drug sensitivity, microRNA, mRNA and proteomic profiling data available.59 Most importantly, their genetic identity can be verified through DNA fingerprinting60, allowing reliable confirmation of their identity.
A number of the cell lines are suitable for development as murine xenografts (Supplementary Table S1, Figure 2A–B). OVCAR-3 has been successfully passaged in mice and developed as an i.p. xenograft that retains many characteristics of its human tumor counterpart with histology similar to a serous OvCa.61 SKOV362 and PEO2363 were collected on relapse after cisplatin and chlorambucil treatment and represent well differentiated serous adenocarcinoma cell lines that form xenograft tumors with a histology that is also very similar to a human serous cancer. Passaging the SKOV3 cells in nude mice allowed the development of several sublines (SKOV3ip.164, SKOV3x65) that grow as disseminated disease in the abdomens of nude mice. This mouse model is characterized by numerous (100–200) small nodules on the surface of the peritoneum, bowel mesentery, and diaphragm and the transformation of the mouse omentum into a larger tumor39,64,65, mimicking human advanced stage serous-papillary OvCa [Figure 2A]. When gene expression in SKOV3 was compared to that of SKOV3ip.1, the latter showed upregulation of v-erb-b2, FGFR1, MMP-19 and downregulation of nm23B66, possibly explaining the rapid growth of these cells as xenografts. OVCAR-367 and OVCAR-5 [Figure 2Ba] have a similar widely disseminated xenograft growth pattern. In contrast, intraperitoneal injection of HeyA8 cells yields a few large tumors that grow in the ovaries and mouse omentum without peritoneal dissemination [Figure 2Bb], mimicking a distinct human OvCa growth pattern.68 Most OvCa cell lines can be successfully transfected or transduced to express fluorescent or bioluminescent vectors to enable longitudinal optical imaging of tumor growth [Figure 2Ab-c, 2Bc]. Furthermore, the i.p. and s.c. take rate of human OvCa cell lines can be improved by mixing them before injection with Matrigel.16
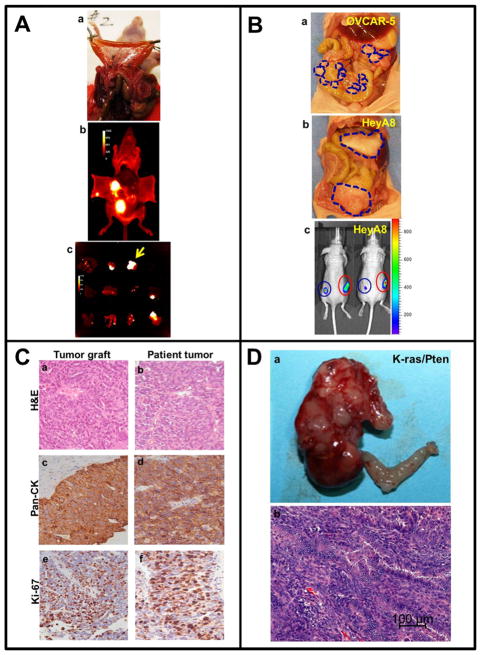
FIGURE 2. Cell line-based and patient-derived in vivo xenograft models.
A) Optical imaging of disseminated i.p. metastasis. (a) SKOV3ip.1 cells were transduced using a lentiviral vector to express red fluorescent protein (RFP), followed by i.p. injection (5×106 cells) into nude mice. After 24 days, mice were sacrificed, the peritoneal cavity opened and metastatic nodules visually enumerated. (b) Fluorescent images of peritoneal organs in situ were taken using a Xenogen IVIS Lumina imaging system. (c) Dissected organs were imaged using a Xenogen IVIS Lumina system. Yellow arrow indicates dissected omentum. Photographs shown are courtesy of Yueying Liu, University of Notre Dame.
B) Distinct in vivo growth patterns of ovarian cancer cells mimic human metastatic dissemination. (a) OVCAR-5 cells (1×106) were injected i.p. into nude mice. After 45 days, mice were sacrificed. Blue lines outline widely disseminated growth of the xenograft as small peritoneal nodules. (b) HeyA8 cells (1×106) were injected i.p. into nude mice. After 28 days, mice were sacrificed. Blue lines outline the growth of large tumors. (c) HeyA8 cells (0.5×105) expressing luciferase were injected s.c. into the mouse flank in the absence (blue circle) or presence (red circle) of carcinoma-associated fibroblasts (CAFs, 1×105). After 14 days, mice were subjected to longitudinal in vivo bioluminescence imaging using the Xenogen IVIS 200 Imaging System. Photographs shown are courtesy of Dr. Marion Zillhardt, University of Chicago.
C) Histological comparison of patient tumor and “xenopatient” graft in the mouse. Following surgical removal of a serous ovarian tumor, a 1 cm fragment was finely minced and injected i.p. into SCID/Beige mice. At about 20 weeks, mice were sacrificed and tumors collected. Mouse tumor grafts (a,c,e) and patient tumors (b,d,f) were subjected to staining with hematoxylin and eosin (H&E, a,b), pan-cytokeratin (c,d), or Ki67 (e,f), as indicated. Photographs shown are courtesy of Saravut John Weroha, Mayo Clinic.
D) Formation of ovarian tumors in LSL-KRasG12D; Ptenfl/fl mice. Tumors were initiated by injection of adenovirus expressing cre recombinase in the right ovarian bursa. The left ovary was not injected and served as an internal control. Mice were sacrificed after 8 weeks and the primary tumor was excised (a), embedded in paraffin, and subjected to staining with H&E (b). Photographs shown are courtesy of Dr. Iris Romero, University of Chicago.
3.2 Clear cell, endometrioid, and mucinous ovarian cancer cell lines
Clear cell carcinoma, which is by definition a poorly differentiated high grade tumor, is represented by several OvCa cell lines [Supplementary Table S1], including ES-269, TOV-21G70, OVISE and OVTOKO71, and TYK-nu and OVMANA.72 ES-2 cells exhibit low to moderate resistance to a number of chemotherapeutic agents including doxorubicin, cisplatin, and etoposide. OVISE and OVTOKO were established from metastatic tumors of two patients after treatment with five to six courses of chemotherapy. Both lines are insensitive to cisplatin, doxorubicin, cyclophosphamide, and etoposide. Interestingly, i.p. injection of the OVTOKO cells yields peritoneal implantation and distant metastasis in a mouse model, whereas OVISE cells showed no dissemination and metastasis. OVISE contains a homozygous ARID1A mutation often observed in human clear cell cancers.6 Both TYK-nu and OVMANA have been fully sequenced and a PIK3CA mutation in OVMANA was identified72 that recapitulates the genetic changes observed in human clear cell OvCa.5
Endometrioid OvCa is represented by several cell lines [Supplementary Table S1]. The first endometrioid cell line was MDAH2774, developed from cells in the ascitic fluid from a patient with endometrioid OvCa and forms tumors in nude mice.73 59M was established from the ascitic fluid sample of patient who previously presented with a synchronous stage IA carcinoma of the ovary and an in situ carcinoma of the endometrium.74 COV362 was established from a pleural effusion, and like its sub-clone COV362.4, shows anchorage-independent growth in agar.75 TOV-112D was derived from a Grade 3, stage FIGO IIIC endometrioid OvCa.70 A mouse endometrioid cancer cell line76 has been established from the LSL-KRasG12D;Ptenfl/fl mouse model.77 EFO-27, representing a mucinous papillary adenocarcinoma, was derived from a solid omental metastasis.47
3.3 Primary ovarian and fallopian tube cells, immortalized lines, and establishing lines from primary ovarian tumors
Establishing primary OvCa cells has remained a challenge as the optimal cell culture medium and ECM to successfully and reproducibly culture primary OvCa has remained elusive even though various methods have been published. During the initial process of establishing a cell line from a patient tumor rapidly growing contaminating cells, such as fibroblastic and stromal cells, can out-compete the cancer cells in culture. Precautions to avoid non-cancer cell contamination include Percoll gradient separation and isolation with CD45 antibodies.78,79 Other methods to isolate primary OvCa cells from ascites and solid tumor tissue include enriching tumor cells using immunomagnetic beads coupled to EpCAM antibodies80 or filtration through a nylon cell strainer81 and the use of optimized media previously shown to enhance the growth of breast cancer cells.82 The primary cells may be characterized by EpCAM and HE4 protein expression and CK7 mRNA expression.81
Since the OvCa cell of origin may be the abdominal peritoneum, the surface epithelium of the ovary7 or the fimbriated end of the fallopian tube83, culturing the primary epithelial cells from these sites is crucial to identifying the pathways involved in the neoplastic transformation of serous OvCa. However, when attempting to propagate these primary epithelia in vitro, culture conditions induce senescence and differentiation. Propagation of human immortalized ovarian surface cells (IOSE)84 and fallopian tube fimbria has been made possible by stable transfection with SV40 T antigen.85,86 For example, IOSE-29 does not form tumors, while its neoplastic counterpart IOSE29EC forms s.c. and i.p. tumors in SCID mice.87 Immortalized fallopian tube cell lines used for i.p. injection retain gross, histological, immunophenotypical and genomic characteristics similar to human high grade OvCa.8,88 When fimbrial cells are cultured on collagen-coated transwells, cell polarization is enhanced, which allows the investigation of DNA damage from chemotherapy and other sources.88 The use of a 3D alginate scaffold also supports the growth of normal mouse oviducts and ovarian surface cells, as well as baboon fimbria.44–46 A human fallopian tube cell line has also been established from tumor tissue.89
3.4 Mouse and rat ovarian cancer cell lines
The syngeneic mouse model allows OvCa initiation directly from mouse OSE (MOSE). MOSE are scraped from the mouse bursa and are passaged in culture on plastic until phenotypic changes occur, such as the loss of cell contact inhibition, which results in cellular mounds and changes in cell morphology.90 During neoplastic progression in the cell culture dish, the tumor-suppressor proteins E-cadherin and connexin-43 are lost, distinguishing early from late MOSE.91 When MOSE are injected i.p. into C57BL6 mice, ascites and metastatic tumors form by about 90 days92, while s.c. injected cells cause solid tumor formation confined to the injection area. When non-transformed cells are injected i.p. and s.c., no tumor formation is observed. The most commonly used mouse OvCa cell line is ID8, developed in vitro from ovaries of the C57BL6 mouse strain.90 The MOSE cells IG10 and IF5 are cognate tumor-inducing clonal cell lines which in vivo have very different survival times and tumor loads (IF5: 182 ±3 days, IG10 72±2 days).
Although the transformation into MOSE occurs in vitro, the MOSE system may to some extent recapitulate progression of normal OSE to an epithelial OvCa. A rat ovarian orthotopic xenograft variant has been reported that uses the spontaneously immortalized rat surface ovarian epithelial cell line NuTu-19 in Fisher 344 immunocompetent rat hosts. In this model, development of pelvic extension and peritoneal metastases were dependent upon the number of injected cells.93 The syngeneic mouse or rat orthotopic models are very useful for studies requiring the preservation of an intact immune system and can be used to confirm results obtained with xenograft models.
3.5 Cell line authentication
Good cell culture practices and the observation, monitoring, and documentation of cell morphology, behavior, and growth rates will reduce the frequency of cell misidentification. Changes may indicate microbial contamination, genotypic drift due to high passage number, or cross-contamination with another cell line. Given these daily risks and the tremendous implications of using the wrong cell line for experiments, the authentication of cell lines is now being emphasized by scientific journals and granting agencies. Human cell lines should be regularly authenticated and identified using DNA short-tandem repeat (STR) profiling.94 Although OvCa cell lines derived from a multitude of sub-types are available, and multiple OvCa cell lines have been assessed in a single study, availability of a collection of regularly authenticated, mycoplasma tested, and well characterized epithelial OvCa cell lines would be optimal, a need in the field highlighted by a recent report on the extent of OvCa cell line misidentification 95. Ideally, the cell lines would be accompanied by extensive genetic/epigenetic and other molecular information.
3.6 Ovarian cancer stem cells
Ovarian cancer stem cells have now been isolated from a number of sources, including primary tumors, ascites, and established OvCa cell lines.53,96–99 The first report of the isolation and identification of stem cells from OvCa patients described two ascites-derived clones able to form multicellular-aggregate, anchorage-independent spheres in culture, and serially propagate xenograft tumors in nude mice that were histopathologically similar to their parental tumors.96 A number of surface markers have been used to isolate stem cells from primary patient OvCa (Supplementary Table S2). In addition, subpopulations of tumorigenic stem-like “side populations” cells in cultures of OVCAR3, IGROV-1, and SK-OV-3 have been reported.98,100 Since these represent a small population of chemoresistant cells in a tumor, the development of model systems that include stem cells may lead to a better understanding of tumorigenesis and drug resistance in OvCa.
3.7 Orthotopic implantation of cell lines and whole tissue tumor grafts (“Xenopatients”)
Early events in metastasis cannot be fully appreciated when i.p. xenografts are used, since they rely on the artificial dispersion of single cell suspensions of cancer cells in the peritoneal cavity, rather than dissociation of metastatic cells from an intact primary tumor tissue. Orthotopic ovarian xenografts may mimic more closely the process of dissemination from the primary ovarian tumor relative to the i.p. xenografts discussed above and are therefore well suited for studying metastasis. However, there are significant anatomic differences between rodents, which have a closed bursa at the end of a long and coiled fallopian tube with longitudinal folds enclosing the ovary101, and humans where the tube is straight, short, and the epithelium is exposed to the peritoneal cavity. Several approaches have been utilized for orthotopic mouse models: injection of cancer cell lines under the ovarian bursa, implantation of human or murine tumor fragments adjacent to the ovary or under the renal capsule, and i.p. injection of minced human tumor.102–105 Critical steps in the metastatic process are invasion through the ovarian bursa followed by peritoneal spread with colonization and invasion of the organs in the peritoneal cavity. Intrabursal injection of OvCa cells causes tumor formation in the ovary, but peritoneal dissemination may not occur. Moreover, only a small volume (>5μl) can be injected under the fat pad without causing mechanical disruption of the bursa and peritoneal spillage.
Implantation of whole human OvCa tissue fragments under or adjacent to the ovarian bursa of SCID mice generates ovarian tumors that histologically resemble the tumor of origin.102 In this model, tumor take rate is approximately 65% at 16 weeks and production of malignant ascites has been recorded, although metastases occurred infrequently. However, in one study using initial implantation of intact human ovarian tumor fragments, metastatic dissemination initiated from orthotopic ovarian tumors was reported following serial passage of human xenografts in the ovarian fat pad.106 After several passages, peritoneal spread was recorded, resembling the clinical features of the human disease. Higher tumor take (~95%) is observed when fragments of human ovarian tumors are implanted under the renal capsule of SCID mice104, perhaps reflecting a more permissive host milieu facilitated by the rich kidney vasculature. While tumor growth was generally slow and observed metastases were rare, the histological and immunophenotypic characteristics of the original tumor are preserved in the tumor graft. Growth of less aggressive tumors, such as benign, borderline, and granulosa tumors is also possible when fragments are implanted under the renal capsule.
An alternative method to implantation of full tissue fragments, which requires mouse surgery, is to inject a finely minced primary human tumor slurry directly i.p in mice.107 Mice are then monitored for engraftment using manual palpation and small animal ultrasound. This methodology has led to a high engraftment rate (~74%) from over 200 consecutive patients with untreated ovarian, primary peritoneal and fallopian tube cancers.105 The “xenopatients” recapitulated the histological [Figure 2C], molecular and platinum-sensitivity characteristics of the source patient tumors. Thus, “xenopatients” could be useful models for assessing response to therapeutic interventions or biomarker discovery in a manner reflecting the behavior of the original tumor. Indeed, response to platinum chemotherapy was recorded in an orthotopic serous fallopian tube explant, mirroring the clinical response of the donor patient.108 These data suggest that these models, albeit very labor-intensive, may be useful tools for individualizing patient therapy.
4 GENETICALLY ENGINEERED MOUSE MODELS OF OVARIAN CANCER
Genetically engineered mouse models (GEMMs) of OvCa, which can closely recapitulate human disease, have the potential to greatly improve our understanding of OvCa biology.109 These models, involving genes expressed or deleted specifically in the ovary or the fallopian tube, allow us to evaluate the physiological relevance of defined genetic changes in OvCa and to better understand the mechanistic significance of genetic lesions revealed in human tumors. Such pre-clinical models are clearly also suited for testing new compounds for cancer prevention76 and therapy110,111, since they can be used to model drug resistance as well as contribute to the discovery and validation of novel serum biomarkers. GEMMs of OvCa are summarized in Supplementary Table S3.
4.1 GEMM using various combinations of p53, RB, Myc, Akt and Pten
Generating GEMMs of OvCa in which the fallopian tube or ovarian surface epithelium (OSE) is specifically targeted has proven difficult due to the lack of defined transcriptional promoters active specifically in these cell types. One of the first animal models of OvCa used the TVA receptor system driven by the epithelial specific cytokeratin 5 (CK5) promoter to delete the p53 tumor suppressor in isolated ovarian surface epithelia in vitro.112 OSE cells in which p53 had been deleted in this manner were then infected in vitro with retroviruses expressing combinations of relevant oncogenes (c-myc, K-ras, and/or Akt). The cells, now with altered gene expression, were transplanted into host mice. This process ensured that tumors were unlikely to arise from tissues other than OSE that express CK5. These mice developed OvCa with papillary structures consistent with serous OvCa and using these mice it was shown that rapamycin inhibits the growth of tumors depending on Akt activity.110 Subsequent work showed that a single oncogene, in this case c-Myc, was sufficient to drive OSE tumorigenesis when both p53 and BRCA1 were deleted using the TVA system.113 Clearly, this model showed that the ovarian surface is capable of forming tumors when the correct oncogene and tumor suppressor gene mutations are introduced.
The lack of defined cell type specific promoters continues to hinder more conventional transgenic approaches to driving the deletion or activation of relevant tumor suppressors and oncogenes in the OSE. Since the promoters used to express genes in OSE are also active in other tissues of Müllerian origin (fallopian tube, uterus, cervix, ovarian granulosa cells), the origin of emerging “ovarian” tumors in the mouse model is open to question. One of the most effective promoters used to drive Cre recombinase or other transgenes has been the promoter of the Müllerian Inhibiting Substance II Receptor (MISRII) gene, also known as the Anti-Müllerian Hormone Type II Receptor (AMHRII). The MISRII promoter primarily drives transgene expression in the ovarian surface and ovarian granulosa cells, but it also expressed the transgene in the mesenchyme of the oviduct. The first transgenic mouse model to utilize the MISRII promoter used it to drive expression of the SV40 large T antigen (SV40-Tag), a viral oncogene that binds to and inactivates both p53 and all members of the retinoblastoma (Rb) pocket protein family of cell cycle regulators. As a result, approximately 50% of the animals developed large undifferentiated ovarian adenocarcinomas by 90–100 days.114 In order to more effectively control the timing of oncogene expression, a Lox-STOP-Lox (LSL) cassette was inserted between the SV40-Tag and the ubiquitous CAG promoter. Oncogene expression was then induced following direct intrabursal injection of adenovirus expressing Cre-recombinase, which, theoretically, only reached oviductal and ovarian surface epithelial cells.115 Approximately 89% of the pCAG-LSL-SV40-Tag mice injected in this way developed poorly differentiated tumors with metastasis throughout the abdominal cavity, similar to the tumor type and incidence observed when SV40-Tag expression was driven by the MISRII promoter.114,115 Thus, intrabursal injection and MISRII both effectively target the ovarian surface, while SV40-Tag expression in the oviductal mesenchyme does not induce oviductal tumors.
Cre-Lox technology has been used more extensively to induce targeted deletion of the Pten tumor suppressor and to activate the K-Ras oncogene. In two separate models, LSL-KRasG12D;Ptenfl/fl mice were induced to develop ovarian tumors either through adenoviral delivery of Cre77 or through expression of a Cre transgene under the control of the aforementioned MISRII promoter.116 The MISRII-Cre; LSL-KRasG12D; Ptenfl/fl mice develop a form of low grade epithelial serous cancer derived from the ovarian surface with 100% penetrance. This is currently the only transgenic mouse that models a low grade serous OvCa.116 In contrast, when tumors were induced in LSL-KRasG12D;Ptenfl/fl mice by infection of the OSE with adenovirus expressing Cre-recombinase, the mice developed endometrioid or poorly differentiated carcinomas [Figure 2D].77 In this model, adenoviral vectors that express Cre recombinase were used to selectively express or delete genes in the ovary. These vectors are injected into the ovarian bursa, theoretically only reaching oviductal, ovarian surface epithelial, and bursa cells. However, in practice they sometimes leak out through the injection site and reach uterine tissue. The difference between these two models, both of which target K-Ras and Pten, is the manner in which Cre is delivered. 77,116 This illustrates the importance of defining the target tissue and developmental timing for deletion/activation of the critical oncogenes and tumor suppressors. For example, follicular depletion and a loss of circulating ovarian hormones such as estrogen are evident in the MISRII-Cre; LSL-KRasG12D;Ptenfl/fl mice116 but not in the Ad-Cre; LSL-KRasG12D;Ptenfl/fl mice.117 To what extent such developmental and follicular effects of transgene expression play a role in tumorigenesis in these mice is not clear.
The Pten tumor suppressor has also been deleted in mice in combination with the Apc tumor suppressor, a signaling molecule in the Wnt/β-catenin pathway.118 The combined deletion of Pten and Apc by intrabursal injection of Cre-recombinase expressing adenovirus resulted in the formation of the endometrioid sub-type OvCa in all injected animals118, similar to that seen in the Ad-Cre; LSL-KRasG12D;Ptenfl/fl mice.77 These animals develop a more aggressive phenotype when additional mutations in p53 and in PI3Kca are introduced.119 Based on the observation that the AKT pathway is up-regulated in human OvCa due to the mutation or amplification of the PIK3CA gene5, a mouse model has also been designed with an activating mutation in PI3K (PIK3CAH1047R) that, when combined with deletion of Pten, led to robust activation of the Akt pathway. After intrabursal adenoviral Cre-recombinase injections, the Ad-Cre; PIK3CAH1047R;Ptenfl/fl mice formed ovarian serous adenocarcinomas or granulosa cell tumors.111 This model has been used to test the efficacy of a PI3K/mTOR pathway inhibitor which was initially effective in inhibiting tumor growth, although the tumors eventually became resistant.111 These distinct mouse models of OvCa in which Pten is deleted demonstrate that Pten deletion alone is not sufficient for tumor formation, but the specific context of Pten deletion in the OSE or in the OSE and granulosa cells impacts the histotype and grade of the cancer formed.
4.2 GEMM of BRCA mutations
The BRCA1 and 2 genes are either mutated or silenced by methylation in approximately 20% of all high grade OvCa.4 Several conditional BRCA1 and BRCA2 mouse models have been generated. In these, the BRCA allele, flanked by LoxP sites, is deleted either by Cre-recombinase expression driven by the MISRII or FSHR promoter, or by adenoviral injection.117,120–122 Targeted deletion of only BRCA1 or BRCA2 in the ovaries is generally not sufficient to induce malignancy, although FSHR promoter driven deletion of only BRCA1 did generate cystic tumors in one model.117 The prolonged estrous phase and enhanced estrogen signaling in this model may account for this phenotype, since FSHR is expressed in granulosa, ovarian surface, and oviductal cells.117 Tumor formation may be influenced either by loss of BRCA1 in cells other than those of the ovarian surface or oviduct or by the effects of increased estrogen signaling in cells without BRCA1. Conditional loss of BRCA1 alone (Cre-recombinase injection into the bursa) was insufficient to generate tumors and resulted in hyperplasia of the OSE. Combining loss of BRCA1 with loss of p53 resulted in either leiomyosarcomas or no tumors at all, unless the Rb family was also inactivated.120 Loss of BRCA1 in combination with p53 (BRCA1LoxP/LoxP p53LoxP/LoxP) led to development of a leiomyosarcoma but not a serous OvCa.120–122 In a recently reported mouse model122 the key event initiating OvCa was the inactivation of the Rb tumor suppressor and related p107/p130 pocket proteins through targeted expression of a mutant form of the SV40-Tag that cannot inactivate p53. This, when combined with the inactivation of p53 and BRCA1 deletion, caused progression to advanced serous OvCa. Similarly, combined loss of p53 and Rb function led to tumor formation.123
4.3 GEMM of fallopian tube cancer
Since the emergence of the hypothesis that the fallopian tubes may be the primary site of origin of high grade serous carcinoma124, a few models have been introduced to help explain potential genetic causes of tubal cancers109. When the oviductal glycoprotein (OVGP) gene promoter was used to drive SV40-Tag expression, mice developed oviductal, uterine and vaginal tumors.125 Tumor cells were occasionally found in the ovaries, but it was not determined if these represented the primary lesion or a metastasis. Recently, MISRII-Cre; Ptenfl/fl;Dicerfl/fl mice have been generated in which both the Pten and the Dicer gene, required for efficient micro-RNA processing, were deleted in all tissues of Müllerian duct origin. These mice developed tumors resembling high-grade serous OvCa in the mesenchyme of the oviduct that metastasized to the peritoneum, the diaphragm, and formed ascites.126 Histologic analysis and gene expression profiling suggested that these tumors resembled high-grade serous cancer in humans; however, the tumors did not have mutations in BRCA, p53 or deficiencies in homologous repair.126 This GEMM consistently develops high-grade serous cancer, which metastasizes within the abdominal cavity in a very similar fashion to human OvCa. Interestingly, a recent abstract suggests that a Ptenfl/fl; Dicerfl/fl/p53LSLR172H/+ triple knock out develops both tubal and ovarian tumors.127 Thus, it remains to be determined whether the epithelial expression of the “p53 signature” in BRCA-mutant human OvCa and the formation of serous cancers from the stroma in this mouse model126 can be reconciled in terms of human disease origin. Lastly, very recent reports suggest that an inducible Pax8-TetON system can generate a high grade serous cancer from the fallopian tube epithelium when p53 and BRCA1 or BRCA2 are simultaneously deleted.128 The tumors were more likely to be found in the proximal rather than the distal portion of the oviduct. It is the distal portion that is anatomically comparable to the fimbriae of the human fallopian tube and is the more likely site of OvCa formation. This discrepancy in tumor localization when compared to human disease may be explained by the relative strength of the Pax8 promoter in transgenic mice.
GEMMs offer the potential to introduce specific modifications to pathways in a time and tissue dependent manner that can help to confirm or deny the importance of genetic mutations in disease onset and chemoresistance. Other advantages of GEMMs are that OvCa develop disease in a shorter time period than non-human primates and chickens allowing studies to be completed quickly. With the use of viral delivery to introduce cre-recombinase, mutations can be turned on and off in different stages of tissue development. Criticism of GEMMs were raised due to concerns that the existing GEMMs do not employ genetic lesions relevant to human high grade serous cancers and, in many instances, may be driven by inappropriate regulatory sequences and promoters, which may explain, in part, their failure to recapitulate the peritoneal dissemination clinically observed in human OvCa. Unfortunately, OSE do not express a tissue specific promoter and a promoter only expressed in specific epithelial cells of the fallopian tube (e.g. secretory cells8) has not yet been uncovered. Another drawback of GEMMs is that they can produce different phenotypes due to the background strain of the mouse model.120,123 Since human OvCa is genetically complex, it is likely that genetic mouse models represent only one particular molecular subtype of OvCa, rather than the entire spectrum of the corresponding human disease.4
5 THE LAYING HEN MODEL OF EPITHELIAL OVARIAN CANCER
One of the major obstacles to progress in OvCa prevention and treatment is a dearth of spontaneous disease models. The primary animal model available for the study of spontaneous epithelial OvCa is the laying hen.129 As early as the 1960s, reports identified domestic egg-laying hens as probable surrogates for human OvCa and suggested that they be investigated as potential models.130,131 A landmark large scale epidemiological study documented an age dependent onset of the disease, with the first occurrence of OvCa observed in hens of 2–2.5 years of age with an incidence of 4%, while in hens aged from 4–6 years the incidence was 30–50%.132 These findings have been confirmed in a recent study demonstrating that the first observable incidence of spontaneous OvCa was in hens aged between 2–2.5 years with an incidence of epithelial OvCa of all stages that reached a peak of nearly 60% at 4 years.133 A distinct advantage of pre-clinical studies in laying hens is that large-scale studies with hundreds of animals, conducted over extended periods of time, can be performed at relatively little cost. After a hen has completed her 2nd year of laying, producing from 300 to 500 eggs, she has ovulated approximately the same number of times as a woman who has undergone menopause, when the risk of OvCa increases dramatically.134 A considerable body of epidemiological data supports the link between the number of lifetime ovulations and OvCa risk. Specifically studies using the domestic hen, support the “incessant ovulation” hypothesis by showing that when ovulation is arrested in hens, either (i) pharmacologically by dosing with progestins135, (ii) using a model of restricted ovulation through nutrient deprivation, or (iii) via genetic selection for low-egg laying variants the incidence of OvCa is lower.135–137 The high rate of OvCa in egg-laying hens could be the consequence of genomic damage to the ovarian surface epithelium associated with incessant ovulations or may indirectly result from facilitated genetic changes or impaired DNA repair which ultimately lead to the development of OvCa in the hen. A direct relationship between ovulation and OvCa remains to be determined.129
5.1 Description of the hen model
Epithelial OvCa in the hen presents with many features of the human disease [Figure 3A, B] including heterogeneity with at least four clearly distinguishable histological subtypes. In contrast to human OvCa, in which serous disease represents 70% of late stage disease, 50% of late stage tumors in the hen have histologic features of endometrioid carcinoma in the primary tumor and corresponding peritoneal metastases.138,139 However, similar to their human counterparts, hens exhibit clear cell, endometrioid, mucinous and serous histological subtypes [Figure 3C]. The clinical presentation in hens is similar to that in women, in that hens develop substantial volumes of ascites fluid, peritoneal dissemination of metastases, and pulmonary emboli. Hens develop solid tumors in the lung parenchyma, which is rare in women with advanced serous OvCa, but may be observed in human endometrioid or clear cell cancers.140
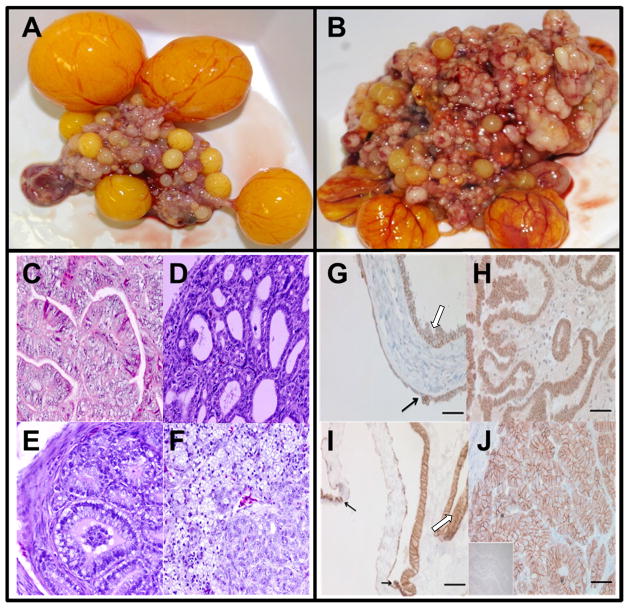
FIGURE 3. The domestic egg laying hen model of ovarian cancer.
A) Normal ovary of a domestic laying hen. The ovary contains a set of 4 large pre-ovulatory hierarchical follicles, small developing follicles, and a post-ovulatory follicle.
B) Primary malignant ovarian tumor in laying hen with stage IV OvCa. The tumor was classified as a serous OvCa on an endometrioid background. The tumor has metastasized to distant organs with profuse ascites and had an endometrioid histotype. Multiple solid tumor masses are seen.
C) Histological types of malignant ovarian tumors in hens. (a) Ovarian serous carcinoma showing sheets of lacelike papillary folding and cells with large pleomorphic nuclei with mitotic bodies. (b) Ovarian endometrioid carcinoma with confluent back-to-back glands. Glands contain a single layer of epithelial cells with sharp luminal margin. (c) Ovarian mucinous carcinoma with crowded glands in clusters without intervening stroma surrounded by a fibromuscular layer. The epithelium contains columnar and intercalated ciliated goblet cells. The nuclei are separated from the basement membrane and have moved toward the apical surface with occasional stratification and luminal secretion. (d) Poorly differentiated ovarian clear cell carcinoma showing vacuolated cells containing high-grade nuclear atypia that invade the stroma and theca layer of stromal follicles. Deposition of eosinophilic hyalinized matrix in the stroma and necrotic bodies are also seen. Hematoxylin and eosin staining (original magnification 40X).
D) Immunohistochemical localization of E-cadherin expression in human (a,b) and laying hen (c,d) normal ovarian and cancerous tissue. The uninvolved epithelial cells in normal human ovarian tissue (a) and in normal laying hen ovarian tissue (c) lack E-cadherin, while early neoplastic transformation (black arrow) of adjacent ‘normal’ ovarian surface epithelial cells and mild to moderate dysplastic cells (white arrow) are marked by an increase in E-cadherin expression. Tumor cells of a human endometrioid type tumor (b) and a primary hen tumor (d) express high E-cadherin levels in distinct patterns throughout the tissue (bar= 50μm).
Certain molecular markers of human OvCa are reproduced in the hen including early expression of E-cadherin [Figure 3D]139, up-regulation of COX-1141, alterations in p53, K-Ras and Her2/neu142; expression of CA125143, and several surrogate immunohistochemical markers, including PCNA, p27, TGFβ1, cytokeratin AE1/AE3, pan cytokeratin, EGFR, Lewis Y, CEA, Tag 72, and erbB-2.144 Gene profiling studies and bioinformatics comparisons between laying hen and human cancer gene expression patterns have further demonstrated the conservation of genetic lesions associated with OvCa and histotype-specific mutations.142,145,146 Because of the importance of the chicken as a meat-animal and its extensive use as a model for developmental studies, robust genomic resources for the chicken have been developed, including cDNA, long-oligo and GeneChip™ genome arrays.147,148 The chicken epi-genome has also been mapped.149 Extensive chicken genomic resources are available via the NCBI150, the Genome Institute at Washington University, and in a recently published collection of chicken genomic resources.151
5.2 Prevention
The first pre-clinical intervention study in the laying hen evaluated the efficacy of medroxyprogesterone acetate (Depo-Provera®) in reducing the frequency of spontaneously developing reproductive tract adenocarcinoma, showing a 15% risk reduction in animals treated with the progestin.135 The authors observed that the high rate of reproductive tract adenocarcinoma further supported the use of the laying hen model of spontaneous ovarian carcinogenesis to test chemoprevention strategies. A recent chemoprevention trial in hens studied treatment with progestin alone, estrogen alone and progestin plus estrogen.152 Results show that treatment with progestin alone and in combination with estrogen decreased the prevalence of OvCa with a concomitant reduction in egg laying.135,152 Both groups concluded that their findings were consistent with the theory that a reduction of ovulatory events may prevent the development of ovarian adenocarcinoma.135,152 The utility of the hen model for pre-clinical evaluation of therapeutic modalities for the prevention of OvCa (reviewed by Rodriguez153) has been demonstrated in studies testing the efficacy of progestins135,152, anti-inflammatory NSAIDs154; and dietary intervention with flaxseed.155
5.3 Studies of early ovarian cancer in hens
A distinct advantage of the hen model of OvCa is that the disease arises spontaneously in chickens and therefore presents investigators with the opportunity to study the initial events leading to OvCa, identify targets for prevention, and devise and test interventions. One example of an early event in OvCa development that has been observed in hens and corroborated in human clinical specimens is the presence of anti-ovarian auto antibodies in the serum of hens with early stage OvCa.156,157 Another example is the increased expression of E-cadherin in hen OvCa, which is clearly seen at the point of neoplastic transformation where normal surface epithelium transitions morphologically into precursor lesions which develop into early primary tumor sites (as shown in Figure 3Da,c).139 Similar patterns of increased E-cadherin in inclusion cysts of human ovarian tumors have been described.7 In addition, the pattern of expression of tubal genes in primary ovarian tumors of the hen 158 and repetition of the “p53-signature” in pre-neoplastic lesions of the tubal and ovarian surface epithelium of the hen 141 are conserved in the hen analogous to humans.
5.4 Imaging in hens
Doppler ultrasonography has been employed to analyze the kinetics of an ultrasound contrast agent used to identify and follow ovarian tumor-associated neo-angiogenesis in early-stage OvCa in the hen. Findings were corroborated on necropsy.155,159 The kinetics of the ultrasound contrast agent was shown to be indicative of ovarian tumor-associated neo-angiogenesis in early stage OvCa. The use of contrast-enhanced ultrasound for the detection of early ovarian tumors demonstrates the potential for the development of new clinical diagnostics tools using the hen model. Moreover, the hen model may also be useful for testing the efficacy of different contrast agents in a preclinical setting.
5.5 Limitations and challenges
There has been very slow progress in the development of stable cell lines derived from chicken OvCa or ascites for mechanistic studies in hens. Ovarian surface epithelial cells, tumor cells from primary cancer, and tumor cells isolated from ascites fluid from hens have been described, but as yet these studies have been limited to primary cultures or cell cultures with low passage numbers.160 Similarly, there has been very little progress in the development of transgenic chicken models for the manipulation of targeted genes by over-expression, mutation, ablation, and silencing. Promising studies, however, have shown that chick embryos can be genetically manipulated, offering hope for the eventual development of transgenic chicken models of OvCa.161,162
Another challenge of working with hens is the limited availability of chicken specific reagents, such as antibodies for immunohistochemical analyses. A panel of commercially available surrogate cancer antigen antibodies has been examined for their cross-reactivity in hens144 and successfully adapted for the use with hen tumor tissue using robust antigen-retrieval procedures. However, despite the clearly demonstrated power of the hen model and its potential for evaluating therapeutics and preventatives, only a handful of investigators have taken advantage of the model. Access to experimental poultry facilities and lack of capacity for chickens in standard biomedical vivarium facilities limit more widespread adoption of the hen as the standard animal model for OvCa research.
6 SUMMARY
Within the last five years, significant advancement has been made in developing a more comprehensive suite of tools with which to experimentally address the pathobiologic mechanisms that underlie the poor survival of OvCa patients. Despite this progress, the conduct of experimental translational research in OvCa remains limited by substantial challenges. For example, a large number of OvCa cell lines have been derived and maintained in culture, some of which exhibit a chemoresistant phenotype. The value of this resource would be substantially enhanced by the establishment of a “biobase” collection of regularly authenticated, well characterized, and well annotated cell lines for distribution to the research community, similar to the resource available to the breast cancer research community.163 Access to patient-matched normal and tumor cells, normal epithelial and mesenchymal cells from other primary sources (for example, fallopian tube), and enhanced access to well-annotated patient tissue would also facilitate research progress. Several complex 3D and organotypic models of OvCa metastasis have been developed that take into account cell and tissue architecture and the contributions of extracellular matrix as well as stromal and inflammatory cells in the tumor microenvironment. Lacking, however, are models that contribute to our understanding of the very earliest events in OvCa metastasis; the shedding of cells from the primary tumor. Use of these models to elucidate molecular events that initiate and regulate the “terminal transition” from free-floating ascitic cell or multi-cellular aggregate to peritoneally anchored metastatic lesion may identify novel therapeutic targets to block metastasis. A challenge will be adaptation of these 3D and organotypic models to a high throughput format to facilitate drug screening. The use of murine OvCa models with orthotopic injection into the peritoneal cavity, ovarian bursa, or kidney capsule has proven utility to address a number of important research questions. However, evolution in our understanding of OvCa initiation, progression and early stages of metastasis is hampered by the need for improved genetic models. Identification of more precise tissue-specific promoters to target genetic lesions (or sets of mutations) identified in human OvCa tumors to specific tissues in the reproductive tract (ovarian epithelium, fimbriae of fallopian tube, etc.) would promote progress. In this regard, the laying hen model of spontaneous OvCa holds promise and the development of additional research tools, reagents, and approaches for use in this model system is warranted. Lastly, as we realize that “ovarian cancer” is many diseases, sub-type specific models will need to be developed and tested. With careful calibration of reagents and experimental models, together with enhanced collaboration, we are poised for significant advancement of our goal to identify novel, effective, and personalized approaches to detect and treat ovarian cancer.
Supplementary Material
Acknowledgments
The impetus for this collaborative compendium arose from the inaugural workshop of the Indiana-Illinois End Epithelial Ovarian Cancer Coalition (IIEEOC) held at the University of Notre Dame in 2012. The IIEEOCC Workshop was supported by the Harper Cancer Research Institute, the University of Notre Dame, the Indiana University Simon Cancer Center and the Indiana Clinical and Translational Sciences Institute. We gratefully acknowledge the lively and informative discussion contributed by all workshop participants.
Funding for our work was provided by National Institutes of Health/National Cancer Institute Research Grants, CA086584 (MSS), CA109545 (MSS); CA085289 (KN), CA085289 (KN), the Integrative Cancer Biology Program CA1113001 (KN), and an Ovarian Cancer Research Fund (PPD/IU) to KN and DM; NIH/National Center for Complementary and Alternative Medicine grants AT00408, AT005295 (BH); National Cancer Institute Award CA133915 (BH); American Institute for Cancer Research 06-A043 (BH); NIH/NCI CA111882 (EL) and an Ovarian Cancer Research Fund (PPD/UofC) to EL. Joanna Burdette was supported by the Ovarian Cancer Research Fund Liz Tilberis Ovarian Cancer Scholar Award (L/T UIC), the American Cancer Society Illinois Division Research Grant RSG-12-230-01-TBG, and the Department of Defense OC110133. Paul Haluska was supported by the Mayo Clinic SPORE in Ovarian Cancer-CA136393.
ABBREVIATIONS
- AMHRII
Anti-Müllerian Hormone Type II Receptor
- CK5
Cytokeratin 5
- ECM
Extracellular Matrix
- GEMM
Genetically Engineered Mouse Model
- GFR
Growth factor-reduced
- IOSE
Immortalized Ovarian Surface Epithelium
- i.p
intraperitoneal
- LSL
Lox-STOP-Lox
- MISRII
Müllerian Inhibiting Substance II Receptor
- MOSE
Mouse Ovarian Surface Epithelium
- MT1-MMP
Membrane Type 1 Matrix Metalloproteinase
- OvCa
Ovarian Cancer
- RB
Retinoblastoma
- s.c
subcutaneous
- STR
Short-Tandem Repeat (STR) profiling
- TME
Tumor Microenvironment
References
- 1.Landen CN, Birrer MJ, Sood AK. Early events in the pathogenesis of epithelial ovarian cancer. J Clin Oncol. 2008;26(6):995–1005. doi: 10.1200/JCO.2006.07.9970. [DOI] [PubMed] [Google Scholar]
- 2.Barbolina MV, Moss NM, Westfall SD, et al. Microenvironmental regulation of ovarian cancer metastasis. Cancer Treat Res. 2009;149:319–334. doi: 10.1007/978-0-387-98094-2_15. [DOI] [PubMed] [Google Scholar]
- 3.Vaughn S, Coward JI, Bast RC, et al. Rethinking ovarian cancer: Recommendations for improving outcomes. Nature Rev. 2011;11:719–725. doi: 10.1038/nrc3144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.The Cancer Genome Atlas Network. Integrated genomic analyses of ovarian carcinoma. Nature. 2011;474:609–615. doi: 10.1038/nature10166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kuo KT, Mao TL, Jones S, et al. Frequent activating mutations of PIK3CA in ovarian clear cell carcinoma. Am J Pathol. 2009;174(5):1597–1601. doi: 10.2353/ajpath.2009.081000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wiegand KC, Shah S, Al-Agha OM, et al. ARID1A mutations in endometriosis associated ovarian carcinomas. N Engl J Med. 2010;363(16):1532–1543. doi: 10.1056/NEJMoa1008433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Auersperg N, Wong AST, Choi K, Kang S, Leung PCK. Ovarian surface epithelium: Biology, endocrinology and pathology. Endocr Rev. 2001;22(2):255–288. doi: 10.1210/edrv.22.2.0422. [DOI] [PubMed] [Google Scholar]
- 8.Karst AM, Levanon K, Drapkin R. Modeling high-grade serous ovarian carcinogenesis from the fallopian tube. Proc Natl Acad Sci USA. 2011;108(18):7547–7552. doi: 10.1073/pnas.1017300108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lengyel E. Ovarian cancer development and metastasis. Am J Pathol. 2010;177(3):1053–1064. doi: 10.2353/ajpath.2010.100105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Symowicz J, Adley BP, Gleason KJ, et al. Engagement of collagen-binding integrins promotes matrix metalloproteinase-9-dependent E-cadherin ectodomain shedding in ovarian carcinoma cells. Cancer Res. 2007;67(5):2030–2039. doi: 10.1158/0008-5472.CAN-06-2808. [DOI] [PubMed] [Google Scholar]
- 11.Hudson LG, Zeineldin R, Stack MS. Phenotypic plasticity of neoplastic ovarian epithelium: Unique cadherin profiles in tumor progression. Clin Exp Metastasis. 2008;25:643–655. doi: 10.1007/s10585-008-9171-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sawada K, Mitra AK, Radjabi AR, et al. Loss of E-cadherin promotes ovarian cancer metastasis via alpha 5-integrin, which is a therapeutic target. Cancer Res. 2008;68(7):2329–2339. doi: 10.1158/0008-5472.CAN-07-5167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burleson KM, Hansen LK, Skubitz AP. Ovarian carcinoma spheroids disaggregate on type I collagen and invade live human mesothelial cell monolayers. Clin Exp Metastasis. 2004;21(8):685–697. doi: 10.1007/s10585-004-5768-5. [DOI] [PubMed] [Google Scholar]
- 14.Burleson KM, Casey RC, Skubitz KM, Pambuccian SE, Oegema TR, Skubitz AP. Ovarian carcinoma ascites spheroids adhere to extracellular matrix components and mesothelial cell monolayers. Gynecol Oncol. 2004;93:170–181. doi: 10.1016/j.ygyno.2003.12.034. [DOI] [PubMed] [Google Scholar]
- 15.Kenny HA, Nieman KM, Mitra AK, Lengyel E. The first line of intra-abdominal metastatic attack: Breaching the mesothelial cell layer. Cancer Discov. 2011;1(2):100–102. doi: 10.1158/2159-8290.CD-11-0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mullen P, Ritchie A, Langsdon SP, Miller WR. Effect of matrigel on the tumorigenicity of human breast and ovarian carcinoma cell lines. Int J Cancer. 1996;67(6):816–820. doi: 10.1002/(SICI)1097-0215(19960917)67:6<816::AID-IJC10>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 17.Adams AT, Auersperg N. A cell line, ROSE 199, derived from normal rat ovarian surface epithelium. Exp Cell Biology. 1985;53(4):181–188. doi: 10.1159/000163311. [DOI] [PubMed] [Google Scholar]
- 18.Barbolina MV, Adley BP, Ariztia EV, Liu Y, Stack MS. Microenvironmental regulation of membrane type 1 matrix metalloproteinase activity in ovarian carcinoma cells via collagen-induced EGR1 expression. J Biol Chem. 2007;282(7):4924–4931. doi: 10.1074/jbc.M608428200. [DOI] [PubMed] [Google Scholar]
- 19.Moss NM, Barbolina MV, Liu Y, Sun L, Munshi HG, Stack MS. Ovarian cancer cell detachment and multicellular aggregate formation are regulated by membrane type 1 matrix metalloproteinase: A potential role in I.p metastatic dissemination. Cancer Res. 2009;69(17):7121–7129. doi: 10.1158/0008-5472.CAN-08-4151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hotary KB, Allen EB, Brooks PC, Datta NS, Long MW, Weiss SJ. Membrane Type I Matrix Metalloproteinase usurps tumor growth control imposed by the three-dimensional extracellular matrix. Cell. 2003 Jul 11;114:33–45. doi: 10.1016/s0092-8674(03)00513-0. [DOI] [PubMed] [Google Scholar]
- 21.Evans CL, Abu-Yousif AO, Park YJ, et al. Killing hypoxic cell populations in a 3D tumor model with EtNBS-PDT. PloS One. 2011;6(8):e23434. doi: 10.1371/journal.pone.0023434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klein OJ, Bhayana B, Park YJ, Evans CL. In vitro optimization of EtNBS-PDT against hypoxic tumor environments with a tiered, high-content, 3D model optical screening platform. Mol Pharm. 2012;9(11):3172–3182. doi: 10.1021/mp300262x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Allen HJ, Porter C, Gamarra M, Piver MS, Johnson EA. Isolation and morphologic characterization of human ovarian carcinoma cell clusters present in effusions. Exp Cell Biology. 1987;55(4):194–208. doi: 10.1159/000163419. [DOI] [PubMed] [Google Scholar]
- 24.Lin RZ, Chang HY. Recent advances in three-dimensional multicellular spheroid culture for biomedical research. Biotechnol J. 2008;9–10:1172–1184. doi: 10.1002/biot.200700228. [DOI] [PubMed] [Google Scholar]
- 25.Gilead A, Neeman M. Dynamic remodeling of the vascular bed precedes tumor growth: MLS ovarian carcinoma spheroids implanted in nude mice. Neoplasia. 1999;1(3):226–230. doi: 10.1038/sj.neo.7900032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zietarska M, Maugard C, Filali-Mouhim A, et al. Molecular description of a 3D in vitro model for the study of epithelial ovarian cancer (EOC) Mol Carcinog. 2007;46:872–885. doi: 10.1002/mc.20315. [DOI] [PubMed] [Google Scholar]
- 27.Niedbala MJ, Crickard K, Bernacki R. Interactions of human ovarian tumor cells with human mesothelial cells grown on extracellular matrix. Experimental Cell Res. 1985;160:499–513. doi: 10.1016/0014-4827(85)90197-1. [DOI] [PubMed] [Google Scholar]
- 28.Lessan K, Aguiar D, Oegema TR, Siebenson L, Skubitz AP. CD44 and β1 integrin mediate ovarian carcinoma cell adhesion to peritoneal mesothelial cells. Am J Pathol. 1999;154(5):1525–1537. doi: 10.1016/s0002-9440(10)65406-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Suzuki N, Aoki D, Tamada Y, et al. HMOCC-1, a human monoclonal antibody that inhibits adhesion of ovarian cancer cells to human mesothelial cells. Gynecol Oncol. 2004;95:290–298. doi: 10.1016/j.ygyno.2004.06.024. [DOI] [PubMed] [Google Scholar]
- 30.Kishikawa T, Sakamoto M, Ino Y, Kubushiro K, Nozawa S, Hirohashi S. Two distinct pattern of peritoneal involvement shown by in vitro and in vivo ovarian cancer dissemination models. Invasion and Metastasis. 1995;15:11–21. [PubMed] [Google Scholar]
- 31.Casey RC, Koch KA, Oegema TR, et al. Establishment of an in vitro assay to measure the invasion of ovarian carcinoma cells through mesothelial cell monolayers. Clin Exp Metastasis. 2003;20:343–356. doi: 10.1023/a:1024009131191. [DOI] [PubMed] [Google Scholar]
- 32.Rieppi M, Vergani V, Gatto C, et al. Mesothelial cells induce the motility of human ovarian carcinoma cells. Int J Cancer. 1999;80:303–307. doi: 10.1002/(sici)1097-0215(19990118)80:2<303::aid-ijc21>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 33.Iwanicki M, Davidowitz RA, Ng MR, et al. Ovarian cancer spheroids use myosin-generated force to clear the mesothelium. Cancer Discov. 2011;2:OF1–OF7. doi: 10.1158/2159-8274.CD-11-0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jones LM, Gardner MJ, Catterall JB, Turner GA. Hyaluronic acid secreted by mesothelial cells: A natural barrier to ovarian cancer cell adhesion. Clin Exp Metastasis. 1995;13(5):373–380. doi: 10.1007/BF00121913. [DOI] [PubMed] [Google Scholar]
- 35.Niedbala MJ, Crickard K, Bernacki R. In vitro degradation of extracellular matrix by human ovarian carcinoma cells. Clin Exp Metastasis. 1987;5(2):181–197. doi: 10.1007/BF00058063. [DOI] [PubMed] [Google Scholar]
- 36.Sawada M, Shii J, Akedo H, Tanizawa O. An experimental model for ovarian tumor invasion of cultured mesothelial cell monolayer. Lab Invest. 1994;70(3):333–338. [PubMed] [Google Scholar]
- 37.Kenny HA, Krausz T, Yamada SD, Lengyel E. Use of a novel 3D culture model to elucidate the role of mesothelial cells, fibroblasts and extra-cellular matrices on adhesion and invasion of ovarian cancer cells. Int J Cancer. 2007;121(7):1463–1472. doi: 10.1002/ijc.22874. [DOI] [PubMed] [Google Scholar]
- 38.Sawada K, Radjabi AR, Shinomiya N, et al. C-Met overexpression is a prognostic factor in ovarian cancer and an effective target for inhibition of peritoneal dissemination and invasion. Cancer Res. 2007;67(4):1670–1680. doi: 10.1158/0008-5472.CAN-06-1147. [DOI] [PubMed] [Google Scholar]
- 39.Kenny HA, Kaur S, Coussens LM, Lengyel E. The initial steps of ovarian cancer cell metastasis are mediated by MMP-2 cleavage of vitronectin and fibronectin. J Clin Invest. 2008;118(4):1367–1379. doi: 10.1172/JCI33775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ko SY, Barengo N, Ladanyi A, Lee JS, Lengyel E, Naora H. HOXA9 promotes ovarian cancer growth by stimulating cancer-associated fibroblasts. J Clin Invest. 2012;122(10):3603–3617. doi: 10.1172/JCI62229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kaur S, Kenny HA, Jagadeeswaran S, et al. β3-integrin expression on tumor cells inhibits tumor progression, reduces metastasis, and is associated with a favorable prognosis in patients with ovarian cancer. Am J Pathol. 2009;175(5):2184–2196. doi: 10.2353/ajpath.2009.090028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nieman KM, Kenny HA, Penicka CV, et al. Adipocytes promote ovarian cancer metastasis and provide energy for rapid tumor growth. Nature Med. 2011;17(11):1498–1503. doi: 10.1038/nm.2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Khan SM, Funk HM, Thiolloy S, et al. In vitro metastatic colonization of human ovarian cancer cells to the omentum. Clin Exp Metastasis. 2012;27:185–196. doi: 10.1007/s10585-010-9317-0. [DOI] [PubMed] [Google Scholar]
- 44.King SM, Quartuccio S, Hillard TS, Inoue K, Burdette JE. Alginate hydrogels for three-dimensional organ culture of ovaries and oviducts. Journal of Visualized Experiments. 2011;52:1–6. doi: 10.3791/2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jackson KS, Inoue K, Davis DA, Hillard TS, Burdette JE. Three-dimensional ovarian organ culture as a tool to study normal ovarian surface epithelial wound repair. Endocrinology. 2009;150(8):3921–3926. doi: 10.1210/en.2008-1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.King SM, Hilliard TS, Wu LY, Jaffe RC, Fazleabas AT, Burdette JE. The impact of ovulation on fallopian tube epithelial cells: evaluating three hypotheses connecting ovulation and serous ovarian cancer. Endocrine-Related Cancer. 2011;18(5):627–642. doi: 10.1530/ERC-11-0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hozel F, Simon WE, Albrecht M, Hansel M, Dietel M. Cell lines derived from human ovarian carcinomas: Growth stimulation by gonadotropic and steroid hormones. J Natl Cancer Inst. 1983;70:839–845. [PubMed] [Google Scholar]
- 48.Buick RN, Pullano R, Trent JM. Comparative properties of five human ovarian adenocarcinoma cell lines. Cancer Res. 1985;45:3668–3676. [PubMed] [Google Scholar]
- 49.Selby PJ, Thomas JM, Monaghan P, Sloane J, Peckham MJ. Human tumour xenografts established and serially transplanted in mice immunologically deprived by thymectomy, cytosine arobinoside and whole-body irradiation. Br J Cancer. 1980;41:52–62. doi: 10.1038/bjc.1980.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Green JA, Vistica DT, Young RC, Hamilton TC, Ozols RF. Potentiation of melphalan cytotoxicity in human ovarian cancer cell lines by gluthatione depletion. Cancer Res. 1984;44:5427–5431. [PubMed] [Google Scholar]
- 51.Karlan BY, Jones JC, Slamon DJ, Lagasse LD. Glucocorticoids stabilize HER-2/neu messenger RNA in human epithelial ovarian carcinoma cells. Gynecol Oncol. 1994;53:70–77. doi: 10.1006/gyno.1994.1090. [DOI] [PubMed] [Google Scholar]
- 52.Duan Z, Feller AJ, Toh HC, Makastorsis T, Seiden MV. TRAG-3 a novel gene, isolated from a taxol-resistant ovarian carcinoma cell line. Gene. 1999;229:75–81. doi: 10.1016/s0378-1119(99)00042-6. [DOI] [PubMed] [Google Scholar]
- 53.Landen CN, Goodman B, Katre AA, et al. Targeting aldehyde dehydrogenase cancer stem cells in ovarian cancer. Mol Cancer Ther. 2010;9(12):3186–3199. doi: 10.1158/1535-7163.MCT-10-0563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Godwin AK, Meister A, O’Dwyer PJ, Huang CS, Hamilton TC, Anderson ME. High resistance to cisplatin in human ovarian cancer cell lines is associated with marked increase of glutathione synthesis. Proc Natl Acad Sci USA. 1992;89:3070–3074. doi: 10.1073/pnas.89.7.3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Johnson SW, Swiggard PA, Handel LM, et al. Relationship between platinum-DNA adduct formation and removal and cisplatin cytotoxicity in cisplatin-sensitive and resistant human ovarian cancer cells. Cancer Res. 1994;54:5911–5916. [PubMed] [Google Scholar]
- 56.Giannakakou P, Sackett D, Kang Y, et al. Pacliaxel-resistant human ovarian cancer cells have mutant β-tubulins that exhibit impaired paclitaxel-driven polymerization. J Biol Chem. 1997;272(27):17118–17125. doi: 10.1074/jbc.272.27.17118. [DOI] [PubMed] [Google Scholar]
- 57.Andrews P, Murphy M, Howell SB. Differential potentiation of alkylating and platinating agent cytotoxicity in human ovarian carcinoma cells by gluthatione depletion. Cancer Res. 1985;45:6250–6253. [PubMed] [Google Scholar]
- 58.Cheng L, Lu W, Kulkarni B, et al. Analysis of chemotherapy response programs in ovarian cancers by the next-generation sequencing technologies. Gynecol Oncol. 2010;117:159–169. doi: 10.1016/j.ygyno.2010.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sunshine M, Varma S, Reinhold W, Pommier Y. CellMiner. 2013 ( http://discover.nci.nih.gov/cellminer/home.do)
- 60.Yip KW, Ito E, Mao X, et al. Potential use of alexidine dihydrochloride as an apoptosis-promoting anticancer agent. Mol Cancer Ther. 2006;5(9):2234–2240. doi: 10.1158/1535-7163.MCT-06-0134. [DOI] [PubMed] [Google Scholar]
- 61.Hu L, Hofman J, Holash J, Yancopoulos G, Sood AK, Jaffe RB. Vascular endothelial growth factor Trap combined with paclitaxel strikingly inhibits tumor and ascites, prolonging survival in a human ovarian cancer model. Clin Cancer Res. 2005;11(19):6966–6972. doi: 10.1158/1078-0432.CCR-05-0910. [DOI] [PubMed] [Google Scholar]
- 62.Hua W, Christianson T, Rougeot C, Rochefort H, Clinton GM. SKOV3 ovarian carcinoma cells have functional estrogen receptor but are growth resistant to estrogens and antiestrogens. J Steroid Biochem Molec Biol. 1995;55:279–289. doi: 10.1016/0960-0760(95)00187-5. [DOI] [PubMed] [Google Scholar]
- 63.Miyajima Y, Nakano R, Morimatsu M. Analysis of expression of matrix metalloproteinases-2 and -9 in hypopharyngeal squamous cell carcinoma by in situ hybridization. Ann Otol Rhino Laryngol. 1995;104:678–684. doi: 10.1177/000348949510400902. [DOI] [PubMed] [Google Scholar]
- 64.Yu D, Wolf JK, Scanlon M, Price JE, Hung M-C. Enhanced c-erb B-2/neu expression in human ovarian cancer cells correlates with more severe malignancy that can be suppressed by E1A. Cancer Res. 1992;53:891–898. [PubMed] [Google Scholar]
- 65.Shao M, Cao L, Shen C, et al. Epithelial-to-mesenchymal transition and ovarian tumor progression induced by tissue transglutaminase. Cancer Res. 2009;69(24):9192–9201. doi: 10.1158/0008-5472.CAN-09-1257. [DOI] [PubMed] [Google Scholar]
- 66.Bai F, Feng J, Cheng Y, Shi J, Yang R, Cui H. Analysis of gene expression patterns of ovarian cancer cell lines with different metastatic potentials. Int J Gynecol Cancer. 2006;16:202–209. doi: 10.1111/j.1525-1438.2006.00296.x. [DOI] [PubMed] [Google Scholar]
- 67.Hu L, Hofmann J, Lu Y, Mills GB, Jaffe RB. Inhibition of phosphatidylinositol 3′-kinase increases efficacy of paclitaxel in in vitro and in vivo ovarian cancer models. Cancer Res. 2002;62(February 15):1087–1092. [PubMed] [Google Scholar]
- 68.Camps J, Chang S, Hsu TC, et al. Fibroblast-mediated acceleration of human epithelial tumor growth in vivo. Proc Natl Acad Sci USA. 1990;87:75–79. doi: 10.1073/pnas.87.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lau DHM, Lewis AD, Ehsan MN, Sikic BI. Multifactorial mechanisms associated with broad cross-resistance of ovarian carcinoma cells selected by cyanomorpholino doxorubicin. Cancer Res. 1991;51:5181–5187. [PubMed] [Google Scholar]
- 70.Provencher DM, Lounis H, Champoux L, et al. Characterization of four novel epithelial ovarian cancer cell lines. In Vitro Cell Dev Biol Anim. 200;36(6):357–361. doi: 10.1290/1071-2690(2000)036<0357:COFNEO>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 71.Gorai I, Nakazawa T, Miyagi E, Hirahara F, Nagashima Y, Minaguchi H. Establishment of two human ovarian clear cell adenocarcinoma lines from metastatic lesions with fifferent properties. Gynecol Oncol. 1995;57:33–46. doi: 10.1006/gyno.1995.1097. [DOI] [PubMed] [Google Scholar]
- 72.Cheung HW, Cowley GS, Weir BA, et al. Systematic investigation of genetic vulnerabilities across cancer cell lines reveals lineage-specific dependencies in ovarian cancer. Proc Natl Acad Sci USA. 2011;108(30):12372–12377. doi: 10.1073/pnas.1109363108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Freedman RS, Pihl E, Kusyk C, Gallager HS, Rutledge F. Characterization of an ovarian carcinoma cell line. Cancer. 1978;42(5):2352–2359. doi: 10.1002/1097-0142(197811)42:5<2352::aid-cncr2820420536>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 74.Wilson AP, Dent M, Pejovic T, Hubbold L, Radford H. Characterisation of seven human ovarian tumour cell lines. Br J Cancer. 1996;74:722–727. doi: 10.1038/bjc.1996.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.van der Berg-Baker CA, Hagemeijer A, Franklin-Postma EM, et al. Establishment and characterization of 7 ovarian carcinoma cell lines and one granulosa tumor cell line: growth features and cytogenetics. Int J Cancer. 1993;53(4):613–620. doi: 10.1002/ijc.2910530415. [DOI] [PubMed] [Google Scholar]
- 76.Romero IL, Gordon I, Jagadeeswaran S, et al. Effects of oral contraceptives or a gonadotropin-releasing hormone agonist on ovarian carcinogenesis in genetically engineered mice. Cancer Prev Res. 2009;2(9):792–799. doi: 10.1158/1940-6207.CAPR-08-0236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dinulescu D, Ince TA, Quade B, Shafer S, Crowley D, Jacks T. Role of K-ras and PTEN in the development of mouse models of endometriosis and endometrioid ovarin cancer. Nature Med. 2005;11(1):63–70. doi: 10.1038/nm1173. [DOI] [PubMed] [Google Scholar]
- 78.Dunfield LD, Shepherd TG, Nachtigal M. Primary culture and mRNA analysis of human ovarian cells. Biol Proced Online. 2002;4(1):55–61. doi: 10.1251/bpo34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shepherd TG, Theriault BL, Campbell EJ, Nachtigal M. Primary culture of ovarian surface epithelial cells and ascites-derived ovarian cancer cells from patients. Nat Protoc. 2006;1(6):2643–2649. doi: 10.1038/nprot.2006.328. [DOI] [PubMed] [Google Scholar]
- 80.Barker S, Casado E, Gomez-Navarro J, et al. An immunomagnetic-based method for the purification of ovarian cancer cells from patient-derived ascites. Gynecol Oncol. 2001;82(1):57–63. doi: 10.1006/gyno.2001.6226. [DOI] [PubMed] [Google Scholar]
- 81.Clauss A, Ng V, Liu J, et al. Overexpression of elafin in ovarian carcinoma is driven by genomic gains and activation of the nuclear factor kB pathway and is associated with poor overall survival. Neoplasia. 2010;12(2):161–172. doi: 10.1593/neo.91542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ince TA, Richardson AL, Bell GW, et al. Transformation of different human epithelial cell types leads to distinct tumor phenotypes. Cancer Cell. 2007;12(2):160–170. doi: 10.1016/j.ccr.2007.06.013. [DOI] [PubMed] [Google Scholar]
- 83.Karst AM, Drapkin R. Primary culture and immortalization of human fallopian tube secretory epithelial cells. Nat Protoc. 2012;7:1755–1764. doi: 10.1038/nprot.2012.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kruk PA, Maines-Bandiera SL, Auersperg N. A simplified method to culture human ovarian surface epithelium. Lab Invest. 1990;63(1):132–136. [PubMed] [Google Scholar]
- 85.Maines-Bandiera S, Kruk PA, Auersperg N. Simian virus 40-transformed human ovarian surface epithelial cells escape normal growth controls but retain morphogenetic responses to extracellular matrix. Am J Obstet Gynecol. 1992;167(3):729–735. doi: 10.1016/s0002-9378(11)91579-8. [DOI] [PubMed] [Google Scholar]
- 86.Ando H, Kobayashi M, Toda S, Kikkawa F, Masahashi T, Mizutani S. Establishment of a ciliated epithelial cell line from human fallopian tube. Hum Reprod. 2000;15(7):1597–1603. doi: 10.1093/humrep/15.7.1597. [DOI] [PubMed] [Google Scholar]
- 87.Auersperg N, Pan J, Grove BD, et al. E-cadherin induces mesenchymal-to-epithelial transition in human ovarian surface epithelium. Proc Natl Acad Sci USA. 1999;96(11):6249–6254. doi: 10.1073/pnas.96.11.6249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Levanon K, Ng V, Piao H, et al. Primary ex vivo cultures of human fallopian tube epithelium as a model for serous ovarian carcinogenesis. Oncogene. 2009:1–11. doi: 10.1038/onc.2009.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Runnebaum IB, Tong XW, Möbus V, Kieback DG, Rosenthal HE, Krell HW. P53 mutant His175 identified in a newly established fallopian tube carcinoma cell line secreting interleukin 6. FEBS Lett. 1994;353:29–32. doi: 10.1016/0014-5793(94)00953-8. [DOI] [PubMed] [Google Scholar]
- 90.Roby KF, Taylor CC, Sweetwood JP, et al. Development of a syngeneic mouse model for events related to ovarian cancer. Carcinogenesis. 2000;21(4):585–591. doi: 10.1093/carcin/21.4.585. [DOI] [PubMed] [Google Scholar]
- 91.Roberts PC, Mottillo EP, Baxa AC, et al. Sequential molecular and cellular events during neoplastic progression: A mouse syngeneic ovarian cancer model. Neoplasia. 2005;7(10):944–956. doi: 10.1593/neo.05358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Greenaway J, Moorehead R, Shaw P, Petrik J. Epithelial-stromal interaction increases cell proliferation, survival and tumorigenicity in a mouse model of human epithelial ovarian cancer. Gynecol Oncol. 2008;108:385–394. doi: 10.1016/j.ygyno.2007.10.035. [DOI] [PubMed] [Google Scholar]
- 93.Sloan Stakleff KD, Rouse AG, Ryan AP, Haller NA, von Gruenigen VE. A novel early-stage orthotopic model for ovarian cancer in the fisher 344 rat. Int J Gynecol Cancer. 2005;15:246–254. doi: 10.1111/j.1525-1438.2005.15211.x. [DOI] [PubMed] [Google Scholar]
- 94.Lorenzi PL, Reinhold W, Varma S, et al. DNA fingerprinting of the NCI-60 cell line panel. Mol Cancer Ther. 2009;8(4):713–724. doi: 10.1158/1535-7163.MCT-08-0921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Korch S, Spillman MA, Jacksion TA, et al. DNA profiling analysis of endometrial and ovarian cell lines reveals misidentification, redundancy and contamination. Gynecol Oncol. 2012;127(1):241–248. doi: 10.1016/j.ygyno.2012.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bapat SA, Mali AM, Koppikar CB, Kurrey NK. Stem and progenitor-like cells contribute to the aggressive behavior of human epithelial ovarian cancer. Cancer Res. 2005;65(8):3025–3029. doi: 10.1158/0008-5472.CAN-04-3931. [DOI] [PubMed] [Google Scholar]
- 97.Zhang S, Balch C, Chan MW, et al. Identification and characterization of ovarian cancer-initiating cells from primary human tumors. Cancer Res. 2008;68(11):4311–5507. doi: 10.1158/0008-5472.CAN-08-0364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Szotek PP, Pieretti-Vanmarcke R, Masiakos PT, et al. Ovarian cancer side populations defines cells with stem cell-like characteristics and Mullerian Inhibiting Substance responsiveness. Proc Natl Acad Sci USA. 2006;103:11154–11159. doi: 10.1073/pnas.0603672103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.McLean K, Gong Y, Choi Y, et al. Human ovarian carcinoma-associated mesenchymal stem cells regulate cancer stem cells and tumorigenesis via altered BMP production. J Clin Invest. 2011;121(8):3206–3219. doi: 10.1172/JCI45273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hu L, McArthur C, Jaffe RB. Ovarian cancer stem-like population cells are tumorigenic and chemoresistant. Br J Cancer. 2010;102:1276–1283. doi: 10.1038/sj.bjc.6605626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Stewart C, Behringer RR. Mouse oviduct development. Results Probl Cell Differ. 2012;55:247–262. doi: 10.1007/978-3-642-30406-4_14. [DOI] [PubMed] [Google Scholar]
- 102.Drew AF, Blick TJ, Lafleur MA, et al. Correlation of tumor-and stromal-derived MT1-MTP expression with progression of human ovarian tumors in SCID mice. Gynecol Oncol. 2004;95:437–448. doi: 10.1016/j.ygyno.2004.08.032. [DOI] [PubMed] [Google Scholar]
- 103.Fu X, Hoffman RM. Human ovarian carcinoma metastatic models constructed in nude mice by orthotopic transplantation of histologically-intact patient specimens. Anticancer Res. 1993;25:283–286. [PubMed] [Google Scholar]
- 104.Lee CH, Xue H, Sutcliffe M, et al. Establishment of subrenal capsule xenografts of primary human ovarian tumors in SCID mice: Potential models. Gynecol Oncol. 2005;96:48–55. doi: 10.1016/j.ygyno.2004.09.025. [DOI] [PubMed] [Google Scholar]
- 105.Weroha SJ, Becker MA, Harrington SC, et al. Ovarian avatar models predictive of platinum response in ovarian cancer patients. Annals of Oncology. 2013;24(suppl 1):16. [Google Scholar]
- 106.Xu Y, Silver DF, Yang NP, et al. Characterization of human ovarian carcinomas in a SCID mouse model. Gynecol Oncol. 1999;72(161):170. doi: 10.1006/gyno.1998.5238. [DOI] [PubMed] [Google Scholar]
- 107.Elkas JC, Baldwin RL, Pegram M, Tseng Y, Slamon DJ, Karlan BY. A human ovarian carcinoma murine xenograft model useful for preclinical trials. Gynecol Oncol. 2002;87:200–206. doi: 10.1006/gyno.2002.6819. [DOI] [PubMed] [Google Scholar]
- 108.Khabele D, Fadare O, Liu AY, et al. An orthotopic model of platinum-sensitive high grade serous fallopian tube carcinoma. Int J Clin Exp Pathology. 2012;5(1):37–45. [PMC free article] [PubMed] [Google Scholar]
- 109.Garson K, Gamwell LF, Pitre EMG, Vanderhyden BC. Technical challenges and limitations of current mouse models of ovarian cancer. Journal of Ovarian Research. 2012;(5):5–39. doi: 10.1186/1757-2215-5-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Xing D, Orsulic S. A genetically defined mouse ovarian carcinoma model for the molecular characterization of pathway-targeted therapy and tumor resistance. Proc Natl Acad Sci USA. 2005;102(19):6936–6941. doi: 10.1073/pnas.0502256102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kinross KM, Montgomery KG, Kleinschmidt M, et al. An activating Pik3ca mutation coupled with Pten loss is sufficient to initiate ovarian tumorigenesis in mice. J Clin Invest. 2012;122(2):553–557. doi: 10.1172/JCI59309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Orsulic S, Li Y, Soslow RA, Vitale-Cross LA, Gutkind S, Varmus HE. Induction of ovarian cancer by defined multiple genetic changes in a mouse model system. Cancer Cell. 2002;1(February):53–62. doi: 10.1016/s1535-6108(01)00002-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Xing D, Orsulic S. A mouse model for the molecular characterization of BRCA1-associated ovarian carcinoma. Cancer Res. 2006;66(18):8949–8953. doi: 10.1158/0008-5472.CAN-06-1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Connolly D, Bao R, Nikitin A, et al. Female mice chimeric for expression of the simian virus 40 TAg under control of the MISIIR promoter develop epithelial ovarian cancer. Cancer Res. 2003;63:1389–1397. [PubMed] [Google Scholar]
- 115.Laviolette LA, Garson K, Macdonald EA, et al. 17 beta-estradiol accelerates tumor onset and decreases survival in a transgenic mouse model of ovarian cancer. Endocrinology. 2010;151(3):929–938. doi: 10.1210/en.2009-0602. [DOI] [PubMed] [Google Scholar]
- 116.Mullany LK, Fan H-Y, Liu Z, et al. Molecular and function characteristics of ovarian surface epithelial cells transformed by KrasG12D and loss of Pten in mouse model in vivo. Oncogene. 2011;30:3522–3536. doi: 10.1038/onc.2011.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chodankar R, Kwang S, Sangiorgi F, et al. Cell-nonautonomous induction of ovarian and uterine serous cystadenomas in mice lacking a functional Brca1 in ovarian granulosa cells. Curr Biol. 2005;15:561–565. doi: 10.1016/j.cub.2005.01.052. [DOI] [PubMed] [Google Scholar]
- 118.Wu R, Hendrix-Lucas N, Kuick R, et al. Mouse model of human ovarian endometrioid adenocarcinoma based on somatic defects in the Wnt/β-catenin and PI3K/Pten signaling pathways. Cancer Cell. 2007;11:321–333. doi: 10.1016/j.ccr.2007.02.016. [DOI] [PubMed] [Google Scholar]
- 119.Wu R, Baker SJ, Hu TC, Norman KM, Fearon ER, Cho KR. Type I to type II ovarian carcinoma progression mutant Trp53 or PIK3CA confers a more aggressive tumor phenotype in a mouse model of ovarian cancer. Am J Pathol. 2013;182(4):1391–1399. doi: 10.1016/j.ajpath.2012.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Clark-Knowles K, Garson K, Jonkers J, Vanderhyden BC. Conditional inactivation of Brca1 in the mouse ovarian surface epithelium results in an increase in preneoplastic changes. Experimental Cell Res. 2007;313:133–145. doi: 10.1016/j.yexcr.2006.09.026. [DOI] [PubMed] [Google Scholar]
- 121.Quinn BA, Brake T, Hua X, et al. Induction of ovarian leiomosarcomas in mice by conditional inativation of Brca1 an p53. PloS One. 2009;4(12):e8404. doi: 10.1371/journal.pone.0008404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Szabova L, Yin C, Bupp S, et al. Perturbation of RB, p53 and Brca1 or Brca2 cooperate in inducing metastatic serous epithelial ovarian cancer. Cancer Res. 2012;72(16):4141–4153. doi: 10.1158/0008-5472.CAN-11-3834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Flesken-Nikitin A, Choi K, Eng JP, Shmidt EN, Nikitin A. Induction of carcinogenesis by concurrent inactivation of p53 and Rb1 in the mouse ovarian surface epithelium. Cancer Res. 2003;63:3459–3463. [PubMed] [Google Scholar]
- 124.Piek JM, van Diest PJ, Zweemer RP, et al. Dysplastic changes in prophylactically removed fallopian tubes of women predisposed to developing ovarian cancer. J Pathology. 2001;195(4):451–456. doi: 10.1002/path.1000. [DOI] [PubMed] [Google Scholar]
- 125.Miyoshi I, Takahashi K, Kon Y, et al. Mouse transgenic for murine oviduct-specific glycoprotein promoter-driven simian virus 40 large t-antigen: tumor formation and it hormonal regulation. Molecular Reproduction and Developement. 2013;63:168–176. doi: 10.1002/mrd.10175. [DOI] [PubMed] [Google Scholar]
- 126.Kim J, Coffey DM, Creighton CJ, Yu Z, Hawkins SM, Matzuk MM. High-grade serous ovarian cancer arises from fallopian tube in a mouse model. Proc Natl Acad Sci USA. 2012;109(10):3921–3926. doi: 10.1073/pnas.1117135109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kim J, Coffey D, Ma L, Matzuk MM. A p53 activating mutation accelerates the progressoin of high-grade serous ovarian cancer arising in the fallopian tube. AACR Annual Meeting. 2013 Abstract #328. [Google Scholar]
- 128.Perets R, Muto KW, Bijron JG, et al. A genetically engineered mouse model for high grade serous “ovarian” carcinoma arising in the fallopian tube; AACR Annual Meeting; 2012. [Google Scholar]
- 129.Johnson PA, Giles JR. The hen as a model of ovarian cancer. Nat Rev Cancer. 2013;13(6):432–436. doi: 10.1038/nrc3535. [DOI] [PubMed] [Google Scholar]
- 130.Papasolomontos PA, Appleby EC, Mayor O. Pathological findings in condemned chickens: A survey of 1,000 carcases. Veterinary Record. 1969;25(17):459–464. doi: 10.1136/vr.84.17.459. [DOI] [PubMed] [Google Scholar]
- 131.Awadhiya RP. Studies on the pathology of neoplasms in animals. I. Ovarian tumors in fowls. Indian Vet J. 1967;44(11):917–920. [PubMed] [Google Scholar]
- 132.Fredrickson TN. Ovarian tumors of the hen. Environ Health Perspect. 1987;73:35–51. doi: 10.1289/ehp.877335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Eilati E, Pan L, Bahr JM, Hales DB. Age dependent increase in prostaglandin pathway coincides with onset of ovarian cancer in laying hens. Prostaglandins, Leukotrienes and Essential Fatty Acids. 2012;87(6):177–184. doi: 10.1016/j.plefa.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Casagrande JT, Louie E, Pike MC, Roy S, Ross R, Henderson B. Incessant ovulation” and ovarian cancer. Lancet. 1979;2:170–173. doi: 10.1016/s0140-6736(79)91435-1. [DOI] [PubMed] [Google Scholar]
- 135.Barnes MN, Berry D, Straughn JM, et al. A pilot study of ovarian cancer chemoprevention using medroxyprogesterone acetate in an avian model of spontaneous ovarian carcinogenesis. Gynecol Oncol. 2002;87(1):57–63. doi: 10.1006/gyno.2002.6806. [DOI] [PubMed] [Google Scholar]
- 136.Giles JR, Elkin RG, Trevino LS, Urick ME, Ramachandran R, Johnson PA. The restricted ovulator chicken: a unique animal model for investigating the etiology of ovarian cancer. Int J Gynecol Cancer. 2010;20(5):738–744. doi: 10.1111/igc.0b013e3181da2c49. [DOI] [PubMed] [Google Scholar]
- 137.Carver DK, Barnes HJ, Anderson KE, et al. Reduction of ovarian and oviductal cancers in calorie-restricted laying chickens. Cancer Prev Res. 2011;4(4):562–567. doi: 10.1158/1940-6207.CAPR-10-0294. [DOI] [PubMed] [Google Scholar]
- 138.Barua A, Bitterman P, Abramowicz JS, et al. Histopathology of ovarian tumors in laying hens: a preclinical model of human ovarian cancer. Int J Gynecol Cancer. 2009;19(4):531–539. doi: 10.1111/IGC.0b013e3181a41613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ansenberger K, Zhuge Y, Lagman JA, et al. E-cadherin expression in ovarian cancer in the laying hen, Gallus domesticus, compared to human ovarian cancer. Gynecol Oncol. 2009;113(3):362–369. doi: 10.1016/j.ygyno.2009.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Alfonso M, Adochiles L, Hendrickson VM, Carver DK, Rodriguez GC, Barnes HJ. Metastatic adenocarcinoma in the lungs of older laying hens. Avian diseases. 2005;49(3):430–432. doi: 10.1637/0005-2086(2005)49[430:MAITLO]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 141.Hales DB, Zhuge Y, Lagman JA, et al. Cyclooxygenases expression and distibution in the normal ovary and their role in ovarian cancer in the domestic hen (Gallus domesticus) Endocrine. 2013;33(3):235–244. doi: 10.1007/s12020-008-9080-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Hakim AA, Barry CP, Barnes HJ, et al. Ovarian adenocarcinomas in the laying hen and women share similar alterations in p53, ras, and Her-2/neu. Cancer Prev Res. 2013;2(2):114–121. doi: 10.1158/1940-6207.CAPR-08-0065. [DOI] [PubMed] [Google Scholar]
- 143.Jackson E, Anderson K, Ashwell C, Petitte J, Mozdziak PE. CA125 expression in spontaneous ovarian adenocarcinomas from laying hens. Gynecol Oncol. 2007;104(1):192–198. doi: 10.1016/j.ygyno.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 144.Rodriguez-Burford C, Barnes MN, Berry W, Partridge EE, Grizzle WE. Immunohistochemical expression of molecular markers in an avian model: A potential model for precliical evaluation of agents for ovarian cancer chemoprevention. Gynecol Oncol. 2001;81(3):373–379. doi: 10.1006/gyno.2001.6191. [DOI] [PubMed] [Google Scholar]
- 145.Gonzalez Bosquet J, Peedicayil A, Maguire J, et al. Comparison of gene expression patterns between avian and human ovarian cancers. Gynecol Oncol. 2011;120(2):256–264. doi: 10.1016/j.ygyno.2010.10.030. [DOI] [PubMed] [Google Scholar]
- 146.Trevino LS, Giles JR, Wang W, Urick ME, Johnson PA. Gene expression profiling reveals differentially expressed genes in ovarian cancer of the hen: Support for oviductal origin? Horm Cancer. 2010;1(4):177–186. doi: 10.1007/s12672-010-0024-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Li X, Chiang HI, Zhu J, Dowd SE, Zhou H. Characterization of a newly developed chicken 44K agilent microarray. BMC Genomics. 2008;9(60) doi: 10.1186/1471-2164-9-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Burnside J, Neiman P, Tang J, et al. Development of a cDNA array for chicken gene expression analysis. BMC Genomics. 2005;6(1):13. doi: 10.1186/1471-2164-6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Li Q, Li N, Hu X, et al. Genome-wide mapping of DNA methylation in chicken. PloS One. 2011;6(5) doi: 10.1371/journal.pone.0019428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.NCBI. Gallus gallus (chicken) genome. 2013 http://www.ncbi.nlm.nih.gov/genome?term=gallus%20gallus.
- 151.Nature. Focus: Chicken Genome. 2004 http://www.nature.com/nature/focus/chickengenome/
- 152.Trevino LS, Buckles EL, Johnson PA. Oral contraceptives decrease the prevalence of ovarian cancer in the hen. Cancer Prev Res. 2012;5(2):343–349. doi: 10.1158/1940-6207.CAPR-11-0344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Hoekstra A, Rodriguez GC. Chemoprevention of ovarian cancer. Cancer Treat Res. 2009;149(2):3–34. doi: 10.1007/978-0-387-98094-2_1. [DOI] [PubMed] [Google Scholar]
- 154.Urick ME, Giles JR, Johnson PA. Dietary aspirin decreases the stage of ovarian cancer in the hen. Gynecol Oncol. 2009;112(1):166–170. doi: 10.1016/j.ygyno.2008.09.032. [DOI] [PubMed] [Google Scholar]
- 155.Ansenberger K, Richards C, Zhuge Y, et al. Decreased severity of ovarian cancer and increased survival in hens fed a flaxseed-enriched diet for 1 year. Gynecol Oncol. 2010;117(2):341–347. doi: 10.1016/j.ygyno.2010.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Barua A, Bradaric MJ, Edassery SL, et al. Anti-tumor antibodies and ovarian cancer in women and hens. AACR International Conference; 2006. [Google Scholar]
- 157.Barua A, Edassery SL, Bitterman P, et al. Prevalence of antitumor antibodies in laying hen model of human ovarian cancer. Int J Gynecol Cancer. 2009;19(4):500–507. doi: 10.1111/IGC.0b013e3181a39db1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Giles JR, Shivaprasad HL, Johnson PA. Ovarian tumor expression of an oviductal protein in the hen: A model for human serous adenocarcinoma. Gynecol Oncol. 2004;95(3):530–533. doi: 10.1016/j.ygyno.2004.07.061. [DOI] [PubMed] [Google Scholar]
- 159.Barua A, Bitterman P, Bahr JM, et al. Detection of tumor-associated neoangiogenesis by doppler ultrasonography during early-stage ovarian cancer in laying hens. J Ultrasound Med. 2010;29(2):173–182. doi: 10.7863/jum.2010.29.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Giles JR, Olson LM, Johnson PA. Characterization of ovarian surface epithelial cells from the hen: A unique model for ovarian cancer. Experimental Biology and Medicine. 2006;231(11):1718–1725. doi: 10.1177/153537020623101108. [DOI] [PubMed] [Google Scholar]
- 161.Kong BW, Carlson DF, Fahrenkrug SC, Foster DN. Application of the sleeping beauty transposon system to avian cells. Anim, Genet. 2008;39(2):180–186. doi: 10.1111/j.1365-2052.2008.01702.x. [DOI] [PubMed] [Google Scholar]
- 162.Tseng CL, Peng CL, Huang JY, Cheng JC, Lin FH. Gelatin nanoparticles as gene carriers for transgenic chicken applications. J Biomater Appl. 2012 doi: 10.1177/0885328211434089. [DOI] [PubMed] [Google Scholar]
- 163.Neve RM, Chin K, Fridlyand J, et al. A collection of breast cancer cell lines for the study of functionally distinct cancer subtypes. Cancer Cell. 2006;10:515–527. doi: 10.1016/j.ccr.2006.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.